Abstract
Candida albicans is an opportunistic human pathogen causing both superficial and disseminated diseases. It is a dimorphic fungus, switching between yeast and hyphal forms, depending on cues from its microenvironment. Hyphae play an important role in the pathogenesis of candidiasis. The host's response to Candida infection is multifaceted and includes the participation of granulocytes as key effector cells. The aim of this investigation was to study host gene expression during granulocyte-Candida interaction. Effector cells were generated by the granulocytic differentiation of HL60 cells. The resulting cell population was shown to be morphologically and functionally equivalent to granulocytes and is therefore referred to as HL60 granulocytoids for the purposes of this study. Gene expression profiles were determined 1 h after hosts were infected with C. albicans. Three Candida-granulocytoid ratios were chosen to reflect different degrees of HL60 granulocytoid inhibition of C. albicans. The data demonstrate that at the high pathogen-host ratio, C. albicans modulated the HL60 granulocytoid's response by downregulating the expression of known antimicrobial genes. In addition, looking at the expression of a large number of genes, not all of which have necessarily been implicated in candidastatic or candidacidal mechanisms, it has been possible to describe the physiological response of the HL60 granulocytoid to an infectious challenge with C. albicans. Finally, some of the observed changes in HL60 granulocytoid gene expression were investigated in freshly isolated human polymorphonuclear leukocytes infected with C. albicans. Similar changes were seen in these primary human cells, lending support to the validity of this model.
Candida albicans is a fungus that is a normal component of the microflora in humans. Although it rarely causes persistent infection in healthy individuals, it can cause either superficial or disseminated disease in immunocompromised patients (23, 59). Immunosuppressive therapy for transplantation, chemotherapy or radiotherapy, genetic disorders of the immune system, and human immunodeficiency virus-associated acquired immunodeficiency are all risk factors that predispose to candidiasis. The infection may be superficial, affecting the mucocutaneous membranes, in which case T-lymphocyte-dependent cell-mediated immune responses appear to be of primary importance in protection, although innate and adaptive systems must communicate efficiently to constitute a functional immune system (8, 38, 45). In systemic infections, a large body of evidence implicates phagocytic cells such as neutrophils in the host's resistance to C. albicans infection. They play a key role in recognition and inhibition of the pathogen and orchestrate the subsequent adaptive immune response (36, 44, 49, 65, 66, 74). A number of studies have explored the Candida-neutrophil interaction in vitro and have demonstrated phagocytosis and killing of C. albicans by neutrophils (1, 2, 19, 21, 50, 69, 73).
C. albicans is a dimorphic yeast that uses environmental cues to switch between yeast and hyphal forms (58). There is a great deal of interest in the relative contribution of the two morphological forms to virulence (39, 48, 52). The hyphal form is thought to be virulent because of its ability to penetrate tissues. Supporting this, deletion of genes that promote hyphal formation result in strains that are reduced in virulence or nonvirulent (42, 47, 63). Peripheral blood leukocytes are known to inhibit C. albicans hyphal elongation (33). Since hyphae represent a virulence factor, the ability of professional phagocytes to inhibit hyphal growth may represent a key element of the defense mechanisms employed by the host. It was therefore of interest to study this phenomenon in greater detail.
Granulocytic differentiation of the HL60 myelomonocytic cell line provides a useful in vitro model of infection (29). HL60 cells were isolated from the peripheral blood of a patient with acute promyelocytic leukemia (11, 25). Although the immature HL60 population is predominantly composed of promyelocytes, there is also a small percentage that are more mature, displaying morphological characteristics of myelocytes, metamyelocytes, and banded and segmented neutrophils. Functionally, however, the population is unable to exert either microbicidal or tumoricidal effects (29). Upon treatment with dimethyl formamide, dimethyl sulfoxide, or retinoic acid, the cells differentiate along the granulocytic pathway. Differentiation is accompanied by striking morphological changes (10, 13, 29, 53). The cells stop proliferating, and the nucleus becomes pyknotic and polymorphic. Furthermore, there is a dramatic increase in the superoxide anion and hydrogen peroxide production capability of the cells, which are among the most important known microbicidal products of granulocytes. There is also a concomitant increase in their ability to phagocytose (12, 24).
In support of the observed microbicidal activity, the cells express a number of genes associated with these functions (61). The neutrophilic differentiation of HL60 cells thus provides an attractive and widely used model for studies of granulocytes (7, 12, 29). However, it is important to recognize that it is a model and along with several similarities with human polymorphonuclear leukocytes (PMNL), there are also qualitative and quantitative differences, for instance, in global gene expression patterns (32) and in the presence of secondary granules (55). Our studies have verified the granulocytic nature of the dimethyl formamide-differentiated cells with respect to their interaction with C. albicans. Therefore, for the purposes of this study, it is justified to use them as a model system to investigate the interaction of granulocytes with Candida. We refer to them as HL60 granulocytoids in this report.
We wanted to determine changes in expression profiles of these granulocyte-like cells during an interaction with C. albicans. Recent reports of the use of such comprehensive approaches to study host-pathogen interactions have provided insight not only into the defense mechanisms used by the host but also strategies used by the pathogen to overcome them (17, 68, 71).
We report a striking change in the host gene expression profile during the interaction with C. albicans. These include a number of changes underlying the induction of an inflammatory response and cell fate determination. Based on experiments at different multiplicities of infection (MOIs), we present evidence that C. albicans may modulate these responses. Furthermore, to examine the validity of the HL60 granulocytoids at the level of gene expression, semiquantitative reverse transcription (RT)-PCR analysis was used to probe the expression of some of the most relevant genes in primary human cells during their interaction with C. albicans.
(Part of this information was presented at the First Montréal Microarray Workshop, 17 September 2001, Montréal, Québec, Canada; no abstracts were published.)
MATERIALS AND METHODS
HL60 culture and differentiation.
HL60, a human promyelocytic leukemia cell line, was obtained from the American Type Culture Collection and was maintained in RPMI 1640 (Gibco-Invitrogen, Burlington, Ontario, Canada) supplemented with 20% heat-inactivated fetal bovine serum (HyClone, Logan, Utah) (growth medium). To differentiate cells along the granulocytic pathway, cells were seeded at a density of 3 × 105 to 4 × 105 cells/ml in growth medium supplemented with 0.7% dimethyl formamide (Sigma-Aldrich, Oakville, Ontario, Canada) and differentiation was allowed to proceed for 4 days. This regimen was chosen for experiments after preliminary studies demonstrated that it was optimal for the generation of a homogeneous population of cells (determined by the expression of cell surface markers, described below) and capable of potent candidacidal activity (microscopic examination of Candida colony formation in the presence and absence of HL60 granulocytoids, data not shown).
Flow cytometry.
The expression of cell surface antigens (CD13, CD14, CD11b, CD16b, CD116, and mannose receptor) was determined during the course of differentiation by measuring the binding of fluorescein isothiocyanate- or phycoerythrin-conjugated antibodies anti-CD13 (MY-7) (Coulter Immunology, Hialeah, Florida) anti-CD116, anti-CD14 anti-CD11b, and anti-CD16b (Cedarlane Laboratories Limited, Hornby, Ontario, Canada), and anti-mannose receptor (Immunotech, Marseille, France) by flow cytometry. Differentiated cells at a density of 106 cells/ml were incubated with antibody according to the manufacturer's instructions. Excess antibody was removed by washing the cells once with phosphate-buffered saline (PBS). Specific antibody binding was measured in terms of total fluorescence of the cell population with an EPICSXL-flow cytofluorometer (Beckman Coulter, Fullerton, Calif.) compared to the binding of a fluorescein isothiocyanate- or phycoerythrin-labeled isotypic negative control (Cedarlane Laboratories Limited, Hornby, Ontario, Canada).
Isolation of human PMNL.
Human PMNL were isolated from pooled venous blood of five healthy human volunteers. All procedures were carried out according to guidelines established by the National Research Council Human Ethics Committee (protocol number 2003-14). PMNL purification was carried out with discontinuous Percoll-serum gradient as described by Read et al. (62). Briefly, whole blood, mixed with 3.8% sodium citrate (10:1), was centrifuged at 220 × g for 20 min at room temperature. The plasma was removed and the cells were resuspended in 5 ml of 6% dextran T-500 (Amersham Biosciences, Uppsala, Sweden) in a final volume of 50 ml of saline solution. Red cells were allowed to settle for 30 min at room temperature. The cells in the red cell-free layer were washed twice with PBS. The washed cell pellet was resuspended in 2 ml of heat-inactivated fetal bovine serum and layered on a discontinuous Percoll-serum gradient with 42% and 51% Percoll (Amersham Biosciences). The gradients were centrifuged at 180 × g for 12 min at room temperature. The PMNL layer was collected, washed twice in PBS, and resuspended in PBS containing 0.1% dextran T-500 to remove contaminating red blood cells. The final cell population was resuspended in growth medium at the required cell density. The cell population was greater than 99% PMNL by differential staining (ProtocolHema 3, Biochemical Sciences, Swedesboro, N.J.) and greater than 99% viable by trypan blue exclusion.
C. albicans culture.
C. albicans wild-type strain SC5314 or a green fluorescent protein (GFP)-expressing strain (a kind gift from Brendan Cormack) (15) was grown overnight in YPD medium (1% yeast extract, 1% Bacto Peptone [Difco Laboratories, Detroit, Mich.], and 2% dextrose [Sigma]) at 30°C and harvested by centrifugation. The blastospores were washed twice in PBS and resuspended in growth medium at the required concentration.
Candida growth inhibition assay.
To quantitate the level of killing, Candida CFU were determined after 5 h of incubation in growth medium or growth medium containing 2 × 106 HL60 cells or HL60 granulocytoids. The incubation was carried out in a six-well dish, with 2.5 ml of growth medium per well. The number of Candida blastospores were added to each well to give MOIs of 0.1, 0.5, and 5. After 5 h of coculture, the entire contents of the well were scraped into the growth medium, mixed thoroughly, and 50 μl was appropriately diluted and plated on a YPD-agar plate. Percent killing was calculated with the formula [1 − (CFU. of C. albicans in coculture/CFU. of C. albicans alone)] × 100.
Microscopy.
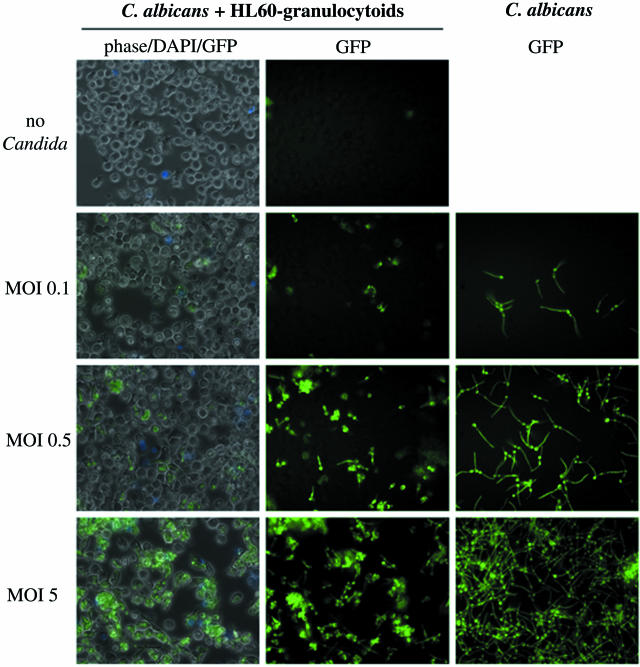
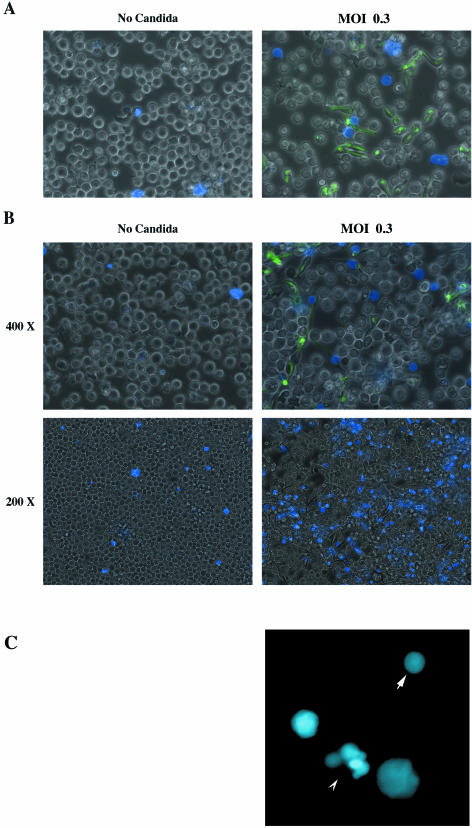
For Fig. 3 and 5, 0.7 × 106 HL60 granulocytoids were infected with C. albicans at MOIs of 0.1, 0.5, and 5 in a 24-well plate (Fig. 3) and an MOI of 0.3 (Fig. 5). An MOI of 0.3 was chosen when longer periods of coculture were required (Fig. 4 and 5, Table 2). At high MOIs, extensive hyphal growth at later times complicates interpretation of the photographs; 0.2 μg of 4′,6′-diamidino-2-phenylindole dihydrochloride (DAPI) (Molecular Probes, Eugene, Oreg.) was added to the culture medium to monitor cell death (Fig. 3 and 5). Phase contrast and epifluorescence pictures were taken with a Leica DMIRE2 inverted microscope (Leica Microsystemes Canada) equipped with a Hamamatsu cooled charge-coupled device camera at 200× or 400× magnification, with the appropriate filters. Openlab software (Improvision, Lexington, Mass.) was used for image acquisition. For each field, three separate pictures were taken: phase contrast, blue fluorescence (for imaging DAPI nuclear staining), and green fluorescence (for imaging the C. albicans expressing GFP) (GFP-C. albicans).
FIG. 3.
HL60 granulocytoid-C. albicans interaction. GFP-expressing C. albicans were cultured either alone (right column) or with HL60 granulocytoids at the indicated MOIs, as described in Materials and Methods. Photographs were taken 1.5 h later at 400× magnification. Each of the panels in the first column is a superimposition of three images: phase contrast, blue fluorescence (to visualize DAPI staining), and green fluorescence (to visualize GFP-C. albicans). Panels in the second column show the images corresponding to the green fluorescence in the first column. Panels in the last column show green fluorescence images to visualize C. albicans cultured alone at densities corresponding to the indicated MOIs.
FIG. 5.
C. albicans-induced mortality in the HL60 granulocytoid population. HL60 granulocytoids were cultured either alone (left column) or with GFP-expressing C. albicans at an MOI of 0.3 (right column) as described in Materials and Methods. Photographs were taken 1.5 h (A) and 6 h (B and C) later. In the first and second rows, images are superimpositions of three photographs: phase contrast, green fluorescence (to visualize GFP-C. albicans), and blue fluorescence (to visualize DAPI staining) at a magnification of 400×. GFP-Candida can be seen engulfed by HL60 granulocytoids at both 1.5 h and 6 h postinfection. The third row shows blue fluorescence images at a magnification of 200×. DAPI-stained blue cells are indicative of cell death. (C) Nuclear morphology visualized by DAPI staining. The arrow points to a typical example of nuclear condensation and the arrowhead points to an example of nuclear fragmentation. A portion of the 400× image was further magnified 4× digitally.
FIG. 4.
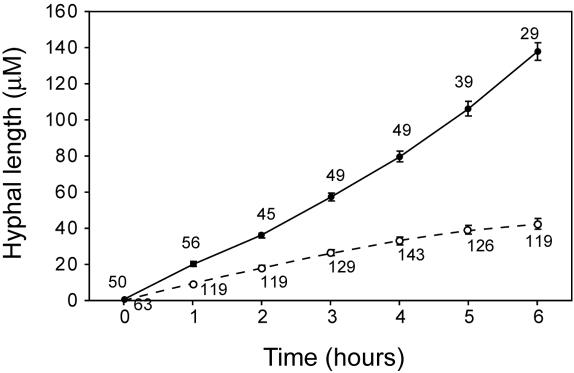
C. albicans hyphal growth in the presence and absence of dimethyl formamide-induced neutrophils. GFP-Candida were cultured with (dotted line) or without (solid line) HL60 granulocytoids in a Bioptechs ΔTC3 petri dish as described in Materials and Methods. Photographs were taken every 60 min with a 20× objective. Hyphal length was measured for all Candida cells in three microscopic fields for each time point. The number of Candida cells counted for each point on the graph is indicated. The figure shows the change in average hyphal length of C. albicans. The standard error is indicated at each time point.
TABLE 2.
Viability of HL60 granulocytoids exposed to C. albicans
| Time postinfection (h) | No. of cells counted | % of cells DAPI positive |
|---|---|---|
| 1 | 179 | 5.02 |
| 2 | 271 | 8.11 |
| 3 | 301 | 13.62 |
| 4 | 129 | 20.93 |
| 5 | 65 | 24.61 |
| 6 | 45 | 44.44 |
| 7 | 46 | 91.30 |
| 16 | 495 | 93.22 |
| Controla | 730 | 15.71 |
Uninfected cells incubated for 16 h.
For time lapse microscopy (Fig. 4 and Table 2), 0.5 × 106 HL60 granulocytoid cells were grown in one half of a partitioned Bioptechs (Butler, Pa.) ΔTC3 petri dish containing 0.75 ml of culture medium. C. albicans was added to HL60 granulocytoids at an MOI of 0.3. The other half contained either C. albicans alone (Fig. 4) or HL60 granulocytoids alone (Table 2). Leibovitz's L-15 medium (Gibco-Invitrogen) supplemented with 10% fetal bovine serum (HyClone) was used to maintain the pH at 7.2 to 7.3 during image acquisition. The temperature was maintained at 37°C with a Bioptechs controller. Still images were captured every 60 min with a Sensys cooled charge-coupled device monochrome camera (Roper Scientific, Trenton, N.J.) mounted on a DM-IRB inverted fluorescence microscope (Leica, Wetzlar, Germany). The microscope was fitted with Ludl (Hawthorne, N.Y.) Bioprecision motorized hardware: stage, focus drive and filter wheels, all hooked up to a Mac2002 controller. All filters used were from Chroma (Brattleboro, Vt.). The entire system was controlled by Openlab software (Improvision, Lexington Mass.) running on a Powermac G4 (Apple, Cupertino, Calif.).
Caspase-3 activity.
Caspase-3 activity was detected in whole-cell lysates from uninfected and infected HL60 granulocytoids. Twenty million HL60-derived granulocytoids were plated in a 150-mm tissue culture dish. An overnight culture of C. albicans was washed, resuspended in growth medium, and added to the HL60 granulocytoids to give an MOI of 0.3. After an incubation of 6 h at 37°C, the cells were washed with PBS and lysed in cell lysis buffer (ApoAlert caspase-3 fluorescent assay kit, Clontech Laboratories, Palo Alto, Calif.). Caspase-3 activity was assayed in the extracts with the substrate N-acetyl-Asp-Glu-Val-Asp-7-amino-4-trifluormethyl coumarin (DEVD-AFC) and inhibitor N-acetyl-Asp-Glu-Val-Asp-aldehyde (DEVD-CHO) according to the manufacturer's instructions.
RNA extraction.
Twenty million HL60-derived granulocytoids were plated in a 150-mm tissue culture dish. An overnight culture of C. albicans was washed, resuspended in growth medium, and added to the HL60 granulocytoids to give the required MOIs. After an incubation of 1 h at 37°C, the cells were scraped, centrifuged at 1,500 rpm for 5 min, and cytoplasmic RNA was prepared with a Qiagen RNeasy kit (Qiagen, Mississauga, Ontario, Canada) according to the manufacturer's instructions. Briefly, the cell pellets were lysed in a mild lysis buffer (50 mM Tris-Cl [pH 8], 140 mM NaCl, 1.5 mM MgCl2 and 0.5% [vol/vol] Nonidet P40). C. albicans does not lyse under these conditions (data not shown). Nuclei and C. albicans were removed by centrifugation. Cytoplasmic RNA was purified with a Qiagen RNeasy column.
RNA extraction was performed in three separate experiments. The three HL60 granulocytoid control RNA samples were pooled and called the HL60 granulocytoid pool. One of the three HL60 granulocytoid samples (extraction 2) was also analyzed on its own and called the HL60 granulocytoid single. Similarly, the three RNA samples of HL60 granulocytoids exposed to C. albicans were pooled and called the HL60 granulocytoid+candida pool. HL60 granulocytoid+candida (extraction 2) was analyzed on its own and called the HL60 granulocytoid+candida single.
Human PMNL.
The RNA isolation method described above was chosen to minimize C. albicans contamination, since intact Candida could be eliminated before the addition of chaotropic agents such as guanidinium hydrochloride. However, we were unable to extract high-quality RNA from freshly isolated human PMNL exposed to C. albicans with this method. Therefore, direct lysis in RLT buffer (Qiagen) was used to minimize RNA degradation. In this case, 20 million human PMNL were plated in a 150-mm tissue culture dish. An overnight culture of C. albicans was washed, resuspended in growth medium, and added to the PMNL to give the required MOIs. After an incubation of 1 h at 37°C, the cells were scraped, centrifuged at 1,500 rpm for 5 min, and cells were lysed directly with buffer RLT (Qiagen RNeasy kit). DNA contamination was removed by with a DNA shredder column (Qiagen), and total RNA was isolated by purification on a Qiagen RNeasy column.
Microarray analysis. The methods for preparation of cRNA directly from total RNA and subsequent steps leading to hybridization and scanning of the Hu-GeneFL gene chip arrays (Affymetrix, Santa Clara, Calif.) were provided by the manufacturer.
cDNA synthesis. Briefly, first-strand cDNA was synthesized from 20 μg of total RNA with a special oligo(dT)24 primer containing a T7 RNA polymerase promoter at its 5′ end (from GenSet; for more information, see www.GenSet.oligos.com) in 20 μl of first-strand reaction mix at 42°C for 1 h. The second strand was then synthesized in second-strand reaction mix for 2 h at 16°C. All enzymes and buffers were from Gibco-Invitrogen except RNase H and T4 DNA polymerase, which were from Fermentas MBI (Burlington, Ontario, Canada). cDNAs were extracted with phenol-chloroform, precipitated with ethanol, and then resuspended in diethylpyrocarbonate-treated water.
In vitro transcription reaction.
After second-strand synthesis, biotin-labeled cRNA was generated from the cDNA sample by an in vitro transcription reaction with the BioArray RNA transcript labeling kit (Enzo Diagnostics, Farmingdale, N.Y.) with biotin-labeled CTP and UTP. The labeled cRNA was purified with RNeasy spin columns (Qiagen). Fifteen micrograms of each cRNA sample was fragmented at 94°C for 35 min in fragmentation buffer (40 mM Tris-acetate, pH 8.1, 100 mM potassium acetate, and 30 mM magnesium acetate) and then used to prepare 300 μl of the hybridization mix. A biotinylated oligonucleotide, B2, that hybridizes to unique features at the center and four corners of each chip was used to orient the probe sets on the chip.
Oligonucleotide array hybridization and scanning.
The cRNA hybridization mix was heated to 94°C for 5 min, equilibrated to 45°C for 5 min, and clarified by centrifugation (14,000 × g) at room temperature for 6 min. Aliquots of each sample (10 μg of cRNA in 200 μl of the mix) were hybridized to HuGeneFL gene chip arrays (Affymetrix) at 45°C for 16 h in a rotisserie oven set at 60 rpm (gene chip hybridization oven 640 from Affymetrix). The arrays were then washed with SSPE (15 mM NaCl, 10 mM NaH2PO4 · H2O, and 1 mM EDTA), stained with streptavidin-phycoerythrin (Molecular Probes), and washed again. The whole procedure of washing and staining was carried out in Affymetrix's gene chip fluidics station 400. Then the microarray was scanned with a gene array scanner (Agilent Technologies, Mississauga, Ontario, Canada). Average difference and expression calls for each feature on the chip were analyzed with Affymetrix gene chip analysis suite version 3.2 with default parameters. A default value of 50 was assigned to any hybridization signal of less than 50. All data points were normalized to the total fluorescence of the chip.
Quantitative RT-PCR analysis.
cDNA was made from 3 to 5 μg of RNA with Superscript II (Gibco-Invitrogen, Burlington, Ontario, Canada) according to the manufacturer's instructions. Quantitative PCR was performed in a Light Cycler (Roche Diagnostics, Laval, Québec, Canada) with the DNA SYBR Green I reaction (Roche Diagnostics). A β-actin control PCR was performed in parallel in the same light cycler run. Quantitation was performed by comparison of crossing points. Thus, RNA levels for gene X were quantified relative to ACTB mRNA levels (relative level of X = crossing point for X/crossing point for ACTB).
For semiquantitative analysis of changes in human PMNL infected with C. albicans, cDNA was made from 1 to 3 μg of RNA with Superscript II (Gibco-Invitrogen) according to the manufacturer's directions. PCR was performed for 15, 20, 25, 30, and 35 cycles, and amplified products were visualized by Southern analysis. Digoxigenin-labeled DNA fragments were used as probes (Roche Diagnostics) for hybridization and detection was performed with a digoxigenin luminescent detection kit (Roche Diagnostics) according to the manufacturer's instructions. Quantitation of the bands was performed with the NIH-IMAGE software (http://ccp14.minerals.csiro.au/ccp/web-mirrors/nih-image/nih-image/).
The sequences of gene-specific primers, MgCl2 concentrations, and annealing temperatures are described in Table 1.
TABLE 1.
PCR primers
| Gene | Primers | Annealing tempa (°C) | MgCl2 concna (mM) | Remarks |
|---|---|---|---|---|
| PAC1 | Forward TTGCCCTACCTGTTCCTGGG | 55/60 | 3/3.5 | |
| Reverse GTCTCAAACTGCAGCAGCTG | ||||
| HBEGF | Forward TGGTGCTGAAGCTCTTTCTGG | 55 | 3 | |
| Reverse TGCGGGACCATGAAGCTGCT | ||||
| HNP1 | Forward ACAGAGGACTGCTGTCTGCC | 55/58 | 3 | |
| Reverse CCAGAGTCTTCCCTGGTAGAT | ||||
| N. E. | Forward CGGAGCCCCAGCCCCACCAT | 55/58 | 2 | 8% dimethyl sulfoxide |
| Reverse TGGCGATCCCACGGTTCCTG | ||||
| PAI2 | Forward CCATGGTCTACATGGGCTCC | 55/60 | 3/4.5 | |
| Reverse TGCGCTGAGCCGAGTTTACA | ||||
| ACTB | Forward GAGCAAGAGAGGCATCCTCA | 55/58 | 3 | |
| Reverse TCAGGCAGCTCGTAGCTCTT | ||||
| TR3 | Forward CAGGGACCAGGCTGAGACTC | 60 | 3.5 | |
| Reverse GAGCAGGGGCTGCCATAGTAG | ||||
| S28 | Forward TTGAAAATCCGGGGGAGAG | 54 | 3 | |
| Reverse ACATTGTTCCAACATGCCAG | ||||
| TNF | Forward GAGGAGGCGCTCCCCAAGAAG | 63 | 4.5 | |
| Reverse GTGAGGAGCACATGGGTGGAG | ||||
| Ca-ACT1 | Forward AAGCCGGTTTTGCCGGTGACT | 62 | 4 | Okeke et al. (60) |
| Reverse TGGTGAACAATGGATGGACACT |
Values are given for PCR/light cycler analysis.
Statistical analysis.
To determine whether killing by HL60 granulocytoids (see Fig. 2) was statistically significant over killing by undifferentiated HL60 cells, the data were subjected to a nonparametric two-sample ranking test. The Mann-Whitney test was applied to C. albicans CFU obtained in the presence of HL60 granulocytoids in comparison with those in the presence of undifferentiated HL60 cells. The same test was then used to compare the level of HL60 granulocytoid-specific killing at two different MOIs.
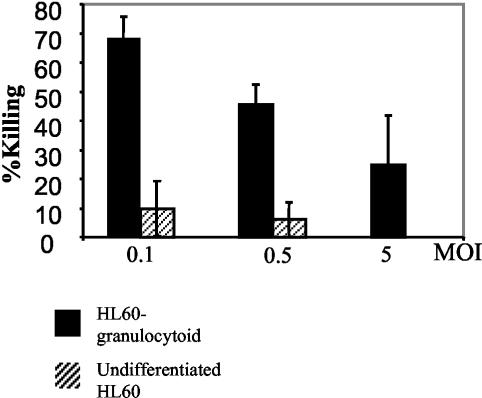
FIG. 2.
Candida colony formation after 5 h of coculture with HL60 or HL60 granulocytoids. C. albicans was cultured alone or with HL60 or HL60-derived granulocytoids for 5 h at the indicated MOIs as described in Materials and Methods. The figure shows percent killing by HL60 granulocytoids (solid bars) or undifferentiated HL60 (hatched bars). The results presented are the means of three independent experiments. Bars represent the standard error.
To determine whether the difference in hyphal growth rates (Fig. 4) in the presence and absence of HL60 granulocytoids was statistically significant, hyphal growth rate was represented by the slope of the graph of hyphal length versus time. The two slopes were compared by the use of Student's t test for comparison of two slopes proposed by Jerrold H. Zar (77).
To evaluate the changes in gene expression determined by quantitative PCR (Fig. 8) statistically, they were analyzed by the Mann-Whitney test.
FIG. 8.
Quantitative RT-PCR analysis. Relative RNA levels of HNP1, N.E., HBEGF, and PAC1 measured by quantitative RT-PCR in RNA from HL60 granulocytoids or HL60 granulocytoids exposed to different MOIs (0.1, 0.5, and 5) of C. albicans for 1 h. RNA levels were measured relative to the amount of ACTB mRNA as described in Materials and Methods. Results are presented as the increase over expression in uninfected HL60 granulocytoids. Bars represent the standard error.
RESULTS
Expression of cell surface markers.
The expression of six cell surface markers was determined during the course of dimethyl formamide-induced differentiation with flow cytometry. These markers were chosen because of their expression on granulocytes and their roles in normal neutrophil function. They are antigens expressed on professional phagocytes (such as CD13 and CD14); antigens implicated in the phagocytosis of Candida, including complement receptor C3R (CD11b), FcγRIIIB (CD16b) and mannose receptor; and receptors for cytokines (granulocyte-macrophage colony stimulating factor) that are known to affect neutrophil function (CD116).
Figure 1 shows the specific binding of fluorescently labeled antibodies to a day 4 differentiated cell population. By comparison to binding of the corresponding idiotypic negative control, the figure shows that the dimethyl formamide-induced neutrophils expressed the CD11b, CD14, CD13, and CD116 antigens. The differentiated cells were uniformly negative for CD16b and mannose receptor expression. Antigen expression was also monitored on days 2 and 7 of dimethyl formamide treatment (data not shown). However, a 4-day differentiation regimen was chosen because the resulting cell population was the most homogeneous in terms of cell surface marker expression and most effective at killing C. albicans (data not shown).
FIG. 1.
Expression of cell surface markers on 4-day dimethyl formamide-treated HL60 cells. Expression of cell surface markers was detected by binding of fluorescently tagged antibodies recognizing the markers in question. The figure shows the number of cells binding antibody (y axis) and the intensity of fluorescence (x axis) determined by flow cytometry. Specific binding was determined by comparing the binding profile of anti-CD11b, anti-CD116, anti-CD14, anti-mannose receptor, and anti-CD16b (filled profile) to the binding profile of the corresponding isotypic negative controls (described in Materials and Methods).
HL60 granulocytoids mediate C. albicans killing.
To determine whether HL60 granulocytoids possess candidacidal properties, Candida colony formation was evaluated after 5 h of coincubation. CFU were determined in Candida cultures grown alone, with undifferentiated HL60 cells, or with HL60 granulocytoids for 5 h (Fig. 2). Statistical analysis of the candidacidal activities of HL60 granulocytoids and of undifferentiated HL60 cells with the Mann-Whitney test revealed that, at MOIs of 0.1 and 0.5, killing by HL60 granulocytoids was significantly elevated over that of undifferentiated cells (P value of 0.01). However, a similar analysis indicated that HL60 granulocytoids were not able to kill C. albicans to significant levels at an MOI of 5 (P value of 0.2). Although it would appear from Fig. 2 that Candida killing is more effective at an MOI of 0.1 (68% killing) than at an MOI of 0.5 (45% killing), the difference in the levels of killing at these MOIs was not statistically significant (P value of 0.2). Results presented are an average of three independent experiments. Within each experiment, each sample was analyzed in triplicate.
HL60 granulocytoids inhibit hyphal elongation.
To further investigate the nature of the host-pathogen interaction, photographs were taken early in the coculture, namely 1.5 h postinfection (Fig. 3). When GFP-expressing C. albicans was cultured at 37°C, 5% CO2 and in serum-containing medium, hyphal formation was induced. The hyphal length of C. albicans cultured alone was not affected by cell density. (Fig. 3, compare GFP images in last column). However, examination of the Candida in a coculture with HL60 granulocytoids revealed a decrease in hyphal growth (Fig. 3, compare GFP images in last column with corresponding images in middle column). The extent of inhibition depended on the ratio of C. albicans cells to HL60 granulocytoid cells. At low MOIs (0.1 and 0.5) the effect was more striking than at the highest ratio tested.
Each of the panels in the first column is the result of superimposing three images: one with phase contrast, one to detect DAPI-positive cells, and one to detect GFP-expressing Candida. These images show that for MOIs of 0.1 and 0.5, most of the C. albicans population has been engulfed by the HL60 granulocytoids, either partially or entirely. At the highest MOI, it is not as easy to determine the degree of Candida internalization or hyphal inhibition, although it is clear that all the C. albicans appear to be closely associated with HL60 granulocytoids. Coculture with undifferentiated HL60 cells had no effect on hyphal growth (data not shown). There was no detectable difference in the mortality of the HL60 granulocytoid population at the three different MOIs, as assessed by DAPI staining.
Hyphal length.
To quantitate the effect of HL60 granulocytoids on Candida hyphal growth, hyphal length was measured by video microscopy at intervals of 60 min. An average hyphal length was determined for all candidal cells in three microscopic fields. Figure 4 provides a quantitative measure of the hyphal growth of GFP-expressing C. albicans in the presence and absence of HL60 granulocytoids (Candida-granulocytoid ratio = 0.3). In contrast to an average length of 137 μm in the absence of HL60 granulocytoids, Candida in the coculture, produced hyphae averaging only 42 μm at the end of the observation period (6 h). To ensure that GFP expression was not affecting the result, the experiment was repeated with wild-type C. albicans (SC5314). Similar results were obtained (data not shown). The differences were shown to be statistically significant with a Student's t test at a P value of 0.001.
HL60 granulocytoid killing by C. albicans.
To determine the fate of HL60 granulocytoids exposed to C. albicans, the viability of the granulocytoid cells was determined every hour after they were infected with the GFP-expressing C. albicans cells at an MOI of 0.3. Table 2 shows that between 6 and 7 h postinfection, Candida induced a significant level of mortality in the HL60 granulocytoids. A control HL60 granulocytoid population had 85% DAPI-negative viable cells even after 16 h in culture. In sharp contrast, only 5% of C. albicans-infected HL60 granulocytoids were viable at this time.
Figure 5 provides a visual appreciation of this interaction after 1.5 h (A) and 6 h (B) of coculture. We note that HL60 granulocytoids that have internalized Candida are not necessarily the ones that have taken up the DAPI stain. Of the cells that have DAPI-positive nuclei, only some have visibly ingested Candida. This would indicate either that cell death is not a direct consequence of phagocytosis of C. albicans or that the ingested particle is no longer visible because it has already been digested by the granulocytoid. In the bottom two panels, images captured at lower magnification (200×) allow the reader to get a more global view of the culture. It is clear that a significantly larger proportion of cells are DAPI positive in the coculture with C. albicans than in the HL60 granulocytoids cultured alone for 6 h.
To determine whether the cause of death was apoptosis, nuclear morphology was examined in all the cells in microscopic fields chosen at random from the two populations of HL60 granulocytoids, uninfected and infected with C. albicans. In the case of the un-infected cells, out of 133 cells counted (three microscopic fields), none were DAPI positive. In contrast, in the infected population, it was not possible to count all the cells in phase contrast due to dense overgrowth by C. albicans hyphae. Therefore DAPI-positive nuclei were counted. In eight microscopic fields, 138 positive nuclei were counted. Thirty-two of these were seen to have nuclear condensation (Fig. 5C, arrow) and in six others, nuclear fragmentation was evident (Fig. 5C, arrowhead). The remaining 100 did not display morphological features indicative of apoptosis, but may well be in the early stages of apoptosis. Nuclear condensation and fragmentation are evident relatively late in the process (16). These changes are clearly indicative of apoptotic death.
In addition, a 2.5- to 3.5-fold increase in caspase-3 activity was detected. Caspase-3 activity of 11.095 ± 0.036 pmol/ml/min in uninfected HL60 granulocytoids increased to 37.27 ± 0.8 pmol/ml/min. in the presence of C. albicans at an MOI of 0.3. Addition of the caspase inhibitor DEVD-CHO decreased enzyme activity to 0.14 ± 0.01 pmol/ml/min, again pointing to apoptosis as the method of cell death.
Determination of granulocytoid expression profiles.
Since HL60 granulocytoids display both candidastatic and candidacidal activities, we wanted to characterize the effect of this pathogen on host global gene expression profiles. HL60 granulocytoids were incubated with growth medium alone or with C. albicans at an MOI of 0.5 for 1 h at 37°C and 5% CO2.
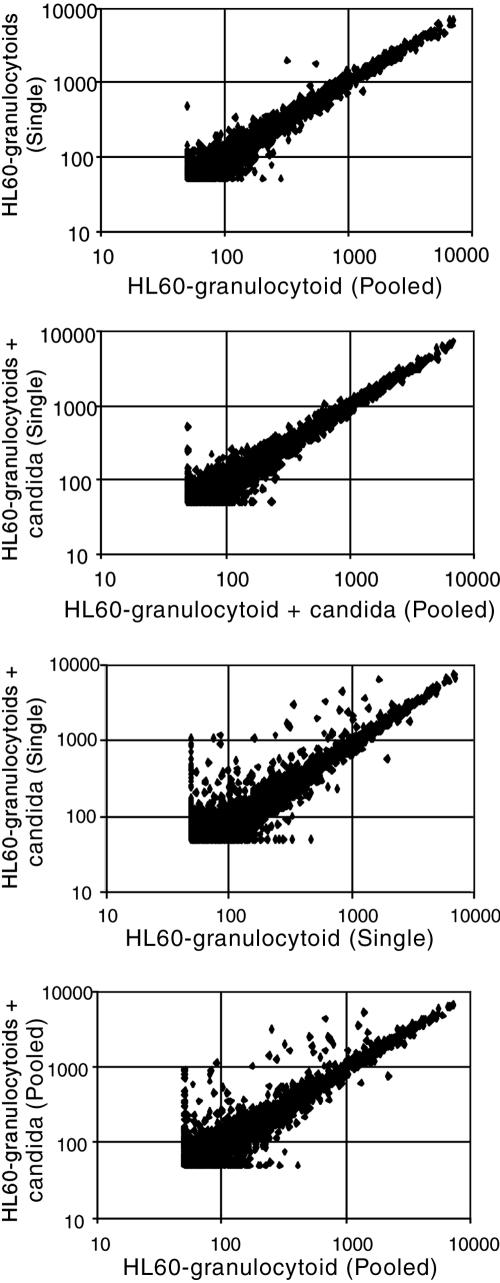
Comparison of the expression profiles indicated that there were very few differences between the pooled and single HL60 granulocytoid RNA samples (Fig. 6A). Only 20 genes out of the 7,000 tested had expression levels that were different by more than 2.5-fold between the two samples. These genes were excluded from further analysis. This suggests that the differentiation regimen is reproducible. Similarly HL60 granulocytoid+candida pooled was not significantly different from HL60 granulocytoid+candida single (Fig. 6B). However, when HL60 granulocytoid single was compared to HL60 granulocytoid+candida single (Fig. 6C) or HL60 granulocytoid pooled was compared to HL60 granulocytoid+candida pooled (Fig. 6D), a large number of changes were observed in both cases.
FIG. 6.
Scatter plot analysis of microarray data. (A) Comparison of the levels of expression of 7,000 genes in HL60 granulocytoids (HL60 granulocytoid single) with levels in pooled RNA from three extractions (HL60 granulocytoid pooled). (B) HL60 granulocytoids exposed to Candida at an MOI of 0.5 for 1 h (HL60 granulocytoid+candida single) with pooled RNA from independent extractions of neutrophils exposed to Candida at an MOI of 0.5:1 for 1 h (HL60 granulocytoid+candida pooled). (C) HL60 granulocytoid single compared to HL60 granulocytoid+candida single. (D) HL60 granulocytoid pooled compared to HL60 granulocytoid+candida pooled.
Table 3 lists the changes in expression in HL60 granulocytoids subjected to an infectious challenge. Only changes greater than 2.5-fold (upregulation) or 1.9-fold (downregulation) that were reproducible in the following experiment (described below) have been listed. The table compares the results from the single versus the pooled experiments. It is important to note that these two comparisons gave very similar changes in gene expression, both qualitatively and quantitatively, again pointing to the reproducibility of the system. Finally, the expression of three genes, β-actin (ACTB), phosphoglycerate kinase (PGK), and human secreted cyclophilin-like protein (SCYLP), was used as an internal control to verify equal input of RNA, labeling, and hybridization. It was considered important to use more than one such RNA because it is not known if they might themselves be affected by exposure to the pathogen. All three remained relatively constant through all the experiments. Therefore, the changes described are significantly over experimental variation.
TABLE 3.
Microarray dataa
| Change | Genes | Change (fold)
|
Ratio, single/pooled | |
|---|---|---|---|---|
| Single | Pooled | |||
| Upregulation | Early response genes | |||
| EGR3 | 20.8 | 17.9 | 1.2 | |
| MINOR | 16.9 | 10.2 | 1.6 | |
| TR3 | 14.2 | 11.0 | 1.3 | |
| NGFIB | 11.6 | 9.3 | 1.2 | |
| EGR1 | 4.0 | 2.7 | 1.5 | |
| EGR2 | 20.7 | 18.6 | 1.1 | |
| GOS8 | 4.7 | 5.3 | 0.9 | |
| GOS3 | 10.4 | 9.9 | 1.0 | |
| GOS2 | 13.8 | 12.7 | 1.1 | |
| PAC1 | 14.1 | 14.4 | 1.0 | |
| PC3 | 6.7 | 5.3 | 1.3 | |
| PPK | 4.8 | 3.8 | 1.3 | |
| C8FW | 4.7 | 4.6 | 1.0 | |
| Inflammatory cytokines and their targets | ||||
| TNFA | 4.7 | 5.9 | 0.8 | |
| IL1B | 5.1 | 4.7 | 1.1 | |
| MCSF1 | 7.4 | 7.1 | 1.0 | |
| COX2 | 17.8 | 15.8 | 1.1 | |
| HBEGF | 7.4 | 7.9 | 0.9 | |
| TSG6 | 4.1 | 4.8 | 0.8 | |
| MIP3A | 6.0 | 4.8 | 1.2 | |
| MIP1A | 8.8 | 12.7 | 0.7 | |
| SCYA2 | 5.3 | 6.5 | 0.8 | |
| MCP2 | 4.1 | 3.0 | 1.4 | |
| T. F. | 6.5 | 6.2 | 1.0 | |
| GROB | 8.4 | 6.7 | 1.2 | |
| Downregulation | CEBPA | 0.18 | 0.17 | 1.0 |
| IFNGR A chain | 0.40 | 0.50 | 0.8 | |
| P. C. | 0.15 | 0.20 | 0.7 | |
| CMYC | 0.32 | 0.20 | 1.3 | |
| Internal Controls | ACTB | 1.0 | 0.8 | 1.2 |
| PGK | 0.9 | 0.9 | 1.0 | |
| SCYLP | 1.2 | 0.9 | 1.3 | |
Changes in expression of individual genes in HL60 granulocytoids exposed to C. albicans at an MOI of 0.5:1 for an hour. Data are expressed as the ratio of expression in HL60 granulocytoids exposed to C. albicans over expression in HL60 granulocytoids. Data in the column single resulted from the ratios of expression in samples HL60 granulocytoid + candida single over HL60 granulocytoid single. Data in the column pooled resulted from the ratios of expression in samples H160 granulocytoid + candida pooled over HL60 granulocytoid pooled.
Inflammatory cytokines and their targets.
As shown in Table 3, expression of both interleukin-1β (IL1B) and tumor necrosis factor alpha (TNFA) was induced in the HL60 granulocytoids within an hour of exposure to C. albicans. A number of genes known to be regulated by these cytokines were also upregulated in response to infection. Cyclooxygenase-2 (COX2, PTGS2) encodes a key enzyme in prostaglandin synthesis and is expressed more abundantly in the infected HL60 granulocytoids. TSG6 was isolated as a TNF-α-inducible gene and its hyaluronate-binding domain implicates it in cell-cell contact. mRNA for proteins playing a role in cell-cell signaling important in the inflammatory process are also more abundant in the presence of C. albicans. The chemokines macrophage inflammatory protein 1-alpha (MIP-1α) and exodus (MIP-3α, SCYA-20) are examples of such proteins.
Cell cycle and apoptosis.
A number of genes encoding products known to mediate a cell-stimulatory signal were upregulated. These gene products include members of the steroid/thyroid nuclear receptor super family such as early growth response 1, 2, and 3 proteins (EGR1, EGR2, and EGR3). Heparin-binding epidermal growth factor-like growth factor (HBEGF), which is a member of the epidermal growth factor (EGF) family of growth factors and that can bind the EGF receptor and induce mitogenic and/or chemotactic activities, was also upregulated. Extracellularly regulated kinase 3 (ERK3), a mitogen-activated protein kinase, was also induced in response to C. albicans. Genes encoding orphan receptors such as nerve growth factor induced-B (NGFI-B, also known as TR3 and Nur77), transcriptionally inducible nuclear receptor (TINUR), mitogen-induced nuclear orphan receptor (MINOR, also known as NOR1), and NOT are upregulated. Members of this family are known to mediate both cell proliferative and apoptotic stimuli.
Downregulation of CEBPA.
As seen in Table 3, CCAAT enhancer binding protein alpha (CEBPA) mRNA levels were 5- to 6-fold lower in HL60 granulocytoids exposed to C. albicans than in uninfected HL60 granulocytoids. C/EBP-α controls the expression of a number of myeloid-specific gene products, including antimicrobial proteins such as the human neutrophil peptide-1 (HNP1, DEFA1) defensin and neutrophil elastase (N.E., ELA2). We therefore expected to see downregulation of these genes as a consequence of the observed downregulation of CEBPA expression. This was not the case for a number of known C/EBP-α targets. We thus determined if exposure to higher MOIs of Candida caused more marked downregulation of the upstream factor. This in turn might result in a more significant decrease in the expression of downstream target genes. In addition, we had observed an MOI-dependent decrease in candidacidal activity. The two factors prompted us to determine the expression profiles of HL60 granulocytoids exposed to two different MOIs of Candida.
HL60 granulocytoid response to different C. albicans MOIs.
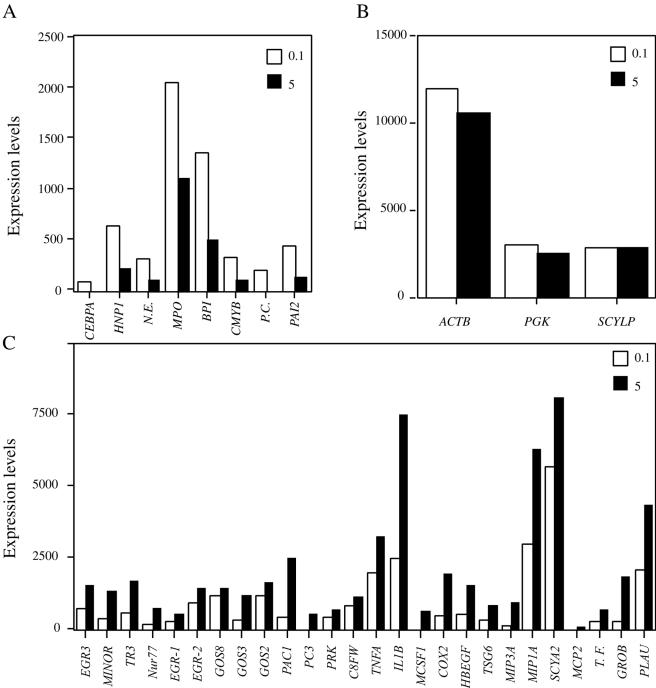
To determine whether exposure of HL60 granulocytoids to higher MOIs of C. albicans may affect the expression of some genes, RNA was prepared from HL60 granulocytoids exposed to C. albicans at MOIs of 0.1 and 5. Comparison of HL60 granulocytoid expression profiles with the Affymetrix gene chips under these two conditions showed that there were in fact genes whose expression was lower at an MOI of 5 than at MOI of 0.1. In support of the data in Table 3, CEBPA, coding for a myeloid-specific transcription factor is one such gene (Fig. 7A). Interestingly the expression of CEBPA target genes encoding antimicrobial proteins such as HNP1, N.E., myeloperoxidase (MPO), and bactericidal/permeability increasing protein (BPI) followed the same pattern. Other genes that had a similar expression profile were the cellular homologue of the avian myeloblastosis virus transforming gene (CMYB), plasminogen activator inhibitor type 2 (PAI2), and protein C (PC). While these results do not allow us to conclude that active downregulation has taken place, they are consistent with the downregulation observed in Table 3.
FIG. 7.
Graphic representation of RNA levels. Panel A shows genes whose expression levels were higher in HL60 granulocytoids exposed to C. albicans at an MOI of 0.1 than in HL60 granulocytoids exposed to C. albicans at an MOI of 5. Panel B shows genes whose expression levels remained unaffected in HL60 granulocytoids on exposure to C. albicans (internal controls). Panel C shows genes whose expression levels were lower in HL60 granulocytoids exposed to C. albicans at an MOI of 0.1 than in HL60 granulocytoids exposed to C. albicans at an MOI of 5.
In contrast, a number of genes were more highly expressed at the high MOI (5) than at the low MOI (0.1). This list was very similar to the one generated when only one Candida MOI was tested (Table 3). Thus, genes implicated in cell fate determination such as the early response genes and nuclear steroid orphan receptors are more highly expressed at the high MOIs. Expression of inflammatory cytokines and their targets follow the same pattern (Fig. 7C).
Figure 7B shows changes in expression levels for ACTB, PGK, and SCYLP. They remained essentially unchanged during the host-pathogen interaction.
Confirmation of microarray data by quantitative RT-PCR.
As confirmation of data generated by microarray analysis, changes in expression of four genes were verified by quantitative PCR with a Light Cycler. As shown in Fig. 8, the expression of HNP1 and N.E. was downregulated upon exposure to C. albicans at all MOIs. By contrast, the expression of HBEGF and PAC1 was induced in an MOI-dependent fashion. The figure combines data from six light cycler experiments representing three different RNA extractions. Changes in gene expression with respect to uninfected HL60 granulocytoids were statistically significant at all the MOIs tested and for all the genes studied (P value of 0.01) with the Mann-Whitney test.
Changes in human PMNL gene expression during an infectious challenge.
Since this study revealed changes in the expression of genes not previously associated with the response of granulocytes to an infectious challenge, it was considered important to verify some of them in primary human cells. As a first step, the human PMNL-C. albicans interaction was studied with the parameters established by HL60 granulocytoid-Candida coculture experiments. Microscopic evaluation of C. albicans colony formation and hyphal growth in the presence and absence of host cells revealed that, at the lower MOIs (0.1 and 0.5), human PMNL expressed stronger antifungal activity than the in vitro model, HL60 granulocytoids. At an MOI of 5, however, these differences were less evident (data not shown).
To measure changes in gene expression, human PMNL were exposed to C. albicans at MOIs of 0.1, 0.5, and 5 under the same conditions that were used for HL60 granulocytoid infection. RNA was extracted after 1 h of coculture as described in Materials and Methods. To confirm that the preparation was free of C. albicans nucleic acid contamination, RT-PCR analysis was performed with primers specific for C. albicans actin (60). Comparison of amplified product from PMNL cDNA with that from known amounts of total C. albicans cDNA indicated that the highest level of contamination was between 1 and 10 ng in 1 μg of PMNL RNA (Fig. 9A). This level of contamination does not change the amount of starting material significantly. In addition, none of the primers used for amplification of human genes shared significant homology with sequences in the C. albicans genome and did not give any specific amplification product when tested in a PCR with C. albicans cDNA (data not shown).
FIG. 9.
Semiquantitative analysis of changes in PMNL gene expression. (A) cDNAs corresponding to 1 μg of RNA extracted from uninfected PMNL or PMNL infected with C. albicans at an MOI of 0.1, 0.5, or 5 were subjected to PCR with primers specific for C. albicans actin. The amount of amplified fragment was compared to that from cDNA corresponding to 1,000, 100, 10, 1, and 0.1 ng of C. albicans RNA. (B) RT-PCR analysis visualized by Southern hybridization. Amplification was carried out for low (15 cycles for PAC1, PAI2, S28, and ACTB, 20 cycles for TNFA and TR3, 25 cycles for N.E., and 30 cycles for HNP1) or high (20 cycles for PAC1, PAI2, S28, and ACTB, 25 cycles for TNFA and TR3, 30 cycles for N.E., and 35 for HNP1) intensity. Numbers indicate the intensity of hybridization relative to that in uninfected PMNL (set to 1).
Human PMNL RNA was used in a semiquantitative RT-PCR analysis where amplification of each gene was monitored at 15, 20, 25, 30, and 35 cycles to determine relative amounts of the relevant transcript under subsaturating conditions of amplification. Shown in Fig. 9B are the results of Southern analysis for two of the most informative conditions. Upregulation of TNFA, TR3, and PAC1 was evident at both 15 and 20 cycles. Southern analysis of PCR-amplified N.E. revealed an upregulation at the lower MOIs, which was absent at the highest MOI, 5. A similar profile was noted for PAI2, although the changes were not as pronounced. HNP1 was downregulated upon the addition of C. albicans, even at the low MOIs. Figure 9 also shows upregulation of ACTB at both 15 and 20 cycles. To determine whether this was truly an upregulation or whether the differences in ACTB levels reflected differences in starting material, another housekeeping gene, S28, was chosen. Figure 9B shows that S28 levels were relatively constant.
DISCUSSION
We have studied the interaction of HL60 granulocytoids with C. albicans in an in vitro infection model. Granulocytic differentiation of HL60 myelomonocytic cells results in a granulocyte-like cell population that has been previously extensively characterized (7, 12, 29, 53). The differentiated cell population expresses a number of cell surface markers implicated in normal granulocyte function. Although one of these, CD16, was not detected, this is not expected to affect our results since antibody-mediated phagocytosis should not play a major role in our system. Importantly, the pattern of cell surface antigen expression shows that these cells are a homogeneous population.
Before studying changes in gene expression upon Candida challenge, we verified that the HL60-derived granulocytoids used in this study are comparable to a true PMNL population, in that they recognize, phagocytose and kill Candida In addition, hyphal growth in a C. albicans population that is cocultured with HL60 granulocytoids is significantly impaired. Finally, our studies show that, like true human neutrophils (67), they themselves undergo apoptosis on exposure to C. albicans. Therefore, in terms of both the homogeneity of the population and their functional characteristics, the HL60-derived granulocytoids provided an attractive model to study granulocyte-Candida interactions. Moreover, undifferentiated HL60 cells do not possess any significant level of microbicidal activity. Therefore, the possibility of using the immature progenitors as a negative control makes it possible to distinguish nonspecific effects of the presence of mammalian cells from the more specific elements of the host-pathogen interaction.
These studies allowed us to identify three Candida-host cell ratios, such that, at the lowest ratio, HL60 granulocytoids were much more effective in killing C. albicans than at the highest one (Fig. 2). To gain insight into the molecular mechanisms underlying these interactions, we determined HL60 granulocytoid gene expression profiles during their interaction with C. albicans under different degrees of infectious challenge. The initial experiments allowed us to determine the time frame in which changes in gene expression may be relevant. We observed that within 5 h of coculture, HL60 granulocytoids can cause significant Candida killing (Fig. 2) and close to this time (6 to 7 h), viability in the HL60 granulocytoid population is significantly reduced (Table 2 and Fig. 5). Therefore all gene expression profiles were measured early in the interaction (1 h postinfection).
The first step was to determine whether the granulocytic differentiation of HL60 cells and their interaction with Candida was reproducible enough to make this study feasible. RNA was analyzed either individually or as a pooled sample. Samples were pooled with the idea that if differentiation was very variable, many differences would be apparent between the individual and the pooled sample. This was not the case. Perhaps even more importantly, pooled samples were used to reduce background biological variation. It has been reported that expression of certain genes is exceptionally sensitive to small changes in the microenvironment such that expression could be upregulated at one time and downregulated at another time, even though identical conditions were used (31, 76). Therefore changes were considered to be significant only if they were seen in the pooled samples also. Furthermore, when additional experiments were carried out with different Candida MOIs, the expression of the same genes was affected. This again indicates that the HL60 granulocytoids were responding similarly and that the hybridization to the microarray was reproducible. Moreover, some of the key changes observed in HL60 granulocytoids were also seen with human PMNL exposed to Candida, pointing to the significance of these changes.
Of the genes tested, the most notable exception was ACTB in that it was upregulated during the human PMNL-Candida interaction (Fig. 9B) but stayed relatively constant in HL60 granulocytoids under similar conditions. Induction of ACTB in the early phase of the host-pathogen interaction is not surprising given its role in microbial pathogenesis (27) and regulation of transcription (35, 51). However, upregulation of ACTB was not detected in HL60 granulocytoids infected with C. albicans by either microarray (Fig. 7B) or quantitative RT-PCR analysis. Whether this reflects a minor difference between the two cell types, for instance, a difference in the time required for induction, or whether it is indicative of a more basic difference in the response of the two host cells to infection with Candida is not clear. However, it is unlikely to represent a major difference in the response of the two cell types given that more similarities (TNFA, PAC1, TR3, HNP1, N.E., PAI2) were detected than differences.
An important part of the host's response to an infectious challenge is to prolong the life of normally short-lived neutrophils (43) and inflammatory mediators such as IL-1β participate in this response (14). The observed increase in IL-1β expression in HL60 granulocytoids exposed to C. albicans may be representative of a similar phenomenon.
IL-1β (20) and TNF-α (3, 22, 75) are well known mediators of the inflammatory process and known to be expressed by neutrophils during an infectious challenge. Therefore, as expected, TNFA expression is upregulated in human PMNL within an hour of infectious challenge with C. albicans (Fig. 9B). IL1B, TNFA and their targets, COX2 (57), MIP1A, MIP3A (64), and tissue factor (TF) (6), are all upregulated in HL60 granulocytoids in response to a C. albicans challenge (Table 3). The observed expected upregulation of an inflammatory response in HL60 granulocytoids further supports their choice as a model for studies on host-pathogen interaction. A similar spectrum of genes was upregulated in human dendritic cells (TNFA, IL1B, COX2, MIP1A) during the early phase of infection by C. albicans, Escherichia coli, and influenza virus (30) and in PMNL (MIP3α, GROB, TNFA and MIP1α) during phagocytosis of immunoglobulin G and complement-coated beads (40).
Thus, there is an induction of an inflammatory response that is directly proportional to MOI. Why then are Candida not being killed as efficiently at the higher MOIs? We note that a number of genes encoding known antimicrobial products are not induced in this MOI-dependent fashion. In fact, the MPO, HNP1, and N.E. genes are less well expressed when neutrophils are exposed to a Candida MOI of 5 than when they are exposed to an MOI of 0.1.
Myeloperoxidase may play a major role in the neutrophils defense against C. albicans by provoking hyphal damage (19) since the hyphal form is more difficult to phagocytose and then kill (47). Interestingly, at higher MOIs, there is less myeloperoxidase RNA. It is tempting to speculate that Candida may have evolved a mechanism to downregulate the expression of proteins such as myeloperoxidase to escape the host cells.
The gene for human neutrophil protein 1 (HNP1), a defensin, is also expressed less well at an MOI of 5 than at an MOI of 0.1. HNP1 is expressed primarily in neutrophils and its expression is driven by the myeloid-specific CCAAT enhancer binding protein C/EBP-α (34). Interestingly, we observed a lower level of CEBPA mRNA at an MOI of 5 than at an MOI of 0.1, and this may be responsible for the observed similarities in the expression pattern of the HNP1 mRNA.
Serprocidin family serine proteases participate in the antimicrobial activity of the neutrophil either through their proteolytic activity, or by perturbation of membranes by direct insertion (72). The serprocidin family member neutrophil elastase (N.E.) has only modest direct antimicrobial effects. However, it can manifest microbicidal activity indirectly by cleaving cathelicidin proforms to generate active antimicrobial peptides (9). It is also expressed less well at an MOI of 5 than at an MOI of 0.1 in our studies with HL60 granulocytoids during an infectious challenge with Candida. CEBP-α and c-Myb, two factors that have been implicated in control of N.E. gene expression (5), share the same expression pattern.
Thus, most known anticandidal genes are not as efficiently expressed in HL60 granulocytoids exposed to a high C. albicans MOI as when the cells are exposed to a low MOI of Candida. This is suggestive of a mechanism by which Candida overcomes the host response. The fact that in primary human cells, too, the expression of both HNP1 and N.E. appears to be modulated by high Candida MOIs makes this observation particularly interesting and worthy of further study. Although HNP1 and N.E. are downregulated at all three MOIs in HL60 granulocytoids (Fig. 8), in human PMNL, HNP1 is downregulated but N.E. appears to be upregulated at MOIs of 0.1 and 0.5 and downregulated only at the highest MOI (MOI = 5).
It is not clear why changes in Candida MOI do not appear to affect N.E. expression in the same way in HL60 granulocytoids and PMNL. It is possible that the higher level of N.E. expression in resting PMNL and/or a stronger induction on infectious challenge require a higher MOI of the pathogen to modulate it. Perhaps, there is in fact an upregulation of N.E. gene expression in HL60 granulocytoids exposed to low MOIs but these MOIs are lower than 0.1 and have therefore been missed in this study. If N.E. was required for growth inhibition of Candida, the observation that at lower MOIs freshly isolated PMNL are more potent than HL60 granulocytoids is consistent with this scenario.
Yet another mRNA whose expression is modulated by Candida is the protease inhibitor PAI2. While the gene for the protease urokinase-type plasminogen activator (PLAU) (4, 41) is more highly expressed in HL60 granulocytoids exposed to a Candida MOI of 5 than in HL60 granulocytoids exposed to an MOI of 0.1, the gene for its inhibitor (PAI2) is not. This situation could lead to enhanced degradation of the extracellular matrix that could be exploited by the pathogen to penetrate host tissue.
Another role for PAI2 is in the inhibition of apoptosis. PAI2 has been implicated in the inhibition of apoptotic death of macrophage infected with Mycobacterium avium (26). Loss of phagocytic cells has obvious deleterious consequences for the innate immune system and therefore molecules such as PAI2 have an important role in preserving it. Perhaps blocking PAI2 induction is a strategy that Candida employs to decrease the anti-apoptotic activity in the cell. Of interest in this respect is a recent study by Kobayashi et al. (40), where global gene expression profiles were measured in human polymorphonuclear leukocytes during phagocytosis of IgG and complement-coated beads. Both in the study by Kobayashi et al. and in our model, the host cells undergo cell death on activation. A family of genes encoding orphan receptors TR3, NOR-1, and NURR1 in PMNL is upregulated in both systems. In addition we have verified that TR3 is also upregulated in primary human cells, PMNL, upon an infectious challenge with C. albicans. TR3 has been reported to mediate both cell proliferative and apoptotic stimuli (46). However, it is tempting to implicate this family of genes in host cell apoptosis as has been done by Kobayashi et al., since they are known to be crucial for T-cell and macrophage (37) apoptosis. Moreover, cell death follows soon after their induction in PMNL (36) during phagocytosis and on exposure to Candida (this study).
A number of pathogens downregulate host processes that are antagonistic to pathogen growth and survival (17, 18, 28, 54). Our studies also indicate that C. albicans may modulate host gene expression to its advantage by inhibiting the activation of microbicidal pathways. In support of this, Cryptococcus neoformans and C. albicans produce immunomodulatory prostaglandins (56) and Candida has been shown to release an immune modulator that blocks neutrophil killing (70). Our studies provide insight into the molecular mechanisms underlying immunosuppression.
In conclusion, our results demonstrate an upregulation of an inflammatory response and a downregulation of the anti-Candida responses of HL60-derived neutrophils in an MOI-dependent fashion. We have also verified some of these changes in freshly isolated human PMNL infected with C. albicans. HL60 granulocytoids thus represent a valid model to study granulocyte-Candida interactions. Our study reveals a number of changes in gene expression that may reflect the survival strategies of the two cell types during host-pathogen interaction.
Acknowledgments
We thank Edwin Chang for help with statistical analysis and Doreen Harcus and Christiane Cantin for helpful suggestions.
This work was supported by grants from the National Research Council.
Editor: T. R. Kozel
Footnotes
This is National Research Council publication number 46168.
REFERENCES
- 1.Belcher, R. W., J. F. Carney, and F. G. Monahan. 1973. An electron microscopic study of phagocytosis of Candida albicans by polymorphonuclear leukocytes. Lab. Investig. 29:620-626. [PubMed] [Google Scholar]
- 2.Bjerknes, R. 1984. Flow cytometric assay for combined measurement of phagocytosis and intracellular killing of Candida albicans. J. Immunol. Methods 72:229-241. [DOI] [PubMed] [Google Scholar]
- 3.Blanchard, D. K., J. Y. Djeu, T. W. Klein, H. Friedman, and W. E. Stuart. 1988. Protective effects of tumor necrosis factor in experimental Legionella pneumophilia infections of mice via activation of PMN function. J. Leukoc. Biol. 43:429-435. [DOI] [PubMed] [Google Scholar]
- 4.Blasi, F., J.-D. Vassali, and K. Dano. 1987. Urokinase-type plasminogen activator: proenzyme, receptor and inhibitors. J. Cell Biol. 104:801-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borregaard, N., and J. B. Cowland. 1997. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood 89:3503-3521. [PubMed] [Google Scholar]
- 6.Carmeliet, P., and D. Collen. 1998. Molecules in focus Tissue factor. Int. J. Biochem. Cell Biol. 30:661-667. [DOI] [PubMed] [Google Scholar]
- 7.Cellier, M., C. Shustik, W. Dalton, E. Rich, J. Hu, D. Malo, E. Schurr, and P. Gros. 1997. Expression of the human NRAMP1 gene in professional primary phagocytes:studies in blood cells and in HL-60 promyelocytic leukemia. J. Leukoc. Biol. 61:96-105. [DOI] [PubMed] [Google Scholar]
- 8.Challacombe, S. J. 1990. Immunology of oral candidosis. In L. P. Samaranayak and T. W. MacFarlane (ed.), Oral candidosis. Wright, London, United Kingdom
- 9.Cole, A. M., J. Shi, A. Ceccarelli, A. P. Kim, and T. Ganz. 2001. Inhibition of neutrophil elastase prevents cathelicidin activation and impairs clearance of bacteria from wounds. Blood 97:297-304. [DOI] [PubMed] [Google Scholar]
- 10.Collins, S. J. 1987. The HL-60 promyelocytic leukemia cell line: proliferation, differentiation, and cellular oncogene expression. Blood 70:1233-1244. [PubMed] [Google Scholar]
- 11.Collins, S. J., R. C. Gallo, and R. E. Gallagher. 1977. Continuous growth and differentiation of human myeloid leukemic cells in suspension culture. Nature 270:347-349. [DOI] [PubMed] [Google Scholar]
- 12.Collins, S. J., F. W. Ruscetti, R. E. Gallagher, and R. C. Gallo. 1979. Normal functional characterizatics of cultured human promyelocytic leukemia cells (HL-60) after induction of differentiation by DMSO. J. Exp. Med. 149:969-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collins, S. J., F. W. Ruscetti, R. E. Gallagher, and R. C. Gallo. 1978. Terminal differentiation of human promyelocytic leukemia cells induced by dimethyl sulfoxide and other polar compounds. Proc. Natl. Acad. Sci. USA 75:2458-2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colotta, F., F. Re, N. Polentarutti, S. Sozanni, and A. Mantovani. 1992. Modulation of granulocyte survival and programmed cell death by cytokines and bacterial products. Blood 80:2012-2020. [PubMed] [Google Scholar]
- 15.Cormack, B. P., G. Bertram, M. Egerton, N. A. R. Gow, S. Falklow, and A. J. P. Brown. 1997. Yeast-enhanced green fluorescent protein (yEGFP): a reporter of gene expression in Candida albicans. Microbiology 143:303-311. [DOI] [PubMed] [Google Scholar]
- 16.Darynkiewicz, Z., G. Juan, X. Li, W. Gorczyca, T. Murakami, and F. Traganos. 1997. Cytometry in cell necrobiology: analysis of apoptosis and accidental cell death (necrosis). Cytometry 27:1-20. [PubMed] [Google Scholar]
- 17.Detweiler, C. S., D. B. Cunanan, and S. Falkow. 2001. Host microarray analysis reveals a role for the Salmonella response regulator phoP in human macrophage cell death. Proc. Natl. Acad. Sci. USA 98:5850-5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Devaney, E., and M. Yazdanbakhsh. 2001. Prospects and challenges in lymphatic filariasis. Parasite Immunol. 23:323-325. [DOI] [PubMed] [Google Scholar]
- 19.Diamond, R. D., S. C. Clark, and C. C. Haudenschild. 1980. Damage to Candida albicans hyphae and pseudohyphae by the myeloperoxidase system and oxidative products of neutrophil metabolism in vitro. J. Clin. Investig. 66:908-917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dinarello, C. A. 1996. Biologic basis for Interleukin-1 in disease. Blood 87:2095-2147. [PubMed] [Google Scholar]
- 21.Djeu, J. Y., D. K. Blanchard, D. Halkias, and H. Friedman. 1986. Growth inhibition of Candida albicans by human polymorphonuclear neutrophils: activation by interferon-γ and tumor necrosis factor. J. Immunol. 137:2980-2984. [PubMed] [Google Scholar]
- 22.Djeu, J. Y., D. K. Blanchard, A. L. Richards, and H. Friedman. 1988. TNF induction by C. albicans from human natural killer cells and monocytes. J. Immunol. 141:4047-4052. [PubMed] [Google Scholar]
- 23.Fridkin, S., and W. R. Jarvis. 1996. Epidemiology of nosocomial fungal infections. Clin. Microbiol. Rev. 9:499-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gahmberg, C. G., K. Nilsson, and L. C. Andersson. 1979. Specific changes in the surface glycoprotein pattern of human promyelocytic leukemia cell line HL-60 during morphologic and functional differentiation. Proc. Natl. Acad. Sci. USA 76:4087-4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gallagher, R., S. Collins, J. Trujillo, K. McCredie, M. Ahearn, S. Tsai, R. Metzger, G. Aulakh, R. Ting, F. Rucetti, and R. Gallo. 1979. Characterization of the continuous differentiating myeloid cell line (HL60) from a patient with acute promyelocytic leukemia. Blood 54:713-733. [PubMed] [Google Scholar]
- 26.Gan, H., G. W. Newman, and H. G. Remold. 1995. Plasminogen activator inhibitor type 2 prevents programmed cell death of human macrophages infected with Mycobacterium avium, serovar 4. J. Immunol. 155:1304-1315. [PubMed] [Google Scholar]
- 27.Gruenheid, S., and B. Finley. 2003. Microbial pathogenesis and cytoskeletal function. Nature 422:775-781. [DOI] [PubMed] [Google Scholar]
- 28.Haig, D. M., and S. Fleming. 1999. Immunomodulation by virulence proteins of the parapoxvirus orf virus. Vet. Immunol. Immunopathol. 72:81-86. [DOI] [PubMed] [Google Scholar]
- 29.Harris, P., and P. Ralph. 1985. Hum. Leukemic models of myelomonocytic development: a review of the HL-60 and U937 cell lines. J. Leukoc. Biol. 37:407-422. [DOI] [PubMed] [Google Scholar]
- 30.Huang, Q., D. Liu, P. Majewski, L. C. Schulte, J. M. Korn, R. A. Young, E. S. Lander, and N. Hacohen. 2001. The plasticity of dendritic cell responses to pathogens and their components. Science 294:870-875. [DOI] [PubMed] [Google Scholar]
- 31.Hughes, T. R., M. J. Marton, A. R. Jones, C. J. Roberts, R. Stoughton, C. D. Armour, H. A. Bennett, E. Coffey, H. Dai, Y. D. He, M. J. Kidd, A. M. King, M. R. Meyer, D. Slade, P. Y. Lum, S. B. Stepaniants, D. D. Shoemaker, D. Gachotte, K. Chakraburtty, J. Simon, M. Bard, and S. H. Friend. 2000. Functional discovery via a compendium of expression profiles. Cell 102:109-126. [DOI] [PubMed] [Google Scholar]
- 32.Itoh, K., K. Okubo, H. Utiyama, T. Hirano, J. Yoshii, and K. Matsubara. 1998. Expression profiles of active genes in granulocytes. Blood 92:1432-1441. [PubMed] [Google Scholar]
- 33.Kagaya, K., and Y. Fukazawa. 1981. Murine defense mechanism against Candida albicans infection. II. Opsonization, phagocytosis and intracellular killing of C. albicans. Microbiol. Immunol. 25:807-818. [DOI] [PubMed] [Google Scholar]
- 34.Kaiser, V., and G. Diamond. 2000. Expression of mammalian defensin genes. J. Leukoc. Biol. 68:779-784. [PubMed] [Google Scholar]
- 35.Kang, K. W., S. J. Lee, J. W. Park, and S. G. Kim. 2002. Phospphatidylinositol 3-kinase regulates nuclear translocation of NF-E2-related factor 2 through actin rearrangements in response to oxidative stress. Mol. Pharmacol. 62:1001-1010. [DOI] [PubMed] [Google Scholar]
- 36.Kim, M. H., G. E. Rodey, R. A. Good, R. A. Chilgren, and P. G. Quie. 1969. Defective candidacidal capacity of polymorphonuclear leukocytes in chronic granulomatous disease of childhood. J. Pediatr. 75:300-303. [DOI] [PubMed] [Google Scholar]
- 37.Kim, S. O., K. Ono, P. S. Tobias, and J. Han. 2003. Orphan nuclear receptor Nur77 is involved in caspase-independent macrophage cell death. J. Exp Med. 197:1441-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kirkpatrick, C. H., R. R. Rich, and J. E. Bennett. 1971. Chronic mucocutaneous candidiasis: model building in cellular immunity. Ann. Intern. Med. 74:955-978. [DOI] [PubMed] [Google Scholar]
- 39.Kobayashi, S. D., and J. E. Cutler. 1998. Candida albicans hyphal formation and virulence: is there a clearly defined role? Trends Microbiol. 6:92-94. [DOI] [PubMed] [Google Scholar]
- 40.Kobayashi, S. D., J. M. Voyich, C. L. Buhl, R. M. Stahl, and F. R. DeLeo. 2002. Global changes in gene expression by human polymorphonuclear leukocytes during receptor-mediated phagocytosis: Cell fate is regulated at the level of gene expression. Proc. Natl. Acad. Sci. USA 99:6901-6906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kruithof, E. K. O., M. S. Baker, and C. Bunn. 1995. Biological and clinical aspects of plasminogen activator inhibitor type 2. Blood 86:4007-4024. [PubMed] [Google Scholar]
- 42.Leberer, E., K. Ziegelbauer, A. Schmidt, D. Harcus, D. Dignard, J. Ash, L. Johnson, and D. Y. Thomas. 1997. Virulence and hyphal formation of Candida albicans require the Ste20p-like protein kinase CaCla4p. Curr. Biol. 7:539-546. [DOI] [PubMed] [Google Scholar]
- 43.Lee, A., M. K. B. Whyte, and C. Haslett. 1993. Inhibition of apoptosis and prolongation of neutrophil functional longevity by inflammatory mediators. J. Leukoc. Biol. 54:283-288. [PubMed] [Google Scholar]
- 44.Lehrer, R., and M. J. Cline. 1969. Leukocyte myeloperoxidase deficiency and disseminated candidiasis: the role of myeloperoxidase in resistance to Candida infection. J. Clin. Investig. 48:1478-1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leigh, J. E., C. Steele, F. L. Wormley, W. Luo, R. A. Clark, W. Gallaher, and P. L. Fidel. 1998. Th1/Th2 cytokine expression in saliva of human immunodeficiency virus-positive and human immunodeficiency virus-negative individuals with oropharyngeal candidiasis. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 19:373-380. [DOI] [PubMed] [Google Scholar]
- 46.Li, H., S. K. Kolluri, J. Gu, M. I. Dawson, X. Cao, P. D. Hobbs, B. Lin, G. Chen, J. Lu, F. Lin, Z. Xie, J. A. Fontana, J. C. Reed, and X. Zhang. 2000. Cytochrome c release and apoptosis induced by mitochondrial targeting of nuclear orphan receptor TR3. Science 289:1159. [DOI] [PubMed] [Google Scholar]
- 47.Lo, H.-J., J. R. Kohler, B. DiDomenico, D. Loebenberg, A. Cacciapuoti, and G. R. Fink. 1997. Nonfilamentous C. albicans mutants are avirulent. Cell 90:939-949. [DOI] [PubMed] [Google Scholar]
- 48.Madhani, H. D., and G. R. Fink. 1998. The control of filamentous differentiation and virulence in fungi. Trends Cell Biol. 8:348-353. [DOI] [PubMed] [Google Scholar]
- 49.Malech, H. L., and J. I. Gallin. 1987. Neutrophils in human disease. N. Engl. J. Med. 317:687-694. [DOI] [PubMed] [Google Scholar]
- 50.Martin, E., and S. Bhakdi. 1991. Quantitative analysis of opsonophagocytosis and of killing of Candida albicans by human peripheral blood leukocytes by with flow cytometry. J. Clin. Microbiol. 29:2013-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miralles, F., G. Posern, A. I. Zaromytidou, and R. Treisman. 2003. Actin dynamics control SRF activity by regulation of its coativator MAL. Cell 113:329-342. [DOI] [PubMed] [Google Scholar]
- 52.Mitchell, A. P. 1998. Dimorphism and virulence in Candida albicans. Curr. Opin. Microbiol. 1:687-692. [DOI] [PubMed] [Google Scholar]
- 53.Mukherjee, A. B., R. J. Czirbik, N. Z. Parsa, and J. R. Testa. 1985. Induction of terminal differentiation and nuclear appendage(s) formation in a human myeloid leukemia cell line (HL60). Cytobios 44:109-118. [PubMed] [Google Scholar]
- 54.Nash, P., A. Whitty, J. Handwerker, J. Macen, and G. McFadden. 1998. Inhibitory specificity of the anti-inflammatory myxoma virus serpin, Serp-1. J. Biol. Chemis. 273:20982-20991. [DOI] [PubMed] [Google Scholar]
- 55.Newburger, P. E., M. E. Chovaniec, J. S. Greenburger, and H. Cohen. 1979. Functional changes in human leukemic cell line HL60. J. Cell Biol. 82:315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Noverr, M. C., S. M. Phare, G. B. Toews, M. J. Coffey, and G. B. Huffnagle. 2001. Pathogenic yeasts Cryptococcus neoformans and Candida albicans produce immunomodulatory prostaglandins. Infect. Immun. 69:2957-2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.O'Banion, M. K., V. D. Winn, and D. A. Young. 1992. cDNA cloning and functional activity of a glucocorticoid-regulated inflammatory cyclooxygenase. Proc. Natl. Acad. Sci. USA 89:4888-4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Odds, F. C. 1988. Candida and candidosis, 2nd ed. Bailliere Tindall, London, United Kingdom
- 59.Odds, F. C. 1996. Epidemiological shifts in opportunistic and nosocomial Candida infections: mycological aspects. Int. J. Antimicrob. Agents 6:141-144. [DOI] [PubMed] [Google Scholar]
- 60.Okeke, C. N., R. Tsuboi, and H. Ogawa. 2001. Quantification of Candida albicans actin mRNA by the light cycler system as a means of assessing viability in a model of cutaneous candidiasis. J. Clin. Microbiol. 39:3491-3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Okubo, K., K. Itoh, A. Fukushima, J. Yoshii, and K. Matsubara. 1995. Monitoring cell physiology by expression profiles and discovering cell type-specific genes by compiled expression profiles. Genomics 30:178-186. [DOI] [PubMed] [Google Scholar]
- 62.Read, R. A., E. E. Moore, F. A. Moore, V. S. Carl, and A. Banerjee. 1993. Platelet-activating factor-induced polymorphonuclear priming independent of CD 11B adhesion. Surgery 114:308-313. [PubMed] [Google Scholar]
- 63.Rocha, C. R., K. Schroppel, D. Harcus, A. Marcil, D. Dignard, B. N. Taylor, D. Y. Thomas, M. Whiteway, and E. Leberer. 2001. Signaling through adenylyl cyclase is essential for hyphal growth and virulence in the pathogenic fungus Candida albicans. Mol. Biol. Cell. 12:3631-3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rollins, B. J. 1997. Chemokines. Blood 90:909-928. [PubMed] [Google Scholar]
- 65.Romani, L. 2001. Innate immunity against fungal pathogens, p. 401-432. In R. C. Calderone (ed.), Fungal pathogenesis: principles and clinical applications. Marcel Dekker Inc., New York, N.Y.
- 66.Romani, L., and S. H. E. Kaufmann. 1998. Immunity to fungi. Res. Immunol. 149:277-281. [DOI] [PubMed] [Google Scholar]
- 67.Rotstein, D., J. Parodo, R. Taneja, and J. C. Marshall. 2000. Phagocytosis of Candida albicans induces apoptosis of human neutrophils. Shock 14:278-283. [DOI] [PubMed] [Google Scholar]
- 68.Saban, M. R., H. Hellmich, N.-B. Nguyen, J. Winston, T. G. Hammond, and R. Saban. 2001. Time course of LPS-induced gene expression in a mouse model of genitourinary inflammation. Physiol. Genomics 5:147-160. [DOI] [PubMed] [Google Scholar]
- 69.Saresella, M., K. Roda, L. Speciale, D. Taramelli, E. Mendozzi, F. Guerini, and P. Ferrante. 1997. A rapid evaluation of phagocytosis and killing of Candida albicans by CD13+ leukocytes. J. Immunol. Methods 210:227-234. [DOI] [PubMed] [Google Scholar]
- 70.Smail, E. H., B. N. Cronstein, T. Meshulam, A. L. Esposito, R. W. Ruggeri, and R. D. Diamond. 1992. In vitro, Candida albicans releases the immune modulator adenosine and a second, high molecular weight agent that blocks neutrophil killing. J. Immunol. 148:3588-3595. [PubMed] [Google Scholar]
- 71.Subrahmanyam, Y. V. B. K., S. Yamaga, Y. Prasher, H. H. Lee, N. P. Hoe, Y. Kluger, M. Gerstein, J. D. Goguen, P. E. Newburger, and S. M. Weissman. 2001. RNA expression patterns change dramatically in human neutrophils exposed to bacteria. Blood 97:2457-2468. [DOI] [PubMed] [Google Scholar]
- 72.Travis, J. 1988. Structure, function and control of neutrophil proteinases. Am. J. Med. 84:37-42. [DOI] [PubMed] [Google Scholar]
- 73.van Spriel, A. B., I. E. van den Herik-Oudjik, N. M. van Sorge, H. A. Vilé, J. A. G. van Strijp, and J. G. J. van de winkel. 1999. Effective phagocytosis and killing of Candida albicans via targeting FcγRI (CD64) or FcαRI (CD89) on neutrophils. J. Infect. Dis. 179:661-669. [DOI] [PubMed] [Google Scholar]
- 74.van't Wout, J. W., I. Linde, P. C. Leijh, and R. van Furth. 1988. Contribution of granulocytes and monocytes to resistance against experimental disseminated Candida albicans infection. Eur. J. Clin. Microbiol. Infect. Dis. 7:736-741. [DOI] [PubMed] [Google Scholar]
- 75.Vassali, P. 1992. The pathophysiology of tumor necrosis factors. Annu. Rev. Immunol. 10:411-452. [DOI] [PubMed] [Google Scholar]
- 76.Wittes, J., and H. P. Friedman. 1999. Searching for evidence of altered gene expression: a comment on statistical analysis of microarray data. J. Natl. Cancer Inst. 91:400-401. [DOI] [PubMed] [Google Scholar]
- 77.Zar, J. H. 1999. Biostatistical analysis, p. 360-363. Prentice Hall, Upper Saddle River, N.J.