Abstract
Aims
This study examined the feasibility and efficacy of behavioral incentives for reducing cigarette smoking among pregnant methadone-maintained patients.
Methods
Participants (N=102) were randomly assigned to: 1) contingent behavioral incentives (CBI: n=42); 2) non-contingent behavioral incentives (NCBI: n=28); or 3) treatment as usual (TAU: n=32). Baseline carbon monoxide (CO) levels were calculated for each participant. Subsequently, breath samples were tested three times weekly to measure changes in smoking behavior. CBI participants received incentives for target reductions from baseline: any reduction (week 1); 10% reduction (weeks 2-4), 25% reduction (weeks 5-7), 50% reduction (weeks 8-9), 75% reduction (week 10-11); abstinence (CO<4ppm) (week 12 until delivery). NCBI participants received incentives independent of smoking CO measurement results. TAU participants received no incentives, the standard treatment at the program.
Results
CBI condition participants submitted significantly lower mean CO values than the NCBI and TAU conditions over the course of the intervention (p<.0001). Nearly half (48%) of the CBI participants, met the 75% smoking reduction target and one third (31%) met the abstinence target at week 12. In contrast, none of the NCBI met either the 75% or abstinence target. Only 2% of the TAU participants met the 75% reduction and none of the TAU participants met the abstinence target. These smoking behavior reductions did not yield significant differences in birth outcomes.
Conclusions
Cigarette smoking may be significantly reduced among pregnant, methadone-maintained women through the use of contingent reinforcement for gradual reductions in breath carbon monoxide levels.
Introduction
An estimated 16.4% of women in the U.S. smoke cigarettes during pregnancy.1 Prenatal cigarette smoking is associated with increased rates of ectopic pregnancy, spontaneous abortion, placenta previa, placental abruption, premature rupture of membranes, preterm delivery, low birth weight, intrauterine growth restriction and Sudden Infant Death Syndrome. 2-8 It is estimated that prenatal cigarette smoking accounts for 10% of all neonatal deaths, 14% of preterm deliveries and 20-30% of low birth weight babies in the US annually. 9 Risks associated with prenatal cigarette smoking may be dose-related with higher smoking rates associated with decreases in gestational age, and increases in incidence of low birth weight, infant mortality and infant morbidity. 10-14
Cigarette Smoking among Pregnant, Drug-Dependent Women
Cigarette smoking among drug dependent pregnant women is alarmingly high, ranging from an estimated 77%- 99% of the population.15-19 Unfortunately, programs that treat pregnant patients for substance use disorders often fail to address cigarette smoking. Thus, a window of opportunity to impact prenatal smoking is lost. Smoking cessation interventions do not appear to adversely impact drug abuse treatment outcomes20-21, and may actually improve drug abstinence rates. 22-25 Simultaneous treatment of drug abuse and cigarette smoking is fitting because of the similarities between the addictive behaviors in initiation, maintenance and relapse. 26-27
Contingency Management (CM) for Treating Cigarette Smoking
CM is an effective intervention for cigarette smoking. 28-30 However, few studies have examined the use of CM for cigarette smoking during pregnancy. In a study of pregnant cigarette smokers, Higgins et al. 31 showed that end-of-pregnancy abstinence rates were higher for participants receiving contingent incentives (n=30) versus non-contingent incentives (n=23). In a later study, Higgins et al. 32 compared the cigarette smoking patterns and birth outcomes of contingent and non-contingent conditions in a sample of pregnant smokers from three controlled trials (N=266). Participants assigned to the contingent condition had significantly greater late pregnancy abstinence rates compared to the non-contingent condition participants. Additionally, infants born to the contingent incentive participants had higher mean birth weights and were less likely to be preterm than the infants born to the mothers in the non-contingent condition.
CM Methods
One particularly effective schedule of reinforcement for maintaining continuous abstinence is the escalating voucher schedule. 33-37 This schedule provides monetary vouchers that increase in value as abstinence continues. Relapse results in zero earnings and a return to the lowest value earned.
More recently, CM shaping procedures have been employed to intervene with populations who have not responded to other treatments. Shaping allows for intermediate behavioral targets for incentive delivery. 38 A series of studies support the efficacy of shaping procedures in reducing CO levels among non-treatment seeking cigarette smokers. 38-40 In a recent study, Lamb et al.38 randomized cigarette smokers identified as hard to treat (HTT n=96) versus easier to treat (ETT: n=50) to receive CM or CM shaping (CMS). The CM condition provided incentives for breath CO levels <4 ppm (i.e., smoking abstinence). The CMS condition participants received incentives for intermediate CO targets (lower than 7th lowest of the participant’s last 9 samples) or <4 ppm. Based on cluster analysis of smoking outcomes, 4 groups were identified: stable successes, improving, deteriorating, and poor outcomes. HTT participants were significantly more likely to belong to one of the unsuccessful clusters compared to ETT participants. However, HTT assigned to the CMS versus the CM condition were significantly more likely to belong to one of the successful clusters. These findings suggest that the shaping procedures are particularly effective for increasing smoking cessation efforts among HTT participants. Studies of CM shaping suggest that less demanding behavioral targets may be more effective than abstinence criteria in hard to treat populations.38, 41-42
CM among Pregnant Women with Substance use Disorders
No studies to date have compared the efficacy of CM shaping procedures among pregnant, drug-dependent cigarette smokers. The primary aim of the current study was to evaluate the feasibility and efficacy of a CBI shaping schedule compared to NCBI and TAU for reducing cigarette smoking in this population. A secondary aim was to examine birth outcomes among the three conditions. It was hypothesized that the CBI condition would be associated with higher smoking reduction rates and improved birth outcomes compared to the NCBI and the TAU conditions.
Methods
Participants and Setting
Figure 1. illustrates participant flow into the study. Participants (N=102) were patients who entered treatment at the Center for Addiction and Pregnancy (CAP) between 05/20/2005 to 01/09/2009. The study was approved by the Johns Hopkins Institutional Review Board (IRB). Individuals eligible for the study were: pregnant, age 18 or older, ≤30 weeks of gestation, nicotine dependent or smoked 10 or more cigarettes daily, and capable of providing informed consent.
Figure 1.
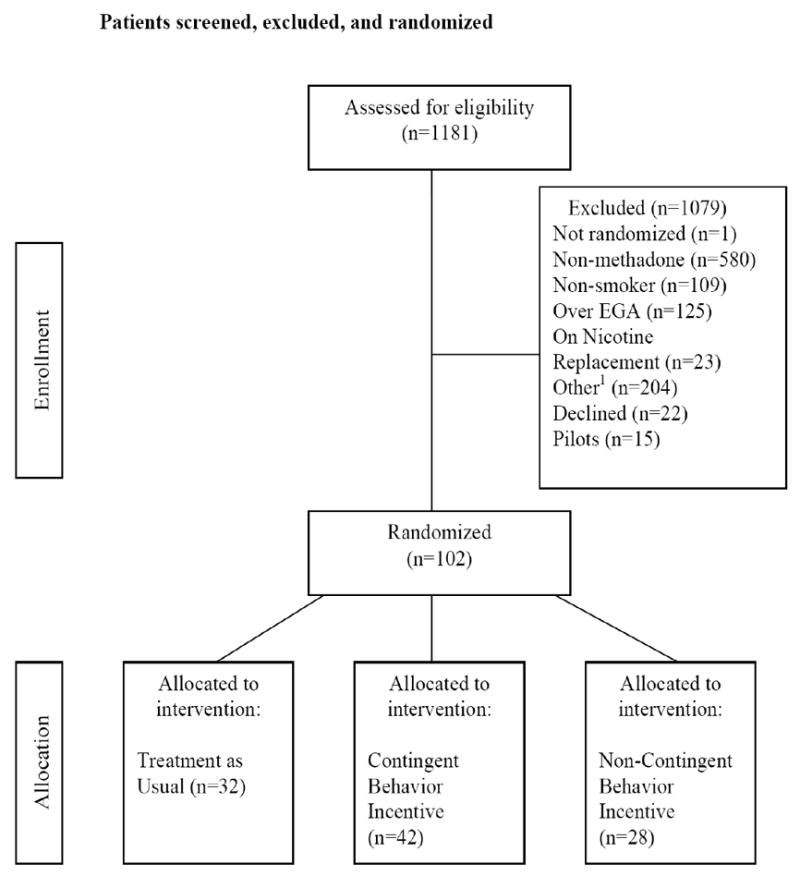
Number of Patients Screened, Excluded, and Randomized to the TAU, CBI, and NCBI Study Conditions
1Other exclusion reasons: Benzodiazepine or alcohol use (n=93); Live outside metro area (n=15); Pregnancy/medical issues (n=40); Psychiatric issues (n=38); In controlled environment (n=6); Under 18 years of age (n=1); Left treatment AMA (n=11)
Center for Addiction and Pregnancy (CAP)
CAP is a comprehensive drug and alcohol treatment program for pregnant women located on the Johns Hopkins Bayview Medical Campus (JHBMC) in Baltimore, MD. Obstetrical care, pediatric services, psychiatric treatment, and methadone medication, when warranted, are provided on site. 43-44 CAP patients first complete an initial 8 day residential stay on an assisted living unit (ALU), and then progress to outpatient treatment.
Study Procedures
Participants were randomly assigned to one of three conditions: 1) contingent behavioral incentives for cigarette smoking reductions (CBI: n=42); 2) behavioral incentives that were not contingent on cigarette smoking reduction (NCBI: n=28); or 3) treatment as usual (TAU: n=32). Participants who provided informed consent provided baseline biological samples and completed an initial assessment battery.
Baseline Biological Samples
Carbon Monoxide
A baseline carbon monoxide (CO) breath sample was collected from participants using a Smokerlyzer® monitor (Bedfont Scientifics, Inc.), which provides carbon monoxide levels from inhaled tobacco smoke in parts per million (ppm). This baseline CO level was used to evaluate changes in CO level during the inpatient treatment phase. A second baseline CO level was determined fro the outpatient treatment phase.
Urinalysis
A baseline urine sample was collected and tested for the metabolites of cocaine (cut-off: 300 ng/ml), opiates (cut-off 300 ng/ml), and cotinine (cut-off 200 ng/ml).
Baseline Assessments
Baseline assessments were typically completed within 7 days of program admission. A description of the assessments is provided below.
The Addiction Severity Index (ASI).45
The ASI is a semi-structured interview that assesses lifetime and past 30 day psychosocial variables in seven domains, including medical, drug and alcohol, employment/support, legal, family/social and psychiatric functioning. Quantity and frequency of cigarette smoking during the 30 days prior to CAP treatment enrollment also was assessed.
The Structured Clinical Interview for DSM-IV Disorders (SCID-I e-module). 46
The SCID-I (e-module) is a structured interview for determining lifetime and current DSM-IV substance abuse/dependence diagnoses.
Assessment training
ASI and SCID training and fidelity procedures for the research team are described elsewhere.47-48
Revised Fagerstrom Test for Nicotine Dependence (FTND).49
The FTND is a six-item questionnaire that assesses for current nicotine dependence in populations of cigarette smokers. Scores of 5 or more on the FTND were considered nicotine dependent for study inclusion purposes.
Weekly Specimen Collection
Carbon Monoxide testing
Breath samples were collected using the Smokerlyzer® CO monitor for all conditions three times per week on Monday, Wednesday, and Friday.
Urine testing
Observed urine samples were collected for all participants three times per week on Monday, Wednesday, and Friday for the nicotine metabolite, cotinine. Additionally, a once per week random urine specimen was tested for cocaine and opiates. All urine specimens were analyzed by FRIENDS, Inc. using enzyme immunoassay testing (cocaine cut-off: 300 ng/ml; opiate cut-off 300 ng/ml; cotinine cut-off 200 ng/ml). Samples testing above the specified cut-off values were also tested using gas chromatography-mass spectrometry (GCMS).
1 Month, 3 Month, and 6 Week Post-Partum Assessment
The ASI was conducted at 1 and 3 months after study enrollment, and at 6 weeks post-partum. At each assessment participants were asked to report the frequency (days) and quantity (daily amount) of cigarettes smoked during the past 30 days. Additionally, participants provided a breath sample for CO measurement and a urine specimen that was tested for cocaine, opiates, and cotinine.
Study Conditions
Study Procedures Consistent for All Conditions
All study conditions followed the same urine and breath specimen collection and assessment schedule. In addition, all conditions received a brief (10 minute) Motivational Interviewing style feedback session.
Contingent Behavioral Incentives (CBI)
In addition to the CAP usual treatment, CBI participants were eligible to earn incentives contingent upon cigarette smoking reduction or abstinence for a period of 12 weeks or until delivery. Smoking targets were minimal during the initial weeks of intervention and increased gradually to ensure adequate learning and reinforcement. Incentives could be earned for each sample left on Monday, Wednesday, and Friday (3 samples per week) if the following reduction and abstinence targets were met: week 1: any reduction; weeks 2-4: 10% reduction; weeks 5-7: 25% reduction; weeks 8-9: 50% reduction; week 10-11: 75% reduction; week 12 until delivery: abstinence (CO <4ppm). Participants had the opportunity to earn a $7.50 voucher for the first smoking reduction target, and the value of the voucher increased by $1/day for each consecutive target met throughout the 12 week incentive period to a maximum of $41.50. If a contingent participant failed to meet the tobacco use reduction target during the 12 week incentive period, she earned $0 for that sample and the incentive schedule was reset to the original voucher value of $7.50. If the participant again met the target reduction on five consecutive occasions, she earned vouchers at the previously attained level.
Separate baseline CO levels were calculated for the inpatient and outpatient phases of treatment.
Non-Contingent Behavioral Incentives (NCBI)
Prior to the study start, a pilot study was implemented to generate a pool of CBI schedules (n=15) (as described above) to which NCBI participants were yoked. These pilot participants were used to generate a yoking schedule for the NCBI condition only and thus were not included in the study. The procedures are best described as “pseudo-yoking,” as the CBI pilot schedule and the NCBI condition did not operate simultaneously (the pilot schedule was previously generated). Participants in the NCBI condition were yoked to a randomly selected individual in the pilot CBI condition who had generated a sufficient schedule for yoking (i.e., submitted CO samples for at least a two week period). Thus, incentives for this condition were not contingent on the NCBI participant’s own behavior, but rather a previously generated schedule of incentives. Participants were informed that they had the chance to earn vouchers but whether they earned a voucher and the amount they earned was determined by an already generated schedule and thus was not linked to their own cigarette smoking. NCBI participants were required to leave CO and urine samples to receive any voucher earnings generated by the yoked schedule. NCBI participants were eligible to receive yoked, non-contingent earnings for 12 weeks or until delivery.
Treatment as usual (TAU)
As part of standard CAP care, information regarding the frequency and quantity of cigarette smoking is gathered at the patient’s initial obstetrical appointment on the first day of treatment. Patients are provided specific information about the adverse effects associated with cigarette smoking for the mother and the infant. In addition, patients are provided with educational materials about risks of smoking during pregnancy. During follow-up obstetrical appointments, patients are routinely asked about their cigarette smoking and commended on efforts to abstain. TAU participants were informed that they would be compensated for providing urine and breath samples, but that they would not earn incentives as part of their study participation.
The CBI, NCBI, and TAU conditions did not differ on inpatient levels of CO or self-reported cigarette smoking. Although patients received incentives for any reduction from baseline during the inpatient phase of the intervention (week 1), smoking behaviors during this period were artificially altered due to the controlled environment of the ALU. Thus, the results presented herein are for the outpatient phase of treatment (weeks 2-12), during which behavior was not artificially subscribed due to programming requirements.
Outcomes Assessed and Data Analysis
Primary outcomes
The following primary outcomes were assessed for the study conditions: 1) mean CO values across the 12 week outpatient intervention period, 2) proportion of participants meeting behavioral smoking reduction/abstinence targets and 3) self-reported number of cigarettes smoked at 1 month, 3 months, and 6 weeks post-partum.
Secondary outcomes
The following secondary outcomes were assessed for the relevant the study conditions: 1) voucher earnings for the CBI and NCBI conditions, and 2) maternal and neonatal outcomes, including proportion of illicit-drug positive urine toxicology tests at delivery, proportion of low birth weight infants, proportion of preterm deliveries, mean infant birth weight, APGAR scores at 1 and 5 minutes, and length of hospital stay.
Data Analysis
Between study condition comparisons on single measurement variables (e.g., baseline, birth outcomes) were conducted with Chi-square tests for dichotomous variables and one-way analysis of variance for continuous variables. Continuous variables assessed repeatedly throughout the intervention were analyzed with a two-factor analysis of variance (Study Condition × Time) with an autoregressive covariance structure using SAS Proc Mixed.50 Significant main and interaction effects were further examined with Tukey post-hoc comparisons.
For dichotomous outcomes assessed repeatedly over the intervention were analyzed with an exchangeable correlation structure using General Estimating Equations (GEE).51 All analyses were conducted using SAS 9.1 for Windows with P value of less than 0.05 indicating statistical significance.
Results
Participant Characteristics
The CBI, NCBI, and TAU conditions did not significantly differ on demographic, pre-treatment or baseline cigarette smoking measures (see table 1). Participants were on average thirty years old, mostly Caucasian, single, unemployed, and had less than a high school education. Participants typically entered drug treatment during the second trimester of pregnancy. In the 30 days before treatment entry, participants averaged 29 days of cigarette smoking and reported smoking approximately a pack of cigarettes a day.
Table 1.
Demographics and Pre-treatment characteristics
| Measure | Total (N =102) | TAU (n =32) | CBI (n = 42) | NCBI (n=28) | Test Statistic χ2(df) or F(df, df) |
|---|---|---|---|---|---|
| (SD or % in parentheses) | |||||
| Demographics | |||||
| Mean age | 30.8 (6.0) | 30.0 (5.6) | 32.2 (6.4) | 29.8 (5.6) | F(2, 99)=1.850 |
| Race n (%) | |||||
| Caucasian | 66 (65.0) | 21 (65.6) | 23 (54.8) | 22 (78.6) | χ2(2)=4.188 |
| African-American / Other | 36 (35.0) | 11 (34.4) | 19 (45.2) | 6 (21.4) | |
| Mean estimated gestational age at entry | 16.6 (6.9) | 17.6 (7.4) | 16.9 (6.2) | 14.9 (7.3) | F(2, 99)=1.250 |
| Mean years of education | 11.1 (1.5) | 11.3 (1.5) | 11.2 (1.5) | 10.8 (1.5) | F(2, 92)=0.900 |
| Currently single n (%) | 81 (85.3) | 23 (76.7) | 33 (89.2) | 25 (89.3) | χ2(2)=2.579 |
| Unemployed n (%) | 90 (94.7) | 28 (93.3) | 35 (94.6) | 27 (96.4) | χ2(2)=0.281 |
| Mean ASI Composite Scores | |||||
| Medical | 0.242 (0.337) | 0.283 (0.375) | 0.221 (0.322) | 0.225 (0.321) | F(2, 92)=0.330 |
| Employment | 0.858 (0.203) | 0.865 (0.201) | 0.845 (0.222) | 0.868 (0.185) | F(2, 92)=0.120 |
| Alcohol | 0.009 (0.042) | 0.003 (0.011) | 0.018 (0.065) | 0.001 (0.007) | F(2, 92)=1.660 |
| Drug Use | 0.322 (0.116) | 0.325 (0.124) | 0.311 (0.104) | 0.334 (0.123) | F(2, 92)=0.300 |
| Legal | 0.196 (0.214) | 0.151 (0.177) | 0.191 (0.235) | 0.251 (0.215) | F(2, 92)=1.630 |
| Family/Social | 0.146 (0.208) | 0.172 (0.216) | 0.137 (0.208) | 0.129 (0.202) | F(2, 92)=0.350 |
| Psychiatric | 0.180 (0.211) | 0.190 (0.216) | 0.166 (0.204) | 0.189 (0.222) | F(2, 92)=0.140 |
| Nicotine Use | |||||
| Mean days of nicotine use (past 30 days) | 29.2 (4.7) | 29.1 (5.1) | 28.7 (5.9) | 30.0 (0.2) | F(2, 92)=0.650 |
| Mean Number of cigarettes smoked per day (past 30 days) | 18.0 (8.6) | 17.9 (7.4) | 17.1 (10.0) | 19.1 (7.9) | F(2, 91)=0.430 |
Participation and Smoking Reduction
The three conditions did not differ on the mean number of weeks of intervention participation (CBI= 12.5; SD±8.3), NCBI=12.6±8.6, TAU= 9.5±6.7). Baseline outpatient CO levels did not differ by study condition. However, as shown in figure 2, there were significant group differences in CO levels across the 11 week outpatient intervention (weeks 2-12) with the CBI condition submitting lower mean CO values than the NCBI and TAU conditions (F=18.050, p<.0001). Overall, the pattern of cigarette smoking fluctuated considerably in the TAU condition from week to week, remained fairly stable for the NCBI condition (with the exception week 9 decrease) and decreased over time in the CBI condition. The mean CO level for the CBI condition decreased from 12.1 at baseline to 4.0(±5.5) at the final study week. Additionally, the CO level for participants in the CBI condition at the final study week (4.0±5.5) was approximately half of the CO levels for the NCBI (8.7±2.8) and TAU (8.4±4.2) conditions. The conditions also differed on the measure of mean cotinine levels during the 11 week intervention. Specifically, the CBI condition had significantly lower mean cotinine values during the intervention than the TAU condition (M=1592 (1000) versus M=1938 (986), respectively; F=4.620, p=.009).
Figure 2.
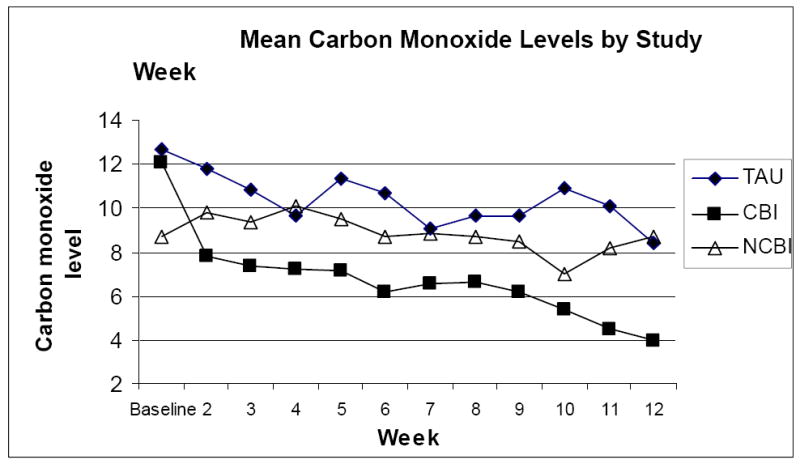
Mean Carbon Monoxide Levels for the TAU, CBI, and NCBI Conditions by Study Week
As can be seen in figure 3, a considerable portion of CBI participants met each of the behavioral targets. A full 48% of CBI participants met the 75% reduction target and 31% of the CBI participants met the abstinence target (cotinine <4ppm) at week 12. Only 2% of the TAU participants met the 75% reduction and none of the participants met the abstinence target. None of the NCBI participants met the 75% reduction or the abstinence targets.
Figure 3.
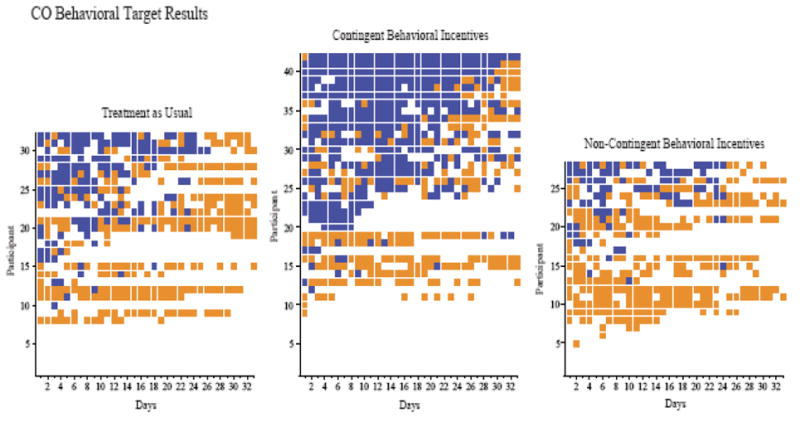
CO behavioral target results across consecutive days during intervention. Within each panel, rows of data represent the CO behavioral target result for each individual participant. Dark filled squares indicate CO targets met and light filled squares indicate CO targets missed. Missing samples are denoted by empty cells. Within each panel, participants are arranged from those showing the fewest targets met on the bottom to those with the most targets met on the top.
Voucher earnings from weeks 2-12 were not significantly different between the voucher conditions (CBI and NCBI). Total mean (±SEM) vouchers received were $156.85 ±30.7 (range: $0 – $736) and $96.98 ±25.18 (range: $0 – $384) in the CBI and NCBI conditions, respectively. NCBI participants had the potential to receive the same amount of incentives received by the CBI participants to whom they were yoked. The lower monetary values received by the NCBI participants reflect that these participants more frequently failed to show for study sessions and thus forfeited vouchers that were available to them.
Smoking at 1 Month, 3 Months and 6 Weeks Post-Partum
Consistent with the objective CO smoking measure, self-report of number of cigarettes smoked during the previous 24 hours significantly differed between the CBI and the TAU conditions (CBI=9.3±5.5 vs TAU=15.3±7.5, p<.0001). There were no significant differences in quantity of cigarettes smoked in the past 24 hours between the CBI and NCBI conditions. Self-reported number of cigarettes smoked also significantly differed between the CBI and TAU conditions at the 1 month (10.0±5.3 vs 17.0±7.6, p=.016) and 3 month assessment (8.7±5.6 vs 16.9±5.8, p=.008). However, there were no significant differences in quantity of cigarettes smoked between the CBI and NCBI conditions at 1 and 3 month assessment. CBI participants continued to report smoking fewer cigarettes compared to both the TAU and NCBI participants at six weeks post-partum, well after the intervention was withdrawn (10.7 cigarettes per day vs 14.0 & 17.8 respectively); however, these differences were no longer statistically significant. The conditions did not differ on the point prevalence measure of mean cotinine level at the 1 month, 3 months, or 6 week post-partum assessment.
Maternal and Neonatal Outcomes
Maternal and neonatal outcomes for a subset of the sample for whom delivery data were available (n=68) are depicted in table 1. The CBI condition had a lower proportion of babies born pre-term compared to the NCBI and the TAU conditions (17%, 25%, and 29%, respectively) and a lower proportion of babies born low birth weight (<2500 g) compared to the NCBI and TAU conditions (20%, 38% and 43% , respectively). These differences on two key infant outcome variables are clinically relevant and may be related to behavior changes, specifically smoking reductions in the CBI condition. However, these differences did not reach statistical significance. Since there were non-significant results for the primary birth outcomes of gestation at delivery and birth weight we conducted post hoc power analysis with power (1 - β) at 0.80, α = 0.05, and observed small effect size, Cohen’s f = 0.13. This analysis showed us that sample sizes would have to increase up to 280 per group for group differences to reach statistical significance at the .05 level (Cohen, 1988).
Discussion
There are several notable findings from this randomized trial examining the feasibility and efficacy of a CM intervention for shaping smoking reduction in drug-dependent pregnant women. First, this was a well-accepted intervention with high rates of participation among all three conditions. Second, the CM procedure demonstrated efficacy in a population not seeking to reduce or quit cigarette smoking. Nearly half of the participants in the CBI condition met the 75% smoking reduction target, and 31% were considered abstinent at some point during the study intervention. The reductions in CO for the CBI group versus TAU were also supported by participant self report for number of cigarettes smoked at the 1 and 3 month assessment visits. The CBI participants continued to self-report fewer daily cigarettes at the 6 week post-partum visit compared to TAU; however, the differences were no longer statistically significant.
Third, the reductions in cigarette smoking among the CBI condition appear to have some effect on birth outcomes, although the differences observed did not reach statistical significance. In a larger study employing CM with non-substance using pregnant cigarette smokers (N=266), Higgins et al.32 found significant differences for the proportion of low birth weight and proportion of pre-term deliveries for participants receiving CM versus control participants. It may be that the small sample size of the current study did not provide sufficient power to detect birth outcome differences. However, it could also be that the reductions in smoking started too late in the pregnancy (mid-second trimester) to have an effect on neonatal outcomes and/or that greater and more rapid smoking reductions are needed to improve birth outcomes. Future studies of shaping procedures in this population are needed to evaluate optimal smoking reduction targets to maximize the intervention’s effect on outcomes. There are several limitations to the study that should be noted. The study utilized carbon monoxide as a measure of smoking reduction and cessation rather than cotinine, which has a longer detection window. Though CO measurement provides for a shorter detection window than cotinine, we feel that CO testing was more consistent with the goal of the intervention, which was to provide of immediate reinforcement for reductions in cigarette smoking. It is well established that the immediacy of reinforcement of target behaviors is critical for effective incentive programs.52 Another study limitation the relatively brief intervention window. The CM intervention was available until delivery, and thus did not extend into the post-partum period when relapse frequently occurs. Optimally, CM interventions should start in the first trimester and extend into the critical post-partum period to sustain any gains made during the pregnancy.
There are several notable strengths of the study. First, the study was a randomized clinical trial, allowing for greater attribution of the observed outcomes to the CBI intervention. Second, the trial is the first to evaluate CM shaping procedures for drug-dependent pregnant cigarette smokers who are not seeking treatment for smoking cessation. Thus, the study provides valuable information on methods for shaping quit behavior in a hard to treat and population. Third, the study intervention employed may be feasible for community translation given the relatively low cost of the incentives and the use of low cost measurement of cigarette smoking.
The study’s findings indicate that the CM procedure is effective for shaping smoking reduction and cessation in drug-dependent pregnant women. It may be that shaping procedures are particularly effective in hard to treat populations that may have more difficulty making substantial behavior change.38 Further research is needed to evaluate the benefits of CM shaping interventions on key maternal and neonatal outcomes. The use of effective CM procedures pregnant drug dependent women may have particular importance given the potential for improved outcomes not only for the mother but the infant as well.
Table 2.
Descriptive Statistics for Maternal and Neonatal Outcomes
| Measure | Total (N = 68) | TAU (n = 21) | CBI (n = 30) | NCBI (n = 17) | Test Statistic χ2(df) or F(df, df) | p-value |
|---|---|---|---|---|---|---|
| (SD or % in parentheses) | ||||||
| Maternal Drug Treatment Outcomes | ||||||
| % Urinalysis Positive | 36.5% | 36.5% | 28.6% | 47.6% | F(2, 97)=2.920 | 0.058 |
| Perinatal and Delivery | ||||||
| % low birthweight (<2500 gm) | 31.3% | 42.9% | 20.0% | 37.5% | χ2(2)=3.369 | 0.186 |
| % pre-term delivery | 25.0% | 28.6% | 16.7% | 35.3% | χ2(2)=2.215 | 0.330 |
| % NICU admissions | 52.2% | 61.9% | 46.7% | 50.0% | χ2(2)=1.192 | 0.551 |
| % Spontaneous abortion | 7.1% | 10% | 3% | 10% | χ2(2)=1.687 | 0.430 |
| Length of maternal hospital stay | 3.7 (4.3) | 3.3 (2.3) | 4.2 (6.0) | 3.1 (1.3) | F(2, 64)=0.440 | 0.646 |
| Gestational age at delivery | 37.5 (3.2) | 37.8 (2.7) | 37.9 (3.6) | 37.0 (3.0) | F(2, 65)=0.510 | 0.601 |
| Urine toxicology tests positive for illicit drugs at delivery n (%) | 13 (19.0%) | 5 (23.8%) | 6 (19.4%) | 2 (12.5%) | χ2(2)=0.753 | 0.686 |
| Mean birthweight grams | 2772.5 (652.1) | 2701.3 (598.3) | 2863.3 (694.3) | 2695.6 (656.9) | F(2, 64)=0.520 | 0.597 |
| Mean APGAR 1 minute | 7.9 (1.6) | 7.9 (1.9) | 8.1 (1.1) | F(2, 62)=0.240 | 0.790 | |
| Mean APGAR 5 minutes | 8.6 (1.4) | 8.4 (1.3) | 8.7 (1.7) | 8.8 (0.6) | F(2, 62)=0.510 | 0.601 |
| Mean length of stay NICU | 5.3 (8.9) | 6.6 (11.0) | 4.0 (7.9) | 5.8 (8.0) | F(2, 64)=0.560 | 0.572 |
| Treated for neonatal abstinence syndrome (NAS) n (%) | 54 (81.0%) | 17 (81.0%) | 27 (87.1%) | 10 (66.7%) | χ2(2)=2.701 | 0.259 |
Acknowledgments
SUPPORT: This work was supported by NIDA grant R01DA12403
Footnotes
DECLARATION: The authors declare no conflict of interest.
References
- 1.Substance Abuse and Mental Health Services Administration. NSDUH Series H-36, HHS Publication No SMA 09-4434. Rockville: MDL Office of Applied Studies; 2009. Results from the 2008 National Survey on Drug Use and Health: National Findings. [Google Scholar]
- 2.O’Campo P, Davis MV, Gielen AC. Smoking cessation interventions for pregnant women: review and future directions. Seminars in Perinatology. 1993;19(4):279–85. doi: 10.1016/s0146-0005(05)80042-4. [DOI] [PubMed] [Google Scholar]
- 3.Williams MA, Mittendorf R, Stubblefield PG, Lieberman E, Schoenbaum SC, Monson RR. Cigarettes, coffee, and preterm premature rupture of the membranes. American Journal of Epidemiology. 1992;135(8):895–903. doi: 10.1093/oxfordjournals.aje.a116385. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong BG, McDonald AD, Sloan M. Cigarette, alcohol, and coffee consumption and spontaneous abortion. American Journal of Public Health. 1992;82(1):85–7. doi: 10.2105/ajph.82.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DiFranza JR, Lew RA. Effect of maternal cigarette smoking on pregnancy complications and sudden infant death syndrome. Journal of Family Practice. 1995;40(4):385–94. [PubMed] [Google Scholar]
- 6.Castles A, Adams EK, Melvin CL, Kelsch C, Boulton ML. Effects of smoking during pregnancy. Five meta-analyses. American Journal of Preventive Medicine. 1999;16(3):208–215. doi: 10.1016/s0749-3797(98)00089-0. [DOI] [PubMed] [Google Scholar]
- 7.Dolan-Mullen P, Ramirez G, Groff JY. A meta-analysis of randomised trials of prenatal smoking cessation interventions. American Journal of Obstetrics and Gynecology. 1994;171:1328–1334. doi: 10.1016/0002-9378(94)90156-2. [DOI] [PubMed] [Google Scholar]
- 8.England LJ, Kendrick JS, Wilson HG, Merritt RK, Gargiullo PM, Zahniser SC. Effects of smoking reduction during pregnancy on the birth weight of term infants. American Journal of Epidemiology. 2001;154:694–701. doi: 10.1093/aje/154.8.694. [DOI] [PubMed] [Google Scholar]
- 9.Floyd RL, Rimer BK, Giovino GA, Mullen PD, Sullivan SE. A review of smoking in pregnancy: Effects on pregnancy outcomes and cessation efforts. Annual Review of Public Health. 1993;14:379–411. doi: 10.1146/annurev.pu.14.050193.002115. [DOI] [PubMed] [Google Scholar]
- 10.Li CQ, Windsor RA, Perkins L, Goldenberg RL, Lowe JB. The impact on infant birth weight and gestational age of cotinine-validated smoking reduction during pregnancy. Journal of the American Medical Association. 1993;269(12):1519–24. [PubMed] [Google Scholar]
- 11.Bardy AH, Seppala T, Lillsunde P, Kataja JM, Koskela P, Pikkarainen J, Hiilesmaa VK. Objectively measured tobacco exposure during pregnancy: Neonatal effects and relation to maternal smoking. British Journal of Obstetrical Gynaecology. 1993;100:721–726. doi: 10.1111/j.1471-0528.1993.tb14262.x. [DOI] [PubMed] [Google Scholar]
- 12.Windsor RA, Orleans CT. Guidelines and methodological standards for smoking cessation intervention research among pregnant women: Improving the science and art. Health Education Quarterly. 1986;13(2):131–162. doi: 10.1177/109019818601300203. [DOI] [PubMed] [Google Scholar]
- 13.Kleinman J, Pierre M, Madans J, Land G, Schramm W. The effects of maternal smoking on fetal and infant mortality. American Journal of Epidemiology. 1988;127:274–282. doi: 10.1093/oxfordjournals.aje.a114803. [DOI] [PubMed] [Google Scholar]
- 14.Andres RL, Day MC. Perinatal complications associated with maternal tobacco use. Seminars in Neonatology. 2000;5(3):231–41. doi: 10.1053/siny.2000.0025. [DOI] [PubMed] [Google Scholar]
- 15.Haller DL, Knisely JS, Dawson KS, Schnoll SH. Perinatal substance abusers psychological and social characteristics. Journal of Nervous and Mental Diseases. 1993;181:509–513. doi: 10.1097/00005053-199308000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Svikis DS, Golden A, Huggins G, Pickens RW, McCaul ME, Velez M, Rosendale T, Brooner R, Gazaway P, Stitzer M, Ball C. Cost effectiveness of treatment for drug-abusing pregnant women. Drug and Alcohol Dependence. 1997;45:105–113. doi: 10.1016/s0376-8716(97)01352-5. [DOI] [PubMed] [Google Scholar]
- 17.Haug NA, Stitzer ML, Svikis DS. Smoking during pregnancy and intention to quit: A profile of methadone-maintained women. Nicotine and Tobacco Research. 2001;3:333–339. doi: 10.1080/14622200110050493. [DOI] [PubMed] [Google Scholar]
- 18.Tuten M, Jones HE, Svikis D. Comparing homeless and domiciled pregnant substance dependent women on psychosocial characteristics and treatment outcomes. Drug and Alcohol Dependence. 2003;69:95–99. doi: 10.1016/s0376-8716(02)00229-6. [DOI] [PubMed] [Google Scholar]
- 19.Jones HE, O’Grady K, Dahne J, Johnson R, Lemoine L, Milio L, Ordeans A, Shelby P. Management of acute postpartum pain in patients maintained on methadone or buprenorphine during pregnancy. American Journal of Drug and Alcohol Abuse. 2009;35(3):151–156. doi: 10.1080/00952990902825413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hughes JR, Kalman D. Do smokers with alcohol problems have more difficulty quitting? Drug & Alcohol Dependence. 2006;82:91–102. doi: 10.1016/j.drugalcdep.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 21.McCarthy WJ, Collins C, Hser YI. Does cigarette smoking affect drug abuse treatment? Journal of Drug Issues. 2002;32:61–80. [Google Scholar]
- 22.Lemon SC, Friedmann PD, Stein MD. The impact of smoking cessation on drug abuse treatment outcome. Addictive Behaviors. 2003;28(7):1323–1331. doi: 10.1016/s0306-4603(02)00259-9. [DOI] [PubMed] [Google Scholar]
- 23.Friend KB, Pagano M. Smoking cessation and alcohol consumption in individuals in treatment for alcohol use disorders. Journal of Addictive Diseases. 2005a;36(2):205–219. doi: 10.1300/J069v24n02_06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friend KB, Pagano ME. Changes in cigarette consumption and drinking outcomes: Findings from Project MATCH. Journal of Substance Abuse Treatment. 2005b;29:221–229. doi: 10.1016/j.jsat.2005.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prochaska JJ, Delucchi K, Hall SM. A meta-analysis of smoking cessation interventions with individuals in substance abuse treatment or recovery. Journal of Consulting and Clinical Psychology. 2004;72:1144–1156. doi: 10.1037/0022-006X.72.6.1144. [DOI] [PubMed] [Google Scholar]
- 26.Irving LM, Seidner AL, Burling TA, Thomas RG, Brenner GF. Drug and alcohol abuse inpatients’ attitudes about smoking cessation. Journal of Substance Abuse. 1994;6:267–278. doi: 10.1016/s0899-3289(94)90458-8. [DOI] [PubMed] [Google Scholar]
- 27.McCool RM, Richter KP. Why do so many drug users smoke? Journal of Substance of Substance Abuse Treatment. 2003;25:43–49. doi: 10.1016/s0740-5472(03)00065-5. [DOI] [PubMed] [Google Scholar]
- 28.Roll JM, Higgins ST, Badger GJ. An experimental comparison of three different schedules of reinforcement of drug abstinence using cigarette smoking as an exemplar. Journal of Applied Behavior Analysis. 1996;29:495–505. doi: 10.1901/jaba.1996.29-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roll JM, Higgins ST. A within-subject comparison of three different schedules of reinforcement of drug abstinence using cigarette smoking as an exemplar. Drug and Alcohol Dependence. 2000;58:103–109. doi: 10.1016/s0376-8716(99)00073-3. [DOI] [PubMed] [Google Scholar]
- 30.Alessi SM, Badger GJ, Higgins ST. An experimental examination of the initial weeks of abstinence in cigarette smokers. Experimental and Clinical Psychopharmacology. 2004;12(4):276–287. doi: 10.1037/1064-1297.12.4.276. [DOI] [PubMed] [Google Scholar]
- 31.Higgins ST, Sigmon SC, Wong CJ, Heil SH, Badger GJ, Donham R, Dantona RL, Anthony S. Community reinforcement therapy for cocaine-dependent outpatients. Archives of General Psychiatry. 2003;60:1043–1052. doi: 10.1001/archpsyc.60.9.1043. [DOI] [PubMed] [Google Scholar]
- 32.Higgins ST, Bernstein IM, Washio Y, Heil SH, Badger GJ, Skelly JM, Higgins TM, Solomon LJ. Effects of smoking cessation with voucher-based contingency management on birth outcomes. Addiction. 2010;105:2023–2030. doi: 10.1111/j.1360-0443.2010.03073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Higgins ST, Budney AJ, Bickel WK, Foerg FE, Donham R, Badger GJ. Incentives improve treatment retention and cocaine abstinence in ambulatory cocaine-dependent patients. Archives of General Psychiatry. 1994;51:568–576. doi: 10.1001/archpsyc.1994.03950070060011. [DOI] [PubMed] [Google Scholar]
- 34.McKay JR, Lynch KG, Coviello D, Morrison R, Cary MS, Skalina L, Plebani J. Randomized trial of continuing care enhancements for cocaine dependent patients following initial engagement. Journal of Consulting and Clinical Psychology. 2010;78(1):111–120. doi: 10.1037/a0018139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silverman K, Wong CJ, Higgins ST, Brooner RK, Montoya ID, Contoreggi C, Preston KL. Increasing opiate abstinence through voucher-based reinforcement therapy. Drug and Alcohol Dependence. 1996a;41:157–165. doi: 10.1016/0376-8716(96)01246-x. [DOI] [PubMed] [Google Scholar]
- 36.Silverman K, Higgins ST, Brooner RK, Montoya ID, Cone EJ, Schuster CR, Preston KL. Sustained cocaine abstinence in methadone maintained patients through voucher-based reinforcement therapy. Archives of General Psychiatry. 1996b;53:409–415. doi: 10.1001/archpsyc.1996.01830050045007. [DOI] [PubMed] [Google Scholar]
- 37.Roll JM, Shoptaw S. Contingency management for the treatment of methamphetamine abuse: Schedule effects. Psychiatric Research. 2006;144:91–93. doi: 10.1016/j.psychres.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 38.Lamb RJ, Kirby KC, Morral AR, Galbicka G, Iguchi MY. Shaping smoking cessation in hard-to-treat smokers. Journal of Consulting and Clinical Psychology. 2010;78(1):62–71. doi: 10.1037/a0018323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lamb RJ, Morral AR, Kirby KC, Iguchi MY, Galbicka G. Shaping smoking cessation using percentile schedules. Drug and Alcohol Dependence. 2004;76:247–259. doi: 10.1016/j.drugalcdep.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 40.Lamb RJ, Morral AR, Galbicka G, Kirby KC, Iguchi MY. Shaping reduced smoking in smokers without cessation plans. Experimental and Clinical Psychopharmacology. 2005;13:83–92. doi: 10.1037/1064-1297.13.2.83. [DOI] [PubMed] [Google Scholar]
- 41.Higgins ST, Silverman K, Heil SH, editors. Contingency management in substance abuse treatment. New York, NY: The Guilford Press; 2008. [Google Scholar]
- 42.Preston KL, Umbricht A, Wong CJ, Epstein DH. Shaping cocaine abstinence by successive approximation. Journal of Consulting and Clinical Psychology. 2001;69:643–654. doi: 10.1037//0022-006x.69.4.643. [DOI] [PubMed] [Google Scholar]
- 43.Jannson L, Svikis DS, Lee J, Paluzzi P, Rutigliano P, Hackerman F. Pregnancy and Addiction: a comprehensive care model. Journal of Substance Abuse Treatment. 1996;13:321–329. doi: 10.1016/s0740-5472(96)00070-0. [DOI] [PubMed] [Google Scholar]
- 44.Jansson LM, Choo RE, Harrow C, Velez M, Schroeder JR, Lowe R, Huestis MA. Concentrations of methadone in breast milk and plasma in the immediate perinatal period. Journal of Human Lactation. 2007;23:184–190. doi: 10.1177/0890334407300336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, et al. The fifth edition of the Addiction Severity Index. Journal of Substance Abuse Treatment. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- 46.First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis-I Disorders. New York, NY: State Psychiatric Institute: Biometrics Research; 1995. [Google Scholar]
- 47.Fitzsimons H, Tuten M, Jones HE. Mood disorders affect drug treatment success of pregnant women. Journal of Substance Abuse Treatment. 2007;32:19–25. doi: 10.1016/j.jsat.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 48.Jones HE, Wong CJ, Tuten M, Stitzer ML. Reinforcement-based therapy: 12 month evaluation of an outpatient drug-free treatment for heroin abusers. Drug and Alcohol Dependence. 2005;79:119–128. doi: 10.1016/j.drugalcdep.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 49.Heatherton T, Kozlowski L, Frecker R, Fagerström K. The Fagerstrom test of nicotine dependence: A revision of the Fagerstrom tolerance questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 50.Singer JD. Using SAS PROC MIXED to fit multilevel models, hierarchical models, and individual growth models. J Educ Behav Stat. 1998;24:323–355. [Google Scholar]
- 51.Zeger SL, Liang KY, Albert P. Models for longitudinal data, a generalized estimating equation approach. Biometrics. 1998;44:1049–1060. [PubMed] [Google Scholar]
- 52.Stitzer M, Petry N. Contingency management for treatment of substance abuse. Annual Review of Clinical Psychology. 2006;2:411–434. doi: 10.1146/annurev.clinpsy.2.022305.095219. [DOI] [PubMed] [Google Scholar]


