Abstract
We performed a longitudinal clinical and parasitological follow-up study in OoDo, a village in southeast Asia in which malaria is hyperendemic, in order to assess the association between protection against malaria attacks and antibodies to three currently evaluated vaccine candidates, merozoite surface protein 1 (MSP1), MSP3, and the 220-kDa glutamate-rich protein (GLURP) from Plasmodium falciparum. Our results showed that the levels of cytophilic immunoglobulin G3 (IgG3) antibodies against conserved regions of MSP3 and GLURP were significantly correlated with protection against clinical P. falciparum malaria. In contrast, the levels of noncytophilic IgG4 antibodies against GLURP increased with the number of malaria attacks. Furthermore, we observed a complementary effect of the MSP3- and GLURP-specific IgG3 antibodies in relation to malaria protection. In the individuals that did not respond to one of the antigens, a strong response to the other antigen was consistently detected and was associated with protection, suggesting that induction of antibodies against both MSP3 and GLURP could be important for the development of protective immunity. The complementarity of the responses to the two main targets of antibody-dependent cellular inhibition identified to date provides the first rational basis for combining these two antigens in a hybrid vaccine formulation.
In regions where malaria is hyperendemic, adults develop potent but nonsterile immunity against malaria in which individuals chronically harbor low-grade parasitemia and only occasionally suffer from mild clinical malaria, a clinical state known as premunition (12, 24). Antibodies have been repeatedly shown to play an important role in the development of premunition (20, 26), and numerous immunological studies have suggested that human antibodies of the cytophilic subclasses (immunoglobulin G1 [IgG1] and IgG3) are particularly critical in this respect (2, 5, 6, 18, 21, 27, 28, 30). This antiparasite immunity is a strain-independent, nonsterilizing type of immunity which is acquired after lengthy exposure (ca. 15 to 20 years) to the parasite. It is commonly observed in Africa and in some parts of Papua New Guinea, but it has only recently been documented in southeast Asia (29). Although antibodies can act directly upon merozoite invasion of red blood cells (reviewed in reference 4), the most efficient in vivo mechanism for antibody-mediated parasite control in areas where the disease is endemic requires the participation of monocytes (16, 17). The antibody-dependent cellular inhibition (ADCI) assay mimics this cooperation between monocytes and cytophilic parasite-specific antibodies, and ADCI appears today to be the best in vitro surrogate marker of acquired immunity against Plasmodium falciparum blood stages (11). Two molecules have been identified as targets of human antibodies that are effective in ADCI; these molecules are the 48-kDa merozoite surface protein 3 (MSP3) (22) and the 220-kDa glutamate-rich protein (GLURP) (31). Several immunoepidemiological studies performed in geographically separated areas of Africa have demonstrated that high levels of GLURP- and MSP3-specific cytophilic antibodies are significant predictors of protection against clinical malaria (10, 23), providing epidemiological support for the concept that antibodies against GLURP and MSP3 can actively control parasite multiplication in vivo by cooperation with cells bearing FcγII receptors (6). These receptors exhibit higher affinity for the IgG3 subclass than for the IgG1 subclass (25). The B-cell epitopes recognized by these human antibodies have been mapped within both the GLURP and MSP3 molecules, and nucleotide sequencing has demonstrated that these important epitopes are highly conserved in a number of P. falciparum laboratory lines and field isolates from Africa and Asia (8, 15, 19). In view of the conservation of the epitopes, we investigated whether cytophilic antibodies against GLURP and MSP3 are involved in the development of immunity to clinical malaria in an Asian population in Myanmar, as they have been reported to be in Africa (i.e., in a different human and parasite genetic background). Since numerous reports have argued that antibodies against the C terminus of MSP1 have a direct role, we also included this molecule in our investigation.
MATERIALS AND METHODS
Study area and population.
The study was conducted in a rural village in Myanmar called OoDo, which is in a resettled forested region with a tropical climate characterized by three seasons: a hot dry season (March to June), a hot wet monsoon season (July to October), and a cool dry season (November to February). Malaria transmission occurs year round, but it peaks after heavy rains (August and September). The estimated annual number of infective bites received by each villager was approximately 10, and the majority of infections were due to P. falciparum (98%); the remaining 2% of infections were due to Plasmodium vivax. OoDo can thus be described as an area where seasonal malaria transmission is hyperendemic (29, 32).
For the present study, a subpopulation consisting of 116 individuals (47 males and 68 females) who were available for a minimum of 30 of the 45 monthly surveys was drawn from a larger cohort of 292 villagers (29). Thus, we included only villagers who were present for more than 66% of the weekly medical examinations to ensure that sufficient follow-up was available. Of the 116 individuals, 12 (10.3%) were <5 years old, 56 (48.3%) were 5 to 14 years old, and 48 (41.4%) were ≥15 years old. The study cohort was followed up by active and passive case detection from 1 April 1995 to 31 December 1998. Serum samples were taken yearly during September throughout the study period and were kept at −20°C until they were used. The samples used for the present study were samples taken in September 1998 (n = 116) and September 1993 (n = 13). The study was approved by the Myanmar National Ethical Committee. In addition to samples from the study cohort, a pool of 10 sera from adults living in an area of Senegal where malaria is holoendemic was used as a positive control. We also systematically included negative controls that consisted of six serum samples from healthy French blood donors who were never exposed to malaria.
Parasitological and clinical surveillance.
Parasitological surveillance for malarial infection was carried out by systematic monthly examinations of thick and thin blood films from finger pricks obtained during weekly visits of a medical team to each household. In addition, blood films were made when individuals complained of fever or other symptoms which could be associated with malaria. Hence, clinical malaria attacks were detected by daily active and passive case detection. A slide was considered negative if no parasite was visualized in 200 oil fields in a Giemsa-stained thick film. The geometric means of the parasite densities for villagers who were <5, 5 to 14, and ≥15 years old were as follows: 141, 150, and 99 parasites/μl of blood, respectively, for asymptomatic healthy villagers and 41,40l, 2,670, and 750 parasites/μl of blood, respectively, for symptomatic villagers (unpublished data).
A malaria attack was defined on the basis of four concomitant criteria: (i) body temperature of ≥38.0°C, (ii) absence of other clinical disease, (iii) parasitemia markedly (more than sevenfold) higher than the geometric mean parasitemia of asymptomatic individuals in the corresponding age group, and (iv) clinical and parasitological improvement after chloroquine treatment. Two febrile attacks were regarded as two different malaria attacks if they were separated by >72 h. Malarial attack rates recorded from 1 January to 31 December 1998 were used for the present study.
Antigens.
The three recombinant GLURP antigens were derived from N-terminal nonrepeat region R0 (GLURP27-500), central repeat region R1 (GLURP489-705), and C-terminal repeat region R2 (GLURP705-1178) of P. falciparum F32 (23). The C-terminal 19-kDa fragment of MSP1, MSP1-19, from the Wellcome strain (MSP1-W-19) was produced as a recombinant glutathione S-transferase fusion protein in Escherichia coli and was a kind gift from A. Holder, London, United Kingdom. The glutathione S-transferase tag was removed by enzymatic cleavage and subsequent affinity chromatography before use. The MSP3b synthetic peptide (184-AKEASSYDYILGWEFGGGVPEHKKEEN-210) contained the MSP3b B-cell epitope which reacts with human antibodies that are effective in ADCI (22).
Antibody assays.
The levels of antibodies to the three P. falciparum-derived antigens were measured by an enzyme-linked immunosorbent assay, as previously described (23). Briefly, microtiter plates (Maxisorb; Nunc, Roskilde, Denmark) were coated overnight at 4°C with 50 μl of recombinant proteins or synthetic peptide at the following concentrations: 0.5 μg/ml (R0 and R2), 1 μg/ml (R1 and MSP1), and 5 μg/ml (MSP3b). For GLURP antigens 0.05 M Na2CO3 (pH 9.6) was used as a coating buffer, and for MSP1 and MSP3 phosphate-buffered saline (PBS) (pH 7.4) was used as a coating buffer. The next day the plates were washed with PBS containing 0.05% Tween 20 (PBST) and blocked with 2.5% nonfat milk in PBS for 2 h. Portions (50 μl) of the sera diluted in PBST containing 1.25% (wt/vol) nonfat milk were added to duplicate wells and incubated for 1 h at room temperature. Various dilutions of sera were used for the antigens; a 1:200 dilution was used for GLURP, a 1:100 dilution was used for MSP1, and a 1:20 dilution was used for MSP3. These dilutions were selected as the dilutions that provided the optimal sensitivity for each antigen after preliminary pilot studies, which revealed that there was a >10-fold difference between control and test samples. Bound antibody was detected by peroxidase-conjugated goat anti-human immunoglobulin (Caltag Laboratories) diluted 1:3,000. Color was revealed by using O-phenylenediamine (Sigma, St. Louis, Mo.) and H2O2 in citrate buffer (pH 5) for 30 min. The optical density at 492 nm was determined with a plate reader (Titertek Multiskan MCC 1340). The plates were washed extensively with PBST between incubation steps. All enzyme-linked immunosorbent assays included one positive and six negative control sera selected because they had a mean optical density plus 3 standard deviations that was similar to that for 100 French blood donors who were never exposed to malaria.
For determination of IgG subclasses (IgG1 to IgG4), the following monoclonal mouse anti-human subclasses were used: clone NL16 for IgG1 (Boehringer), clone HP6002 for IgG2 (Sigma), clone Zg4 for IgG3 (Immunotech), and clone RJ4 for IgG4 (Immunotech). The preparations were diluted 1:2,000, 1:10,000, 1:10,000, and 1:1,000, respectively, in 1.25% (wt/vol) nonfat milk in PBST and incubated for 1 h at room temperature. Goat anti-mouse IgG conjugated to peroxidase (Caltag Laboratories), diluted 1:3,000 in 1.25% (wt/vol) nonfat milk in PBST, was added and incubated for 1 h. Bound labeled antibody was revealed as described above. The dilutions of each isotype-specific monoclonal antibody were determined previously as the dilutions that discriminated between human IgG subclasses (i.e., resulted in no cross-reactions between subclasses) (23). The results for total IgG, as well as subclass antibody levels, were expressed as ratios (or arbitrary units), which were calculated by dividing the mean optical density of a test preparation by the mean optical density plus 3 standard deviations for the six normal controls tested simultaneously. For determination of prevalence the cutoff ratio was 1. For statistical analysis the arbitrary units were used as a continuous variable.
Statistical analysis.
The Mann-Whitney U test and Spearman's rank order correlation coefficient were used to calculate differences between two groups of observations and the association between two variables, respectively. The association between the risk of malaria attack and the levels of antibodies (expressed as log antibody ratios in order to linearize relationships and optimize the analysis of data) was tested with JMP software by using a stepwise regression model in which the effect of confounding factors such as age (or time spent in the village) and gender were controlled for. An analysis of variance was applied to the regression model. A test of the null hypothesis was based on the variance ratio designated F, and departures from the null hypothesis tended to give values of F greater than unity.
RESULTS
P. falciparum infections in the study cohort.
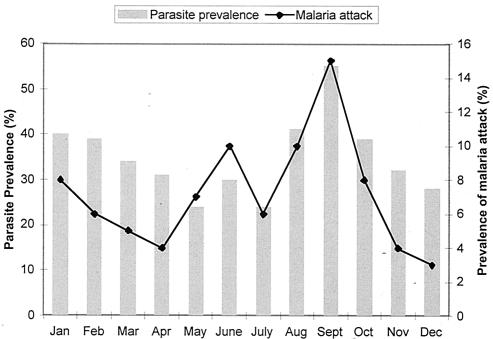
All 116 subjects in the study cohort were from OoDo village in Myanmar in southeast Asia, where malaria is hyperendemic (29). The prevalence of P. falciparum parasitemia fluctuated around 24% from May to July and increased to around 40% from August to October in 1998. The incidence of clinical malaria, which was calculated by determining the average number of attacks per month in the study cohort and was expressed as a percentage, varied considerably over the year, peaking in September (Fig. 1). The infective inoculation rate has not been determined for OoDo; however, Tun-Lin et al. (32) found 13.7 infective bites per person per year in a village which is located 15 km east of OoDo. This finding agrees well with the estimated number of bites calculated by the method of Beier et al. (3), 11 infective bites per person per year. Most infections (98%) were due to P. falciparum (29). During the 12-month period of continuous clinical surveillance, 86 (74%) of the 116 villagers had at least one malaria attack, as defined in Materials and Methods, and these individuals were considered to be susceptible to malaria. During the same 12-month period, 30 (26%) of the villagers had no episode of clinical malaria, and these individuals were considered clinically protected.
FIG. 1.
Prevalence of P. falciparum parasitemia and clinical malaria attacks in the 116 villagers in the OoDo study cohort during the 1998 surveillance period.
Antibody recognition of P. falciparum-derived MSP3, GLURP, and MSP1 antigens.
The ratios of IgG and IgG subclasses to the MSP3b184-210 peptide (MSP3b) and the four recombinant proteins representing the GLURP27-500 (R0), GLURP489-705 (R1), GLURP705-1179 (R2), and MSP1-19 C-terminal regions were determined for the 116 sera collected during September 1998. R2 was the antigen that was most frequently recognized by IgG antibodies (67.2%), followed by R1, MSP3b, and MSP1 (all 62%) and finally R0 (58.6%). The ratios of IgG to all three GLURP regions and MSP1 were significantly associated with age; the Spearman's rank order correlation coefficients (R) were 0.51, 0.26, 0.41, and 0.43 for R0, R1, R2, and MSP1, respectively (P < 0.05). Several of the subclass responses were found to be associated with age; these included the responses of IgG2 (R = 0.344; P = 0.0002) and IgG4 (R = 0.309; P = 0.0009) against MSP1, IgG2 (R = 0.243; P = 0.009) against MSP3, IgG1 (R = 0.214; P = 0.022) and IgG3 (R = 0.268; P = 0.004) against R0, IgG2 (R = 0.266; P = 0.004) and IgG3 (R = 0.313; P = 0.0008) against R1, and IgG4 (R = 231; P = 0.014) against R2. Neither the level nor the prevalence of the positive antibody response varied with gender for any of the antigens tested.
Identification of antibody responses associated with protection against clinical malaria.
A striking difference between IgG subclass responses and protection was observed for the three different antigens (Table 1). For example, the IgG response against the C-terminal 19-kDa fragment of MSP1 was almost exclusively an IgG1 subclass response, with a median value that was 8.6 times higher in the protected group than in the susceptible group, whereas IgG3 antibodies were the predominant antibodies against the MSP3b epitope in protected individuals and the median value was 6.5 times higher than the value obtained for susceptible individuals. Although less pronounced, a similar pattern of cytophilic IgG subclass responses was observed for different regions of GLURP; IgG1 antibodies were the predominant antibodies against the nonrepeat R0 region, and IgG3 antibodies were the predominant antibodies against the R2 repeat region.
TABLE 1.
Median ratios and 95% confidence intervals of IgG subclass antibodies to MSP1, MSP3, and GLURP antigens found in villagers from OoDo considered to be either protected from or susceptible to P. falciparum malaria attacks over 1 year of active and continuous follow-upa
| Antigen | IgG subclass | Median ratios (95% confidence intervals)
|
P value | |
|---|---|---|---|---|
| Protected group (n = 30) | Susceptible group (n = 86) | |||
| MSP1 | IgG | 7.70 (3.41-9.90) | 2.53 (1.59-4.52) | 0.0005b |
| IgG1 | 9.5 (1.4-20.8) | 1.1 (0.6-3.2) | 0.003b | |
| IgG2 | 0.9 (0.9-1.1) | 0.9 (0.8-0.9) | 0.163 | |
| IgG3 | 0.9 (0.05-1.7) | 0.4 (0.2-0.7) | 0.106 | |
| IgG4 | 1.2 (0.8-2.1) | 1.0 (0.7-1.1) | 0.013 | |
| MSP3 | IgG | 2.88 (1.52-3.79) | 2.75 (1.28-3.18) | 0.277 |
| IgG1 | 1.4 (0.8-2.2) | 0.7 (0.5-0.9) | 0.0001b | |
| IgG2 | 1.1 (0.9-1.3) | 0.8 (0.8-0.9) | 0.0001b | |
| IgG3 | 6.5 (3.1-10.7) | 1.0 (0.9-1.2) | 0.0001b | |
| IgG4 | 1.3 (1.0-1.8) | 1.0 (0.9-1.1) | 0.0042 | |
| R0 | IgG | 17.47 (11.97-23.87) | 8.85 (5.21-13.99) | 0.0034 |
| IgG1 | 3.9 (2.8-5.9) | 1.8 (1.3-2.3) | 0.0001b | |
| IgG2 | 0.9 (0.5-2.3) | 0.8 (0.5-1.1) | 0.219 | |
| IgG3 | 1.3 (0.7-3.0) | 0.9 (0.5-1.1) | 0.019 | |
| IgG4 | 0.2 (0.2-1.4) | 0.6 (0.3-0.8) | 0.867 | |
| R1 | IgG | 3.30 (2.52-5.56) | 1.50 (0.98-2.43) | 0.0003b |
| IgG1 | 0.4 (0.2-0.7) | 0.2 (0.1-0.3) | 0.0252 | |
| IgG2 | 0.9 (0.4-1.6) | 0.6 (0.4-0.6) | 0.0236 | |
| IgG3 | 1.2 (0.5-2.6) | 0.5 (0.4-0.7) | 0.0177 | |
| IgG4 | 0.2 (0.2-0.5) | 0.7 (0.3-0.8) | 0.038 | |
| R2 | IgG | 10.53 (5.71-15.47) | 3.15 (1.55-5.33) | 0.0004b |
| IgG1 | 2.0 (1.0-5.0) | 1.0 (0.5-2.1) | 0.010 | |
| IgG2 | 2.0 (1.1-3.8) | 0.9 (0.6-1.3) | 0.0002b | |
| IgG3 | 6.4 (2.1-8.2) | 0.9 (0.6-1.8) | 0.0001b | |
| IgG4 | 1.0 (0.7-1.1) | 0.6 (0.5-0.8) | 0.003 | |
The statistics were not adjusted for age, and P values were determined by the nonparametric Mann-Whitney U test. Nonparametric calculations of two-sided confidence intervals for the median were based on order statistics (the method provides a confidence interval for the upper and lower boundaries of the rank order of the value; the symmetric intervals whose confidence coefficients were 95% or more are indicated). Given the number of statistical tests carried out, the Bonferroni's correction factor was applied to determine the level of significance, and only P values of <0.002 were considered significant.
Significant.
Since the antibody responses against GLURP and MSP1 increased as a function of age, the clinical status of the villagers was reexamined in a stepwise-regression model in which age and all IgG subclass ratios (log transformed) for all antigens were considered explanatory variables. When all these parameters were tested in the model and particularly when age was controlled for, among all the antibody responses the predictors of malaria protection identified were high ratios of IgG3 against MSP3b (F ratio, 67.5; P < 0.0001) and against GLURP R0 (F ratio, 23.1; P < 0.0001). None of the other antibody ratios were significantly associated with protection in this model (and therefore the F ratios were low and not significant). In contrast, the analysis indicated that the levels of IgG4 against R0 increased with the number of malaria attacks (F ratio, 4.4; P = 0.038) and R1 (F ratio, 3.9; P = 0.051); i.e., they were to some extent predictive of susceptibility to malaria.
Antigen specificity of IgG3 responses in protected villagers.
Table 1 shows the general pattern of IgG3 antibody responses against the different blood stage antigens in OoDo. The range of values was large for most antigen-specific antibody responses, and this suggested that there might be different subgroups of responders. Sera of villagers who were protected against clinical malaria did not all have high IgG3 reactivity against both MSP3b and GLURP R0. Some individuals displayed unexpectedly low IgG3 reactivity against one of these two antigens. In an attempt to understand how these villagers were protected against malaria attacks, we analyzed two subgroups characterized by (i) low IgG3 responses against MSP3 (7 of 30 cases) or (ii) low IgG3 responses against R0 (15 of 30 cases). The ratios of IgG3 antibodies against the other three antigens were measured (Table 2). The seven protected individuals (ages, 33.9 ± 7.0 years [mean ± standard error]) with low IgG3 responses to MSP3b (median IgG3 ratio, 1.2) were found to have a strong IgG3 response to R0 (median IgG3 ratio, 10.4). In the second subgroup, containing 15 other individuals (ages, 24.7 ± 4.3 years) who were also protected despite a low IgG3 response to GLURP R0 (median IgG3 ratio, 0.6), the reverse situation was found: there was a high IgG3 antibody response against MSP3b (median IgG3 ratio, 10.0) and to a lesser extent against GLURP R2 (median IgG3 ratio, 4.1). The antibody ratios for the individuals who responded to only one antigen tended to be higher than the antibody ratios for the individuals who responded to both antigens. The numbers of years spent in OoDo did not differ significantly for the groups of low responders to MSP3 (20.43 ± 4.70 years of residence) and R0 (18.7 ± 10.6 years of residence).
TABLE 2.
Median ratios and 95% confidence intervals of antigen-specific IgG3 responses in villagers who were protected from clinical malaria but who had a low IgG3 response against either MSP3 or R0a
| Low IgG3 response antigenb | No. of villagers | Median antibody ratio (95% confidence interval) of IgG3 antibody responses to:
|
||||
|---|---|---|---|---|---|---|
| MSP3 | MSP1 | R0 | R1 | R2 | ||
| MSP3 | 7 | 1.2 (0.4-1.6) | 1.2 (0.3-3.8) | 10.4 (0.4-25.4)c | 1.0 (0.1-1.7) | 1.7 (0.3-43.0) |
| R0 | 15 | 10.0 (3.0-15.3)c | 0.0 (0.0-1.9) | 0.6 (0.3-0.7) | 2.1 (0.5-3.8) | 4.1 (1.3-7.8)c |
Nonparametric calculations of two-sided confidence intervals for the median were based on order statistics (the method provides a confidence interval for the upper and lower boundaries of the rank order of the value; the symmetric intervals whose confidence coefficients were 95% or more are indicated).
Low responders were defined as individuals with a specific IgG3 response below the lower 95% confidence interval limit of the median IgG3 ratio. The lower 95% confidence interval limits were 2.30 and 1.38 for MSP3 and R0, respectively.
The specific median antibody ratio is significantly (P < 0.05) higher than the median IgG3 ratio for a similar-size group of age-matched susceptible individuals. P was determined by the Mann-Whitney U test.
Serum samples from 13 of the 30 individuals considered protected in 1998 had also been obtained in 1993 and therefore were used to compare at a 5-year interval the relative levels of anti-MSP3 and anti-R0 IgG1 and IgG3 antibodies. No major change in the levels of specific IgG1 against the two antigens was detected. The seven individuals with high IgG3 responses against MSP3 in 1998 also had high titers 5 years earlier. In contrast, for GLURP the six individuals with a strong anti-R0 IgG3 response in 1998 had substantially lower anti-R0 IgG3 responses 5 years earlier (P = 0.0157), when they were 23.7 ± 6.9 years old, but they had high IgG3 responses against MSP3. Thus, there was a drastic change in these six individuals, whose protection status was associated with IgG3 to R0 in 1998 and only with IgG3 to MSP3 5 years earlier.
DISCUSSION
Several independent seroepidemiological studies have demonstrated that there is a significant association between high IgG1 and/or IgG3 antibody ratios against GLURP and MSP3 and protection against clinical P. falciparum malaria. Since these studies were performed in African locations where premunition is well established, it was important to examine whether such associations also occur in other areas of the world where malaria is endemic and where premunition has been documented. Our study is the first study to show that there is an association between antigen-specific antibody responses and protection against clinical malaria in southeast Asia. The prevalence of positive IgG antibody responses against GLURP and MSP3 was high in OoDo, ranging from 58.6% (R0) to 67.2% (R2). This observation is in keeping with the finding that B-cell epitopes within GLURP and MSP3 are highly conserved among P. falciparum laboratory lines and field isolates from Africa and Asia (8, 15, 19). The prevalence of antibodies to MSP1-W-19 was also high, and the values were almost twice the values found in The Gambia and Sierra Leone (14) and in Ghana (9), suggesting that strains related to the Wellcome strain might be prevalent in OoDo. Antibodies against MSP1-19 were predominantly of the IgG1 isotype, in agreement with previous reports from Africa (7, 14). In some studies researchers have found that high levels of anti-MSP1 IgG1 antibodies are associated with lower parasitemia (28) and protection against malaria attacks (14), whereas in other studies the researchers failed to observe such an association (9). In the present study, none of the IgG subclass responses against MSP1-19 were associated with protection against clinical malaria when specific antibody responses were corrected for the confounding effect of age-dependent exposure to P. falciparum malaria.
The ratios of IgG against all GLURP regions and MSP1 were significantly associated with age (P < 0.05), and the levels of several IgG subclasses also increased with age, a phenomenon which has been frequently reported for antibody responses to various malarial antigens (1, 2, 13, 23), reflecting most likely cumulative exposure to malaria parasites and possibly gradual maturation of the immune system over time.
There was a statistically significant difference between the ratios of IgG3 against R0 and MSP3 for the protected individuals living in OoDo and the ratios for the nonprotected individuals, and this was true when the data were adjusted for age. These results are in agreement with those of Dodoo et al. (10), who found that cytophilic antibody responses against R0 and R2 were strong predictors of protection in Ghanaian children, and those of Oeuvray et al. (23), who found that there was a consistent association between protection and elevated levels of IgG3 against GLURP R0 and R2 in Dielmo, West Africa. Together, these results suggest that the response of the same subclass of IgG to the same critical epitopes might be involved in the gradual development of protection in African populations as well as in Asian populations living in areas where malaria is endemic, against African and Asian P. falciparum isolates. In addition, in the present study we found that there was a significant negative association between the levels of noncytophilic antibodies against R0 and R1 and clinical protection. Therefore, on the one hand, there was a positive association between cytophilic IgG subclass responses and protection, and on the other hand, there was a negative association between noncytophilic subclass responses with the same epitope specificity and protection. Our finding is in agreement with the results of Ndungu et al. (21), who reported that elevated levels of IgG1 in relation to levels of IgG2 and IgG4 antibodies against a crude extract of a P. falciparum lysate were associated with protection against severe malaria. Conversely, elevated levels of IgG2 in relation to IgG1 and IgG3 antibodies were associated with a higher risk of severe malaria in Kenyan children. These epidemiological results are in agreement with the in vitro observation that noncytophilic antibodies can inhibit the bridging of merozoites to human monocytes by cytophilic antibodies against the same antigenic target and thereby reduce the ability of the latter to control parasite multiplication by the ADCI mechanism (5).
Whereas most of the protected residents of OoDo exhibited high IgG3 responses against both MSP3 and GLURP, a number of individuals with low or undetectable IgG3 responses against either one of these antigens also appeared to be protected. We found that all the protected individuals with low GLURP R0-specific IgG3 responses had significantly high ratios of specific anti-MSP3 IgG3 antibodies and vice versa. This observation suggests that antibodies against GLURP and MSP3 may act in a complementary manner to control parasite multiplication in immune individuals. This is relevant when the role of these antibodies in the ADCI mechanism is considered. Indeed, only simultaneous assessment of responses to several antigens revealed this complementary effect. This finding supports testing simultaneously several antigens for evaluating complementary as well as possible antagonistic antibody interactions that could have consequences for the design of combined vaccines.
In conclusion, in the present study we showed that (i) IgG3 antibodies against conserved regions of MSP3 and GLURP R0 are strong surrogates of protection against clinical malaria in an area of southeast Asia where malaria is endemic; (ii) to reach a state of premunition in Asia as well as in Africa, it is necessary to preferentially produce a cytophilic subclass of antibody against critical antigens (namely, MSP3b and GLURP, both of which induce antibodies active in ADCI); (iii) there appears to be a complementation effect between humoral responses specific for these two antigens, and IgG3 responses might have similar activities against the risk of malarial attack, provided that they are present against one antigen when responses to the other antigen are low or almost absent; and (iv) the responses to different B-cell epitopes on a given antigen appear to have evolved independently, and the level of recognition can change over time.
The complementarity of the responses to the two main targets of ADCI identified to date provides the first rational basis for combining these two antigens in a hybrid vaccine formulation.
Acknowledgments
We express our gratitude to Total Myanmar Exploration and Production and WHO/TDR for their contributions to implementation of the field study in OoDo, and we acknowledge the support of the P. A. Messerschmidt and Wife's Foundation, the Lundbeck Foundation, the NOVO Nordisk Foundation, the Hans and Nora Buchard's Foundation, the King Christian the Ninth and Queen Louise's Jubilee Fund, and the Foundation for Advancement of the Health Sciences.
Editor: J. M. Mansfield
REFERENCES
- 1.al-Yaman, F., B. Genton, K. J. Kramer, S. P. Chang, G. S. Hui, M. Baisor, and M. P. Alpers. 1996. Assessment of the role of naturally acquired antibody levels to Plasmodium falciparum merozoite surface protein-1 in protecting Papua New Guinean children from malaria morbidity. Am. J. Trop. Med. Hyg. 54:443-448. [DOI] [PubMed] [Google Scholar]
- 2.Aribot, G., C. Rogier, J. L. Sarthou, J. F. Trape, A. T. Balde, P. Druilhe, and C. Roussilhon. 1996. Pattern of immunoglobulin isotype response to Plasmodium falciparum blood-stage antigens in individuals living in a holoendemic area of Senegal (Dielmo, West Africa). Am. J. Trop. Med. Hyg. 54:449-457. [DOI] [PubMed] [Google Scholar]
- 3.Beier, J. C., G. F. Killeen, and J. I. Githure. 1999. Entomologic inoculation rates and Plasmodium falciparum malaria prevalence in Africa. Am. J. Trop. Med. Hyg. 61:109-113. [DOI] [PubMed] [Google Scholar]
- 4.Bolad, A., I. Nebie, and K. Berzins. 2000. Analysis of antibody mediated invasion/growth inhibition in vitro of wild isolates of Plasmodium falciparum. Scand. J. Immunol. 52:332. [Google Scholar]
- 5.Bouharoun-Tayoun, H., and P. Druilhe. 1992. Antibodies in falciparum malaria: what matters most, quantity or quality? Mem. Inst. Oswaldo Cruz 87:229-234. [DOI] [PubMed] [Google Scholar]
- 6.Bouharoun-Tayoun, H., C. Oeuvray, F. Lunel, and P. Druilhe. 1995. Mechanisms underlying the monocyte-mediated antibody-dependent killing of Plasmodium falciparum asexual blood stages J. Exp. Med. 182:409-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cavanagh, D. R., C. Dobano, I. M. Elhassan, K. Marsh, A. Elhassan, L. Hviid, E. A. Khalil, T. G. Theander, D. E. Arnot, and J. S. McBride. 2001. Differential patterns of human immunoglobulin G subclass responses to distinct regions of a single protein, the merozoite surface protein 1 of Plasmodium falciparum. Infect. Immun. 69:1207-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Stricker, K., J. Vuust, S. Jepsen, C. Oeuvray, and M. Theisen. 2000. Conservation and heterogeneity of the glutamate-rich protein (GLURP) among field isolates and laboratory lines of Plasmodium falciparum. Mol. Biochem. Parasitol. 111:123-130. [DOI] [PubMed] [Google Scholar]
- 9.Dodoo, D., T. G. Theander, J. A. Kurtzhals, K. Koram, E. Riley, B. D. Akanmori, F. K. Nkrumah, and L. Hviid. 1999. Levels of antibody to conserved parts of Plasmodium falciparum merozoite surface protein 1 in Ghanaian children are not associated with protection from clinical malaria. Infect. Immun. 67:2131-2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dodoo, D., M. Theisen, J. A. L. Kurtzhals, B. D. Akanmori, K. A. Koram, S. Jepsen, F. K. Nkrumah, T. G. Theander, and L. Hviid. 2000. Naturally acquired antibodies to the glutamate-rich protein are associated with protection against Plasmodium falciparum malaria. J. Infect. Dis. 181:1202-1205. [DOI] [PubMed] [Google Scholar]
- 11.Druilhe, P., and J. L. Perignon. 1997. A hypothesis about the chronicity of malaria infection. Parasitol. Today 13:353-357. [DOI] [PubMed] [Google Scholar]
- 12.Druilhe, P., and J. L. Perignon. 1994. Mechanisms of defense against Plasmodium falciparum asexual blood stages in humans. Immunol. Lett. 41:115-120. [DOI] [PubMed] [Google Scholar]
- 13.Dziegiel, M., P. Rowe, S. Bennett, S. J. Allen, O. Olerup, A. Gottschau, M. Borre, and E. M. Riley. 1993. Immunoglobulin M and G antibody responses to Plasmodium falciparum glutamate-rich protein: correlation with clinical immunity in Gambian children. Infect. Immun. 61:103-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Egan, A. F., J. Morris, G. Barnish, S. Allen, B. M. Greenwood, D. C. Kaslow, A. A. Holder, and E. M. Riley. 1996. Clinical immunity to Plasmodium falciparum malaria is associated with serum antibodies to the 19-kDa C-terminal fragment of the merozoite surface antigen, PfMSP-1. J. Infect. Dis. 173:765-769. [DOI] [PubMed] [Google Scholar]
- 15.Huber, W., I. Felger, H. Matile, H. J. Lipps, S. Steiger, and H. P. Beck. 1997. Limited sequence polymorphism in the Plasmodium falciparum merozoite surface protein 3. Mol. Biochem. Parasitol. 87:231-234. [DOI] [PubMed] [Google Scholar]
- 16.Khusmith, S., and P. Druilhe. 1983. Cooperation between antibodies and monocytes that inhibit in vitro proliferation of Plasmodium falciparum. Infect. Immun. 41:219-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lunel, F., and P. Druilhe. 1989. Effector cells involved in nonspecific and antibody-dependent mechanisms directed against Plasmodium falciparum blood stages in vitro. Infect. Immun. 57:2043-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luty, A. J., J. Mayombo, F. Lekoulou, and R. Mshana. 1994. Immunologic responses to soluble exoantigens of Plasmodium falciparum in Gabonese children exposed to continuous intense infection. Am. J. Trop. Med. Hyg. 51:720-729. [DOI] [PubMed] [Google Scholar]
- 19.McColl, D. J., and R. F. Anders. 1997. Conservation of structural motifs and antigenic diversity in the Plasmodium falciparum merozoite surface protein-3 (MSP-3). Mol. Biochem. Parasitol. 90:21-31. [DOI] [PubMed] [Google Scholar]
- 20.McGregor, I. A., S. P. Carrington, and S. Cohen. 1963. Treatment of East African Plasmodium falciparum with West African human gammaglobulin. Trans. R. Soc. Trop. Med. Hyg. 57:170-175. [Google Scholar]
- 21.Ndungu, F. M., P. C. Bull, A. Ross, B. S. Lowe, E. Kabiru, and K. Marsh. 2002. Naturally acquired immunoglobulin (Ig)G subclass antibodies to crude asexual Plasmodium falciparum lysates: evidence for association with protection for IgG1 and disease for IgG2. Parasite Immunol. 24:77-82. [DOI] [PubMed] [Google Scholar]
- 22.Oeuvray, C., H. Bouharoun-Tayoun, H. Gras-Masse, E. Bottius, T. Kaidoh, M. Aikawa, M. C. Filgueira, A. Tartar, and P. Druilhe. 1994. Merozoite surface protein-3: a malaria protein inducing antibodies that promote Plasmodium falciparum killing by cooperation with blood monocytes. Blood 84:1594-1602. [PubMed] [Google Scholar]
- 23.Oeuvray, C., M. Theisen, R. Christophe, J. F. Trape, S. Jepsen, and P. Druilhe. 2000. Cytophilic immunoglobulin responses to Plasmodium falciparum glutamate-rich protein are correlated with protection against clinical malaria in Dielmo, Senegal. Infect. Immun. 68:2617-2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perignon, J. L., and P. Druilhe. 1994. Immune mechanisms underlying the premunition against Plasmodium falciparum malaria. Mem. Inst. Oswaldo Cruz 89(Suppl. 2):51-53. [DOI] [PubMed] [Google Scholar]
- 25.Pleass, R. J., and J. M. Woof. 2001. Fc receptors and immunity to parasites. Trends Parasitol. 17:545-551. [DOI] [PubMed] [Google Scholar]
- 26.Sabchareon, A., T. Burnouf, D. Ouattara, P. Attanath, H. Bouharoun-Tayoun, P. Chantavanich, C. Foucault, T. Chongsuphajaisiddhi, and P. Druilhe. 1991. Parasitological and clinical human response to immunoglobulin administration in falciparum malaria. Am. J. Trop. Med. Hyg. 45:297-308. [DOI] [PubMed] [Google Scholar]
- 27.Sarthou, J. L., G. Angel, G. Aribot, C. Rogier, A. Dieye, A. T. Balde, B. Diatta, P. Seignot, and C. Roussilhon. 1997. Prognostic value of anti-Plasmodium falciparum-specific immunoglobulin G3, cytokines, and their soluble receptors in West African patients with severe malaria. Infect. Immun. 65:3271-3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi, Y. P., U. Sayed, S. H. Qari, J. M. Roberts, V. Udhayakumar, A. J. Oloo, W. A. Hawley, D. C. Kaslow, B. L. Nahlen, and A. A. Lal. 1996. Natural immune response to the C-terminal 19-kilodalton domain of Plasmodium falciparum merozoite surface protein 1. Infect. Immun. 64:2716-2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soe, S., Khin-Saw-Aye, Htay-Aung, Nay-Win, Tin-Aung, Than-Swe, C. Roussilhon, J. L. Pérignon, and P. Druilhe. 2001. Premunition against Plasmodium falciparum: demonstration in a malaria hyperendemic village in Myanmar. Trans. R. Soc. Trop. Med. Hyg. 95:81-84. [DOI] [PubMed] [Google Scholar]
- 30.Taylor, R. R., T. Allen, and M. Aikawa. 1998. IgG3 antibodies to Plasmodium falciparum merozoite surface protein 2 (MSP2): increasing prevalence with age and association with clinical immunity to malaria. Am. J. Trop. Med. Hyg. 58:406-413. [DOI] [PubMed] [Google Scholar]
- 31.Theisen, M., S. Soe, C. Oeuvray, A. W. Thomas, J. Vuust, S. Daneelsen, S. Jepsen, and P. Druilhe. 1998. The glutamate-rich protein (GLURP) of Plasmodium falciparum is a target for antibody-dependent monocyte-mediated inhibition of parasite growth in vitro. Infect. Immun. 66:11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tun-Lin, W., M. M. Thu, S. M. Than, and M. M. Mya. 1995. Hyperendemic malaria in a forested hilly Myanmar village. J. Am. Mosq. Control Assoc. 11:401-407. [PubMed] [Google Scholar]