Abstract
Since 2004 it become clear that atypical bovine spongiform encephalopthies (BSEs) exist in cattle. Whenever their detection has relied on active surveillance plans implemented in Europe since 2001 by rapid tests, the overall and inter-laboratory performance of these diagnostic systems in the detection of the atypical strains has not been studied thoroughly to date. To fill this gap, the present study reports on the analytical sensitivity of the EU-approved rapid tests for atypical L- and H-type and classical BSE in parallel. Each test was challenged with two dilution series, one created from a positive pool of the three BSE forms according to the EURL standard method of homogenate preparation (50% w/v) and the other as per the test kit manufacturer's instructions. Multilevel logistic models and simple logistic models with the rapid test as the only covariate were fitted for each BSE form analyzed as directed by the test manufacturer's dilution protocol. The same schemes, but excluding the BSE type, were then applied to compare test performance under the manufacturer's versus the water protocol. The IDEXX HerdChek ® BSE-scrapie short protocol test showed the highest sensitivity for all BSE forms. The IDEXX® HerdChek BSE-scrapie ultra short protocol, the Prionics® - Check WESTERN and the AJ Roboscreen® BetaPrion tests showed similar sensitivities, followed by the Roche® PrionScreen, the Bio-Rad® TeSeE™ SAP and the Prionics® - Check PrioSTRIP in descending order of analytical sensitivity. Despite these differences, the limit of detection of all seven rapid tests against the different classes of material set within a 2 log10 range of the best-performing test, thus meeting the European Food Safety Authority requirement for BSE surveillance purposes. These findings indicate that not many atypical cases would have been missed surveillance since 2001 which is important for further epidemiological interpretations of the sporadic character of atypical forms.
Introduction
Transmissible spongiform encephalopathies (TSEs) or prion diseases include a group of progressive, neurodegenerative as yet untreatable disorders affecting several mammalian species, including Creutzfeldt-Jakob disease (CJD) in humans, bovine spongiform encephalopathy (BSE) in cattle and scrapie in small ruminants. TSEs are characterized by the concentration of an anomalous isoform (PrPRes) of the natural prion protein (PrPc) in the central nervous system (CNS) and peripheral tissues. PrPres differs from PrPc in its aggregated state and partial protease resistance. These characteristics are exploited by the majority of the methods currently used for TSE diagnosis. The protease resistant disease related PrP entity, varies in its extent of degradation by proteinase K (PK) which is influenced by the strain-dependent conformational variations of the secondary and tertiary structure of PrPres. In different TSE strains, the pathological prion protein displays disease-specific features such as different cleavage sites after proteolytic treatment, glycosylation profile, and deposition patterns, which make strain identification possible [1].
The existence of different BSE strains was discovered in 2004. The classical form (C-BSE) coexists with the atypical H-type BSE (H-BSE) originally described in France [2] and the L-type BSE (L-BSE), also known as bovine amyloidotic spongiform encephalopathy (BASE), an unusual form of BSE first identified in Italy [3]. Their diagnostic differentiation is based mainly on the molecular features of the PrPres identified by Western blot analysis. After PK digestion, PrPres shows a triplet of non-, mono-, and diglycoforms, which, while expressing their quantitative ratio and migration positions, peculiarly typify the original BSE strain [4]. H- and L-BSE have a higher or a lower discernible molecular mass of unglycosylated PrPres, respectively, at Western blot analysis; in addition, L- BSE has smaller proportion of diglycosylated PrPres with levels between 40–55% than C-BSE where values range between 60–80%. Subsequently, occurrence of the two atypical forms to several European countries, Japan and North America has been reported. The origin of different BSE forms is still cryptic. C-BSE, which was isolated during the epizootic disease, was postulated to have occurred after the recycling of a scrapie agent insufficiently deactivated in destructor plants [5], [6]. However, this has been questioned after the detection of atypical BSE forms that seem to occur spontaneously in older cattle [7] and that, under certain circumstances, are able to change their biochemical properties into those of classical BSE. The accidental use of bovine material derived from an animal that had succumbed to a spontaneous form of BSE in the feed and food production may therefore also have been the origin of the BSE crisis. Such hypothesis seems to be also compatible with the peculiar distribution by year of birth of cattle affected by atypical BSEs, in comparison to bovines affected by C-type BSE observed in France [8]. Moreover, it has been shown in several transmission experiments to primates [9], [10] and in human and bovine PrP transgenic mice [11], [12] that L-type BSE seems to have a higher zoonotic potential than C-type BSE. The use of rapid tests that are able to reliably detect such cases is therefore crucial in the frame of the protection of the consumer from an accidental exposure to the BSE agent.
Until 1999 EU surveillance systems for bovine spongiform encephalopathy (BSE) were primarily passive, i.e. relying on the examination of diseased adult cattle showing clinical signs reported to the veterinary authorities, in compliance with the Decision 98/272/EC which modifies Decision 94/474/EC [13]. Brains were examined by histopathology and immunhistochemistry for PrPres identification. Rapid molecular diagnostic assays became officially available in the late 1990s. With the enforcement of Regulation (EC) No. 999/2001 [14] the use of rapid tests became mandatory: a large number of countries subsequently detected the first BSE cases.
To provide dependable tools for an active surveillance system, in 1999 the European Commission (EC) carried out the first scientific evaluation of four new rapid post mortem BSE tests to assess their diagnostic accuracy and analytical sensitivity on brain tissue from clinically affected bovines [15]. Subsequent EU validation exercises enhanced the estimating parameters, including test robustness on autolyzed samples and testing of negative field samples to address the test specificity and to simulate routine activity [16], [17], [18].
To date, the EC has assessed 19 rapid tests in the frame of three “successive” evaluations and approved 9 for survey purposes [19].
In 2009 the Community Reference Laboratory (EURL) for TSEs assessed the analytical sensitivity of all the currently approved TSE rapid tests to determine their continued suitability for active surveillance plans [20]. The analytical sensitivity study was then evaluated by the European Food Safety Authority (EFSA) [21], [22] on the basis of current EFSA requirements for the evaluation of TSE rapid post mortem tests [23].
In that context, the lowest limit of detection (LOD) of rapid tests approved for the diagnosis of TSEs in bovines was assessed. The pre-prepared positive and negative dilution series (EURL protocol) were compared with the manufacturer's dilution series. The rapid tests with a LOD poorer than 2 log10 as compared to the best-performing assay could not be recommended for use in the frame of BSE monitoring in cattle and TSE in small ruminants within the EU.
At same time, the BIOHAZ Panel recommended that a similar study should have been conducted with regard to the other TSE strains. Furthermore, because experimental transmission of atypical BSE prions suggests that they might be more insidious than classical BSE [24], the assessment of approved rapid-test performance on detecting atypical BSE strains remains a priority.
The aim of this study was to compare the analytical sensitivity of all presently EU-approved rapid post mortem tests for the detection of atypical BSE forms in bovines by assessing their lower LOD against atypical L- and H-type BSE. The outcome will be of interest for the interpretation of epidemiological surveillance data of all three BSE types.
Materials and Methods
Study Design
Consistent with the methodology of the EFSA analytical sensitivity study, the strategy of the Italian TSE National Reference Laboratory (NRL) was to compare the performance of all approved rapid tests against the same sample pools. Thus, the test results could be directly compared and their performance ranked according to their respective LOD.
Each test was challenged with decreasing amounts of confirmed BSE-positive material in a consistent background of negative material prepared following two protocols: the one using the EURL standard method of homogenate preparation (50% w/v protocol) [23] and the other as directed by the test kit manufacturer's instructions. This was done to permit comparison between the two preparation protocols.
The study design was set up to account for several confounders (e.g., the operator, the day of the test, plates, etc.). Factors were controlled by minimizing variability (e.g., all tests were performed by only two operators) and fitting multilevel logistic models in which the confounders were set as random or fixed effects [25], [26], [27].
The first step was a basic descriptive analysis. The second involved only the manufacturer's protocol, and a multilevel logistic model was fitted for each BSE type in order to compare test kit performance. The date of testing execution constituted the first level of the models (level 1); replicated crossover and nesting of the plates were the second level (level 2). The intraclass correlation coefficient (ICC) of the replicates obtained from these models allowed us to verify that their residual variance was due only to the replicates [25], [26], [27]. In this case, when the number of positive replicates was monotone decreasing in the dilutions, we could neglect the fixed effect of the dilution and focus instead on the effect of the different test kits. In the third step, simple logistic models [28], [29] were fitted with the specific test kit as the only covariate.
Finally, the test results obtained under the two preparation protocols were compared. As mentioned, we fitted the multilevel logistic models not referring to each BSE type but instead to each test kit. Seven simple logistic models [28], [29] one for each test, were then fitted: the interaction between BSE type and protocol was the only covariate in the model.
Tissue Background
Briefly, atypical L-BSE tissue was obtained from two Italian field cases. The atypical H-BSE tissue pool was provided to the Italian NRL by the Friedrich-Loeffler-Institut (FLI) (Germany) and originated from German calves experimentally inoculated intracranially [24]. Two C-BSE pools were included in the study, one strongly reacting at confirmatory Western blot, the second weakly. This was done in order to have reference data on known matrices for the comparison of unexplored results with atypical BSE. The strong C-BSE type tissue included a pool of five Dutch regularly slaughtered field cases (collected and tested in the frame of statutory BSE surveillance plane) provided by Central Veterinary Institute of Wageningen UR (CVI). The weak C-BSE tissue was a mixture of two Italian natural cases. All positive tissues originated from brain stem area and were confirmed by discriminatory Western blot analysis [4]. The negative tissue was created from 30 bovine brainstems randomly selected from Italian slaughtered surveillance samples which had tested negative at the IDEXX® HerdCheck BSE-scrapie ultra short protocol test [30] and confirmatory Western blot.
All details pertaining to sample origin were recorded.
Preparation of Diagnostic Test Material
To ensure that the samples would be homogeneous, they were prepared using the Veterinary Laboratory Agency (VLA) standard methods for TSE QA sample production (Veterinary Laboratory Agency, Standard Operating Procedure, “Instruction for the homogenisation and dilution of brainstem for preparation of QA Samples” – personal communication).
Accordingly, four CNS tissue pools (L, H, C strong, and C weak) were prepared from L-BSE-positive, H-BSE-positive, C-BSE strong and C-BSE weak positive tissues, respectively. The 100% CNS tissues were trimmed, pooled, mildly minced with scalpels, and then treated with a low-speed hand-held homogenizing unit for 30 s. A negative pool was prepared as described above. Each BSE-positive macerate pool was diluted in pre-homogenized negative tissue to obtain 2 base logarithm dilutions series down to 1∶1024. As the 1∶1024 dilution of the C-BSE strong pool tested positive at the IDEXX® HerdCheck BSE-scrapie short protocol, further 1∶2048 and 1∶4096 dilutions of the same tissue were investigated, with negative results.
To set up the dilution series, one half of the BSE-positive pools was prepared under the EURL homogenization protocol in nuclease-free water (50% w/v) using a low-speed hand-held homogenizing unit for a total of 90 s in three successive treatments. Each dilution underwent a final homogenization cycle to ensure the preparation was mixed thoroughly. All the homogenates were aliquoted into test-specific pre-labelled grinding tubes as directed by the manufacturer's instructions and stored at −20°C.
The other half of the BSE-positive and negative starting tissue pools was distributed in the manufacturer's tissue-disruption supports for the different test kits and then immediately submitted to the specific protocols as per the manufacturer's instructions.
Each dilution was tested in triplicate by each rapid test. The test panel consisted of 150 aliquots, with 30 samples per pool, for each dilution protocol.
Testing Exercise
The tests included in the study were those approved according to Regulation (EC) No. 999/2001 amended by Regulation 162/2009. Enfer Scientific [31] declined to participate in the study. The Prionics® - Check LIA BSE Antigen Test Kit [32] had been withdrawn from the market at the time the study was conducted.
A unique batch of each rapid test specifically provided for this study by the manufacturers largely before the relative expiring date was used for all the analyses. One rapid test was performed per day and all the dilution series were tested in triplicate. One out of three positive results interpreted according to the test specifications was selected as the criterion for judging the overall result as positive. For the evaluation of the Prionics® - Check WESTERN [33], the samples were considered positive if they exhibited a signal with a three-band pattern. A more diffuse pattern of PrPres with the top band clearly visible, as reported by the manufacturer, was considered positive as well.
The laboratory test exercise was completed within 15 days from the starting point of generating and freezing the aliquots.
Results
The analyses of the different BSE samples – C-type strong, C-type weak, H-type and L-type – under both the EURL protocol or the manufacturers' protocol indicated that in principle, all tests were able to detect the different types of BSE though at different sensitivity (Table 1).
Table 1. Detection limits obtained by the different rapid tests for the different BSE forms. The number of positives out of three replicates is also reported.
| Test | Weak C – BSE | Strong C – BSE | L – BSE | H – BSE | Number of false positive/number of negative samples tested | ||||
| Manufacturer prepared dilutions | 50% w/v | Manufacturer prepared dilutions | 50% w/v | Manufacturer prepared dilutions | 50% w/v | Manufacturer prepared dilutions | 50% w/v | ||
| IDEXX® HerdCheck BSE-scrapie Short | 1∶64 | 1∶16 | 1∶1024 | 1∶512 | 1∶512 | 1∶512 | 1∶256 | 1∶512 | 0/30 |
| 3/3 | 3/3 | 2/3 | 3/3 | 3/3 | 2/3 | 3/3 | 2/3 | ||
| IDEXX® HerdCheck BSE-scrapie Ultra Short | 1∶32 | 1∶16 | 1∶512 | 1∶512 | 1∶256 | 1∶128 | 1∶128 | 1∶64 | 0/30 |
| 3/3 | 3/3 | 3/3 | 3/3 | 3/3 | 3/3 | 1/3 | 3/3 | ||
| Bio-Rad ® TeSeE TM SAP | 1∶2 | - | 1∶64 | 1∶4 | 1∶16 | 1∶4 | 1∶32 | 1∶32 | 0/30 |
| 3/3 | 0/3 | 3/3 | 3/3 | 3/3 | 3/3 | 2/3 | 3/3 | ||
| Prionics®-Check Western | 1∶32 | 1∶16 | 1∶128 | 1∶64 | 1∶256 | 1∶32 | 1∶128 | 1∶128 | 0/30 |
| 1/3 | 3/3 | 3/3 | 2/3 | 1/3 | 3/3 | 3/3 | 1/3 | ||
| Prionics®-Check PrioSTRIP | 1∶4 | 1∶2 | 1∶32 | 1∶16 | 1∶16 | 1∶16 | 1∶16 | 1∶16 | 0/30 |
| 1/3 | 1/3 | 2/3 | 2/3 | 3/3 | 3/3 | 3/3 | 3/3 | ||
| AJ Roboscreen® BetaPrion | 1∶32 | 1∶16 | 1∶128 | 1∶64 | 1∶512 | 1∶128 | 1∶128 | 1∶32 | 0/30 |
| 2/3 | 3/3 | 2/3 | 2/3 | 2/3 | 3/3 | 3/3 | 3/3 | ||
| Roche PrionScreen® | 1∶8 | 1∶2 | 1∶64 | 1∶16 | 1∶16 | 1∶16 | 1∶32 | 1∶64 | 0/30 |
| 3/3 | 3/3 | 2/3 | 3/3 | 3/3 | 1/3 | 3/3 | 2/3 | ||
The ability of the rapid tests to identify positive replicates clearly differed between the tests when increasing dilutions were compared within the manufacturers' protocol and under the EURL 50% w/v protocol. Under the manufacturer's dilution protocol, the sensitivity of the IDEXX® HerdCheck BSE-scrapie short protocol test for all BSE types was higher than that of the other rapid tests. The sensitivity of the IDEXX® HerdCheck BSE-scrapie ultra short protocol, Prionics® - Check WESTERN, and AJ Roboscreen® BetaPrion [34] was similar, followed in decreasing order by the Roche® PrionScreen [35] Bio-Rad® TeSeE™ SAP [36] and Prionics® - Check PrioSTRIP [37], the last two of which displayed the lowest analytical sensitivity, notably for L-BSE.
The multilevel models fitted in the second step of the statistical analysis confirmed that the residual variance was almost entirely due to the replicates. For all BSE types, the ICC of the replicates was higher than 0.99. Therefore, apart from a few exceptions, it was assumed that the three replicates for each dilution would have the same result, whereupon a simplified logistic model was adopted. The IDEXX® HerdCheck BSE-scrapie short protocol was taken as the reference test, as it provided the highest analytical sensitivity for all BSE forms.
The logistic models showed that a loss of sensitivity up to two dilutions lower than the best-performing test was not statistically significant. Testing with the C-BSE strong pool showed that only the IDEXX® HerdCheck BSE-scrapie ultra short protocol test compared favourably with the IDEXX® HerdCheck BSE-scrapie short protocol test; while the sensitivity of the AJ Roboscreen® BetaPrion, IDEXX® HerdCheck BSE-scrapie ultra short protocol, Prionics® - Check WESTERN for detecting the other BSE types was not statistically different from that of the reference test.
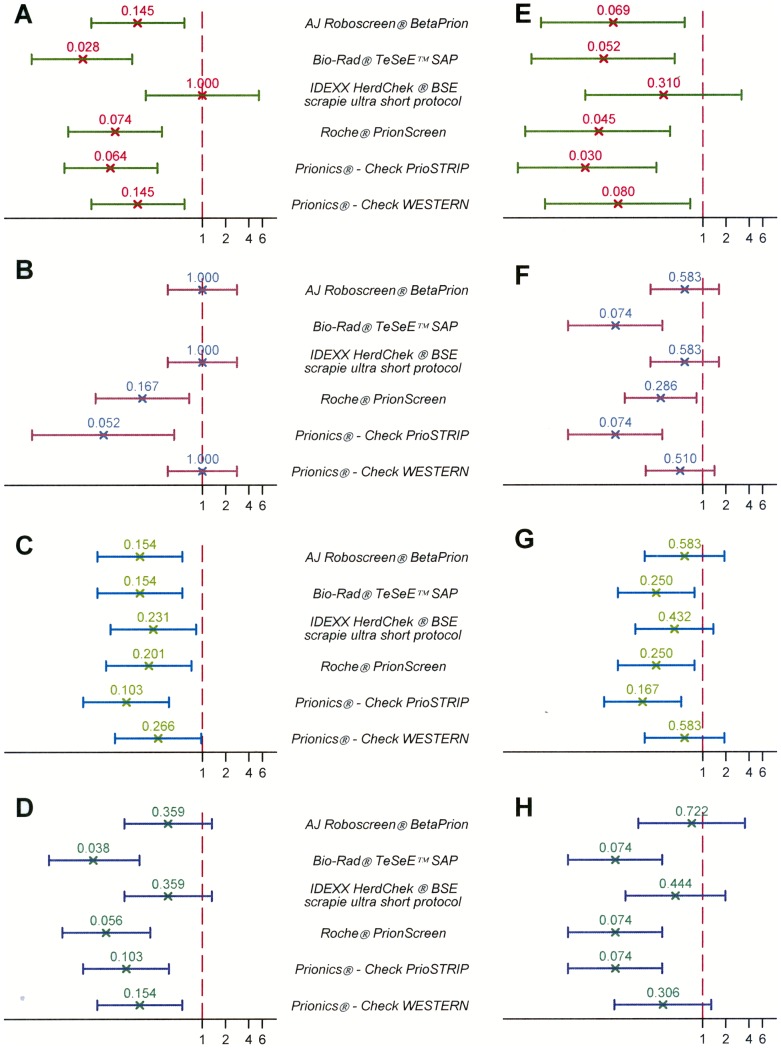
To further discriminate between the quality of performance of the different test systems, logistic models were applied on the data obtained. On the basis of the odds ratio (OR) magnitude, each test can be ranked using the IDEXX® HerdCheck BSE-scrapie short protocol as reference test and it can be concluded that a higher OR is related to higher sensitivity. Ranking obtained under the manufacturer's protocol did not differ from that obtained under the water protocol (Figure 1).
Figure 1. Test performance compared to the IDEXX® HerdCheck BSE-scrapie short protocol.
The vertical axis reports the different rapid tests challenged. The horizontal axis reflects the odds ratio magnitude using IDEXX® HerdCheck BSE-scrapie short protocol as reference test. Panels A, B, C, D (left column) report the results obtained under the w/v protocol; panels E, F, G, H (right column) display the results under the manufacturers' instructions. BSE forms studied: panels A, E: strong C-type; panels B, F: weak C-type; panels C, G: H-type; panels D, H: L-type. All the weak C type water dilutions series tested negative with Bio-Rad® TeSeE™ SAP (notably, the optical densities were for all the three replicates of the 1∶2 dilution just under the cut-off value), thereby, the odd ratio could not be calculated (Subfigure B).
Comparison of test performance under the two dilution protocols based on the descriptive analysis (Table 1) showed that all seven tests had a higher analytical sensitivity for the BSE-positive samples prepared under the manufacturer's protocol than those prepared as per the water protocol for all BSE types, except for H-BSE, toward which the tests performed generally better with the 50% w/v homogenates.
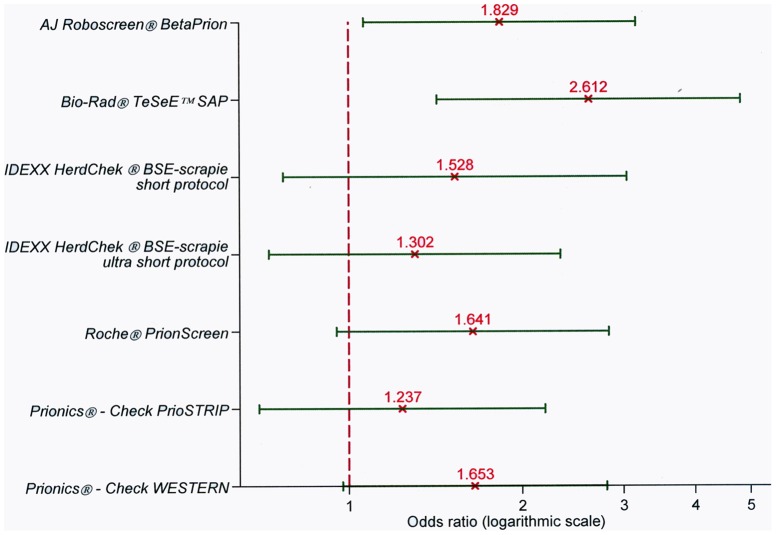
The same scheme to compare the tests under the manufacturers' protocol was then applied to compare their performance under the water protocol. A simple logistic model was fitted for each test. All seven rapid tests performed better under the manufacturers' dilution protocol than under the 50% w/v protocol (Figure 2), as already suggested by the descriptive analysis, however, upon the least approach, this result appeared to be statistically significant only for the AJ Roboscreen® BetaPrion and the Bio-Rad® TeSeE™ SAP kits.
Figure 2. Comparison of test performance under the manufacturer's dilution protocol versus the 50% w/v protocol.
The vertical axis reports the different rapid tests challenged. The horizontal axis reflects the odds ratio magnitude.
Assessment of test specificity was not within the scope of this study; nevertheless, appropriate BSE-negative tissue amounts tested on each test platform displayed negative results.
Discussion
In this study we have evaluated the analytical sensitivity of approved rapid tests for the current known atypical BSEs detection. It is to be noted that Seuberlich et al. [38] raised the possibility that a new prion disease not previously encountered and distinct from the known types of BSEs exists. Nevertheless, the information is really limited and the puzzle of the different observations has still to be assembled, considering that the results described remind the features of poorly digested normal PrP (known as the physiologically C2 fragment of PrP [39], [40]).
Referring to the tissues origin, is to be remarked that the investigated H-BSE tissues originated from intracranially challenged cattle, whereas the three other forms derived from field cases. Nevertheless, recent studies showed that biochemical and histopathological features of experimental H-type BSE animals were identical to that found with field H-type [12], [24], [41].
According to our results, all tests were able to detect both H- and L-BSE types at a 1∶16 dilution prepared as directed by the manufacturer's instructions, with the same performance as for classical BSE.
The LOD varied across the tests. The IDEXX® HerdCheck BSE-scrapie short protocol showed the highest analytical sensitivity, as previously reported in a EURL study on classical BSE [21]. The performance of the AJ Roboscreen® BetaPrion, IDEXX® HerdCheck BSE-scrapie ultra short protocol, and Prionics® - Check WESTERN compared favourably with one another at our statistical analysis. The Prionics® - Check PrioSTRIP, Bio-Rad® TeSeE™ SAP and Roche® PrionScreen tests showed the lowest sensitivities for all the BSE types analyzed. These results were confirmed also using other explorative statistical approaches (e.g., Poisson models for number of positive replicates, receiver operating characteristic [ROC] curves) which we had initially applied (results not reported).
The analytical sensitivity of the tests was investigated in accordance with the requirements set by the relevant evaluation protocols established by the European Commission, the SSC and EFSA, using serial dilutions of sample replicates.
Test differences between the last positive dilutions of weak and strong C-BSE samples varies among the different systems from two to four factors (2 base logarithm) for buffer dilutions and from two to five factors for water dilutions. In this context, the different tests showed parallel results between the dilutions prepared following the two protocols. The dynamic range of each rapid test or rather the concentration range of PrPres that results in a change in response is a specific peculiarity of each diagnostic system.
The rate of conversion of substrate to coloured product should be proportional to the amount of PrPres within the well, but there are many limits to this depending on the analyte itself, that tends to aggregate rapidly in solution, and on the combination of methods and materials used within the test kits other than on the equipments.
A gradual stratification of the signal represents a surplus value for TSE rapid assays.
In our study, the Bio-Rad® TeSeE™ SAP test could surprisingly detect only the 1∶2 dilution when challenged with positive C BSE weak samples. A loss of analytical sensitivity for this test was observed also during the active surveillance activity carried out from 2004 to 2008 by the Italian Reference Center for TSEs applying Bio-Rad® TeSeE™ test. In that context, a National batch testing was performed on every new batch prior to commercialization to provide reassurance that BSE rapid test kits were fit for the survey purpose. As a consequence, distribution of some kit batches was precluded because of the lack of signal showed on positive reference samples. Further to the unexpected poor performance of Bio-Rad® TeSeE™ within this study even after test repetition, the same Bio-Rad® homogenate sample set, according to previous studies in which its suitability for the IDEXX test was shown, was challenged with the last test revealing signals miming the ones reported for IDEXX test (data not shown). The question of whether the specific kit batch affected the test performance is of concern, but it is noteworthy that all producers were asked to provide a kit for this evaluation. Thereby, our results represent a picture of the kits available on the market.
The seven simple logistic models showed a meaningful difference between the dilution protocols only for the AJ Roboscreen® BetaPrion and Bio-Rad® TeSeE™ SAP. The lower bounds of the 95% confidence intervals for the Roche® PrionScreen and Prionics® - Check WESTERN tests approached 1 (0.9528 and 0.9755, respectively); for the remaining tests, there was no statistical evidence of a higher test sensitivity between the manufacturer's dilution protocol and the 50% w/v protocol (Figure 2).
Whenever in order to evaluate the field performances of BSE rapid post mortem tests the manufacturers' protocol represents the term of reference, the relevance of water dilution-based results relies on the specific Annex X of Regulation (EC) 999/2001 requirements. NRLs for TSE periodically have to verify national diagnostic standards and methods by means of comparative trials. The objectives are to monitor national rapid test activity and to demonstrate to the EC that the rapid surveillance system is effective. EURL itself annually verifies the interlaboratory agreement of the rapid systems used by the NRLs.
As previously reported in the EURL study [20], the analytical sensitivity values obtained under the 50% w/v protocol were from one to three dilutions inferior to those obtained under the specific homogenization protocol. For all the tests except one, the discrepancies between the two modes of dilutions were similar whatever the sample tested. Particularly with the Bio-Rad test the strong positive C -BSE sample was four factors lower when the water protocol was applied. Anyway, this is congruent with the EFSA 2009 results [21], where the discrepancy set at three logarithms. This difference needs to be taken into account when organizing ring trials, during which a less sensitive test could be penalized.
To rule out a possible decrement of the signal related to the storage of the water aliquots, and because of the scarcity of atypical BSE material, the laboratory test exercise was completed within a 15-day period. This precautionary approach was taken as no data exist on the stability of atypical BSE homogenates, whereas differences in stability have been observed for atypical versus classical scrapie [21], [42], [43], [44]. Further, as it is indeed known that the results of some tests can lapse while approaching the expiring date of kit batches, the kits provided for the evaluation were expected to expire from three to six months after the date of testing. Table S1 lists the kit batches used, the expiring dates and the days of testing.
With regard to the homogeneity of serial dilutions, as PrPres is amyloidogenic, the fibrils tend to aggregate in solution [45], thus potentially hindering a real homogeneity of dilution series. In our study, the ICC of the replicates was higher than 0.99. This ensured that, whenever the amounts of BSE tissues available were extremely limited, the material tested was homogeneous.
When considering the working principle of rapid tests, summarized in the Text S1, all approved tests include a PK digestion step to unmask cryptic epitopes, except for the IDEXX HerdChek® BSE-scrapie EIA, which relies on conformational detection technology using a specific aggregate specific capture ligand on a dextran polymer (Seprion ligand technology, Microsens Biotechnologies, London, UK) [46]. The severe effects of proteinase K (PK) in digesting atypical PrPres are well known. Depending on the PK concentration, signal loss after atypical BSE-related PrPres PK digestion varies from less than 20% for the C-type isolates to more than 50% for both L- and H-type BSE tissues [4]. This could be the reason for the higher sensitivity of the IDEXX test in detecting atypical BSEs compared to the others. However, the type of detergent used in homogenates and the type of TSE strain used do affect the extent of PrPres degradation, and this remains a matter of further study [47].
With regard to the interpretation of results, five of the rapid tests in this study are based on semi-quantitative ELISA methods that produce a qualitative result relative to a cut-off value. To minimize subjectivity, the study's Prionics® - Check PrioSTRIP results were interpreted with the use of the computerized PrioSCAN® software, although visual interpretation by two independent readers was also validated. The Prionics® - Check Western is both a qualitative and quantitative test, as it distinguishes PrPres in non-, mono-, and diglycoforms while expressing their respective quantitative ratio and migration positions. The diagnostic criteria for positive results are based on the exhibition of a three-band signal, the top one corresponding to a protein with an approximate molecular weight of 30 kD. Signal intensity decreases from top to bottom, but the higher band should be clearly visible immediately under the PK band. Significant blot images of atypical BSE dilution series obtained on in the frame of this study are presented in the Figure S1. In addition, extremely weak samples, notably for atypical BSE strains, can vary in their conventional blot pattern that fit positive criteria. Glycoform separation on the Sodium Dodecyl Sulphate PolyAcrylamide gel by electrophoresis causes the PrPres signal to thin out along the migration line rather than concentrate in a narrow area, as occurs with ELISA and immunochromatographic methods. This means that if the relative non-, mono-, and diglycoform immunoreactivity ratios of L-BSE are taken as corresponding roughly to 39%, 35%, and 26% [48], the blot signal characterizing the last tissue ratio meeting the non-negative criteria generates from only 39% of the total prion protein on the migration line. Despite this, the Prionics® - Check Western was found to be among the more sensitive systems, indicating that the interpretation of a specific PrPres marker by an expert reader can increase the test's sensitivity.
In conclusion, despite the evidence of clear differences in relative analytical sensitivity, the LOD of all seven rapid tests included in this study, against all the classes of material used, was within a 2 log10 range of the best-performing test, thus meeting EFSA criteria for rapid tests for BSE monitoring.
No certain conclusions on the field of diagnostic performance of these rapid-test kits can be drawn from our results on their analytical sensitivity, as the two parameters are not directly linked, anyway samples from animals exhibiting subclinical signs [24], could be expected to behave similarly to extremely diluted CNS tissues used in analytical sensitivity studies.
The outcome of this study endorses the current epidemiological follow up and interpretation of all three BSE forms prevalence [49], [50] and means that for epidemiological studies the data obtained in the different countries and regions of EU can be considered equally, as plausibly, most stronger atypical cases have been detected by the different rapid tests.
Supporting Information
Prionics ® - Check WESTERN Rapid Test results.
(DOC)
Kit batches, expiring dates and days of testing.
(DOC)
Brief description of the principles of the different rapid tests.
(DOC)
Acknowledgments
We would like to thank Maria Mazza, Danilo Pitardi and Antonio Longo (Istituto Zooprofilattico Sperimentale del Piemonte Liguria e Valle d'Aosta, Torino, Italy) for their helpful contribution, the AHVLA group for the advises towards the design of this study.
Funding Statement
The authors have no funding or support to report.
References
- 1. Bessen RA, Marsh RF (1994) Distinct PrP properties suggest the molecular basis of strain variation in transmissible mink encephalopathy. J Virol 68: 7859–7868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Biacabe AG, Laplanche JL, Ryder S, Baron T (2004) Distinct molecular phenotypes in bovine prion diseases. EMBO Rep 5: 110–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Casalone C, Zanusso G, Acutis P, Ferrari S, Capucci L, et al. (2004) Identification of a second bovine amyloidotic spongiform encephalopathy: molecular similarities with sporadic Creutzfeldt-Jakob disease. Proc Natl Acad Sci U S A 101: 3065–3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jacobs JG, Langeveld JPM, Biacabe AG, Acutis PL, Polak MP, et al. (2007) Molecular discrimination of atypical bovine spongiform encephalopathy strains from a geographical region spanning a wide area in Europe. J Clin Microbiol 45 (6) 182–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wilesmith JW, Wells GA, Cranwell MP, Ryan JB (1988) Bovine spongiform encephalopathy: epidemiological studies. Vet Rec 123: 638–644. [PubMed] [Google Scholar]
- 6. Baron T, Biacabe AG (2006) Origin of bovine spongiform encephalopathy. Lancet 367: 297–298; author reply 298–299. [DOI] [PubMed] [Google Scholar]
- 7. Brown P, Mc Shane LM, Zanusso G, Detwile L (2006) On the question of sporadic or atypical bovine spongiform encephalopathy and Creutzfeldt-Jakob disease. Emerging Infectious Diseases 12: 1816–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Biacabe AG, Morignat E, Vulin J, Calavas D, Baron TGM (2008) Atypical bovine spongiform encephalopathies, France, 2001–2007. Emerging Infectious Diseases 14: 298–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Comoy EE, Casalone C, Lescoutra-Etchegaray N, Zanusso G, Freire S, et al. (2008) Atypical BSE (BASE) transmitted from asymptomatic aging cattle to a primate. PLoS One 20 3 (8) e3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ono F, Tase N, Kurosawa A, Hiyaoka A, Ohyama A, et al. (2011) Atypical L-type bovine spongiform encephalopathy (L-BSE) transmission to cynomolgus macaques, a non-human primate. Jpn J Infect Dis 64 (1) 81–4. [PubMed] [Google Scholar]
- 11. Kong Q, Zheng M, Casalone C, Qing L, Huang S, et al. (2008) Evaluation of the human transmission risk of an atypical bovine spongiform encephalopathy prion strain. J Virol 82: 3697–3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Buschmann A, Gretzschel A, Biacabe AG, Schiebel K, Corona C, et al. (2006) Atypical BSE in Germany–proof of transmissibility and biochemical characterization. Vet Microbiol 117 (2–4) 103–16. [DOI] [PubMed] [Google Scholar]
- 13.(1998) European Commission Decision 98/272/CE which is related to epidemiological surveillance of spongiform encephalopathies and which modifies Decision 94/474/CE. Official Journal of the European Union L 122 p. 59. [Google Scholar]
- 14.(1999b) European Commission Regulation (EC) No. 999/2001 of the European Parliament and of the Council of 22 May 2001 laying down rules for the prevention, control and eradication of certain transmissible spongiform encephalopathies. Official Journal of the European Union L 147 pp 1–40. [Google Scholar]
- 15. Moynagh J, Schimmel H, Kramer GN (1999) The evaluation of tests for the diagnosis of transmissible spongiform encephalopathy in bovines. Nature 400: 105–105. [DOI] [PubMed] [Google Scholar]
- 16.(2003) Opinion of the Scientific Steering Committee on the field trial evaluation of the evaluation of two new rapid BSE post mortem tests. Available: http://ec.europa.eu/food/fs/sc/ssc/out316_en.pdf.
- 17.Wolfang P, Pavel V (2004) The field trial of seven new rapid post mortem tests for the diagnosis of bovine spongiform encephalopathy in bovines. Available: http://irmm.jrc.ec.europa.eu/activities/TSE_testing/Documents/globalreportphaseii.pdf.
- 18. Scientific Report of the European Food Safety Authority on the evaluation of two rapid post mortem BSE tests. EFSA Journal 48: 1–10. [Google Scholar]
- 19.(2010) European Commission Regulation (EC) No 956/2010 of the European Parliament and of the Council amending Annex X to Regulation (EC) No. 999/2001 of the European Parliament and of the Council as regards the list of rapid tests. Official Journal of the European Union L 279 pp 10–12. [Google Scholar]
- 20.Webster K, Flowers M, Cassar C, Bayliss D (2009) Determination of analytical sensitivity (detection limit) for currently approved TSE rapid tests. Available: http://www.efsa.europa.eu/de/scdocs/doc/1436.pdf
- 21. Scientific Opinion of the European Food Safety Authority on the analytical sensitivity of approved TSE rapid tests. EFSA Journal 7 (12) 1436. [Google Scholar]
- 22. Scientific Opinion of the European Food Safety Authority on the analytical sensitivity of approved TSE rapid tests - new data for assessment of two rapid tests. EFSA Journal 8: 1591. [Google Scholar]
- 23. Scientific Opinion of the European Food Safety Authority on a protocol for the evaluation of new rapid BSE post mortem tests. The EFSA Journal 508 1–20. [Google Scholar]
- 24. Balkema-Buschmann A, Ziegler U, Mc Intyre L, Keller M, Hoffmann C, et al. (2011) Experimental challenge of cattle with German atypical bovine spongiform encephalopathy (BSE) isolates. Journal of Toxicology and Environmental Health, Part A 74: 2, 103–109. [DOI] [PubMed] [Google Scholar]
- 25.Armstrong BK, White E, Saracci R (2001) In:Principle of exposure measurement in epidemiology. Oxford: Oxford University Press. 351 p. [Google Scholar]
- 26.Rabe-Hesketh S, Skrondal A (2008) In:Multilevel and longitudinal modelling using Stata. College Station, Texas: S. Stata Press. 562 p. [Google Scholar]
- 27.Szklo M, Nieto FJ (2006) Quality assurance and control. In: Epidemiology: beyond the basics. Sudbury, Massachusetts: Jones and Bartlett Publishers. 297–350 pp. [Google Scholar]
- 28.Altman DG (1991) In:Practical Statistics for Medical Research. London: Chapman & Hall/CRC. 611 p. [Google Scholar]
- 29.Fleiss JL, Levin B, Paik MC (2003) In:Statistical Methods for Rates and Proportions. New York: John Wiley & Sons. 760 p. [Google Scholar]
- 30.IDEXX® HerdCheck Bovine Spongiform Encephalopathy Antigen Test Kit, EIA. IDEXX Laboratories, Westbrook, ME, USA. [Google Scholar]
- 31.Enfer Scientific®, Newhall, Naas, County Kildare, Ireland. [Google Scholar]
- 32.Prionics® - Check LIA BSE Antigen Test Kit, Prionics AG, Schlieren-Zurich, Switzerland. [Google Scholar]
- 33.Prionics® - Check WESTERN Prionics AG, Schlieren-Zurich, Switzerland [Google Scholar]
- 34.BetaPrion® BSE EIA Test Kit, AJ Roboscreen, Leipzig, Germany. [Google Scholar]
- 35.PrionScreen® Test, Roche Diagnostics, Mannheim, Germany. [Google Scholar]
- 36.TeSeE™ Purification-Detection SAP Test Kit, Bio-Rad Laboratories, Marnes-La-Coquette, France. [Google Scholar]
- 37.Prionics® - Check PrioSTRIP, Prionics AG, Schlieren-Zurich, Switzerland. [Google Scholar]
- 38. Seuberlich T, Gsponer M, Drögemüller C, Polak PM, McCutcheon S, et al. (2012) Novel prion protein in BSE-affected cattle. Switzerland Emerging Infectious Diseases 18: 1, 158–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pirisinu L, Di Bari M, Marcon S, Vaccari G, D'Agostino C, et al. (2010) A new method for the characterization of strain-specific conformational stability of protease-sensitive and protease-resistant PrPSc . PLoS ONE 5 (9) e12723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kittelberger R (2012) Novel prion protein in BSE-affected cattle, Switzerland. Emerg Infect Dis 18: 890–2 doi: 10.3201/eid1805.111824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dobly A, Langeveld JPM, van Keulen L, Rodeghiero C, Durand S, et al. (2010) No H- and L-type cases in Belgium in cattle diagnosed with bovine spongiform encephalopathy (1999–2008) aging seven years and older. BMC Veterinary Research 6: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Everest SJ, Thorne L, Barnicle DA, Edwards JC, Elliott H, et al. (2006) Atypical prion protein in sheep brain collected during the British scrapie-surveillance programme. J Gen Virol 87: 471–477. [DOI] [PubMed] [Google Scholar]
- 43. Gretzschel A, Buschmann A, Langeveld JPM, Groschup M (2006) Immunological characterization of abnormal prion protein from atypical scrapie cases in sheep using a panel of monoclonal antibodies. J Gen Virol 87: 3715–3722. [DOI] [PubMed] [Google Scholar]
- 44. Klingeborn M, Wik L, Simonsson M, Renstrom LH, Ottinger T, et al. (2006) Characterization of proteinase K-resistant N- and C-terminally truncated PrP in Nor98 atypical scrapie. J Gen Virol 87: 1751–1760. [DOI] [PubMed] [Google Scholar]
- 45. Caughey B, Baron GS, Chesebro B, Jeffrey M (2009) Getting a grip on prions oligomers, amyloids, and pathological membrane interactions. Annual Review of Biochemistry 78: 177–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Grassi J, Maillet S, Simon S, Morel N (2008) Progress and limits of TSE diagnostic tools. Vet Res 39: 33. [DOI] [PubMed] [Google Scholar]
- 47. Breyer J, Wemheuer WM, Wrede A, Graham C, Benestad SL, et al. (2012) Detergents modify proteinase K resistance of PrPres in different transmissible spongiform encephalopathies (TSEs). Vet. Microbiol Doi:10.1016/j.vetmic.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dudas S, Yang J, Graham C, Czub M, McAllister TA, et al. 2010 Molecular, biochemical and genetic characteristics of BSE in Canada. PLoS ONE 5 (5) e10638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Langeveld JPM, Erkens JHF, Rammel I, Jacobs JG, Davidse A, et al. (2011) Four independent molecular prion protein parameters for discriminating new cases of C, L, and H bovine spongiform encephalopathy in cattle. Journal of Clinical Microbiology 49: 8, 3026–3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Polak MP, Zmudzinski JF (2012) Distribution of a pathological form of prion protein in the brainstem and cerebellum in classical and atypical cases of bovine spongiform encephalopathy. The Veterinary Journal 191: 128–130. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Prionics ® - Check WESTERN Rapid Test results.
(DOC)
Kit batches, expiring dates and days of testing.
(DOC)
Brief description of the principles of the different rapid tests.
(DOC)