Abstract
Because of its ease of dispersal and high lethality, Bacillus anthracis is one of the most feared biowarfare agents. A better understanding of anthrax pathogenesis is urgently needed to develop new therapies for systemic disease that is relatively unresponsive to antibiotics. Although experimental evidence has implicated a role for macrophages in anthrax pathogenesis, clinical and pathological observations suggest that a direct insult to the host vasculature may also be important. Two bacterial toxins, lethal toxin and edema toxin, are believed to mediate the clinical sequelae of anthrax. Here, I examined whether these toxins are directly toxic to endothelial cells, the cell type that lines the interior of blood vessels. I show for the first time that lethal toxin but not edema toxin reduces the viability of cultured human endothelial cells and induces caspase-dependent endothelial apoptosis. In addition, this toxicity affects both microvascular and large vessel endothelial cells as well as endothelial cells that have differentiated into tubules within a type I collagen extracellular matrix. Finally, lethal toxin induces cleavage of mitogen-activated protein kinase kinases in endothelial cells and inhibits phosphorylation of ERK, p38, and JNK p46. Based on the contributions of these pathways to endothelial survival, I propose that lethal toxin-mediated cytotoxicity/apoptosis results primarily through inhibition of the ERK pathway. I also hypothesize that the observed endothelial toxicity contributes to vascular pathology and hemorrhage during systemic anthrax.
Bacillus anthracis is a gram-positive, spore-forming bacterium that causes anthrax in humans and animals (20). Spores from this organism may lie dormant in the environment for years. Once exposed, humans develop three distinct forms of disease depending on the route of acquisition, known as cutaneous, inhalational, and gastrointestinal anthrax. Systemic spread of organisms during infection is almost uniformly fatal.
Two Bacillus anthracis toxins, lethal toxin (LT) and edema toxin (ET), are primary mediators of disease (2, 35). LT alone is capable of causing rapid death in rodent models in a manner that was reported to be dependent on host macrophage function (15). Macrophage-dependent lethality was attributed to rapid release of interleukin-1 and tumor necrosis factor alpha, although a reduction in lipopolysaccharide-induced cytokine expression after LT treatment was noted by other investigators (9). In contrast to LT, ET does not cause lethality but induces transient edema when injected into animals (34).
LT and ET each consist of two components, an enzymatic activity, lethal factor (LF) and edema factor (EF), respectively, and a shared cofactor, protective antigen. Protective antigen is responsible for delivery of LF and EF to their site of action in the host cytoplasm. Functionally, LF is a zinc-dependent endopeptidase that inactivates mitogen-activated protein kinase kinases (MKKs) (7, 37). It has been shown to cleave the N terminus of MKKs 1, 2, 3, 4, 6, and 7 and thereby suppress phosphorylation of downstream mitogen-activated protein kinases (MAP kinases) including ERK (extracellular signal-regulated kinase), p38, and JNK/SAPK (c-Jun NH2-terminal kinase/stress-activated protein kinase). However, additional host targets have not been ruled out. Recently, inhibition of the p38 MAP kinase pathway by LT has been associated with the induction of apoptosis in macrophages (28). In contrast to LF, EF is a calmodulin-dependent adenylate cyclase (26) that has been shown to raise intracellular levels of cyclic AMP in a number of cell types. However, the manner in which this activity leads to edema formation is not yet known.
Although much attention has been paid to the role of host macrophages in anthrax pathogenesis, clinical, pathological, and experimental observations suggest that a direct insult to the host vasculature may also be important. Bleeding symptoms, including hemorrhagic lymphadenitis, mediastinitis, pericarditis, tracheobronchitis, and meningoencephalitis, tissue hemorrhage, and bleeding into the gastrointestinal tract, are often prominent findings associated with significant morbidity (1, 11). Autopsy studies show an underlying destruction of both large and small vessels with associated endothelial necrosis and vessel inflammation (1, 11, 13).
Since LT and ET have been implicated as primary mediators of anthrax pathology, I wondered whether these toxins might also contribute to vascular damage. To address this possibility, I developed an in vitro system to examine the effect of toxins on primary human endothelial cells. This cell type was examined specifically because endothelial cells line the interior of all blood vessels and are often primary mediators of vascular pathology in disease states (3). I found that LT but not ET was toxic to endothelial cells and propose that LT may contribute in this manner to the vascular pathology observed during anthrax.
MATERIALS AND METHODS
Cytotoxicity experiments.
Pooled human umbilical vein endothelial cells (HUVEC; Clonetics Corp.) and pooled neonatal dermal microvascular endothelial cells (DMVEC, Clonetics Corp.) were grown in EGM-2 MV (Clonetics) and used at passages 5 and 7, respectively. For cytotoxicity experiments, cells were resuspended in medium 199 with Earle's salts and 10% fetal bovine serum (Life Technologies), and seeded at 25,000 cells/cm2 on human collagen I (Vitrogen, Cohesion, Inc.)-treated plasticware.
Purified lethal factor (LF), protective antigen, and edema factor (EF) (kindly provided by Rachel Legmann and R. John Collier, Harvard Medical School) were added to a final concentrations of 100 ng per ml (LF and EF) and 500 ng per ml (protective antigen) unless otherwise indicated. Cell viability was assessed with the CellTiter 96 Aqueous One Solution cell proliferation assay (Promega). In this assay, viable cells cleave a formazan-based dye [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfonyl)-2H-tetrazolium inner salt (MTS)], allowing quantification of relative cell number. The MAP kinase pathway inhibitors PD98059 (Calbiochem), SB202190, and SP600125 (Biomol) and the caspase inhibitor Z-Val-Ala-Asp (OCH3)-fluoromethylketone (Z-VAD-FMK; Biomol) were dissolved in dimethyl sulfoxide and diluted from 1,000× stock solutions for assays in which controls were adjusted to contain the same final concentration of dimethyl sulfoxide. Statistical analysis was performed with an unpaired, two-tailed Student's t test.
For annexin V experiments, cells from culture supernatant and adherent cells collected after trypsinization were combined; stained with annexin V, Alexa Fluor 488 conjugate (Molecular Probes) and 1 μg of propidium iodide per ml; and incubated for 15 min at 22°C. Alternatively, to assess caspase activation, endothelial cells were stained with 10 μM fluorescein isothiocyanate-VAD-FMK (Promega) for 30 min at 22°C, washed twice in phosphate-buffered saline, and fixed in 0.4% formaldehyde in phosphate-buffered saline according to the manufacturer's recommendations (Promega Notes, volume 76). Flow cytometry was then performed with a Becton Dickinson FACSCalibur and data were analyzed (5,000 cells per assay) with the BD CellQuest software package. This software was also used to perform pairwise comparisons of histograms with Kolmogorov-Smirnov statistics.
In vitro vessel formation assays.
Three dimensional and two-dimensional collagen assays were performed as described previously (18, 38). For three-dimensional assays, HUVEC were resuspended at 2E6 cells per ml in 2 mg of ice-cold rat tail collagen (BD Biosciences) per ml containing 16 nM phorbol myristate acetate (PMA, Fisher Scientific), RPMI 1640 (Invitrogen), 10% fetal bovine serum (In Vitrogen), and indicated amounts of toxins. This collagen preparation gels rapidly at 37°C, trapping HUVEC within the three-dimensional extracellular matrix. At the end of the experiment, gels were fixed in 4% paraformaldehye-1% glutaraldehyde in Dulbecco's phosphate-buffered saline and either stained with diamidino-2-phenylindole dihydrochloride (DAPI, Sigma) for 10 min or processed for histology. The glutaraldehyde was found to be useful in adding physical rigidity to gels before tissue processing. Photomicrography of cultures or histology slides was performed with an inverted Nikon Eclipse TE100 microscope equipped with a color charge-coupled device camera and IPLab imaging software (Scanalytics). For two-dimensional assays, confluent HUVEC monolayers were overlaid with 0.5 mg of of rat tail collagen per ml containing RPMI, 10% fetal bovine serum, and 16 nM PMA and/or toxins as indicated.
Western blots.
HUVEC were resuspended in EBM-2 containing 2% fetal bovine serum and plated at 90% confluence. After 48 h, they were washed four times with phosphate-buffered saline and incubated in EBM-2 containing 1% tissue culture grade bovine serum albumin (Sigma-Aldrich) as previously described (40). After addition of toxins and incubation for the indicated times, cells were washed once in ice-cold phosphate-buffered saline and harvested in protein loading buffer containing 2% sodium dodecyl sulfate. Samples were then boiled, sonicated for 2 s to shear DNA, and loaded on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) precast 8 to 16% gradient gels (Bio-Rad Life Sciences). Western transfers were to 0.45-μm nitrocellulose membranes (Bio-Rad Life Sciences).
Antibodies to both phosphorylated and native forms of ERK, p38, JNK/SAPK, MEK1/2, and the N terminus of MEK4 were from Cell Signaling Technology. Antibodies to MEK3 and α-tubulin were obtained from Santa Cruz Biotechnology, and the antibody to the N terminus of MEK1 was from Upstate Biotechnology. Blots were developed with Pica West or Dura extended signal substrates (Pierce Chemical) and where indicated were stripped for reprobing with restore buffer (Pierce Chemical) at 60°C for 15 min.
RESULTS
LT is cytotoxic to endothelial cells.
I first assessed the effects of LT and ET on primary endothelial cells grown as tissue culture monolayers. After a 24-h treatment, I found that exposure to LT containing as little as 5 ng of LF per ml led to a significant decrease in endothelial viability (P < 0.001, Student's t test). This effect was seen both for endothelial cells derived from large and small vessels, human umbilical vein (HUVEC), and neonatal dermal microvascular endothelial cells (DMVEC). Importantly, loss in cell viability was only observed in the presence of B. anthracis protective antigen. Protective antigen is a protein cofactor necessary for delivery of both LF and EF to their site of action in the host cytoplasm. Therefore, dependence on protective antigen provides an important control indicating that reduced endothelial viability was a result of toxin activity.
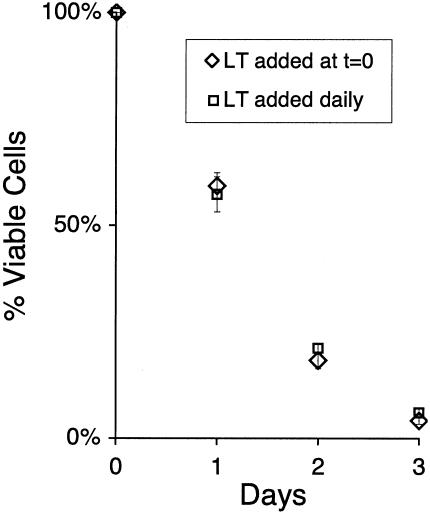
Half-maximal effects of LT were observed with approximately 10 ng of LF per ml and plateaued at concentrations above 50 ng per ml. A time course analysis with saturating amounts of LT (100 ng of LF per ml) showed a gradual decrease in cell viability over 3 days (Fig. 1), with a 95% decrease in viability by day 3. The rate of cell death was not markedly different whether the toxin was added once at the beginning of the assay or daily (Fig. 1), suggesting that a loss of toxin activity at later time points did not account for the gradual progression of cell death. Furthermore, a small number of HUVEC appeared relatively refractory to toxin, maintaining viability 4 days after toxin treatment, a result confirmed by microscopic inspection of cultures (data not shown). In contrast to LT, ET neither reduced endothelial viability with concentrations of EF as high as 100 ng per ml nor potentiated the toxicity of LT (data not shown). These findings established for the first time a direct toxic effect of LT on endothelial cells.
FIG. 1.
Kinetics of cell death. HUVEC were either treated with LT (100 ng of LF + 500 ng of protective antigen per ml) at the start of the experiment only (diamonds) or treated with toxin at the start of the experiment and then daily thereafter with fresh toxin and medium (squares). At the indicated time points, the viability of toxin-treated cells was determined with a formazan-based substrate as described in the text. Percent viability was determined by normalizing results to otherwise identically treated control assays in which LT was not added. Shown are mean and standard deviations of assays performed in octuplicate.
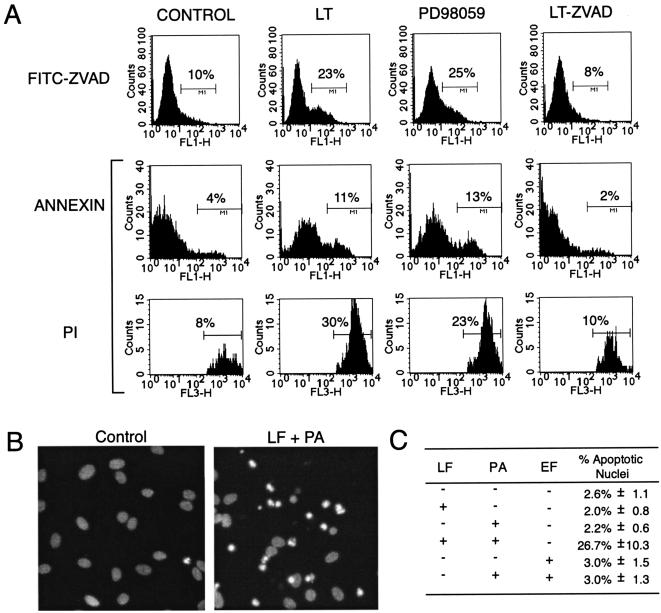
The loss of viable endothelial cells in the presence of LT could be the result of apoptosis or other forms of cell death. I therefore examined whether endothelial cells demonstrated biochemical and morphological hallmarks of apoptosis. First, I tested whether LT-treated cells showed increased levels of annexin V staining, a marker for phosphatidylserine exposure found on the surface of early apoptotic cells. In this assay, unfixed cells are treated concurrently with fluorochrome conjugated annexin V and propidium iodide. Early apoptotic cells stain with annexin but not propidium iodide. However, as a result of the loss of cell membrane integrity, late apoptotic and necrotic cells stain with both. As assessed by flow cytometry, LT induced a >2-fold increase in annexin positivity (early apoptotic cells; P < = 0.001 by Kolmogorov-Smirnov statistics) and >3-fold increase in propidium iodide-positive cells (late apoptotic or necrotic cells; P < = 0.001) relative to controls in which no (Fig. 2A) or single toxin components were added (data not shown). Similar results were found after treatment with PD98059, a known apoptotic stimulus in endothelial cells (14, 18, 24). Therefore, the annexin data suggest an induction of early apoptotic events.
FIG. 2.
LT induces apoptosis. (A) After 18 h of treatment with LT, 20 μM PD98059, or LT and 50 μM Z-VAD-FMK, HUVEC were stained with annexin V, Alexa Fluor 488 conjugate, and propidium iodide (PI). Following flow cytometry, histograms were generated by gating on propidium iodide-negative cells (annexin) and observing green fluorescence (FL-1) or by gating on propidium iodide-positive cells (propidium iodide) and observing red fluorescence (FL-3). Alternatively, HUVEC were stained with 10 μM fluorescein isothiocyanate-ZVAD-FMK, a fluorescent probe that binds to activated caspases. Following flow cytometry, histograms (FITC-ZVAD) were generated by observing green fluorescence (FL-1) for the entire cell population. Percentages are the number of cells within the marked histogram region expressed as a fraction of all cells analyzed. Results are representative of at least three different experiments. (B) After 18 h of treatment with anthrax factors, HUVEC were fixed, permeabilized, stained with DAPI, and examined by fluorescent microscopy. Shown is a representative field from LF- and protective antigen (PA)-treated and untreated controls. Apoptotic nuclei were scored based on characteristic apoptotic features of increased fluorescence (indicative of chromatin condensation) and nuclear fragmentation. A large number of apoptotic nuclear fragments are present in the LT-treated sample. (C) Data presented are the mean and standard deviation of one randomly chosen 100× field from four parallel assays with apoptotic nuclei expressed as a percentage of total HUVEC per field. LT-treated cells showed a significantly increased level of apoptotic nuclei (P < 0.01; Student's t test) compared to all other conditions.
To further assess the importance of apoptosis, I examined the involvement of caspases, enzymes that act early in apoptosis to amplify apoptotic signals and activate downstream apoptotic events. First, activation of caspases was directly examined by staining with fluorescein isothiocyanate-VAD-FMK, a cell-permeating fluorescent probe that binds to activated caspases. As shown in Fig. 2A, LT induced a twofold increase (P < = 0.001) in the number of cells staining strongly with fluorescein isothiocyanate-VAD-FMK in comparison with controls in which no toxin (Fig. 2A) or single toxin components (data not shown) were added. This modest increase in caspase activation likely reflects the gradual nature of endothelial cell death (i.e., Fig. 1). Since caspase activity (36) is lost in late apoptotic cells, only a small cohort of endothelial cells in earlier stages of apoptosis are expected to contribute substantially to caspase activity at a given time point. Therefore, I expected and indeed found the magnitude of induction in these assays to be relatively small in comparison to cumulative loss of viable endothelial cells.
Second, I tested the effect of caspase inhibition. In annexin studies (Fig. 2A), I found that 50 μM Z-VAD-FMK, a broad-spectrum caspase inhibitor, reduced both annexin and propidium iodide positivity nearly to control levels. Furthermore, in cell viability assays, ZVAD-FMK reduced LT-mediated cytotoxicity by over 60% (P < 1E-6; data not shown). Therefore, taken together, these results support a critical role for caspases in LT-mediated cell death.
Finally, the presence of nuclear changes specific for late apoptotic cells was assessed. After fixation, permeabilization, and staining with DAPI (4′,6′-diamidino-2-phenylindole dihydrochloride, Sigma-Aldrich), LT-treated HUVEC showed an approximately 10-fold increase in the number of cells with apoptotic nuclear changes (nuclear fragmentation and increased fluorescence resulting from chromatin condensation) compared to controls (Fig. 2C). Furthermore, in unfixed cultures, cells stained by propidium iodide showed apoptotic nuclear characteristics rather than the un-fragmented, uncondensed nuclei associated with necrosis (data not shown). Therefore, in multiple assays, I found that LT induces apoptosis and conclude that decreased endothelial viability must occur in large part through a caspase-dependent apoptotic process.
In vitro vessel formation assays.
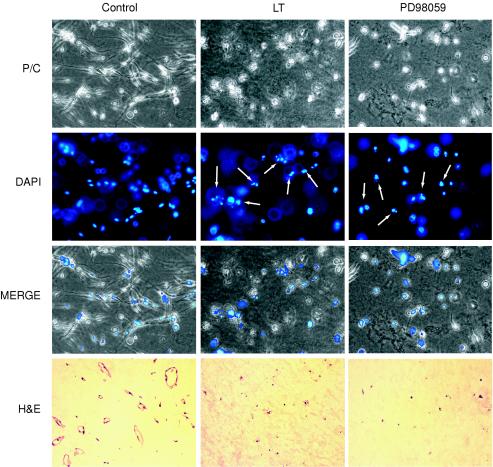
I next explored whether the effects of LT extended to endothelial cells within more complex organizational structures that are thought to model early stages of blood vessel development. In the first model, the three-dimensional collagen assay, HUVEC suspended in type I collagen and treated with phorbol myristate acetate (PMA) reorganized into hollow branched tubules that resembled capillaries (Fig. 3) (18). PMA, a potent inducer of protein kinase C, is thought to promote tubule formation through a number of activities, including the inhibition of apoptosis that otherwise occurs within this extracellular matrix. In this model, I found that LT blocked tubule formation (Fig. 3, phase contrast micrographs). After 3 days of LT treatment, endothelial cells also appeared uniformly nonviable and apoptotic by morphological criteria, demonstrating rounding and cellular fragmentation by phase contrast microscopy and chromatin condensation and nuclear fragmentation by DAPI staining. In addition, while hematoxylin- and eosin-stained control sections showed typical cross sections of hollow, endothelium-lined tubules (Fig. 3), LT-treated samples showed an absence of tubules as well as fragmented endothelial cells with pyknotic nucleic. Finally, when LT was added after tubules had already formed, destruction of endothelial cells also occurred (data not shown), indicating that the toxin was also active after endothelial tubular differentiation. However, in contrast to LT, ET did not appear to negatively affect tubule formation or stability (data not shown).
FIG. 3.
LT inhibits tubule formation. HUVEC were embedded in type I collagen gel containing 16 nM phorbol myristate acetate and treated with either LT or 20 μM PD98059 (an inhibitor of MEK1/2) or left untreated. After 2 days, cells were fixed, stained with DAPI, and photographed with phase contrast (P/C, 200×) and fluorescent (DAPI, 200×) microscopy. Alternatively, cultures were fixed on day 3, paraffin embedded, sectioned, and stained with hematoxylin and eosin (H&E, 100×). Note the presence of tubules in untreated controls and their total disruption in LT- and PD98059-treated cultures. In addition, DAPI images of LT- and PD98059-treated cultures show endothelial cells with morphological hallmarks of apoptosis, i.e., condensed chromatin and nuclear fragmentation. Examples of apoptotic nuclei within the plane of focus in this three-dimensional assay are indicated by arrows.
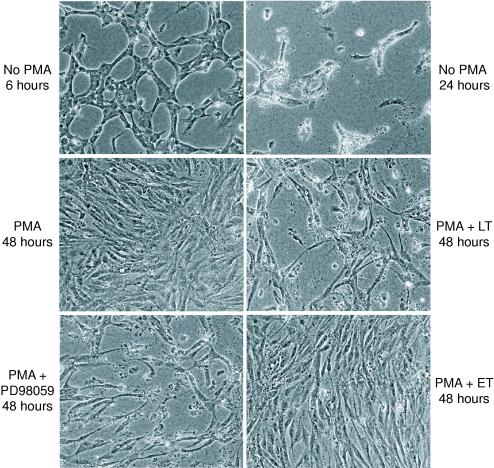
In the second model, the two-dimensional collagen assay, confluent monolayers of endothelial cells rapidly reorganize into a web-like network of interconnected cells, known as cords, after being overlaid with type I collagen gel. Cords are believed to model vessel precursors that arise prior to capillary lumen formation (33, 38). While cords develop within 6 to 12 h of collagen overlay (Fig. 4), in a process involving endothelial migration and reorganization, these structures are not stable and disintegrate almost completely over the following 24 h. Because of the rapid death of endothelial cells that normally occurs in this assay (33, 38, 39), I neither expected nor observed a markedly increased toxicity from the more gradually acting LT.
FIG. 4.
LT induces endothelial death in two-dimensional cord assays. Confluent monolayers of HUVEC were overlaid with 0.5 mg of type I collagen per ml and treated with16 nM phorbol myristate acetate (PMA), LT, ET, and/or 20 μM PD98059. In the absence of PMA, typical cords form 6 h after collagen overlay (upper left panel, phase contrast, 100×); however, by 24 h (upper right panel), most of the endothelial cells are no longer viable. In contrast, in the presence of PMA, HUVEC remained viable as confluent monolayers even after 48 h (middle left panel). However, despite the presence of PMA, a 48-h treatment with LT (middle right panel) or PD98059 (lower left panel) led to significant cell death, leaving HUVEC in atypical cord-like arrangements. In contrast, ET did not have a deleterious effect on endothelial survival (lower right panel).
It is presumed that cells die in two-dimensional assays because of a lack of survival or mitogenic signals that normally accompany vessel development in vivo. Therefore, I checked to see if this limitation could be overcome by addition of PMA, a known inhibitor of endothelial apoptosis in three-dimensional assays. Interestingly, when PMA was added at the start of the experiment, I found that endothelial cells did not reorganize into cords in the presence of PMA even after 48 h, but instead remained as a confluent monolayer with prolonged viability. However, as in the three-dimensional assays, the cytoprotective effect of PMA could not overcome the toxic effect of LT, which led to the destruction of the endothelial monolayer over 1 to 2 days (Fig. 4) with most cells demonstrating morphological hallmarks of apoptotic cell death (data not shown). Interestingly, the remaining intact endothelial cells (i.e., intact nuclei by DAPI staining and extended cellular morphology by phase contrast microscopy) were organized in a somewhat atypical cord-like geometry with elongated endothelial cells touching one another and forming web-like structures. The effect of LT contrasts with that of ET, which did not appear to have a deleterious effect.
LT inhibits MAP kinase pathways in endothelial cells.
To address potential mechanisms for the cytotoxic action of LT, I examined its effects on mitogen-activated protein kinase (MAP kinase) pathways. In response to different stimuli, including cytokines, growth factors, and cellular stress, MAP kinase pathways transmit signals through a stepwise series of kinases (25). In each of these pathways, an upstream MAP kinase kinase kinase (MKKK) becomes phosphorylated and in turn phosphorylates a downstream MAP kinase kinase (MKK), which in turn phosphorylates a MAP kinase. The activated MAP kinase then phosphorylates transcription factors or other protein kinases to alter cellular function. Each of the three major MAP kinase pathways is named for the terminal MAP kinase, i.e., ERK (extracellular signal-regulated kinase), p38, and JNK/SAPK (c-Jun NH2-terminal kinase/stress-activated protein kinase).
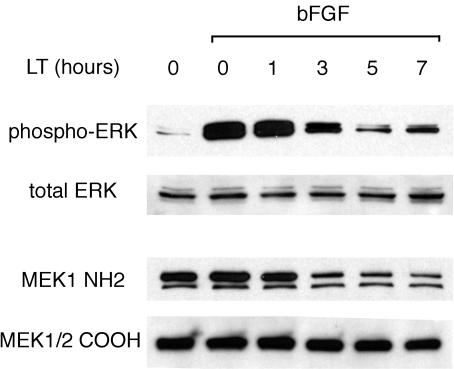
LT has previously been shown to cleave the N terminus of several MKKs (7, 37). Since the N-terminal region cleaved by LT mediates both interaction with upstream MAP kinase kinase kinases (MKKKs) and downstream MAP kinases, this cleavage might block endothelial MAP kinase activation, as it has been shown to do previously in macrophages (29). I therefore first sought to establish whether MAP kinase kinases were in fact cleaved in endothelial cells. To do this, I probed the integrity of MAP kinase kinases by reaction with antibodies that recognize epitopes N-terminal and/or C-terminal to known LT cleavage sites, a strategy previously described by Duesbery et al. (7) and Vitale et al. (37). With an antibody to the N terminus of MEK1, I found that most of MEK1 lost its N-terminal epitope within 5 h of toxin addition (Fig. 5). In contrast, an antibody against a C-terminal epitope, which should react with both native and cleaved forms, recognized approximately the same level of total protein in untreated and LT-treated samples. Furthermore, with the C-terminal antibody, a slight decrease in the molecular weight of MEK1/2 could be appreciated in toxin-treated samples in comparison with the α-tubulin reference band (see Fig. 7). These results are consistent with LT-mediated cleavage of the N terminus of MEK1/2 in HUVEC.
FIG. 5.
Time course of ERK inhibition pathway by LT. HUVEC were serum starved and then treated with LT for the indicated number of hours. They were then treated with 30 ng of bFGF per ml, an activator of the ERK pathway, for 5 min, and lysed in protein sample buffer. Western blots were probed with antibody against phosphorylated ERK (phospho-Thr202/Tyr204), then stripped and probed with antibody to total ERK. Alternatively, blots were probed with an antibody against the N terminus (NH2) of MEK1, then stripped and probed with an antibody (COOH) against a sequence C-terminal to the LT cleavage site in MEK1/2.
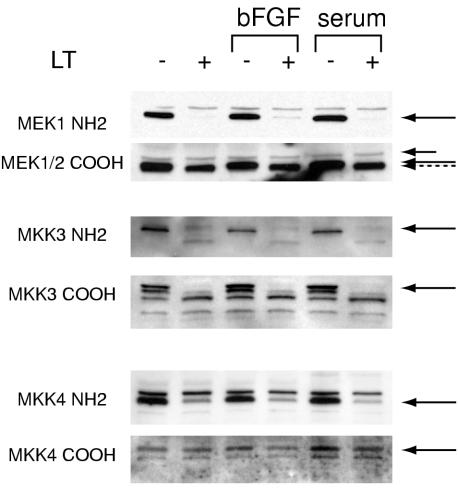
FIG. 7.
LT cleaves multiple MAP kinase kinases that signal through the ERK, p38, and JNK/SAPK pathways. Serum-starved HUVEC were treated with LT for 7 h and 30 ng of FGF per ml or 10% fetal bovine serum for 5 min prior to preparation of cell lysates. Western blots were probed with antibodies against epitopes N-terminal (NH2) or C-terminal (COOH) to previously described LT cleavage sites within the indicated MKKs. As expected, a short treatment with bFGF or serum did not alter MKK cleavage patterns. The MEK1/2 COOH blot was also probed with an antibody to α-tubulin (short arrow). This protein serves as a reference point to appreciate the slightly reduced molecular weight (dashed arrow) of MEK1/2 observed after LT treatment. Solid arrows point to the untreated MKKs. The band marked by the arrow in the MKK-4 COOH blot is exactly superimposable on the band marked in the NH2 blot. For unknown reasons, a cleaved form was not observed with the COOH antibody.
As MEK1 and MEK2 phosphorylate and thereby activate ERK, I next examined the phosphorylation status of ERK. As expected, basic fibroblastic growth factor (bFGF), a potent activator of the ERK pathway, greatly stimulated ERK phosphorylation (Fig. 5). However, pretreatment with LT inhibited this phosphorylation. The extent of inhibition correlated with the length of pretreatment and the degree of MEK1 cleavage (Fig. 5). Taken together, these results are consistent with LT's inhibiting phosphorylation of ERK through cleavage and inactivation of its upstream MKK.
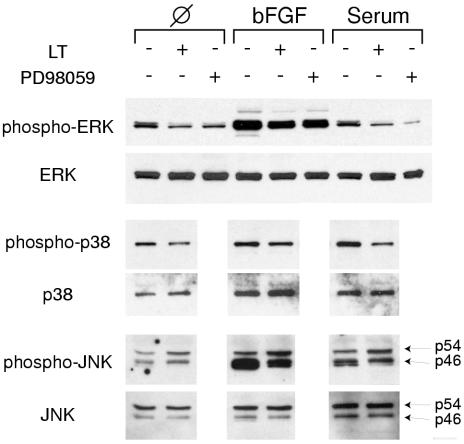
I next examined the effect of LT on the other two major MAP kinase pathways, p38 and JNK/SAPK. I found that LT inhibited the phosphorylation of p38. In addition, it also suppressed phosphorylation of p46 isoforms of JNK/SAPK, evident after pathway stimulation by bFGF or serum treatment (Fig. 6). I then examined whether upstream MKKs involved in phosphorylation of p38 and JNK/SAPK were also affected. With N-terminal and C-terminal antibodies, I found that LT cleaved most of MKK3, an MKK that specifically phosphorylates p38 (Fig. 7), with a cleavage pattern essentially identical to that observed previously (37). In addition, I found evidence for MKK4 cleavage with an N-terminal antibody to this protein. In vivo, MKK4 specifically phosphorylates JNK/SAPK, although it is also noted to phosphorylate both p38 and JNK in vitro (reviewed in reference 25). Therefore, in summary, LT suppresses phosphorylation of the three major MAP kinases, ERK, p38, and JNK/SAPK, as well as cleaves and thereby presumably inactivates upstream MKKs.
FIG. 6.
LT inhibits phosphorylation of ERK, p38, and JNK/SAPK. HUVEC were serum starved and then treated with LT for 7 h or 20 μM PD98059 for 2 h. Cells were then exposed to 30 ng of bFGF per ml or 10% serum for 5 min and immediately harvested. Western blots were probed with phosphorylation-specific antibodies for ERK, p38 and JNK/SAPK. Blots were then stripped and probed with antibodies against total ERK, p38, and JNK/SAPK.
I next explored the connection between MAP kinase inhibition and endothelial cell death. Blockade of the ERK pathway through pharmacological inhibition has previously been shown to induce apoptotic cell death in endothelial cells (14, 18, 24). I also found this to be the case in standard tissue culture assays (Fig. 2A and 8) and three-dimensional (Fig. 3) and two-dimensional (Fig. 4) collagen assays, where 20 μM PD98059, a specific inhibitor of MEK1,2, was nearly as effective in inducing cell death as LT. Western blots documented a concomitant suppression of ERK phosphorylation, which was similar in extent to the effect of LT (Fig. 6).
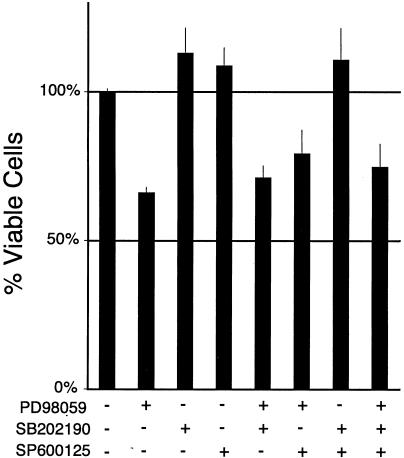
FIG. 8.
ERK pathway inhibition leads to decreased endothelial viability. HUVEC were treated for 24 h with inhibitors of the ERK (20 μM PD98059), p38 (3 μM SB202190), and JNK/SAPK (5 μM SP600125) pathways. Values shown are mean and standard deviations for sextuplicate cell viability assays normalized to controls incubated in the absence of inhibitors.
To probe potential contributions of other MAP kinase pathways towards endothelial apoptosis, I used two specific inhibitors of p38 MAP kinase and JNK/SAPK. In contrast to PD98059, treatment with the p38 MAP kinase inhibitor SB202190 led to increased viability of HUVEC in tissue culture (Fig. 8) and three-dimensional collagen assays (data not shown). Similar effects of this drug on HUVEC were described previously (12, 18, 41). Likewise, treatment with the JNK/SAPK inhibitor SP600125 led to a slightly increased viability of HUVEC, at the previously reported 50% inhibitory concentration (5 μM) for this drug in Jurkat T cells (4). This is consistent with previous findings that downmodulation of the JNK pathway is cytoprotective in endothelial cells (10, 16, 17, 42). It should be noted that at higher concentrations (20 μM), SP600125 was toxic for endothelial cells; however, the interpretation of this result is unclear, since others have recently found inhibition of additional pathways with high concentrations of this drug (21). Importantly, when PD98059 was added in combination with the p38 and JNK/SAPK inhibitors, it still exerted significant toxicity. Therefore, inhibition of the ERK pathway appeared to account for a significant part of LT's toxicity towards endothelial cells.
DISCUSSION
I found that LT but not ET induced endothelial apoptosis. However, the effects of LT on endothelial cells differed in a number of ways from its previously described effects on macrophages. First, in endothelial cells, cytotoxicity occurred gradually over 3 days, in contrast to rapid cell death of macrophages that occurs within hours (28). This slowly progressive cytotoxicity occurred despite almost complete cleavage of MAP kinase kinases within 5 h of toxin addition and an apparently nonlimiting amount of toxin. The gradual nature of cell death likely reflects heterogeneity within primary endothelial cultures (e.g., multiple subtypes of cells) (31), so that subpopulations of cells show differential susceptibility to the effects of toxin, i.e., MAP kinase inhibition. Therefore, some endothelial cells die quickly, some more slowly, and a small number appear resistant. The underlying reason for this differential susceptibility may prove a useful area further investigation, since it may suggest ways to induce a greater level of resistance to toxin.
Second, endothelial cells appeared to respond differently to MAP kinase inhibition than macrophages. Park et al. (28) found that inhibition of the p38 kinase pathway was the primary event leading to macrophage apoptosis after treatment with LT. In contrast, p38 inhibition promoted endothelial survival, an observation that has been made previously in a number of endothelial types, including HUVEC (12, 18, 41). Therefore, I conclude that p38 inhibition does not contribute to LT cytotoxicity in our studies. Third, in macrophage studies, apoptosis was only induced efficiently in the presence of lipopolysaccharide and lipoteichoic acid (28). In contrast, in endothelial cells, cytotoxicity was efficiently induced in the absence of these other stimuli (data not shown). Furthermore, lipopolysaccharide and lipoteichoic acid from Bacillus subtilis did not potentiate the effects of LT (data not shown). Finally, polymyxin B at 25 μg per ml, an inhibitor of lipopolysaccharide activity, did not diminish the effects of LT, arguing against the contribution of potential contamination with lipopolysaccharide to the cytotoxic response.
Finally, inhibition of the ERK pathway appeared to have profound effects on the viability of endothelial cells. This is consistent with a large number of studies indicating the importance of the ERK kinase pathway in endothelial proliferation and survival (14, 18). In the present study, both LT and a specific pharmacological inhibitor of MEK-1 and MEK-2 inhibited phosphorylation of ERK to a similar extent. Furthermore, toxin and drug had similar detrimental effects on endothelial survival in all endothelial models tested. Therefore, I conclude that in contrast to macrophages, the major toxic effect of LT results from the inhibition of the ERK pathway. However, I cannot completely exclude the contribution of other pathways not yet considered, since LT may interact with other cellular substrates and thereby decrease survival in multiple ways, or effects from differential inhibition of JNK isoforms. Ultimately, the effects of LT must represent the combinatorial effects of multiple pathways including the ERK kinase pathway.
Interestingly, the toxic effects of LT were apparent on physiologically different types of endothelial cells. LT affected both HUVEC and DMVEC, cells derived from large vessels and microvessels, respectively. These cell types differ markedly with respect to their sensitivity to stress, response to cytokine stimulation, motility, and contributions to angiogenesis (30). In addition, LT was toxic to HUVEC in tissue culture monolayers and to differentiated HUVEC forming tubular structures. Under these two conditions, endothelial cells are known to differ physiologically in a number of ways including their response to external stimuli and their ability to enter the cell cycle (18, 19, 23).
This general toxicity of LT towards diverse types and states of endothelial cells led us to consider on a theoretical basis whether LT might similarly damage endothelial cells in vivo. Clearly, the destruction of vessels observed during anthrax is not typically observed with severe systemic infections caused by other gram-positive or gram-negative bacterial pathogens. This suggests that vessel damage results from unique attributes of Bacillus anthracis that may relate to toxin expression and/or other factors. However, to date, hemorrhage has not yet been described after experimental injection with LT. This negative finding may potentially result from the lack of necessary cofactors present during an actual infection. Alternatively, experimental injection strategies described in previously reported studies may not have been as efficient as live bacteria in delivering toxic doses of LT to susceptible vascular beds. During the septic phase of anthrax, bacteria replicate to large numbers (22) and may therefore deliver larger amounts of toxin in a sustained manner. Intriguingly, purified LT has recently been found to inhibit tumor angiogenesis in a mouse model, providing the first experimental evidence for an in vivo effect on host vasculature (6). Inhibition of endothelial survival is a well-established mechanisms for suppressing angiogenesis (5), especially since endothelial cells involved in angiogenesis are particularly susceptible to apoptotic induction. This observation now forms the basis for a number of therapies targeting angiogenesis in tumors. Therefore, the cytotoxic activity observed in our studies could potentially help explain LT's previously observed antiangiogenic effects. Moreover, these studies suggest a possible mechanism of action. Pharmacological inhibition of the ERK kinase pathway is known to suppress new vessel formation in in vivo angiogenesis assays (8, 32). Therefore, LT's inhibition of the ERK pathway in endothelial cells may have similar effects. Interestingly, an antiangiogenic effect even without the overt destruction of vessels could be an important cause of morbidity during anthrax, since repair of tissue injury, which must occur during systemic anthrax, depends on new vessel formation (27).
I have now provided evidence that LT is directly toxic to endothelial cells in vitro in both cell monolayer and in vitro angiogenesis models. Through its toxic action on endothelial cells, LT may therefore contribute to the devastating hemorrhagic complications of anthrax, a hypothesis that needs to be examined in future studies in animal models. If this hypothesis is correct, strategies aimed at promoting endothelial survival may prove useful in treating this highly fatal disease.
Acknowledgments
I thank Rachel Legmann and R. John Collier for the generous gift of anthrax toxin components; Jeannie T. Lee and Shirley Stiver for critical reading of the manuscript; and Robin Bachelder for teaching me how to use the flow cytometer.
This work was supported by the Beth Israel Hospital Pathology Foundation.
Editor: J. T. Barbieri
REFERENCES
- 1.Abramova, F. A., L. M. Grinberg, O. V. Yampolskaya, and D. H. Walker. 1993. Pathology of inhalational anthrax in 42 cases from the Sverdlovsk outbreak of 1979. Proc. Natl. Acad. Sci. USA 90:2291-2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beall, F. A., M. J. Taylor, and C. B. Thorne. 1961. Rapid lethal effect in rats of a third component found upon fractionating the toxin of Bacillus anthracis. J. Bacteriol. 83:1274-1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Behrendt, D., and P. Ganz. 2002. Endothelial function. From vascular biology to clinical applications. Am. J. Cardiol. 90:40L-48L. [DOI] [PubMed] [Google Scholar]
- 4.Bennett, B. L., D. T. Sasaki, B. W. Murray, E. C. O'Leary, S. T. Sakata, W. Xu, J. C. Leisten, A. Motiwala, S. Pierce, Y. Satoh, S. S. Bhagwat, A. M. Manning, and D. W. Anderson. 2001. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc. Natl. Acad. Sci. [DOI] [PMC free article] [PubMed]
- 5.Dimmeler, S., and A. M. Zeiher. 2000. Endothelial cell apoptosis in angiogenesis and vessel regression. Circ. Res. 87:434-439. [DOI] [PubMed] [Google Scholar]
- 6.Duesbery, N. S., J. Resau, C. P. Webb, S. Koochekpour, H. M. Koo, S. H. Leppla, and G. F. Vande Woude. 2001. Suppression of ras-mediated transformation and inhibition of tumor growth and angiogenesis by anthrax lethal factor, a proteolytic inhibitor of multiple MEK pathways. Proc. Natl. Acad. Sci. 98:4089-4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duesbery, N. S., C. P. Webb, S. H. Leppla, V. M. Gordon, K. R. Klimpel, T. D. Copeland, N. G. Ahn, M. K. Oskarsson, K. Fukasawa, K. D. Paull, and G. F. Vande Woude. 1998. Proteolytic inactivation of MAP-kinase-kinase by anthrax lethal factor. Science 280:734-737. [DOI] [PubMed] [Google Scholar]
- 8.Eliceiri, B. P., R. Klemke, S. Stromblad, and D. A. Cheresh. 1998. Integrin alphavbeta3 requirement for sustained mitogen-activated protein kinase activity during angiogenesis. J. Cell Biol. 140:1255-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erwin, J. L., L. M. DaSilva, S. Bavari, S. F. Little, A. M. Friedlander, and T. C. Chanh. 2001. Macrophage-derived cell lines do not express proinflammatory cytokines after exposure to Bacillus anthracis lethal toxin. Infect Immun. 69:1175-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esen, M., B. Schreiner, V. Jendrossek, F. Lang, K. Fassbender, H. Grassme, and E. Gulbins. 2001. Mechanisms of Staphylococcus aureus induced apoptosis of human endothelial cells. Apoptosis 6:431-439. [DOI] [PubMed] [Google Scholar]
- 11.Gleiser, C. A. 1967. Pathology of anthrax infection in animal hosts. Fed. Proc. 26:1518-1521. [PubMed] [Google Scholar]
- 12.Gratton, J. P., M. Morales-Ruiz, Y. Kureishi, D. Fulton, K. Walsh, and W. C. Sessa. 2001. Akt down-regulation of p38 signaling provides a novel mechanism of vascular endothelial growth factor-mediated cytoprotection in endothelial cells. J. Biol. Chem. 276:30359-30365. [DOI] [PubMed] [Google Scholar]
- 13.Grinberg, L. M., F. A. Abramova, O. V. Yampolskaya, D. H. Walker, and J. H. Smith. 2001. Quantitative pathology of inhalational anthrax I: quantitative microscopic findings. Mod. Pathol. 14:482-495. [DOI] [PubMed] [Google Scholar]
- 14.Gupta, K., S. Kshirsagar, W. Li, L. Gui, S. Ramakrishnan, P. Gupta, P. Y. Law, and R. P. Hebbel. 1999. VEGF prevents apoptosis of human microvascular endothelial cells via opposing effects on MAP kinase/ERK and SAPK/JNK signaling. Exp. Cell Res. 247:495-504. [DOI] [PubMed] [Google Scholar]
- 15.Hanna, P. C., D. Acosta, and R. J. Collier. 1993. On the role of macrophages in anthrax. Proc. Natl. Acad. Sci. 90:10198-10201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu, Y. L., S. Li, J. Y. Shyy, and S. Chien. 1999. Sustained JNK activation induces endothelial apoptosis: studies with colchicine and shear stress. Am. J. Physiol. 277:1593-1599. [DOI] [PubMed] [Google Scholar]
- 17.Hull, C., G. McLean, F. Wong, P. J. Duriez, and A. Karsan. 2002. Lipopolysaccharide signals an endothelial apoptosis pathway through TNF receptor-associated factor 6-mediated activation of c-Jun NH2-terminal kinase. J. Immunol. 169:2611-2618. [DOI] [PubMed] [Google Scholar]
- 18.Ilan, N., S. Mahooti, and J. A. Madri. 1998. Distinct signal transduction pathways are utilized during the tube formation and survival phases of in vitro angiogenesis. J. Cell Sci. 111:3621-3631. [DOI] [PubMed] [Google Scholar]
- 19.Ingber, D. E. 2002. Mechanical signaling and the cellular response to extracellular matrix in angiogenesis and cardiovascular physiology. Circ. Res. 91:877-887. [DOI] [PubMed] [Google Scholar]
- 20.Inglesby, T. V., T. O'Toole, D. A. Henderson, J. G. Bartlett, M. S. Ascher, E. Eitzen, A. M. Friedlander, J. Gerberding, J. Hauer, J. Hughes, J. McDade, M. T. Osterholm, G. Parker, T. M. Perl, P. K. Russell, and K. Tonat. 2002. Anthrax as a biological weapon, 2002: updated recommendations for management. JAMA 287:2236-2252. [DOI] [PubMed] [Google Scholar]
- 21.Jiang, G., Q. Dallas-Yang, F. Liu, D. E. Moller, and B. B. Zhang. 2003. Salicylic acid reverses phorbol 12-myristate-13-acetate (PMA)- and tumor necrosis factor alpha (TNFalpha)-induced insulin receptor substrate 1 (IRS1) serine 307 phosphorylation and insulin resistance in human embryonic kidney 293 (HEK293) cells. J. Biol. Chem. 278:180-186. [DOI] [PubMed] [Google Scholar]
- 22.Klein, F., J. S. Walker, D. F. Fitzpatrick, R. E. Lincoln, B. G. Mahlandt, J. Jones, W. I., J. P. Dobbs, and K. J. Hendrix. 1966. Pathophysiology of Anthrax. J. Infect Dis. 116:123-138. [DOI] [PubMed] [Google Scholar]
- 23.Kuzuya, M., S. Satake, H. Miura, T. Hayashi, and A. Iguchi. 1996. Inhibition of endothelial cell differentiation on a glycosylated reconstituted basement membrane complex. Exp. Cell Res. 226:336-345. [DOI] [PubMed] [Google Scholar]
- 24.Kuzuya, M., S. Satake, M. A. Ramos, S. Kanda, T. Koike, K. Yoshino, S. Ikeda, and A. Iguchi. 1999. Induction of apoptotic cell death in vascular endothelial cells cultured in three-dimensional collagen lattice. Exp. Cell Res. 248:498-508. [DOI] [PubMed] [Google Scholar]
- 25.Kyriakis, J. M., and J. Avruch. 2001. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol. Rev. 81:807-869. [DOI] [PubMed] [Google Scholar]
- 26.Leppla, S. H. 1982. Anthrax toxin edema factor: a bacterial adenylate cyclase that increases cyclic AMP concentrations of eukaryotic cells. Proc. Natl. Acad. Sci. 79:3162-3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li, J., Y. P. Zhang, and R. S. Kirsner. 2003. Angiogenesis in wound repair: angiogenic growth factors and the extracellular matrix. Microsc. Res. Tech. 60:107-114. [DOI] [PubMed] [Google Scholar]
- 28.Park, J. M., F. R. Greten, Z. W. Li, and M. Karin. 2002. Macrophage apoptosis by anthrax lethal factor through p38 MAP kinase inhibition. Science 297:2048-2051. [DOI] [PubMed] [Google Scholar]
- 29.Pellizzari, R., C. Guidi-Rontani, G. Vitale, M. Mock, and C. Montecucco. 2000. Lethal factor of Bacillus anthracis cleaves the N terminus of MAPKKs: analysis of the intracellular consequences in macrophages. Int. J. Med. Microbiol. 290:421-427. [DOI] [PubMed] [Google Scholar]
- 30.Ribatti, D., B. Nico, A. Vacca, L. Roncali, and F. Dammacco. 2002. Endothelial cell heterogeneity and organ specificity. J. Hematother. Stem Cell Res. 11:81-90. [DOI] [PubMed] [Google Scholar]
- 31.Rone, J. D., and A. L. Goodman. 1987. Heterogeneity of rabbit aortic endothelial cells in primary culture. Proc. Soc. Exp. Biol. Med. 184:495-503. [DOI] [PubMed] [Google Scholar]
- 32.Rusnati, M., C. Urbinati, B. Musulin, D. Ribatti, A. Albini, D. Noonan, C. Marchisone, J. Waltenberger, and M. Presta. 2001. Activation of endothelial cell mitogen activated protein kinase ERK(1/2) by extracellular HIV-1 Tat protein. Endothelium 8:65-74. [DOI] [PubMed] [Google Scholar]
- 33.Segura, I., A. Serrano, G. G. De Buitrago, M. A. Gonzalez, J. L. Abad, C. Claveria, L. Gomez, A. Bernad, A. C. Martinez, and H. H. Riese. 2002. Inhibition of programmed cell death impairs in vitro vascular-like structure formation and reduces in vivo angiogenesis. FASEB J. 16:833-841. [DOI] [PubMed] [Google Scholar]
- 34.Smith, H. 2002. Discovery of the anthrax toxin: the beginning of studies of virulence determinants regulated in vivo. Int. J. Med. Microbiol. 291:411-417. [DOI] [PubMed] [Google Scholar]
- 35.Smith, H., J. Keppie, and J. L. Stanley. 1955. The chemical basis of the virulence of Bacillus anthracis. V. The specific toxin produced by B. anthracis in vivo. Br. J. Exp. Pathol. 36:460-472. [PMC free article] [PubMed] [Google Scholar]
- 36.Smolewski, P., J. Grabarek, H. D. Halicka, and Z. Darzynkiewicz. 2002. Assay of caspase activation in situ combined with probing plasma membrane integrity to detect three distinct stages of apoptosis. J. Immunol Methods. 265:111-121. [DOI] [PubMed] [Google Scholar]
- 37.Vitale, G., L. Bernardi, G. Napolitani, M. Mock, and C. Montecucco. 2000. Susceptibility of mitogen-activated protein kinase kinase family members to proteolysis by anthrax lethal factor. Biochem. J. 352:739-745. [PMC free article] [PubMed] [Google Scholar]
- 38.Whelan, M. C., and D. R. Senger. 2003. Collagen I initiates endothelial cell morphogenesis by inducing actin polymerization through suppression of cyclic AMP and protein kinase A. J. Biol. Chem. 278:327-334. [DOI] [PubMed] [Google Scholar]
- 39.Wright, T. J., L. Leach, P. E. Shaw, and P. Jones. 2002. Dynamics of vascular endothelial-cadherin and beta-catenin localization by vascular endothelial growth factor-induced angiogenesis in human umbilical vein cells. Exp. Cell Res. 280:159-168. [DOI] [PubMed] [Google Scholar]
- 40.Wu, L. W., L. D. Mayo, J. D. Dunbar, K. M. Kessler, M. R. Baerwald, E. A. Jaffe, D. Wang, R. S. Warren, and D. B. Donner. 2000. Utilization of distinct signaling pathways by receptors for vascular endothelial cell growth factor and other mitogens in the induction of endothelial cell proliferation. J. Biol. Chem. 275:5096-5103. [DOI] [PubMed] [Google Scholar]
- 41.Yu, Y., and J. D. Sato. 1999. MAP kinases, phosphatidylinositol 3-kinase, and p70 S6 kinase mediate the mitogenic response of human endothelial cells to vascular endothelial growth factor. J. Cell Physiol. 178:235-246. [DOI] [PubMed] [Google Scholar]
- 42.Zhang, C., J. Kawauchi, M. T. Adachi, Y. Hashimoto, S. Oshiro, T. Aso, and S. Kitajima. 2001. Activation of JNK and transcriptional repressor ATF3/LRF1 through the IRE1/TRAF2 pathway is implicated in human vascular endothelial cell death by homocysteine. Biochem. Biophys. Res. Commun. 289:718-724. [DOI] [PubMed] [Google Scholar]