Abstract
BACKGROUND
Reports from US, UK and European drug policy entities, and ongoing media accounts, show increasing recreational use of 4-methylmethcathinone (4-MMC, mephedrone) and 3,4-methylenedioxypyrovalerone (MDPV). Severe sympathomimetic symptoms, hallucinations, psychoses, and even deaths have been reported, yet little scientific information is available on the effects of these compounds in laboratory models. Available studies on the neurochemistry of these drugs show that 4-MMC and MDPV enhance DA neurotransmission, while 4-MMC additionally enhances 5-HT neurotransmission- a pattern much like that reported for methamphetamine vs. 3,4-methylenedioxymethamphetamine (MDMA). As is the case for designer amphetamines, these neurochemical distinctions may predict differential potential for repetitive versus episodic abuse and distinct lasting toxicities.
METHODS
This study determined relative locomotor stimulant effects of 4-MMC (1–10 mg/kg, s.c.) and MDPV (0.5–5.6 mg/kg, s.c.), in comparison with d-methamphetamine (MA; 0.5–5.6 mg/kg, s.c.) and MDMA (1–7.5 mg/kg, s.c.) on a measure of locomotor activity – voluntary wheel running – in male Wistar rats (N=8).
RESULTS
Compared to counts of wheel rotations after saline, a biphasic change in the pattern of counts was observed after injections of MA and MDPV, with relatively higher counts following lower doses and lower counts following the highest dose. However, monophasic, dose-dependent reductions in counts were observed in response to injections of MDMA and 4-MMC.
CONCLUSION
Thus, voluntary wheel running yielded the same categorical distinctions for these drugs as did prior experiments testing the effects of these drugs on monoaminergic neurotransmission. These data indicate that MDPV produces prototypical locomotor stimulant effects whereas 4-MMC is more similar to the entactogen MDMA.
Keywords: cathinones, stimulants, activity wheel, Ecstasy, bath salts
1. Introduction
A recent DEA report (DEA, 2011a) and ongoing media accounts show increasing recreational use of 4-methylmethcathinone (4-MMC, mephedrone) and 3,4-methylenedioxypyrovalerone (MDPV) in the US. Little direct information is available on the effects of these novel, cathinone derivative compounds in the scientific literature, as was reflected in recent European public health / public policy reports (Iversen et al., 2010; Sedefov et al., 2010). Nevertheless, a growing literature continues to confirm that within a diversity (Reitzel et al., 2011) of compounds termed “plant food” and “bath salts” by popular media, 4-MMC and MDPV are major components (Borek and Holstege, 2012; Kyle et al., 2011; Murray et al., 2012; Rust et al., 2012; Spiller et al., 2011; Thornton et al., 2012). Some 4-MMC users reported similar sympathomimetic and entactogenic effects as MDMA (Winstock et al., 2011a; 2011b); however, high rates of intranasal administration combined with subjective reports of craving and addiction suggest that 4-MMC may have a greater potential for repetitive, compulsive use than does MDMA (Brunt et al., 2010; Carhart-Harris et al., 2011; Dargan et al., 2010). One factor thought to have contributed to the rise in popularity of 4-MMC was a decrease in the purity of MDMA, and especially worrisome is the idea that recreational drug users may be replacing MDMA with a potentially more addictive compound. Similarly, concern is elevated by emerging clinical case studies that reported MDPV-induced psychotic symptoms reminiscent of methamphetamine (MA) use and MA-induced psychosis (Antonowicz et al., 2011; Orikabe et al., 2011; Thornton et al., 2012).
Online user-reports reveal potential differences in subjective and physiological effects which may result from distinct neuropharmacological properties (Bluelight, 2006, 2008; Geezaman, 2009; MephTest, 2009) of substituted cathinones. Such data are limited in utility since, for example, 4-MMC has been reported to be both similar to MDMA and “better than cocaine” in different subpopulations (Geezaman, 2009; Winstock et al., 2011b) and in any case, even drug-experienced subjects are poor at distinguishing MDMA from d-methamphetamine (MA) under blinded conditions in human laboratory studies (Kirkpatrick et al., 2012). These differences are critical to explore since prior experience with designer amphetamines such as MDMA and d-methamphetamine (MA) can reveal distinct constellations of potential health threats with respect to liability for acute behavioral effects, compulsive versus episodic use and lasting neurochemical toxicities (De La Garza et al., 2006; Fantegrossi et al., 2009; Kitamura et al., 2006; Ricaurte et al., 1988; Yuan et al., 2006). There are also case reports of Ecstasy/MDMA use patterns that are daily or at least several times per week (Hurault de Ligny et al., 2005; Jansen, 1999; Kouimtsidis et al., 2006), highly consistent with compulsive use patterns that are common to reference standard drugs of abuse such as methamphetamine Such differences may be important in the development of evidence-based public policy actions such as the preliminary action to add cathinones to the list of controlled substances within the United States (DEA, 2011b).
Recent neurochemical studies provide evidence that 4-MMC may produce effects that are similar to those of amphetamines on behavioral assays (e.g., horizontal locomotor ambulation) but dissimilar to those of amphetamines on neurochemical assays (e.g., extrasynaptic serotonin concentrations). Specifically, 4-MMC produces a stronger relative overflow of serotonin versus dopamine in the nucleus accumbens, a pattern which is dissimilar to those of amphetamine or d-methamphetamine but similar to those of MDMA (Baumann et al., 2011; 2008a; 2008b; Kehr et al., 2011a). Moreover, one recent report found that, although the potency of 4-MMC for inhibiting dopamine uptake in synaptosomes was on the order of that of MA, the potency to inhibit serotonin uptake was similar to that of MDMA (Hadlock et al., 2011).
However, 4-MMC has been shown to have locomotor stimulant effects similar to those of amphetamine/methamphetamine; at least over a restricted dose range of 1–3 mg/kg (Baumann et al., 2012; Kehr et al., 2011b). Yet, selective serotonin reuptake inhibitors suppress wheel activity (Haug et al., 1990; Weber et al., 2009) as does MDMA at moderate doses (Gilpin et al., 2011). Thus, for 4-MMC there appears to be a categorical distinction implicated by neurochemistry – particularly that of serotonin – that is not reflected in currently available reports of horizontal locomotor ambulation.
On the other hand, in mice, the effects of MDPV on neurochemistry and locomotor activity appear to be more similar to those of MA than those of MDMA – increased striatal overflow of dopamine, but not serotonin, and increased horizontal locomotor ambulation (Fuwa et al., 2007). However, this report only compared locomotor stimulant effects of single doses of MDPV, MA and MDMA. And, caution should be taken regarding these limited data on neurochemistry. There is currently no information available on the precise pharmacological mechanism of MDPV; although structurally related compounds which lack the 3,4-methylenedioxy moiety appear to function as monoamine transporter inhibitors (Meltzer et al., 2006).
The goal of the present investigation was to determine the acute effects of MDPV, 4-MMC, MDMA and d-methamphetamine (MA) on a type of locomotor activity – voluntary wheel running – that appears to discriminate MA and MDMA in manner concordant with differences in their respective serotonin neurochemistry (Baumann et al., 2012; Kehr et al., 2011b); a discrimination not observed for measures of horizontal ambulatory locomotor activity (cites). Specifically, it is predicted that the two drugs with strong serotoninergic actions – 4-MMC and MDMA – will decrease wheel running at all effective doses while those drugs without this action – MDPV and MA – will increase wheel running at low/moderate doses and decrease running at high doses.. In addition, we extend upon the dose-range limitations of previous studies (Baumann et al., 2012; Kehr et al., 2011).
In summary, we present evidence that voluntary wheel running may represent an improved locomotor model for distinguishing MDMA-like from MA-like drugs over current measures of horizontal ambulation.
2. Materials and Methods
2.1 Subjects
Eight male Wistar rats (Charles River, NY, USA) weighing between 250–300 g and approximately 9 weeks old on arrival at the lab (and 18 weeks at the start of the current study) were used. These rats were trained to operate response levers for food reward and given the opportunity to run on a wheel in hour long sessions to serve as a control group for a previous study (Miller et al., 2011). The animals were housed in groups of two in a temperature controlled vivarium (23 ± 1 °C) with a 12-hr:12-hr light:dark cycle. Food and water were available ad libitum for the entire length of the study except during lever training (overall feeding was restricted) and within the daily behavioral testing sessions. Studies were conducted under protocols approved by the Institutional Animal Care and Use Committee (IACUC) of The Scripps Research Institute in a manner consistent with the recommendations of the NIH Guide for the Care and Use of Laboratory Animals (Clark et al., 1996).
2.2 Apparatus
Experimental sessions were conducted in procedure rooms using specialized operant chambers with an integrated activity wheel (Med Associates; Model ENV-045; 12-inch inner diameter wheel, thus ~1 meter traveled per revolution). The chambers were enclosed in individual sound-attenuating cubicles and all devices were recorded from by using MED-PC IV software (Med Associates). Counts of wheel rotations within a session were collected into 12 sequential 5-min bins for analysis.
2.3 Drugs
D-methamphetamine (MA) and 3,4-methylenedioxymethamphetamine (MDMA) were provided by RTI under contract to the National Institute on Drug Abuse Drug Supply Program. 3,4-methylenedioxypyrovalerone (MDPV) and 4-methylmethcathinone (4-MMC) were synthesized according to literature precedent (Camilleri et al., 2010). Drugs were dissolved in sterile saline and administered subcutaneously in a volume of 1 ml/kg for acute challenges.
2.4 Experimental Procedure
Drug challenges
Subjects were provided with access to activity wheels for one hour per day, 5 days a week, Monday thru Friday, starting approximately one hour into the dark cycle. Drug doses (or vehicle) were administered subcutaneously immediately prior to the start of Tuesday and Friday sessions. For the remaining 3 sessions, rats were untreated. Five blocks of treatment for four compounds were administered in the following order: MA (vehicle, 0.56 mg/kg, 1.0 mg/kg, 5.6 mg/kg), MDMA (vehicle, 1.0 mg/kg, 5.6 mg/kg), MDPV (vehicle, 0.5 mg/kg, 1.0 mg/kg, 5.6 mg/kg), and 4-MMC (vehicle, 1.0 mg/kg, 5.6 mg/kg, 10.0 mg/kg), MDMA (vehicle, 7.5 mg/kg). Drug dose was approximately balanced within order and fully balanced across subjects for each treatment block. The highest dose of MDMA (7.5 mg/kg) was afforded its own block at the end of the experiment to avoid potential fatalities under these conditions (Gilpen et al., 2011). Summary data for the MA challenges were previously reported (Miller et al., 2011).
Animals were assessed behaviorally at the conclusion of each drug-treatment session for repetitive sniffing, licking and/or circular head motion, as well as a lack of orienting response to a finger tap on the side of the home cage. This assessment was scored as the presence or absence of this set of observations and that score was used to generate a value for a dichotomous, categorical variable for stereotypy.
2.5 Data Analysis
To compare wheel rotations as a function of drug dose, for each treatment block (except MDMA, see below), wheel rotations were analyzed by repeated-measure analysis of variance (rmANOVA) using drug dose and time bin as within-subjects factors. To compare wheel rotations after vehicle injections as a function of treatment block, wheel rotations were analyzed by rmANOVA using treatment block and time bin as within-subjects factors.
Preliminary analysis comparing wheel counts between the vehicle conditions of the two MDMA treatment blocks found neither a main effect of treatment block nor an interaction between treatment block and time bin. Thus, the vehicle conditions of the two MDMA treatment blocks were combined (average values) to simplify analysis.
Counts of wheel rotations are presented as the mean ± the standard error of the mean (SEM) and rmANOVA were followed with post hoc paired-means comparisons to delineate simple effects using the Tukey-Kramer method (Tukey's HSD). For all analyses, the criterion for significant difference was set at p < 0.05. StatView 5.0 software (SAS Institute, Cary, NC, USA) was used for analyses and graph production.
3. Results
3.1 Effect of d-methamphetamine (MA) on wheel activity
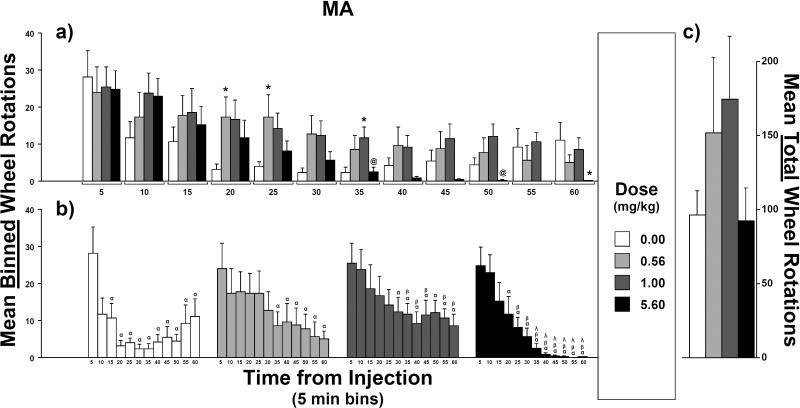
Compared to saline, counts of wheel rotations were higher early in the session after injections of the two lowest doses of MA (0.56 and 1.0 mg/kg, s.c.) but were lower later in the session after the highest dose of MA (5.6 mg/kg) (Figure 1). A rmANOVA confirmed an interaction between MA dose and time from injection (F33,231 = 2.0, p < 0.01) as well as a main effect of time [F11,77 = 12.2, p < 0.0001] and a trend toward a main effect of dose [F3,21 = 2.8, p = 0.065].
Fig. 1.
Mean counts of wheel rotations after injections of d-methamphetamine (MA; N = 8): a) Effect of injection dose on mean binned-counts (5-min intervals) as a function of time bin. b) Effect of time-from-injection on mean binned-counts as a function of injection dose. c) Effect of injection dose on mean total-counts (i.e., sum of the twelve binned-counts). For the effect of dose, symbols over bars indicate a mean difference from vehicle (*) or from 1.0 mg/kg (@). For the effect of time-from-injection, symbols over bars indicate a mean difference from the 5-min bin (α), from the 10-min bin (β) or from the 15-min bin (λ). Error bars represent SEM.
For post hoc comparisons between mean counts of wheel rotations after varied MA doses, mean total-counts (i.e., counts summed across all 5-min sampling intervals or “time bins”; Figure 1c) where not found statistically different. However, compared to mean binned-counts (Figure 1a) after vehicle injections, means were higher after 0.56 mg/kg injections in the 20- and 25-min time bins and higher after 1.0 mg/kg injections in the 35-min time bin but lower after 5.6 mg/kg injections in the 60-min time bin. Compared to mean binned-counts after 1 mg/kg injections, means were reliably lower after 5.6 mg/kg injections in the 35- and 50-min time bins. Lastly, within each time bin, all other means-pairs of binned-counts did not differ.
For post hoc comparisons of means at varied time intervals after injection, after vehicle injections, mean binned-counts (Figure 1b) of wheel rotations was higher for the 5-min post-injection time bin than means for the 15- through 60-min time bins. After injections of 0.56 mg/kg MA, the mean was higher in the 5-min time bin than the means for the 35- through 60-min time bins. After injections of 1.0 mg/kg, the mean was higher in the 5-min time bin than the means for the 30- through 60-min time bins and the mean was higher in the 10-min time bin than the means for the 35- through 45-min as well as the 55- and 60-min time bins. After injections of 5.6 mg/kg, the mean was higher in the 5-min time bin than the means for the 20-through 60-min time bins, the mean was higher in the 10-min time bin than the means for the 25- through 60-min time bins and the mean was higher in the 15-min time bin than the means for the 35- through 60-min time bins. Lastly, within each dose, all other means-pairs of binned-counts were equivalent.
Stereotypy was scored as “present” in all of the rats at the end of the session following the highest dose of MA but was scored “absent” after all other sessions.
3.2 Effects of 3,4-methylenedioxymethamphetamine (MDMA) on wheel activity
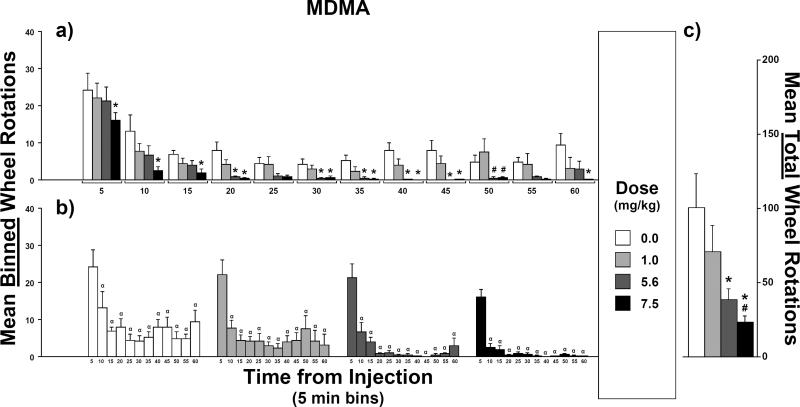
Mean counts of wheel rotations were lower after injections of MDMA for all doses tested in comparison with the saline condition (Figure 2). A rmANOVA confirmed a main effect of dose (F3,21 = 12.8, p < 0.0001) as well as a main effect of time (F11,77 = 27.0, p < 0.0001) but not an interaction between dose and time (F33,231 = 1.1, p = 0.4).
Fig. 2.
Mean counts of wheel rotations after injections of 3,4-methylenedioxymethamphetamine (MDMA; N = 8): a) Effect of injection dose on mean binned-counts (5-min intervals) as a function of time bin. b) Effect of time-from-injection on mean binned-counts as a function of injection dose. c) Effect of injection dose on mean total-counts (i.e., sum of the twelve binned-counts). For the effect of dose, symbols over bars indicate a mean difference from vehicle (*) or from 1.0 mg/kg (#). For the effect of time-from-injection, symbols over bars indicate a mean difference from the 5-min bin (α). Error bars represent SEM.
For post hoc comparisons between mean counts of wheel rotations after varied MDMA doses, compared to vehicle, mean total-counts (i.e., counts summed across all 5-min sampling intervals or “time bins”; Figure 2c) were lower after 5.6 mg/kg and 7.5 mg/kg MDMA injections. Mean total-counts were also lower after 7.5 mg/kg than after 5.6 mg/kg. Additionally, compared to mean binned-counts (Figure 2a) after vehicle injections, means were lower after 5.6 mg/kg injections in the 20- and 30- through 45-min time bins as well as lower after 7.5 mg/kg injections in the 5- through 20-, 30- through 45- and 60-min time bins. Compared to mean binned-counts after 1 mg/kg injections, means were lower after 5.6 mg/kg and 7.5 mg/kg injections in the 50-min time bin. Lastly, within each time bin, all other means-pairs of binned-counts were equivalent.
For post hoc comparisons of means at varied time intervals after injection, regardless of dose, within each dose, mean binned-counts (Figure 2b) of wheel rotations were higher in the 5-min post-injection time bin than for all other time bins. Within each dose, all other means-pairs of binned-counts were equivalent.
Stereotypy was scored as “absent” in all of the rats at the end of the session following the all doses of MDMA.
3.3 Effects of 3,4-methylenedioxypyrovalerone (MDPV) on wheel activity
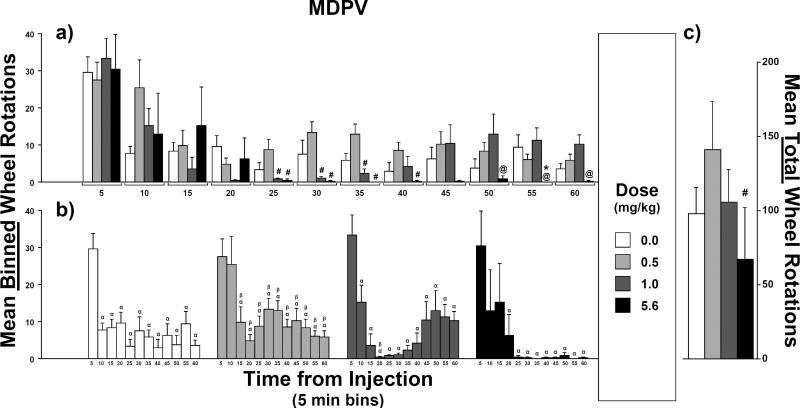
Counts of wheel rotations were higher early in the session after injections of MDPV at all doses tested (0.5, 1.0 and 5.6 mg/kg, s.c.) compared with the saline condition, but were lower in the middle of the session after the two highest doses (Figure 3). For the highest dose, counts remained lower than those for vehicle for the remainder of the session. A rmANOVA confirmed an interaction between dose and time (F33,231 = 1.9, p < 0.01) as well as main effects of dose (F3,21 = 3.2, p < 0.05) and time (F11,77 = 16.5, p < 0.0001).
Fig. 3.
Mean counts of wheel rotations after injections of 3,4-methylenedioxypyrovalerone (MDPV; N = 8): a) Effect of injection dose on mean binned-counts (5-min intervals) as a function of time bin. b) Effect of time-from-injection on mean binned-counts as a function of injection dose. c) Effect of injection dose on mean total-counts (i.e., sum of the twelve binned-counts). For the effect of dose, symbols over bars indicate a mean difference from vehicle (*), from 0.5 mg/kg (#) or from 1.0 mg/kg (@). For the effect of time-from-injection, symbols over bars indicate a mean difference from the 5-min bin (α) or from the 10-min bin (β). Error bars represent SEM.
For post hoc comparisons between mean counts of wheel rotations after varied MDPV doses, mean total-counts (i.e., counts summed across all 5-min sampling intervals or “time bins”; Figure 3c) after MDPV injections, regardless of dose, where not different from the mean after vehicle injections. However, mean total-counts after 5.6 mg/kg were lower than those after 0.5 mg/kg. Compared to mean binned-counts (Figure 3a) after vehicle injections, the mean was lower after 5.6 mg/kg MDPV injections in the 55-min time bin only. Compared to mean binned-counts after 0.5 mg/kg MDPV injections, counts were lower after 1 mg/kg in the 25-through 35-min time bins and lower after 5.6 mg/kg in the 25- through 40-min time bins. Compared to mean binned-counts after 1 mg/kg injections, means were lower after 5.6 mg/kg for the 50- and 60-min time bins. Lastly, within each time bin, all other means-pairs of binned-counts were not reliably different.
For post hoc comparisons of means at varied time intervals after injection, after vehicle injections, mean binned-counts (Figure 3b) of wheel rotations was higher in the 5-min post-injection time bin than the means for the 10- through 60-min time bins. After injections of 0.5 mg/kg MDPV, the mean was higher in the 5-min and 10-min time bins than the means for the 15- through 60-min time bins. After injections of 1.0 mg/kg, the mean was higher in the 5-min time bin than the means for the 10- through 60-min time bins and the mean was higher in the 10-min time bin than the mean for the 20-min time bin. After injections of 5.6 mg/kg, the mean was higher in the 5-min time bin than the means for the 20- through 60-min time bins. Lastly, within each dose, all other means-pairs of binned-counts were not statistically different.
Stereotypy was scored as “present” in all of the rats at the end of the session following the highest dose of MDPV but was scored “absent” after all other sessions. The pattern of post-session stereotypy after MDPV was similar to that of MA. For both drugs, post-session stereotypy was only observed after doses that caused late-session vehicle-relative decreases in counts of wheel rotations.
3.4 Effects of 4-methylmethcathinone (4-MMC) on wheel activity
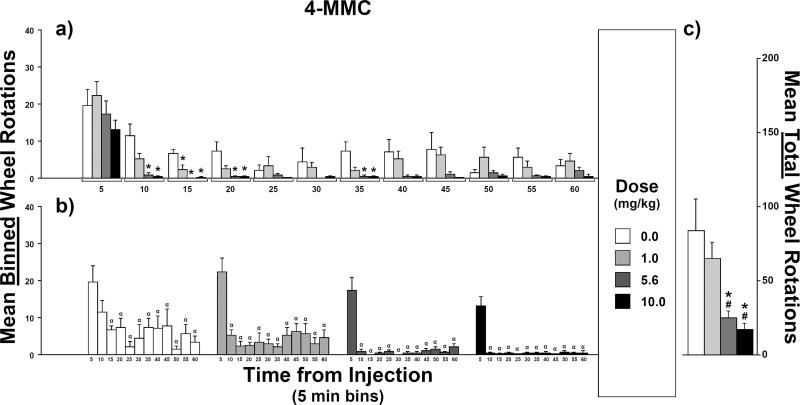
Counts of wheel rotations were lower after injections of 4-MMC for all doses tested (Figure 4) compared with the saline condition. A rmANOVA confirmed main effects of dose (F3,21 = 10.3, p < 0.001) and time (F11,77 = 21.1, p < 0.0001) but no interaction between dose and time (F33,231 = 1.2, p = 0.2).
Fig. 4.
Mean counts of wheel rotations after injections of 4-methylmethcathinone (4-MMC; N = 8): a) Effect of injection dose on mean binned-counts (5-min intervals) as a function of time bin. b) Effect of time-from-injection on mean binned-counts as a function of injection dose. c) Effect of injection dose on mean total-counts (i.e., sum of the twelve binned-counts). For the effect of dose, symbols over bars indicate a mean difference from vehicle (*) or from 1.0 mg/kg (#). For the effect of time-from-injection, symbols over bars indicate a mean difference from the 5-min bin (α). Error bars represent SEM.
For post hoc comparisons between mean counts of wheel rotations after varied 4-MMC doses, compared to either vehicle or 1.0 mg/kg 4-MMC, mean total-counts (i.e., counts summed across all 5-min sampling intervals or “time bins”; Figure 4c) were lower after 5.6 mg/kg and 10 mg/kg. Additionally, compared to mean binned-counts (Figure 4a) after vehicle injections, means were lower after 5.6 mg/kg and 10 mg/kg in the 10- through 20-min and 35-min time bins. Means were also lower after 1.0 mg/kg than after vehicle for the 15 min time bin. Lastly, within each time bin, all other means-pairs of binned-counts were equivalent.
For post hoc comparisons of means at varied time intervals after injection, regardless of dose, within each dose, mean binned-counts (Figure 4b) of wheel rotations were higher in the 5-min time bin than for all other time bins with the one exception of the mean for the 10-min time bin after vehicle. Within each dose, all other means-pairs of binned-counts were not reliably different.
For all doses of 4-MMC, stereotypy was scored as “absent” in all of the rats at the end of all sessions. Thus, 4-MMC and MDMA were equivalent with regard to post-session stereotypy.
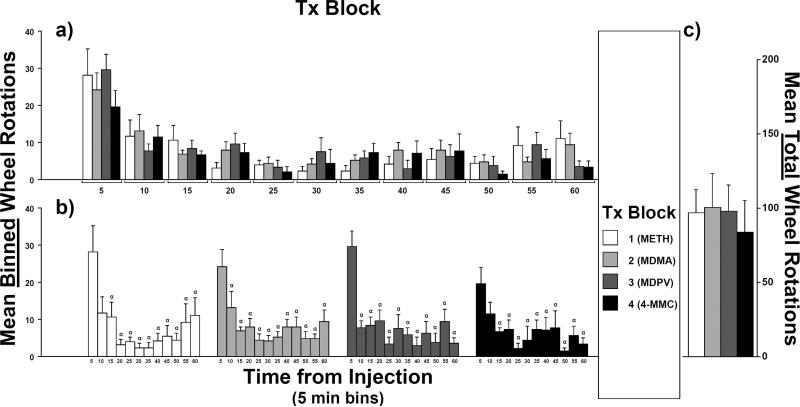
3.5 Effects of treatment block (Tx Block) on wheel activity after vehicle injections
Counts of wheel rotations after vehicle injections did not differ as a function of Tx Block (Figure 5a–c). A rmANOVA confirmed no main effect of treatment block (F3,21 = 0.6, p = 0.6) nor an interaction between treatment block and time bin (F33,231 = 1.2, p = 0.3). There was, however, a main effect of time bin (F11,77 = 13.7, p < 0.0001). Post hoc comparisons of means at varied time intervals after injection for each Tx Block are described above in the Results section of that respective Tx Block.
Fig. 5.
Mean counts of wheel rotations after injections of vehicle only for each treatment block (Tx Block; N = 8): a) Effect of Tx Block on mean binned-counts (5-min intervals) as a function of time bin. b) Effect of time-from-injection on mean binned-counts as a function of Tx Block. c) Effect of Tx Block on mean total-counts (i.e., sum of the twelve binned-counts). Means did not differ as a function of Tx Block for either binned-counts or total-counts. For the effect of time-from-injection, symbols over bars indicate a mean difference from the 5-min bin (α). Error bars represent SEM.
4. Discussion
The data from this study identify distinct behavioral responses for two compounds that are commonly linked together in the popular media under one street description, i.e., “bath salts.” The novel cathinone derivatives 4-methylmethcathinone (4-MMC; “mephedrone”) and 3,4-methylenedioxypyrovalerone (MDPV) have also been included together in a recent preliminary control action by the US Drug Enforcement Agency (DEA, 2011b). In the current study, the locomotor effects of MDPV were biphasic, leading to increased wheel activity at lower doses and suppressed activity at higher doses. This pattern of results was generally very similar to the pattern produced by d-methamphetamine (MA). It may be the case that MDPV is more potent in decreasing wheel activity (vehicle-relative decreases in counts at lower doses; 1.0 for MDPV vs. for 5.6 MA) but is less efficacious at increasing wheel activity (maximum total-counts after 1.0 mg/kg MA = 175 and after 0.5 mg/kg MDPV = 141). In contrast, 4-MMC produced monophasic decreases in activity levels on the wheel, a pattern which was similar to the effects of 3,4-methylenedioxymethamphetamine (MDMA; “Ecstasy”). Each drug caused linear, dose-dependent, vehicle-relative decreases in counts at doses of 5.6 mg/kg and higher. Together with recent neurochemical information (Baumann et al., 2011; Fuwa et al., 2007; Kehr et al., 2011a) these data underline the need to consider effects and risks of 4-MMC and MDPV to be distinct.
The findings for 4-MMC appear superficially to be discordant with recent reports of increased ambulatory activity (relative to vehicle treatment) in adult rats that were tethered for microdialysis and dosed (0.3–3.0 mg/kg) during the inactive (light) part of the diurnal cycle (Baumann et al., 2012; Kehr et al., 2011b). The factors of tethering and light-cycle phase at the time of injection can cause differences in activity. However, the difference in the direction of the observed effects of 4-MMC on “locomotor activity” likely is a result of considering wheel running and horizontal ambulation together as “locomotor activity” (Novak et al., 2012). Moreover, there is a parallel in the locomotor stimulant effects of MDMA. Prior reports have found that low to moderate doses of MDMA increase horizontal ambulation (Bankson and Cunningham, 2002; Bubar et al., 2004; Gold and Koob, 1988; Gold et al., 1988; Herin et al., 2005) yet we have shown previously that wheel activity is suppressed by 5 mg/kg MDMA (Gilpin et al., 2011). Interestingly, MDMA suppresses spontaneous activity in nonhuman primates (Crean et al., 2007; Fantegrossi et al., 2009; Von Huben et al., 2007).
Increased open field activity was also reported for adolescent rats treated with 15–30 mg/kg 4-MMC (Motbey et al., 2011). However, Baumann and colleagues have argued effectively that for MDMA, “effect scaling” (e.g., neurochemical effects) generates the most relevant dose ranges for animal models (Baumann et al., 2007). Given significant neurochemical and behavioral effects reported by Kehr et al. (2011), Baumann et al (2012) and the present study, at much lower doses, the relevance of 15–30 mg/kg 4-MMC for the human condition is unclear.
A more general contribution of the present work is the determination of locomotor effects of stimulant drugs on voluntary activity on a wheel. Although laboratory rats will spontaneously use wheels when they are provided (Sherwin, 1998), will make operant responses for wheel access (Hundt and Premack, 1963; Premack et al., 1964) and may reduce intravenous drug intake when provided with concurrent wheel access (Cosgrove et al., 2002; Miller et al., 2011) there are relatively few data available on the effects of psychoactive drugs on wheel activity. Thus, it is unclear whether effects of drugs which alter locomotion in an open field will translate directly to a somewhat more intentional, voluntary behavior such as running on an activity wheel. There is limited prior evidence, indeed, that activity on wheels may be suppressed by treatment with stimulants that increase locomotor activity in the home cage or open field (Bradbury et al., 1987; Della Maggiore and Ralph, 2000). Similarly, selective serotonin reuptake inhibitors decrease wheel running in mice but may increase open field locomotion (Haug et al., 1990; Weber et al., 2009). In this study, both MA and MDPV increased wheel activity, consistent with similar increases produced by each drug when assessed telemetrically in the homecage (Aarde et al., 2012) and with our prior observation for MA (Gilpin et al., 2011). In total, the data suggest that activity wheels function as an improved model (compared with open-field ambulation) for discriminating MDMA-like from MA-like behavioral effects in rodents.
Although the assessment of stereotyped behavior was not sophisticated in this study the outcome was consistent with what would be expected in the case of methamphetamine with both stereotyped behavior and reduced locomotor activity on the wheel found at higher doses. MDMA appears to be much less potent than amphetamine or methamphetamine in inducing stereotypy in a traditional open field/activity monitoring preparations (Fantegrossi et al., 2008; O'Loinsigh et al., 2001; Walker et al., 2010) and the lack of any significant stereotypy noted in the present study is consistent with what would be predicted given the dose range used.
One potential limitation to the present study was the repeated-measures design and the fixed testing order for the four compounds. It is theoretically possible that some degree of plasticity of the response across drugs may have influenced the outcome. However, this is unlikely based on the consistency of wheel running after vehicle injections across the study period and on related studies with these compounds. Additionally, the effects are consistent with the profiles and dose effect relationships observed for MDPV, 4-MMC and MA in naïve groups of animals when assessed with radiotelemetry for homecage activity (Aarde et al., 2012; Wright et al., 2011). Thus it is concluded that any possible effects of the order in which the compounds were evaluated is unlikely to have qualitatively altered the results.
In conclusion, this study underlines the error of assuming all novel cathinone derivative stimulants that become popular with recreational users will share neuropharmacological or biobehavioral properties. There may be some derivatives that are most similar to prototypical stimulants, some that are similar to MDMA and some that may afford a unique constellation of desired effects. These results encourage additional study to delineate similarities and differences between the different cathinones and to contrast effects with the better-studied amphetamine derivatives. Such data would provide an improved basis on which to infer relative health risks conferred by specific compounds which may be grouped, inaccurately, as if they were of common effect.
Acknowledgements
This work was supported by US Public Health Service / National Institutes of Health grants DA018418, DA024705 and DA024105. This is publication # 21652 from The Scripps Research Institute.
Role of Funding Source The US Public Health Service / National Institutes of Health / National Institute on Drug Abuse had no further role in the study beyond the financial support provided.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors The design for this study was created by MAT, PKH and SMA as a result of collaborative interest in the cathinones by the labs of KLH, TJD and MAT.
DMA and TJD designed synthesis routes, synthesized and validated the 4-MMC and MDPV compounds.
KLH provided pharmacokinetic and metabolic data on 4-MMC not reported herein which was critical to study design.
PKH conducted experiments and generated preliminary data summaries with SMA.
Primary statistical analysis was by SMA in consultation with PKH and MAT, graph creation by PKH and SMA and the initial draft of the manuscript written by PKH, SMA and MAT.
All authors approved the manuscript draft.
Conflict of Interest The authors declare no conflicts of interest for this work
References
- Aarde SM, Huang P-K, Creehan KM, Vaillancourt BD, Vandewater SA, Wright MJ, Miller ML, Taffe MA. Methylenedioxypyrovalerone (MDPV): Self-administration and acute drug challenges in rats. FASEB J. 2012;26:1040–1045. [Google Scholar]
- Antonowicz JL, Metzger AK, Ramanujam SL. Paranoid psychosis induced by consumption of methylenedioxypyrovalerone: two cases. Gen. Hosp. Psychiatr. 2011;33:640, e645–646. doi: 10.1016/j.genhosppsych.2011.04.010. [DOI] [PubMed] [Google Scholar]
- Bankson MG, Cunningham KA. Pharmacological studies of the acute effects of (+)-3,4-methylenedioxymethamphetamine on locomotor activity: role of 5-HT(1B/1D) and 5-HT(2) receptors. Neuropsychopharmacology. 2002;26:40–52. doi: 10.1016/S0893-133X(01)00345-1. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Ayestas MA, Jr., Partilla JS, Sink JR, Shulgin AT, Daley PF, Brandt SD, Rothman RB, Ruoho AE, Cozzi NV. The Designer Methcathinone Analogs, Mephedrone and Methylone, are Substrates for Monoamine Transporters in Brain Tissue. Neuropsychopharmacology. 2012;37:1192–1203. doi: 10.1038/npp.2011.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Clark RD, Franken FH, Rutter JJ, Rothman RB. Tolerance to 3,4-methylenedioxymethamphetamine in rats exposed to single high-dose binges. Neuroscience. 2008a;152:773–784. doi: 10.1016/j.neuroscience.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Clark RD, Rothman RB. Locomotor stimulation produced by 3,4-methylenedioxymethamphetamine (MDMA) is correlated with dialysate levels of serotonin and dopamine in rat brain. Pharmacol. Biochem. Behav. 2008b;90:208–217. doi: 10.1016/j.pbb.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Wang X, Rothman RB. 3,4-Methylenedioxymethamphetamine (MDMA) neurotoxicity in rats: a reappraisal of past and present findings. Psychopharmacology (Berl.) 2007;189:407–424. doi: 10.1007/s00213-006-0322-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluelight [accessed on 1/3/2012];MDPV Megathread. 2006 http://www.bluelight.ru/vb/threads/278421-MDPVMegathread.
- Bluelight [accessed on 9/28/10];(RC's) Big mephedrone thread. 2008 http://www.bluelight.ru/vb/showthread.php?t=400517.
- Borek HA, Holstege CP. Hyperthermia and multiorgan failure after abuse of “bath salts” containing 3,4-methylenedioxypyrovalerone. Ann. Emerg. Med. 2012 doi: 10.1016/j.annemergmed.2012.01.005. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Bradbury AJ, Costall B, Naylor RJ, Onaivi ES. 5-Hydroxytryptamine involvement in the locomotor activity suppressant effects of amphetamine in the mouse. Psychopharmacology (Berl.) 1987;93:457–465. doi: 10.1007/BF00207235. [DOI] [PubMed] [Google Scholar]
- Brunt TM, Poortman A, Niesink RJ, van den Brink W. Instability of the ecstasy market and a new kid on the block: mephedrone. J. Psychopharmacol. 2010;25:1543–1547. doi: 10.1177/0269881110378370. [DOI] [PubMed] [Google Scholar]
- Bubar MJ, Pack KM, Frankel PS, Cunningham KA. Effects of dopamine D1- or D2-like receptor antagonists on the hypermotive and discriminative stimulus effects of (+)-MDMA. Psychopharmacology (Berl.) 2004;173:326–336. doi: 10.1007/s00213-004-1790-1. [DOI] [PubMed] [Google Scholar]
- Camilleri A, Johnston MR, Brennan M, Davis S, Caldicott DGE. Chemical analysis of four capsules containing the controlled substance analogues 4-methylmethcathinone, 2-fluoromethamphetamine, alpha-phthalimidopropiophenone and N-ethylcathinone. Forens. Sci. Int. 2010;197:59–66. doi: 10.1016/j.forsciint.2009.12.048. [DOI] [PubMed] [Google Scholar]
- Carhart-Harris RL, King LA, Nutt DJ. A web-based survey on mephedrone. Drug Alcohol Depend. 2011;118:19–22. doi: 10.1016/j.drugalcdep.2011.02.011. [DOI] [PubMed] [Google Scholar]
- Cosgrove KP, Hunter RG, Carroll ME. Wheel-running attenuates intravenous cocaine self-administration in rats: sex differences. Pharmacol. Biochem. Behav. 2002;73:663–671. doi: 10.1016/s0091-3057(02)00853-5. [DOI] [PubMed] [Google Scholar]
- Crean RD, Davis SA, Taffe MA. Oral administration of (+/−)3,4-methylenedioxymethamphetamine and (+)methamphetamine alters temperature and activity in rhesus macaques. Pharmacol. Biochem. Behav. 2007;87:11–19. doi: 10.1016/j.pbb.2007.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dargan PI, Albert S, Wood DM. Mephedrone use and associated adverse effects in school and college/university students before the UK legislation change. QJM. 2010;103:875–879. doi: 10.1093/qjmed/hcq134. [DOI] [PubMed] [Google Scholar]
- De La Garza R, 2nd, Fabrizio KR, Gupta A. Relevance of rodent models of intravenous MDMA self-administration to human MDMA consumption patterns. Psychopharmacology (Berl.) 2006;189:425–434. doi: 10.1007/s00213-005-0255-5. [DOI] [PubMed] [Google Scholar]
- DEA Request for information on synthetic cathones. Microgram Bull. 2011a;44:31–34. [Google Scholar]
- DEA Schedules of controlled substances: temporary placement of three synthetic cathinones in Schedule I. Final Order. Federal register 76. 2011b:65371–65375. [PubMed]
- Della Maggiore V, Ralph MR. The effect of amphetamine on locomotion depends on the motor device utilized. The open field vs. the running wheel. Pharmacol. Biochem. Behav. 2000;65:585–590. doi: 10.1016/s0091-3057(99)00260-9. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Bauzo RM, Manvich DM, Morales JC, Votaw JR, Goodman MM, Howell LL. Role of dopamine transporters in the behavioral effects of 3,4-methylenedioxymethamphetamine (MDMA) in nonhuman primates. Psychopharmacology (Berl.) 2009;205:337–347. doi: 10.1007/s00213-009-1545-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi WE, Ciullo JR, Wakabayashi KT, De La Garza R, 2nd, Traynor JR, Woods JH. A comparison of the physiological, behavioral, neurochemical and microglial effects of methamphetamine and 3,4-methylenedioxymethamphetamine in the mouse. Neuroscience. 2008;151:533–543. doi: 10.1016/j.neuroscience.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuwa T, Fukamori N, Tanaka T, Kubo Y, Ogata A, Uehara S, Honda Y, Kodama T. Microdialysis study of drug effectgs on central nervous system - Changes of dopamine levels in mice striatum after oral administration of methylenedioxypyrovalerone. Tokyo-to Kenko Anzen Kenkyu Senta Kenkyu Nenpo. 2007;58:287–292. [Google Scholar]
- Geezaman DF. [accessed on 29 Sep 2010>];Surprisingly like E. 2009 http://www.erowid.org/experiences/exp.php?ID=77952.
- Gilpin NW, Wright MJ, Jr., Dickinson G, Vandewater SA, Price JU, Taffe MA. Influences of activity wheel access on the body temperature response to MDMA and methamphetamine. Pharmacol. Biochem. Behav. 2011;99:295–300. doi: 10.1016/j.pbb.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold LH, Koob GF. Methysergide potentiates the hyperactivity produced by MDMA in rats. Pharmacol. Biochem. Behav. 1988;29:645–648. doi: 10.1016/0091-3057(88)90034-2. [DOI] [PubMed] [Google Scholar]
- Gold LH, Koob GF, Geyer MA. Stimulant and hallucinogenic behavioral profiles of 3,4-methylenedioxymethamphetamine and N-ethyl-3,4-methylenedioxyamphetamine in rats. J. Pharmacol. Exp. Ther. 1988;247:547–555. [PubMed] [Google Scholar]
- Hadlock GC, Webb KM, McFadden LM, Chu PW, Ellis JD, Allen SC, Andrenyak DM, Vieira-Brock PL, German CL, Conrad KM, Hoonakker AJ, Gibb JW, Wilkins DG, Hanson GR, Fleckenstein AE. 4-Methylmethcathinone(mephedrone): neuropharmacological effects of a designer stimulant of abuse. J. Pharmacol. Exp. Ther. 2011;339:530–536. doi: 10.1124/jpet.111.184119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haug M, Wallian L, Brain PF. Effects of 8-OH-DPAT and fluoxetine on activity and attack by female mice towards lactating intruders. Gen. Pharmacol. 1990;21:845–849. doi: 10.1016/0306-3623(90)90443-p. [DOI] [PubMed] [Google Scholar]
- Herin DV, Liu S, Ullrich T, Rice KC, Cunningham KA. Role of the serotonin 5-HT2A receptor in the hyperlocomotive and hyperthermic effects of (+)-3,4-methylenedioxymethamphetamine. Psychopharmacology (Berl.) 2005;178:505–513. doi: 10.1007/s00213-004-2030-4. [DOI] [PubMed] [Google Scholar]
- Hundt AG, Premack D. Running as both a positive and negative reinforcer. Science. 1963;142:1087–1088. doi: 10.1126/science.142.3595.1087. [DOI] [PubMed] [Google Scholar]
- Hurault de Ligny B, El Haggan W, Comoz F, Lobbedez T, Pujo M, Griveau AM, Bottet P, Bensadoun H, Ryckelynck JP. Early loss of two renal grafts obtained from the same donor: role of ecstasy? Transplantation. 2005;80:153–156. doi: 10.1097/01.tp.0000158713.70266.06. [DOI] [PubMed] [Google Scholar]
- Iversen L, Adebowale V, Abdulrahim D, Arr-Jones G, Barnes M, Birtwistle M, Bray S, Carlin E, Clancy C, Crome I, Doran R, Gibbons S, Hargreaves P, Healy C, Hickman M, Measham F, Liddell D, Mathewson H, Pearce T, Philips J, Phillips R, Roberts H, Rowlands M, Tomlinson M, Wing A. Consideration of the Cathinones. Advisory Council on the Misuse of Drugs. 2010 [Google Scholar]
- Jansen KL. Ecstasy (MDMA) dependence. Drug Alcohol Depend. 1999;53:121–124. doi: 10.1016/s0376-8716(98)00111-2. [DOI] [PubMed] [Google Scholar]
- Kehr J, Ichinose F, Yoshitake S, Goiny M, Sievertsson T, Nyberg F, Yoshitake T. Mephedrone, compared to MDMA (ecstasy) and amphetamine, rapidly increases both dopamine and serotonin levels in nucleus accumbens of awake rats. Br J Pharmacol. 2011a;164:1949–1958. doi: 10.1111/j.1476-5381.2011.01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehr J, Ichinose F, Yoshitake S, Goiny M, Sievertsson T, Nyberg F, Yoshitake T. Mephedrone, compared with MDMA (ecstasy) and amphetamine, rapidly increases both dopamine and 5-HT levels in nucleus accumbens of awake rats. Br. J. Pharmacol. 2011b;164:1949–1958. doi: 10.1111/j.1476-5381.2011.01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick MG, Gunderson EW, Perez AY, Haney M, Foltin RW, Hart CL. A direct comparison of the behavioral and physiological effects of methamphetamine and 3,4-methylenedioxymethamphetamine (MDMA) in humans. Psychopharmacology (Berl.) 2012;219:109–122. doi: 10.1007/s00213-011-2383-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura O, Wee S, Specio SE, Koob GF, Pulvirenti L. Escalation of methamphetamine self-administration in rats: a dose-effect function. Psychopharmacology (Berl.) 2006;186:48–53. doi: 10.1007/s00213-006-0353-z. [DOI] [PubMed] [Google Scholar]
- Kouimtsidis C, Schifano F, Sharp T, Ford L, Robinson J, Magee C. Neurological and psychopathological sequelae associated with a lifetime intake of 40,000 ecstasy tablets. Psychosomatics. 2006;47:86–87. doi: 10.1176/appi.psy.47.1.86. [DOI] [PubMed] [Google Scholar]
- Kyle PB, Iverson RB, Gajagowni RG, Spencer L. Illicit bath salts: not for bathing. J. MS State Med. Assoc. 2011;52:375–377. [PubMed] [Google Scholar]
- Meltzer PC, Butler D, Deschamps JR, Madras BK. 1-(4-Methylphenyl)-2-pyrrolidin-1-ylpentan-1-one (Pyrovalerone) analogues: a promising class of monoamine uptake inhibitors. J. Med. Chem. 2006;49:1420–1432. doi: 10.1021/jm050797a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MephTest [accessed on 29 Sep 2010];Good Alternative to MDMA. 2009 http://www.erowid.org/experiences/exp.php?ID=82321.
- Miller ML, Vaillancourt BD, Wright MJ, Jr., Aarde SM, Vandewater SA, Creehan KM, Taffe MA. Reciprocal inhibitory effects of intravenous d-methamphetamine self-administration and wheel activity in rats. Drug Alcohol Depend. 2011;121:90–96. doi: 10.1016/j.drugalcdep.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motbey CP, Hunt GE, Bowen MT, Artiss S, McGregor IS. Mephedrone (4-methylmethcathinone, 'meow'): acute behavioural effects and distribution of Fos expression in adolescent rats. Addict. Biol. 2011;2:409–422. doi: 10.1111/j.1369-1600.2011.00384.x. [DOI] [PubMed] [Google Scholar]
- Murray BL, Murphy CM, Beuhler MC. Death following recreational use of designer drug “bath salts” containing 3,4-Methylenedioxypyrovalerone (MDPV) J. Med. Toxicol. 2012;8:69–75. doi: 10.1007/s13181-011-0196-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Loinsigh ED, Boland G, Kelly JP, O'Boyle KM. Behavioural, hyperthermic and neurotoxic effects of 3,4-methylenedioxymethamphetamine analogues in the Wistar rat. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2001;25:621–638. doi: 10.1016/s0278-5846(00)00179-2. [DOI] [PubMed] [Google Scholar]
- Orikabe L, Yamasue H, Inoue H, Takayanagi Y, Mozue Y, Sudo Y, Ishii T, Itokawa M, Suzuki M, Kurachi M, Okazaki Y, Kasai K. Reduced amygdala and hippocampal volumes in patients with methamphetamine psychosis. Schizophr. Res. 2011;132:183–189. doi: 10.1016/j.schres.2011.07.006. [DOI] [PubMed] [Google Scholar]
- Premack D, Schaeffer RW, Hundt A. Reinforcement of drinking by running: effect of fixed ratio and reinforcement time. J. Exp Anal. Behav. 1964;7:91–96. doi: 10.1901/jeab.1964.7-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitzel LA, Dalsgaard PW, Muller IB, Cornett C. Identification of ten new designer drugs by GC-MS, UPLC-QTOF-MS, and NMR as part of a police investigation of a Danish Internet company. Drug Test Anal. 2011 doi: 10.1002/dta.358. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Ricaurte GA, Forno LS, Wilson MA, DeLanney LE, Irwin I, Molliver ME, Langston JW. (+/−)3,4-Methylenedioxymethamphetamine selectively damages central serotonergic neurons in nonhuman primates. JAMA. 1988;260:51–55. [PubMed] [Google Scholar]
- Rust KY, Baumgartner MR, Dally AM, Kraemer T. Prevalence of new psychoactive substances: a retrospective study in hair. Drug Test Anal. 2012 doi: 10.1002/dta.1338. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Sedefov R, Solberg U, Gallegos A, Almeida A. European Monitoring Centre for Drugs and Drug Addiction. Lisbon, Portugal: 2010. Europol–EMCDDA Joint Report on a New Psychoactive Substance: 4-methylmethcathinone (mephedrone) [Google Scholar]
- Sherwin CM. Voluntary wheel running: a review and novel interpretation. Anim. Behav. 1998;56:11–27. doi: 10.1006/anbe.1998.0836. [DOI] [PubMed] [Google Scholar]
- Spiller HA, Ryan ML, Weston RG, Jansen J. Clinical experience with and analytical confirmation of “bath salts” and “legal highs” (synthetic cathinones) in the United States. Clin. Toxicol. (Phila) 2011;49:499–505. doi: 10.3109/15563650.2011.590812. [DOI] [PubMed] [Google Scholar]
- Thornton SL, Gerona RR, Tomaszewski CA. Psychosis from a bath salt product containing flephedrone and MDPV with serum, urine, and product quantification. J. Med. Toxicol. 2012 doi: 10.1007/s13181-012-0232-4. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Huben SN, Lay CC, Crean RD, Davis SA, Katner SN, Taffe MA. Impact of ambient temperature on hyperthermia induced by (+/−)3,4-methylenedioxymethamphetamine in rhesus macaques. Neuropsychopharmacology. 2007;32:673–681. doi: 10.1038/sj.npp.1301078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker QD, Morris SE, Arrant AE, Nagel JM, Parylak S, Zhou G, Caster JM, Kuhn CM. Dopamine uptake inhibitors but not dopamine releasers induce greater increases in motor behavior and extracellular dopamine in adolescent rats than in adult male rats. J. Pharmacol. Exp. Ther. 2010;335:124–132. doi: 10.1124/jpet.110.167320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M, Talmon S, Schulze I, Boeddinghaus C, Gross G, Schoemaker H, Wicke KM. Running wheel activity is sensitive to acute treatment with selective inhibitors for either serotonin or norepinephrine reuptake. Psychopharmacology. 2009;203:753–762. doi: 10.1007/s00213-008-1420-4. [DOI] [PubMed] [Google Scholar]
- Winstock A, Mitcheson L, Ramsey J, Davies S, Puchnarewicz M, Marsden J. Mephedrone: use, subjective effects and health risks. Addiction. 2011a;106:1991–1996. doi: 10.1111/j.1360-0443.2011.03502.x. [DOI] [PubMed] [Google Scholar]
- Winstock AR, Mitcheson LR, Deluca P, Davey Z, Corazza O, Schifano F. Mephedrone, new kid for the chop? Addiction. 2011b;106:154–161. doi: 10.1111/j.1360-0443.2010.03130.x. [DOI] [PubMed] [Google Scholar]
- Wright MJ, Angrish D, Aarde SM, Creehan KM, Vandewater SA, Dickerson TJ, Taffe MA. College on Problems of Drug Dependence, Annual Meeting. Hollywood, FL: 2011. Effect of Rat Strain and Ambient Temperature on the Hypothermic and Locomotor Stimulant Effects of 4-methylmethcathinone (4-MMC) [Google Scholar]
- Yuan J, Hatzidimitriou G, Suthar P, Mueller M, McCann U, Ricaurte G. Relationship between temperature, dopaminergic neurotoxicity, and plasma drug concentrations in methamphetamine-treated squirrel monkeys. J. Pharmacol. Exp. Ther. 2006;316:1210–1218. doi: 10.1124/jpet.105.096503. [DOI] [PubMed] [Google Scholar]