Abstract
Group I metabotropic glutamate receptors (mGluR1 and 5) are G protein coupled receptors that regulate neuronal activity in a number of ways. Some of the most well studied functions of group I mGluRs, such as initiation of multiple forms of mGluR-dependent long term depression, require receptor localization near the postsynaptic density (PSD). This localization is in turn dependent on the Homer family of scaffolding proteins which bind to a small motif on the distal C-termini of mGluR1 and 5, localize the receptors near the PSD, strengthen coupling to postsynaptic effectors and simultaneously uncouple the mGluRs from extra-synaptic effectors such as voltage dependent ion channels. Here the selectivity of this uncoupling process was examined by testing the ability of Homer-2b to uncouple mGluR1 from multiple voltage dependent calcium channels including CaV2.2 (N-typ e), CaV3.2 (T-type), and CaV2.1 (P/Q-type) expressed in rat sympathetic neurons from the superior cervical ganglion (SCG). Of these, only the mGluR1-CaV2.1 modulatory pathway was insensitive to Homer-2b expression. Uncoupling from this channel was achieved by coexpression of an mGluR1 C-terminal protein designed to disrupt a previously described direct interaction between these two proteins, suggesting that this interaction allows incorporation of CaV2.1 into the mGluR1/Homer signaling complex, thereby preserving modulation in the presence of scaffolding Homer proteins.
Keywords: G protein coupled receptor, calcium channel, sympathetic neuron, pharmacology
1. Introduction
1.1
Metabotropic glutamate receptors (mGluRs) are a family of G protein coupled receptors activated by the excitatory neurotransmitter glutamate. There are 8 mammalian mGluR genes with widespread expression in the central nervous system, and more limited expression peripherally (Schoepp, 2001). Two of the mGluRs (mGluR1 and 5) comprise the group I mGluR subfamily, which couple to the Gq/11 and Gi/o proteins and commonly exhibit postsynaptic expression at glutamatergic synapses (Hay and Kunze, 1994; Choi and Lovinger, 1996; Kammermeier and Ikeda, 1999; Schoepp, 2001).
Group I mGluRs localize near the postsynaptic density (PSD) through their interaction with the Homer family of scaffolding proteins, which anchor not only mGluR1/5 to the PSD but also several other proteins including IP3 receptors (IP3Rs) (Brakeman et al., 1997; Ehrengruber et al., 2004; Duncan et al., 2005), some transient receptor potential (TRP) cation channels (Yuan et al., 2003), dynamin (Gray et al., 2003), and others (Ehrengruber et al., 2004; Shiraishi-Yamaguchi et al., 2009). Interestingly, a natural dominant negative Homer subtype has been described which interacts with Homer binding partners, but does not act as a scaffold (Brakeman et al., 1997; Xiao et al., 1998; Tu et al., 1999). These so called “short” Homers (Homer-1a and Ania-3), exhibit regulated expression, and as such are upregulated following periods of strong neuronal activity (Brakeman et al., 1997; Kato et al., 1997). Interaction with the scaffolding, or “long,” Homers induces strong mGluR coupling to PSD localized effectors such as AMPA receptors (Kammermeier and Worley, 2007), and to synaptic responses such as mGluR-dependent forms of long-term plasticity (Kirschstein et al., 2007; Ronesi and Huber, 2008; Ueta et al., 2008). At the same time, the scaffolding Homers uncouple group I mGluRs from other effectors such as the plasma membrane ion channels CaV2.2 and KCNQ potassium channels (Kammermeier et al., 2000).
To date, long Homer protein mediated uncoupling from mGluR signaling has been demonstrated only for CaV2.2 channels and KCNQ channels (Kammermeier et al., 2000), but that uncoupling should occur from any effector localized outside the mGluR-Homer-PSD signaling complex. As such, any effector localized within this complex should remain coupled to mGluR1/5 in the presence of long Homers, even if these effectors are closely related to CaV2.2. Recently, a direct interaction between mGluR1 and CaV2.1 has been reported by Kitano et al. (Kitano et al., 2003), who showed that these proteins directly interact in expression systems and in cerebellar neurons. This interaction allowed us the opportunity to test the dogmatic model explaining the regulation of mGluR signaling by Homer proteins. Specifically, we hypothesized that the mGluR1-CaV2.1 interaction should allow incorporation of this channel into the mGluR-Homer signaling complex and thus preserve its modulation by mGluR1 when Homer proteins are expressed, in contrast to the mGluR1-CaV2.2 pathway (Kammermeier et al., 2000; Kammermeier, 2008). If this were the case, it would represent the first demonstration of group I mGluR mediated modulation of a voltage dependent channel that remains robust when scaffolding Homers are abundantly expressed. Thus, modulation of CaV2.1 currents by mGluR1, and its sensitivity to Homer protein over-expression, was examined in rat sympathetic neurons from the superior cervical ganglion (SCG), a system in which Homer-dependent regulation of mGluR signaling has been demonstrated (Kammermeier et al., 2000; Kammermeier, 2008; Won et al., 2009).
2. Materials and Methods
2.1 Electrophysiology and data analysis
Pipettes for patch-clamp experiments were generated using a Sutter (Novato, CA) P-97 horizontal puller with 8250 glass (Garner Glass, Claremont, CA) and had resistances of 1–3 MΩ. Series resistances were 1–5 MΩ prior to electronic compensation of 80%. Whole-cell patch-clamp recordings were made with an EPC-7 (Heka Elektronik, Germany) or Axon 200B (Molecular Devices, Sunnyvale, CA) patch clamp amplifier. Voltage protocol generation and data acquisition were performed using custom software (courtesy Stephen R. Ikeda, NIAAA, Rockville, MD) on a Macintosh G3 or G4 computer (Apple Computer, Cupertino, CA) with an InstruTech (Heka Elektronik) ITC-16 or ITC-18 data acquisition board. Currents were low-pass filtered at 3–5 kHz using the 4-pole Bessel filter in the patch clamp amplifiers, digitized at 2–5 kHz and stored on the computer for later analysis. Experiments were performed at 21–24 °C (room temperature). Patch-clamp data analysis was performed using the Igor Pro software package (Wavemetrics, Lake Oswego, OR).
The external (bath) recording solution contained (in mM): 155 tris hydroxymethyl aminomethane, 20 4-(2-Hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 10 glucose, 10 CaCl2, and 0.0003 tetrodotoxin (TTX), pH 7.4. The internal (pipette) solution contained: 120 N-methyl-D-glucamine (NMG) methanesulfonate, 20 TEA, 11 EGTA, 10 HEPES, 10 sucrose, 1 CaCl2, 4 MgATP, 0.3 Na2GTP, and 14 tris creatine phosphate, pH 7.2. L-Glutamate (Sigma) was used as the agonist for mGluRs. SNX482 (SNX) and ω-conotoxin GVIA (CTX) were obtained from Tocris Bioscience (Ellisville, MO). L-Glutamate was obtained from Sigma-Aldrich (St. Louis, MO). All drugs and control solutions were applied to cells using a custom, gravity-driven perfusion system positioned ~100 μm from the cell, allowing rapid solution exchange (≤250 ms). The degree of calcium current inhibition was calculated as the maximum current inhibition in the presence of drug compared to the last current measurement prior to drug application.
2.2 Primary culture preparation, cDNA injection, antibodies, and plasmid generation
A description of cell isolation and cDNA injection is found elsewhere (Ikeda et al., 1995; Lu et al., 2009). Animal protocols were approved by the university committee on animal resources (UCAR). Briefly, SCGs were removed from adult male Wistar rats (175–225 g) after CO2 euthanasia and decapitation, then incubated in Earle’s balanced salt solution (InVitrogen, Life Technologies Carlsbad, CA) containing 0.6 mg/ml trypsin (Worthington Biochemicals, Freehold, NJ) & 0.8 mg/ml collagenase D (Boehringer Mannheim Biochemicals, Indianapolis, IN) for 60 minutes at 35 °C. Cells were transferred to minimum essential medium (InVitrogen/Gibco), plated on poly-L-lysine (Sigma Chemical Co., St. Louis, MO) coated culture dishes and incubated at 37 °C for 2–4 hours before cDNA injection. Injected cells were incubated overnight at 37 °C (95% air and 5% CO2; 100% humidity) and patch clamp experiments were performed the next day. Anti-CaV2.1 antibody was obtained from Abcam (Cabbridge, MA). Anti-HA was obtained from Covance (Princeton, NJ). Both were used at a dilution of 1:500 for experiments shown in Fig. 1.
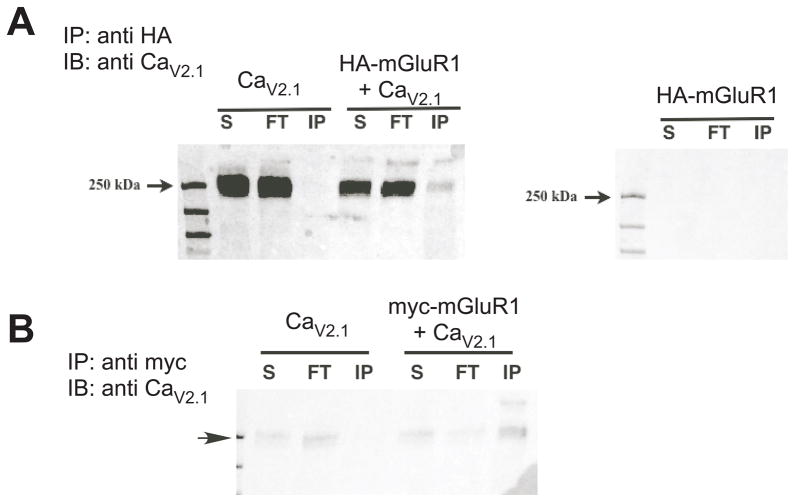
Fig. 1.
CaV2.1 co-immunoprecipitates with mGluR1 in HEK293 cells. A, Anti-CaV2.1 (1:500) immunoblot showing protein from supernatant (S), flow-through (FT) and from the anti-HA immunoprecipitation (1:500) experiment (IP), with expression conditions indicated above: CaV2.1 alone and HA-mGluR1 + CaV2.1 (left) and HA-mGluR1 alone (right). B, Similar to A, but using an anti-myc antibody paired with myc-mGluR1 for the IP. Arrow indicates 250 kd marker.
Injection of cDNA was performed with an Eppendorf 5247 microinjector and InjectMan NI 2 micromanipulator (Madison, WI) 3–5 hours following cell isolation. Injection electrodes were made with a Sutter P-97 horizontal electrode puller (Novato, CA) from thin-walled, borosilicate glass (World Precision Instruments, Sarasota, FL). Plasmids were stored at −20 °C as a 0.4 – 1 μg/μl stock solution in TE buffer (10 mM TRIS, 1 mM EDTA, pH 8). All mGluR constructs were injected at 100–130 ng μl−1 (pCDNA3.1+; InVitrogen). All neurons were co-injected with “enhanced” green fluorescent protein cDNA (0.02 μg/μl; pEGFPN1; BD Biosciences-Clontech, Palo Alto, CA) for identification of successfully injected cells.
Constructs were all sequence confirmed, and PCR products were purified with Qiagen (Valencia, CA) silica membrane spin columns prior to restriction digestion and ligation. Midipreps were prepared using Qiagen anion exchange columns, and amplified in either Top10 or DH5α E. coli. (InVitrogen).
3. Results
3.1 mGluR1 and CaV2.1 interact
To verify that an interaction between mGluR1 and CaV2.1 could be detected, we expressed in HEK293 cells a hemagglutinin (HA)- tagged mGluR1, CaV2.1 (with β2A and α2δ), or both constructs together and performed co-immunporecipitation experiments. Cell lysates were immunoprecipitated (IP) with an anti-HA antibody and blots probed using an anti CaV2.1 antibody. Results of these experiments are shown in Fig. 1, which also illustrates samples from the supernatant (S) and flow-through (FT), as well as the IP for each expression condition. A prominent band just under 250 kd, the expected size of CaV2.1, was evident in the lysates of CaV2.1 expressing cells and also appeared (although less prominently) in the IP only when the channel was co-expressed with HA-mGluR1 (Fig. 1A, HA-mGluR1a + CaV2.1, “IP” lane). Analogous experiments were performed using myc-tagged mGluR1 with CaV2.1 with similar results (Fig. 1B). These data confirm the findings of Kitano et al. (Kitano et al., 2003) of an interaction between mGluR1 and CaV2.1.
3.2 CaV2.1 channels can be expressed in rat sympathetic neurons and modulated by mGluR1
The long forms of the Homer scaffolding proteins (Homer 1b, 1c, 2 and 3) bind to a proline rich motif near the C-terminus of mGluR1 and 5 (Brakeman et al., 1997; Xiao et al., 1998; Tu et al., 1999), causing these receptors to form clusters (Kammermeier et al., 2000; Kammermeier, 2006), and organize around the post-synaptic density (Ango et al., 2000). As a result of this interaction, mGluR1/5-mediated modulation of voltage dependent, N-type calcium channels (CaV2.2) and M-type potassium channels (KV7) is disrupted (Kammermeier et al., 2000). The simplest explanation for this Homer-mediated uncoupling is that clustering of mGluR1/5 by Homers physically sequesters the receptors away from the voltage gated channels such that subsequent coupling is impaired. To date however, this model has not been directly tested. We reasoned that interaction between mGluR1 and CaV2.1 may preserve mGluR1-CaV2.1 modulation even in the presence of Homer protein over-expression (and mGluR1 clustering) since the channels may be incorporated into the mGluR1/Homer signaling domains that putatively exclude other voltage dependent channels, such as CaV2.2 and M-channels. To test this, we examined mGluR1 modulation of calcium currents in rat sympathetic neurons from the superior cervical ganglion (SCG), a system in which Homer-mediated uncoupling of mGluR1 from calcium current modulation has been demonstrated (Kammermeier et al., 2000; Kammermeier, 2008). Since these neurons do not express CaV2.1 channels natively, it was necessary to express them heterologously.
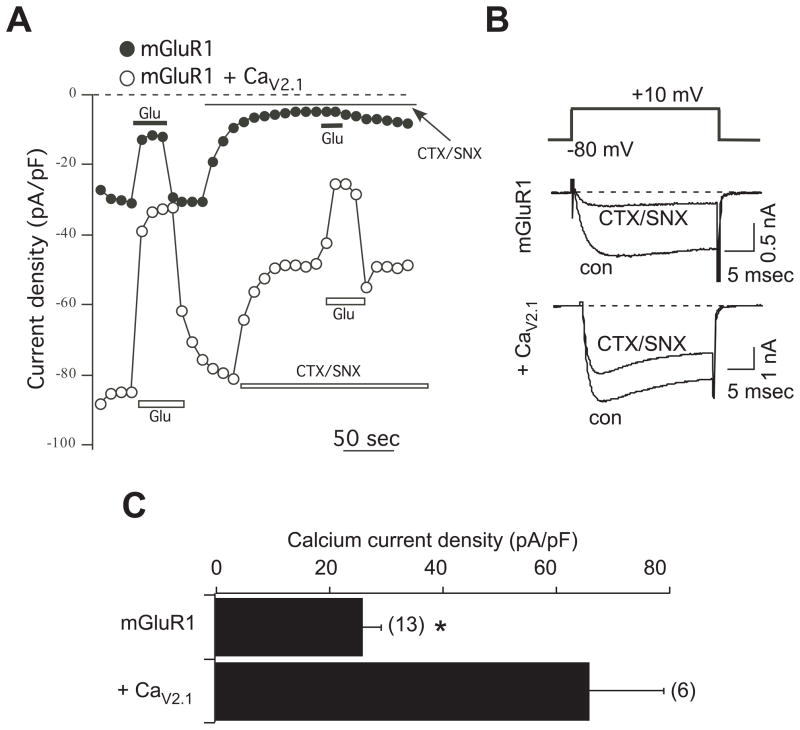
Calcium currents in SCG neurons expressing mGluR1 alone were strongly inhibited by the cocktail of 1 μM ω-conotixin GVIA, a selective CaV2.2 blocker (Reynolds et al., 1986), and 500 nM SNX482, a selective CaV2.3 blocker (Newcomb et al., 1998) (Fig. 2A). The toxin cocktail reduced calcium current density in 13 cells from 26 ± 3 pA/pF to 6 ± 2 pA/pF, a reduction of about 80% (Fig. 2B). In neurons co-expressing the αsubunitof CaV2.1, the toxin insensitive fraction of the current was greatly enhanced. In these cells, the toxin cocktail reduced the current from 66 ± 13 pA/pF to 37 ± 9 pA/pF in 6 cells, an inhibition of only 44% (Fig. 2B). Surprisingly, it was not necessary to co-express calcium current accessory subunits to induce CaV2.1 expression. Nevertheless, these data confirm that CaV2.1 channels can be effectively expressed in SCG neurons.
Fig. 2.
CaV2.1 expression in SCG neurons. A, Sample current density time courses from cells expressing mGluR1 alone (filled circles), or mGluR1 plus CaV2.1 (open circles). Current inhibition by 100 μM glutamate (Glu) is evident under both conditions. CTX/SNX indicates application of 1 μM ω-conotoxin GVIA and 500 nM SNX482. Current measurements were obtained 10 msec into a test pulse to +10 mV. B, Sample current traces from the same cells as in A. The voltage protocol is shown above, and consisted of a 25 msec step from a −80 mV holding potential to + 10 mV. Control (uninhibited) currents are shown (con) as are currents maximally inhibited by the CTX-SNX toxin combination. C, Average (± SEM) current densities for SCG neurons expressing mGluR1 alone or mGluR1 plus CaV2.1. Asterisk indicates that current densities are significantly different (T-test, p < 0.05).
Next, the ability of mGluR1 activation to modulate CaV2.1 currents was examined. As shown in the current density time courses in Fig. 2A, both the total current (before toxin application under both expression conditions) and the more isolated “CaV2.1 current” (in the presence of toxins in CaV2.1 expressing cells) was rapidly and reversibly inhibited upon application of 100 μM glutamate, demonstrating that CaV2.1 currents are modulated by mGluR1 in SCG neurons, which do not natively express any mGluRs (Kammermeier and Ikeda, 1999; Kammermeier and Yun, 2005). Indeed, in 5 uninjected control SCG neurons, application of 100 μM glutamate failed to inhibit the current, producing 1.2 ± 1.6% inhibition. These data confirm that SCG neurons represent a null mGluR background.
3.3 CaV2.1 current modulation is insensitive to Homer protein expression
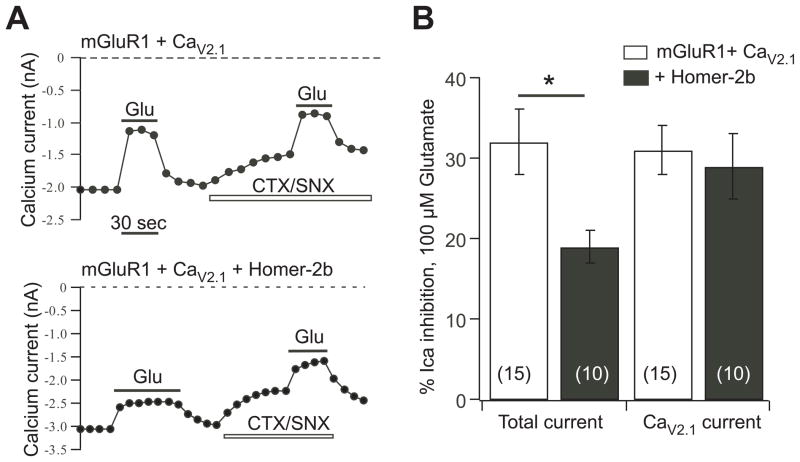
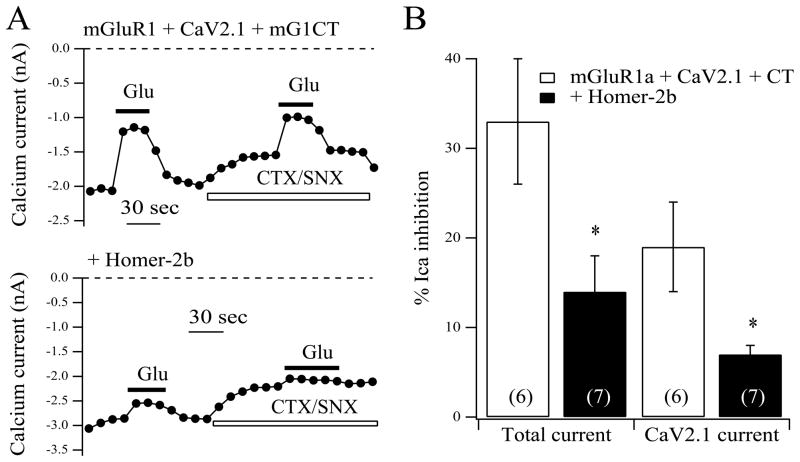
To test whether CaV2.1 currents are, by virtue of their interaction with mGluR1, insensitive to uncoupling by Homer protein expression, we examined their modulation via mGluR1 in the absence and presence of over-expressed Homer-2b, which strongly uncouples mGluR1 from modulating the native SCG calcium currents (Kammermeier et al., 2000; Won et al., 2009). Indeed, in control cells expressing mGluR1 and CaV2.1, the isolated CaV2.1 currents (in the presence of toxins) were inhibited by 31 ± 3% (n=15), and by 29 ± 4% (n=10) in Homer-2b co-expressing cells (Fig. 3A, B). In the same cells, the magnitude of the total calcium current inhibition was also examined, which includes CaV2.1 currents as well as the native CaV2.2 and CaV2.3 currents (Zhu and Ikeda, 1994), as an internal control for Homer-2b expression. Inhibition of the total current was significantly reduced by Homer-2b, from 32 ± 4% in the 15 control cells to 19 ± 2% in the 10 Homer-2b expressing cells (Fig. 3A, B). These data demonstrate that modulation of CaV2.1 currents in SCG neurons by mGluR1 is insensitive to Homer protein expression.
Fig. 3.
CaV2.1 current modulation by mGluR1 is insensitive to Homer protein expression. A, current amplitude time courses showing modulation by glutamate (Glu) of the total current and the isolated CaV2.1 current (in the presence of CTS/SNX) for SCG neurons expressing mGluR1 and CaV2.1 (upper) or mGluR1, CaV2.1, and Homer-2b (lower). B, average (± SEM) calcium current inhibition by 100 μM glutamate of the total current (left) and isolated CaV2.1 current (right) for neurons with the indicated expression. Number of cells for each condition shown in parentheses.
3.4 Over-expression of other voltage dependent calcium channels in SCG neurons does not result in Homer insensitivity
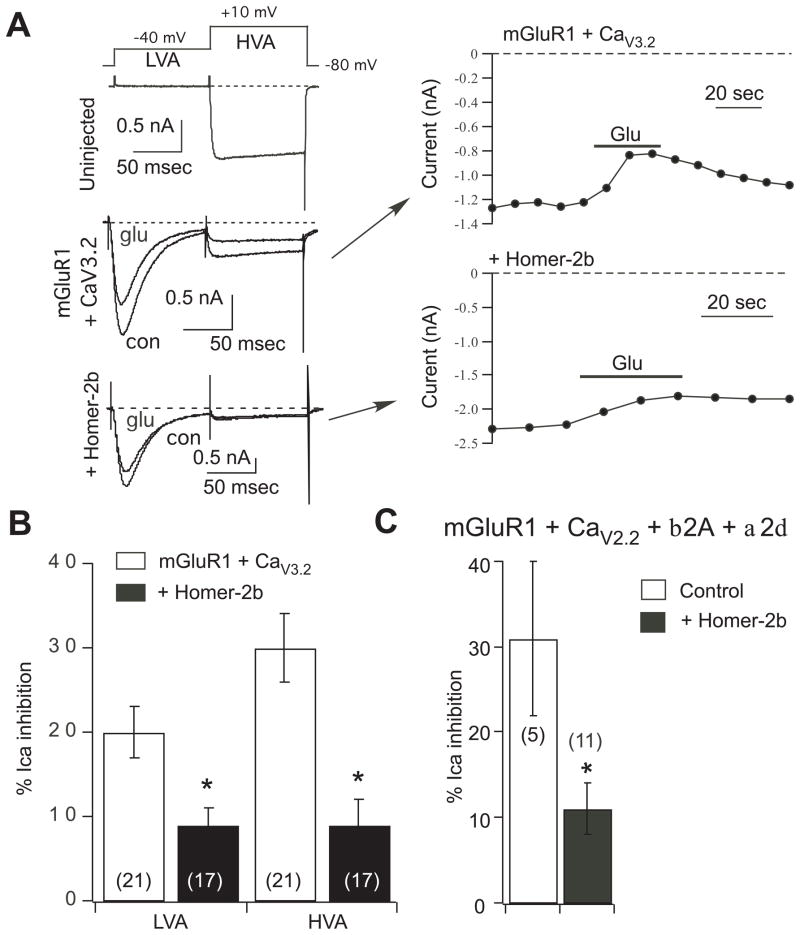
To ascertain whether the modulation of CaV2.1 currents by mGluR1 in the presence of Homer-2b was the result of channel over-expression, modulation of two other over-expressed voltage dependent calcium channels in SCG neurons was examined in the absence and presence of Homer-2b (Fig. 4). First, the expression and mGluR1 modulation of the T-type calcium channel CaV3.2 (Perez-Reyes et al., 1998) was examined. As expected, co-expression of CaV3.2 in SCG neurons resulted in a prominent, low-voltage activated, rapidly inactivating component of the calcium current (Fig. 4A). These currents were elicited and separated from the native calcium currents using a two step voltage protocol shown in Fig. 4A (upper). From a holding potential of −80 mV, 100 msec voltage steps were applied to −40 mV to elicit and inactivate the low-voltage activated CaV3.2 current (“LVA”), then to +10 mV to elicit the native, high-voltage activated currents (“HVA”). A sample current trace from an uninjected control cell (Fig. 4A) shows that the LVA step did not elicit any current. However, when CaV3.2 channels were expressed a prominent, rapidly inactivating current was evident during the LVA step. The amplitude of this current varied widely from less than 10 to about 55 pA/pF, but was detectable in each CaV3.2 expressing cell examined, and never seen in uninjected cells. By the end of the 100 msec LVA step, nearly all of the CaV3.2 current was inactivated, and therefore the current elicited during the HVA step was made up of almost entirely natively expressed calcium currents. Note that the driving force for calcium current is considerably smaller at +10 mV than at −40 mV, so the contribution of CaV3.2 channels to the HVA current is much smaller than appears from a cursory glance at the current traces in Fig. 4A.
Fig. 4.
Overexpression of other voltage dependent calcium channels in SCG neurons does not preserve coupling to mGluR1 in the presence of Homer-2b. A, sample current traces (left) and time courses (right) illustrating the two-step voltage protocol (top) used to separate low voltage activated (CaV3.2) currents (LVA) from the native high voltage activated currents (HVA), and inhibition by 100 μM glutamate. Uninjected SCG neurons (upper left) showed no LVA currents. Neurons injected with mGluR1 and CaV3.2 (middle left and upper right) had prominent LVA and HVA currents, both of which were modulated by 100 μM glutamate (Glu). Additional co-expression of Homer-2b (lower left and lower right) uncoupled both the LVA and HVA currents from glutamate modulation. B, Average (± SEM) LVA and HVA calcium current inhibition by 100 μM glutamate in cells with the indicated expression. Number of cells is shown in parentheses. C, Average (± SEM) calcium current inhibition by 100 μM glutamate in control cells (expressing mGluR1, CaV2.2, and accessory proteins) and in cells co-expressing Homer-2b. Asterisk indicates a statistically significant difference from paired control (without Homer-2b; p ≤ 0.05). Number of cells is shown in parentheses.
Next, the ability of CaV3.2 currents to be modulated by mGluR1 was examined by applying 100 μM glutamate to SCG neurons co-expressing mGluR1 and CaV3.2. Indeed, a rapid and reversible inhibition of the current was observed, averaging 20 ± 3% (n=21). Although there are a plethora of T-type calcium modulatory pathways described in the literature, most of the CaV3.2 inhibitory mechanisms described are G protein mediated (Chemin et al., 2006), and thus appropriate for comparison to the CaV2 modulation studied here. In the same cells, the native HVA currents were inhibited by 30 ± 4%. Interestingly, in neurons in which Homer-2b was co-expressed, modulation of both the LVA and HVA components was significantly reduced to 9 ± 2% and 9 ± 3%, respectively (n=17). These data confirm that mGluR1-mediated modulation of an over-expressed calcium channel in SCG neurons can be effectively uncoupled by Homer protein expression.
To compare the Homer protein sensitivity of mGluR1 modulation of a more closely related, over-expressed calcium channel, experiments were performed in SCG neurons over-expressing CaV2.2 (beyond native expression levels). Interestingly, co-injection of just the CaV2.2 subunit cDNA was not sufficient to increase the current density in SCG neurons (not shown). Therefore, it was necessary to co-inject small amounts of cDNA for the calcium channel accessory subunits β2A and α2δ (Campbell et al., 1988; Ellis et al., 1988) in each of the CaV2.2-expressing cells. In these cells, the average calcium current density was increased to 118 ± 14 pA/pF (n=16) from the typical value of around 30 pA/pF for control cells measured under identical conditions. As expected, the over-expressed CaV2.2 currents appeared to be modulated to a similar degree as the native currents, and were significantly uncoupled upon Homer-2b expression. 100 μM glutamate applied to cells expressing mGluR1 and CaV2.2 in the absence and presence of Homer-2b were inhibited by 31 ± 9% (n=5) and 11 ± 3% (n=11), respectively. These data provide further evidence that the observed insensitivity to Homer-mediated uncoupling from mGluR1 modulation of CaV2.1 is not the result of channel over-expression.
3.5 Expression of an mGluR1 C-terminal construct imparts Homer sensitivity to CaV2.1 modulation
To determine whether the interaction between mGluR1 and CaV2.1 was responsible for preserving coupling in the presence of Homer-2b, an mGluR1 C-terminal protein was co-expressed to disrupt the interaction. Kitano et al. (Kitano et al., 2003) demonstrated that the interaction between the two proteins was localized to their C-termini, so over-expression of the cytoplasmic C-tail of mGluR1 should compete for binding to the CaV2.1 tail and prevent or reduce binding of the full length mGluR1. Thus a construct (mG1-CT) consisting of the C-tail of mGluR1, truncated just before the canonical Homer binding site (amino acids 842–1150 of mGluR1a) and fused to CFP to verify expression at its N-terminus, was made and expressed in SCG neurons with mGluR1 or mGluR1 + Homer-2b (Fig. 5). Deletion of the Homer binding site from the CT construct was necessary to insure that it did not act to sequester expressed Homer-2b. When mG1-CT was co-expressed with mGluR1 and similar experiments as those reported in Fig. 3 (above) were performed, both the total calcium current and the isolated CaV2.1 currents (in the presence of the toxin cocktail) were similar to control conditions. 100 μM glutamate produced an average inhibition in these cells of 33 ± 7% and 19 ± 5%, respectively (n=6; Fig. 5A, B). However, when Homer-2b was co-expressed with mGluR1 and mG1-CT, glutamate-mediated inhibition of both the total current and the isolated CaV2.1 current was significantly reduced to 14 ± 4% and 7 ± 1% (n=7), respectively (Fig. 5A, B). These data support the hypothesis that modulation of CaV2.1 by mGluR1 is protected from Homer-mediated uncoupling by virtue of its interaction with the C-tail of mGluR1. Further, since CaV2.1 modulation by mGluR1 was not significantly reduced upon expression of mG1-CT (in the absence of Homer-2b), it may be concluded that modulation per se does not require direct association between receptor and channel, but association seems to be required for modulation only when mGluR1 is organized into clusters by Homer-2b. Finally, because Homer-2b expression can uncouple the native currents from modulation by mGluR1 in the presence of CaV2.1, it can be inferred indirectly that association with the channel does not prevent Homer-induced clustering of mGluR1. That is, it appears unlikely that the mGluR1-CaV2.1 interaction prevents Homer binding to the mGluR1 C-tail.
Fig. 5.
CaV2.1 current modulation by mGluR1 is rendered sensitive to Homer protein expression when an mGluR1-CT construct is co-expressed to prevent the receptor-channel interaction. A, Current amplitude time courses showing modulation by glutamate (Glu) of the total current and the isolated CaV2.1 current (in the presence of CTS/SNX) for SCG neurons expressing mGluR1, mG1-CT and CaV2.1 (upper) or mGluR1,, mG1-CT, CaV2.1, and Homer-2b (lower). B, average (± SEM) calcium current inhibition by 100 μM glutamate of the total current (left) and isolated CaV2.1 current (right) for neurons with the indicated expression. Number of cells for each condition shown in parentheses. Asterisk indicates a statistically significant difference from paired control (without Homer-2b; p ≤ 0.05).
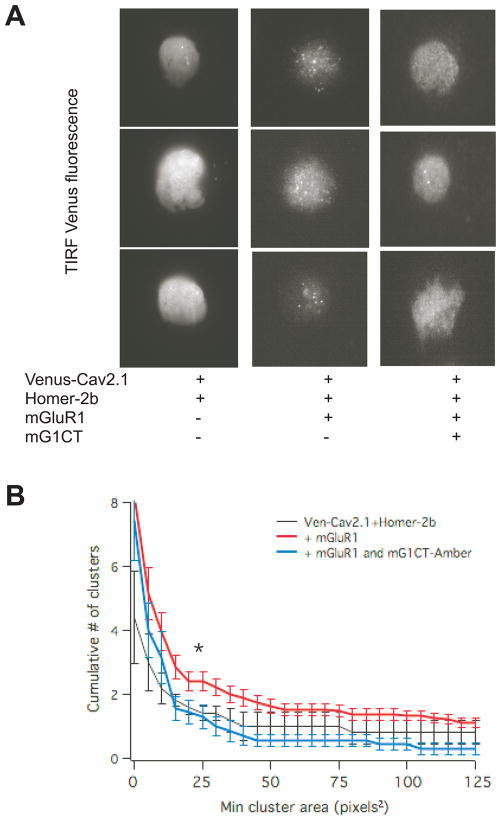
To independently verify that the mG1-CT construct could disrupt the interaction between mGluR1 and CaV2.1 in SCG neurons, a clustering assay was employed using a fluorescently tagged channel (Venus-CaV2.1). We hypothesized that since Homer proteins organize mGluR1 into clusters when co-expressed in SCG neurons (Kammermeier, 2006), clustering of Venus-CaV2.1 should be apparent when co-expressed with Homer-2b and mGluR1, but not with Homer-2b alone. Further, if mG1-CT prevents mGluR1-CaV2.1 association, it should also disrupt Venus-CaV2.1 clustering. For this experiment, a non-fluorescent version of mG1-CT was used. This construct was similar to mG1-CT but was tagged to the non-fluorescent FP variant Amber. The results of these experiments are shown in Fig. 6. Fig. 6A shows three sample total internal reflection fluorescence (TIRF) images of SCG neurons expressing Venus-CaV2.1 with Homer-2b (left), Homer-2b and mGluR1 (center), and with Homer-2b, mGluR1 and mG1-CT-Amber (right). In nearly all cells expressing Venus-CaV2.1 with Homer-2b and mGluR1, a punctate expression pattern was evident that was largely absent in cells from the other expression groups. However, perhaps predictably this clustering was less complete and less consistent than the clustering of mGluR1-GFP in the presence of Homer proteins reported previously (Kammermeier, 2006). Thus to quantify the observed clustering, a cumulative histogram of cluster number vs. size was generated from all of the TIRF images in each group. These data are shown in Fig. 6B. A detailed description of the quantification procedure is provided elsewhere (Kammermeier, 2006). A significant reduction in the number of clusters per cell was evident in neurons expressing mGluR1-CT-Amber from this analysis, of clusters ranging from 25 to 50 pixels2. Similarly sized clusters of mGluR1-GFP were evident in SCG neurons co-expressing Homer proteins in our previous report (Kammermeier, 2006). These data support our prediction that expression of mG1-CT can effectively disrupt the interaction between mGluR1 and CaV2.1 in SCG neurons, and support the interpretation that the loss of mGluR1-CaV2.1 coupling when Homer-2b and mG1-CT are co-expressed is due to this disruption.
Fig. 6.
Coexpression of mGluR1 and Homer-2b induces Venus-CaV2.1 clustering, which is reduced by expression of an untagged mGluR1-CT construct. A, TIRF fluorescence images showing three sample images each of SCG neurons with the indicated expression. B, Clusters were quantified with a cumulative histogram of cluster number vs. size (in pixels2). * indicates statistically significant difference between the number of clusters in cells expressing Venus-CaV2.1, mGluR1 and Homer-2b (red; n=19) compared to cells also expressing mGluR1-CT-Amber (blue; n=7). Cluster histogram for neurons expressing Ven-CaV2.1 + Homer-2b (n=5) are shown in black. Lines represent average clusters ± SEM at 5 pixels2 bins.
4. Discussion
4.1
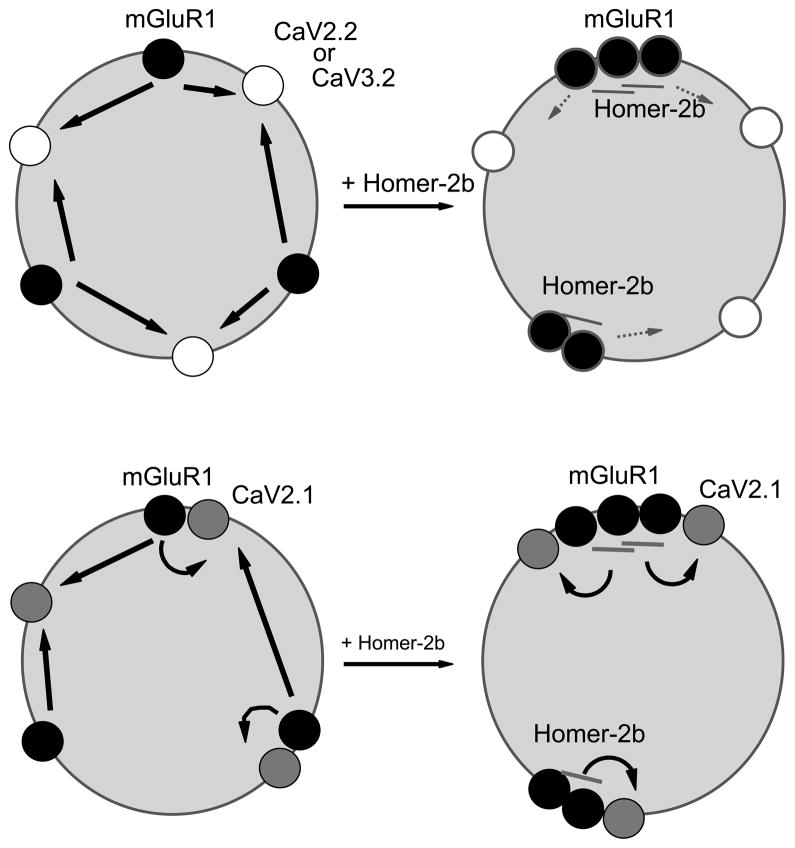
The dogmatic model to explain how Homer proteins regulate mGluR-effector coupling suggests that long, scaffolding Homers (Homer-1b, 1b, 2 and 3) bind to mGluR1 and 5 and organize the receptors into clusters (Kammermeier, 2006) in neuronal expression systems, or near the postsynaptic density (PSD) in native neurons (Ango et al., 2000). Clustered mGluRs represent a signaling mode in which coupling to effectors outside the clusters, specifically the voltage dependent ion channels CaV2.2 and KCNQ channels (Kammermeier et al., 2000), is disrupted while coupling to IP3 receptors (which are also Homer binding proteins) or other effectors within the PSD is strengthened. The second mode is represented by mGluR1 or 5 unbound to Homer proteins or bound to the natural dominant negative splice variants Homer-1a or Ania-3. Such receptors are unclustered and localized apart from the PSD. As such, they couple strongly to plasma membrane ion channels and are uncoupled from PSD localized effectors, such as AMPA receptors (Kammermeier and Worley, 2007), and functions, such as induction of multiple forms of mGluR-dependent plasticity (Ronesi and Huber, 2008; Ueta et al., 2008). The data in the present study further the model by illuminating a mechanism by which one voltage dependent calcium channel, CaV2.1, is selectively rendered exempt from mGluR1 uncoupling by Home protein expression by virtue of a direct interaction between the receptor and the channel (Fig. 7). In addition, these data show for the first time a Homer-dependent uncoupling of mGluR1 from the T-type calcium channel CaV3.2 (Fig. 7). This uncoupling is predicted by the dogmatic model but had not been previously demonstrated.
Fig. 7.
Revised model describing the regulation of mGluR signaling by Homer proteins. Upper model shows the traditional model in which unclustered, Homer-free mGluR1 couples strongly to voltage dependent calcium channels (CaV2.2 and CaV3.2). Interaction with scaffolding Homer proteins (Homer-2b) results in mGluR1 clustering and uncoupling from channel modulation. Lower model shows revised scenario in which interaction with and coupling to CaV2.1 is preserved in the presence and absence of clustering and Homer expression.
We have not addressed the possibility of an analogous interaction with mGluR5, nor did the Nakanishi group who first described the mGluR1-CaV2.1 interaction (Kitano et al., 2003), but these receptors share fairly high homology in the proximal C-tail believed to participate in the interaction (55% sequence identity in the first 94 amino acids of the respective C-tails). Thus, one may predict that a similar interaction could occur with mGluR5, the only other member of the group I mGluR family and only other Homer interacting mGluR (Brakeman et al., 1997).
It is interesting to speculate about the implications of these findings, particularly in the context of cerebellar Purkinje neurons, which express both mGluR1 and CaV2.1 at high levels (McDonough et al., 1997; Knöpfel and Grandes, 2002). CaV2.x channels are widely known as presynaptic calcium channels that mediate calcium entry into synaptic terminals during depolarization and thus trigger neurotransmitter release (Catterall, 2011). Indeed G protein mediated inhibition of the CaV2.x channels is a principally important pathway for presynaptic inhibition of neurotransmitter release. In cerebellar Purkinje neurons however, CaV2.1 channel expression is clearly not limited to presynaptic terminals. At least one study describes an apparent postsynaptic localization of CaV2.1 in Purkinje neurons (Kulik et al., 2004), in addition to its expression elsewhere in these cells. It is possible that the direct interaction between mGluR1 and CaV2.1 may be necessary for localization near the PSD. If so, one may extrapolate that this localization is thus indirectly dependent on Homer protein scaffolding, and that the principal site of importance of the findings in the present study would be at this site where we predict that strong voltage dependent calcium channel modulation via mGluR1 would be maintained. This would be in contrast to other neuronal subtypes, few of which express both mGluR1 and CaV2.1 at such high levels.
It is difficult to predict the precise physiological role of postsynaptic mGluR1-CaV2.1 coupling in Purkinje cells or why it is necessary to maintain with this specific receptor-channel pair, but it is possible that CaV2.1 channels strongly influence dendritic electrical properties and that this negative feedback pathway is necessary for proper dendritic signal integration. Alternatively, calcium levels near the PSD may require fairly tight regulation in Purkinje cells, as calcium entry through CaV2.1 has been linked to calcium-calmodulin dependent protein kinase II (CaMKII) activation. Further, this Purkinje cell CaV2.1-CaMKII pathway has been shown to regulate Homer phosphorylation which in turn can alter the apparent affinity for scaffolding Homer proteins for mGluRs (Mizutani et al., 2008). Thus it is possible that both CaV2.1 localization near the PSD in Purkinje cells as well as the negative feedback provided by mGluR1-mediated CaV2.1 inhibition postsynaptically may be an important feature of Purkinje cell physiology.
Highlights.
Modulation of CaV2.1 by mGluR1 is insensitive to Homer-2b expression.
Other calcium channels are uncoupled from mGluR1 by Homer-2b.
Preventing the interaction between mGluR1 and CaV2.1 permits Homer-uncoupling.
Acknowledgments
We thank D. I. Yule (University of Rochester) for reagents and D. Morrow (University of Rochester) for reagents and assistance with protein chemistry experiments.
Abbreviations
- mGluR
metabotropic glutamate receptor
- SCG
superior cervical ganglion
- Glu
L-glutamate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ango F, Pin JP, Tu JC, Xiao B, Worley PF, Bockaert J, Fagni L. Dendritic and axonal targeting of type 5 metabotropic glutamate receptor is regulated by homer1 proteins and neuronal excitation. Journal of Neuroscience. 2000;20:8710–8716. doi: 10.1523/JNEUROSCI.20-23-08710.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brakeman PR, Lanahan AA, O’Brien R, Roche K, Barnes CA, Huganir RL. Worley P.F. Homer: a protein that selectively binds metabotropic glutamate receptors. Nature. 1997;386:284–288. doi: 10.1038/386284a0. [DOI] [PubMed] [Google Scholar]
- Campbell KP, Leung AT, Sharp AH. The biochemistry and molecular biology of the dihydropyridine-sensitive calcium channel. Trends in Neurosciences. 1988;11:425–430. doi: 10.1016/0166-2236(88)90193-2. [DOI] [PubMed] [Google Scholar]
- Catterall WA. Voltage-gated calcium channels. Cold Spring Harb Perspect Biol. 2011;3:a003947. doi: 10.1101/cshperspect.a003947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemin J, Traboulsie A, Lory P. Molecular pathways underlying the modulation of T-type calcium channels by neurotransmitters and hormones. Cell Calcium. 2006;40:121–134. doi: 10.1016/j.ceca.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Choi S, Lovinger DM. Metabotropic glutamate receptor modulation of voltage-gated Ca2+ channels involves multiple receptor subtypes in cortical neurons. J Neurosci. 1996;16:36–45. doi: 10.1523/JNEUROSCI.16-01-00036.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan RS, Hwang S-Y, Koulen P. Effects of Vesl/Homer proteins on intracellular signaling. Exp Biol Med (Maywood) 2005;230:527–535. doi: 10.1177/153537020523000803. [DOI] [PubMed] [Google Scholar]
- Ehrengruber MU, Kato A, Inokuchi K, Hennou S. Homer/Vesl proteins and their roles in CNS neurons. Mol Neurobiol. 2004;29:213–227. doi: 10.1385/MN:29:3:213. [DOI] [PubMed] [Google Scholar]
- Ellis SB, Williams ME, Ways NR, Brenner R, Sharp AH, Leung AT, Campbell KP, McKenna E, Koch WJ, Hui A. Sequence and expression of mRNAs encoding the alpha 1 and alpha 2 subunits of a DHP-sensitive calcium channel. Science. 1988;241:1661–1664. doi: 10.1126/science.2458626. [DOI] [PubMed] [Google Scholar]
- Gray NW, Fourgeaud L, Huang B, Chen J, Cao H, Oswald BJ, Hémar A, McNiven MA. Dynamin 3 is a component of the postsynapse, where it interacts with mGluR5 and Homer. Curr Biol. 2003;13:510–515. doi: 10.1016/s0960-9822(03)00136-2. [DOI] [PubMed] [Google Scholar]
- Hay M, Kunze DL. Glutamate metabotropic receptor inhibition of voltage-gated calcium currents in visceral sensory neurons. J Neurophysiol. 1994;72:421–430. doi: 10.1152/jn.1994.72.1.421. [DOI] [PubMed] [Google Scholar]
- Ikeda SR, Lovinger DM, McCool BA, Lewis DL. Heterologous expression of metabotropic glutamate receptors in adult rat sympathetic neurons: subtype-specific coupling to ion channels. Neuron. 1995;14:1029–1038. doi: 10.1016/0896-6273(95)90341-0. [DOI] [PubMed] [Google Scholar]
- Kammermeier PJ, Ikeda SR. Expression of RGS2 alters the coupling of metabotropic glutamate receptor 1a to M-type K+ and N-type Ca2+ channels. Neuron. 1999;22:819–829. doi: 10.1016/s0896-6273(00)80740-0. [DOI] [PubMed] [Google Scholar]
- Kammermeier PJ, Worley PF. Homer 1a uncouples metabotropic glutamate receptor 5 from postsynaptic effectors. Proc Natl Acad Sci USA. 2007;104:6055–6060. doi: 10.1073/pnas.0608991104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammermeier PJ, Xiao B, Tu JC, Worley PF, Ikeda SR. Homer proteins regulate coupling of group I metabotropic glutamate receptors to N-type calcium and M-type potassium channels. Journal of Neuroscience. 2000;20:7238–7245. doi: 10.1523/JNEUROSCI.20-19-07238.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammermeier PJ, Yun J. Activation of metabotropic glutamate receptor 1 dimers requires glutamate binding in both subunits. J Pharmacol Exp Ther. 2005;312:502–508. doi: 10.1124/jpet.104.073155. [DOI] [PubMed] [Google Scholar]
- Kammermeier PJ. Surface clustering of metabotropic glutamate receptor 1 induced by long Homer proteins. BMC Neurosci. 2006;7:1. doi: 10.1186/1471-2202-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammermeier PJ. Endogenous homer proteins regulate metabotropic glutamate receptor signaling in neurons. Journal of Neuroscience. 2008;28:8560–8567. doi: 10.1523/JNEUROSCI.1830-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato A, Ozawa F, Saitoh Y, Hirai K, Inokuchi K. vesl, a gene encoding VASP/Ena family related protein, is upregulated during seizure, long-term potentiation and synaptogenesis. FEBS Letters. 1997;412:183–189. doi: 10.1016/s0014-5793(97)00775-8. [DOI] [PubMed] [Google Scholar]
- Kirschstein T, Bauer M, Muller L, Ruschenschmidt C, Reitze M, Becker AJ, Schoch S, Beck H. Loss of Metabotropic Glutamate Receptor-Dependent Long-Term Depression via Downregulation of mGluR5 after Status Epilepticus. Journal of Neuroscience. 2007;27:7696–7704. doi: 10.1523/JNEUROSCI.4572-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitano J, Nishida M, Itsukaichi Y, Minami I, Ogawa M, Hirano T, Mori Y, Nakanishi S. Direct interaction and functional coupling between metabotropic glutamate receptor subtype 1 and voltage-sensitive Cav2.1 Ca2+ channel. J Biol Chem. 2003;278:25101–25108. doi: 10.1074/jbc.M303266200. [DOI] [PubMed] [Google Scholar]
- Knöpfel T, Grandes P. Metabotropic glutamate receptors in the cerebellum with a focus on their function in Purkinje cells. Cerebellum (London, England) 2002;1:19–26. doi: 10.1007/BF02941886. [DOI] [PubMed] [Google Scholar]
- Kulik A, Nakadate K, Hagiwara A, Fukazawa Y, Luján R, Saito H, Suzuki N, Futatsugi A, Mikoshiba K, Frotscher M, Shigemoto R. Immunocytochemical localization of the alpha 1A subunit of the P/Q-type calcium channel in the rat cerebellum. Eur J Neurosci. 2004;19:2169–2178. doi: 10.1111/j.0953-816X.2004.03319.x. [DOI] [PubMed] [Google Scholar]
- Lu VB, Williams DJ, Won Y-J, Ikeda SR. Intranuclear microinjection of DNA into dissociated adult mammalian neurons. Journal of Visualized Experiments: JoVE. 2009 doi: 10.3791/1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough SI, Mintz IM, Bean BP. Alteration of P-type calcium channel gating by the spider toxin omega-Aga-IVA. Biophysical Journal. 1997;72:2117–2128. doi: 10.1016/S0006-3495(97)78854-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani A, Kuroda Y, Futatsugi A, Furuichi T, Mikoshiba K. Phosphorylation of Homer3 by Calcium/Calmodulin-Dependent Kinase II Regulates a Coupling State of Its Target Molecules in Purkinje Cells. Journal of Neuroscience. 2008;28:5369–5382. doi: 10.1523/JNEUROSCI.4738-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb R, Szoke B, Palma A, Wang G, Chen XH, Hopkins W, Cong R, Miller J, Urge L, Tarczy-Hornoch K, Loo JA, Dooley DJ, Nadasdi L, Tsien RW, Lemos J, Miljanich G. Selective peptide antagonist of the class E calcium channel from the venom of the tarantula Hysterocrates gigas. Biochemistry. 1998;37:15353–15362. doi: 10.1021/bi981255g. [DOI] [PubMed] [Google Scholar]
- Perez-Reyes E, Cribbs LL, Daud A, Lacerda AE, Barclay J, Williamson MP, Fox M, Rees M, Lee JH. Molecular characterization of a neuronal low-voltage-activated T-type calcium channel. Nature. 1998;391:896–900. doi: 10.1038/36110. [DOI] [PubMed] [Google Scholar]
- Reynolds IJ, Wagner JA, Snyder SH, Thayer SA, Olivera BM, Miller RJ. Brain voltage-sensitive calcium channel subtypes differentiated by omega-conotoxin fraction GVIA. Proc Natl Acad Sci USA. 1986;83:8804–8807. doi: 10.1073/pnas.83.22.8804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronesi JA, Huber KM. Homer Interactions Are Necessary for Metabotropic Glutamate Receptor-Induced Long-Term Depression and Translational Activation. Journal of Neuroscience. 2008;28:543–547. doi: 10.1523/JNEUROSCI.5019-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoepp DD. Unveiling the functions of presynaptic metabotropic glutamate receptors in the central nervous system. J Pharmacol Exp Ther. 2001;299:12–20. [PubMed] [Google Scholar]
- Shiraishi-Yamaguchi Y, Sato Y, Sakai R, Mizutani A, Knöpfel T, Mori N, Mikoshiba K, Furuichi T. Interaction of Cupidin/Homer2 with two actin cytoskeletal regulators, Cdc42 small GTPase and Drebrin, in dendritic spines. BMC Neurosci. 2009;10:25. doi: 10.1186/1471-2202-10-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu JC, Xiao B, Naisbitt S, Yuan JP, Petralia RS, Brakeman P, Doan A, Aakalu VK, Lanahan AA, Sheng M, Worley PF. Coupling of mGluR/Homer and PSD-95 complexes by the Shank family of postsynaptic density proteins. Neuron. 1999;23:583–592. doi: 10.1016/s0896-6273(00)80810-7. [DOI] [PubMed] [Google Scholar]
- Ueta Y, Yamamoto R, Sugiura S, Inokuchi K, Kato N. Homer 1a suppresses neocortex long-term depression in a cortical layer-specific manner. J Neurophysiol. 2008;99:950–957. doi: 10.1152/jn.01101.2007. [DOI] [PubMed] [Google Scholar]
- Won Y-J, Puhl HL, Ikeda SR. Molecular reconstruction of mGluR5a-mediated endocannabinoid signaling cascade in single rat sympathetic neurons. Journal of Neuroscience. 2009;29:13603–13612. doi: 10.1523/JNEUROSCI.2244-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao B, Tu JC, Petralia RS, Yuan JP, Doan A, Breder CD, Ruggiero A, Lanahan AA, Wenthold RJ, Worley PF. Homer regulates the association of group 1 metabotropic glutamate receptors with multivalent complexes of homer-related, synaptic proteins. Neuron. 1998;21:707–716. doi: 10.1016/s0896-6273(00)80588-7. [DOI] [PubMed] [Google Scholar]
- Yuan JP, Kiselyov K, Shin DM, Chen J, Shcheynikov N, Kang SH, Dehoff MH, Schwarz MK, Seeburg PH, Muallem S, Worley PF. Homer binds TRPC family channels and is required for gating of TRPC1 by IP3 receptors. Cell. 2003;114:777–789. doi: 10.1016/s0092-8674(03)00716-5. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Ikeda SR. Modulation of Ca(2+)-channel currents by protein kinase C in adult rat sympathetic neurons. J Neurophysiol. 1994;72:1549–1560. doi: 10.1152/jn.1994.72.4.1549. [DOI] [PubMed] [Google Scholar]