Abstract
The initial events predisposing to loss of tolerance in patients with systemic lupus erythematosus (SLE) are largely unknown, as are the events that precipitate the transition from preclinical to overt disease. We hypothesized that induction of murine SLE would require tipping the balance between tolerance and immunity in two ways: 1) an immunogen that could take advantage of apoptotic cells as a scaffold for epitope spread, and 2) an immune activator that would generate a strong and persistent T cell response to the inciting immunogen. We show that immunization of C57BL/6 and BALB/c mice with human β2-glycoprotein I, an apoptotic cell-binding protein, in the presence of LPS induces a long-lived, potent response to β2-glycoprotein I that results in epitope spread to multiple SLE autoantigens. SLE-specific autoantibodies emerged in a sequential manner that recapitulated the order seen in human SLE. Moreover, immunized mice developed overt glomerulonephritis closely resembling human lupus nephritis.
The initial events predisposing to loss of self-tolerance in patients with systemic lupus erythematosus (SLE)5 are largely unknown. The events that precipitate the transition from preclinical to overt disease are equally unclear, but there is a general consensus that, as the disease progresses, the autoimmune response spreads to involve not only an increased number of autoantigens but also more epitopes within each autoantigen. This concept of a sequential spread of the autoimmune response, leading to the ordered emergence of autoreactivity to multiple autoantigens, is known as “epitope spread”. Epitope spread can be both intramolecular, involving multiple epitopes on the same molecule, and intermolecular, involving epitopes on different molecules that are physically associated as part of a macromolecular complex. Intermolecular epitope spread provides an elegant explanation for how autoantibodies to non-protein Ags, such as DNA and phospholipid, can occur. Strong support for epitope spread in human SLE comes from recent data showing that autoantibodies emerge in a remarkably consistent order and precede the development of clinical disease by up to 9 years, although the Ag initiating the immune response remain an enigma (1). The autoantibodies appear in a sequential order with antiphospholipid (aPL) Ab and antinuclear Ab (ANA) appearing first, followed by anti-Ro/SS-A, anti-La/SS-B, and anti-dsDNA, and subsequently by anti-Smith Ag (anti-Sm) and anti-nuclear ribonucleoprotein (anti-nRNP) (1).
Many of the autoantigens in SLE can be found on the surface of apoptotic cells, suggesting that apoptotic cells may constitute the cellular platform or “scaffold” upon which epitope spread occurs. Normally, intermolecular epitope spread occurs in molecules whose physical association or complexing permits their simultaneous uptake by an APC. Uptake of an apoptotic cell by an APC should similarly result in presentation of all Ags within or on the surface of the apoptotic cell by the APC. In this way, the apoptotic cell serves as a scaffold physically linking multiple autoantigens that are eventually targeted by the autoimmune response. Indeed, targeted deletion of several, but not all, receptors or molecules involved in the clearance of apoptotic cells leads to the development of multiple SLE-related autoantibodies and systemic autoimmunity (2– 6). Despite this strong connection between apoptotic cells and SLE, immunization of normal mice with apoptotic cells has failed to reproduce the renal pathology and panoply of autoantibodies observed in SLE patients (7, 8). In these studies, immunization with apoptotic cells induced primarily autoantibodies of IgM isotype (ANA, anticardiolipin (aCL) Abs, and anti-ssDNA Abs), without detectable anti-dsDNA or pathologic evidence of nephritis (7). Although immunization with dendritic cells, either alone or containing apoptotic or necrotic cells, induced significantly elevated titers of circulating IgG anti-dsDNA Abs and glomerular IgG deposits, the mice did not develop overt nephritis (8). The failure to induce overt disease in these experimental models is most likely attributable to the fact that immunization with apoptotic cells is a double-edged sword. On the one hand, apoptotic cells represent a cellular platform containing nearly all the autoantigens targeted in SLE. Conversely, there is clear evidence that apoptotic cells can suppress the immune response, especially through release of anti-inflammatory cytokines from phagocytic cells (9, 10).
We hypothesized that induction of murine SLE would require tipping the normal balance of tolerance vs immunity in two ways. First, it was essential to select an immunogen that could take advantage of apoptotic cells as a scaffold for epitope spread, but at the same time avoid the immunosuppression associated with administration of apoptotic cells. Second, it was necessary to generate a strong and persistent T cell response to the inciting immunogen. To address the first issue, we selected human β2-glycoprotein I (β2GPI), a heterologous protein that readily binds to apoptotic cells and appears to be one of the first autoantigens targeted in humans developing SLE (1, 11). To minimize the effects of apoptotic cells, we immunized normal mice with soluble heterologous β2GPI and relied on the fact that heterologous β2GPI would interact with endogenous apoptotic cells. To address the second issue, we coimmunized mice with LPS. Activators of the innate immune system and mediators of inflammation, such as LPS (12, 13), are among the most powerful stimuli leading to up-regulation of CD80 and CD86. LPS may also have additional effects that tip the response toward potent immunogenicity, such as enhanced survival of memory T cells (14). We hypothesized that stimulation with LPS at the time of immunization would lead to more potent Th cell activation, with subsequent augmentation of the anti-β2GPI response and intermolecular epitope spread to other apoptotic cell-associated autoantigens. We show that induction of a strong and long-lived response to a heterologous apoptotic cell-binding protein (i.e., β2GPI) in the presence of LPS results both in epitope spread to multiple autoantigens targeted in SLE and in the development of glomerulonephritis.
Materials and Methods
Materials
Unless stated otherwise, all chemicals were obtained commercially and used without further purification. β2GPI (apolipoprotein H) purified from human serum was obtained from Crystal Chem. All phospholipids were obtained from Avanti Polar Lipids. Escherichia coli DNA was obtained from Worthington Biochemical. LPS (E. coli-derived, serotype O111:B4) was obtained from List Biological Laboratories. Murine TNF-α was obtained from BioSource International.
Mice and preparation of thymocytes
All animal experiments were approved by the McGill University Animal Care Committee. Pathogen-free BALB/c and C57BL/6 mice were obtained from Harlan Sprague Dawley, and were maintained under specific pathogen-free conditions for all experiments. Specific pathogen-free CD28−/− mice on a C57BL/6 background were rederived from CD28−/− mice obtained from The Jackson Laboratory. Syngeneic thymocytes (or congenic thymocytes in the case of CD28−/− mice) were used in all immunizations. Apoptosis was induced in freshly isolated BALB/c or C57BL/6 thymocytes by incubation for 8 h at 37°C with 5 × 10−6 M hydrocortisone in FBS-free medium, composed of DMEM plus 1% penicillin-streptomycin solution and 0.01% BSA, as previously described (15, 16). Under these conditions, thymocytes are >75% apoptotic and the remaining cells are all viable with no evidence of necrotic cells (15, 16). Cells were cultured in the absence of FBS to avoid exposure to bovine β2GPI. The cell suspension was gently mixed every 30 min. Human β2GPI (20 μg per 107 cells) or normal mouse serum (NMS) (20% v/v, diluted in 10 mM HEPES buffer (150 mM NaCl (pH 7.2)); 20 μl of NMS per 107 cells), as a source of murine β2GPI, was added to the cell suspension for the last 1 h of the 8-h incubation with hydrocortisone. The cells were washed three times with HEPES buffer before use for immunization.
Immunization with soluble or apoptotic cell-bound β2GPI in the presence of LPS
After being bled for preimmune sera, 8- to 10-wk-old female BALB/c, C57BL/6, or CD28−/− mice were divided into groups of either 5 or 10 mice per immunogen. Mice received 100-μl i.v. injections of immunogen. Soluble and cell-bound immunogens included HEPES buffer, human β2GPI (20 μg), and NMS (20% v/v). Cell-bound immunogens were prepared as earlier described for mice and preparation of thymocytes. The mice also received an i.v. injection of either HEPES buffer, LPS (10 –50 μg per mouse), TNF-α (1.0 μg per mouse), or levan (10 μg per mouse) ~10 min following injection with immunogen. The range in the dose of LPS reflects the fact that the LPS dose was reduced in later experiments, as repeated immunization with 50 μg of LPS resulted in frequent mortalities. In these later experiments, mice received 25 μg of LPS for the first two immunizations, followed by 10 μg of LPS for subsequent immunizations. For LPS and TNF-α, the doses and schedule of injections were selected based on earlier studies (12, 13, 17, 18). All groups of mice received four injections at 2-wk intervals, followed by a fifth injection 3 mo later. Mice were bled 12–14 days after injection. In some studies, mice were bled after each immunization, whereas in others, mice were bled following the penultimate and final immunizations.
aPL and anti-β2GPI ELISA
In the present study, Ab reactivity to bovine heart cardiolipin (CL), dioleoyl phosphatidylserine (PS), dioleoyl phosphatidylcholine (PC), or egg phosphatidylethanolamine (PEth) (referred to as aCL, anti-PS, anti-PC, or anti-PEth, respectively) was determined in the presence of 10% FBS (bovine β2GPI). Ab reactivity to human β2GPI-coated plates (in the absence of phospholipid) was defined as anti-β2GPI. ELISA on the different phospholipids was performed as previously described (15), with the following modifications. CL, PS, PEth, and PC prepared at 90 μg/ml in 0.01 M PBS (pH 7.3), were plated (50 μl/well) in Immulon 2 plates (Dynatech Laboratories) and dried for 16 h at 37°C. For the anti-β2GPI ELISA, human β2GPI was coated at 15 μg/ml in PBS in Greiner ELISA (high binding) plates (Bellco Glass). All coated plates were blocked with PBS plus 0.5% gelatin and 10% FBS for 2 h at 4°C, and washed three times with 0.01 M TBS (pH 7.4). However, in certain experiments (e.g., Fig. 5), CL-coated plates were blocked with PBS plus 0.3% gelatin for 2 h at 4°C and incubated with bovine β2GPI (10% FBS), human β2GPI (10 μg/ml), or murine β2GPI (10% NMS) in PBS plus 0.3% gelatin for 16 h at 37°C before the addition of plasma or serum samples diluted in PBS containing 0.3% gelatin. In all other ELISA, plasma or sera were diluted 1/100 (unless otherwise noted) in PBS containing 0.3% gelatin and 10% FBS. Plasma or serum samples were incubated in duplicate coated wells for 3 h at 25°C, and the plates were then washed three times with TBS. Alkaline phosphatase-conjugated goat anti-mouse IgG (Southern Biotechnology Associates) and/or goat anti-mouse IgM (Sigma-Aldrich) were incubated for 16 h at 4°C. The plates were washed three times with TBS, developed with p-nitrophenol phosphate for ~20 min at 37°C, and the OD at 405 nm was read using an ELISA reader (model EAR, 400 AT; SLT Labinstruments). MRL/MpJ-Tnfrsf6lpr (MRL-lpr/lpr) mouse sera, murine hybridoma aPL, and murine anti-human β2GPI Ab served as positive controls for all ELISA. In all ELISA, one-half of the plate was coated with 90 μg/ml gelatin in PBS to ensure specificity of Ab binding.
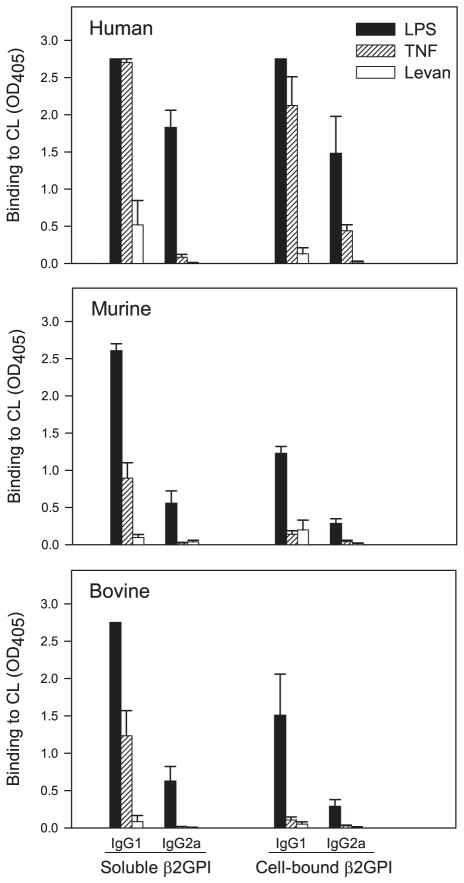
FIGURE 5.
LPS and TNF-α induce loss of self-tolerance to β2GPI and production of autoreactive aCL. BALB/c mice were immunized with soluble or apoptotic cell-bound human β2GPI in the presence of LPS, TNF-α, or levan. Diluted (1/100) serum or plasma of the immunized mice was evaluated in duplicate for IgG1 or IgG2a aCL in the presence of human (upper), murine (middle), or bovine (lower) β2GPI. Bovine (FBS) is the most commonly used form of β2GPI for aCL ELISA. CL-coated plates were incubated with β2GPI before the addition of plasma or serum samples. Data are the mean OD405 ± SE for each group of mice (n = 5), and 2.75 represents the maximal OD405 value. Data shown without SE indicate that all mice in that group had maximal OD405 values. Ab reactivity is shown at a 1/100 dilution for all sera to allow for comparison between the different conditions, but titration of aCL reactivity with human β2GPI demonstrated that the IgG1 response exceeded the IgG2a response by greater than 5-fold. In the presence of LPS or TNF-α, soluble or apoptotic cell-bound human β2GPI induced aCL IgG (predominantly IgG1) that reacted not only with human, but also with murine and bovine β2GPI. These data are representative of three independent experiments (n = 5–10 mice per group).
Lupus anticoagulant activity
Blood collected from immunized mice, using glass Pasteur pipettes pre-coated with sodium citrate, was anticoagulated with 3.8% buffered sodium citrate (9:1, blood to sodium citrate). Platelet poor plasma was isolated by centrifugation for 3 min at 11,600 × g in a tabletop microfuge (Micro-Centaur; Accurate Chemical and Scientific). Murine plasma was diluted 1/1 with citrated normal human plasma and was tested for lupus anticoagulant activity in a dilute activated partial thromboplastin time (aPTT) assay, as previously described (19, 20). aPTT values were considered significantly prolonged if they exceeded the mean plus 2 SE of the aPTT value of plasma from control mice immunized with HEPES or cells plus HEPES.
Anti-dsDNA ELISA and ANA test
Immulon 2 plates were coated with E. coli DNA (dsDNA) at 2.5 μg/ml in PBS (50 μl/well) and dried for 16 h at 37°C. The coated plates were blocked with PBS containing 0.5% gelatin and 10% FBS for 2 h at 4°C, and washed three times with TBS. Murine plasma or sera diluted 1/100 in PBS containing 0.3% gelatin and 10% FBS were incubated in duplicate coated wells for 3 h at 25°C. The remainder of the assay was performed as described for the aPL and anti-β2GPI ELISA. For ANA testing, murine sera diluted at 1/80 and 1/160 were incubated on HEp-2 cell line substrate (Kallestad; Bio-Rad), followed by detection of Ab binding with Alexa Fluor 488-conjugated goat anti-mouse IgG (1/100 dilution; Molecular Probes) according to the manufacturer’s instructions. MRL-lpr/lpr and BALB/c mouse sera served as positive and negative controls, respectively, for all assays.
Anti-Ro/SS-A, anti-La/SS-B, anti-nRNP, and anti-Sm ELISA
Immulon 2 plates were coated with Ro/SS-A, La/SS-B, nRNP, or Sm (Immunovision) at 4 μg/ml in PBS (50 μl/well) and dried for 16 h at 4°C. The coated plates were blocked with PBS containing 0.5% gelatin and 10% FBS for 2 h at 4°C, and washed three times with TBS. Murine plasma or sera diluted 1/100 in PBS containing 0.3% gelatin and 10% FBS were incubated in duplicate coated wells for 3 h at 25°C. The remainder of the assay was performed as described for the aPL and anti-β2GPI ELISA. Human Abs to Ro/SS-A, La/SS-B, nRNP, or Sm (Immunovision) and normal human IgG served as positive and negative controls, respectively, for the assays and were detected using alkaline phosphatase-conjugated goat anti-human IgG (Sigma-Aldrich).
Histology
Mice (C57BL/6 and BALB/c (n = 6 for each)) were reimmunized 3.5 mo after the fifth immunization, and sacrificed 2 days after this injection. For light microscopy, tissues (kidney, liver, spleen, heart, lung, brain, pancreas, and bone marrow) were fixed in 10% formalin. Following automated dehydration through a graded alcohol series, tissue slices were embedded in paraffin, sectioned at 3 microns (kidney) or 5 microns (all other organs), and stained with H&E. Renal sections were also stained with periodic acid-Schiff, trichrome, and Lendrum’s Martius Scarlet Blue (fibrin).
Multiple small sections of renal cortex were fixed in 4% glutaraldehyde and stored in PBS at 4°C for electron microscopy. Electron microscopic examination was performed on two β2GPI plus LPS-immunized mice (one with a focal proliferative pattern of glomerulonephritis by light microscopy, and one with a diffuse mesangial proliferative pattern of glomerulo-nephritis by light microscopy) using a Hitachi-300 electron microscope.
Immunofluorescent staining of kidney sections
Kidneys were snap frozen in liquid nitrogen and stored at −70°C. Frozen kidneys were mounted in OCT compound (Sakura Finetek) and sectioned by cryostat. Sections (6 μm) were fixed in acetone, washed with PBS, and stained with FITC-conjugated goat anti-mouse IgG F(ab′)2 (1/50 dilution; Caltag Laboratories), goat anti-mouse IgM F(ab′)2 (1/200 dilution; Caltag Laboratories), goat anti-mouse IgA (1/100 dilution; Cappel/MP Biomedicals), or goat anti-mouse C3 (1/100 dilution; Cappel/MP Biomedicals). The slides were mounted with Mowiol (Calbiochem) and reviewed by a renal pathologist (G. Lajoie) who was blinded as to the origin of the tissue section. Slides were photographed with a Leica DC200 attached to a Nikon E600 microscope using the same exposure time (600 ms) and magnification (×400).
Statistical analysis
Statistical significance was determined by a two-tailed unpaired t test with Welch correction, using InStat 3.0 (GraphPad Software).
Results
LPS augments the immune response to soluble and apoptotic cell-bound β2GPI
Our initial studies focused on the effects of LPS-induced costimulation of the immune response to β2GPI. LPS was administered i.v. on the same day as the immunogen using a dose and schedule previously shown to be effective in inducing costimulatory activity (12, 13). Mice were immunized i.v. with β2GPI in one of two forms: 1) as soluble protein (soluble), or 2) bound to the surface of syngeneic apoptotic thymocytes (cell-bound). Both heterologous (human) and autologous (mouse) β2GPI were used.
In agreement with our previous data (15), immunization with heterologous β2GPI (soluble or cell-bound) alone resulted in elevated titers of anti-β2GPI Abs (IgG plus IgM) (Fig. 1A, anti-β2GPI). The addition of LPS markedly enhanced the immune response to both forms of β2GPI, whereas LPS alone was only minimally immunogenic. In contrast to heterologous β2GPI, autologous β2GPI (NMS) did not induce a statistically significant anti-β2GPI response under any conditions.
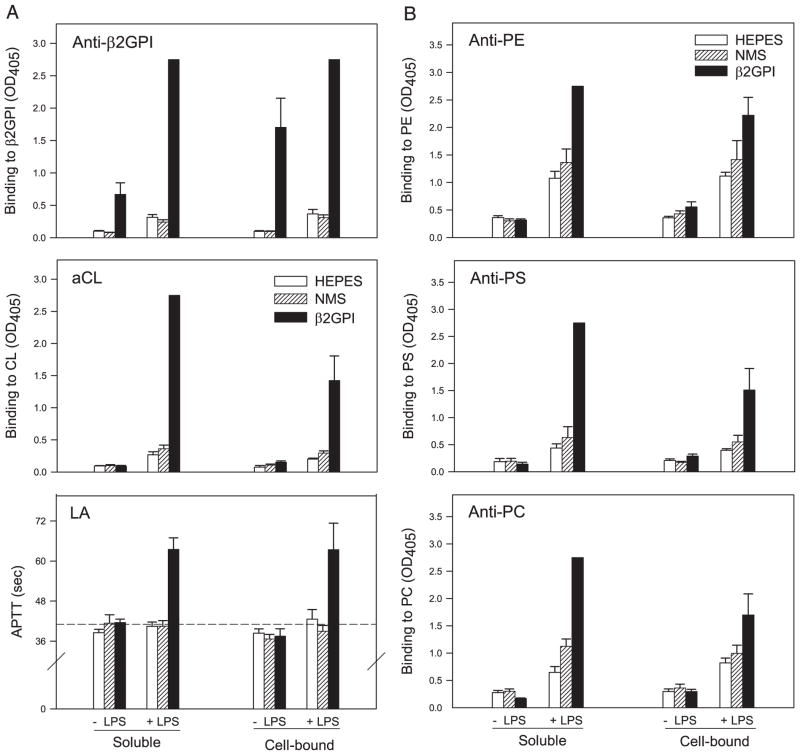
FIGURE 1.
LPS augments the immune response to soluble or apoptotic cell-bound β2GPI and induces aPL Abs. BALB/c mice were immunized with soluble or apoptotic cell-bound Ag (HEPES buffer, NMS, or human β2GPI) in the absence (−) or the presence (+) of LPS. A, Diluted (1/100) serum or plasma of the immunized mice was evaluated in duplicate for Ab (IgG plus IgM) to β2GPI (upper) or to CL (middle) by ELISA. Data are the mean binding (OD405) ± SE for each group of mice (n = 5), and 2.75 represents the maximal OD405 value. Data shown without SE indicate that all mice in that group had maximal OD405 values. Sera with maximal binding at a 1/100 dilution were further titrated (1/400, 1/1600, and 1/6400 dilutions). Anti-β2GPI Ab titers for mice immunized with β2GPI in the presence of LPS were 1/6400 (soluble β2GPI) and 1/400 (cell-bound β2GPI). Lupus anticoagulant (LA) activity (lower) was detected by dilute aPTT assay (APTT) on duplicate samples of undiluted plasma from the same mice. Data are the mean aPTT ± SE (in seconds) for each group of mice (n = 5). The dashed line represents the mean aPTT plus 2 SE (41.0 s) of plasma from control mice (HEPES control in the soluble or cell-bound group), above which aPTT values were considered positive. In the presence of LPS, soluble and cell-bound human β2GPI induced high levels of all three aPL. p < 0.0001 for anti-β2GPI (soluble or cell-bound β2GPI); p < 0.0001 for aCL (soluble β2GPI); and p < 0.04 for aCL (cell-bound β2GPI), compared with the relevant LPS control group. B, Diluted (1/00) serum or plasma of the immunized mice was evaluated in duplicate for Ab (IgG plus IgM) to PEth (upper), PS (middle), or PC (lower) by ELISA. In the presence of LPS, soluble or apoptotic cell-bound human β2GPI induced high levels of all aPL, compared with the relevant LPS control group (with the exception of anti-PC for cell-bound β2GPI): p < 0.0002 for anti-PEth, and p < 0.0001 for anti-PS and anti-PC for soluble β2GPI; and p < 0.03 for anti-PEth and p < 0.05 for anti-PS for cell-bound β2GPI. Soluble autologous β2GPI (NMS), in the presence of LPS, induced a significant aPL response for anti-PC only (p < 0.02). These data are representative of three independent experiments (n = 5–10 mice per group). PE, PEth; anti-PE, anti-PEth.
LPS had a similarly dramatic effect on the aCL response (Fig. 1A, aCL). In the absence of LPS, only cell-bound β2GPI induced a low, but significant, aCL response. The presence of LPS not only increased the magnitude of the aCL response to apoptotic cell-bound β2GPI several-fold, but also resulted in a significant aCL response to soluble β2GPI. Of note, autologous cell-bound β2GPI (NMS) also induced low, but significant, levels of aCL in the presence, but not the absence, of LPS. The responses to the anionic phospholipid, PS, and to the neutrally charged phospholipids, PEth and PC, were similar to that of aCL (Fig. 1B).
We also evaluated the presence of lupus anticoagulant Abs. Lupus anticoagulant Abs, as opposed to anti-β2GPI and aCL, are measured by functional prolongation of an in vitro coagulation assay. Induction of lupus anticoagulant activity, as detected by significant prolongation of coagulation in the aPTT assay, was observed in mice immunized with soluble or cell-bound β2GPI in the presence, but not the absence, of LPS (Fig. 1A, LA). Thus, the addition of LPS not only augmented the response to soluble and cell-bound β2GPI, but also led to broadening of the response to include other β2GPI-dependent aPL (as assessed by binding to multiple phospholipids and in vitro coagulation).
LPS increases the IgG and IgM responses to β2GPI via distinct mechanisms, while TNF-α affects only the IgG response
LPS increases the immune response in at least three ways. First, LPS provides a nonspecific stimulatory signal to B cells, independent of Ag specificity, thereby bypassing the need for specific T cell help (21). This response is predominantly of IgM isotype, and is typically transient and of low affinity. Second, LPS up-regulates the expression of costimulatory molecules on APC, thereby enhancing the activation of Ag-specific Th cells (12, 13). The specific help provided by these Th cells leads to an increased response by Ag-specific B cells that is of high affinity and contains significant IgG. Third, LPS results in a tight coupling of T cells and dendritic cells in the spleen and lymph nodes, and promotes differentiation of Ag-specific T cells into memory cells (14), resulting in a response that is long-lived.
To assess the relative contributions of the first two mechanisms to the heightened anti-β2GPI and aCL responses, we determined the isotype distribution of the induced Abs. Consistent with its role as a nonspecific polyclonal activator of B cells, LPS alone caused a slight, but significant, increase in the IgM response for both anti-β2GPI (Fig. 2A, anti-β2GPI IgM) and aCL (Fig. 2B, aCL IgM). Furthermore, the IgM anti-β2GPI responses following immunization with soluble or cell-bound β2GPI in the presence of LPS were both dramatically increased compared with the relevant LPS controls. In sharp contrast to its Ag-nonspecific effect on the IgM response, the effect of LPS on the IgG response was Ag-specific. LPS significantly increased the IgG anti-β2GPI (Fig. 2A, anti-β2GPI IgG) and aCL (Fig. 2B, aCL IgG) responses following immunization with β2GPI (soluble or apoptotic cell-bound), but not HEPES or apoptotic cells alone. Thus, our data provide evidence for a role for LPS in both polyclonal activation (IgM response) and costimulation (IgG response) of the β2GPI-specific response.
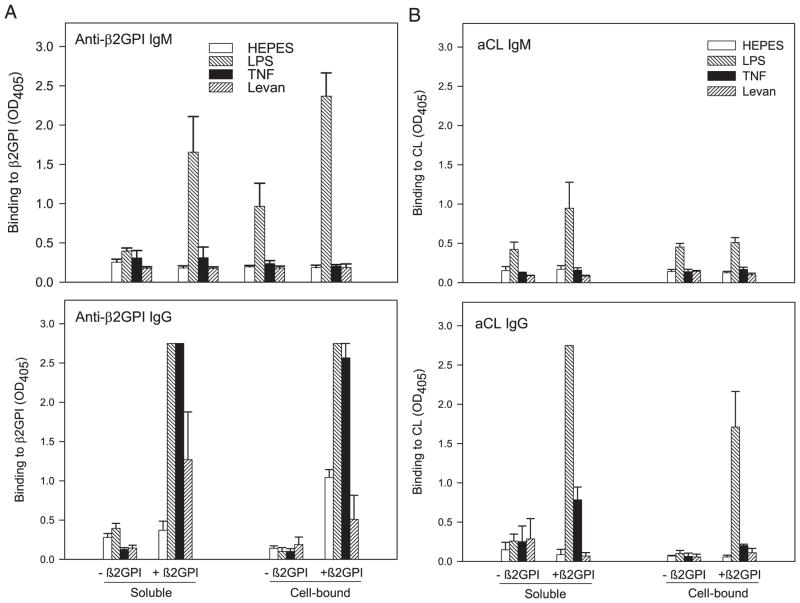
FIGURE 2.
LPS and TNF-α differentially affect the IgG and IgM anti-β2GPI responses to soluble and apoptotic cell-bound β2GPI. BALB/c mice were immunized with soluble or apoptotic cell-bound HEPES buffer without (−) or with (+) human β2GPI in the presence of HEPES buffer, LPS, TNF-α, or levan. Data are the mean binding (OD405) ± SE for each group of mice (n = 5), and 2.75 represents the maximal OD405 value. Data shown without SE indicate that all mice in that group had maximal OD405 values. A, Diluted (1/100) serum or plasma of the immunized mice was evaluated in duplicate for IgM (top) or IgG (bottom) Ab binding to human β2GPI by ELISA. In the presence of LPS, both soluble and cell-bound β2GPI induced a significant anti-β2GPI IgM response (p < 0.05 for soluble; p μ0.02 for cell-bound), and a dramatically elevated anti-β2GPI IgG response (p < 0.0001) compared with the relevant LPS control group. TNF-α significantly enhanced the anti-β2GPI IgG response to soluble (p < 0.0001) and apoptotic cell-bound (p < 0.0002) β2GPI, but had no effect on the IgM responses. Bacterial levan had no significant effect on the anti-β2GPI IgM or IgG responses. Sera with maximal IgG binding at a 1/100 dilution were further titrated (1/400, 1/1600, and 1/6400 dilutions). Anti-β2GPI IgG Ab titers were 1/6400 (soluble β2GPI) and 1/400 (cell-bound β2GPI) for mice immunized in the presence of LPS; and 1/400 (soluble β2GPI) and 1/100 (cell-bound β2GPI) for mice immunized in the presence of TNF-α. B, Diluted (1/00) serum or plasma of the immunized mice was evaluated in duplicate for IgM (top) or IgG (bottom) Ab binding to CL by ELISA. In the presence of LPS, soluble (p < 0.0001) and cell-bound (p < 0.03) β2GPI induced a significant aCL IgG response but no significant IgM response, compared with the relevant LPS control group. TNF-α significantly enhanced the aCL IgG response to apoptotic cell-bound (p < 0.001), but not soluble, β2GPI, and had no effect on IgM aCL. Bacterial levan had no significant effect on the aCL IgM or IgG responses. These data are representative of three independent experiments (n = 5–10 mice per group).
TNF-α, an inflammatory cytokine induced by LPS, mimics the Ag-specific costimulatory effect of LPS for both soluble Ag (17) and superantigen (18). However, LPS-induced memory T cell survival is not dependent on TNF-α (22). We therefore examined the effect of TNF-α on the induction of Abs to both soluble and apoptotic cell-bound Ag. Like LPS, the presence of TNF-α significantly increased the IgG anti-β2GPI response (Fig. 2A) to soluble and cell-bound β2GPI and the aCL response (Fig. 2B) to cell-bound β2GPI. In contrast to LPS, TNF-α did not significantly affect the IgM anti-β2GPI (Fig. 2A) or IgM aCL (Fig. 2B) responses. This result is consistent with the role of TNF-α as an inducer of costimulatory activity without nonspecific polyclonal B cell activation. To further address this issue, we studied the effect of bacterial levan, a thymus-independent Ag that has minimal co-stimulatory activity but can act as a polyclonal activator of B cells (23, 24). Bacterial levan had no significant effect on the IgM or IgG anti-β2GPI (Fig. 2A) and aCL (Fig. 2B) responses. Taken together, these data suggest that it is the costimulatory, and not the nonspecific, polyclonal effects of LPS that are essential to the development of the IgG anti-β2GPI and IgG aCL responses.
Enhancement of the immune response to β2GPI by LPS and TNF-α is dependent on CD28-mediated costimulation
The predominant costimulatory signal for activation of naive T cells occurs through the interaction of CD28 on T cells with CD80 or CD86 on APC (18). To determine whether CD28 was involved in mediating the costimulatory effects of LPS and TNF-α, we repeated our immunization protocol in CD28−/− mice. CD28−/− mice failed to develop anti-β2GPI IgG, aCL IgG, and lupus anti-coagulant responses, even in the presence of LPS (Fig. 3). In contrast, CD28+/+ (C57BL/6) mice produced high levels of all three aPL in response to soluble β2GPI plus LPS. These data demonstrate that CD28 is required for the LPS-induced augmentation of the IgG response to β2GPI. Of note, the initial IgM response to β2GPI and LPS, which is independent of memory, did not differ significantly between CD28+/+ and CD28−/− mice (data not shown). However, upon subsequent immunization, the IgM response in CD28+/+ mice exceeded that in CD28−/− mice (data not shown), suggesting that the presence of CD28 contributes to the memory response for IgM as well.
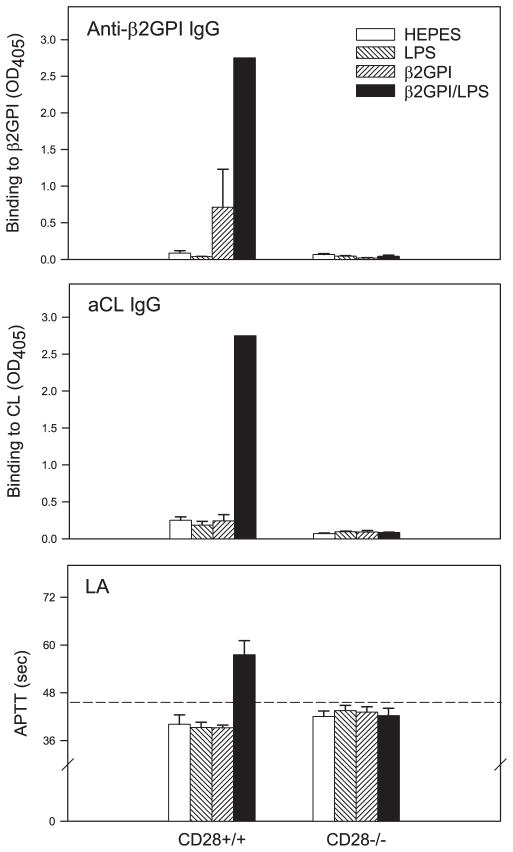
FIGURE 3.
Enhancement of the immune response to β2GPI by LPS is dependent on CD28-mediated costimulation. CD28+/+ (C57BL/6) or CD28−/− mice were immunized with soluble Ag (HEPES buffer, LPS, human β2GPI in the absence of LPS, or human β2GPI in the presence of LPS). Diluted (1/100) serum or plasma of the immunized mice was evaluated in duplicate for IgG Ab to β2GPI (upper) or CL (middle) by ELISA. Lupus anticoagulant (LA) activity (lower) was detected by dilute aPTT assay (APTT) on duplicate samples of undiluted plasma. Data are the mean assay values ± SE for each group of mice (n = 5), and 2.75 represents the maximal OD405 value. Data shown without SE indicate that all mice in that group had maximal OD405 values. The dashed line represents the mean aPTT + 2 SE (45.0 s) for plasma from control mice (HEPES-immunized), above which aPTT values were considered positive. In the absence of CD28, there was no IgG aPL response. These data are representative of three independent experiments (n = 5 mice per group).
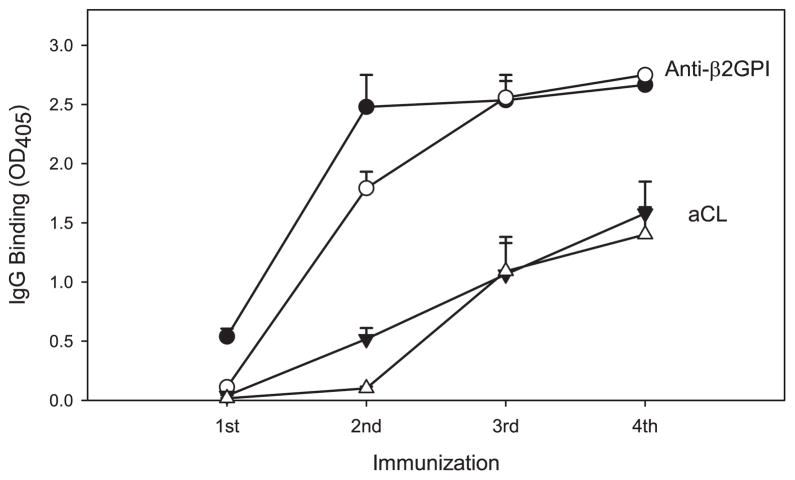
As C57BL/6 and BALB/c mice are known to differ in the inducibility of autoantibodies and the manifestations of autoimmune disease (25, 26), we compared the immune response to β2GPI plus LPS in these two strains (Fig. 4). Although maximal levels of IgG and anti-β2GPI Abs were attained in both strains by the third immunization, C57BL/6 mice had significantly higher Ab titers at earlier time points. The findings were similar for aCL. These data indicate that the kinetics of the aPL response may be more rapid in C57BL/6 as compared with BALB/c mice.
FIGURE 4.
LPS augments the immune response to β2GPI in both C57BL/6 and BALB/c mice. C57BL/6 or BALB/c mice were immunized with soluble Ag (HEPES buffer, LPS, human β2GPI in the absence of LPS, or human β2GPI in the presence of LPS), as in Fig. 3. The kinetics of the response are shown only for the mice immunized with human β2GPI in the presence of LPS. Diluted (1/8000) serum or plasma of the immunized mice was evaluated in duplicate for IgG Ab to β2GPI (anti-β2GPI) and aCL by ELISA following the first through fourth immunizations with immunogen. Data are the mean OD405 ± SE for each group of C57BL/6 (●, ▼) or BALB/c (○, △) mice (n = 10). Data shown without SE indicate that all mice in that group had maximal OD405 values. Ab reactivity is shown at a 1/8000 dilution of serum for all bleeds to allow a comparison of the sera at submaximal binding (OD405 < 2.75, except for anti-β2GPI following the fourth immunization). However, this high dilution results in the apparent negativity of early bleeds (first and second). In fact, these sera had significant Ab titers (anti-β2GPI ≥ 1/2000 for the first and second bleed, and aCL ≥1/2000 for the second bleed). Statistical comparisons of titers between strains were made using appropriate dilutions between 1/100 and 1/8000. Titers of anti-β2GPI and aCL IgG were significantly higher in C57BL/6 than BALB/c mice following the first (p < 0.0001) and second (p < 0.05 for anti-β2GPI and p < 0.002 for aCL) immunizations, but both strains had similar aPL levels by the third immunization. These data are representative of three independent experiments (n = 5–10 mice per group).
LPS and TNF-α induce loss of self-tolerance to β2GPI and production of autoimmune aPL
LPS promotes Ag-specific Ig class switching, leading to both IgG1 (Th2-dependent) and IgG2a (Th1-dependent) responses (12, 17). Of the inflammatory cytokines induced by LPS, TNF-α enhances IgG1 production, but only minimally enhances IgG2a production (17). As shown in Fig. 5, both LPS and TNF-α promoted a strong IgG1 aCL response to soluble and apoptotic cell-bound β2GPI with a more limited (~5-fold lower) IgG2a response. Similar findings were observed for the anti-β2GPI response (data not shown).
LPS can not only increase the magnitude of individual Ab responses, but also lead to disruption of T cell tolerance (27). To determine whether immunization with human β2GPI in the presence of LPS resulted in spread of the immune response to epitopes present on autologous murine β2GPI, we compared the β2GPI-dependent aCL reactivity of sera from immunized mice using human and murine sources of β2GPI as Ag in the assay. As expected, the β2GPI-dependent aCL response was greatest for human β2GPI (Fig. 5, upper panel). However, there was also significant aCL reactivity to murine β2GPI (Fig. 5, middle panel). Thus, immunization with human β2GPI, soluble or cell-bound, in the presence of LPS or TNF-α resulted in a loss of self-tolerance to β2GPI and the production of autoimmune aPL.
β2GPI immunization in the presence of LPS induces epitope spread of the immune response and recapitulates the sequence of autoantibody emergence in human SLE
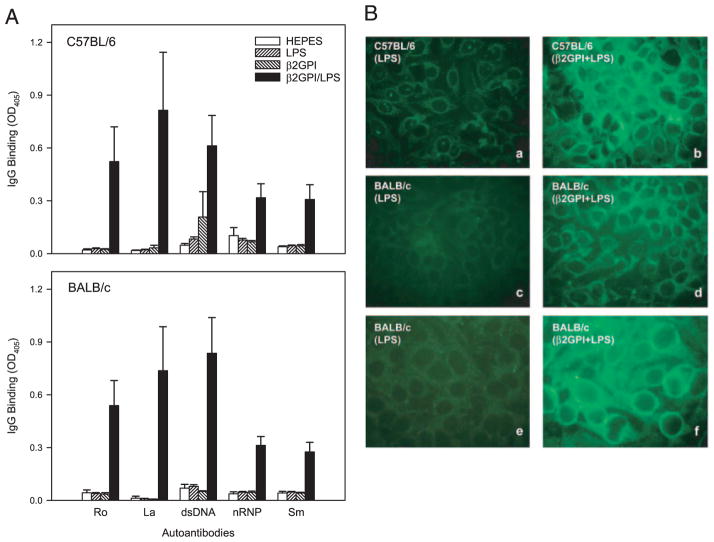
To determine whether the immune response in the presence of LPS had also spread to other apoptotic cell-associated autoantigens, we evaluated serum reactivity to a panel of autoantigens relevant to SLE. We focused, in particular, on IgG autoantibodies that are characteristic of SLE and have been shown to precede the development of clinical disease, namely, anti-Ro/SS-A, anti-La/SS-B, anti-dsDNA, anti-nRNP, and anti-Sm (1). Titers of all five SLE-related autoantibodies were significantly elevated in C57BL/6 and BALB/c mice immunized with soluble β2GPI in the presence of LPS (Fig. 6A). In the absence of LPS, the immune response to β2GPI did not spread to Ro/SS-A, dsDNA, La/SS-B, nRNP, or Sm.
FIGURE 6.
Immunization with β2GPI in the presence of LPS induces a spread of the immune response to autoantibodies typically seen in human SLE. C57BL/6 or BALB/c mice were immunized with soluble Ag (HEPES buffer, LPS, human β2GPI in the absence of LPS, or human β2GPI in the presence of LPS). A, Diluted (1/100) serum or plasma of the immunized mice was evaluated in duplicate for IgG Ab to Ro/SS-A (Ro), La/SS-B (La), dsDNA, nRNP, and Sm by ELISA after four immunizations with immunogen. Data are the mean OD405 ± SE for each group of mice (n = 10). Both C57BL/6 and BALB/c mice immunized with β2GPI in the presence of LPS produced significantly elevated levels of all five SLE-related autoantibodies, compared with LPS-immunized controls. For C57BL/6 mice, p < 0.04 for anti-Ro/SS-A and anti-La/SS-B, and p < 0.02 for the other autoantibodies. For BALB/c mice, p < 0.01 for anti-Ro/SS-A, p < 0.02 for anti-La/SS-B, p < 0.005 for anti-dsDNA, p < 0.0005 anti-nRNA, and p < 0.003 for anti-Sm. These data are representative of three independent experiments (n = 5–10 mice per group). B, Diluted sera (1/80 for panels c and d, or 1/160 for panels a, b, e, and f) from immunized mice were evaluated for ANA staining using Alexa Fluor 488-conjugated goat anti-mouse IgG Ab. A strong and predominantly granular cytoplasmic pattern, indicative of mitochondrial staining and consistent with aCL, was observed in β2GPI plus LPS-immunized C57BL/6 and BALB/c mice (panels b and d), compared with LPS-immunized control mice (panels a and c). Some β2GPI plus LPS-immunized mice exhibited a nuclear envelope pattern of staining suggestive of Abs to the nuclear lamina (panel f), compared with the LPS-immunized control (panel e). The original magnification (×60) is the same for all slides, and slides were photographed using the same exposure time (330 ms).
The presence of cell-reactive autoantibodies was confirmed by the ANA test. A predominantly granular cytoplasmic pattern indicative of mitochondrial staining was observed in both C57BL/6 and BALB/c mice immunized with soluble β2GPI in the presence of LPS, compared with little or no staining by the LPS-immunized control sera (Fig. 6B, panels a– d). Mitochondrial staining is consistent with the presence of aCL, as CL is a major component of the mitochondrial membrane. Staining by sera from some β2GPI plus LPS-immunized mice exhibited a nuclear envelope pattern suggestive of Abs to the nuclear lamina (e.g., anti-lamin B1), and consistent with the presence of ANA (Fig. 6B, panels e and f).
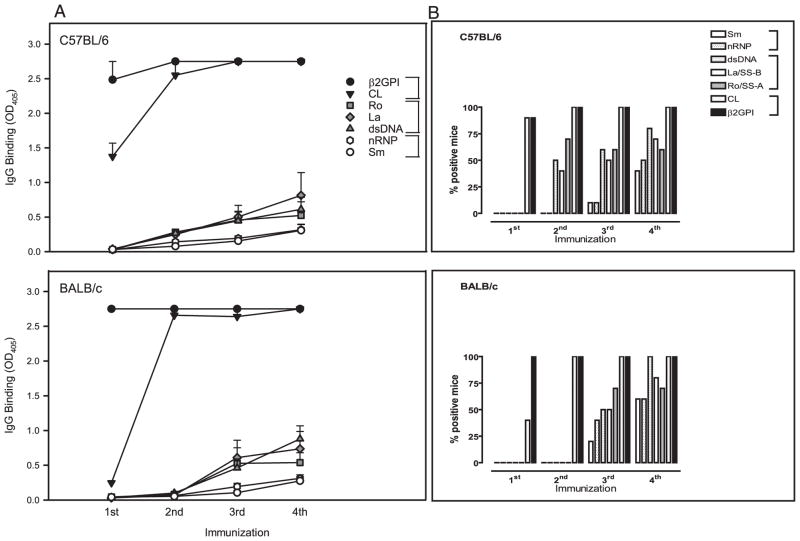
We next looked at the sequence in which the SLE-related autoantibodies arose in C57BL/6 and BALB/c mice immunized with soluble β2GPI in the presence of LPS (Fig. 7A). The autoantibodies emerged in the following sequence: anti-β2GPI, aCL, anti-Ro/SS-A and anti-La/SS-B, anti-dsDNA, anti-nRNP, and anti-Sm. They fall into three sequential groups: 1) anti-β2GPI and aCL Abs (aPL); 2) anti-Ro/SS-A, anti-La/SS-B, and anti-dsDNA; and 3) anti-nRNP and anti-Sm. This kinetic pattern is analogous to that seen in human SLE (1). Although C57BL/6 and BALB/c mice showed a similar sequence of autoantibody emergence, SLE-related autoantibodies appeared earlier in C57BL/6 mice (Fig. 7B). Thus, immunization with β2GPI in the presence of LPS induces a spread of the immune response that not only encompasses multiple diverse autoantigens but also recapitulates the sequence of auto-antibody emergence in human SLE (1).
FIGURE 7.
Immunization with β2GPI in the presence of LPS induces a spread of the immune response that recapitulates the sequence of autoantibody emergence in human SLE. C57BL/6 or BALB/c mice were immunized with soluble Ag (HEPES buffer, LPS, or human β2GPI in the absence or presence of LPS), as in Fig. 6. The kinetics of the response are shown for mice immunized with human β2GPI in the presence of LPS. A, Diluted (1/100) serum or plasma of the immunized mice was evaluated in duplicate for IgG Ab to β2GPI, CL, Ro/SS-A (Ro), La/SS-B (La), dsDNA, nRNP, and Sm by ELISA following the first through fourth immunization. Data represent the mean OD405 ± SE for each group of mice (n = 10) with error bars, and 2.75 represents the maximal OD405 value. Data shown without SE indicate that all mice in that group had maximal OD405 values. The SLE-related autoantibodies emerged in the following sequence: anti-β2GPI, aCL, anti-Ro/SS-A and anti-La/SS-B, anti-dsDNA, anti-nRNP, and anti-Sm. Basically, they fall into three sequential groups (grouped by brackets): 1) anti-β2GPI and aCL Abs (aPL) (filled symbols); 2) anti-Ro/SS-A, anti-La/SS-B, and anti-dsDNA (grayed symbols); and 3) anti-nRNP and anti-Sm (open symbols). B, The percentage (%) of C57BL/6 and BALB/c mice positive for each of the SLE-related IgG autoantibodies following the first through fourth immunization with β2GPI in the presence of LPS. Autoantibody positivity for duplicate serum samples assayed by ELISA was defined as a mean OD405 ≥ 0.25. C57BL/6 and BALB/c mice show a similar sequence of autoantibody emergence, but SLE-related autoantibodies emerged earlier in the C57BL/6 mice. These data are representative of three independent experiments (n = 10 mice per group).
β2GPI immunization in the presence of LPS induces the production of autoantibodies with pathological consequence
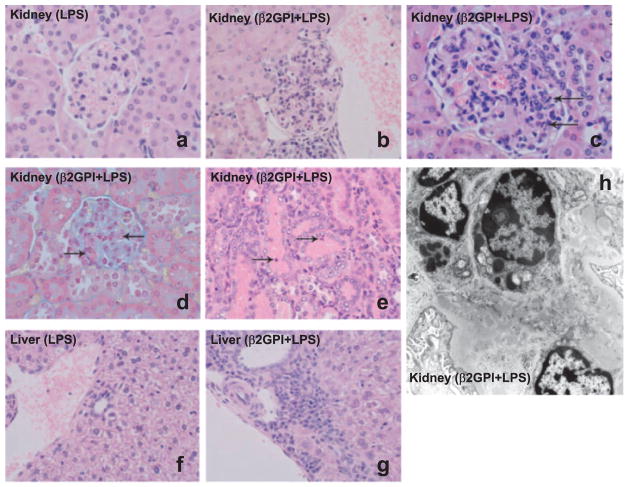
The presence of SLE-like autoantibodies in these mice was associated with significant organ damage resembling that seen in human SLE. Light microscopy of the kidneys of mice immunized with β2GPI in the presence of LPS showed two histological patterns consistent with lupus nephritis. The first pattern, mesangial proliferative glomerulonephritis, was characterized by a segmental to global increase in the number of mesangial cells. Mesangial proliferative glomerulonephritis was observed in both C57BL/6 (Fig. 8b) and BALB/c mice (data not shown). One of the C57BL/6 mice with mesangial proliferative glomerulonephritis also showed acute tubular necrosis (Fig. 8e), characterized by focal epithelial cell necrosis, granular casts, tubular epithelial simplification, loss of brush border, and increased epithelial cell mitotic figures. The second pattern, focal proliferative glomerulonephritis, was characterized by all of the following: segmental endocapillary hypercellularity, including infiltrating neutrophils and monocytes; segmental fibrinoid necrosis with karyorrhexis and apoptosis of infiltrating neutrophils; and deposition of intracapillary and extracapillary fibrin (Fig. 8, c and d). Focal proliferative glomerulonephritis was observed in the kidneys of C57BL/6, but not BALB/c, mice. All LPS-immunized mice examined (n = 4) showed normal histology, and had no evidence of glomerulonephritis or acute tubular necrosis (Fig. 8a).
FIGURE 8.
Immunization with β2GPI in the presence of LPS induces lupus nephritis and autoimmune hepatitis. a, A representative glomerulus from the kidney of a LPS-immunized C57BL/6 control mouse. The glomerulus is normocellular and has no evident pathology (H&E stained, original magnification, ×600). b, A representative glomerulus from the kidney of a β2GPI plus LPS-immunized C57BL/6 mouse showing marked global mesangial hypercellularity. A collection of plasma cells is seen below the glomerulus (H&E stained, original magnification, ×400). c, A representative glomerulus from the kidney of a β2GPI plus LPS-immunized C57BL/6 mouse showing segmental endocapillary hypercellularity, with occlusion of capillary lumina and some apoptotic bodies (arrows) (H&E stained; original magnification, ×600). d, A representative glomerulus from the kidney of a β2GPI plus LPS-immunized C57BL/6 mouse showing segmental necrosis with fibrin deposition (arrows). The fibrin stains red (Lendrum’s Martius Scarlet Blue stained; original magnification, ×600). e, A representative section from the kidney of a β2GPI plus LPS-immunized C57BL/6 mouse showing tubular necrosis, intraluminal granular casts, and tubular cell apoptosis (arrows) (H&E stained; original magnification, ×400). f, A representative section from the liver of an LPS-immunized C57BL/6 control mouse. No portal or lobular inflammatory infiltrate is seen (H&E stained; original magnification, ×400). g, A representative section from the liver of a β2GPI plus LPS-immunized C57BL/6 mouse showing marked portal inflammatory infiltrate with abundant plasma cells and focal interface hepatitis (H&E stained; original magnification, ×400). h, Ultrastructure of a glomerulus from a β2GPI plus LPS-immunized C57BL/6 mouse showing mesangial proliferative glomerulonephritis. There are abundant immune complex-type electron-dense mesangial deposits as well as an increase in mesangial cell number (uranyl acetate, lead citrate; original magnification, ×17,500). All data are representative of four control LPS-immunized mice (two C57BL/6 mice, two BALB/c mice) and eight β2GPI plus LPS-immunized mice (four C57BL/6 mice and four BALB/c mice). None of the four control mice developed pathological findings, whereas all eight β2GPI plus LPS-immunized mice developed autoimmune hepatitis (7/8) and/or glomerulonephritis (7/8).
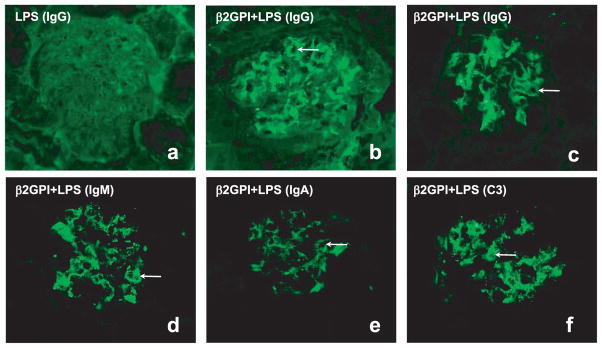
Immunofluorescent microscopy of kidneys from mice immunized with β2GPI in the presence of LPS showed segmental to usually diffuse mesangial and capillary wall deposition of IgG, IgM, IgA, and complement C3 (Fig. 9, b–f). Such a “full house” of staining is characteristic of human SLE-associated glomerulonephritis. In sharp contrast, LPS-immunized mice showed neither mesangial nor capillary wall deposition of IgG (Fig. 9a). Although IgM, IgA, and C3 were found in the mesangium of LPS-immunized mice, only IgM was deposited in the capillary walls, and its staining was rare to segmental (data not shown) as opposed to the diffuse staining observed in mice immunized with β2GPI in the presence of LPS (Fig. 9, d–f).
FIGURE 9.
Immunization with β2GPI in the presence of LPS induces immune deposits in the kidney. a– c, Representative glomeruli from the kidneys of a LPS-immunized C57BL/6 control mouse (B4) (a) and two β2GPI plus LPS-immunized C57BL/6 (DD2 and DD3) mice (b and c) stained for IgG. d–f, Representative glomeruli from the kidney of a β2GPI plus LPS-immunized C57BL/6 (DD3) mouse stained for IgM (d), IgA (e), or C3 (f). Sections were stained with FITC-conjugated goat anti-mouse Abs to IgG, IgM, IgA, or C3. Original magnification (×400) is the same for all slides, and slides were photographed using the same exposure time (600 ms). Arrows indicate capillary loop staining. The kidneys of mice immunized with β2GPI plus LPS showed segmental to often diffuse mesangial and capillary wall deposition of IgG, IgM, IgA, and complement C3, whereas LPS-immunized mice showed neither mesangial nor capillary wall deposition of IgG.
Pathology was also observed in the liver of mice immunized with β2GPI in the presence of LPS. Seven of eight of these mice showed features suggestive of autoimmune hepatitis (Fig. 8g), characterized by a mild to marked portal inflammatory infiltration by lymphocytes and plasma cells, piecemeal necrosis (inflammation or necrosis of hepatic parenchyma at its interface with portal tracts), and mild lobular inflammation. In contrast, none of the control LPS-immunized mice (n = 4) displayed such changes. (Fig. 8f)
Other H&E-stained tissues were also examined by light microscopy. No significant pathology was seen in any of the other organs examined (spleen, bone marrow, pancreas, brain, lungs, and heart).
Ultrastructural examination (electron microscopy) of the kidneys from two C57BL/6 mice immunized with β2GPI plus LPS revealed glomerular mesangial expansion with an increased number of mesangial cells and the presence of abundant electron-dense deposits. (Fig. 8h). Small subendothelial immune deposits were also seen in some glomerular peripheral capillaries. The presence of electron-dense mesangial and subendothelial deposits is typical of immune complex-mediated glomerulonephritis. The light and electron microscopic features closely resemble those seen in human lupus nephritis (World Health Organization class II and class III).
Discussion
We hypothesized that immunization with an apoptotic cell-binding protein (β2GPI) in the presence of LPS would induce not only a strong autoantibody response to β2GPI, but also a breakdown in tolerance to other apoptotic cell-associated autoantigens. In this study, we confirm this hypothesis in mice immunized with human β2GPI and LPS. Breakdown in tolerance arose upon repeated exposure to human β2GPI, but only in the presence of LPS. Upon repeated immunization, we observed not only the sequential emergence of multiple SLE-related autoantibodies, but also the development of overt lupus nephritis, closely resembling the mesangial and focal proliferative forms of glomerulonephritis seen in human lupus nephritis.
We selected LPS because of the multiple ways in which LPS can enhance the immune response. These include: polyclonal activation of B cells (21); up-regulation of costimulatory molecules on APC (12, 13); induction of proinflammatory cytokines (TNF-α, IL-1, and IL-6) (28); and promotion of the differentiation of Ag-specific Th cells into memory cells, resulting in a long-lived Ag-specific response (14). Although our data are consistent with the possibility that all of the effects of LPS are important, it is clear that polyclonal B cell activation itself was insufficient to induce the effects achieved by LPS. First, the Abs induced were primarily IgG, indicating a T cell-dependent response. Second, bacterial levan, a polyclonal B cell activator with minimal costimulatory activity (23, 24), had no significant effect on the anti-β2GPI and aCL responses. Third, TNF-α, a cytokine that induces costimulatory activity without polyclonal activation, was able to promote aCL production, albeit less effectively than LPS. Although it is unclear precisely how LPS enhances T cell survival, it is known that LPS induces tight coupling of Ag-specific T cells with APC, resulting in T cell clones that are conditioned to survive, expand, and differentiate into long-lived memory T cells (14). In the absence of LPS or another adjuvant, the same T cells undergo clonal deletion.
Several results suggest that we generated long-lived memory T cells. First, extremely high titers (>1/1000) of IgG anti-β2GPI and aCL were present in sera of these mice as early as 2 wk after the first immunization and persisted for several months following the fourth immunization. Second, β2GPI-reactive B and T cells were readily isolated from these mice 2–3 mo following the final immunization (A. Rico de Souza, T. Tolomeo, M. Dieudé, T. Shi, B. Meulenkamp, J. S. Levine, and J. Rauch, manuscript in preparation). Third, and most important, we observed intermolecular epitope spread to other autoantigens (Ro/SS-A, La/SS-B, dsDNA, nRNP, and Sm), consistent with the induction of β2GPI-specific memory T cells capable of providing help to other autoantigen-specific B cells (Fig. 10). All three findings were exclusive to mice immunized with β2GPI plus LPS, and were not found in mice immunized with either β2GPI or LPS alone, or in CD28-deficient mice.
FIGURE 10.
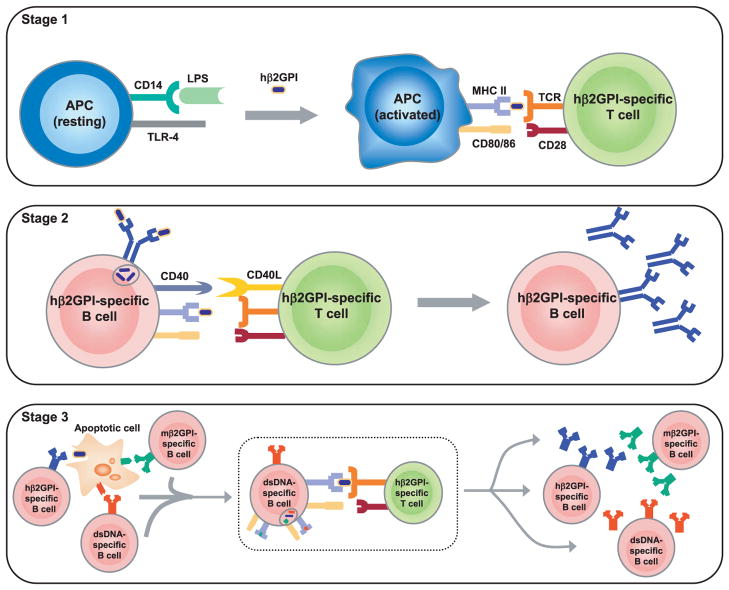
Model for epitope spread of the response from human β2GPI to multiple SLE autoantigens. This illustration outlines a minimal, but sufficient, model by which immunization of mice with human β2GPI in the presence of LPS leads to a break in tolerance and epitope spread to multiple SLE autoantigens. Stage 1, Activation of APC and human β2GPI-specific T cells: APCs interact with LPS via its receptor, CD14, leading to signaling through TLR4. This results in APC activation and the production of multiple proteins that contribute to inflammation and enhance adaptive immunity. We highlight the effects of LPS on up-regulation of MHC class II and costimulatory molecules CD80/86 by the APC, but do not exclude other direct effects of LPS on APC, B cells, or T cells. In the presence of human β2GPI, the activated APC presents human β2GPI-derived peptide to the human β2GPI-specific T cell, resulting in activation of this T cell. Stage 2, Activation of human β2GPI-specific B cells: The activated human β2GPI-specific T cell expresses CD40L on its surface and engages its receptor, CD40, on the human β2GPI-specific B cell, resulting in proliferation and differentiation of human β2GPI-specific B cells. As human β2GPI contains multiple epitopes, multiple B cell clones are likely activated (not shown). B cells with high and specific affinity for human β2GPI dominate the response, but clones that cross-react with murine β2GPI and/or possess aCL reactivity are also generated. Stage 3, Generation of B cell autoimmunity: B cells specific for apoptotic cell-associated surface autoantigens, including murine β2GPI and dsDNA, take up apoptotic cells via their surface Ig. Uptake of apoptotic cells leads to the presentation of multiple apoptotic cell-derived peptides, arising not only from the target of the surface Ig but also from any other proteins associated with the apoptotic cell. In this setting, the human β2GPI-specific T cell can provide help to any B cell that internalizes an apoptotic cell having human β2GPI bound to its surface. This effect is shown for human β2GPI-specific, murine β2GPI-specific, and dsDNA-specific B cells, but would also apply to B cells specific for other autoantigens (e.g., Ro/SS-A, La/SS-B, nRNP, and Sm) expressed on the apoptotic cell surface. The enclosed process in Stage 3 shows the interaction of the human β2GPI-specific T cell with a dsDNA-specific B cell, but a similar interaction would occur for any B cell presenting the human β2GPI peptide recognized by this human β2GPI-specific T cell. The affinity of the B cell surface Ig for Ag, and the functional status and number of self-reactive B cells are among the factors that likely influence the kinetics of epitope spread for each autoantigen. We do not exclude the possibility that T cells specific for murine β2GPI or other apoptotic cell-associated autoantigens may also be activated, or that diminished activity of regulatory T cells and other tolerogenic constraints may contribute to the observed autoimmune response. In this manner, multiple autoreactive B cells can be activated by a single human β2GPI-specific T cell and lead to a panoply of autoantibodies and SLE-like disease. hβ2GPI, human β2GPI; mβ2GPI, murine β2GPI.
Mice immunized with β2GPI plus LPS initially produced Abs to human β2GPI, but the response quickly expanded to include autoantibodies reactive with murine β2GPI. Ultimately, the autoimmune response evolved to target multiple SLE autoantigens. Fig. 10 outlines a minimal model for the hypothetical stages that lead to a break in tolerance and epitope spread to multiple SLE autoantigens. This model presumes that B cells specific for an apoptotic cell surface Ag can bind and internalize apoptotic cells via their surface Ig. The model also presumes that uptake of apoptotic cells will result in the presentation of multiple apoptotic cell-derived peptides, arising not only from the specific target of the surface Ig but also from any other proteins associated with the apoptotic cell. In this setting, a human β2GPI-specific T cell can therefore provide help to any B cell that internalizes an apoptotic cell having human β2GPI bound to its cell surface. Although these stages represent a minimal, but sufficient, model to explain epitope spread to different SLE autoantigens, they do not exclude the possible involvement of T cells reactive with murine β2GPI or other autoantigens, and/or diminishment of specific tolerogenic restraints (29).
Our demonstration that mice immunized with β2GPI plus LPS produced significantly elevated IgG autoantibodies to multiple SLE autoantigens (all shown to be present on the surface of apoptotic cells (16, 30, 31)) is consistent with this proposed model of epitope spread. Our data indicate that a long-lived T cell response to a single heterologous protein (e.g., human β2GPI) can give rise to autoimmunity in a healthy nonautoimmune host if this protein interacts with a scaffold containing multiple autoantigens (i.e., the apoptotic cell) on which epitope spread can occur. It is notable that β2GPI represents one of the first autoantigens targeted in human SLE (1), and that the presence of aPL predicts an earlier appearance of anti-dsDNA and anti-Sm Abs, as well as a more severe clinical outcome (11). Autoantibodies can be detected in SLE patients up to 9.4 years before diagnosis, with the number of autoantibodies increasing over time (1). The autoantibodies appear in a sequential order with aPL and ANA appearing first, followed by anti-Ro/SS-A, anti-La/SS-B, and anti-dsDNA, and subsequently by anti-Sm and anti-nRNP (1). It is therefore striking that autoantibodies in our immunized mice emerged in a very similar pattern and fell into three sequential groups: 1) anti-β2GPI and aCL Abs (aPL); 2) anti-Ro/SS-A, anti-La/SS-B, and anti-dsDNA; and 3) anti-nRNP and anti-Sm. This finding was reproduced in two strains of mice (C57BL/6 and BALB/c), despite their different haplotypes and Th cell orientations (Th1 vs Th2), although aPL and other autoantibodies appeared earlier in C57BL/6 mice. C57BL/6 also developed pathology earlier than BALB/c mice, suggesting that, as in human SLE (11), earlier appearance of aPL and Ab persistence may be as important as titer.
Immunization with β2GPI in the presence of LPS induced a pattern of glomerulonephritis closely resembling that seen in human lupus nephritis, including mesangial proliferative and focal proliferative patterns. These histologic findings, together with our autoantibody data, demonstrate that we were able to induce SLE-like disease in nonautoimmune mice immunized with β2GPI in the presence of LPS. The appearance of autoantibodies mimicked the sequence observed in human SLE, with β2GPI-dependent autoantibodies emerging early followed by the progressive accumulation of other autoantibodies and culminating in the onset of disease. It is intriguing that immunization of normal mice with an apoptotic cell-binding protein produces lupus-like disease, whereas immunization with apoptotic cells, alone (7, 8) or in the presence of an apoptotic cell-binding protein (15, 32), failed to reproduce the overt renal pathology and panoply of autoantibodies observed in SLE patients. In the present study, we compared the immune responses to both soluble and apoptotic cell-bound β2GPI. The immune response to soluble β2GPI exceeded that to apoptotic cell-bound β2GPI by greater than 10-fold. Although we cannot exclude the possibility that the lower response to apoptotic cell-bound β2GPI was due to a lesser quantity of β2GPI (see Materials and Methods), we believe that coinjection of apoptotic cells likely inhibited the immune response to β2GPI. Indeed, the inhibitory effects of apoptotic cells on the immune response are well known (9, 10, 33). Thus, although a weak to moderate immune response may be induced by immunization with apoptotic cells, either alone (7) or complexed with protein (15, 32), these immunizations did not result in the hallmark features of human lupus: strong IgG auto-reactivity, epitope spread to multiple autoantigens (particularly, dsDNA), and renal disease. Similarly, immunization with β2GPI (15, 34 –36) or other apoptotic cell-binding proteins (30) does not typically give rise to lupus-specific autoantibodies (e.g., anti-dsDNA) or lupus nephritis, although transient anti-dsDNA (34) and sustained ANA (32) responses have been observed.
The present study contrasts with others in that we were able to induce not only multiple SLE IgG autoantibodies of moderate to high titer, but also significant organ damage comparable to that seen in human lupus. To do so, we took advantage of apoptotic cells as a scaffold for epitope spread to multiple SLE-associated autoantigens, while avoiding the anti-inflammatory effect and possible tolerogenicity of apoptotic cells. To our knowledge, our study is the first model to show that overt autoimmune disease, mirroring both the panoply of autoantibodies and the spectrum of glomerulonephritis observed in human SLE, can be induced by immunization of nonautoimmune mice with an apoptotic cell-binding protein. We propose that induction of murine SLE requires tipping the balance from tolerance to immunity in a minimum of two ways: 1) use of an immunogen that can take advantage of apoptotic cells as a scaffold for epitope spread, but avoids the immunosuppression associated with apoptotic cells; and 2) induction of a strong and persistent T cell response to the inciting immunogen, for example, by the addition of LPS. Our data clearly show that a strong and persistent immune response to an apoptotic cell-binding protein (e.g., β2GPI) is sufficient to break tolerance and induce SLE-like disease. Furthermore, our findings provide clear evidence that epitope spread initiated by a single Ag is sufficient to produce multiple SLE autoantibodies and overt lupus nephritis, providing an experimental model for the epitope spread that may occur in human SLE.
Acknowledgments
We are grateful to Janet Laganière for expertise and assistance with early histology studies; Dr. Wolfgang Schneider and Lisa Murphy of the Clinical Biochemistry Laboratory of the Montreal General Hospital for testing of murine samples in clinical assays; Daniel Houle for expert assistance in rederiving murine strains; and Gabriel Bonventi for immunofluorescent staining of kidney sections. We thank Dr. Angela Rico de Souza, Cheryl Robinson, Dr. Mélanie Dieudé, Dr. Pascal Amireault, and Tanya Tolomeo for review of the manuscript, and Drs. Samuel Behar, Julie Desbarats, Angelika Longacre, David Ucker, Marie-Laure Brisson, and Hong Xiao for helpful discussions. We are indebted to Dr. Mélanie Dieudé for an important contribution to Fig. 10, and to Dr. Jean-Luc Senécal for expert advice on the interpretation of the ANA staining.
Footnotes
This work was supported by Grants DK59793 and HL69722 from the National Institutes of Health (to J.S.L.), a Young Investigator Award from the National Kidney Foundation of Illinois (to J.S.L.), Grant TAS97/0009 from the Arthritis Society of Canada (to J.R.), and Grant MOP-67101 from the Canadian Institutes for Health Research (to J.R.).
Abbreviations used in this paper: SLE, systemic lupus erythematosus; CL, cardiolipin; aCL, anticardiolipin; β2GPI, β2-glycoprotein I; ANA, antinuclear Ab; nRNP, nuclear ribonucleoprotein; PC, phosphatidylcholine; PEth, phosphatidylethanolamine; PS, phosphatidylserine; Sm, Smith Ag; aPL, antiphospholipid; aPTT, activated partial thromboplastin time; NMS, normal mouse serum.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, Harley JB. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349:1526–1533. doi: 10.1056/NEJMoa021933. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell DA, Pickering MC, Warren J, Fossati-Jimack L, Cortes-Hernandez J, Cook HT, Botto M, Walport MJ. C1q deficiency and autoimmunity: the effects of genetic background on disease expression. J Immunol. 2002;168:2538–2543. doi: 10.4049/jimmunol.168.5.2538. [DOI] [PubMed] [Google Scholar]
- 3.Cohen PL, Caricchio R, Abraham V, Camenisch TD, Jennette JC, Roubey RA, Earp HS, Matsushima G, Reap EA. Delayed apoptotic cell clearance and lupus-like autoimmunity in mice lacking the c-mer membrane tyrosine kinase. J Exp Med. 2002;196:135–140. doi: 10.1084/jem.20012094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanayama R, Tanaka M, Miyasaka K, Aozasa K, Koike M, Uchiyama Y, Nagata S. Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science. 2004;304:1147–1150. doi: 10.1126/science.1094359. [DOI] [PubMed] [Google Scholar]
- 5.Devitt A, Parker KG, Ogden CA, Oldreive C, Clay MF, Melville LA, Bellamy CO, Lacy-Hulbert A, Gangloff SC, Goyert SM, Gregory CD. Persistence of apoptotic cells without autoimmune disease or inflammation in CD14−/− mice. J Cell Biol. 2004;167:1161–1170. doi: 10.1083/jcb.200410057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stuart LM, Takahashi K, Shi L, Savill J, Ezekowitz RA. Mannose-binding lectin-deficient mice display defective apoptotic cell clearance but noautoimmune phenotype. J Immunol. 2005;174:3220–3226. doi: 10.4049/jimmunol.174.6.3220. [DOI] [PubMed] [Google Scholar]
- 7.Mevorach D, Zhou JL, Song X, Elkon KB. Systemic exposure to irradiated apoptotic cells induces autoantibody production. J Exp Med. 1998;188:387–392. doi: 10.1084/jem.188.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Georgiev M, Agle LM, Chu JL, Elkon KB, Ashany D. Mature dendritic cells readily break tolerance in normal mice but do not lead to disease expression. Arthritis Rheum. 2005;52:225–238. doi: 10.1002/art.20759. [DOI] [PubMed] [Google Scholar]
- 9.Voll RE, Herrmann M, Roth EA, Stach C, Kalden JR. Immunosuppressive effects of apoptotic cells. Nature. 1997;390:350–351. doi: 10.1038/37022. [DOI] [PubMed] [Google Scholar]
- 10.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-β, PGE2, and PAF. J Clin Invest. 1998;101:890– 898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McClain MT, Arbuckle MR, Heinlen LD, Dennis GJ, Roebuck J, Rubertone MV, Harley JB, James JA. The prevalence, onset, and clinical significance of antiphospholipid antibodies prior to the diagnosis of systemic lupus erythematosus. Arthritis Rheum. 2004;50:1226–1232. doi: 10.1002/art.20120. [DOI] [PubMed] [Google Scholar]
- 12.Khoruts A, Mondino A, Pape KA, Reiner SL, Jenkins MK. A natural immunological adjuvant enhances T cell clonal expansion through a CD28-dependent, interleukin (IL)-2-independent mechanism. J Exp Med. 1998;187:225–236. doi: 10.1084/jem.187.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vella AT, McCormack JE, Linsley PS, Kappler JW, Marrack P. Lipopolysaccharide interferes with the induction of peripheral T cell death. Immunity. 1995;2:261–270. doi: 10.1016/1074-7613(95)90050-0. [DOI] [PubMed] [Google Scholar]
- 14.Maxwell JR, Rossi RJ, McSorley SJ, Vella AT. T cell clonal conditioning: a phase occurring early after antigen presentation but before clonal expansion is impacted by Toll-like receptor stimulation. J Immunol. 2004;172:248–259. doi: 10.4049/jimmunol.172.1.248. [DOI] [PubMed] [Google Scholar]
- 15.Levine JS, Subang R, Koh JS, Rauch J. Induction of anti-phospholipid autoantibodies by β2-glycoprotein I bound to apoptotic thymocytes. J Autoimmun. 1998;11:413– 424. doi: 10.1006/jaut.1998.0235. [DOI] [PubMed] [Google Scholar]
- 16.Price BE, Rauch J, Shia MA, Walsh MT, Lieberthal W, Gilligan HM, O’Laughlin T, Koh JS, Levine JS. Antiphospholipid autoantibodies bind to apoptotic, but not viable, thymocytes in a [beta] 2-glycoprotein I-dependent manner. J Immunol. 1996;157:2201–2208. [PubMed] [Google Scholar]
- 17.Pape KA, Khoruts A, Mondino A, Jenkins MK. Inflammatory cytokines enhance the in vivo clonal expansion and differentiation of antigen-activated CD4+ T cells. J Immunol. 1997;159:591–598. [PubMed] [Google Scholar]
- 18.Vella AT, Mitchell T, Groth B, Linsley PS, Green JM, Thompson CB, Kappler JW, Marrack P. CD28 engagement and proinflammatory cytokines contribute to T cell expansion and long-term survival in vivo. J Immunol. 1997;158:4714– 4720. [PubMed] [Google Scholar]
- 19.Rauch J, Tannenbaum M, Janoff AS. Distinguishing plasma lupus anticoagulants from anti-factor antibodies using hexagonal (II) phase phospholipids. Thromb Haemost. 1989;62:892– 896. [PubMed] [Google Scholar]
- 20.Rauch J, Janoff AS. Phospholipid in the hexagonal phase is immunogenic: evidence for immunorecognition of nonbilayer lipid phases in vivo. Proc Natl Acad Sci USA. 1990;87:4112– 4114. doi: 10.1073/pnas.87.11.4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Granholm NA, Cavallo T. Autoimmunity, polyclonal B-cell activation and infection. Lupus. 1992;1:63–74. doi: 10.1177/096120339200100203. [DOI] [PubMed] [Google Scholar]
- 22.Maxwell JR, Ruby C, Kerkvliet NI, Vella AT. Contrasting the roles of costimulation and the natural adjuvant lipopolysaccharide during the induction of T cell immunity. J Immunol. 2002;168:4372– 4381. doi: 10.4049/jimmunol.168.9.4372. [DOI] [PubMed] [Google Scholar]
- 23.Pricop L, Hatakeyama A, Moran T, Bona C. Antibody response against poly (Glu60Ala20Tyr10) terpolymer and bacterial levan in κ-deficient mice. Eur J Immunol. 1995;25:1039–1043. doi: 10.1002/eji.1830250427. [DOI] [PubMed] [Google Scholar]
- 24.Boswell CM, Stein KE. Avidity maturation, repertoire shift, and strain differences in antibodies to bacterial levan, a type 2 thymus-independent polysaccharide antigen. J Immunol. 1996;157:1996–2005. [PubMed] [Google Scholar]
- 25.Bolland S, Ravetch JV. Spontaneous autoimmune disease in Fcγ RIIB-deficient mice results from strain-specific epistasis. Immunity. 2000;13:277–285. doi: 10.1016/s1074-7613(00)00027-3. [DOI] [PubMed] [Google Scholar]
- 26.Fukuyama H, Nimmerjahn F, Ravetch JV. The inhibitory Fcγ receptor modulates autoimmunity by limiting the accumulation of immunoglobulin G+ anti-DNA plasma cells. Nat Immunol. 2005;6:99–106. doi: 10.1038/ni1151. [DOI] [PubMed] [Google Scholar]
- 27.Chiller JM, Weigle WO. Termination of tolerance to human gamma globulin in mice by antigen and bacterial lipopolysaccharide. J Exp Med. 1973;137:740–750. doi: 10.1084/jem.137.3.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Waage A, Brandtzaeg P, Halstensen A, Kierulf P, Espevik T. The complex pattern of cytokines in serum from patients with meningococcal septic shock. J Exp Med. 1989;169:333–338. doi: 10.1084/jem.169.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 30.Casciola-Rosen LA, Anhalt G, Rosen A. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J Exp Med. 1994;179:1317–1330. doi: 10.1084/jem.179.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qian Y, Wang H, Clarke SH. Impaired clearance of apoptotic cells induces the activation of autoreactive anti-Sm marginal zone and B-1 B cells. J Immunol. 2004;172:625– 635. doi: 10.4049/jimmunol.172.1.625. [DOI] [PubMed] [Google Scholar]
- 32.Asano K, Miwa M, Miwa K, Hanayama R, Nagase H, Nagata S, Tanaka M. Masking of phosphatidylserine inhibits apoptotic cell engulfment and induces autoantibody production in mice. J Exp Med. 2004;200:459– 467. doi: 10.1084/jem.20040342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cvetanovic M, Ucker DS. Innate immune discrimination of apoptotic cells: repression of proinflammatory macrophage transcription is coupled directly to specific recognition. J Immunol. 2004;172:880– 889. doi: 10.4049/jimmunol.172.2.880. [DOI] [PubMed] [Google Scholar]
- 34.Blank M, Faden D, Tincani A, Kopolovic J, Goldberg I, Gilburd B, Allegri F, Balestrieri G, Valesini G, Shoenfeld Y. Immunization with anticardiolipin cofactor ([beta]-2-glycoprotein I) induces experimental antiphospholipid syndrome in naive mice. J Autoimmun. 1994;7:441– 455. doi: 10.1006/jaut.1994.1032. [DOI] [PubMed] [Google Scholar]
- 35.Silver RM, Pierangeli SS, Gharavi AE, Harris EN, Edwin SS, Salafia CM, Branch DW. Induction of high levels of anticardiolipin antibodies in mice by immunization with β2-glycoprotein I does not cause fetal death. Am J Obstet Gynecol. 1995;173:1410–1415. doi: 10.1016/0002-9378(95)90626-6. [DOI] [PubMed] [Google Scholar]
- 36.Tincani A, Gilburd B, Abu-Shakra M, Blank M, Allegri F, Ottaviani R, Riboni M, Meroni PL, Balestrieri G, Shoenfeld Y. Immunization of naive BALB/c mice with human β2-glycoprotein I breaks tolerance to the murine molecule. Arthritis Rheum. 2002;46:1399–1404. doi: 10.1002/art.10304. [DOI] [PubMed] [Google Scholar]