Abstract
Purpose
The aim of this study was to compare the clinical outcome of open flap debridement (OFD) with a biphasic calcium phosphate (BCP) graft to that of OFD without BCP graft for the treatment of intrabony periodontal defects (IBDs).
Methods
The study included 25 subjects that had at least one intrabony defect of 2- or 3-wall morphology and an intrabony component≥4 mm as detected radiographically. Subjects were randomly assigned to treatment with (BCP group, n=14) or without BCP (OFD group, n=11). Clinical parameters were recorded at baseline and 6 months after surgery and included the plaque index, gingival index, probing depth (PD), clinical attachment level (CAL), and gingival recession (REC). A stringent plaque control regimen was enforced for all of the patients during the 6-month observation period.
Results
In all of the treatment groups, significant PD reductions and CAL gains occurred during the study period (P<0.01). At 6 months, patients in the BCP group exhibited a mean PD reduction of 3.7±1.2 mm and a mean CAL gain of 3.0±1.1 mm compared to the baseline. Corresponding values for the patients treated with OFD were 2.5±0.8 mm and 1.4±1.0 mm, respectively. Compared to OFD group, the additional CAL gain was significantly greater in the patients in BCP group (P=0.028). The additional PD reduction was significant for the BCP group (P=0.048). The REC showed a significant increase in both groups, and the amount of recession was significantly smaller in the BCP group than OFD group (P=0.023). In radiographic evaluation, the height of the bone fill in the BCP group was significantly greater than OFD group.
Conclusions
The clinical benefits of BCP found in this study indicate that BCP may be an appropriate alternative to conventional graft materials.
Keywords: Alveolar bone loss, Bone transplantation, Calcium phosphates, Chronic periodontitis, Hydroxyapatites
INTRODUCTION
Various studies have investigated the regenerative treatment of periodontal intrabony defects, and numerous treatment methods have been suggested to restore lost periodontal structures. These include the use of guided tissue regeneration (GTR), bone replacement grafts, and biologic factors such as enamel matrix proteins and growth factors, or a combination of these techniques.
Although barrier membranes are used to allow periodontal ligament (PDL) progenitor cells to selectively repopulate on root surfaces in traditional methods, the efficacy of bioactive agents is mainly based on mitogenic and chemotactic effects on PDL and alveolar bone cells. Overall, the use of these approaches resulted in additional benefits in terms of clinical attachment level (CAL) gain and probing depth (PD) reduction compared to open flap debridement (OFD) alone. However, the magnitude of CAL and PD changes differed between the studies. In particular, the success of GTR is known to be technically sensitive and dependent on various confounders [1,2].
Regarding bone replacement grafts, from a biologic point of view, autogenous bone grafts have been considered the gold-standard material with the most predictable outcomes [3,4], but they require a second surgical site to harvest the graft from a donor area. Alternatively, xenografts and allografts have been well documented to be suitable as bone replacement grafts [5-7]. However, the incomplete resorption of these materials has frequently been reported [8-10]. Moreover, although statistically negligible, a risk of transmitting diseases still exists from the use of allografts and xenografts.
The use of alloplastic materials, which are synthetic, inorganic, and biocompatible bone-graft substitutes, may be an alternative for the treatment of intrabony periodontal defects (IBDs). The advantages are easier accessibility, eliminating the need of a donor site as is necessary with autogenous grafts, and no risk for disease transmission, which may accompany the use of allografts and xenografts. Hydroxyapatite (HA) and β-tricalcium phosphate (β-TCP) have shown significant clinical improvements in grafted sites compared to non-grafted sites in controlled clinical studies [11-13]. A new biomaterial was introduced recently for periodontal regenerative therapy. This fully synthetic bone graft substance, termed HA/β-TCP, is a composite of medical purity biphasic calcium phosphate (BCP): a mixture of 60% HA, which is 100% crystalline, and 40% of the β form of TCP in particulate form. A previous study demonstrated the advantages of using a composite of these two materials compared to the use of either material alone [14]. However, the number of controlled clinical studies on the use of the HA/β-TCP as a bone replacement graft in the treatment of periodontal is limited so far.
Therefore, the aim of this study was to compare the clinical outcomes of OFD with BCP graft to that of OFD without BCP graft for the treatment of IBD.
MATERIALS AND METHODS
Study design and population
The present study was designed as a prospective controlled clinical trial. This study included 25 subjects (13 males and 12 females; age range, 31 and 64 years; mean age, 46.3±8.5 years) who were referred for treatment of moderate or severe chronic periodontitis to the Department of Periodontology, Chosun University Dental Hospital.
These patients had received non-surgical periodontal therapy without systemic or locally delivered antibiotics from the same periodontist 10 to 14 weeks before being enrolled in this study.
All participants were informed about the risks and benefits of the procedure and signed informed consent. The two different therapeutic modalities for the treatment of deep intraosseous periodontal defects were compared. BCP group was treated with BCP (GENESIS-BCP, DIO Co., Busan, Korea)graft after OFD. OFD group was treated without BCP graft after OFD.
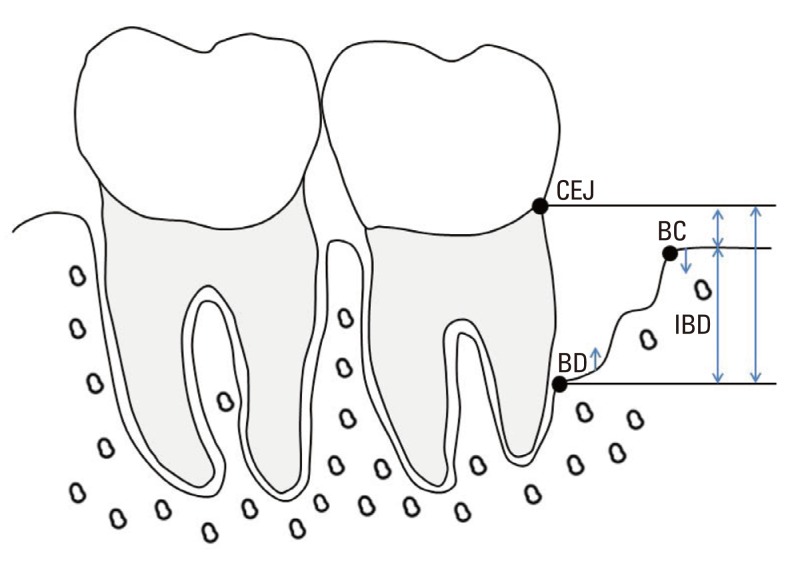
The inclusion criteria were as follows: no systemic diseases or pregnancy, no smoking habit, no regular use of medication for any reason, chronic generalized periodontitis, completion of the initial treatment (including subgingival scaling and root planing), compliance with the maintenance program, presence of inter-proximal IBDs≥4 mm deep (distance between the alveolar crest and the base of the defect on intraoral periapical radiograph) along with an inter-proximal PD≥6 mm following the initial treatment (Fig. 1); absence of furcation involvement or vertical defects extending into the furcation area, the teeth vital and free of radiographic signs of periapical abscesses, and the teeth free of carious lesions in the region of the defect.
Figure 1.
Schematic drawing illustrating the anatomical landmarks and linear measurements taken from digitized radiographs. CEJ: cemento-enamel junction, BC: bone crest, BD: bottom of the defect, IBD, intrabony defect.
This study protocol was approved by the Chosun University Dental Hospital Institutional Review Board (CDMDIRB-1112-56).
Clinical measurements
Complete oral and periodontal examinations of each subject were carried out at baseline (prior to the surgical procedure) and at 6 months postsurgery. Clinical parameters included plaque index (PI) [15], gingival index (GI) [15], PD, clinical attachment lever (CAL), and gingival recession (REC). For CAL and REC, the cemento-enamel junction (CEJ) was used as the reference point. All clinical measurements were made at 6 sites per tooth (mesio-facial, mid-facial, disto-facial, mesio-lingual, mid-lingual, and disto-lingual) using customized acrylic stents with grooves to ensure a reproducible placement of a pressure-sensitive probe (Hawe Click-Probe; Kerr Hawe, Bioggio, Switzerland) set to a probing force of 0.25 N. Measurements were rounded up to the nearest millimeter. A examiner who experienced the calibration procedure, and was not aware of the surgical procedure to be performed, recorded all of the measurements. Periodontal probing or recording of attachment levels should not be performed prior to 6 months postsurgery, because the probing force may damage the healing site, thereby diminishing the regenerative outcome.
Surgical procedure
At the time of the surgical procedure, subjects were randomly allocated to one of two experimental groups, the BCP group or the OFD group. All treatments were performed by the same surgeon.
Occlusal therapy consisting of adjustment or splinting of the teeth should be accomplished prior to surgery to reduce or eliminate excessive mobility or fremitus patterns. The literature suggests that teeth with demonstrable mobility have a poorer long-term outlook after surgery [16].
In both groups, the defects were accessed using papilla preservation flaps. The simplified papilla preservation flap was used to gain access to the root surface and the marginal alveolar bone in areas where the interproximal space had a mesio-distal width≤2 mm measured at the level of the interproximal soft tissue [17]. The modified papilla preservation technique [18] was used in areas with a mesio-distal width of the interproximal space>2 mm. Flaps were extended horizontally (mesially and distally) to obtain complete access to the IBD.
Alveolar bone was exposed about 3 mm beyond the defect's edge. Additional care should be taken to avoid flap perforation or loss of the papilla due to granulomatous tissue from the lesion that adheres to the inner aspect of the flap.
The exposed root surfaces were scaled and planed with ultrasonic instruments and hand curettes. Following the defect debridement, a saturated solution of tetracycline may be applied to the root surface to biologically enhance regeneration through removal of the smear layer and residual colonies of bacteria, including possible exposure of collagen fibrils. Subsequently, the defects and adjacent mucoperiosteal flaps were rinsed thoroughly with sterile saline.
At this stage, the following intrasurgical measurements were performed: the distance from the CEJ to the bottom of the defect (CEJ-BD) and the distance from the CEJ to the most coronal extension of the interproximal bone crest (CEJ-BC). The intrabony component (INTRA) of the defects was defined as (CEJ-BD) - (CEJ-BC). Furthermore, the defects were categorized according to the number of surrounding osseous walls.
Afterwards, random assignment to the respective treatment was performed. A sealed envelope, with a card indicating the surgical procedure to be applied was opened by the surgeon immediately after debridement and defect measurements. In the BCP group, the BCP material was prepared according to the manufacturer's instructions and filled into the defects up to the level of the surrounding bony walls. In the OFD group, no filler was used.
A periosteal releasing incision was performed to ensure primary closure. Finally, the flaps were coronally advanced to obtain complete coverage of the defect. Care was taken to secure an adequate tension-free interproximal closure. Interdental closure was accomplished first with an interdental suture positioned between the apical part of the buccal gingiva and an apical area of the lingual/palatal flap; in addition, an inverted vertical mattress suture was used (Fig. 2).
Figure 2.
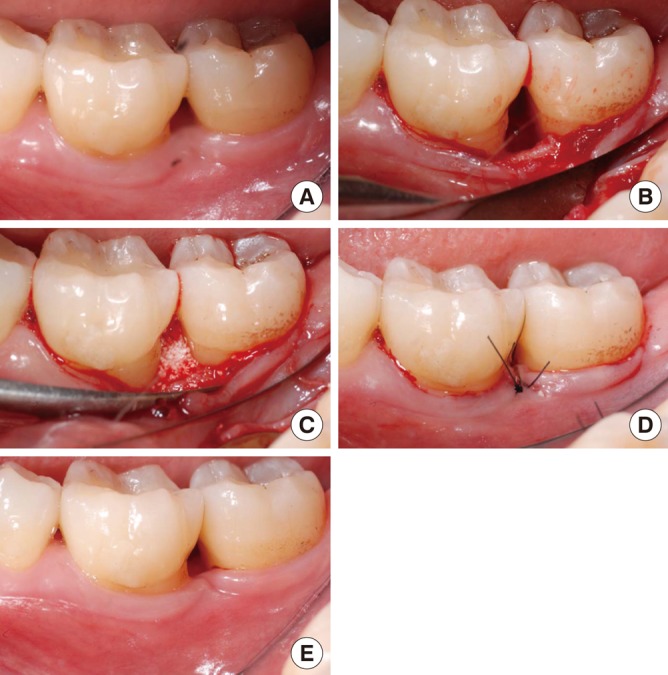
Treatment of an intrabony defect with biphasic calcium phosphate (BCP) on the distal aspect of an lower left first molar. (A) Preoperative clinical view. (B) Intraoperative view of the debrided intrabony defect. (C) Defect filled with the BCP biomaterial. (D) Sutures immediately after flap closure. (E) Clinical view of healing result after 6 months.
Radiographic evaluation
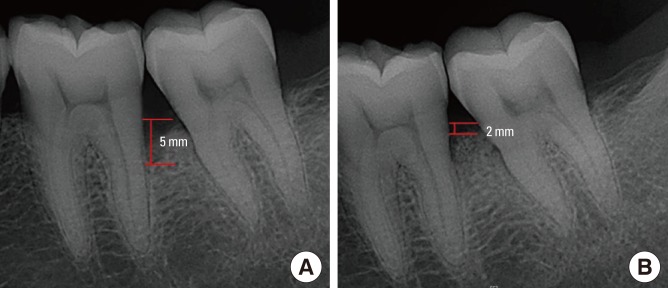
Pre- and postoperative standardized radiographs were taken for diagnostic purposes. Individually customized bite blocks and a parallel angle technique were used to obtain standardized radiographs. IBD was evaluated at baseline and after 6 months. The radiographic IBD depth (vertical distance from the crest of the alveolar bone to the base of the defect) was measured using the Infinitt π-ViewStar calipers (Starpacs, Infinitt, Seoul, Korea) (Fig. 3); Pradeep and Thorat [19] had previously introduced this method.
Figure 3.
Radiographic evaluation. (A) Baseline radiograph showing intrabony periodontal defect (IBD)=5.0 mm with linear measurement. (B) Radiograph after 6 months showing IBD=2.0 mm with linear measurement.
Medication and maintenance
All subjects were instructed to rinse with 0.2% chlorhexidine gluconate mouthrinse twice a day until postoperative four weeks. No periodontal dressings were placed. Antiphlogistic medication (ibuprofen 400 mg, three times a day) was prescribed and used by subjects if necessary. No systemic antibiotics were prescribed. The sutures were removed 10 days after surgery. Subjects were not allowed to brush or floss their teeth in the surgical area for 4 weeks after surgery. Within the first 2 months, oral hygiene control and professional supragingival tooth cleaning were conducted every 2 weeks. Thereafter, recall visits for postoperative hygiene were performed monthly. Postoperative visits included plaque removal (both mechanically and with topical chlorhexidine), selective stain removal, and reinforcement of oral hygiene.
Statistical analyses
For all of the treatment groups, primary values of continuous variables were recorded as the mean and standard deviation. In all of the calculations, the deepest site of the tooth was included. Comparison of age among the groups was performed using the independent t-test. For the data of the categorical variables (bone-wall characteristics), absolute and relative frequencies were calculated and compared among groups using the χ2 test. Comparisons of all other variables (PI, GI, PD, CAL, REC) among the groups were performed using the Wilcoxon test. For differences of these parameters from baseline to 6 months in each treatment group, the Wilcoxon signed-rank test was used. The radiographic IBD depths were also performed using the same method. A P-value of <0.05 was considered statistically significant. Data processing and all statistical analyses were performed using a statistical software package SPSS ver. 12.0.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
None of the patients enrolled in this study reported any unusual pain or discomfort, abscess formation, swelling, or allergic reactions during the course of treatment. Closure was achieved in all of the treated defects and maintained for the entire healing period. No radiographic signs of root resorption were observed at the final control.
No statistically significant differences were found among the two groups for any of the subject characteristics at baseline. Also, there were no statistical differences between the locations of the defects; 15 defects were treated in the lower jaw, and 10 defects were treated in the upper jaw.
In the two groups, the initial depth of the IBDs was similar. The distributions of wall-defect type (2- or 3-wall) were not significantly different between the two groups (Table 1).
Table 1.
Subjects and their periodontal defect characteristics at baseline.
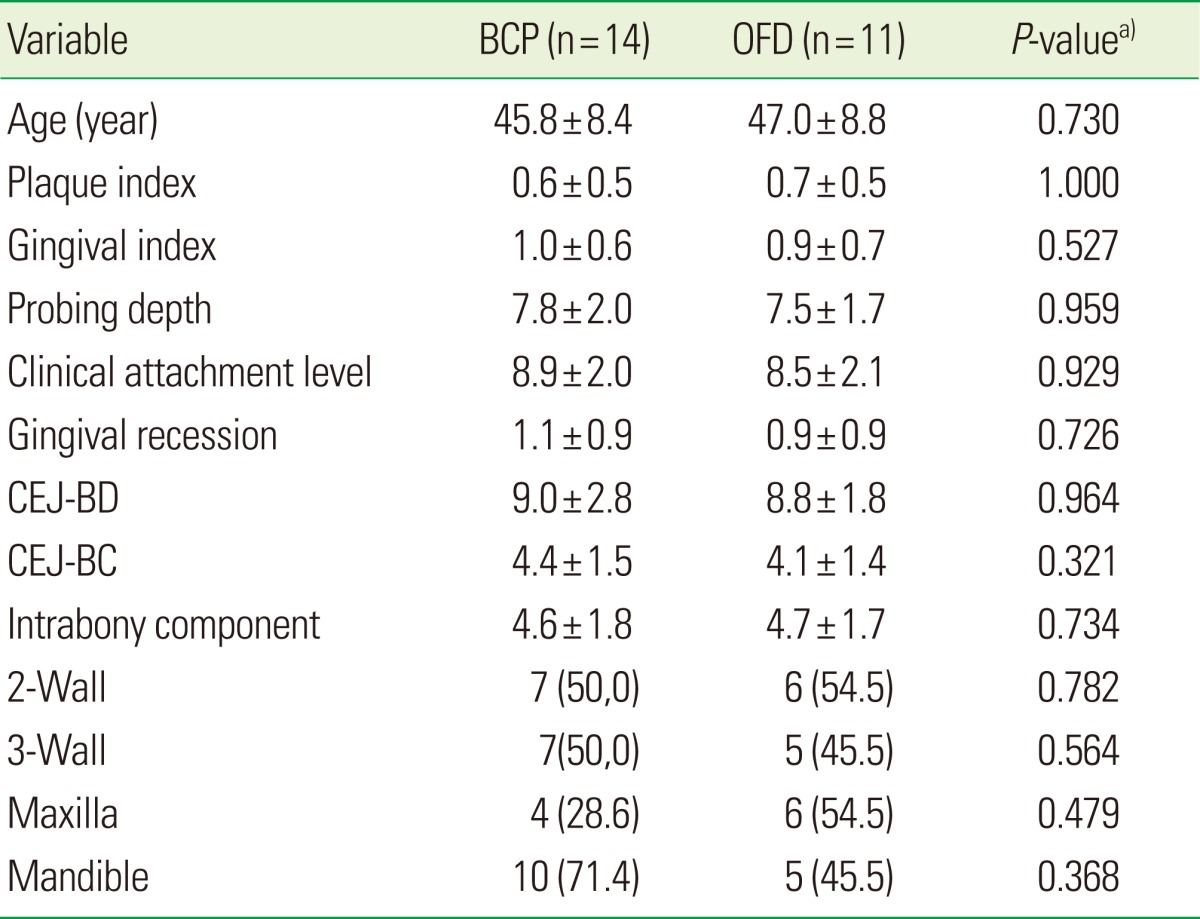
Values are presented as mean±SD or number (%).
BCP: biphasic calcium phosphate, OFD: open flap debridement, CEJ-BD: the distance from the cemento-enamel junction (CEJ) to the bottom of the defect, CEJ-BC: the distance from the CEJ to the most coronal extension of the interproximal bone crest.
a)BCP vs. OFD.
Hygiene indices in the two groups at baseline and 6 months are presented in Table 2. The PI scores remained low from baseline throughout the study period. No statistically significant differences were found among the groups. The GI scores, 6 months after surgery, were not a statistically significant, but all groups showed somewhat improved results. In the hygiene indices other than GI, the difference in scores between two groups was not significant.
Table 2.
Plaque index and gingival index scores at baseline and 6-month after surgery.
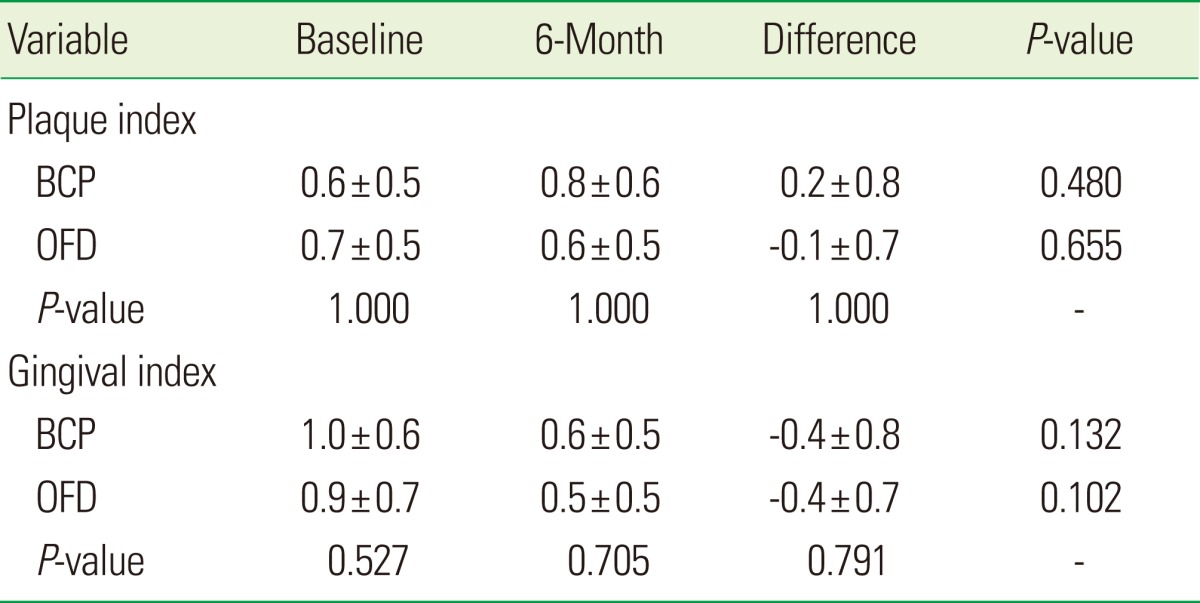
Values are presented as mean±SD.
BCP: biphasic calcium phosphate, OFD: open flap debridement.
All clinical parameter changes are summarized in Table 3. There were no significant differences in the initial PD, CAL, or REC measurements within the two groups. Compared to baseline data, all cohorts exhibited a significant reduction of PD values and CAL gain 6 months after surgery (P<0.007). The groups treated with BCP produced a significantly higher gain of CAL than patients treated with OFD alone. Compared to OFD, the amount of additional CAL gain was 1.6±0.4 mm in the BCP group (P=0.028). Regarding changes of PDs compared to OFD, the BCP group yielded a significantly greater change of PD values (additional PD reduction, 1.2±0.4 mm; P=0.048). REC significantly increased in the two groups 6 months after surgery. The change of REC values in the BCP group (0.4±0.5 mm) was lower than patients treated with OFD alone (1.2±0.8 mm, P=0.023).
Table 3.
Clinical parameters at baseline and 6-month after surgery.
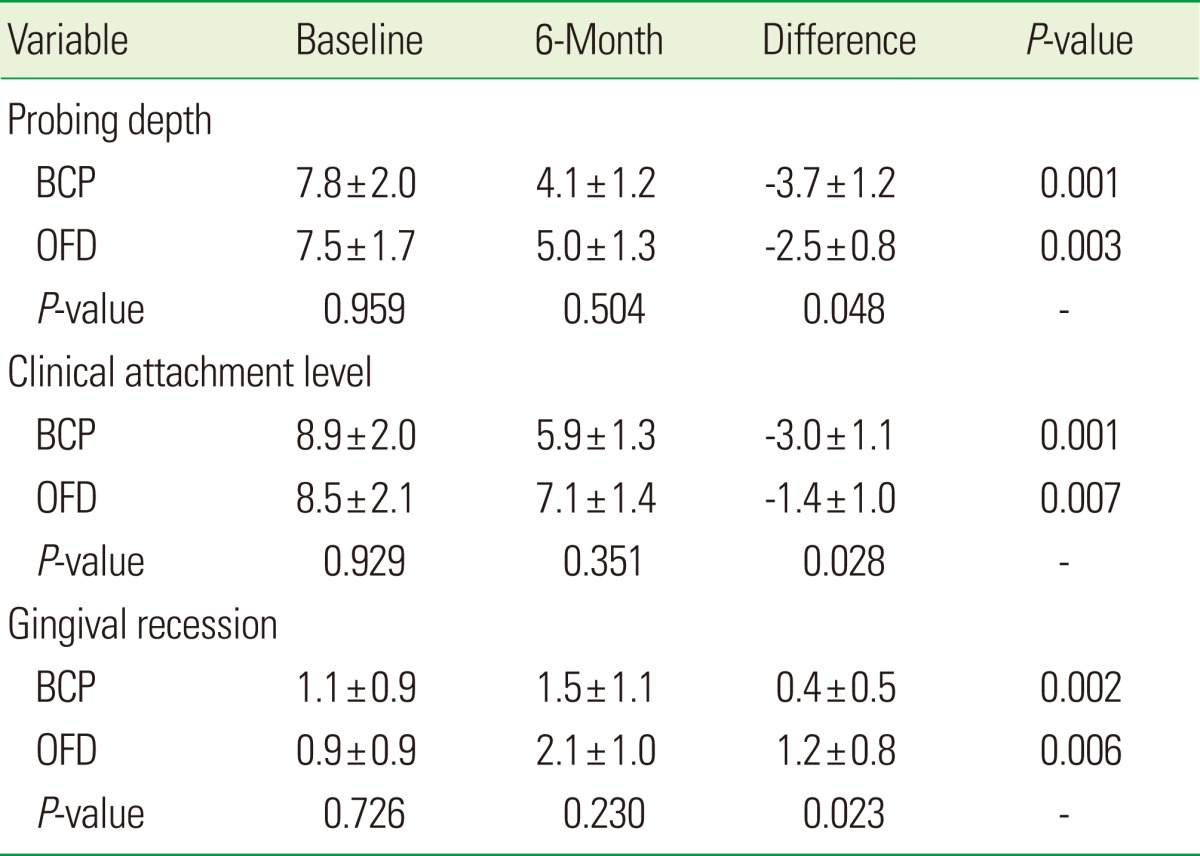
Values are presented as mean±SD.
BCP: biphasic calcium phosphate, OFD: open flap debridement.
Variations in clinical parameters based on wall defects had no statistically significant differences. Also variations of clinical parameters based on the maxilla and mandible had no statistically significant differences (Tables 4 and 5).
Table 4.
Changes in the clinical parameters depending on the wall defect.
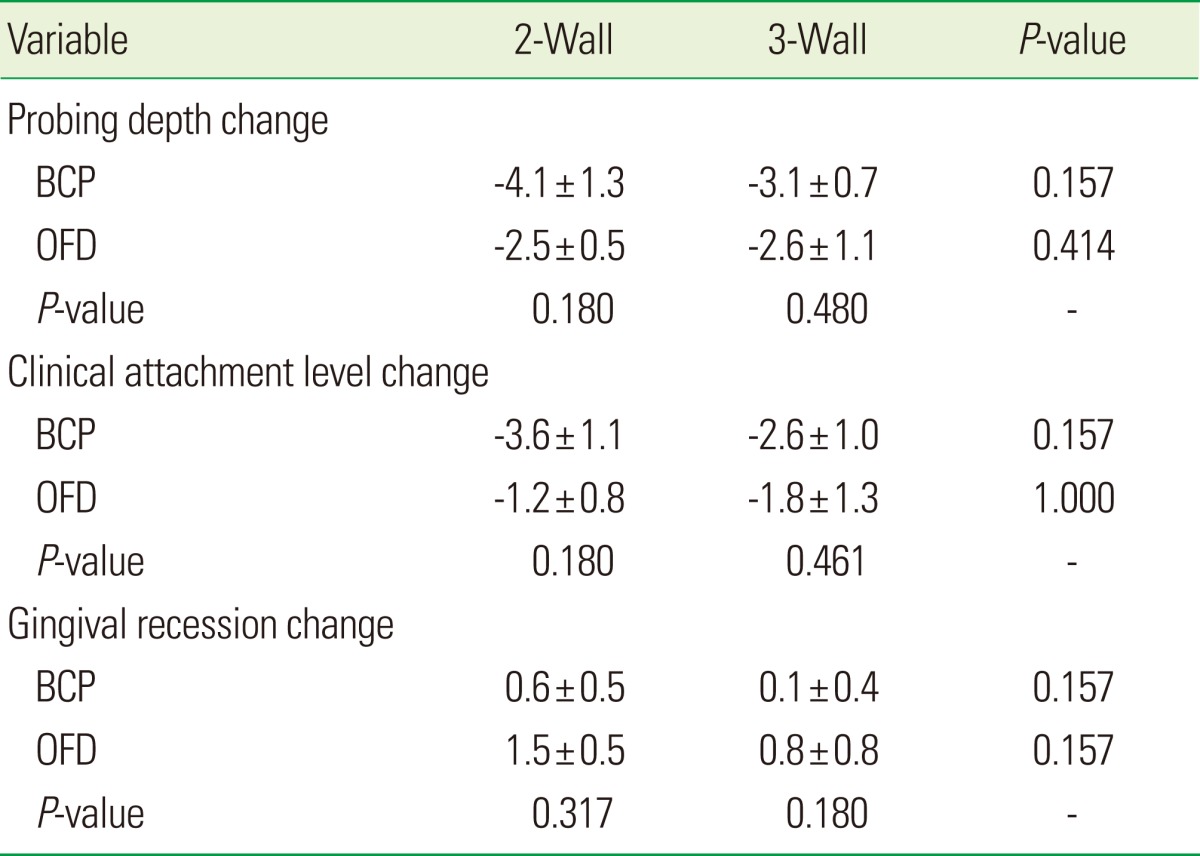
Values are presented as mean±SD.
BCP: biphasic calcium phosphate, OFD: open flap debridement.
Table 5.
Changes in the clinical parameters between maxilla and mandible.
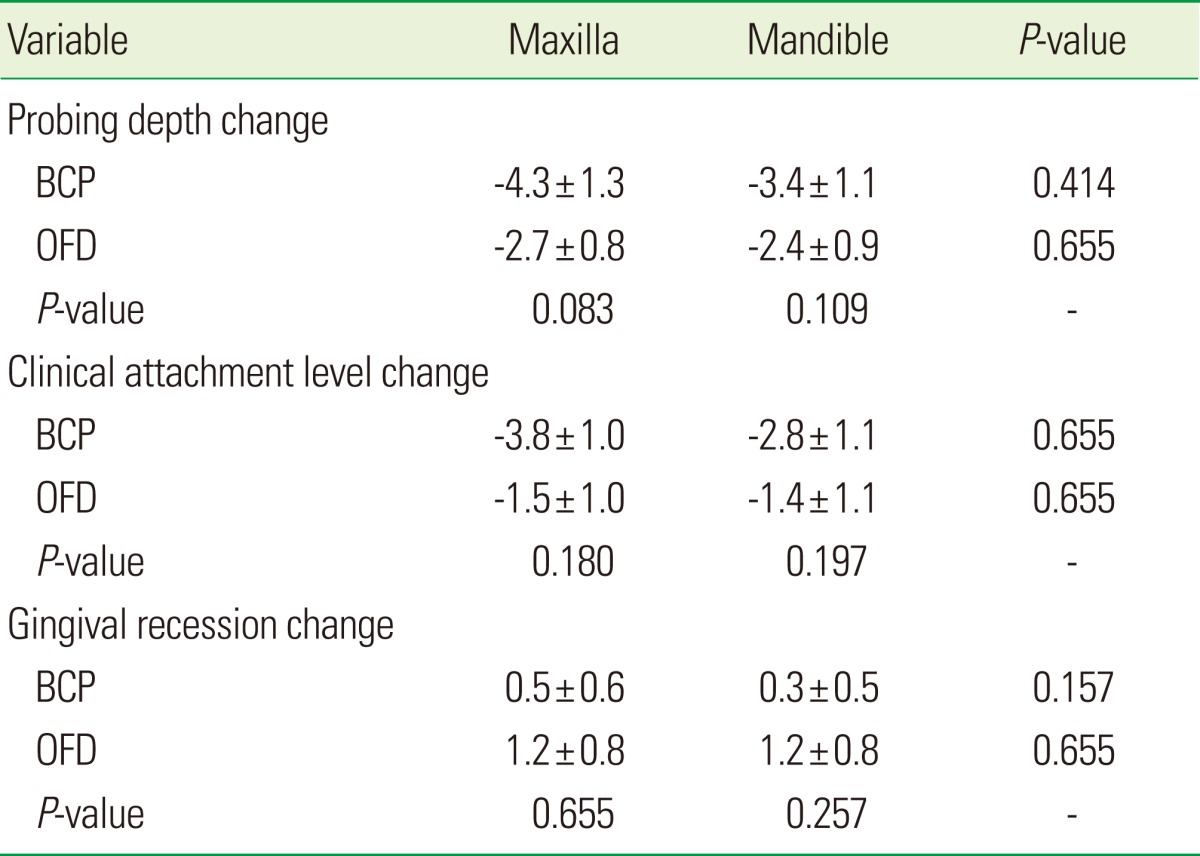
Values are presented as mean±SD.
BCP: biphasic calcium phosphate, OFD: open flap debridement.
Variations in IBD scores between the BCP and OFD groups are summarized in Table 6. The initial IBD scores had no significant difference. Compared to baseline data, the decrease in IBD in the 6 months after surgery had a statistically significant difference between the two groups. Compared to the OFD group, decrement of IBD was significantly larger in the BCP group (P=0.045).
Table 6.
Intrabony defect scores at baseline and 6-month after surgery.
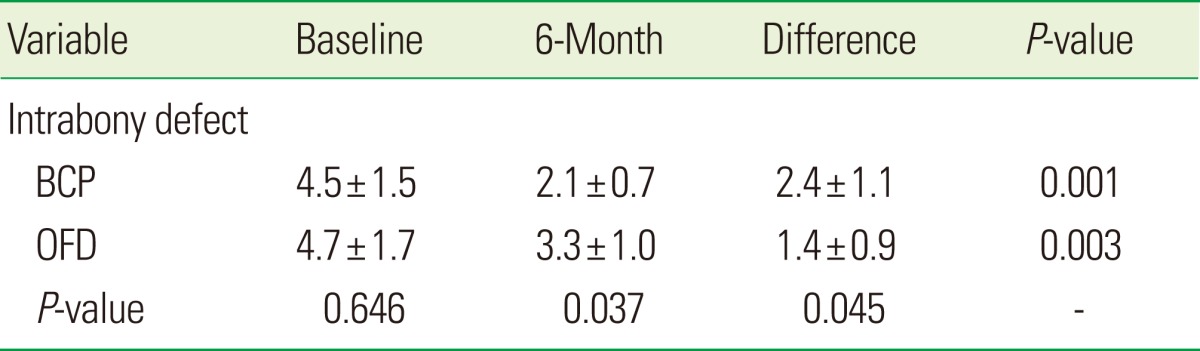
Values are presented as mean±SD.
BCP: biphasic calcium phosphate, OFD: open flap debridement.
DISCUSSION
Bone replacement grafts are widely used for bone formation and periodontal regeneration. Conventional surgical approaches, such as OFD, provide critical access to evaluate and detoxify root surfaces as well as establish improved periodontal form and architecture; however, these surgical techniques offer only limited potential in restoring or reconstituting component periodontal tissues.
Bone grafting materials function, in part, as structural scaffolds and matrices to allow attachment and proliferation of anchorage-dependent osteoblasts. A wide range of bone grafting materials including bone grafts and bone graft substitutes have been applied and evaluated clinically, including autografts, allografts, xenografts, and alloplasts. Although not all bone grafting materials support the formation of a new periodontal attachment apparatus, there is conclusive evidence that periodontal regeneration is achievable with bone replacement grafts in humans.
Among the graft materials, autogenous bone grafts have been known to be the gold standard among bone replacement grafts because of their osteoconductive ability and cell viability, including osteoblasts and osteoprogenitor stem cells, which facilitate osteogenesis [20-22]. The use of autografts in IBDs has been reported to result in a reduction of PD and an increase of CAL values relative to OFD. Although there is no risk for cross-infection or immunogenic reaction without autogenous materials, their limited availability and the necessity of a donor site, and thus, often a second surgical site, may limit their use [20]. In addition, autogenous bone grafts also have the potential disadvantage of involving a higher degree of resorption of the graft material.
For these reasons, alloplastic materials are used. The biologic function of alloplastic materials is similar to that of autogenous bone grafts and sometimes may be superior clinically without the need for a second surgical site. The major advantage of alloplasts over autogenous grafts is their easy availability, and no risk for cross-infection exists.
The mixture of HA and β-TCP has been studied extensively as a new alloplastic material [23]. Among the many kinds of BCP materials that have been produced, a new synthetic bone substitute, was manufactured and used for the present study. Most of BCP materials were mixed randomly and had properties independently due to a process of normally composing it to a mixture form. However, the BCP in this study has the form of a compound with a regular molecule structure [24-30]. The clinical effectiveness of a BCP graft was tested in the treatment of IBDs in comparison to OFD without BCP graft.
To limit patient- and defect-based factors, which might affect the outcome of periodontal surgery, the study was only conducted in non-smokers, in compliant patients with good oral hygiene, and with comparable subjects and defect characteristics among the cohorts (Table 1). Furthermore, during the study period, changes in PI did not differ among the groups.
In this study, the depth of IBDs had to be ≥4 mm because the amount of bone fill that evidently can be achieved using OFD alone, together with crestal resorption and the residual defect, totaled 3.5 mm. Thus, to benefit from the extra cost and time related to adjunctive therapy such as bone graft and GTR, the depth of an IBD should equal or exceed 4 mm [31].
In a previous study by Zafiropoulos et al. [32], it was reported that applying BCP resulted in a significantly greater gain of clinical attachment and hard tissue formation using the combination of autogenous bone with BCP. Research has shown that applying novel biphasic calcium composite (BCC) grafting material comprised of a porous β-TCP and calcium sulfate phase to IBDs, the clinical benefits of BCC were similar to those of autogenous bone, but it has also shown improved results when compared to OFD alone [33].
The application of the BCP biomaterial provided significantly greater PD reductions and CAL gains than OFD alone. The recession increments in the BCP group were lower than the control group treated with OFD alone. The additional CAL gain in the BCP group compared to the OFD group was 1.6±0.4 mm, which corresponds well with the findings of several other studies [26-28,30] on alloplastic bone replacement materials. It is also consistent with the results of a meta-analysis in which the additional CAL gain for alloplastic materials ranged from 1.0 mm for bioactive glass to 1.4 mm for HAs.
The CAL change showed a range from 1.1 to 2.8 mm in 9 studies, of which a demineralized freeze-dried bone allograft was transplanted at the IBD, with an initial PD of more than 6 mm, and the clinical value was evaluated after a 6-month healing period. The statistical significance between other studies and this study was not investigated; however, there was no considerable difference between our research results and the other studies [34].
On the other hand, there are a few studies that did not show a clinical benefit of synthetic graft materials compared to OFD [29,35,36]. There might be several reasons for the discrepancies in the clinical outcomes. First, differences in defect characteristics and the surgical methods used might have led to different results. In the present study, papilla preservation techniques, which are known to improve primary closure and postoperative healing results in regenerative procedures, were applied in all the treated sites. In the cited studies, this approach was not considered [29,35,36]. Second, the overfilling of the defects with the biomaterial, as reported by Shirakata et al. [36], may impair primary wound closure and cause different results. Further, contrary to other studies [21,36], the high frequency of maintenance and the level of plaque control, which are known to correlate with beneficial results in periodontal surgery, may have additionally supported post-operative healing in our study population.
Ellegaard and Löe [37] also reported that defect resolution was greater in a three-wall defect site than that in 2-wall or combination 3-wall and 2-wall defects. Hiatt and Schallhorn [38] reported that the degree of bone fill was associated with the morphology of the IBD (the number of remaining bony walls). In this study, the clinical results according to the 2-wall, 3-wall or mandible, and maxilla did not show statistically significant differences. It is probably because the sample size was too small to show statistical significance.
In this study, the application of the BCP material was well tolerated and led to superior PD and CAL changes in BCP group, when compared to OFD without BCP. Notwithstanding, it remains unclear to what extent the CAL gains obtained represent the regeneration of periodontal attachment. Because histologic specimens were not obtained in this study, inferences about the quality of bone formation at any given point in time or the type of healing attachment gained cannot be made.
In the standardized radiographs, 6 months after treatment with BCP, the filling of radiographic defects with bone-like radiopaque tissue, was observed and indistinguishable from native bone. Nonetheless, the clinical findings of this study must be validated by reentry and/or by histologic analysis in future studies to examine the quality of the defect fill. Moreover, the results of the present study are only applicable to IBDs. For other types of defects, no results are available from this study. Finally, it must be emphasized that the sample size of this trial was limited. Further studies with a higher number of subjects and long-term observations are necessary to verify the findings presented here.
In conclusion, the clinical benefits of BCP found in this study indicate that BCP may be an appropriate alternative to conventional graft materials.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Tonetti MS, Prato GP, Cortellini P. Factors affecting the healing response of intrabony defects following guided tissue regeneration and access flap surgery. J Clin Periodontol. 1996;23:548–556. doi: 10.1111/j.1600-051x.1996.tb01823.x. [DOI] [PubMed] [Google Scholar]
- 2.Machtei EE. The effect of membrane exposure on the outcome of regenerative procedures in humans: a meta-analysis. J Periodontol. 2001;72:512–516. doi: 10.1902/jop.2001.72.4.512. [DOI] [PubMed] [Google Scholar]
- 3.Stahl SS, Froum SJ, Kushner L. Healing responses of human intraosseous lesions following the use of debridement, grafting and citric acid root treatment. II. Clinical and histologic observations: one year postsurgery. J Periodontol. 1983;54:325–338. doi: 10.1902/jop.1983.54.6.325. [DOI] [PubMed] [Google Scholar]
- 4.Froum SJ, Kushner L, Stahl SS. Healing responses of human intraosseous lesions following the use of debridement, grafting and citric acid root treatment. I. Clinical and histologic observations six months postsurgery. J Periodontol. 1983;54:67–76. doi: 10.1902/jop.1983.54.2.67. [DOI] [PubMed] [Google Scholar]
- 5.Bowers GM, Chadroff B, Carnevale R, Mellonig J, Corio R, Emerson J, et al. Histologic evaluation of new attachment apparatus formation in humans. Part II. J Periodontol. 1989;60:675–682. doi: 10.1902/jop.1989.60.12.675. [DOI] [PubMed] [Google Scholar]
- 6.Camelo M, Nevins ML, Schenk RK, Simion M, Rasperini G, Lynch SE, et al. Clinical, radiographic, and histologic evaluation of human periodontal defects treated with Bio-Oss and Bio-Gide. Int J Periodontics Restorative Dent. 1998;18:321–331. [PubMed] [Google Scholar]
- 7.Camargo PM, Lekovic V, Weinlaender M, Nedic M, Vasilic N, Wolinsky LE, et al. A controlled re-entry study on the effectiveness of bovine porous bone mineral used in combination with a collagen membrane of porcine origin in the treatment of intrabony defects in humans. J Clin Periodontol. 2000;27:889–896. doi: 10.1034/j.1600-051x.2000.027012889.x. [DOI] [PubMed] [Google Scholar]
- 8.Caplanis N, Lee MB, Zimmerman GJ, Selvig KA, Wikesjo UM. Effect of allogeneic freeze-dried demineralized bone matrix on regeneration of alveolar bone and periodontal attachment in dogs. J Clin Periodontol. 1998;25:801–806. doi: 10.1111/j.1600-051x.1998.tb02373.x. [DOI] [PubMed] [Google Scholar]
- 9.Yildirim M, Spiekermann H, Biesterfeld S, Edelhoff D. Maxillary sinus augmentation using xenogenic bone substitute material Bio-Oss in combination with venous blood. A histologic and histomorphometric study in humans. Clin Oral Implants Res. 2000;11:217–229. doi: 10.1034/j.1600-0501.2000.011003217.x. [DOI] [PubMed] [Google Scholar]
- 10.Sartori S, Silvestri M, Forni F, Icaro Cornaglia A, Tesei P, Cattaneo V. Ten-year follow-up in a maxillary sinus augmentation using anorganic bovine bone (Bio-Oss). A case report with histomorphometric evaluation. Clin Oral Implants Res. 2003;14:369–372. doi: 10.1034/j.1600-0501.2003.140316.x. [DOI] [PubMed] [Google Scholar]
- 11.Dori F, Arweiler N, Gera I, Sculean A. Clinical evaluation of an enamel matrix protein derivative combined with either a natural bone mineral or beta-tricalcium phosphate. J Periodontol. 2005;76:2236–2243. doi: 10.1902/jop.2005.76.12.2236. [DOI] [PubMed] [Google Scholar]
- 12.Kim SK, Choi EH, Lee JS, Kim TG, Choi SH, Cho KS, et al. Evaluating intra- and inter-examiner reproducibility in histometric measurement: one-wall intrabony periodontal defects in beagle dogs. J Periodontal Implant Sci. 2010;40:172–179. doi: 10.5051/jpis.2010.40.4.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yukna RA, Harrison BG, Caudill RF, Evans GH, Mayer ET, Miller S. Evaluation of durapatite ceramic as an alloplastic implant in periodontal osseous defects. II. Twelve month reentry results. J Periodontol. 1985;56:540–547. doi: 10.1902/jop.1985.56.9.540. [DOI] [PubMed] [Google Scholar]
- 14.Nery EB, LeGeros RZ, Lynch KL, Lee K. Tissue response to biphasic calcium phosphate ceramic with different ratios of HA/beta TCP in periodontal osseous defects. J Periodontol. 1992;63:729–735. doi: 10.1902/jop.1992.63.9.729. [DOI] [PubMed] [Google Scholar]
- 15.Loe H. The gingival index, the plaque index and the retention index systems. J Periodontol. 1967;38:Suppl:610–Suppl:616. doi: 10.1902/jop.1967.38.6.610. [DOI] [PubMed] [Google Scholar]
- 16.Wang HL, Burgett FG, Shyr Y, Ramfjord S. The influence of molar furcation involvement and mobility on future clinical periodontal attachment loss. J Periodontol. 1994;65:25–29. doi: 10.1902/jop.1994.65.1.25. [DOI] [PubMed] [Google Scholar]
- 17.Cortellini P, Prato GP, Tonetti MS. The simplified papilla preservation flap. A novel surgical approach for the management of soft tissues in regenerative procedures. Int J Periodontics Restorative Dent. 1999;19:589–599. [PubMed] [Google Scholar]
- 18.Cortellini P, Prato GP, Tonetti MS. The modified papilla preservation technique. A new surgical approach for interproximal regenerative procedures. J Periodontol. 1995;66:261–266. doi: 10.1902/jop.1995.66.4.261. [DOI] [PubMed] [Google Scholar]
- 19.Pradeep AR, Thorat MS. Clinical effect of subgingivally delivered simvastatin in the treatment of patients with chronic periodontitis: a randomized clinical trial. J Periodontol. 2010;81:214–222. doi: 10.1902/jop.2009.090429. [DOI] [PubMed] [Google Scholar]
- 20.Nasr HF, Aichelmann-Reidy ME, Yukna RA. Bone and bone substitutes. Periodontol 2000. 1999;19:74–86. doi: 10.1111/j.1600-0757.1999.tb00148.x. [DOI] [PubMed] [Google Scholar]
- 21.Hanes PJ. Bone replacement grafts for the treatment of periodontal intrabony defects. Oral Maxillofac Surg Clin North Am. 2007;19:499–512. vi. doi: 10.1016/j.coms.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 22.Froum SJ, Ortiz M, Witkin RT, Thaler R, Scopp IW, Stahl SS. Osseous autografts III Comparison of osseous coagulum-bone blend implants with open curetage. J Periodontol. 1976;47:287–294. doi: 10.1902/jop.1976.47.5.287. [DOI] [PubMed] [Google Scholar]
- 23.Daculsi G, LeGeros RZ, Nery E, Lynch K, Kerebel B. Transformation of biphasic calcium phosphate ceramics in vivo: ultrastructural and physicochemical characterization. J Biomed Mater Res. 1989;23:883–894. doi: 10.1002/jbm.820230806. [DOI] [PubMed] [Google Scholar]
- 24.Zaner DJ, Yukna RA. Particle size of periodontal bone grafting materials. J Periodontol. 1984;55:406–409. doi: 10.1902/jop.1984.55.7.406. [DOI] [PubMed] [Google Scholar]
- 25.Galgut PN, Waite IM, Brookshaw JD, Kingston CP. A 4-year controlled clinical study into the use of a ceramic hydroxylapatite implant material for the treatment of periodontal bone defects. J Clin Periodontol. 1992;19:570–577. doi: 10.1111/j.1600-051x.1992.tb00685.x. [DOI] [PubMed] [Google Scholar]
- 26.Yukna RA, Callan DP, Krauser JT, Evans GH, Aichelmann-Reidy ME, Moore K, et al. Multi-center clinical evaluation of combination anorganic bovine-derived hydroxyapatite matrix (ABM)/cell binding peptide (P-15) as a bone replacement graft material in human periodontal osseous defects. 6-month results. J Periodontol. 1998;69:655–663. doi: 10.1902/jop.1998.69.6.655. [DOI] [PubMed] [Google Scholar]
- 27.Kasaj A, Rohrig B, Zafiropoulos GG, Willershausen B. Clinical evaluation of nanocrystalline hydroxyapatite paste in the treatment of human periodontal bony defects--a randomized controlled clinical trial: 6-month results. J Periodontol. 2008;79:394–400. doi: 10.1902/jop.2008.070378. [DOI] [PubMed] [Google Scholar]
- 28.Froum SJ, Weinberg MA, Tarnow D. Comparison of bioactive glass synthetic bone graft particles and open debridement in the treatment of human periodontal defects. A clinical study. J Periodontol. 1998;69:698–709. doi: 10.1902/jop.1998.69.6.698. [DOI] [PubMed] [Google Scholar]
- 29.Nevins ML, Camelo M, Nevins M, King CJ, Oringer RJ, Schenk RK, et al. Human histologic evaluation of bioactive ceramic in the treatment of periodontal osseous defects. Int J Periodontics Restorative Dent. 2000;20:458–467. [PubMed] [Google Scholar]
- 30.Kim CK, Kim HY, Chai JK, Cho KS, Moon IS, Choi SH, et al. Effect of a calcium sulfate implant with calcium sulfate barrier on periodontal healing in 3-wall intrabony defects in dogs. J Periodontol. 1998;69:982–988. doi: 10.1902/jop.1998.69.9.982. [DOI] [PubMed] [Google Scholar]
- 31.Laurell L, Gottlow J, Zybutz M, Persson R. Treatment of intrabony defects by different surgical procedures. A literature review. J Periodontol. 1998;69:303–313. doi: 10.1902/jop.1998.69.3.303. [DOI] [PubMed] [Google Scholar]
- 32.Zafiropoulos GG, Hoffmann O, Kasaj A, Willershausen B, Weiss O, Van Dyke TE. Treatment of intrabony defects using guided tissue regeneration and autogenous spongiosa alone or combined with hydroxyapatite/beta-tricalcium phosphate bone substitute or bovine-derived xenograft. J Periodontol. 2007;78:2216–2225. doi: 10.1902/jop.2007.070146. [DOI] [PubMed] [Google Scholar]
- 33.Stein JM, Fickl S, Yekta SS, Hoischen U, Ocklenburg C, Smeets R. Clinical evaluation of a biphasic calcium composite grafting material in the treatment of human periodontal intrabony defects: a 12-month randomized controlled clinical trial. J Periodontol. 2009;80:1774–1782. doi: 10.1902/jop.2009.090229. [DOI] [PubMed] [Google Scholar]
- 34.Rosen PS, Reynolds MA, Bowers GM. The treatment of intrabony defects with bone grafts. Periodontol 2000. 2000;22:88–103. doi: 10.1034/j.1600-0757.2000.2220107.x. [DOI] [PubMed] [Google Scholar]
- 35.Dybvik T, Leknes KN, Boe OE, Skavland RJ, Albandar JM. Bioactive ceramic filler in the treatment of severe osseous defects: 12-month results. J Periodontol. 2007;78:403–410. doi: 10.1902/jop.2007.060263. [DOI] [PubMed] [Google Scholar]
- 36.Shirakata Y, Setoguchi T, Machigashira M, Matsuyama T, Furuichi Y, Hasegawa K, et al. Comparison of injectable calcium phosphate bone cement grafting and open flap debridement in periodontal intrabony defects: a randomized clinical trial. J Periodontol. 2008;79:25–32. doi: 10.1902/jop.2008.070141. [DOI] [PubMed] [Google Scholar]
- 37.Ellegaard B, Löe H. New attachment of periodontal tissues after treatment of intrabony lesions. J Periodontol. 1971;42:648–652. doi: 10.1902/jop.1971.42.10.648. [DOI] [PubMed] [Google Scholar]
- 38.Hiatt WH, Schallhorn RG. Intraoral transplants of cancellous bone and marrow in periodontal lesions. J Periodontol. 1973;44:194–208. doi: 10.1902/jop.1973.44.4.194. [DOI] [PubMed] [Google Scholar]