Abstract
BALB/c interleukin-4 (IL-4−/−) or IL-4 receptor-α (IL-4rα−/−) knockout (KO) mice were used to assess the roles of the IL-4 and IL-13 pathways during infections with the blood or liver stages of plasmodium in murine malaria. Intraperitoneal infection with the blood-stage erythrocytes of Plasmodium berghei (ANKA) resulted in 100% mortality within 24 days in BALB/c mice, as well as in the mutant mouse strains. However, when infected intravenously with the sporozoite liver stage, 60 to 80% of IL-4−/− and IL-4rα−/− mice survived, whereas all BALB/c mice succumbed with high parasitemia. Compared to infected BALB/c controls, the surviving KO mice showed increased NK cell numbers and expression of inducible nitric oxide synthase (iNOS) in the liver and were able to eliminate parasites early during infection. In vivo blockade of NO resulted in 100% mortality of sporozoite-infected KO mice. In vivo depletion of NK cells also resulted in 80 to 100% mortality, with a significant reduction in gamma interferon (IFN-γ) production in the liver. These results suggest that IFN-γ-producing NK cells are critical in host resistance against the sporozoite liver stage by inducing NO production, an effective killing effector molecule against Plasmodium. The absence of IL-4-mediated functions increases the protective innate immune mechanism identified above, which results in immunity against P. berghei infection in these mice, with no major role for IL-13.
Both cell-mediated immunity and humoral immunity play important roles in the mechanisms of defense against human and animal malaria. These defense mechanisms largely depend on early innate responses and antigen-specific T helper (Th) cell activation. The importance of the Th1 cytokine gamma interferon (IFN-γ) for protection against malaria has been shown in murine models of malaria (1, 11, 47, 50, 54, 55, 57), with vaccinated animals lacking IFN-γ showing greatly increased susceptibility, increased parasitemia, and a shorter life span.
The role of nitric oxide (NO) in the defense against malaria infection seems to be dependent on the stage of the malaria parasite. In a blood-stage infection with Plasmodium berghei, it was shown that neither NO nor NK cells were important for the control of parasitemia (12, 55). In contrast, compared to the liver-phase stage of sporozoite infection, NK cells were shown to be activated by sporozoites (37) and produced IFN-γ (31, 37). A clear role for NO as a defense mechanism against the liver stage of P. berghei was found only in vaccinated animals (25, 36, 45, 48).
Concerning Th2 response, it was shown that a lack of interleukin-4 (IL-4) in the murine infection with Plasmodium chabaudi chabaudi results in a course of infection similar to that seen in the wild-type mice (2, 50, 51), with slightly different parasitemia kinetics. Moreover the in vitro Th1 immune responses were sustained in the IL-4-deficient mice (51). All of these studies were done by infection of mice with parasitized erythrocytes, but no study has been performed that focused on the role of IL-4 during the liver stage of a malaria infection. When the role of IL-4 for the Th2 immune response is to be analyzed, it is important to take into account the possible involvement of IL-13. Indeed, IL-4 has many overlapping functions with IL-13 (5, 58), since both cytokines use the IL-4 receptor α (IL-4rα) chain, with IL-4 able to signal via the type 1 IL-4r, consisting of IL-4rα and -γ chains or the type 2 IL-4r, consisting of the IL-4rα and IL-13rα chains (21, 34). IL-4rα−/− mice were unresponsive to both IL-4 and IL-13, as recently demonstrated (32). Comparative infection studies using IL-4rα−/− and IL-4−/− mice showed distinctive IL-13 functions in several experimental models, including leishmaniasis (27, 32), schistosomiasis (33), and other helminth infections (3). A possible role of the IL-13 pathway has never been analyzed in murine malaria infection.
It is well known, especially for vaccine development, that the most important defense mechanism of the host against malaria is the early immune response against the liver stages (10, 13, 18, 22, 30). In the present report, studies were performed with sporozoites that were dissected from the intermediate host, the Anopheles stephensi mosquito. This study used mice deficient for IL-4 or IL-4rα. By infecting naive mice with sporozoites, the importance of IL-4 and IL-4rα for the control of the liver and the blood stage of a P. berghei infection was analyzed. The use of sporozoites has the advantage of allowing examination of the very early immune response in the liver and more closely mimics the natural infection cycle.
We report that IL-4−/− or IL-4rα−/− knockout (KO) mouse strains are more resistant and able to survive P. berghei infection when infected with sporozoites. Evidence for the protective mechanism is provided and involves increased NK-mediated IFN-γ production and NO killing effector function in the livers of IL-4- and IL-4rα-deficient mice.
(This work was part of the diploma thesis of Sandra Arriens.)
MATERIALS AND METHODS
Mice.
Six- to eight-week-old female BALB/c mice, BALB/c IL-4−/− mice (26) and BALB/c IL-4rα−/− (32) maintained under specific-pathogen-free conditions were used for all experiments.
Infection with P. berghei.
The P. berghei ANKA strain was used in all experiments. It was maintained by periodic passages through the vector A. stephensi. For infection with sporozoites, mosquitoes were prepared that had been infected from the identical frozen aliquots of parasites. Mice were infected by intravenous (i.v.) injection into the tail of a phosphate-buffered saline (PBS) suspension of 400 or 800 sporozoites per animal. For infection with parasitized erythrocytes, mice were infected with the identical frozen aliquots of parasite. The percentage of parasitemia was calculated by examining Giemsa-stained smears under a microscope with an oil immersion lens (×1,000). The parasitized blood was diluted in PBS and injected intraperitoneally (i.p.) into naive groups of mice.
Flow cytometric analysis.
After liver perfusion with 1 ml of RPMI 1640 (Gibco, Eggenstein, Germany), the liver was removed, and the cells were gently dissociated from the tissue matrix and flushed through a 0.2-mm-pore-diameter sieve in a petri dish. The cell suspension was collected without debris and layered over a Ficoll gradient (Biochrom KG, Berlin, Germany) with a density of 1.077. Centrifugation was performed for 20 min at 1,200 × g. The white layer containing the lymphocytes was removed and washed several times with RPMI 1640 (Gibco). The cells were adjusted to 1 × 106 cells/ml.
One hundred microliters was incubated with a 1:100 dilution of a fluorescein isothiocyanate (FITC)-conjugated rat monoclonal antibody specific for CD4 (YTS 191.1) and an R-phycoerythrin-conjugated anti-CD8 antibody (YTS 169.4) (both from Medac, Hamburg, Germany). Double staining was performed with a 1:100 dilution of an anti-DX5 R-phycoerythrin-conjugated antibody (DX5) and an anti-CD3e FITC-conjugated antibody (145-2C11) (both from Pharmingen, Heidelberg, Germany) to detect DX5+/CD3− NK cells and DX5+/CD3+ T cells. For B-cell staining, an R-phycoerythrin-conjugated rat monoclonal antibody specific for CD45R (RA3-7B2) was used. An FITC-conjugated CD11b (M1/70.15) antibody was used as macrophage marker (both from Medac, Hamburg, Germany). The FITC-conjugated CD11b (M1/70.15) macrophage marker was used to define single-positive (CD45R) B cells by exclusion.
The samples were measured by fluorescence-activated cell sorting (FACS) with a FACScan (Becton Dickinson, Heidelberg, Germany) and analyzed with Cell Quest software (Becton Dickinson).
Immunohistological staining of iNOS in the liver.
Livers were removed 3 days postinfection (p.i.) and fixed in 10% buffered formaldehyde. The livers were embedded in paraffin and cut into 4-μm-thick sections with a microtome (Reichert-Jung, Hamburg Germany). In order to block nonspecific binding of antibodies to FcγRII or -III, sections were incubated for 1 h with anti-CD16/CD32 antibody (5 μg/ml; Pharmingen, Heidelberg, Germany) in 1% (wt/vol) bovine serum albumin (BSA) in PBS. To detect inducible NO synthase (iNOS), paraffin sections were incubated with rabbit antiserum to murine iNOS enzyme (Alexis, Gruenberg, Germany) at 1:500 in a mixture of 1% BSA and 0.05 M PBS at room temperature in a humidified chamber for 1 h. Thereafter, biotinylated goat anti-rabbit immunoglobulin (1:100; Calbiochem, Schwalbach, Germany) was added for 1 h. The color reaction was developed after incubation with diluted alkaline phosphatase-conjugated streptavidin (1:100; Calbiochem) using Sigma Fast Red (Sigma, Munich, Germany), counterstained with hematoxylin for 15 min, and destained with tap water. For analyses, liver sections were examined for positive iNOS staining with a light microscope at a magnification of ×100.
PCR analyses of P. berghei parasites in the blood and liver.
Blood was collected by orbital puncture from mice that survived P. berghei infection. Fifty microliters of blood was taken to extract DNA with the Qiagen Dneasy blood kit (Qiagen, Hilden, Germany). As a positive control, we took blood from a mouse with 70% parasitemia. The concentration of DNA was calculated with the help of a photometer. DNA was adjusted to 0.01 μg/μl, and 4 μl was taken for PCR. The primers for P. berghei detection were specific for the small subunit of the P. berghei rRNA gene (M14599) with the following sequences: P1 (forward), 5′ CACGCGTGCTACACTGATATG; and P2 (reverse), 5′ AGGCATTCCTCGTTCAAGATT.
The primers amplify a 177-bp fragment.
PCR was run with the following program in a 50-μl reaction tube with Hot Star Taq DNA polymerase from Qiagen, after 15 min at 95°C to activate the enzyme: 35 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 30 s.
We analyzed hepatic parasites by reverse transcriptase PCR (RT-PCR) using the primers for P. berghei described above.
Primers for β-actin were used to control the efficiency of the reverse transcription and had the following sequences: B1 (forward), 5′ ATGGATGACGATATCGCT; and B2 (reverse), 5′ ATGAGGTAGTCTGTCAGGT.
The primers amplify a 568-bp fragment.
In detail, livers were dissected 42 h p.i., and each liver was cut into three pieces of the same size and immediately frozen in liquid nitrogen. The livers were homogenized in Trizol (Life Technologies, Karlsruhe, Germany) with 1 ml of Trizol per 100 mg of liver by using a homogenizer (Braun, Melsungen, Germany). Total RNA was purified according to the standard protocol specified for Trizol by the manufacturer and stored at −70°C.
The RNA was quantified, and 2 μg of RNA was reverse transcribed with Omniscript (Qiagen, Hilden, Germany) and 500 ng of random hexamer (Life Technologies) according to the manufacturer's protocol. Four microliters of the template DNA was amplified with Hot Star Taq DNA polymerase (Qiagen) in a 50-μl reaction volume following the standard protocol supplied by the company. Reactions were carried out with the following programs for 35 cycles, after 15 min at 95°C to activate enzyme in a thermal cycler (Biometra, Göttingen, Germany): for P. berghei, 94°C at 30 s, 55°C for 30s, and 72°C for 30 s; and for β-actin, 94°C for 30 s, 56°C for 30 s, and 72°C for 30 s.
Lightcycler real-time PCR for quantitative analyses of iNOS mRNA expression.
The principle of TaqMan quantitative real-time PCR has been described in detail (17). After liver perfusion with 1 ml of RPMI 1640 (Gibco), the liver was removed and snap frozen in liquid nitrogen. The mRNA extraction was done with the RNeasy kit (Qiagen). The extracted liver mRNA was transcribed into cDNA with the Omniscript kit (Qiagen). One PCR unit was defined as the amount of cDNA that yields 50% positive samples in a limiting dilution PCR. The PCR units of β-actin and iNOS mRNA in the samples were calculated by standard curves of β-actin and iNOS. The ratio between the iNOS units and the β-actin units of each sample was then calculated. Each group of mice analyzed contained four animals. Significant differences (P < 0.05) between the groups were calculated by Mann-Whitney U test.
The sequences for all primers and fluorescently labeled probes used in this study are as follows: β-actin forward primer, 5′ AGAGGGAAATCGTGCGTGAC, and reverse primer, 5′ CAATAGTGATGACCTGGCCGT; β-actin hybridization probe, 5′ CACTGCCGCATCCTCTTCCTCCC; iNOS forward primer, 5′ CAGCTGGGCTGTACAAACCTT, and reverse primer, 5′ CATTGGAAGTGAAGCGTTTCG; and iNOS hybridization probe, 5′ CGGGCAGCCTGTGAGACCTTT.
PCR was performed in a 12.5-μl reaction mixture that contained 0.05 ng of the reverse-transcribed cDNA, 3.75 pM forward primer, 3.75 pM reverse primer, and 1.25 pM fluorescent hybridization probe. For each sample, two PCRs were performed. The resulting relative increase in reporter fluorescent dye emission was monitored by the Lightcycler system (Roche, Mannheim, Germany).
Detection of IFN-γ in the liver.
The liver was removed after liver perfusion with 1 ml of RPMI 1640 (Gibco) and homogenized with a pestle in a petri dish. The homogenized liver was resuspended in 2 ml of PBS and centrifuged. IFN-γ concentrations in the supernatant were detected by specific two-site enzyme-linked immunosorbent assay (ELISA) from Becton Dickinson. Purified R4-6A2 was used as a capture antibody in combination with biotinylated XMG1.2 for detection. The ELISA was developed after incubation with streptavidin-peroxidase complex (1:5,000; Roche), using 3,5,3′,5′-tetramethylbenzidine as a substrate (Roth, Karlsruhe, Germany [dissolved at 6 mg/ml in dimethyl sulfoxide]). The sensitivity was 20 pg/ml.
Treatment with L-NIL or aminoguanidine.
Mice were treated with 3 mM l-N6-(1-iminoethyl)-lysine (L-NIL) (Alexis) or 90 mM aminoguanidine (Sigma, Munich, Germany) in drinking water. This was the only source of fluid intake during the treatment period. The treatment was started 1 day before the infection and ended 2 days p.i. The period of treatment was correlated with the time period of the liver stage of P. berghei, which ends 50 h p.i.
Depletion of NK cells.
The treatment with anti-asialo GM1 antibody (Wako BioProducts, Richmond, Va.) to deplete NK cells was started 1 day before the infection. Each mouse was treated once with 20 μl of the rabbit serum stock solution i.v. diluted 1/5 in 1× PBS. Control mice were given normal rabbit serum i.v. FACS analyses of the liver 3 days after treatment with anti-asialo GM1 antibody and 2 days after infection with 400 sporozoites showed a significant decrease of NK cells in comparison to infected control groups (see Table 3). Analyses of NK cell depletion in the blood 1 day after anti-asialo GM1 antibody treatment resulted in reduction (>90%) in NK cells in comparison to the level in control groups (data not shown).
TABLE 3.
Analyses of NK cell depletion with anti-asialo GM1 antibody in the liver 2 days after infection with 400 sporozoites
| Mouse straina | % of NK cells (CD3+/DX5+) | Total no. of NK cellsb |
|---|---|---|
| BALB/c | ||
| Uninfected | 2.5 ± 0.6 | 4.2 ± 1.7† |
| Infected | 3.0 ± 0.2 | 4.4 ± 0.8* |
| Infected + anti-asialo GM1 | 1.3 ± 0.5 | 2.0 ± 0.6*† |
| IL-4−/− | ||
| Uninfected | 3.0 ± 0.6 | 5.3 ± 1.7‡ |
| Infected | 5.2 ± 2.1 | 8.9 ± 3.9*‡ |
| Infected + anti-asialo GM1 | 3.5 ± 0.2 | 5.4 ± 0.5* |
| IL-4rα−/− | ||
| Uninfected | 2.9 ± 0.6 | 5.1 ± 1.4‡ |
| Infected | 4.2 ± 1.0 | 7.4 ± 0.8*‡ |
| Infected + anti-asialo GM1 | 2.5 ± 0.6 | 4.6 ± 0.8* |
Each group contains four animals.
*, significantly different from the infected but normal rabbit serum-treated strain; †, significantly different from the uninfected and normal rabbit serum-treated strain; ‡, significantly different between the infected and uninfected groups (P < 0.05, Mann-Whitney U test).
Statistical analysis.
Statistical analyses were performed by Mann-Whitney U test or Kaplan-Meier test. P values of <0.05 were considered statistically significant.
RESULTS
Resistance of IL-4−/− and IL-4rα−/− mice to the sporozoite stage but not the blood stage of P. berghei infection.
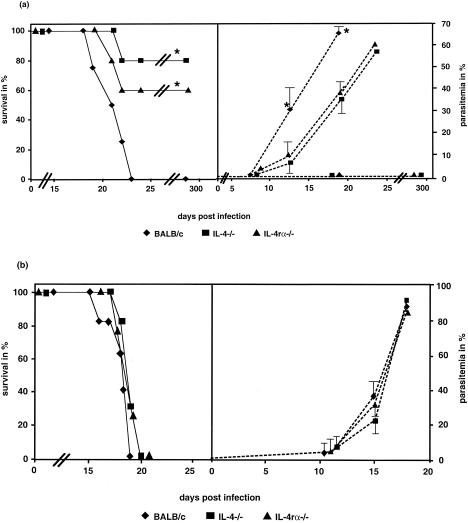
To determine whether Th2 deficiency affects resistance to blood-stage malaria, wild-type mice as well as IL-4 and IL-4rα−/− mice were infected i.v. with 400 or 800 sporozoites or i.p. with 102, 104, or 106 parasitized erythrocytes from P. berghei, and the course and outcome of the infection were compared. After i.v. infection with 400 sporozoites, the wild-type mice developed high parasitemia (Fig. 1a), and all wild-type mice succumbed within 23 days p.i. (Fig. 1a). In contrast, 4 of 5 IL-4−/− mice and 3 of 5 IL-4rα−/− mice survived (Fig. 1a). The IL-4−/− and IL-4rα−/− mice that survived the infection developed no detectable parasitemia (Fig. 1a) during infection. Analyses of the blood from these survivors with PCR confirmed the absence of parasites (data not shown). Those KO animals that succumbed developed parasitemia (Fig. 1a), but showed an increased life span compared tothe wild-type mice (Fig. 1a). Infection with 800 sporozoites resulted in death of all mouse groups without any significant differences in the survival rate or parasitemia (Fig. 1b). These results point out the small window of differential outcome in the absence of IL-4 function. Analyses of hepatic parasites by RT-PCR 42 h after an infection dose of 400 sporozoites resulted in no positive signal in all groups of mice (data not shown).
FIG. 1.
Survival (solid lines) and parasitemia (dotted lines) of mice infected with the P. berghei ANKA strain. The mice were infected i.v. in the tail with 400 (a) or 800 (b) sporozoites. The data are representative of two independent experiments with five animals per group. (a) The wild-type BALB/c mice showed a 100% mortality rate, while 80% of the IL-4−/− mice and 60% of the IL-4rα−/− mice survived the infection. PCR analyses of the blood from mice that survived the infection showed that no parasites were detectable after 20 days p.i. (data not shown). Statistical analyses of the survival rates were done with the Kaplan-Meier test, and differences were found between the wild-type control versus the IL-4−/− and IL-4 α−/− mice (P < 0.05). The values for parasitemia represent the arithmetic means ± standard deviations. The susceptible animals died and had a high parasitemia (up to 70% parasitized erythrocytes). The BALB/c group showed a significantly higher parasitemia at 13 and 18 days p.i. than the KO groups. (b) All groups of died with high parasitemia, and there were no significant differences between their survival rates and parasitemia. The sporozoites were prepared from one batch of infected A. stephensi mosquitoes. All groups of mice were infected with this batch to avoid differences in one experiment due to different viabilities of the sporozoites.
Together, these results show that the absence of IL-4 or its receptor leads to increased resistance to the sporozoite form of P. berghei infection.
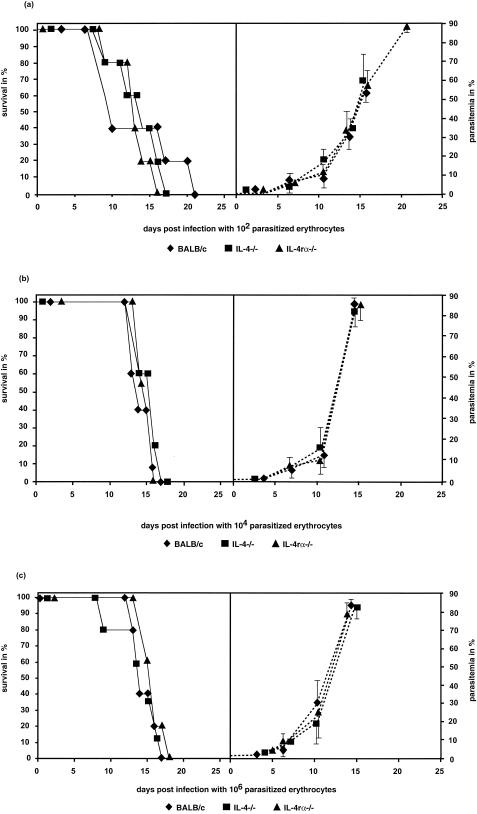
No significant difference in the survival rates was detectable during an infection with 102, 104, or 106 parasitized erythrocytes (Fig. 2). All animals succumbed within 21 days p.i., concomitant with high parasitemia, demonstrating that the absence of IL-4 or IL-4r and IL-13r was not beneficial for resistance against an infection with parasitized erythrocytes of P. berghei. Comparison of the parasitemia shown in Fig. 1a with that in Fig. 2 showed significantly higher parasitemia on days 13 and 18 p.i. in wild-type mice than in KO mouse strains when the infection was done with sporozoites (Fig. 1a). However, no significant differences in parasitemia were found during the time course of infections as determined during three different infection doses with parasitized erythrocytes (Fig. 2).
FIG. 2.
Survival (solid lines) and parasitemia (dotted lines) of BALB/c, IL-4−/−, and IL-4rα−/− mice infected with 102 (a), 104 (b), or 106 (c) parasitized erythrocytes of the P. berghei ANKA strain. All groups showed a 100% mortality rate. Increases in the infection dose led to higher parasitemia (b and c) but not to significantly earlier death due to cerebral malaria. Statistical analyses of the survival rates were done with the Kaplan-Meier test. No differences were found between the wild-type control versus the IL-4−/− and IL-4rα−/− mice. Each group contained five animals. The data are representative of two independent experiments. The parasitized erythrocytes were prepared from one highly infected BALB/c mouse. All groups of mice were infected with this batch to avoid differences in the survival rates in one experiment due to different viabilities of the parasites.
Resistance of IL-4−/− and IL-4rα−/− mice is associated with higher numbers of NK cells in the liver.
The liver is the first target during a sporozoite infection, and the liver phase lasts a very short period of time (50 h) (28). Under the assumption that the immune cell populations in the liver 48 h p.i. (at the end of the liver stage) may be important for parasite control, lymphocytes were analyzed after liver perfusion and compared with the lymphocyte population in the livers from uninfected mice. The total number of cells isolated from each liver was multiplied by the percentage of each cell type determined by FACS in order to obtain the respective total numbers of the subpopulations.
No significant differences in the numbers of NK cells in the liver were detectable between all groups of uninfected animals (Table 1). The population of the NK cells known to be activated by sporozoites (37) was shown to be significantly increased in the livers of the IL-4−/− (9.5 × 104 cells) and IL-4rα−/− (8.4 × 104 cells) mice 2 days p.i. (Table 1) compared to that in the uninfected IL-4−/− (5.3 × 104 cells) and IL-4rα−/− (5.1 × 104 cells) mice. The NK cell population was also significantly higher in the infected IL-4−/− (9.5 × 104 cells) and IL-4rα−/− (8.4 × 104 cells) mice than in the infected wild-type control (5.8 × 104 cells) mice (Table 1). No significant differences were detectable between the populations of CD4+ T cells, CD8+ T cells, B cells, and NK T cells in all strains of mice.
TABLE 1.
Number of lymphocytes in the liver
| Cell type | Mouse straina | Mean ± SD no. of cells (104)b
|
|
|---|---|---|---|
| Uninfected mice | 2 days p.i. | ||
| CD4 | BALB/c | 42.7 ± 6.5 | 44.9 ± 11.4 |
| IL-4−/− | 46.2 ± 17.8 | 42.7 ± 8.6 | |
| IL-4rα−/− | 45.2 ± 11.7 | 41.7 ± 4.4 | |
| CD8 | BALB/c | 4.7 ± 0.9 | 6.5 ± 1.9 |
| IL-4−/− | 5.1 ± 2.8 | 4.4 ± 1.9 | |
| IL-4rα−/− | 4.9 ± 2.5 | 4.6 ± 0.8 | |
| NK cells (CD3− DX5+) | BALB/c | 4.2 ± 1.7 | 5.8 ± 1.6 |
| IL-4−/− | 5.3 ± 1.7 | 9.5 ± 3.1* | |
| IL-4rα−/− | 5.1 ± 1.4 | 8.4 ± 0.3* | |
| NK T cells (CD3+/DX5+) | BALB/c | 24.8 ± 9.4 | 22.1 ± 7.1 |
| IL-4−/− | 25 ± 8.5 | 15.4 ± 4.1 | |
| IL-4rα−/− | 26.6 ± 14.3 | 17.7 ± 2.7 | |
| B cells | BALB/c | 13.9 ± 5.1 | 12.5 ± 5.2 |
| IL-4−/− | 15.8 ± 11.7 | 12.4 ± 6.6 | |
| IL-4rα−/− | 16.9 ± 13.4 | 10.2 ± 3.3 | |
Each group contains four animals.
*, significantly different from the uninfected mice strains and infected BALB/c mice (P < 0.05, Mann-Whitney U test).
iNOS expression in the liver correlates with resistance of KO mice.
Expression of iNOS in livers differed between the wild-type and the KO mice. In wild-type controls, the numbers of iNOS-positive cells per objective field counted were significantly lower than those in the KO groups (Table 2).
TABLE 2.
Number of iNOS-positive cells per objective field at a magnification of ×100
| Expt | Mouse strain | Mean no of iNOS-positive cells | No. of objective fields counted | Quotient (mean no. of iNOS-positive cells/objective field ± SD)a |
|---|---|---|---|---|
| P. berghei infection | BALB/c | 4 | 56 | 0.07 ± 0.123 |
| IL-4−/− | 34 | 35 | 1.1 ± 0.333* | |
| IL-4rα−/− | 33 | 33 | 1.1 ± 0.644* | |
| NK cell depletion + P. berghei infection | BALB/c | 0 | 29 | 0 |
| IL-4−/− | 0 | 35 | 0 | |
| IL-4rα−/− | 0 | 28 | 0 |
*, significantly different from BALB/c control mice (P < 0.05, Mann-Whitney U test).
Analysis of liver mRNA by real-time PCR supported the histological data. At day 3 p.i., IL-4rα−/− mice showed a significantly higher iNOS/β-actin ratio of 6.57 × 106 ± 2.8 × 106 than the wild-type ratio of 2.33 × 106 ± 1.4 × 106. IL-4−/− mice showed a significantly higher mean ratio of 9.45 × 106 ± 3.0 × 106 than the wild-type mean ratio of 3.22 × 106 ± 2.4 × 106 1 day p.i., with each group containing four animals. Differences due to histology demonstrated a much clearer result than PCR analyses, since mRNA levels and expressed proteins do not correlate 1:1.
Blockade of NO production during the liver stage of infection reverses resistance of KO mice.
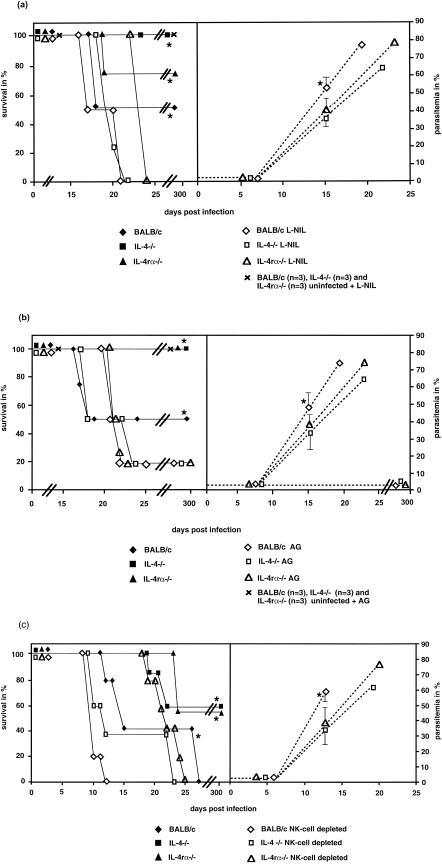
To verify if NO was responsible for the higher resistance of the KO mice, we treated mice with the iNOS inhibitor L-NIL or aminoguanidine prior to i.v. infection with 400 sporozoites. Untreated but infected IL-4−/−, IL-4rα−/−, and BALB/c mice showed 100, 75, and 50% survival, respectively. In contrast, all L-NIL-treated mouse strains died (Fig. 3a). Similar results were observed by using aminoguanidine instead of L-NIL (Fig. 3b). Infected IL-4−/−, IL-4rα−/−, and BALB/c mice, which were not treated with aminoguanidine, showed a 100% survival rate for the KO groups and a 50% survival rate for the BALB/c mice. Treatment with aminoguanidine results in 20% survival in all groups of mice (Fig. 3b). The mice that survived the infection had no detectable parasitemia in the blood. A reason for the lower mortality rate of aminoguanidine-treated mice compared to L-NIL-treated mice may be the lower infectivity rate or the weaker specificity of aminoguanidine to block NO in comparison to L-NIL. The levels of parasitemia in L-NIL (Fig. 3a)- and aminoguanidine (Fig. 3b)-treated KO mice are significantly lower at day 15 p.i. than those in NO-blocked BALB/c mice. No significant differences concerning the parasitemia were detectable at 8 days p.i. in L-NIL- and aminoguanidine-treated mice (Fig. 3a and b).
FIG.3.
Survival (solid lines) and parasitemia (dotted lines) of BALB/c, IL-4−/−, and IL-4rα−/− mice i.v. infected with 400 sporozoites and treated with L-NIL (a) or aminoguanidine (AG) (b) 1 day before infection until 2 days p.i. All animals treated with L-NIL died (a). Statistical analyses of the survival rates were done with the Kaplan-Meier test, and significant differences were found between the L-NIL-treated groups and the untreated groups. Similar results were observed by using aminoguanidine instead of L-NIL (b). The survival rate of the untreated BALB/c wild type mice in this experiment was 50%. This result was different from the survival rates in the experiments shown in Fig. 1, due to lower infectivity of this sporozoite preparation, and represents the interexperimental variation. Analyses of the parasitemia from the L-NIL (a)- or aminoguanidine (b)-treated groups of mice showed no significant differences at day 8 p.i. The BALB/c mouse group treated with L-NIL (a) or aminoguanidine (b) developed a significantly higher parasitemia at day 15 p.i. than the L-NIL (a)- or aminoguandine (b)-treated KO groups. Each group contained four animals. The data are representative of two independent experiments. The sporozoites were prepared from one batch of infected A. stepehensi mosquitoes. All groups of mice were infected with this batch to avoid differences in the survival rates in one experiment due to different viabilities of the sporozoites. (c) Survival (solid lines) and parasitemia (dotted lines) of BALB/c, IL-4−/−, and IL-4rα−/− mice i.v. infected with 400 sporozoites. Depletion of NK cells was achieved by i.v. treatment with anti-asialo GM1 antibody 1 day before the infection. Control groups of mice were given normal rabbit serum. Parasitemia of anti-asialo GM1 antibody-treated BALB/c mice demonstrated no significant differences at day 8 p.i. versus NK cell-depleted KO groups. NK cell-depleted BALB/c mice developed a significantly higher parasitemia at day 12 p.i. than the NK cell-depleted KO mouse groups. Statistical analyses of the survival rates were done with the Kaplan-Meier test. The NK cell-depleted groups showed significantly higher lethality than the normal rabbit serum-treated groups. Each group contained five animals. The data are representative of two independent experiments. The sporozoites were prepared from one batch of infected A. stepehensi mosquitoes. All groups of mice were infected with this batch to avoid differences in the survival rates in one experiment due to different viabilities of the sporozoites.
In summary, the treatment with the iNOS inhibitors L-NIL and aminoguanidine showed that NO plays a key function during resistance against the liver stage of P. berghei.
Depletion of NK cells in the KO mice results in increased susceptibility.
To investigate whether NK cells play an important role in the defense mechanism against the liver stage of P. berghei, we depleted NK cells in vivo with anti-asialo GM1 antibody 1 day prior to infection. More than 90% of blood NK cells were depleted at the day of infection (data not shown). Analyses of liver NK cells 2 days after infection with 400 sporozoites demonstrated a significant inhibition of NK cell increase in the treated IL-4−/− and IL-4rα−/− groups (Table 3) compared to infected but normal rabbit serum-treated mutant mice. Infected and treated wild-type mice showed a decrease in NK cells in the liver in comparison to the infected and uninfected wild-type groups (Table 3).
NK cell-depleted wild-type mice died significantly earlier than the normal rabbit serum-treated wild-type controls (Fig. 3c). All NK cell-depleted KO mice died, while 60% of the normal rabbit serum-treated KO mice survived infection. NK cell-depleted KO mice had a significantly higher parasitemia at day 12 p.i. compared to NK cell-depleted BALB/c mice (Fig. 3c), while parasitemia at day 6 p.i. was not significantly different (Fig. 3c).
Taken together, these data strongly suggest that NK cells are the important cellular mediators for increased resistance of KO mice against malaria.
NK cell in vivo depletion leads to a drastic reduction of liver IFN-γ and iNOS expression.
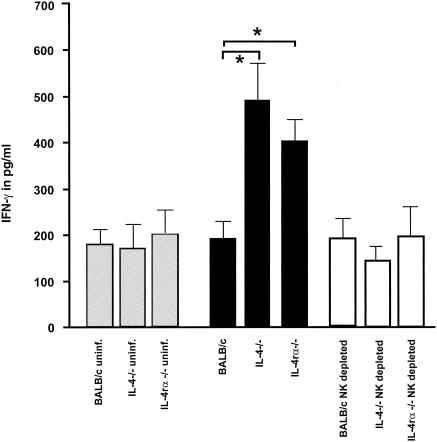
Induced NO production by classical macrophages and other cells is catalyzed by iNOS and crucially dependent on IFN-γ activation (23). Since NK cells are one of the main IFN-γ producers, it is possible that the increased susceptibility observed during NK depletion is triggered by the subsequent lack of IFN-γ production. In order to determine this possibility directly, IFN-γ concentrations were determined ex vivo in liver homogenates. At 3 days p.i., significantly higher IFN-γ concentrations were observed in the livers of non-NK-depleted KO mice than in those from non-NK-depleted wild-type mice (Fig. 4). NK depletion resulted in a reduction of IFN-γ concentration in KO mouse strains only (Fig. 4). These results suggest that the absence of IL-4, IL-4rα, or IL-13rα leads to enhanced IFN-γ production.
FIG. 4.
Detection of IFN-γ in the liver 3 days p.i. in mice infected with 400 sporozoites. The KO mouse groups showed significantly higher concentrations of IFN-γ than the wild-type BALB/c mice, while the NK cell-depleted KO groups demonstrated a significant reduction in IFN-γ in the liver. Uninfected mice of all groups had only a low concentration of IFN-γ in the liver. The data are representative of two independent experiments.
In addition, immunohistological analysis of livers 3 days p.i. showed that iNOS expression was absent in NK-depleted compared to untreated mice (Table 2). Together, these results demonstrate the importance of IFN-γ for protection against the P. berghei liver stage of infection and suggested that NK cells might play a role in IFN-γ up-regulation in the liver.
DISCUSSION
In this report, evidence is provided that IL-4 is a susceptibility factor in P. berghei infections with sporozoites, the natural infective stage of the parasite. IL-4−/− or IL-4rα−/− mice both showed increased resistance to P. berghei compared to BALB/c controls, with reduced parasite burden and increased survival when infected with sporozoites i.v. This was in striking contrast to i.p. infections with parasitized erythrocytes, where neither a difference in parasitemia nor life span was observed between IL-4−/− or IL-4rα−/− mice or BALB/c controls. This differential outcome, depending on the use of parasitized erythrocytes or sporozoites, significantly extends the findings of earlier studies with i.p.-injected parasitized erythrocytes of P. chabaudi, in which no differences between BALB/c IL-4−/− mice and controls were reported (2, 50, 51). The data underscore that it is important to examine the immune mechanisms in the liver. The data in this study indicate that these mechanisms could be responsible for the observed increased resistance of the IL-4−/− and IL-4rα−/− mice.
IL-4rα-deficient mice are impaired in both IL-4 and IL-13 functions (32), whereas IL-4-deficient mice are responsive to IL-13. Since no differences between the two strains regarding host protection against P. berghei were observed, one may conclude that the IL-13-mediated effects via IL-4rα do not play a major role in the disease outcome. However, NK cell-depleted IL-4rα−/− mice survived significantly longer than the NK cell-depleted IL-4−/− mice, which may indicate that IL-13 does have some detrimental effects in the absence of NK cells.
Especially CD8+ T cells play an important role in vaccination studies in immunity, targeting the liver stages of the disease and preventing the development of the pathogenic blood stages (19, 20, 39, 43, 45, 52). Increased protection of mice was achieved by coadministration of dendritic cell-derived CC chemokine 1 with irradiated sporozoites (7). Earlier studies showed that IL-4-producing CD4 T cells in the liver were important for the development of a protective CD8+ T-cell response against the malaria liver stage (8). The liver stage antigen-activated CD8+ T-cell population inhibits the priming of naive CD8+ T cells (16).
These studies do not contrast with our results, because in vaccinated animals, CD4 and CD8 T cells have already been primed, while in our study using a primary infection, the lack of IL-4 or IL-4rα apparently leads to increased innate immunity in the liver. The development of an activated CD8+ T-cell population is detectable as early as 24 h after immunization with sporozoites, followed 24 to 48 h later by an accelerated process of clonal CD8+ T-cell expansion. This acceleration reaches a peak after 4 to 5 days, and after 8 days, the parasite-specific CD8+ T-cell population is stabilized, and memory populations begin to be established (16, 40). Protection in vaccinated animals is largely mediated by IFN-γ-secreting rather than lytic CD8 T lymphocytes (14, 15, 38, 42, 44).
However, due to the rather long period of CD8+ T-cell activation in primary infection, it is unlikely that these cells had a major impact on the differential outcome observed in the liver stage of P. berghei, which lasts only during the first 50 h p.i.
Our observation rather pointed toward NK cells as the major cell type involved in the increased resistance in the absence of IL-4rα responsiveness, with a significant shift toward increased NK cell infiltration and IFN-γ production found in the livers of the infected mutant mouse strains in comparison to that in uninfected controls. Subsequent in vivo depletion studies further demonstrated that NK cells were crucial for protection, and our results suggest that they are the main IFN-γ producers within the liver.
Compared to normal rabbit serum-treated mice, in vivo NK cell depletion of IL-4−/− and IL-4rα−/− mice resulted in the inhibition of NK cell increase in the liver after sporozoite infection (Table 3), while NK cells were depleted (>90%) in the blood (data not shown). These data suggested that NK cells migrate from the blood to the liver during the liver-stage infection. No significant increases in NK cells were observed in wild-type mice after sporozoite infection (Tables 1 and 3). In these mice, treatment with anti-asialo GM1 antibody resulted in a decrease in liver NK cells (Table 3).
IFN-γ is known to be crucial for iNOS-induced NO production by macrophages and hepatocytes, the main killing effector molecule against the liver stage of plasmodium (24, 25, 36, 41, 45, 49, 56). We were able to directly demonstrate this cellular interaction by an observed upregulation of liver-specific iNOS in infected mice that was no longer present in NK cell-depleted animals. In addition, in vivo treatment with the iNOS inhibitor L-NIL or aminoguanidine during the liver stage of a primary infection resulted in exacerbated mortality in all mouse strains used, confirming the protective function of iNOS-catalyzed NO production. Comparison of the survival rates of untreated BALB/c wild-type mice in Fig. 3a and c showed that the survival rate of the BALB/c wild-type mice in Fig. 3a was 50%, while the survival rate in Fig. 3c of the BALB/c wild-type mice was 0%. The reason for this difference was the lower infectivity of the sporozoite preparation used in Fig. 3a and represents the interexperimental variation. On the other hand, the experiment in Fig. 3a pointed out the strong effect of the iNOS inhibitor L-NIL. Although the infectivity of this parasite preparation was lower, all NO-depleted mice died after injection of sporozoites, as shown in Fig. 3a. The batch of sporozoites used for all groups of mice shown in Fig. 3a demonstrated the important role for NO as a defense mechanism against the liver stage.
Probably, IFN-γ-stimulated hepatocytes are the source of NO production. Indeed, previous studies have shown that sporozoites infect hepatocytes (46) and are the main cellular source of NO production (25, 29). The induction of iNOS in the liver by live sporozoites observed in this study is in line with the literature from vaccination studies with experimental animal models in which irradiated sporozoites have been used (24, 25, 36, 41, 45, 49, 56). Attenuated sporozoites still had the ability to invade the hepatocytes and persisted in these cells. This resulted in an upregulation of iNOS in hepatocytes (25) and therefore led to a hepatocyte refractory status (36). Here we show that iNOS is produced by hepatocytes also during a primary infection.
The vaccination protection could be reversed by treating the animals at the time of sporozoite challenge with a competitive inhibitor of the NO pathway (36, 41). Since the irradiated sporozoites were also strong activators of NK cells (37), IFN-γ may have induced infected hepatocyte iNOS to produce NO in order to prevent the development of erythrocytic stages (45). This mechanism is also of importance for the understanding of immune defense mechanisms during human malaria. NO can be produced by human hepatocytes (35), and high iNOS expression from patients infected with P. falciparum correlates with a mild infection (9). Since the liver stage of P. berghei infection only lasts for 2 days, it may be of advantage to develop vaccines that increase liver-specific cell responses and do not induce IL-4 or IL-13.
Analyses of hepatic parasites by RT-PCR resulted in no detectable signal at an infection dose of 400 sporozoites (data not shown). From the literature, the detection limit for Plasmodium yolii hepatic parasites is described in a range from 25 to 6,250 sporozoites (4, 6, 53). However, Plasmodium yoelii and P. berghei showed a large difference in infectivities (4). Moreover, comparing the increase in rRNA of P. yoelii and P. berghei in the liver between 20 and 40 h p.i., it was shown that P. yoelii rRNA increases 11 times, and P. berghei rRNA increases only 1.6 times (4). The conclusions of these data are that the detection limit of P. berghei hepatic stages is much higher than that from P. yoelii. In summary, it was not possible to measure differences in hepatic parasites between mutant and wild-type mouse strains by RT-PCR at an infection dose of 400 sporozoites, while an increase in the infection dose to 800 sporozoites led to high parasitemia and the death of all animals, with no significant differences concerning the survival rates (Fig. 1b).
We have used mice deficient for IL-4 instead of depleting wild-type mice with anti-IL-4 antibodies, because application of anti IL-4 antibodies doesn't lead to a complete deficiency of IL-4 in all tissues.
Taking these findings together, we have shown the importance of using sporozoites for studies of defense mechanisms against malaria parasites. Our study extends the role for IL-4 in immune defense against the liver stage of a sporozoite infection. While earlier analyses with parasitized erythrocytes in the P. chabaudi model showed no differences in the absence of IL-4, our model demonstrated the detrimental role of IL-4 during the liver stage of infection with P. berghei sporozoites. Furthermore, this study has provided strong hints that NK cells were involved in the induction of iNOS in the livers of the KO mice and played an important role in the survival of infection with P. berghei sporozoites.
Acknowledgments
We thank T. Schüler, B. Richter, Y. Richter, and H. Fasel for organization of the animal facility.
A. Hoerauf is supported by the Deutsche Forschungsgemeinschaft (Ho 2009/1-3). F. Brombacher is holder of a Wellcome Trust Research Senior Fellowship for Medical Science in South Africa (grant no. 056708/Z/99).
Editor: J. M. Mansfield
REFERENCES
- 1.Amani, V., A. M. Vigario, E. Belnoue, M. Marussig, L. Fonseca, D. Mazier, and L. Renia. 2000. Involvement of IFN-gamma receptor-mediated signalling in pathology and anti-malarial immunity induced by Plasmodium berghei infection. Eur. J. Immunol. 30:1646-1655. [DOI] [PubMed] [Google Scholar]
- 2.Balmer, P., J. Alexander, and R. S. Phillips. 2000. Protective immunity to erythrocytic Plasmodium chabaudi AS infection involves IFNgamma-mediated responses and a cellular infiltrate to the liver. Parasitology 212:473-482. [DOI] [PubMed] [Google Scholar]
- 3.Barner, M., M. Mohrs, F. Brombacher, and M. Kopf. 1998. Differences between IL-4Ra-deficient and IL-4-deficient mice reveal a role for IL-13 in the regulation of Th2 response. Curr. Biol. 8:669-672. [DOI] [PubMed] [Google Scholar]
- 4.Briones, M. R., M. Tsuji, and V. Nussenzweig. 1996. The large difference in infectivity for mice of Plasmodium berghei and Plasmodium yoelii sporozoites cannot be correlated with their ability to enter into hepatocytes. Mol. Biochem. Parasitol. 77:7-17. [DOI] [PubMed] [Google Scholar]
- 5.Brombacher, F. 2000. The role of interleukin-13 in infectious disease and allergy. Bioessays 22:646-656. [DOI] [PubMed] [Google Scholar]
- 6.Bruna-Romero, O., J. C. Hafalla, G. Gonzalez-Aseguinolaza, G. Sano, M. Tsuji, and F. Zavala. 2001. Detection of malaria liver-stages in mice infected through the bite of a single Anopheles mosquito using a highly sensitive real-time PCR. Int. J. Parasitol. 31:1499-1502. [DOI] [PubMed] [Google Scholar]
- 7.Bruna-Romero, O., J. Schmieg, M. Del Val, M. Buschle, and M. Tsuji. 2003. The dendritic cell-specific chemokine, dendritic cell-derived CC chemokine 1, enhances protective cell-mediated immunity to murine malaria. J. Immunol. 170:3195-3203. [DOI] [PubMed] [Google Scholar]
- 8.Carvalho, L. H., G. I. Sano, J. C. R. Hafala, A. Morrot, M. A. Curotto de Lafaille, and F. Zavala. 2002. IL-4-secreting CD4+ T cells are crucial to the development of CD8+ T-cell response against malaria liver stages. Nat. Med. 8:166-170. [DOI] [PubMed] [Google Scholar]
- 9.Chiwakata, C. B., C. J. Hemmer, and M. Dietrich. 2000. High levels of inducible nitric oxide synthase mRNA are associated with increased monocyte counts in blood and have a beneficial role in Plasmodium falciparum malaria. Infect. Immun. 68:394-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daubersies, P., A. W. Thomas, P. Millet, K. Brahimi, J. A. Langermans, B. Ollomo, L. BenMohamed, B. Slierendregt, W. Eling, A. Van Belkum, G. Dubreuil, J. F. Meis, C. Guerin-Marchand, S. Cayphas, J. Cohen, H. Gras-Masse, P. Druilhe, and L. B. Mohamed. 2000. Protection against Plasmodium falciparum malaria in chimpanzees by immunization with the conserved pre-erythrocytic liver-stage antigen 3. Nat. Med. 6:1258-1263. [DOI] [PubMed] [Google Scholar]
- 11.Favre, N., B. Ryffel, G. Bordmann, and W. Rudin. 1997. The course of Plasmodium chabaudi chabaudi infections in interferon-gamma receptor deficient mice. Parasite Immunol. 19:375-383. [DOI] [PubMed] [Google Scholar]
- 12.Favre, N., B. Ryffel, and W. Rudin. 1999. Parasite killing in murine malaria does not require nitric oxide production. Parasitology 118:139-143. [DOI] [PubMed] [Google Scholar]
- 13.Fidock, D. A., H. Gras-Masse, J. P. Lepers, K. Brahimi, L. Benmohamed, S. Mellouk, C. Guerin-Marchand, A. Londono, L. Raharimalala, J. F. Meis et al. 1994. Plasmodium falciparum liver stage antigen-1 is well conserved and contains potent B and T cell determinants. J. Immunol. 153:190-204. [PubMed] [Google Scholar]
- 14.Gilbert, S. C., M. Plebanski, S. J. Harris, C. E. Allsopp, R. Thomas, G. T. Layton, and A. V. Hill. 1997. A protein particle vaccine containing multiple malaria epitopes. Nat. Biotechnol. 15:1280-1284. [DOI] [PubMed] [Google Scholar]
- 15.Good, M. F., and D. L. Doolan. 1999. Immune effector mechanisms in malaria. Curr. Opin. Immunol. 11:412-419. [DOI] [PubMed] [Google Scholar]
- 16.Hafalla, J. C., A. Morrot, G. I. Sano, G. Milon, J. J. Lafaille, and F. Zavala. 2003. Early self-regulatory mechanisms control the magnitude of CD8(+) T cell responses against liver stages of murine malaria. J. Immunol. 171:964-970. [DOI] [PubMed] [Google Scholar]
- 17.Heid, C. A., J. Stevens, and P. M. Williams. 1996. Real time quantitative PCR. Genome Res. 6:986-994. [DOI] [PubMed] [Google Scholar]
- 18.Herrington, D. A., G. A. Losonsky, G. Smith, F. Volvovitz, M. Cochran, K. Jackson, S. L. Hoffman, D. M. Gordon, M. M. Levine, and R. Edelman. 1992. Safety and immunogenicity in volunteers of a recombinant Plasmodium falciparum circumsporozoite protein malaria vaccine produced in Lepidopteran cells. Vaccine 10:841-846. [DOI] [PubMed] [Google Scholar]
- 19.Hill, A. V., J. Elvin, A. C. Willis, M. Aidoo, C. E. Allsopp, F. M. Gotch, X. M. Gao, M. Takiguchi, B. M. Greenwood, A. R. Townsend et al. 1992. Molecular analysis of the association of HLA-B53 and resistance to severe malaria. Nature 360:434-439. [DOI] [PubMed] [Google Scholar]
- 20.Hill, A. V., A. Jepson, M. Plebanski, and S. C. Gilbert. 1997. Genetic analysis of host-parasite coevolution in human malaria. Philos. Trans. R. Soc. Lond. B Biol. Sci. 352:1317-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hilton, D. J., J. G. Zhang, D. Metcalf, W. S. Alexander, N. A. Nicola, and T. A. Willson. 1996. Cloning and characterization of a binding subunit of the interleukin 13 receptor that is also a component of the interleukin 4 receptor. Proc. Natl. Acad. Sci. USA 93:497-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffman, S. L., and D. L. Doolan. 2000. Malaria vaccines targeting infected hepatocytes. Nat. Med. 6:1218-1219. [DOI] [PubMed] [Google Scholar]
- 23.Hölscher, C., G. Köhler, U. Müller, H. Mossmann, G. A. Schaub, and F. Brombacher. 1998. Defective nitric oxide effector functions lead to extreme susceptibility of Trypanosoma cruzi-infected mice deficient in gamma interferon receptor or inducible nitric oxide synthase. Infect. Immun. 66:1208-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khan, Z. M., C. Ng, and J. P. Vanderberg. 1991. Early hepatic stages of Plasmodium berghei: release of circumsporozoite protein and host cellular inflammatory response. Infect. Immun. 60:264-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klotz, F., L. Scheller, M. Seguin, N. Kumar, M. Marletta, S. Green, and A. Azad. 1995. Co-localization of inducible-nitric oxide synthase and Plasmodium berghei in hepatocytes from rats immunized with irradiated sporozoites. J. Immunol. 154:3391-3395. [PubMed] [Google Scholar]
- 26.Kopf, M., F. Brombacher, G. Kohler, G. Kienzle, K. H. Widmann, K. Lefrang, C. Humborg, B. Ledermann, and W. Solbach. 1996. IL-4-deficient Balb/c mice resist infection with Leishmania major. J. Exp. Med. 184:1127-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matthews, D. J., C. L. Emson, G. J. McKenzie, H. E. Jolin, J. M. Blackwell, and A. N. McKenzie. 2000. IL-13 is a susceptibility factor for Leishmania major infection. J. Immunol. 164:1458-1462. [DOI] [PubMed] [Google Scholar]
- 28.Meis, J. F. G. M., J. P. Verhave, P. H. K. Jap, R. E. Sinden, and J. H. E. T. Meuwissen. 1983. Malaria parasites: discovery of the early liver form. Nature 302:424-426. [DOI] [PubMed] [Google Scholar]
- 29.Mellouk, S., S. J. Green, C. A. Nacy, and S. L. Hoffman. 1991. IFN-gamma inhibits development of Plasmodium berghei exoerythrocytic stages in hepatocytes by an l-arginine-dependent effector mechanism. J. Immunol. 146:3971-3976. [PubMed] [Google Scholar]
- 30.Mellouk, S., F. Lunel, M. Sedegah, R. L. Beaudoin, and P. Druilhe. 1990. Protection against malaria induced by irradiated sporozoites. Lancet 335:4066-4068. [DOI] [PubMed] [Google Scholar]
- 31.Mohan, K., P. Moulin, and M. M. Stevenson. 1997. Natural killer cell cytokine production, not cytotoxicity, contributes to resistance against blood-stage Plasmodium chabaudi AS infection. J. Immunol. 159:4990-4998. [PubMed] [Google Scholar]
- 32.Mohrs, M., B. Ledermann, G. Kohler, A. Dorfmuller, A. Gessner, and F. Brombacher. 1999. Differences between IL-4- and IL-4 receptor alpha-deficient mice in chronic leishmaniasis reveal a protective role for IL-13 receptor signaling. J. Immunol. 162:7302-7308. [PubMed] [Google Scholar]
- 33.Mountford, A. P., K. G. Hogg, P. Coulson, and F. Brombacher. 2001. Signaling via interleukin-4 receptor α chain is required for successful vaccination against schistosomiasis in BALB/c mice. Infect. Immun. 69:228-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nelms, K., A. D. Keegan, J. Zamorano, J. J. Ryan, and W. E. Paul. 1999. The IL-4 receptor: signaling mechanisms and biological functions. Annu. Rev. Immunol. 17:701-738. [DOI] [PubMed] [Google Scholar]
- 35.Nussler, A. K., M. Di Silvio, T. R. Billiar, R. A. Hoffman, D. A. Geller, R. Selby, J. Madariaga, and R. L. Simmons. 1992. Stimulation of the nitric oxide synthase pathway in human hepatocytes by cytokines and endotoxin. J. Exp. Med. 176:261-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nussler, A. K., L. Renia, V. Pasquetto, F. Miltgen, H. Matile, and D. Mazier. 1993. In vivo induction of the nitric oxide pathway in hepatocytes after injection with irradiated malaria sporozoites, malaria blood parasites or adjuvants. Eur. J. Immunol. 23:882-887. [DOI] [PubMed] [Google Scholar]
- 37.Ojo-Amaize, E. A., J. Vilcek, A. H. Cochrane, and R. S. Nussenzweig. 1984. Plasmodium berghei sporozoites are mitogenic for murine T cells, induce interferon, and activate natural killer cells. J. Immunol. 133:1005-1009. [PubMed] [Google Scholar]
- 38.Plebanski, M., S. C. Gilbert, J. Schneider, C. M. Hannan, G. Layton, T. Blanchard, M. Becker, G. Smith, G. Butcher, R. E. Sinden, and A. V. Hill. 1998. Protection from Plasmodium berghei infection by priming and boosting T cells to a single class I-restricted epitope with recombinant carriers suitable for human use. Eur. J. Immunol. 28:4345-4355. [DOI] [PubMed] [Google Scholar]
- 39.Rodrigues, M. M., A. S. Cordey, G. Arreaza, G. Corradin, P. Romero, J. L. Maryanski, R. S. Nussenzweig, and F. Zavala. 1991. CD8+ cytolytic T cell clones derived against the Plasmodium yoelii circumsporozoite protein protect against malaria. Int. Immunol. 3:579-585. [DOI] [PubMed] [Google Scholar]
- 40.Sano, G., J. C. Hafalla, A. Morrot, R. Abe, J. J. Lafaille, and F. Zavala. 2001. Swift development of protective effector functions in naive CD8(+) T cells against malaria liver stages. J. Exp. Med. 194:173-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scheller, L. F., S. J. Green, and A. F. Azad. 1997. Inhibition of nitric oxide interrupts the accumulation of CD8+ T cells surrounding Plasmodium berghei-infected hepatocytes. Infect. Immun. 65:3882-3888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schneider, J., S. C. Gilbert, T. J. Blanchard, T. Hanke, K. J. Robson, C. M. Hannan, M. Becker, R. Sinden, G. L. Smith, and A. V. Hill. 1998. Enhanced immunogenicity for CD8+ T cell induction and complete protective efficacy of malaria DNA vaccination by boosting with modified vaccinia virus Ankara. Nat. Med. 4:397-402. [DOI] [PubMed] [Google Scholar]
- 43.Schofield, L., J. Villaquiran, A. Ferreira, H. Schellekens, R. Nussenzweig, and V. Nussenzweig. 1987. Gamma interferon, CD8+ T cells and antibodies required for immunity to malaria sporozoites. Nature 330:664-666. [DOI] [PubMed] [Google Scholar]
- 44.Sedegah, M., T. R. Jones, M. Kaur, R. Hedstrom, P. Hobart, J. A. Tine, and S. L. Hoffman. 1998. Boosting with recombinant vaccinia increases immunogenicity and protective efficacy of malaria DNA vaccine. Proc. Natl. Acad. Sci. USA 95:7648-7653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seguin, M. C., F. W. Klotz, I. Schneider, J. P. Weir, M. Goodbary, M. Slayter, J. J. Raney, J. U. Aniagolu, and S. J. Green. 1994. Induction of nitric oxide synthase protects against malaria in mice exposed to irradiated Plasmodium berghei infected mosquitoes: involvement of interferon gamma and CD8+ T cells. J. Exp. Med. 180:353-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shin, S. C., J. P. Vanderberg, and J. A. Terzakis. 1982. Direct infection of hepatocytes by sporozoites of Plasmodium berghei. J. Protozool. 29:448-454. [DOI] [PubMed] [Google Scholar]
- 47.Su, Z., and M. M. Stevenson. 2000. Central role of endogenous gamma interferon in protective immunity against blood-stage Plasmodium chabaudi AS infection. Infect. Immun. 68:4399-4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsuji, M., Y. Miyahira, R. Nussenzweig, M. Aguet, M. Reichel, and F. Zavala. 1995. Development of antimalaria immunity in mice lacking IFN-gamma receptor. J. Immunol. 154:5338-5344. [PubMed] [Google Scholar]
- 49.Vanderberg, J. P., Z. M. Khan, and M. J. Stewart. 1993. Induction of hepatic inflammatory response by Plasmodium berghei sporozoites protects BALB/c mice against challenge with Plasmodium yoelii sporozoites. J. Parasitol. 79:763-767. [PubMed] [Google Scholar]
- 50.van der Heyde, H. C., B. Pepper, J. Batchelder, F. Cigel, and W. P. Weidanz. 1997. The time course of selected malarial infections in cytokine-deficient mice. Exp. Parasitol. 85:206-213. [DOI] [PubMed] [Google Scholar]
- 51.von der Weid, T., M. Kopf, G. Kohler, and J. Langhorne. 1994. The immune response to Plasmodium chabaudi malaria in interleukin-4-deficient mice. Eur. J. Immunol. 24:2285-2293. [DOI] [PubMed] [Google Scholar]
- 52.Weiss, W. R., M. Sedegah, R. L. Beaudoin, L. H. Miller, and M. F. Good. 1988. CD8+ T cells (cytotoxic/suppressors) are required for protection in mice immunized with malaria sporozoites. Proc. Natl. Acad. Sci. USA 85:573-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Witney, A. A., D. L. Doolan, R. M. Anthony, W. R. Weiss, S. L. Hoffman, and D. J. Carucci. 2001. Determining liver stage parasite burden by real time quantitative PCR as a method for evaluating pre-erythrocytic malaria vaccine efficacy. Mol. Biochem. Parasitol. 118:233-245. [DOI] [PubMed] [Google Scholar]
- 54.Yoneto, T., S. Waki, T. Takai, Y. Tagawa, Y. Iwakura, J. Mizuguchi, H. Nariuchi, and T. Yoshimoto. 2001. A critical role of Fc receptor-mediated antibody-dependent phagocytosis in the host resistance to blood-stage Plasmodium berghei XAT infection. J. Immunol. 166:6236-6241. [DOI] [PubMed] [Google Scholar]
- 55.Yoneto, T., T. Yoshimoto, C.-R. Wang, Y. Takahama, M. Tsuji, S. Waki, and H. Nariuchi. 1999. Gamma interferon production is critical for protective immunity to infection with blood-stage Plasmodium berghei XAT, but neither NO production nor NK cell activation is critical. Infect. Immun. 67:2349-2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yoshida, S., S. I. Kashiwamura, Y. Hosoya, E. Luo, H. Matsuoka, A. Ishii, A. Fujimura, and E. Kobayashi. 1993. Direct immunization of malaria DNA vaccine into the liver by gene gun protects against lethal challenge of Plasmodium berghei sporozoite. Biochem. Biophys. Res. Commun. 271:107-115. [DOI] [PubMed] [Google Scholar]
- 57.Yoshimoto, T., T. Yoneto, S. Waki, and H. Nariuchi. 1998. Interleukin-12-dependent mechanisms in the clearance of blood-stage murine malaria parasite Plasmodium berghei XAT, an attenuated variant of P. berghei NK65. J. Infect. Dis. 177:1674-1681. [DOI] [PubMed] [Google Scholar]
- 58.Zurawski, G., and J. E. Vries. 1994. Interleukin 13, an interleukin 4-like cytokine that acts on monocytes and B cells, but not on T cells. Immunol. Today 15:19-26. [DOI] [PubMed] [Google Scholar]