Abstract
Preeclampsia (PE) affects 5–8% of all pregnancies and is associated with significant maternal and fetal morbidity and mortality. Placental mitochondrial dysfunction has been reported in PE. MicroRNAs (miRNA) are small non-coding RNAs that regulate gene expression through mRNA degradation and translational repression. MiR-210 has been previously shown to be up regulated in placentas from pregnancies complicated by PE. We hypothesized that placental mitochondrial dysfunction during PE can be mediated by miR-210. Placentas were collected at term from normotensive pregnancies (CTRL) and those complicated by severe PE (n=6 each) following c-section (no labor). Villous tissue from PE showed significantly increased levels of HIF-1α compared to CTRL with no change in corresponding mRNA expression but with reduced DNA-binding activity. Mitochondrial complex III was significantly decreased in PE along with significantly reduced protein expression in complex I and IV during PE. Among the four miRNAs tested, miR-210 showed significant up regulation in PE and significant down regulation of its target, ISCU mRNA. To understand the role of miR-210 in PE, loss- and gain-of-function studies were performed using primary trophoblasts. Trophoblasts were transfected with miR-210 inhibitor or pre-miR-210 and mitochondrial function was measured using Seahorse Extracellular Flux Analyzer. Cells transfected with pre-miR-210 showed significant reduction in oxygen consumption. In contrast, transfection of trophoblast with AntagomiR-210 was sufficient to prevent the DFO-mediated respiratory deficiency. These data collectively suggest that miR-210 over expression during PE could be responsible for placental mitochondria dysfunction.
Keywords: placenta, mitochondria, miR-210, preeclampsia
Introduction
Preeclampsia (PE), a multi-system disorder that affects 5-8% of pregnancies worldwide (1), is manifest as maternal hypertension, proteinuria and edema and is associated with significant fetal and maternal morbidity and mortality. Severe PE is associated with fetal growth restriction, indicating placental dysfunction, and with preterm birth and perinatal death. Although the etiology of PE is unknown, the presence of the placenta is a prerequisite for the development of the syndrome. Current dogma is that preeclampsia is associated with inadequate trophoblast invasion, aberrant spiral arterial remodeling and decreased uteroplacental perfusion leading to a relative placental hypoxia/ischemia and release of mediators that damage the maternal endothelium (2, 3).
Placental development is profoundly influenced by oxygen tension (4). Human cytotrophoblast proliferate in vitro under low O2 conditions but differentiate at higher O2 levels, mimicking the developmental transition they undergo as they invade the placental bed to establish the maternal-fetal circulation in vivo (5). Hypoxia-inducible factor-1α (HIF) plays a role in the regulation of trophoblast function (6). HIF-1α is expressed in the villous cytotrophoblast and decreases with gestational age in normal pregnancies (7), however, increased stabilization of HIF-1α is observed in pregnancies complicated by preeclampsia (8). In addition to hypoxia, inflammatory cytokines, estrogen and reactive oxygen species (ROS) are involved in the stabilization of HIF-1α during normoxia in a number of tissue types including placenta (9).
Normal pregnancy is a state of oxidative stress, which is heightened in preeclampsia (10), with placental mitochondria, an important source of reactive oxygen species (ROS), contributing to generation of oxidative stress (11). Mitochondria are the major oxygen consuming organelles in the cell and play a crucial role in sensing the cellular oxygen concentration (12). When dysfunctional, mitochondria generate excessive amounts of ROS which may be involved in the triggering of preeclampsia (13).
Regulation of cell proliferation, mitochondrial metabolism, oxygen sensing and apoptosis in placenta has been recently found to be regulated by small (22 nucleotide) non-coding RNAs-microRNAs (miRNAs) (14). MiRNAs are highly conserved, regulatory molecules that play an important role in the post-transcriptional regulation of gene expression by promoting RNA instability or translational inhibition (15). Enquobahire et al.(16) have shown eight differentially expressed placental miRNAs in pregnancies complicated by preeclampsia with miR-34C, 139 and 328 being downregulated and miR-210 upregulated (16). Upregulation of placental miR-210 has been also described in preterm and severe preeclampsia (17-21). Zhang et al. found that elevated miR-210 during preeclampsia suppresses trophoblast cell migration and invasion by targeting Homeobox-A9 (HOXA9) and Erphin-A3 (EFNA3).(22). MiR-210 is known to be involved in the hypoxic response and has been shown to be over-expressed in a HIF-1α-dependent manner in many types of tumors. It is purported to be involved in the shift of tumor metabolism from oxidative phosphorylation to glycolysis (Warburg effect) (23). In addition a mechanistic link between miR-210, HIF-1α, mitochondria associated proteins, and mitochondrial function has been identified in cancer cells (24, 25). Based on these observations, we hypothesized that mitochondrial dysfunction seen in the placenta of pregnancies complicated by preeclampsia is mediated by overexpression of miR-210. We demonstrate here that exogenous overexpression of miR-210 in cultured trophoblasts represses mitochondrial respiration, whereas inhibition of miR-210 was able to protect the mitochondria from oxidative damage.
Materials and Methods (see supplemental data for detailed description of Materials and Methods)
Ethical Approval and Study Participants
Placentas were collected from the Labor and Delivery Unit at University Hospital under a protocol approved by the Institutional Review Board of the University of Texas Health Science Center at San Antonio, with informed consent from the patients. Placentas were collected immediately following delivery at term by cesarean section (with no labor) from uncomplicated pregnancies (CTRL) and from pregnancies complicated by severe preeclampsia (PE) (n=6 each group). Severe preeclampsia was defined as hypertension (systolic blood pressure >160 mmHg or diastolic >110 mmHg on two occasions at least 2 hours apart), and proteinuria (>3+ protein on dip stick) occurring after 20 weeks of gestation in a previously normotensive woman.
Tissue processing and sampling
A random sampling technique was used to collect tissue from 5 sites in the placenta. Villous tissue was dissected out from beneath the chorionic plate, avoiding the basal plate, flash frozen and stored at –80°C. Tissue was subsequently thawed, the 5 samples from each placenta combined and homogenized by mini bead beater (Biospec products, USA) in lysis buffer as described earlier (26). Total protein in the homogenates was estimated using Bradford's reagent (BioRad).
Isolation of Primary Trophoblasts
Primary trophoblast cells were isolated from placentas from normotensive women delivered by c-section at term in the absence of labor as described before (27). The trophoblast cells were transfected with premiR-210 for overexpression and antagomiR-210 (Applied Biosystems) for inhibition studies.
Statistical Analysis
Data are reported as mean ± SEM. Comparisons between two groups were performed with Student's t-test. One-way Analyses of Variance (ANOVA) with Tukey's post hoc test was used where appropriate. P value <0.05 was considered as significant.
Results
Clinical data
Clinical characteristics of the patient groups are shown in Table I. There were no significant differences in maternal age, gestational age, maternal body mass index (BMI) or fetal birth weight between groups (Table 1). All preeclamptic pregnancies were defined as severe according to related systolic BP≥160 or diastolic BP≥110 and proteinuria >3+ protein on dip stick.
Table I.
Clinical characteristics of study patients.
| Characteristics | Control (n=6) | Preeclampsia (n=6) | ||
|---|---|---|---|---|
| Mean ± SD | Range | Mean ± SD | Range | |
| Maternal Age at delivery (yrs) | 28.6±2.6 | 27-32 | 32.6±3.6 | (24 -38) |
| Body mass index (kg/m2) | 26.6± 4.9 | 21.9-36 | 30.1±7.5 | 22.2-43.5 |
| Gestational age (weeks) | 38.7±1.1 | 37.2-40.2 | 38.1±1.3 | 37-39.5 |
| BP systolic (mm Hg) | 114±9 | 103-126 | 159±9* | 143-192 |
| BP diastolic (mm Hg) | 71±8 | 61-79 | 99±3* | 92-116 |
| Fetal weight (grams) | 3342±328 | 2778-3629 | 2763±364 | 1300-3595 |
Values are mean ± SD.
P<0.05 vs. control group.
Activation of oxidative stress in placentas with preeclampsia
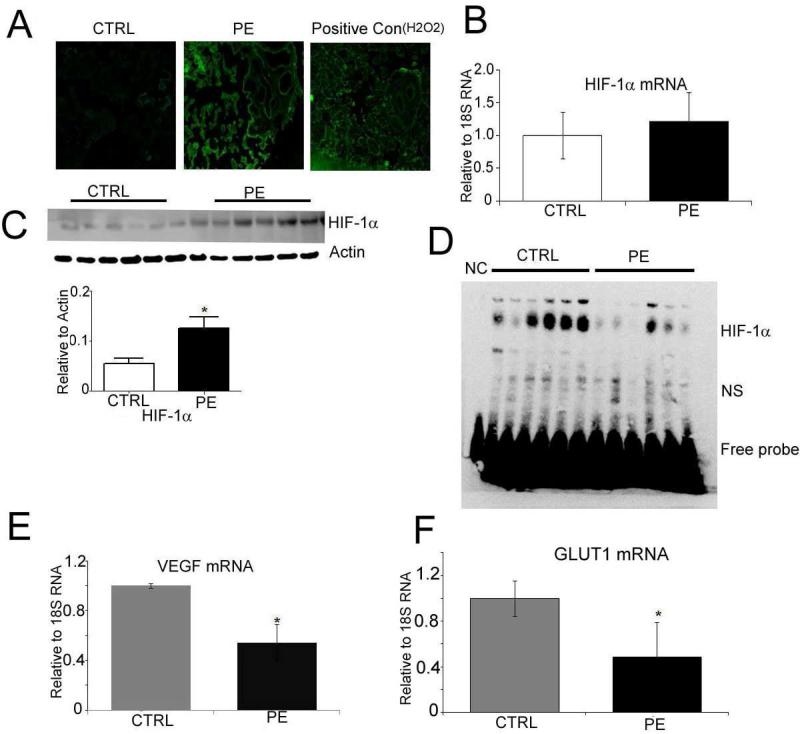
Production of ROS was significantly greater in PE placentas compared to CTRL as measured by DCF staining (Fig. 1A). While there was no significant difference in HIF-1α mRNA expression (Fig 1B), HIF-1α protein expression was 2.5-fold higher in PE compared to CTRL (p=0.01, Fig. 1C). Increased levels of HIF-1α in PE did not lead to a corresponding increase in DNA-binding activity. Electrophoretic mobility shift assay (EMSA, Fig. 1D) showed that HIF-1α binding to hypoxia-responsive element was lower in the PE group than in the CTRL. Consistent with EMSA data, the expression of VEGF and GLUT1 mRNA, downstream target genes of HIF-1α, was significantly lower in PE compared to CTRL group (Fig. 1E and 1F).
Fig. 1. Activation of oxidative stress in placentas with preeclampsia.
(A) DCF staining showing ROS production in cryosections of control and PE placentas. H2O2 treatment of control sections was used as positive control (B) mRNA expression of HIF-1α measured in placental villous tissue, normalized to 18SRNA (C) Western Blots showing HIF-1α expression in control and PE placentas. β-actin was used as the loading control. (D) EMSA showing HIF-1alpha DNA binding activity in control and PE placentas. NC-negative control (probe only without nuclear protein), NS, non-specific signal (E). mRNA expression of VEGF and (F) Glut1 (both measured in the villous placental villous tissue and normalized to 18SRNA). Values shown are mean± SEM, *P<0.05 vs. control, (n=6 each group).
Mitochondrial dysfunction in preeclampsia
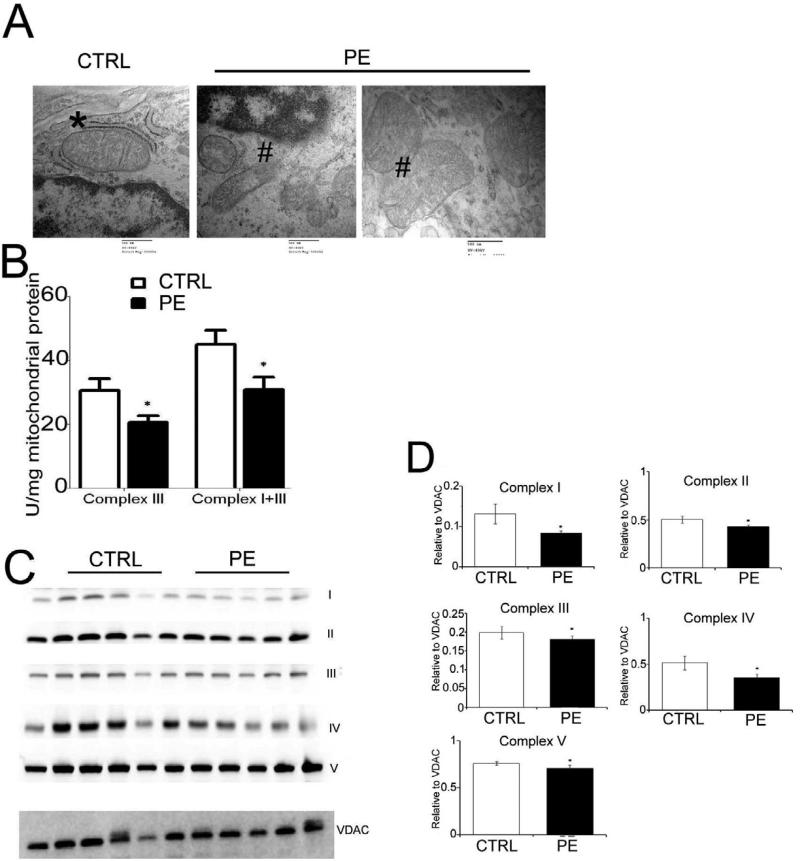
Transmission electron microscopy showed obvious morphological changes in the gross architecture of the mitochondria characterized by mitochondrial swelling and broken cristae (#) in placentas from pregnancies complicated by severe PE suggesting extensive mitochondrial damage compared to CTRL which show intact mitochondria ( *) (Fig. 2A). Measurement of combined Complex I+III activity revealed significantly lower activity in PE compared to CTR, however addition of rotenone (complex I inhibitor) revealed that the reduction was due to lower complex III activity (Fig. 2B). Western Blot for mitochondrial complexes showed a significant reduction in protein expression of complexes I and IV in PE compared to CTRL, with no difference in complexes II, III and V (Fig. 2C).
Fig. 2. Mitochondrial dysfunction in preeclampsia.
(A) Electron micrograph illustrating the morphological changes in the mitochondria of control and preeclamptic placenta (magnification × 50,000). # indicate mitochondrial swelling and broken cristae in placentas from PE and * showing intact mitochondria form CTRL placenta. (B) Mitochondrial complex activity measured calorimetrically in the isolated mitochondria from flash frozen villous tissue of control and preeclampsia. (C) Immunoblot showing expression of mitochondrial complex proteins. VDAC was used as loading control. (D) Band intensity of the mitochondrial complexes is normalized to the intensity of VDAC Values shown are mean± SEM, *p<0.05 vs. control, (n=6 each group).
Placental miR-210 and target expression
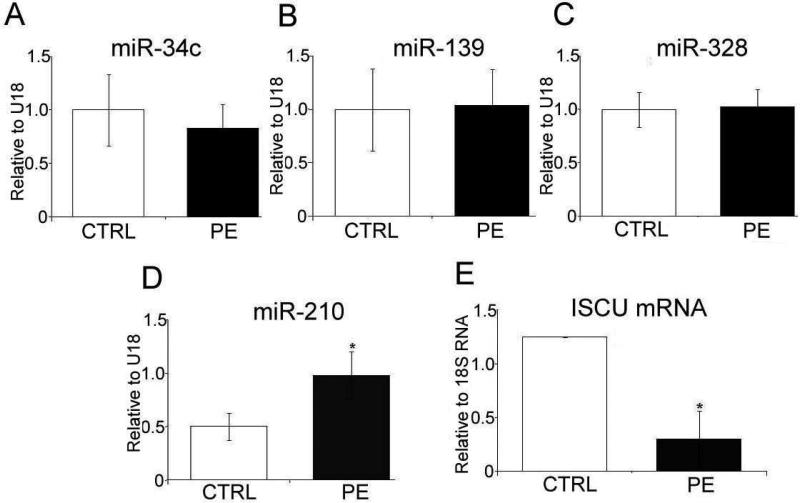
Expression of miR-34c, miR-129 and miR-328 has been previously shown to be altered in PE placentas (16). However, in our cohort there were no differences in their expression between CTRL and PE placentas (Fig. 3A, B, and C respectively). However, in agreement with previous studies, miR-210 showed a 2-fold increased expression in PE samples (p<0.05, Fig. 3D) compared to CTRL. Concomitantly, the mRNA expression of a miR-210 target protein, ISCU, was significantly reduced in preeclampsia compared to CTRL (Fig. 3E).
Fig. 3. Expression of miRs and target gene in villous tissue.
(A) Expression of miR-34C (B) miR139, (C) miR-328 (D) miR-210, normalized to U18. (D) mRNA expression of ISCU normalized to 18SRNA measured in the villous tissue of control pregnancies and those complicated by preeclampsia. Values shown are mean± SEM, *P<0.05 vs control, (n=6 each group)
Gain-of-function studies
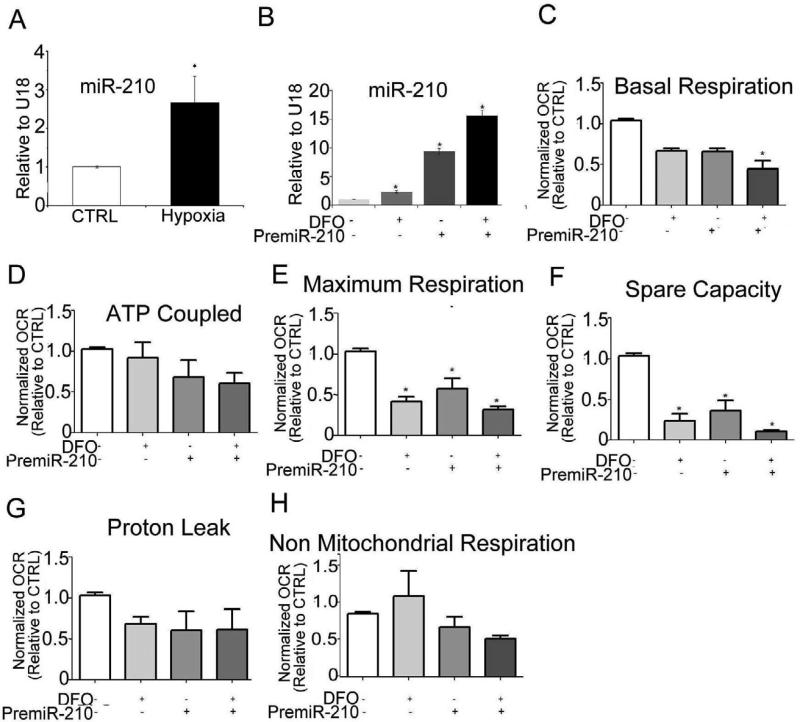
In order to define the role of miR-210 in placental mitochondrial function, we developed an in vitro system using isolated trophoblast. The expression of miR-210 was significantly up-regulated in syncytiotrophoblast as result of exposure to low levels of oxygen (3% O2 for 8h), suggesting that miR-210 expression is triggered by hypoxic stress (Fig. 4A). To explore the role of miR-210 in mitochondrial dysfunction, isolated trophoblast cells were transfected with pre-miR-210 (1nM) for 48h in the presence or absence of the iron chelator DFO (100μM for 24 hours), a known stabilizer of HIF-1α. MiR-210 expression was increased 8-fold in the pre-miR-210 transfected trophoblast cells and increased 12-fold after additional DFO treatment (Fig. 4B). MiR-210 transfection or DFO treatment did not alter either the syncytialization rate or viability of trophoblast cells (not shown). We also confirmed the levels of HIF-1α expression in the trophoblast cells subjected to either hypoxia or DFO by Western Blot (Supplemental Fig. 1A and B respectively). Subsequently we measured the respiration rates of cultured trophoblasts before and after transfection with premiR-210. The basal, maximum respiration and reserve respiratory capacity were significantly reduced in miR-210-overexpressing cells, and further reduced by additional exposure to DFO (Fig. 4C, E and F). No difference in ATP-coupled, proton leak and nonmitochondrial respiration was observed (Fig. 4D, G and H).
Fig. 4. miR-210 over expression inhibits trophoblast mitochondrial respiration.
(A) Isolated trophoblast cells from control placenta were subjected to hypoxia (3%O2 for 8h) and the expression of miR-210 was measured. (B) CTs were transfected with premiR-210 (1nM for 48h) and expression of miR-210 measured before and after exposure to DFO (100μm for 24h). CT values were normalized to U18 expression. The parameters calculated from XF24 measurements: (C) Basal respiration, (D) ATP generation, (E) Maximum respiration, (F) Spare capacity, (G) Proton leak and (H) non mitochondrial respiration in trophoblast cells. Relative OCR is expressed as the fold change with respect to the untreated control cells. Values are mean ± SEM from 8-10 technical replicates from n=3 experiments (separate placentas) performed on different days. *, p<0.05 vs. control.
Inhibition of miR-210 protects mitochondrial respiration
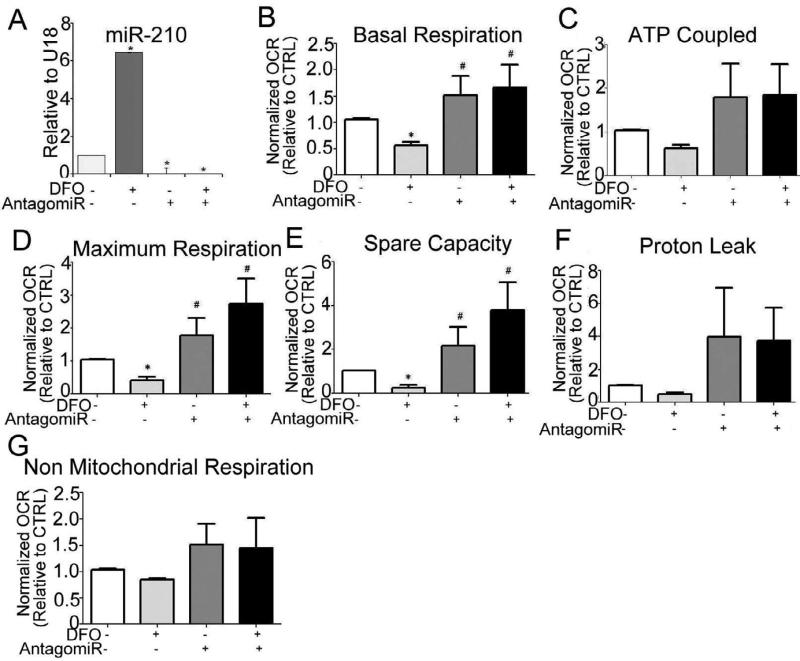
To investigate the role of miR-210 in mitochondrial dysfunction, trophoblast was transfected with a miR-210 inhibitor and cells were further challenged by exposure to a concentration of DFO (200 μM for 24 h), at which we observed significant reduction in respiration (not shown). As shown in Fig. 5A, the transfected cells showed 90% and 98% reduction in expression of miR-210 before and after exposure to DFO respectively when compared to untreated cells. Furthermore, trophoblasts transfected with miR-210 inhibitor showed an elevated basal respiration, which was maintained following DFO treatment (Fig. 5B). Protection of mitochondrial function was observed in miR-210 inhibitor transfected cells as evident by preservation of basal respiration (Fig. 5C), maximum respiration (Fig. 5E) and reserve capacity (Fig. 5F) following DFO treatment, suggesting that miR-210 is involved in mitochondrial dysfunction in trophoblasts under conditions of oxidative stress. Again, no significant difference in ATP coupled respiration (Fig. 5D), proton leak (Fig. 5G) or non-mitochondrial respiration (Fig. 5H) was observed between experimental groups.
Fig. 5. Inhibition of miR-210 reverses trophoblast mitochondrial respiration.
(A) miR-210 expression in trophoblast cells pre-transfected with antagomiR-210 (100nM for 48h) and treated with DFO (200μM for 24h). CT values were normalized to U18 expression. The parameters calculated from XF24 measurements: (B) Basal respiration (C) ATP coupled respiration. (D) Maximum respiration, (E) Spare capacity, (F) Proton leak, and (G) Non mitochondrial respiration. Relative OCR is expressed as the fold change with respect to the untreated control cells. Values are mean ± SEM from 8-10 technical replicates from n=3 experiments (separate placentas) performed on different days*, P<0.05 vs. control; #, p<0.05 vs. 200μM DFO.
Discussion
The etiology of preeclampsia, a serious pregnancy disorder, is still elusive with the only treatment being delivery of the placenta. The placenta is thought to contribute to appearance of the maternal syndrome via aberrant trophoblast invasion and spiral arterial remodeling leading to placental oxidative stress (2). However placental dysfunction can also compromise fetal growth and development. In this study, we used villous tissue from women with severe preeclampsia to be certain we had a well-defined phenotype. The primary findings of the study are: i) we showed mitochondrial dysfunction in placentas from pregnancies complicated by preeclampsia, which was associated with elevated ROS production and HIF1-α stabilization, ii) we confirmed the upregulation of miR-210 and downregulation of ISCU in PE placentas; and iii) we showed that increased miR-210 is necessary and sufficient for mitochondrial dysfunction in PE
Previous reports from our laboratory show elevated placental oxidative and nitrative stress during preeclampsia and significant alterations in antioxidant defenses (28). Consistent with these reports ROS levels (as evidenced by DCF staining) were significantly elevated in placenta from preeclamptic compared to normotensive pregnancies. Elevated ROS may partially account for the stabilization of otherwise short lived HIF-1α in placenta during preeclampsia (9). The highly conserved HIF family is a major player in the physiological response to chronic and acute hypoxia. In general, HIF-1 α binds to the consensus HRE and activates transcription of downstream targets such as VEGF and GLUT-1. Consistent with an existing report (6), we found increased placental HIF-1α protein levels with preeclampsia, but no change in HIF-1α mRNA indicating that HIF-1α protein is stabilized during preeclampsia. Previous studies have also described a possible defect in the oxygen sensing mechanism and consequently higher HIF-1 α protein during preeclampsia in placental villous tissue (29). Moreover, we observed low binding of HIF-1α to the HRE and thus significantly lower levels of VEGF and GLUT1 mRNA levels with preeclampsia compared to normotensive controls. Rajakumar et al found increased HIF-1α binding activity in term villous explants subjected to 1% hypoxic incubation but not with 2% hypoxic incubation (6). Because we do not know the exact oxygen tension in preeclamptic placenta we cannot compare our findings to these previous findings. Thus, our data indicate that HIF1α is elevated but not able to productively bind and stimulate the transcription of some genes involved in the adaptive response to hypoxia (e.g., VEGF) in preeclampsia. This lack of adaptation of placental cells to a hypoxic insult may lead to tissue damage underlying the pathology observed in preeclampsia.
Although the involvement of placental mitochondrial dysfunction in the pathogenesis of preeclampsia was first indicated by Torbergsen in a cohort of mitochondrial disorder patients (13) and since reinforced (30), the exact mechanism remains unclear. Mitochondria exist as dynamic networks and dramatic alterations in mitochondrial morphology during apoptotic cell death, fragmentation of the network and the remodeling of the cristea have been reported (31, 32). In our study, significant morphological abnormalities at the ultrastructural level, reduction in complex III activity and reduction in the protein expression of complexes I and IV were observed in the preeclamptic placentas. Reduction in complex activity suggests altered electron flow through complex III and perhaps damage to other complexes, which may contribute to an increase in ROS production. Future studies will characterize the scope and nature of the decreased activity in complex III in PE.
MiRNAs have been identified as essential mediators of numerous cellular processes (33). Interestingly, a number of hypoxia-related miRNAs (hypoxamirs) such as miR-23, miR-24, miR-26, miR-107 and miR-210 have been identified; most of them demonstrate modest induction under normoxic conditions but are significantly upregulated in hypoxia (34). MiR-210 expression is under direct control of HIF-1α and is downregulated in HIF-1α knockout cell lines (35). Many of these studies have identified miR-210 as a unique hypoxamir that is robustly induced in the hypoxic state in several tissues (36). MiR-210 is also reported to be involved in mitochondrial dysfunction in various types of cancer (37). MiR-210 delivery alone under normal oxygen conditions was sufficient to inhibit mitochondrial energy production, impair oxygen consumption (38), induce lactate accumulation (24, 25), alter mitochondrial membrane potential and disrupt mitochondrial structure in cancer cells (39). Increased miR-210 expression might be a potential mediator of mitochondrial dysfunction during preeclampsia. Microarray studies comparing normotensive and severe preeclamptic placentas have revealed several differentially expressed miRNAs (16-21). These findings strongly suggest the link between elevated miR-210 during preeclampsia and its role in mitochondrial function. Consistent with previous reports, we observed a 2-fold increase in the expression of miR-210 during preeclampsia. A good correlation was also noted between miR-210 expression and hypoxic markers in our cohort of patients, confirming that miR-210 expression was likely driven by hypoxia in vivo, as previously shown in other solid tumors.
Our findings appear particularly interesting in the light of the report published by Lee et al, that miR-210 targets ISCU in trophoblast cell lines (18). More interestingly Chan et al, has also showed that miR-210 targets the transcript coding for ISCU, which facilitate the assembly of iron–sulfur clusters that are incorporated into enzymes involved in energy production, including mitochondrial respiratory complexes and provided evidence that the lower levels of expression of ISCU mediated by miR-210 contribute to a decrease in the activity of the TCA cycle (through aconitase targeting) and the electron transport chain (through complex I destabilization) (38). ISCU are a prosthetic group that promotes electron transport and oxidation-reduction in mitochondria cluster assembly protein (40). 2Fe-S and 4Fe-S clusters are common and play a critical role in a wide range of cellular activities especially electron transfer in oxidative phosphorylation and catalysis of enzymatic reactions (41). Specifically, it was shown that mir-210 regulates the expression of ISCU, a key factor in the assembly of Fe-S cluster subunits of complexes I, II and III in the mitochondria of cancer cells (25). Consistent with this finding, we observed a reduction in placental ISCU mRNA expression during preeclampsia. Repression of ISCU by miR-210 could alter the stoichiometry and function of complexes in electron transport and oxygen consumption leading to an increase in toxic ROS production and decreased protein expression of complex I and activity of complex III as corroborated by our data. These findings strongly suggest the link between elevated miR-210 during preeclampsia and its role in mitochondrial dysfunction. However, further transcriptome studies are under way to define the miR-210 target proteins associated with mitochondrial function.
To explore the outcome of hypoxic conditions thought to be an underlying mechanism in preeclampsia and mitochondrial function, we utilized an in vitro system. Gain- and loss-of-function experiments with miR-210 were used for the in vitro manipulations. Using extracellular flux measurements, mitochondrial function of isolated trophoblast was determined by sequentially adding pharmacological inhibitors of the respiratory chain similar to previous approaches (42). Over expression of miR-210 caused a 50% reduction in maximum respiration and 60% reduction in reserve (spare) capacity of trophoblast cells. Furthermore, miR-210 inhibition rescued the mitochondrial respiration in the presence of DFO. When cells are subjected to stress, mitochondria are capable of drawing upon their reserve capacity to serve the increasing energy demands for maintenance of organ function, cellular repair or ROS detoxification (43). This suggests that mir-210 acts to regulate mitochondrial function during hypoxia to prevent the cells depleting their spare capacity in order to preserve ATP production. We speculate that short-term hypoxia treatment does not alter cellular ATP production, since we did not find a significant effect on the ATP coupled respiration. We can speculate that long-term hypoxia or continuous cycles of ischemia/reoxygenation will result in further depletion of mitochondrial spare capacity and an inability of the cells to maintain ATP production resulting in excessive ROS production and increase in mitochondrial damage.
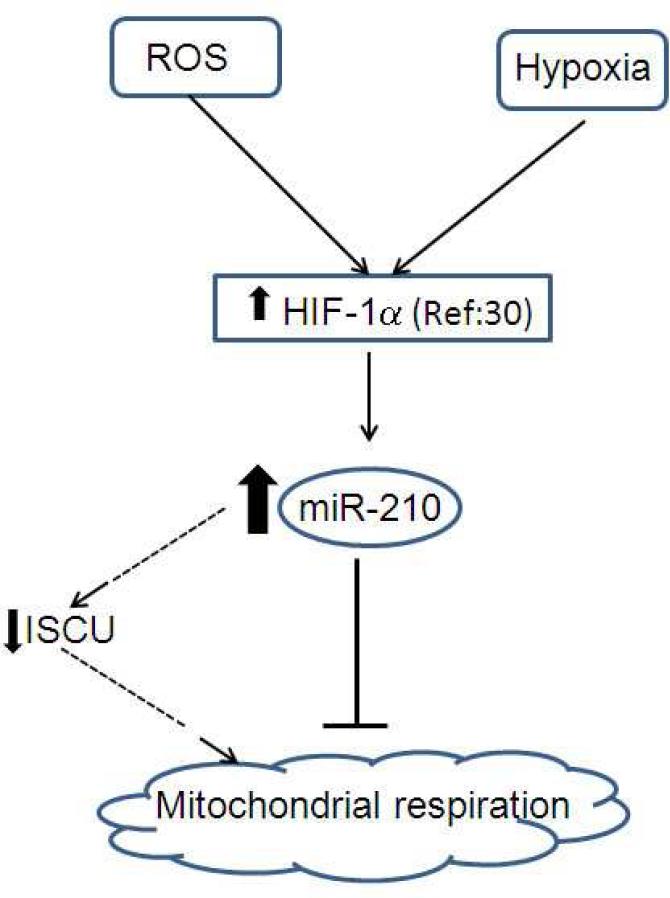
In our model, (Fig. 6) we propose that HIF-1α is stabilized by different co-factors of PE including elevated ROS, inflammation and relative hypoxia. As a result, miR-210 expression is upregulated in the placenta causing repression of mitochondria associated ISCU, potentially leading to mitochondrial dysfunction. In conclusion, based on our in vivo and in vitro functional studies, we suggest that miR-210 exerts a major influence on placental mitochondrial function during preeclampsia.
Fig. 6. Proposed model for the role of miR-210 in mitochondrial function during preeclampsia.
HIF-1α is stabilized by different factors including elevated ROS and relative hypoxia during preeclampsia. MiR-210 expression is up-regulated in placental villous tissue via HIF-1α. Increased miR-210 expression causes repression of mitochondria associated ISCU, thereby repressing mitochondrial respiration during preeclampsia. Respiratory deficiency and up regulation of ROS production in placental tissue might damage mitochondria during preeclampsia.
Supplementary Material
Acknowledgements
Authors are thankful to Elizabeth Dudley for the technical assistance. Funding sources NIH HL075297 (LM); CTSA grant (UL1RR025767) from Institute for Integration of Medicine and Science (IIMS) at UTHSCSA (AM).
Abbreviations
- CTRL
normotensive control
- DCF
Dicholoro Flourescine
- DFO
desferroxamine
- EMSA
Electrophoretic mobility shift assay
- FCCP
(p-trifluoromethoxy carbonyl cyanide phenyl hydrazone)
- GLUT1
glucose transporter 1
- HIF
hypoxia indicible factor
- ISCU
iron sulfur cluster
- miR
microRNA
- PE
preeclampsia
- VEGF
Vascular endothelial growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hogberg U. The World Health Report 2005: “make every mother and child count” - including Africans. Scand J Public Health. 2005;33(6):409–11. doi: 10.1080/14034940500217037. [DOI] [PubMed] [Google Scholar]
- 2.Myatt L. Role of placenta in preeclampsia. Endocrine. 2002 Oct;19(1):103–11. doi: 10.1385/ENDO:19:1:103. [DOI] [PubMed] [Google Scholar]
- 3.Rampersad R, Nelson DM. Trophoblast biology, responses to hypoxia and placental dysfunction in preeclampsia. Front Biosci. 2007;12:2447–56. doi: 10.2741/2246. [DOI] [PubMed] [Google Scholar]
- 4.Adelman DM, Gertsenstein M, Nagy A, Simon MC, Maltepe E. Placental cell fates are regulated in vivo by HIF-mediated hypoxia responses. Genes Dev. 2000 Dec 15;14(24):3191–203. doi: 10.1101/gad.853700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Genbacev O, Zhou Y, Ludlow JW, Fisher SJ. Regulation of human placental development by oxygen tension. Science. 1997 Sep 12;277(5332):1669–72. doi: 10.1126/science.277.5332.1669. [DOI] [PubMed] [Google Scholar]
- 6.Rajakumar A, Conrad KP. Expression, ontogeny, and regulation of hypoxia-inducible transcription factors in the human placenta. Biol Reprod. 2000 Aug;63(2):559–69. doi: 10.1095/biolreprod63.2.559. [DOI] [PubMed] [Google Scholar]
- 7.Caniggia I, Winter JL, Adriana and Luisa Castellucci Award lecture 2001 Hypoxia inducible factor-1: oxygen regulation of trophoblast differentiation in normal and pre-eclamptic pregnancies--a review. Placenta. 2002 Apr;23(Suppl A):S47–57. doi: 10.1053/plac.2002.0815. [DOI] [PubMed] [Google Scholar]
- 8.Rajakumar A, Brandon HM, Daftary A, Ness R, Conrad KP. Evidence for the functional activity of hypoxia-inducible transcription factors overexpressed in preeclamptic placentae. Placenta. 2004 Nov;25(10):763–9. doi: 10.1016/j.placenta.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 9.Patel J, Landers K, Mortimer RH, Richard K. Regulation of hypoxia inducible factors (HIF) in hypoxia and normoxia during placental development. Placenta. 2010 Nov;31(11):951–7. doi: 10.1016/j.placenta.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 10.Myatt L. Review: Reactive oxygen and nitrogen species and functional adaptation of the placenta. Placenta. 2010 Mar;31(Suppl):S66–9. doi: 10.1016/j.placenta.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y, Walsh SW. Increased superoxide generation is associated with decreased superoxide dismutase activity and mRNA expression in placental trophoblast cells in pre-eclampsia. Placenta. 2001 Feb-Mar;22(2-3):206–12. doi: 10.1053/plac.2000.0608. [DOI] [PubMed] [Google Scholar]
- 12.Van Houten B, Woshner V, Santos JH. Role of mitochondrial DNA in toxic responses to oxidative stress. DNA Repair (Amst) 2006 Feb 3;5(2):145–52. doi: 10.1016/j.dnarep.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 13.Torbergsen T, Oian P, Mathiesen E, Borud O. Pre-eclampsia--a mitochondrial disease? Acta Obstet Gynecol Scand. 1989;68(2):145–8. doi: 10.3109/00016348909009902. [DOI] [PubMed] [Google Scholar]
- 14.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009 Jan 23;136(2):215–33. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inui M, Martello G, Piccolo S. MicroRNA control of signal transduction. Nat Rev Mol Cell Biol. 2010 Apr;11(4):252–63. doi: 10.1038/nrm2868. [DOI] [PubMed] [Google Scholar]
- 16.Enquobahrie DA, Abetew DF, Sorensen TK, Willoughby D, Chidambaram K, Williams MA. Placental microRNA expression in pregnancies complicated by preeclampsia. Am J Obstet Gynecol. 2011 Feb;204(2):178, e12–21. doi: 10.1016/j.ajog.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishibashi O, Ohkuchi A, Ali MM, Kurashina R, Luo SS, Ishikawa T, et al. Hydroxysteroid (17-beta) Dehydrogenase 1 Is Dysregulated by Mir-210 and Mir-518c That Are Aberrantly Expressed in Preeclamptic Placentas: A Novel Marker for Predicting Preeclampsia. Hypertension. 2012 Feb;59(2):265–73. doi: 10.1161/HYPERTENSIONAHA.111.180232. [DOI] [PubMed] [Google Scholar]
- 18.Lee DC, Romero R, Kim JS, Tarca AL, Montenegro D, Pineles BL, et al. miR-210 targets iron-sulfur cluster scaffold homologue in human trophoblast cell lines: siderosis of interstitial trophoblasts as a novel pathology of preterm preeclampsia and small-for-gestational-age pregnancies. Am J Pathol. 2011 Aug;179(2):590–602. doi: 10.1016/j.ajpath.2011.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu C, Zhou Y, Zhang Z. MiR-210: an important player in the pathogenesis of preeclampsia? J Cell Mol Med. 2011 Jun 21; doi: 10.1111/j.1582-4934.2011.01370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pineles BL, Romero R, Montenegro D, Tarca AL, Han YM, Kim YM, et al. Distinct subsets of microRNAs are expressed differentially in the human placentas of patients with preeclampsia. Am J Obstet Gynecol. 2007 Mar;196(3):261, e1–6. doi: 10.1016/j.ajog.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 21.Zhu XM, Han T, Sargent IL, Yin GW, Yao YQ. Differential expression profile of microRNAs in human placentas from preeclamptic pregnancies vs normal pregnancies. Am J Obstet Gynecol. 2009 Jun;200(6):661, e1–7. doi: 10.1016/j.ajog.2008.12.045. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Fei M, Xue G, Zhou Q, Jia Y, Li L, et al. Elevated levels of hypoxia-inducible microRNA-210 in pre-eclampsia: new insights into molecular mechanisms for the disease. J Cell Mol Med. 2012 Feb;16(2):249–59. doi: 10.1111/j.1582-4934.2011.01291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang X, Ding L, Bennewith KL, Tong RT, Welford SM, Ang KK, et al. Hypoxia-inducible mir-210 regulates normoxic gene expression involved in tumor initiation. Mol Cell. 2009 Sep 24;35(6):856–67. doi: 10.1016/j.molcel.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Z, Li Y, Zhang H, Huang P, Luthra R. Hypoxia-regulated microRNA-210 modulates mitochondrial function and decreases ISCU and COX10 expression. Oncogene. 2010 Jul 29;29(30):4362–8. doi: 10.1038/onc.2010.193. [DOI] [PubMed] [Google Scholar]
- 25.Favaro E, Ramachandran A, McCormick R, Gee H, Blancher C, Crosby M, et al. MicroRNA-210 regulates mitochondrial free radical response to hypoxia and krebs cycle in cancer cells by targeting iron sulfur cluster protein ISCU. Plos One. 2010;5(4):e10345. doi: 10.1371/journal.pone.0010345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roberts VH, Smith J, McLea SA, Heizer AB, Richardson JL, Myatt L. Effect of increasing maternal body mass index on oxidative and nitrative stress in the human placenta. Placenta. 2009 Feb;30(2):169–75. doi: 10.1016/j.placenta.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bax CM, Ryder TA, Mobberley MA, Tyms AS, Taylor DL, Bloxam DL. Ultrastructural changes and immunocytochemical analysis of human placental trophoblast during short-term culture. Placenta. 1989 Mar-Apr;10(2):179–94. doi: 10.1016/0143-4004(89)90039-8. [DOI] [PubMed] [Google Scholar]
- 28.Myatt L, Eis AL, Brockman DE, Kossenjans W, Greer IA, Lyall F. Differential localization of superoxide dismutase isoforms in placental villous tissue of normotensive, pre-eclamptic, and intrauterine growth-restricted pregnancies. J Histochem Cytochem. 1997 Oct;45(10):1433–8. doi: 10.1177/002215549704501012. [DOI] [PubMed] [Google Scholar]
- 29.Rolfo A, Many A, Racano A, Tal R, Tagliaferro A, Ietta F, et al. Abnormalities in oxygen sensing define early and late onset preeclampsia as distinct pathologies. Plos One. 2010;5(10):e13288. doi: 10.1371/journal.pone.0013288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Widschwendter M, Schrocksnadel H, Mortl MG. Pre-eclampsia: a disorder of placental mitochondria? Mol Med Today. 1998 Jul;4(7):286–91. doi: 10.1016/s1357-4310(98)01293-3. [DOI] [PubMed] [Google Scholar]
- 31.Bereiter-Hahn J, Voth M. Dynamics of mitochondria in living cells: shape changes, dislocations, fusion, and fission of mitochondria. Microsc Res Tech. 1994 Feb 15;27(3):198–219. doi: 10.1002/jemt.1070270303. [DOI] [PubMed] [Google Scholar]
- 32.Karbowski M, Youle RJ. Dynamics of mitochondrial morphology in healthy cells and during apoptosis. Cell Death Differ. 2003 Aug;10(8):870–80. doi: 10.1038/sj.cdd.4401260. [DOI] [PubMed] [Google Scholar]
- 33.Ivan M, Harris AL, Martelli F, Kulshreshtha R. Hypoxia response and microRNAs: no longer two separate worlds. J Cell Mol Med. 2008 Sep-Oct;12(5A):1426–31. doi: 10.1111/j.1582-4934.2008.00398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kulshreshtha R, Ferracin M, Wojcik SE, Garzon R, Alder H, Agosto-Perez FJ, et al. A microRNA signature of hypoxia. Mol Cell Biol. 2007 Mar;27(5):1859–67. doi: 10.1128/MCB.01395-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakada C, Tsukamoto Y, Matsuura K, Nguyen TL, Hijiya N, Uchida T, et al. Overexpression of miR-210, a downstream target of HIF1alpha, causes centrosome amplification in renal carcinoma cells. J Pathol. 2011 Jun;224(2):280–8. doi: 10.1002/path.2860. [DOI] [PubMed] [Google Scholar]
- 36.Chan YC, Banerjee J, Choi SY, Sen CK. miR-210: the master hypoxamir. Microcirculation. 2011 Dec 15; doi: 10.1111/j.1549-8719.2011.00154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Devlin C, Greco S, Martelli F, Ivan M. miR-210: More than a silent player in hypoxia. Iubmb Life. 2011 Feb;63(2):94–100. doi: 10.1002/iub.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chan SY, Zhang YY, Hemann C, Mahoney CE, Zweier JL, Loscalzo J. MicroRNA-210 controls mitochondrial metabolism during hypoxia by repressing the iron-sulfur cluster assembly proteins ISCU1/2. Cell Metab. 2009 Oct;10(4):273–84. doi: 10.1016/j.cmet.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Puissegur MP, Mazure NM, Bertero T, Pradelli L, Grosso S, Robbe-Sermesant K, et al. miR-210 is overexpressed in late stages of lung cancer and mediates mitochondrial alterations associated with modulation of HIF-1 activity. Cell Death Differ. 2011 Mar;18(3):465–78. doi: 10.1038/cdd.2010.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harner M, Korner C, Walther D, Mokranjac D, Kaesmacher J, Welsch U, et al. The mitochondrial contact site complex, a determinant of mitochondrial architecture. EMBO J. 2011 Nov 2;30(21):4356–70. doi: 10.1038/emboj.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rustin P, Munnich A, Rotig A. Succinate dehydrogenase and human diseases: new insights into a well-known enzyme. Eur J Hum Genet. 2002 May;10(5):289–91. doi: 10.1038/sj.ejhg.5200793. [DOI] [PubMed] [Google Scholar]
- 42.Moran M, Rivera H, Sanchez-Arago M, Blazquez A, Merinero B, Ugalde C, et al. Mitochondrial bioenergetics and dynamics interplay in complex I-deficient fibroblasts. Biochim Biophys Acta. 2010 May;1802(5):443–53. doi: 10.1016/j.bbadis.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 43.Dranka BP, Hill BG, Darley-Usmar VM. Mitochondrial reserve capacity in endothelial cells: The impact of nitric oxide and reactive oxygen species. Free Radic Biol Med. 2010 Apr 1;48(7):905–14. doi: 10.1016/j.freeradbiomed.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.