Abstract
Rationale
Repeated exposure to psychostimulants alters behavioral responses to reward-related cues; however, the motivational underpinnings of this effect have not been fully characterized.
Objectives
The following study was designed to examine how amphetamine sensitization affects performance in rats on a series of Pavlovian and operant tasks that distinguish between general-incentive and outcome-selective forms of conditioned responses.
Methods
Adult male rats underwent Pavlovian and instrumental training for food pellet rewards. Following training, rats were sensitized to d-amphetamine (2 mg/kg for 7 days). Rats were subsequently tested on an outcome-selective Pavlovian-instrumental transfer (PIT) task, an outcome-reinstatement task, and an outcome devaluation task. Additionally, in a separate experiment PIT was assessed in amphetamine-sensitized and control rats using a Pavlovian backward-conditioned stimulus.
Results
Repeated amphetamine exposure sensitized locomotor activity to acute amphetamine challenge. Amphetamine altered responses to CS presentations by increasing conditioned approach. During tests of PIT amphetamine-treated rats showed no outcome-selectivity in their responding, responding to a CS whether or not it shared a common outcome with the instrumental response. No effect of amphetamine sensitization was observed on tests of outcome-selective reinstatement by outcome delivery, or action selection based on outcome value. Amphetamine-sensitized rats showed impaired outcome-selective PIT to a backward CS but were unaltered in conditioned approach.
Conclusions
Amphetamine sensitization prevents outcome-selective responding during PIT, which is dissociable from amphetamine’s effects on conditioned approach. These data suggest fundamental alterations in how stimuli motivate action in addiction.
Keywords: Sensitization, incentive, devaluation, reinstatement, operant
Introduction
Cues associated with drug delivery are a major source of craving and relapse among addicts, and much attention has focused on discovering the factors responsible for increasing relapse vulnerability. Studies have shown that a history of exposure to drugs of abuse increases the propensity of drug-taking and susceptibility to reinstatement by drug-associated cues (Mendrek et al. 1998; Pierre and Vezina 1997; Sutton et al. 2000; Vezina et al. 1999). Furthermore, repeated exposure to certain abused substances, such as psychostimulants, enhances craving elicited by natural (i.e., non-drug) reward-associated cues. For example, prior repeated exposure to amphetamine enhances approach and incentive-motivational responses to a conditioned stimulus (CS) that is associated in a Pavlovian manner with delivery of natural rewards such as food or access to mates (Afonso et al. 2009; Harmer and Phillips 1998; Mendez et al. 2009; Simon et al. 2009; Taylor and Jentsch 2001; Wyvell and Berridge 2001).
The findings described above support the hypothesis that neuroadaptations caused by repeated exposure to abused substances enhances the motivational responses elicited by reward-associated cues (Everitt and Wolf 2002; Hyman et al. 2006; Robinson and Berridge 2003). To further test this hypothesis, it is important to recognize that the response elicited by a CS depends on distinct incentive processes; CS’s associated with rewards may elicit a general affective or motivational response as well as a specific response based on a representation of the associated outcome (Balleine and Killcross 2006; Delamater and Oakeshott 2007; Konorski 1967). For example, a cue predicting food and one predicting access to mates may each elicit an increase in arousal (e.g., increased heart rate), but will separately elicit distinct conditioned responses appropriate to the two types of outcomes. In support of this distinction in incentive processing, parallel neural systems have been identified that are involved in generating outcome-selective or general incentive-motivational responses elicited by the CS (Balleine and Killcross 2006; Blundell et al. 2001; Corbit and Balleine; 2005; 2011; Delamater and Oakeshott 2007).
The effects of repeated drug exposure on general and outcome-selective incentive processes have not been fully explored. Some evidence suggests that drug-associated cues utilize general incentive motivation (Corbit and Janak 2007). Furthermore, psychostimulant sensitization has been shown to reduce outcome sensitivity in a Pavlovian task, suggesting that conditioned responses in sensitized animals are motivated primarily by general incentive properties of the CS (Schoenbaum and Setlow 2005). The present study employed a Pavlovian-instrumental transfer (PIT) paradigm to examine outcome-selective incentive motivation in rats that were repeatedly exposed to the psychostimulant amphetamine. PIT measures the effects of a Pavlovian CS on instrumental behavior and is a key tool in understanding incentive motivation (Colwill and Motzkin 1994; Holmes et al. 2010; Kruse et al. 1983). In Experiments 1 and 2, outcome-selective PIT, outcome reinstatement and outcome devaluation paradigms were used to characterize the ability of amphetamine-sensitized and control rats to use outcome information to guide instrumental responding. In Experiment 3, a Pavlovian backward conditioning procedure was used to dissociate the effects of amphetamine exposure on conditioned approach from its effects on PIT.
Materials and Methods
Experiment 1: Effects of amphetamine sensitization on outcome-selective PIT, outcome reinstatement and devaluation
Subjects and Apparatus
Subjects were 32 adult male Long Evans Blue Spruce rats from Harlan Laboratories (Indianapolis IN, USA). Rats were housed in pairs in 47.6 cm × 20.3 cm × 26 cm (w/h/d) polycarbonate containers with Alpha Chip bedding material (Northeastern Products Corp, Warrensburg NY) and had free access to water. One week after arrival, rats were placed on a restricted food diet of approximately 20 g of standard rat pellets per day (Purina, St. Louis MO, USA). Rats were fed after their daily behavioral training session. Food restriction continued for the duration of the experiment. All procedures were approved by the Rutgers University Institutional Animal Care and Use Committee.
Behavioral training and testing took place in twelve identical rat operant conditioning chambers (Med Associates, St. Albans VT, USA). A food cup with infrared detectors was centered on one wall of the chamber. Retractable levers were situated to the left and right of the food cup. Responses on these levers delivered one food pellet from a pellet dispenser mounted outside the chamber. Two types of pellets were used in the experimental procedures: 45-mg grain-based pellets and chocolate-flavored purified pellets (Bio-serv, Frenchtown NJ USA). Three audio generators were located in the chambers, a sonalert, a clicker module and a white-noise generator. Each generator produced sounds at approximately 80 db. A 28 V chamber light was located on the opposite wall from the food cup and illuminated the chamber during behavioral procedures. Each chamber was housed in a sound-attenuating shell and equipped with a ventilation fan that was activated during behavioral procedures.
Behavioral Procedures
General procedures
A timeline of behavioral procedures is depicted in Table 1. Behavioral procedures commenced after one week of food restriction. Rats were provided with one 30-min session to habituate to the testing chamber, after which they began behavioral training. Amphetamine sensitization was carried out between training and tests, in order minimize the effects of amphetamine exposure on behavior that is attributable to alterations in learning (Blaiss and Janak 2007; Hall and Gulley 2010; Oscos et al. 1988).
Table 1. Timeline of experimental activities.
Pavlovian and instrumental training was following by amphetamine sensitization for half the rats, while the remaining rats received saline injections. This was followed by reminder Pavlovian and instrumental session and tests of PIT, and, in Experiment 1, outcome reinstatement and outcome devaluation. Sensitization to the locomotor effects of amphetamine was confirmed after test completion in Experiment 2.
| Behavioral Training | Drug admin | Reminder sessions | PIT test | Outcome reinstatement | Devaluation test | Locomotor test | |
|---|---|---|---|---|---|---|---|
| Expt 1 | |||||||
| Day | 1–14 | 15–35 | 36–37 | 38–40 | 41–42 | 43–44 | |
| Expt 2 | |||||||
| Day | 1–14 | 15–35 | 36–37 | 38–40 | 41–42 | ||
| Expt 3 | |||||||
| Day | 1–17 | 18–38 | 39–40 | 41–42 | |||
Pavlovian conditioning
Rats were trained to associate one auditory stimulus with delivery of chocolate pellets and a second stimulus with delivery of grain pellets. A third stimulus was used as a neutral stimulus and was unpaired with food delivery. During the training session, each auditory stimulus was presented four times per session for 120 s with an inter-stimulus interval that averaged 3 min (range 2–4 min). During CS presentation, food pellets were dispensed according to the following probability: for each second during CS presentation there was a probability of p = 0.06 of pellet delivery, which on average resulted in 7.2 pellets delivered per stimulus presentation. Rats received one training session per day for 6 d. During training the number of head entries that rats made was measured during stimulus presentation or during a 2 min period preceding stimulus onset. Head insertions were not counted for 5 sec after pellet delivery during the CS to minimize the effects of food pellet retrieval on head insertion counts.
Instrumental Conditioning
For each training session, one lever was inserted into the chamber and responses the rats made on the lever delivered a single food pellet. The session terminated when rats earned 20 pellets or 15 min had elapsed. Rats were trained daily on each lever in separate sessions, with a 30 min interval between sessions. Training lasted for 8 days: on days 1–2, each response on the lever resulted in pellet delivery. On days 3–4, pellets were delivered according to a variable-ratio (VR) 5 schedule, which required, on average, 5 responses to earn a pellet reward. On days 5–8, pellets were delivered according to a VR-15 schedule.
Pavlovian and instrumental reminder sessions
These training sessions were conducted two weeks after the final amphetamine injection. Rats received one Pavlovian session and two instrumental training sessions (one session with each lever). These sessions were conducted in the same manner as the training sessions.
Pavlovian-instrumental transfer (PIT) test
The PIT test was conducted in extinction, such that lever pressing and CS presentation produced no outcomes. During the test, one lever was inserted into the operant conditioning chamber and each CS was presented four times in pseudorandom order, with a 3 min interval between CS presentations. The neutral stimulus in the PIT test was not included, as the primary interest was in comparing instrumental response rates during presentation of the two CSs. Prior to the first CS presentation, rats underwent 10 min of instrumental extinction. The following day the alternate lever was inserted into the chamber and the PIT test was repeated. The order of lever testing was randomized across conditions.
Outcome reinstatement test
During the outcome reinstatement test, one lever was inserted into the operant conditioning chamber and rats underwent 6 min of instrumental extinction, after which grain or chocolate pellets were delivered into the chamber, with a 5 min interval between presentations. Each of the two outcomes was presented four times. The following day the opposite lever was inserted and the test repeated.
Outcome devaluation test
Rats were placed in individual cages and provided with 25 g of one of the instrumental outcomes (grain pellets or chocolate-flavored pellets). After 45 min, rats were placed in the operant conditioning chamber and both levers were inserted. Rats had the opportunity to respond on either lever for 10 min. The test was repeated the following day with the opposite outcome devalued.
Amphetamine sensitization
Following Pavlovian and instrumental training and prior to PIT, outcome reinstatement and devaluation tests, rats were divided into two groups of 16 animals, which were matched based on their rate of instrumental responding on the final day of training. Rats assigned to the amphetamine group received an intraperitoneal injection of d-amphetamine (Sigma, St. Louis MO, USA) daily for 7 days at a dose of 2 mg/kg body weight, diluted in 0.8% saline to 2 mg/ml. Control rats received the same volume of saline injections. Rats were sensitized in their home cage. After the final injection, rats remained in their home cage for 14 days.
Statistical analysis
For the PIT test, lever presses during the different CS’s were categorized as Match or Mismatch depending on whether the instrumental outcome was the same or different from the CS-associated outcome. CS responding was expressed relative to baseline during the PIT test by subtracting lever presses made during a pre-CS interval from lever presses during CS presentation. For the outcome reinstatement test, lever presses during a two-min interval following outcome delivery were tallied and categorized as Match or Mismatch, depending on whether the instrumental outcome was the same or different from the freely-delivered outcome. As with the PIT test, baseline responding was subtracted from responding following outcome delivery. For the outcome devaluation test, responses on the two levers were categorized as devalued or valued, depending on whether the rat was pre-fed the instrumental outcome. For all analyses, reinforcer type (chocolate or grain pellet) was collapsed across conditions, as no effect of reinforcer type was observed on measures of instrumental or Pavlovian responding, nor did amphetamine exposure interact with reinforcer type to influence responding. ANOVA’s and t-tests with Bonferroni correction were carried out using statistical analysis software (SPSS, Chicago IL).
Experiment 1 Results
Pavlovian training
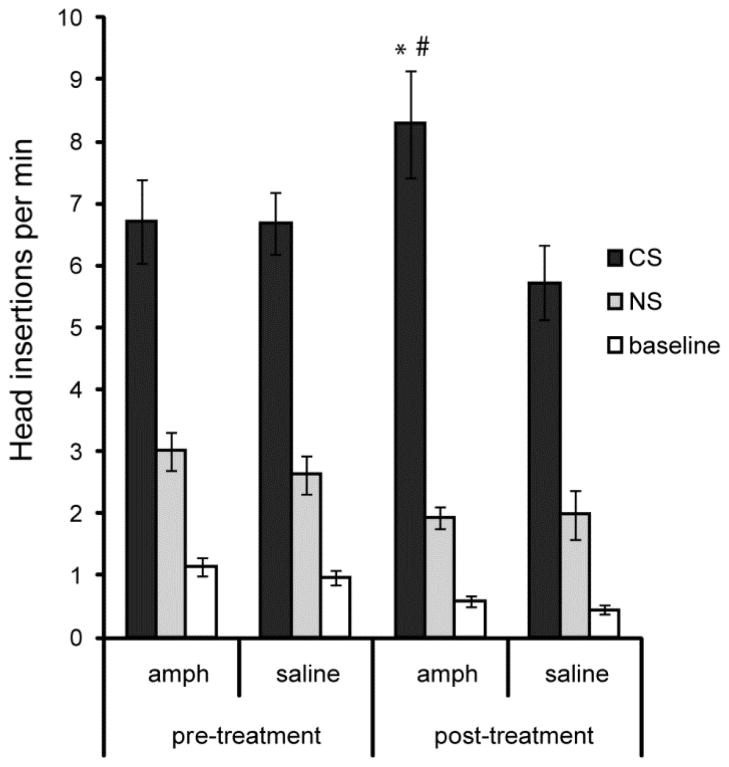
Rats acquired a conditioned approach response that was significantly altered by previous amphetamine exposure (Figure 1). Regardless of drug condition, rats made significantly more approach to the food cup during the CS compared to the neutral stimulus or baseline (F2, 60 = 200.87, P < 0.01). Amphetamine exposure significantly interacted with CS presentation to alter approach behavior (F2, 60 = 10.99 P < 0.01). Rats treated with amphetamine approached the food cup significantly more often during the CS compared to their approach rate before amphetamine exposure (paired t-test: t15 = 2.6 P < 0.05). Furthermore, amphetamine-treated rats approached the food cup significantly more often than saline-treated rats during the reminder session (independent samples t-test: t14 = 2.43 P < 0.05).
Figure 1. Pavlovian approach.
Rats were trained to associate the presence of a conditioned stimulus (CS) with delivery of food pellets and the number of head insertions into the food cup was measured during the CS, a neutral stimulus (NS) unpaired with food, and when no stimulus was present (baseline). The left column depicts head insertions in the final training session prior to drug treatment. The right column depicts head insertions during a reminder training session, which occurred after a subset of rats underwent a sensitizing regimen of amphetamine exposure and withdrawal. * = significantly different from amphetamine pre-treatment CS rate; # = significantly different from saline post-treatment CS rate. Error bars = ± 1 SEM.
Instrumental training and PIT test
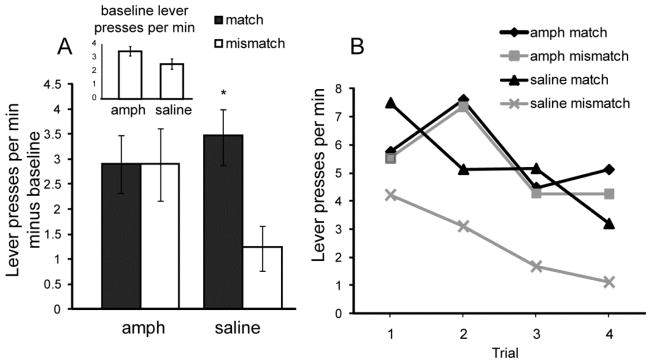
All rats acquired an instrumental response. Amphetamine exposure had no effect on overall instrumental response rates during the reminder session; however, amphetamine significantly altered instrumental responses during the PIT test (Figure 2A). Amphetamine significantly interacted with CS presentation to alter instrumental performance during PIT (ANOVA: F 1, 30 = 6.22, P < 0.05). Outcome-selective transfer was observed among saline-treated rats, as shown by their performing significantly fewer Mismatch responses compared to Match responses (paired t-test: t15 = 4.24 P < 0.01). Among amphetamine-treated rats a similar rate of Match and Mismatch responding was observed, indicating a lack of outcome-selective responding. Furthermore, unlike control rats, amphetamine-treated rats made significantly more Mismatch responses compared to baseline (t15 = 5.97, P < 0.01).
Figure 2. Pavlovian-instrumental transfer (PIT) performance.
(A) Responses on the PIT test were classified as a Match if the CS and instrumental response shared a common outcome, whereas responses were classified as a Mismatch if the response and the CS did not share an outcome. Responses during an equivalent pre-CS (baseline) period were subtracted from responses made during CS presentation, and are presented in the inset. (B) Match and Mismatch responses are presented for each of the four test trials. * = significantly different from saline Mismatch rate. Error bars = ± 1 SEM.
To further illustrate the effect of amphetamine on PIT, responses are depicted for each trial in Figure 2B, and show a consistent difference between Match and Mismatch response rates among saline-treated rats. In amphetamine-sensitized rats, response rates are similar across each type of CS presentation. A 3-factor ANOVA that includes trial (1–4), trial type (match or mismatch) and drug (amphetamine or saline) revealed significant effects of trial (F3, 90 = 3.58 P = 0.01), trial type (F1, 90 = 7.44, P < 0.01) and drug (F1, 30 = 7.6, P < 0.01) and interactions of drug and trial type (F1, 90 = 4.19 P < 0.05).
Outcome reinstatement test
Rats made more instrumental responses following free delivery of the outcome compared to baseline; furthermore, these responses were outcome selective, with greater Match compared to Mismatch responses (ANOVA: F1, 30 = 9.45, P < 0.01; paired t-test: amph- t15 = 2.39, P < 0.05; saline- t15 = 2.10, P = 0.05) (Figure 3). However, no effects of amphetamine were observed on outcome reinstatement.
Figure 3. Instrumental responses following outcome delivery.
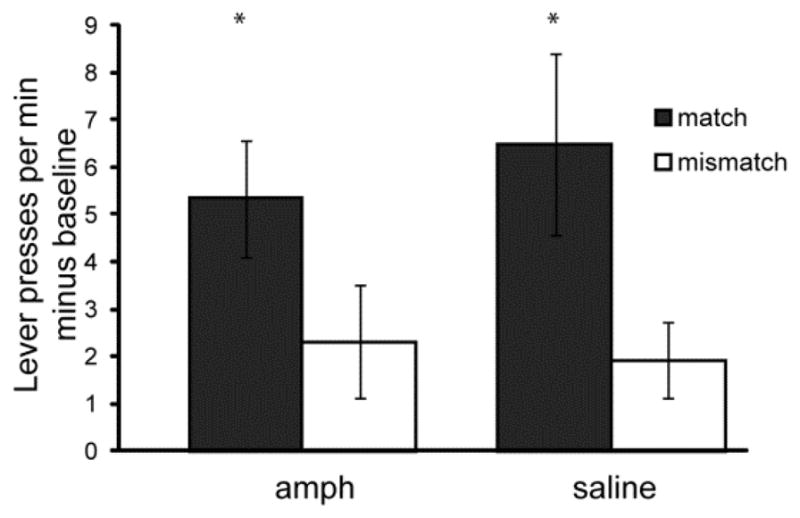
Instrumental response rates were measured during a 2-min interval following free delivery of food pellets in the chamber. Responses were classified as a Match if the outcome of the instrumental response matched the freely delivered food pellet, whereas responses were classified as a Mismatch if the outcome of the instrumental response did not match the freely delivered food pellet. Response rates prior to outcome delivery (baseline) were subtracted from responses following outcome delivery. * = Match significantly greater than Mismatch response rate. Error bars = ± 1 SEM.
Outcome devaluation test
Rats in both conditions made more instrumental responses on the lever that, during training, delivered the valued outcome, compared to the devalued outcome (ANOVA: F1, 30 = 42.26, P < 0.01; paired t-tests amph- t15 = 3.41, P < 0.01; saline- t15 = 6.18, P < 0.01) (Figure 4). However, no effect of was observed on instrumental performance following outcome devaluation.
Figure 4. Instrumental responses following a change in outcome value.
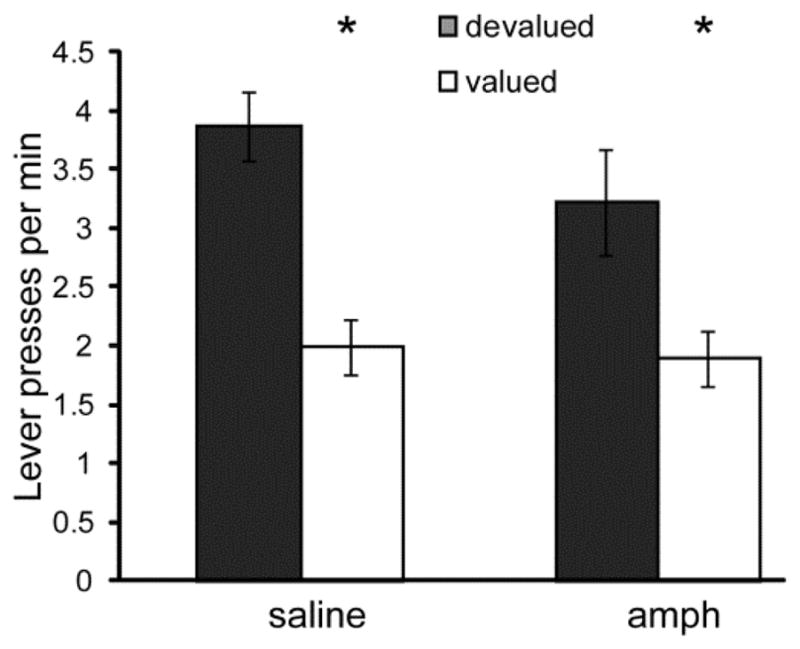
Instrumental response rates were measured in extinction following specific satiety of one instrumental outcome. Responses were classified as Devalued or Valued if the instrumental response previously delivered the same or different outcome from the sated outcome. * = valued significantly greater than devalued response rate. Error bars = ± 1 SEM.
Experiment 2: Effects of amphetamine sensitization on outcome-selective PIT and locomotor activity
Rationale
The purpose of Experiment 2 was to verify that repeated amphetamine exposure sensitized rats to the locomotor effects of acute amphetamine exposure, and that sensitized rats demonstrated alterations in PIT. Subjects were trained using identical procedures to Experiment 1. Following completion of the PIT test, locomotor activity was assessed in all rats following injections of saline and amphetamine.
Subjects and Apparatus
Subjects were 24 experimentally naïve adult male rats, of which 12 were exposed to amphetamine and 12 to saline injections. Housing, diet and drug administration were identical to Experiment 1.
Behavioral Procedures
Pavlovian conditioning, instrumental conditioning and PIT tests were carried out using identical procedures to Experiment 1.
Amphetamine administration
As in experiment 1, rats were given 7 daily amphetamine injections between training and the PIT test. Control animals received saline injections.
Locomotor activity assay
Rats were individually placed in an activity monitoring arena equipped with an automated locomotor activity detection system (Accuscan, Columbus OH, USA). Rats were placed in the arena for a 30 min habituation session. Activity was monitored for 30 min after rats were injected with saline or with 1 mg/kg amphetamine.
Results
Pavlovian conditioning
Rats acquired a conditioned approach response. As in Experiment 1, rats repeatedly exposed to amphetamine demonstrated a greater rate of food-cup approach during the CS compared to saline-treated rats during the reminder test (independent samples t-test: t22 = 2.30, P < 0.05) (Figure 5).
Figure 5. Pavlovian approach (Experiment 2).

Head insertions during CS presentation were significantly greater in amphetamine-sensitized rats compared to control rats. Data are depicted as CS minus baseline response rates. Error bars = ± 1 SEM.
Instrumental conditioning and PIT test
Rats acquired an instrumental response, and, as in Experiment 1, no effect of amphetamine was observed on instrumental responding. Similar to Experiment 1, previous amphetamine exposure significantly interacted with CS presentation to influence instrumental performance during PIT (ANOVA: F 1, 22 = 5.98, P < 0.05) (Figure 6). Outcome-selective transfer was observed among saline-treated rats, as shown by their performing significantly fewer Mismatch responses compared to Match responses (paired t-test: t11 = 2.69 P < 0.05). Among amphetamine-treated rats a similar rate of Match and Mismatch responding was observed, indicating a lack of outcome-selective PIT.
Figure 6. Pavlovian-instrumental transfer (PIT) performance (Experiment 2).
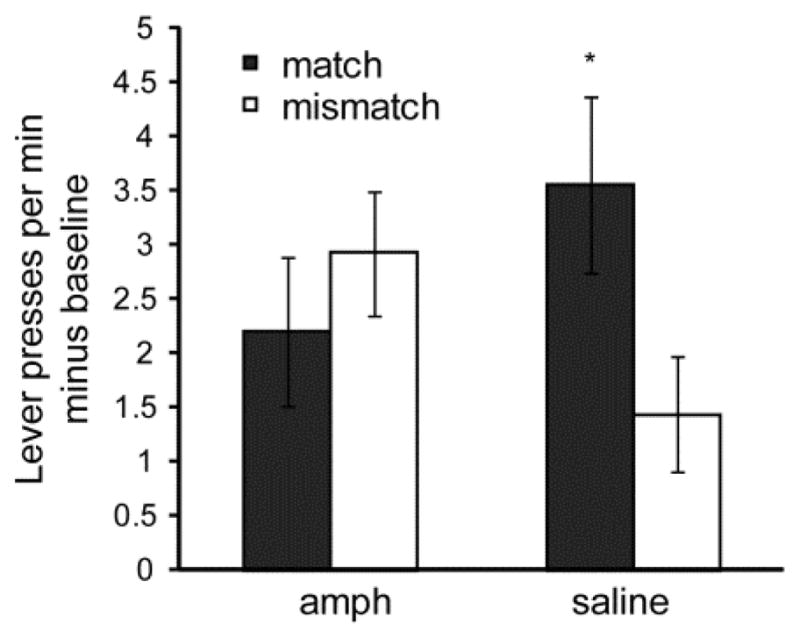
Response rates minus baseline for Match and Mismatch trials are shown for amphetamine-sensitized and control rats. * = significantly different from saline Mismatch rate. Error bars = ± 1 SEM.
Sensitization test
Amphetamine challenge significantly increased locomotor activity in all rats (ANOVA: F1, 22 = 74.05, P < 0.01) (Figure 7). Previous exposure to amphetamine significantly interacted with amphetamine challenge to alter locomotor activity (F1, 22 = 5.99 P < 0.05). Amphetamine-exposed rats demonstrated significantly greater locomotor activity following amphetamine challenge compared to drug-naïve rats (independent samples t-test: t22 = 2.43, P < 0.05).
Figure 7. Locomotor responses to amphetamine challenge.
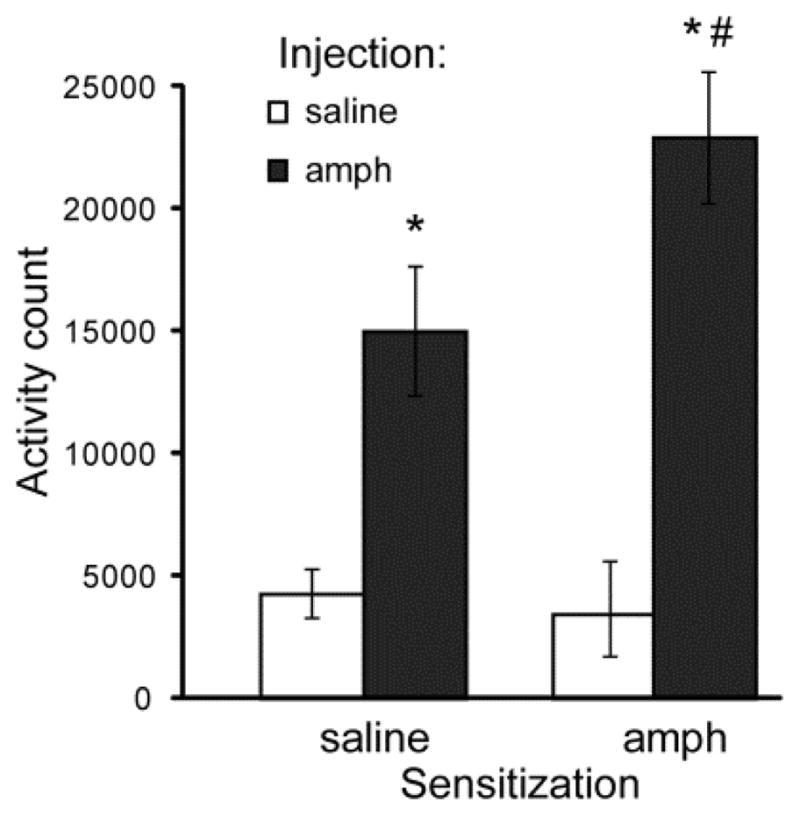
Locomotor activity was assessed in amphetamine-exposed rats or control rats after injections of saline (white bars) and after 1.0 mg/kg amphetamine (black bars). * = significantly different from saline injection. # = significantly different from control rats injected with amphetamine. Error bars = ± 1 SEM.
Experiment 3: Effects of amphetamine sensitization on Pavlovian backward conditioning and outcome-selective PIT
Rationale
The results of Experiments 1 and 2 demonstrate enhanced conditioned approach and impaired outcome-selective PIT following amphetamine sensitization. Experiment 3 was carried out to determine whether these two effects on behavior are related. To achieve this, a backward conditioning procedure was used, in which outcome delivery precedes CS presentation. This form of conditioning produces excitatory or inhibitory associations between the CS and the outcome, depending on the amount of training and the UCS-CS interval (Barnet and Miller 1996; Delamater et al. 2003; Heth 1976; Maier et al. 1976; Tait and Saladin 1986). Although the CS may not elicit conditioned approach following backward conditioning, rats are still capable of associating a CS with its specific outcome. Given these properties, the effects of amphetamine sensitization on PIT using a backward CS were used to dissociate the effects of amphetamine sensitization on conditioned approach from its effects on outcome-selective responding during PIT.
Subjects and apparatus
Subjects were 32 experimentally naïve adult male rats, of which 17 were exposed to amphetamine and 15 to saline injections. Housing, diet and drug administration were identical to Experiment 1.
Behavioral Procedures
Pavlovian backward conditioning
Rats first underwent a habituation session to familiarize themselves with the test chamber, after which they underwent 9 daily training sessions. During each training session, a volley of three food pellets (either chocolate or grain) was dispensed, and each volley was dispensed according to a variable-time schedule that averaged 4 min. Ten sec after food pellet delivery an auditory stimulus was presented for 60 sec. For half the rats, the tone was presented after grain pellet delivery and the white-noise after chocolate pellet delivery, whereas the remaining rats experienced the opposite stimulus-outcome pairings. Rats experienced 8 trials per session, four with each type of food pellet. For each trial, the number of head insertions into the food magazine was recorded during the 60 sec prior to food delivery and during the 60 sec auditory stimulus presentation.
Instrumental conditioning and reminder sessions
Instrumental conditioning took place following Pavlovian conditioning. Instrumental training was performed identically to Experiment 1.
PIT test
The PIT test was conducted identically to Experiment 1, except that the auditory stimulus was 1 min in length and the interval between stimulus presentations was 2 min. Lever presses were recorded during stimulus presentations and for a 1 min period prior to stimulus presentations.
Results
Pavlovian backward conditioning
On the final training trial, rats made fewer head insertions into the food magazine during the CS compared to the pre-CS period (F1, 30 = 39.62, P < 0.01) (Figure 8). However, amphetamine exposure did not alter approach behavior during the reminder session (P > 0.1).
Figure 8. Approach to a backward conditioned stimulus.
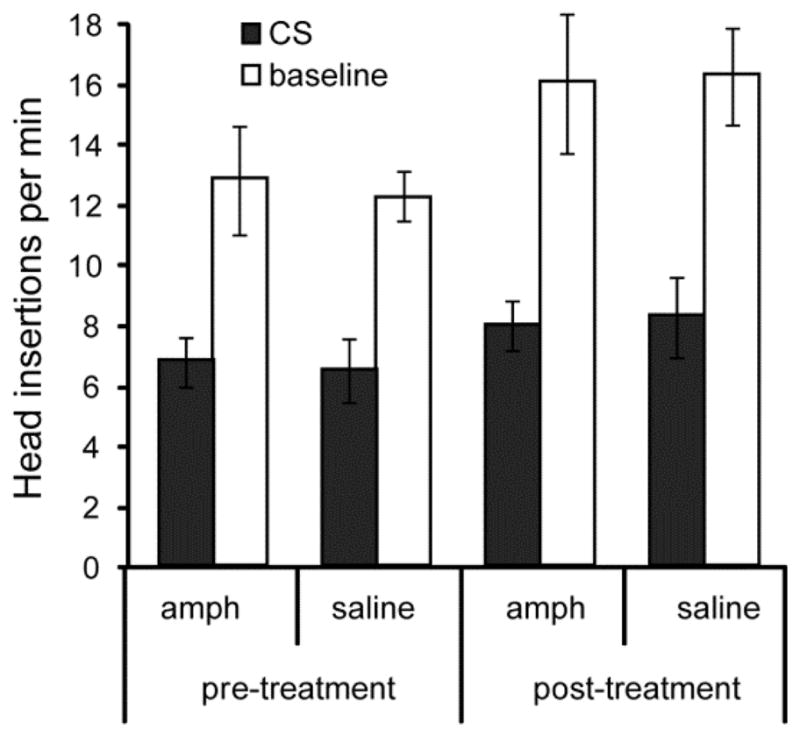
Food cup approach was measured during the CS which preceded food pellet delivery, or during baseline. The left set of bars depicts approach behavior prior to amphetamine treatment, whereas the right set of bars depicts approach behavior following amphetamine or saline treatment. * = significantly different from baseline responding. Error bars = ± 1 SEM.
Outcome-selective PIT test
As in Experiments 1 and 2, responses during the PIT test were categorized as Match or Mismatch, depending on whether the instrumental outcome was the same or different from the outcome associated with the auditory stimulus during Pavlovian backward conditioning. Amphetamine significantly interacted with CS presentation to alter performance during PIT (ANOVA: F1, 30 = 15.40, P < 0.01) (Figure 9). Outcome-selective transfer was observed among saline-treated rats, as shown by their performing significantly fewer Mismatch responses compared to Match responses (Paired t-test: t14 = 3.09, P < 0.01). In contrast, amphetamine-treated rats showed no evidence of outcome-selective transfer, performing Match and Mismatch responses at similar rates.
Figure 9. PIT performance to a backward CS.
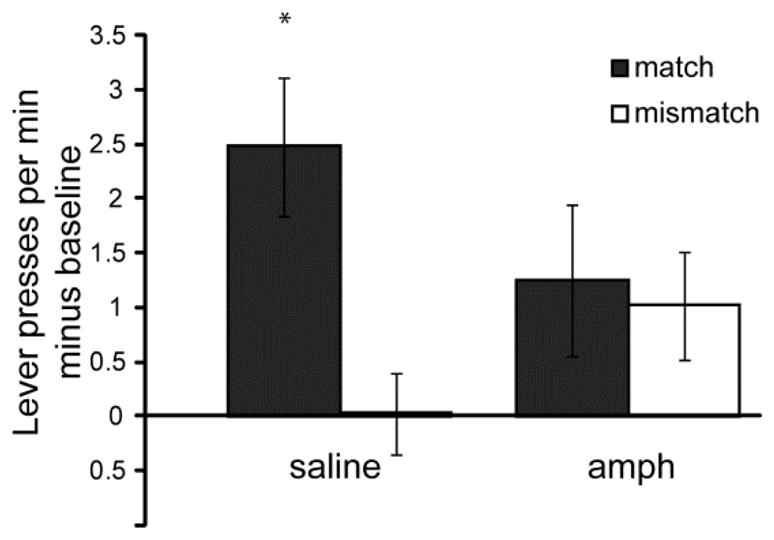
(A) Instrumental responses were classified as a Match if the CS and instrumental response shared a common outcome, whereas responses were classified as a Mismatch if the response and the CS did not share an outcome. Responses during an equivalent pre-CS (baseline) period were subtracted from responses made during CS presentation. * = significantly different from saline Mismatch rate. Error bars = ± 1 SEM.
Discussion
The findings from this study indicate that amphetamine sensitization alters the manner in which Pavlovian associations influence reward-seeking behavior. Specifically, amphetamine sensitization prevented outcome-selective responding during PIT tests. Similar outcome-insensitive behavior has been observed in cocaine-sensitized rats performing a Pavlovian outcome devaluation task (Schoenbaum and Setlow 2005). One explanation for these results is that amphetamine sensitization may enhance control by the system responsible for general incentive motivation, and this enhancement may supersede the performance of stimulus-outcome based responding. This explanation is consistent with the notion that amphetamine sensitization involves a broadening of incentive motivation to non-drug reinforcers, which has been observed in animal studies (Afonso et al. 2009; Corbit and Janak 2007; Fiorino and Phillips 1999; Wyvell and Berridge 2001). In the present experiments, the PIT test was conducted with a single lever, and therefore rats had a single response option, which may have promoted responding in an outcome-insensitive manner. Nevertheless, control animals still showed a reduction in responding during Mismatch presentations, indicating that outcome-sensitive responding is not dependent on having two response options.
The results from Experiment 3 suggest that amphetamine sensitization has distinct effects on conditioned approach and outcome-selective responding during PIT. Following training in a backward conditioning procedure, the CS was found to have opposite effects on conditioned approach and responding during PIT, and amphetamine sensitization appears to have affected only the latter form of responding. These results indicate that the enhanced conditioned magazine approach observed in Experiments 1 and 2 is likely to be independent of the effects of amphetamine sensitization on outcome-selective PIT. Indeed, a number of studies have shown a lack of correspondence between measures of conditioned approach and responding during PIT (Delamater and Holland 2008; Delamater and Oakeshott 2007; Holmes et al. 2010; van den Bos et al. 2004), suggesting that these forms of responses have different neural underpinnings. The lack of effect of amphetamine sensitization on conditioned inhibition of magazine approach following backward conditioning may be because inhibitory and excitatory effects of stimuli appear to rely on separate neural circuits and are differentially affected by amphetamine exposure (Murphy et al. 2008).
In contrast to our results, previous studies have found outcome-selective conditioned inhibition during PIT following backward conditioning (Delamater et al. 2003). Backward conditioning can produce inhibitory or excitatory associations between the stimulus and the outcome, depending on the amount of training, the interval between outcome and CS presentation, and the size of the reinforcer (Barnet and Miller 1996; Delamater et al. 2003; Heth 1976; Maier et al. 1976; Tait and Saladin 1986). The inhibitory effects of the CS on approach and excitatory effects on PIT observed in Experiment 3 may be a consequence of the number of backward conditioning training trials and the differential rates of acquisition of conditioned approach and stimulus-outcome learning. With extended training, the CS in backward conditioning switches from having an excitatory to an inhibitory effect on behavior (Heth 1976). Given that conditioned approach and outcome-selective PIT are acquired at different rates (Delamater and Oakeshott 2007), it may be that in the present study the switch from excitatory to inhibitory effects occurred only for the associations underlying approach but not for the associations guiding PIT.
Rats showed no effects of amphetamine exposure on an outcome-reinstatement paradigm or on outcome-sensitive responding following devaluation. These results eliminate alternative explanations for the lack of outcome-selective PIT. For example, impairments in outcome-selective PIT could be attributed to impaired use of outcome-response associations, based on the fact that outcome-selective responding during PIT requires the use of outcome information supplied by the CS to select the appropriate instrumental action (Balleine and Ostlund 2007; Delamater 1997). However, when provided with the outcome itself, amphetamine-sensitized rats showed normal outcome-selective reinstatement. Additionally, amphetamine-sensitized rats were unimpaired on a choice task following outcome devaluation, indicating intact response-outcome associations. Our findings are similar to previous reports that when amphetamine sensitization occurs after learning it has no effect on choice performance following devaluation (Nelson and Killcross 2006; Nordquist et al. 2007). Amphetamine sensitization prior to learning, however, has been reported to impact subsequent choice behavior (Nelson and Killcross 2006; Nordquist et al. 2007). The paradigm used in the present studies sought to avoid an effect due to altered learning by having sensitization occur between training and test; however, rats did receive re-training sessions after amphetamine exposure, which allows for the possibility that altered learning may have played some role. Arguing against this possibility, however, enhanced conditioned approach was observed on the first trial of the Pavlovian reminder session, which suggests that the rats possessed an altered response to the CS prior to any re-training sessions. Unlike previous studies, no effects of amphetamine sensitization on instrumental response rates were observed (Klein et al. 2007; Mendez et al. 2009; Nordquist et al. 2007). One possibility for this difference is that the response requirement used here was not appropriate to detect effects of amphetamine sensitization.
Alterations in behavior to drugs of abuse reflect, in part, an underlying change in how stimuli motivate action. PIT provides a measure of how much a cue can induce a state of “wanting” for a particular outcome; hence, an enhancement of PIT may be interpreted in terms of drug exposure sensitizing the salience of incentives, thus making animals more responsive to reward-associated stimuli (Robinson and Berridge 1993). The results presented here suggest that part of the sensitization process may involve an attenuation of control over behavior by stimulus-outcome representations, brought about by enhancing general incentive motivation elicited by the CS.
Acknowledgments
This research was supported by grant 026559 from the National Institute on Drug Abuse. I would like to thank Sara Ferreira, Meaghan Riccie and Claudia DeAngelo for assistance with collecting instrumental data and Joan Morrell, Julia Basso, and Leah Dziopa for assistance with collecting locomotor activity data.
Footnotes
The author reports no conflict of interest.
References
- Afonso VM, Mueller D, Stewart J, Pfaus JG. Amphetamine pretreatment facilitates appetitive sexual behaviors in the female rat. Psychopharmacology (Berl) 2009;205:35–43. doi: 10.1007/s00213-009-1511-x. [DOI] [PubMed] [Google Scholar]
- Balleine BW, Killcross S. Parallel incentive processing: an integrated view of amygdala function. Trends Neurosci. 2006;29:272–279. doi: 10.1016/j.tins.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Balleine BW, Ostlund SB. Still at the choice-point: action selection and initiation in instrumental conditioning. Ann N Y Acad Sci. 2007;1104:147–71. doi: 10.1196/annals.1390.006. [DOI] [PubMed] [Google Scholar]
- Barnet RC, Miller RR. Second-order excitation mediated by a backward conditioned inhibitor. J Exp Psychol Anim Behav Process. 1996;22:279–96. doi: 10.1037//0097-7403.22.3.279. [DOI] [PubMed] [Google Scholar]
- Blaiss CA, Janak PH. Post-training, but not post-reactivation, administration of amphetamine and anisomycin modulates Pavlovian conditioned approach. Neurobiol Learn Mem. 2007;87:644–58. doi: 10.1016/j.nlm.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blundell P, Hall G, Killcross S. Lesions of the Basolateral Amygdala Disrupt Selective Aspects of Reinforcer Representation in Rats. J Neurosci. 2001;21:9018–9026. doi: 10.1523/JNEUROSCI.21-22-09018.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwill RM, Motzkin DK. Encoding of the unconditioned stimulus in Pavlovian conditioning. Anim Learn Behav. 1994;22:384–394. [Google Scholar]
- Corbit LH, Balleine BW. The General and Outcome-Specific Forms of Pavlovian-Instrumental Transfer Are Differentially Mediated by the Nucleus Accumbens Core and Shell. The Journal of Neuroscience. 31:11786–11794. doi: 10.1523/JNEUROSCI.2711-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Balleine BW. Double Dissociation of Basolateral and Central Amygdala Lesions on the General and Outcome-Specific Forms of Pavlovian-Instrumental Transfer. J Neurosci. 2005;25:962–970. doi: 10.1523/JNEUROSCI.4507-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Balleine BW. The General and Outcome-Specific Forms of Pavlovian-Instrumental Transfer Are Differentially Mediated by the Nucleus Accumbens Core and Shell. The Journal of Neuroscience. 2011;31:11786–11794. doi: 10.1523/JNEUROSCI.2711-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Janak PH. Ethanol-Associated Cues Produce General Pavlovian-Instrumental Transfer. Alcoholism: Clinical and Experimental Research. 2007;31:766–774. doi: 10.1111/j.1530-0277.2007.00359.x. [DOI] [PubMed] [Google Scholar]
- Delamater AR. Selective reinstatement of stimulus-outcome associations. Anim Learn Behav. 1997;25:400–412. [Google Scholar]
- Delamater AR, Holland PC. The influence of CS-US interval on several different indices of learning in appetitive conditioning. Journal of Experimental Psychology-Animal Behavior Processes. 2008;34:202–222. doi: 10.1037/0097-7403.34.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delamater AR, LoLordo VM, Sosa W. Outcome-specific conditioned inhibition in Pavlovian backward conditioning. Learning & Behavior. 2003;31:393–402. doi: 10.3758/bf03196000. [DOI] [PubMed] [Google Scholar]
- Delamater AR, Oakeshott S. Learning about multiple attributes of reward in Pavlovian conditioning. Ann N Y Acad Sci. 2007 doi: 10.1196/annals.1390.008. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Wolf ME. Psychomotor Stimulant Addiction: A Neural Systems Perspective. J Neurosci. 2002;22:3312–3320. doi: 10.1523/JNEUROSCI.22-09-03312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorino DF, Phillips AG. Facilitation of sexual behavior and enhanced dopamine efflux in the nucleus accumbens of male rats after D-amphetamine-induced behavioral sensitization. J Neurosci. 1999;19:456–63. doi: 10.1523/JNEUROSCI.19-01-00456.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall DA, Gulley JM. Disruptive effect of amphetamines on Pavlovian to instrumental transfer. Behav Brain Res. 2010;216:440–445. doi: 10.1016/j.bbr.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmer CJ, Phillips GD. Enhanced appetitive conditioning following repeated pretreatment with d-amphetamine. Behav Pharmacol. 1998;9:299–308. [PubMed] [Google Scholar]
- Heth CD. Simultaneous and backward fear conditioning as a function of number of CS-UCS pairings. J Exp Psychol Anim Behav Process. 1976;2:117–29. doi: 10.1037//0097-7403.2.2.117. [DOI] [PubMed] [Google Scholar]
- Holmes NM, Marchand AR, Coutureau E. Pavlovian to instrumental transfer: A neurobehavioural perspective. Neurosci Biobehav Rev. 2010;34:1277–1295. doi: 10.1016/j.neubiorev.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–98. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Klein ED, Gehrke BJ, Green TA, Zentall TR, Bardo MT. Repeated cocaine experience facilitates sucrose-reinforced operant responding in enriched and isolated rats. Learn Motiv. 2007;38:44–55. doi: 10.1016/j.lmot.2006.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konorski . Integrative Activity of the Brain. University of Chicago Press, University of Chicago Press; 1967. [Google Scholar]
- Kruse JM, Overmier JB, Konz WA, Rokke E. Pavlovian conditioned stimulus effects upon instrumental choice behavior are reinforcer specific. Learn Motiv. 1983;14:165–181. [Google Scholar]
- Maier SF, Rapaport P, Wheatley KL. Conditioned inhibition and the UCS-CS interval. Anim Learn Behav. 1976;4:217–20. doi: 10.3758/bf03214039. [DOI] [PubMed] [Google Scholar]
- Mendez IA, Williams MT, Bhavsar A, Lu AP, Bizon JL, Setlow B. Long-lasting sensitization of reward-directed behavior by amphetamine. Behavioural Brain Research. 2009;201:74–79. doi: 10.1016/j.bbr.2009.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendrek A, Blaha CD, Phillips AG. Pre-exposure of rats to amphetamine sensitizes self-administration of this drug under a progressive ratio schedule. Psychopharmacology (Berl) 1998;135:416–22. doi: 10.1007/s002130050530. [DOI] [PubMed] [Google Scholar]
- Murphy ER, Robinson ESJ, Theobald DEH, Dalley JW, Robbins TW. Contrasting effects of selective lesions of nucleus accumbens core or shell on inhibitory control and amphetamine-induced impulsive behaviour. European Journal of Neuroscience. 2008;28:353–363. doi: 10.1111/j.1460-9568.2008.06309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson A, Killcross S. Amphetamine Exposure Enhances Habit Formation. J Neurosci. 2006;26:3805–3812. doi: 10.1523/JNEUROSCI.4305-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordquist RE, Voorn P, de Mooij-van Malsen JG, Joosten RNJMA, Pennartz CMA, Vanderschuren LJMJ. Augmented reinforcer value and accelerated habit formation after repeated amphetamine treatment. European Neuropsychopharmacology. 2007;17:532–540. doi: 10.1016/j.euroneuro.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Oscos A, Martinez JL, Jr, McGaugh JL. Effects of post-training d-amphetamine on acquisition of an appetitive autoshaped lever press response in rats. Psychopharmacology (Berl) 1988;95:132–4. doi: 10.1007/BF00212781. [DOI] [PubMed] [Google Scholar]
- Pierre PJ, Vezina P. Predisposition to self-administer amphetamine: The contribution of response to novelty and prior exposure to the drug. Psychopharmacology. 1997;129:277–284. doi: 10.1007/s002130050191. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–91. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction. Annu Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B. Cocaine makes actions insensitive to outcomes but not extinction: Implications for altered orbitofrontal-amygdalar function. Cereb Cortex. 2005;15:1162–1169. doi: 10.1093/cercor/bhh216. [DOI] [PubMed] [Google Scholar]
- Simon N, Mendez I, Setlow B. Effects of prior amphetamine exposure on approach strategy in appetitive Pavlovian conditioning in rats. Psychopharmacology. 2009;202:699–709. doi: 10.1007/s00213-008-1353-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton MA, Karanian DA, Self DW. Factors that determine a propensity for cocaine-seeking behavior during abstinence in rats. Neuropsychopharmacology. 2000;22:626–41. doi: 10.1016/S0893-133X(99)00160-8. [DOI] [PubMed] [Google Scholar]
- Tait R, Saladin M. Concurrent development of excitatory and inhibitory associations during backward conditioning. Learning & Behavior. 1986;14:133–137. [Google Scholar]
- Taylor JR, Jentsch JD. Repeated intermittent administration of psychomotor stimulant drugs alters the acquisition of pavlovian approach behavior in rats: Differential effects of cocaine, d-amphetamine and 3,4-methylenedioxymethamphetamine (“ecstasy”) Biol Psychiatry. 2001;50:137–143. doi: 10.1016/s0006-3223(01)01106-4. [DOI] [PubMed] [Google Scholar]
- van den Bos R, van der Harst J, Vijftigschild N, Spruijt B, van Luijtelaar G, Maes R. On the relationship between anticipatory behaviour in a Pavlovian paradigm and Pavlovian-to-Instrumental Transfer in rats (Rattus norvegicus) Behavioural Brain Research. 2004;153:397–408. doi: 10.1016/j.bbr.2003.12.017. [DOI] [PubMed] [Google Scholar]
- Vezina P, Pierre PJ, Lorrain DS. The effect of previous exposure to amphetamine on drug-induced locomotion and self-administration of a low dose of the drug. Psychopharmacology. 1999;147:125–134. doi: 10.1007/s002130051152. [DOI] [PubMed] [Google Scholar]
- Wyvell CL, Berridge KC. Incentive sensitization by previous amphetamine exposure: increased cue-triggered “wanting” for sucrose reward. J Neurosci. 2001;21:7831–40. doi: 10.1523/JNEUROSCI.21-19-07831.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]