Abstract
The contribution of T cells and graft-reactive antibodies to acute allograft rejection is widely accepted but the role of graft-infiltrating B and plasma cells is controversial. We examined 56 consecutive human renal transplant biopsies classified by Banff schema into T cell-mediated (N=21), antibody-mediated (N=18) and mixed (N=17) acute rejection, using standard immunohistochemistry for CD3, CD20, CD138, and CD45. In a predominantly African-American population (75%), neither Banff classification nor C4d deposition predicted the return to dialysis. Immunohistochemical analysis revealed CD3+ T cells as the dominant cell type, followed by CD20+ B cells and CD138+ plasma cells in all acute rejection types. Using univariate Cox Proportional Hazard analysis, plasma cell density significantly predicted graft failure while B cell density trended towards significance. Surprisingly T cell density did not predict graft failure. The estimated glomerular filtration rate (eGFR) at diagnosis of acute rejection also predicted graft failure, while baseline eGFR ≥6 months prior to biopsy did not. Using multivariate analysis, a model including eGFR at biopsy and plasma cell density was most predictive of graft loss. These observations suggest that plasma cells may be a critical mediator and/or an independently sensitive marker of steroid-resistant acute rejection.
Keywords: B cells, plasma cells, allograft rejection, immunohistochemistry
Introduction
The success of standard immunosuppressive regimens that have been primarily directly at T cell-mediated responses (1-5) has decreased incidence of T cell-mediated acute rejection (TCMR) but has resulted the emergence of donor-specific alloantibodies (DSA) as mediators of acute and chronic allograft rejection (6, 7). The staining for complement 4d (C4d), a degradation product of C4b indicating activation of the classical complement pathway and subsequent deposition on the allograft endothelium, allows for easier diagnosis of antibody mediated rejection (AMR) and was first incorporated into the Banff classification in 2003 (8, 9). C4d-positive kidney biopsies have been associated with poor outcome and a worse prognosis when compared to pure TCMR (10-13). Subsequently, the diagnosis of AMR has led to an increased utilization of immunosuppression therapies that target alloantibody production and removal, including plasmapheresis, intravenous immunoglobulin (IVIG) and the humanized, chimeric monoclonal anti-CD20 antibody, rituximab (14-18).
Recent studies have questioned the value of C4d staining for predicting allograft outcome (10, 19-23), and alternative approaches have been developed to probe the contribution of B cells and antibodies to acute allograft rejection. Sarwal et al. observed that the presence of dense CD20+ B cell clustersin the absence of C4d in acutely rejecting renal allografts correlated with glucocorticoid resistance and accelerated graft failure (24). However, subsequent studies into the role of intra-graft CD20+ B cells in acute renal allograft rejection have yielded conflicting results (25-30). Proposed reasons for these differences include the heterogeneity of the patient populations and immunosuppression regimens, timing of the allograft biopsy, the lack of consensus regarding the definition of ‘CD20+’, and variability in the intra-graft B cell population during acute rejection (reviewed by Zarkhin et al (31)). The aim of this study is to quantify the cellular composition of renal allograft biopsies identified as having acute rejection and to test the hypothesis that B cells and plasma cells independently predict both short- and long-term clinical outcomes.
Materials and Methods
Consecutive human adult renal allograft biopsies with the diagnosis of acute rejection were identified in the University of Chicago Medical Center pathology archives between 2005 and 2009. We examined 56 renal transplant biopsies classified by Banff schema into cell-mediated (N=21), antibody-mediated (N=18) and mixed (N=17) acute rejection. A control group of consecutive biopsies taken for cause (n=33) without evidence of rejection was also examined; these were classified as normal (N=8), acute tubular injury (N=8), interstitial fibrosis and tubular atrophy (N=15) or calcineurin inhibitor toxicity (N=2). Only biopsies with adequate tissue (at least 6 glomeruli and at least 5 mm in length) were studied. No isolated v (intimal arteritis) or borderline lesions were included in the study. Each biopsy was evaluated by standard light microscopy using hematoxylin & eosin and periodic acid-Schiff stains and by indirect immunofluorescence microscopy for C4d (Biogenesis, Sandown, NH). Biopsy interpretation was conducted by a renal pathologist (AC or SMM) and diagnoses were rendered according to 2009 Banff criteria (32). All patient charts were reviewed and relevant clinical data including the presence or absence of DSA was collected when available. Estimated glomerular filtration rate was calculated by the 4-variable Modification of Diet in Renal Disease Equation (33, 34). This study was approved by the University of Chicago Medical Center institutional review board.
Immunohistochemistry
Using standard immunohistochemical methods, sequential 2 μm-thick tissue sections from each biopsy were stained for CD3, CD20, CD138, and CD45, The number of interstitial cells showing strong circumferential membranous staining was counted manually by a single pathologist (JMM or AC) without prior knowledge of the clinical or pathologic data. CD138+ epithelial cells were identifiable and were excluded. The width of every biopsy tissue core was 1 mm and the length was measured to calculate cell densities per square millimeter.
Statistical Analysis
Continuous variables with normal distribution were analyzed by unpaired, two-tailed Student's test or ANOVA and Bonferroni post-hoc test, with p<0.05 indicative of significant differences. Wilcoxon rank-sum or Mann-Whitney test was used for data with skewed distribution, and chi-square or Kruskal-Wallis equality-of-populations rank test was used for categorical variables. Graft survival was analyzed with the Kaplan-Meier and Cox Proportional Hazards models. Statistical analyses were performed with STATA v. 11 (Statacorp, College Station TX) and GraphPad Prism software, version 4 (GraphPad Software, Inc., San Diego, CA)
Results
Clinical Patient Data and Kidney Allograft Biopsy Pathology
The demographic data of patients from both the study and kidney transplant center are summarized in Table 1. Gender and underlying cause of end-stage renal disease in both acute rejection and control (indication biopsies without acute rejection) populations were comparable, but patients with acute rejection were significantly younger than controls (39.3 vs. 51.9 years; p<0.001). The acute rejection group was significantly over-represented with African-Americans (75%) compared to the control group (44%) and overall transplant population (52%). The mean time from transplant to biopsy, also referred to as the post-transplant interval, was comparable for both groups (949 ± 1570 days for the acute rejection, and 1101 ± 1678 days for the control group, p-value = 0.9), while the overall follow-up time from transplantation was significantly shorter for the acute rejection group (1517 ± 1601 days) compared to the control group (2310 ± 1701 days) (p=0.0008). The TCMR biopsies were categorized as Banff type IA (N=5), type IB (N=9), and type IB+IIA (N=7). The mixed rejection biopsies were categorized as Banff type IA+C4d-positive (N=5), type IA+IIB+C4d-positive (N=1), type IB+C4d-positive (N=9), and type IB+IIA+C4d-positive (N=2). Not surprising, the majority of patients diagnosed with AMR and mixed rejection were PRA+ and DSA+ while a lower frequency of those diagnosed with TCMR were DSA+ (Supplemental Table 1). Interstitial fibrosis (ci score) and tubular atrophy (ct score) in the rejection and control groups were comparable (p-value = 0.5 and 0.4, respectively). DSA and PRA
Table 1.
Patient Demographics and Clinical and Pathologic Data
| Acute Rej (n=56) | Control (n=33) | UC Tx (n=530) | p-value Rej vs. Total | p-value Rej vs. Ctrl | |
|---|---|---|---|---|---|
| Age | 39.3 ±13.9 | 51.6 ± 12.5 | 47.74 ± 15.1 | <0.0001 | <0.0001*** |
| Gender | |||||
| Male | 62.5% | 66.7% | 62.0% | 1.0 | 0.1* |
| Female | 37.5% | 33.3% | 38.0% | ||
| Race† | |||||
| African American | 75.0% | 44.0% | 51.6% | 0.006 | 0.001* |
| Caucasian | 19.6% | 48.0% | 31.7% | ||
| Hispanic | 5.4% | 8.0% | 13.8% | ||
| Diagnosis† | |||||
| Hypertension | 39.2% | 43.3% | 30.6% | 0.006 | 0.8* |
| Diabetes | 23.2% | 36.6% | 33.2% | 0.3 | 0.2* |
| FSGS | 10.7% | 6.7% | 6.4% | 0.4 | 0.3* |
| Lupus | 5.3% | 0% | 3.6% | 0.2 | 0.7* |
| Others | 32.1% | 33.3% | 27.0% | 0.3 | 1.0* |
| Follow-up Days | Mean ± SD (Median,) | ||||
|---|---|---|---|---|---|
| From Tx | 1517 ±1601 (1139) | 2370 ±1701 (1559) | 0.052** | ||
| From Tx to Bx | 949 ±1570 (366) | 1101 ±1678 (322) | 0.9** | ||
| From Bx | 568 ± 477 (440) | 1269 ±251 (1261) | <0.0001** | ||
| Mean ± SD | |||||
|---|---|---|---|---|---|
| Acute Rej | Control | ||||
| Interstitial fibrosis (ci score) | 1.2 ± 0.78 | 1.13 ± 0.18 | 0.5*** | ||
| Tubular atrophy (ct score) | 1.2 ± 0.82 | 1.13 ±0.18 | 0 4*** | ||
| GFR Pre-Bx | 51.7 ± 25.3 (49.0) | 48.4 ± 3.3 (46.0) | 0 4**** | ||
| GFR at Bx | 24.2 ± 12.6 (22.1) | 39.3 ± 3.7 (35.0) | 0.002**** | ||
| GFR at 4 weeks post-Bx | 31.4 ± 16.5 (31.0) | 40.0 ± 4.7 (42.0) | 0 1**** | ||
chi-square test.
Wilcoxon rank-sum test.
Mann-Whitney test.
2-tailed t-test.
For the acute rejection group, n=53 for this variable.
Abbreviations: Tx: transplant; Rej: rejection; Bx: biopsy; FSGS: focal segmental glomerulosclerosis; UC Tx: Total University of Chicago Transplants from 2004-2009.
Baseline eGFR, determined approximately 6 months prior to biopsy, were comparable in both rejection and control groups (51.7 vs. 48.4 ml/min) (p=0.5), but the mean eGFR at the time of biopsy and at 4 weeks post-biopsy were significantly lower for the acute rejecting group (Table 1). All patients received high dose corticosteroids to treat acute rejection. In addition, thymoglobulin was used predominantly to treat the majority TCMR (63.2%) while B cell directed therapies of IVIG, plasmapheresis and/or rituximab were used to treat the majority of mixed rejection (70.6%) and AMR (80%) (Supplemental Table 2).
Banff classification or C4d deposition were not significant predictors of allograft survival
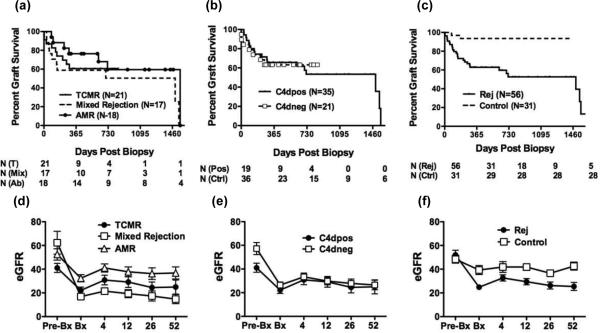
With a mean follow-up of 568 ± 477 days from biopsy to the end of study, Kaplan-Meier analysis for the return to dialysis was performed on biopsies categorized by either Banff classification or C4d peritubular capillary deposition status (Figure 1). While a trend towards a worse outcome was observed for patients with mixed rejection, overall Banff classification and C4d deposition was not significantly predictive of graft outcome (p=0.3 & 0.9, respectively).
Figure 1.
Banff classification (a, d) and C4d deposition (b, e) in biopsies diagnosed with acute rejection do not predict long-term graft survival. For cause biopsies diagnosed as non-acute rejection were used as controls (c, f). Graft was survival determined by Kaplan-Meier analysis and eGFR were determined at the indicated weeks post-biopsy. N represents the numbers of study subjects at the indicated time points.
Because long-term graft survival could be affected by events unrelated to the acute rejection event, we evaluated sequential eGFRs in the year following the diagnosis of acute rejection (Figure 1). In all rejection types, we observed a drop in eGFR at the time of biopsy, which was significantly larger for mixed rejection (45.0 ml/min/1.73 m2) compared to TCMR or AMR (20.9 & 18.5 ml/min/1.73 m2, respectively; p=0.01. A modest increase in eGFR was observed within 4 weeks post-biopsy; however, this was comparable among all the rejection types (2.1, 8.7 and 7.1 ml/min/1.73 m2). Neither the drop in eGFR nor it recovery were significantly different between the C4d-positive or C4d-negative groups (p=0.3). There also was no association between the age, race or gender of recipient and the likelihood of graft loss after a diagnosis of acute rejection (Table 2).
Table 2.
Association of Demographic, Renal, and Pathological Variables with Renal Graft Failure, Univariate Cox Proportional Hazard Analysis (n=56)
| Variable | Hazard Ratio | Std. Error | p-value | 95% Confidence Interval |
|---|---|---|---|---|
| Age | 0.99 | 0.013 | 0.4 | 0.96-1.02 |
| Gender | 0.97 | 0.40 | 0.9 | 0.44-2.16 |
| Post-transplant interval (log) | 1.18 | 0.13 | 0.1 | 0.96-1.45 |
| AA vs. other race | 2.12 | 1.16 | 0.2 | 0.73-6.21 |
| CD45 density (log) | 1.65 | 0.38 | 0.03 | 1.06-2.60 |
| CD3 density (log) | 1.27 | 0.23 | 0.2 | 0.89-1.82 |
| CD138 density (log) | 1.27 | 0.13 | 0.02 | 1.05-1.54 |
| CD20 density (log) | 1.36 | 0.23 | 0.07 | 0.98-1.90 |
| GFR baseline* | 1.0 | 0.01 | 1.0 | 0.98-1.02 |
| GFR at biopsy** | 0.94 | 0.02 | 0.001 | 0.91-0.98 |
| GFR at 4 wks*** | 0.89 | 0.02 | <0.001 | 0.85-0.94 |
| GFR at 12 wks& | 0.91 | 0.02 | <0.001 | 0.88-0.95 |
| GFR at 26 wks&& | 0.91 | 0.02 | <0.001 | 0.87-0.95 |
| GFR at 52 wks^ | 0.86 | 0.04 | 0.004 | 0.78-0.95 |
| Change GFR, baseline to biopsy$ | 1.02 | 0.008 | 0.006 | 1.006-1.04 |
| Change GFR, biopsy to 4 wks$$ | 0.95 | 0.02 | 0.02 | 0.90-0.99 |
| Model 1 | ||||
| CD138 density (log) | 1.29 | 0.19 | 0.08 | 0.97-1.72 |
| CD3 density (log) | 0.84 | 0.23 | 0.5 | 0.49-1.44 |
| CD20 density (log) | 1.12 | 0.29 | 0.7 | 0.68-1.86 |
| Model 2 | ||||
| CD20 density (log) | 1.12 | 0.33 | 0.7 | 0.62-2.01 |
| CD3 density (log) | 0.74 | 0.22 | 0.3 | 0.41-1.33 |
| CD138 density (log) | 1.30 | 0.19 | 0.08 | 0.97-1.74 |
| eGFR at Bx | 0.94 | 0.02 | 0.004 | 0.91-0.98 |
| Model 3 | ||||
| CD138 density (log) | 1.22 | 0.12 | 0.04 | 1.01-1.48 |
| eGFR at Bx | 0.94 | 0.02 | 0.004 | 0.91-0.98 |
n=46
n=54
n=52
n=50
n=44
n=32
n=45
n=51
Abbreviations: AA: African-American; GFR: glomerular filtration rate; wks: weeks
Interstitial inflammatory cell densities in renal biopsies with acute rejection
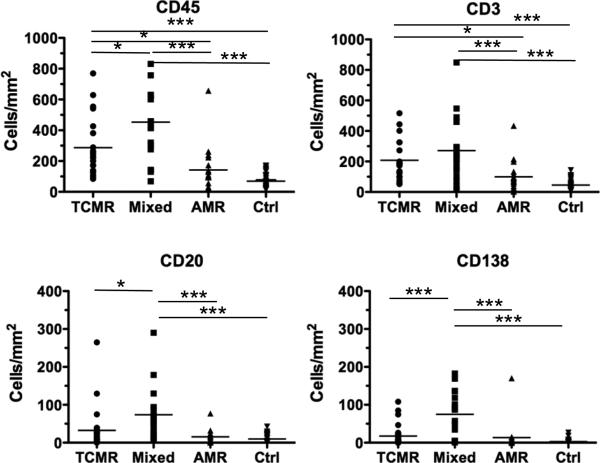
We next tested whether total cellular infiltrate or the density of B or plasma cells specifically were predictive of poor graft outcome. There were significant differences in the densities of CD45+ and CD3+ T cells among the rejection types, with TCMR and mixed rejection biopsies having significantly higher densities compared to AMR (Figure 2). We also analyzed 33 control indication biopsies that were diagnosed as not having acute rejection. These control biopsies were C4d negative and had significantly lower densities of CD45+ and T cell infiltrate compared to mixed and cellular rejection, and had cell densities similar to AMR (Figure 2).
Figure 2.
Cell Types and Densities in Renal Biopsies Grouped by Banff Classification of TCMR, Mixed, AMR and control non-rejection biopsies. Statistically significant comparisons by ANOVA and post-hoc Bonferroni tests between rejection types are indicated by lines; * p<0.05; **p<0.01; ***p<0.005.
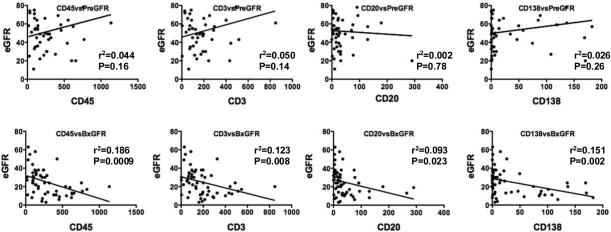
Because the density of the cellular infiltrate at biopsy had previously been reported to correlate with the post-transplant time interval, we investigated whether this was the case in our cohort. It was notable that the time post-transplantation when the rejection occurred was comparable in all groups, with 35-48% and 12-29% of the grafts <1 year and <90 days post-transplantation, respectively. There was no significant correlation between the density of the cellular infiltrate at the time of acute rejection and the transplant-to-biopsy interval (Figure 1). Also there was no correlation between the density of the cellular infiltrate, including B and plasma cells, during acute rejection and the baseline eGFR (Figure 3). The post-transplant interval did not predict loss of graft following a diagnosis of acute rejection, as determined by univariate Cox Proportional Hazard Analysis (Table 2). Finally we observed that neither the eGFRs at baseline nor at the time of biopsy were significantly associated with the age (transplant-to-biopsy interval) of the graft.
Figure 3.
eGFR at biopsy (BxGFR), but not baseline eGFR (PreGFR) at ≥6 months prior to biopsy, were inversely correlated with interstitial leukocyte (CD45), T (CD3), B (CD20) and plasma (CD138) cell densities. Regression analysis was performed, r2 and p-values are indicated.
Einecke et al. (35) reported an association between the post-transplant interval and expression of B cell and immunoglobulin transcripts. Because the majority (77%) of their biopsies were classified as non-acute rejection, we examined whether increased post-transplant interval (age of the graft from the time of transplantation to biopsy) in our control, non-acute rejection, group had increased B cells and/or plasma cell densities. We observed that the densities of CD138+ plasma cells (p<0.004), but not CD45+, CD3+ or CD20+ cells, correlated with the post-transplant time interval. Noteworthy is that the densities of the cellular infiltrate, including the CD138+ cells, were 4 to 12 fold lower in the control biopsies compared with those diagnosed with acute rejection.
Interstitial inflammatory cell densities predict graft loss and return to dialysis
We tested whether increased densities of CD45+, CD3+ T, CD20+ B or CD138+ plasma cells were predictive of graft failure and return to dialysis. When these cell densities were individually analyzed by univariate Cox Proportional Hazard Analysis, CD45+ (p=0.03) and CD138+ (p=0.02) densities were significantly associated with graft failure while CD20+ density approached significance (p=0.07). Surprisingly, CD3+ (p=0.2) density at acute rejection did not predict the return to dialysis.
Because biopsies that have higher density of CD45+ cell infiltrate also tended to have higher densities of T cells, B cell and plasma cells (Figure 2), the densities of these three cell subsets were analyzed in a single multivariate model, to determine whether B cells or plasma cells were independently predictive of graft failure (Table 2, Model 1). We found that T and B cell densities were not independently associated with failure (p=0.5 and 0.7, respectively), and that plasma cell densities approached significance (p=0.08). These data likely reflect the tight association between high T, B and plasma cell densities in the biopsies, and support the notion that increased overall infiltration is strongly associated with the return to dialysis (Figure 2).
eGFRs and Interstitial inflammatory cell densities during acute rejection predict graft loss and return to dialysis
We performed univariate Cox Proportional Hazard Analysis to test whether graft failure and return to dialysis was independently predicted by eGFR at biopsy or at 4, 12, 26 and 52 weeks after biopsy. The eGFR at each of these time points was predictive of graft loss (Table 2) as were the magnitude of drop in eGFR from baseline to biopsy and of increase in eGFR from biopsy to 4 weeks post-biopsy. We focused subsequent analysis on eGFR at biopsy (p=0.001; Table 2) because it was the most straightforward predictor of graft failure.
We observed a significant negative correlation between eGFRs at biopsy and the densities of CD45+, CD3+, CD20+ and CD138+ cells (Figure 3) (p=0.023-0.0009). Multivariate Cox analysis with eGFR values and CD3+, CD20+ and CD138+ cell densities (Model 2, Table 2) determined that a lower eGFR was strongly predictive of graft failure (HR=0.94; p=0.004), a higher CD138+ cell density trended to significance (HR=1.30, p=0.08) while CD20+ and CD3+ cell densities were not. When CD20+ and CD3+ cell densities were removed in a step-wise fashion to create the most parsimonious model, higher CD138+ plasma cell density became a significant independent predictor of failure (HR=1.22, p=0.041) while eGFR retained a strong negative association (Model 3, Table 2). Furthermore, Model 3 (p=0.0002) was better at predicting failure than a univariate Cox Model either with eGFR at biopsy or CD138+ density alone, underscoring the significance of higher plasma cell density as a predictor of graft failure. Of note, interstitial fibrosis was not predictive of graft failure in this model (analysis not shown).
Discussion
Despite the critical diagnostic role of allograft biopsies, controversy persists regarding their ability to predict graft outcomes accurately. Our study is consistent with recent reports that neither Banff class nor C4d status predicted short-term or long-term graft outcome and that the overall degree of inflammation in the biopsies and the post-transplant interval is better at predicting graft loss (19-22). When CD45+, T, B and plasma cell densities were examined, CD45+ and CD138+ densities were significantly associated with graft failure while CD20+ density approached significance, while surprisingly, CD3+ densities did not.
Using a microarray approach of testing biopsies from unselected renal allografts, Einecke et al. (35) reported that increased B cell and immunoglobulin gene transcripts were predictive of reduced recovery of renal function. Because elevated B cell and plasma cell transcripts also correlated with the post-transplant interval (>5 months), Einecke et al. (35) concluded that B cells and plasma cells were not directly mediating rejection but were simply indicative of older grafts with reduced renal function. In contrast, we did not observe a significant correlation between B and plasma cell densities in the acute rejection biopsy and the age of the graft. Since the majority (77%) of the biopsies examined in Einecke et al. (35) were diagnosed as non-rejection, we considered the possibility that their conclusions largely reflected the non-rejecting, and not the acutely rejecting, biopsies. Indeed, when our control biopsies were examined, a strong correlation between the age of the biopsy and the density of plasma cells was observed. However, the densities of overall cellular infiltrate, and of plasma cells specifically, in the non-acute rejection biopsies were 4 to 11 fold lower than those in the acute rejection biopsies. Thus during acute rejection, the composition and increased density of infiltrating plasma cells can play important roles in graft pathogenesis and in determining graft outcome.
At the diagnosis of acute rejection, the CD45+ cell density was a good predictor of poor graft outcome. When T cell, B cell and plasma cell subsets were investigated, T cell densities were not predictive, B cells trended to being significantly predictive and only plasma cell densities significantly correlated with graft failure. Our observations that B cells are weakly predictive of poor graft outcomes straddle previous reports that B cells demarcate a subset of steroid-resistant acute rejection and reports that B cells do not (25-30). Even though plasma cells represent the minority subset (≤16%) of total infiltrating cells in the rejecting biopsies, their densities were most predictive of long-term graft outcome, an observation consistent with previous reports of plasma cell-rich acute rejection having worse graft outcome (36-38). Because mixed rejection biopsies with the highest densities of T cells and C4d deposition also had the highest plasma cell densities, these observations raise the possibility that plasma cells are being generated in situ and they contribute to the rejection process by the secretion of graft-reactive antibodies.
Univariate Cox proportional hazard analyses revealed that eGFR at the diagnosis of acute rejection/biopsy, as well as eGFR post-biopsy, but not eGFR at baseline, were strong predictors of graft failure. While this is not an unexpected finding, it underscores the critical effect acute rejection over other parameters such as the time since transplantation (age of the graft) or its functional capacity prior to rejection has on the long-term outcome of the allograft. Furthermore, in a multivariate analysis, eGFR at biopsy and densities of plasma cells were independent predictors of graft failure, and the combination of eGFR at biopsy and increased densities of plasma cells considered in a single model was an even stronger predictor of graft failure than either alone.
We speculate that the type of rejection occurring with plasma cells present in the parenchyma may differ from an acute rejection event that does not include plasma cells or may represent a temporally more advanced stage of rejection. In either case, if the antibodies secreted by these plasma cells are graft-reactive, they can bind to the allograft to elicit humoral rejection and also generate opsonins that enhance T cell-mediated rejection (39, 40). The lack of therapeutic agents that can successfully control plasma cell secretion of antibodies may explain why rejection episodes with dense plasma cell infiltrate are associated with graft failure and return to dialysis. The alternative interpretation is that plasma cells are simply a marker of a temporally more advanced rejection process, and that they are simply recruited by the inflammatory milieu. If this is true then targeting the plasma cells would be irrelevant and targeting the underlying cause of rejection would be necessary. Both scenarios are not mutually exclusive, and it is possible that some plasma cells are generated in situ and are graft-specific, while others are recruited into the graft in a non-antigen-specific manner. A demonstration that the graft infiltrating plasma cells are enriched for graft-reactivity will strongly support a conclusion that plasma cells and the antibodies they secrete in situ directly contribute to acute rejection.
In summary, our study confirm that the combination of low eGFR and high density of plasma cells provides the most strongly predictive model of allograft outcome. Resolving whether plasma cells are directly contributing to rejection, or if they are being recruited in an antigen-independent manner, is now critical since the former would mandate the targeting of plasma cells while the latter suggests an optimal strategy of targeting the acute rejection process.
Supplementary Material
Acknowledgement
We thank Marcus Clark for assistance in the immunohistochemical studies and helpful discussions.
This work was funded by in part by National Institutes of Health grants, R03AI069284 and R01AI083452, to ASC.
Abbreviations
- AMR
antibody mediated rejection
- C4d
complement 4d
- DSA
donor-specific alloantibodies
- eGFR
estimated glomerular filtration rate
- IVIG
intravenous immunoglobulin
- TCMR
T cell-mediated acute rejection
Footnotes
The authors of this manuscript have no conflicts of interests to declare as described by Transplant International.
A.C., A.S.C. participated in research design
A.C., J.M.M., M.L.C., M.A.J., W.J.C., R.S., Z.D., S.R.M., S.M.M., M.M., M.Z.D., J.W.W., A.S.C. participated in performance of the research
A.C., M.Z.D., A.S.C. participated in data analysis
A.C., J.M.M., M.L.C., A.S.C. participated in writing the manuscript
References
- 1.Lakkis FG, Arakelov A, Konieczny BT, Inoue Y. Immunologic ‘ignorance’ of vascularized organ transplants in the absence of secondary lymphoid tissue. Nat Med. 2000;6(6):686. doi: 10.1038/76267. [DOI] [PubMed] [Google Scholar]
- 2.Arakelov A, Lakkis FG. The alloimmune response and effector mechanisms of allograft rejection. Semin Nephrol. 2000;20(2):95. [PubMed] [Google Scholar]
- 3.Game DS, Warrens AN, Lechler RI. Rejection mechanisms in transplantation. Wien Klin Wochenschr. 2001;113(20-21):832. [PubMed] [Google Scholar]
- 4.Taylor AL, Watson CJ, Bradley JA. Immunosuppressive agents in solid organ transplantation: Mechanisms of action and therapeutic efficacy. Crit Rev Oncol Hematol. 2005;56(1):23. doi: 10.1016/j.critrevonc.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 5.Tonshoff B, Hocker B. Treatment strategies in pediatric solid organ transplant recipients with calcineurin inhibitor-induced nephrotoxicity. Pediatr Transplant. 2006;10(6):721. doi: 10.1111/j.1399-3046.2006.00577.x. [DOI] [PubMed] [Google Scholar]
- 6.Lamb KE, Lodhi S, Meier-Kriesche HU. Long-term renal allograft survival in the United States: a critical reappraisal. Am J Transplant. 11(3):450. doi: 10.1111/j.1600-6143.2010.03283.x. [DOI] [PubMed] [Google Scholar]
- 7.Meier-Kriesche HU, Schold JD, Srinivas TR, Kaplan B. Lack of improvement in renal allograft survival despite a marked decrease in acute rejection rates over the most recent era. Am J Transplant. 2004;4(3):378. doi: 10.1111/j.1600-6143.2004.00332.x. [DOI] [PubMed] [Google Scholar]
- 8.Feucht HE, Schneeberger H, Hillebrand G, et al. Capillary deposition of C4d complement fragment and early renal graft loss. Kidney Int. 1993;43(6):1333. doi: 10.1038/ki.1993.187. [DOI] [PubMed] [Google Scholar]
- 9.Racusen LC, Colvin RB, Solez K, et al. Antibody-mediated rejection criteria - an addition to the Banff 97 classification of renal allograft rejection. Am J Transplant. 2003;3(6):708. doi: 10.1034/j.1600-6143.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- 10.Haas M. The significance of C4d stainingf with minimal histologic abnormalities. Curr Opin Organ Transplant. 15(1):21. doi: 10.1097/MOT.0b013e3283342ebd. [DOI] [PubMed] [Google Scholar]
- 11.Regele H, Exner M, Watschinger B, et al. Endothelial C4d deposition is associated with inferior kidney allograft outcome independently of cellular rejection. Nephrol Dial Transplant. 2001;16(10):2058. doi: 10.1093/ndt/16.10.2058. [DOI] [PubMed] [Google Scholar]
- 12.Nickeleit V, Zeiler M, Gudat F, Thiel G, Mihatsch MJ. Detection of the complement degradation product C4d in renal allografts: diagnostic and therapeutic implications. J Am Soc Nephrol. 2002;13(1):242. doi: 10.1681/ASN.V131242. [DOI] [PubMed] [Google Scholar]
- 13.Bohmig GA, Exner M, Watschinger B, et al. C4d deposits in renal allografts are associated with inferior graft outcome. Transplant Proc. 2001;33(1-2):1151. doi: 10.1016/s0041-1345(00)02467-2. [DOI] [PubMed] [Google Scholar]
- 14.Sureshkumar KK, Hussain SM, Carpenter BJ, Sandroni SE, Marcus RJ. Antibody-mediated rejection following renal transplantation. Expert Opin Pharmacother. 2007;8(7):913. doi: 10.1517/14656566.8.7.913. [DOI] [PubMed] [Google Scholar]
- 15.Venetz JP, Pascual M. New treatments for acute humoral rejection of kidney allografts. Expert Opin Investig Drugs. 2007;16(5):625. doi: 10.1517/13543784.16.5.625. [DOI] [PubMed] [Google Scholar]
- 16.Becker YT, Samaniego-Picota M, Sollinger HW. The emerging role of rituximab in organ transplantation. Transpl Int. 2006;19(8):621. doi: 10.1111/j.1432-2277.2006.00345.x. [DOI] [PubMed] [Google Scholar]
- 17.Singh N, Pirsch J, Samaniego M. Antibody-mediated rejection: treatment alternatives and outcomes. Transplant Rev (Orlando) 2009;23(1):34. doi: 10.1016/j.trre.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 18.Jordan SC, Peng A, Vo AA. Therapeutic strategies in management of the highly HLA-sensitized and ABO-incompatible transplant recipients. Contrib Nephrol. 2009;162:13. doi: 10.1159/000170864. [DOI] [PubMed] [Google Scholar]
- 19.Loupy A, Hill GS, Suberbielle C, et al. Significance of C4d Banff scores in early protocol biopsies of kidney transplant recipients with preformed donor-specific antibodies (DSA). Am J Transplant. 11(1):56. doi: 10.1111/j.1600-6143.2010.03364.x. [DOI] [PubMed] [Google Scholar]
- 20.El Karoui K, Hill GS, Karras A, et al. Focal segmental glomerulosclerosis plays a major role in the progression of IgA nephropathy. II. Light microscopic and clinical studies. Kidney Int. 79(6):643. doi: 10.1038/ki.2010.460. [DOI] [PubMed] [Google Scholar]
- 21.Haririan A, Kiangkitiwan B, Kukuruga D, et al. The impact of c4d pattern and donor-specific antibody on graft survival in recipients requiring indication renal allograft biopsy. Am J Transplant. 2009;9(12):2758. doi: 10.1111/j.1600-6143.2009.02836.x. [DOI] [PubMed] [Google Scholar]
- 22.Mannon RB, Matas AJ, Grande J, et al. Inflammation in areas of tubular atrophy in kidney allograft biopsies: a potent predictor of allograft failure. Am J Transplant. 10(9):2066. doi: 10.1111/j.1600-6143.2010.03240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sis B, Jhangri GS, Bunnag S, Allanach K, Kaplan B, Halloran PF. Endothelial gene expression in kidney transplants with alloantibody indicates antibody-mediated damage despite lack of C4d staining. Am J Transplant. 2009;9(10):2312. doi: 10.1111/j.1600-6143.2009.02761.x. [DOI] [PubMed] [Google Scholar]
- 24.Sarwal M, Chua MS, Kambham N, et al. Molecular heterogeneity in acute renal allograft rejection identified by DNA microarray profiling. N Engl J Med. 2003;349(2):125. doi: 10.1056/NEJMoa035588. [DOI] [PubMed] [Google Scholar]
- 25.Hippen BE, DeMattos A, Cook WJ, Kew CE, 2nd, Gaston RS. Association of CD20+ infiltrates with poorer clinical outcomes in acute cellular rejection of renal allografts. Am J Transplant. 2005;5(9):2248. doi: 10.1111/j.1600-6143.2005.01009.x. [DOI] [PubMed] [Google Scholar]
- 26.Kayler LK, Lakkis FG, Morgan C, et al. Acute cellular rejection with CD20-positive lymphoid clusters in kidney transplant patients following lymphocyte depletion. Am J Transplant. 2007;7(4):949. doi: 10.1111/j.1600-6143.2007.01737.x. [DOI] [PubMed] [Google Scholar]
- 27.Muorah MR, Brogan PA, Sebire NJ, Trompeter RS, Marks SD. Dense B cell infiltrates in paediatric renal transplant biopsies are predictive of allograft loss. Pediatr Transplant. 2009;13(2):217. doi: 10.1111/j.1399-3046.2008.00943.x. [DOI] [PubMed] [Google Scholar]
- 28.Scheepstra C, Bemelman FJ, van der Loos C, et al. B cells in cluster or in a scattered pattern do not correlate with clinical outcome of renal allograft rejection. Transplantation. 2008;86(6):772. doi: 10.1097/TP.0b013e3181860a74. [DOI] [PubMed] [Google Scholar]
- 29.Tsai EW, Rianthavorn P, Gjertson DW, Wallace WD, Reed EF, Ettenger RB. CD20+ lymphocytes in renal allografts are associated with poor graft survival in pediatric patients. Transplantation. 2006;82(12):1769. doi: 10.1097/01.tp.0000250572.46679.45. [DOI] [PubMed] [Google Scholar]
- 30.Bagnasco SM, Tsai W, Rahman MH, et al. CD20-positive infiltrates in renal allograft biopsies with acute cellular rejection are not associated with worse graft survival. Am J Transplant. 2007;7(8):1968. doi: 10.1111/j.1600-6143.2007.01885.x. [DOI] [PubMed] [Google Scholar]
- 31.Zarkhin V, Li L, Sarwal M. “To B or not to B?” B-cells and graft rejection. Transplantation. 2008;85(12):1705. doi: 10.1097/TP.0b013e318177793e. [DOI] [PubMed] [Google Scholar]
- 32.Sis B, Mengel M, Haas M, et al. Banff '09 meeting report: antibody mediated graft deterioration and implementation of Banff working groups. Am J Transplant. 2010;10(3):464. doi: 10.1111/j.1600-6143.2009.02987.x. [DOI] [PubMed] [Google Scholar]
- 33.Myers GL, Miller WG, Coresh J, et al. Recommendations for improving serum creatinine measurement: a report from the Laboratory Working Group of the National Kidney Disease Education Program. Clin Chem. 2006;52(1):5. doi: 10.1373/clinchem.2005.0525144. [DOI] [PubMed] [Google Scholar]
- 34.Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function--measured and estimated glomerular filtration rate. N Engl J Med. 2006;354(23):2473. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- 35.Einecke G, Sis B, Reeve J, et al. Antibody-mediated microcirculation injury is the major cause of late kidney transplant failure. Am J Transplant. 2009;9(11):2520. doi: 10.1111/j.1600-6143.2009.02799.x. [DOI] [PubMed] [Google Scholar]
- 36.Charney DA, Nadasdy T, Lo AW, Racusen LC. Plasma cell-rich acute renal allograft rejection. Transplantation. 1999;68(6):791. doi: 10.1097/00007890-199909270-00011. [DOI] [PubMed] [Google Scholar]
- 37.Gartner V, Eigentler TK, Viebahn R. Plasma cell-rich rejection processes in renal transplantation: morphology and prognostic relevance. Transplantation. 2006;81(7):986. doi: 10.1097/01.tp.0000215014.40595.ab. [DOI] [PubMed] [Google Scholar]
- 38.Meehan SM, Domer P, Josephson M, et al. The clinical and pathologic implications of plasmacytic infiltrates in percutaneous renal allograft biopsies. Hum Pathol. 2001;32(2):205. doi: 10.1053/hupa.2001.21574. [DOI] [PubMed] [Google Scholar]
- 39.Burns AM, Chong AS. Alloantibodies prevent the induction of transplantation tolerance by enhancing alloreactive T cell priming. J Immunol. 2011;186(1):214. doi: 10.4049/jimmunol.1001172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burns AM, Ma L, Li Y, et al. Memory alloreactive B cells and alloantibodies prevent anti-CD154-mediated allograft acceptance. J Immunol. 2009;182(3):1314. doi: 10.4049/jimmunol.182.3.1314. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.