Abstract
Transplantation of neural precursor cells (NPCs) is a promising therapeutic strategy in CNS injury. However, the adult CNS lacks instructive signals present during development and, depending on the region and type of transplant, may be inhibitory for neuron generation and axonal growth. We examined the effects of the white matter in different regions of the adult CNS on the properties of NPC transplants with respect to cell survival, differentiation, migration, and axonal growth. NPCs were prepared from day 13.5 embryonic spinal cord of transgenic rats that express the human placental alkaline phosphatase (AP) reporter. These NPCs were injected unilaterally into the cervical spinal cord white matter and into the corpus callosum of adult rats and were analyzed immunohistochemically 2 weeks later. NPCs survived in both regions and differentiated into astrocytes, oligodendrocytes, and neurons, with no apparent differences in survival or phenotypic composition. However, in the spinal cord white matter, graft-derived cells, identified as precursors and glial cells, migrated from the injection site rostrally and caudally, while in the corpus callosum, graft-derived cells did not migrate and remained at the injection site. Importantly, graft-derived neurons extended axons from the grafting site along the corpus callosum past the midline, entering into the contralateral side of the corpus callosum. These results demonstrate dramatic differences between white matter regions in the spinal cord and brain with respect to cell migration and axonal growth and underscore the importance of considering the effects of the local CNS environment in the design of effective transplantation strategies.
Keywords: axonal growth, cell migration, corpus callosum, spinal cord, white matter
INTRODUCTION
Cell transplantation is a promising therapeutic strategy for the treatment of CNS disorders and injury. Various types of cells have been studied in different diseases and injury models including Schwann cells (Xu et al., 1997; Hill et al., 2006), olfactory ensheathing cells (Bretzner et al., 2010), and genetically modified fibroblasts (Liu et al., 1999; Jin et al., 2002). At the same time, interest has been growing in the therapeutic application of neural stem cells (NSCs) because they can also be used for glial and neuronal cell replacement (Lu et al., 2003; Singec et al., 2007; Karimi-Abdolrezaee et al., 2010; Ruff and Fehlings, 2010) and for restoring connectivity (Bonner et al., 2011). Previous studies have shown that multipotent NSCs have the potential to generate the three major cell types in vitro—astrocytes, oligodendrocytes, and neurons—but that their fate following transplantation depends not only on their intrinsic properties but also on environmental cues. For example, NSCs grafted into adult spinal cord differentiate into glia but not into neurons (Cao et al., 2001; Lepore et al., 2004), demonstrating the restrictive nature of the nonneurogenic environment. In contrast, grafting NSCs into the neurogenic environment of the hippocampus (dentate gyrus), a region that continues adult neurogenesis, allows for the generation of neurons (Shihabuddin et al., 2000). Neural precursor cells (NPCs), composed of neuronal restricted precursors (NRPs) and glial restricted precursors (GRPs), have a more limited self-renewal and differentiation potential, but they are committed to neuronal and glial fates, respectively (Rao, 2004; Lepore et al., 2006). Consequently, lineage-restricted precursors have been used to generate distinct populations of neural cells, but their specific properties following transplantation depend not only on their intrinsic differentiation potential but also on the specific cues present in the grafting region. For example, when NRPs from embryonic day 13.5 spinal cord were grafted into intact adult spinal cord, the cells differentiated into mature neurons, extended neurites, and integrated with the host (Han et al., 2002) whereas GRPs transplanted into adult spinal cord differentiated into astrocytes and oligodendrocytes and migrated extensively along the white matter but not in the gray matter (Han et al., 2004). Transplantation of NPCs composed of NRPs/GRPs into different regions of the adult CNS, such as spinal cord, hippocampus, and striatum, confirmed the ability of lineage-restricted precursors to generate neurons, astrocytes, and oligodendrocytes in all three regions (Lepore et al., 2004). However, these studies revealed regional differences, underscoring the need for a better understanding of the properties of NPCs and their response to the specific host environment. In this study, we examined the effects of different areas of the adult CNS on the properties of NPC transplants, focusing our analysis on cell migration and axonal growth. Our results demonstrated dramatic differences between white matter regions in the spinal cord and brain with respect to cell migration and axonal growth, highlighting the importance of considering the local CNS environment in the design of effective transplantation strategies.
MATERIALS AND METHODS
NPC Preparation
Lineage-restricted NPCs composed of NRPs and GRPs were isolated from embryonic day 13.5 transgenic Fischer 344 rats that express the marker gene human placental AP (alkaline phosphatase) as described previously (Lepore and Fischer, 2005). Briefly, cords were dissociated using a 0.05% trypsin/EDTA solution for 20 min at 37°C. Cells were plated in NRP complete medium (Dulbecco modified Eagle medium/F12, bovine serum albumin [1 mg/ml, Sigma-Aldrich, St. Louis, MO], B27, basic fibroblast growth factor [10 μg/ml], NT-3 [10 μl/ml], pen-strep [100 IU/ml]) on poly-L-lysine (13.3 μg/ml, Sigma)- and laminin (20 μg/ml, Invitrogen, Carlsbad, CA)-coated dishes. Following embryonic dissection, NPCs were cultured for 5 days prior to transplantation. Cells were dissociated from culture flasks using 0.05% trypsin/EDTA, washed, and resuspended at a concentration of 12,500 cells/μl in NRP basal medium (NRP complete medium without basic fibroblast growth factor and NT-3) for transplantation. Cells were placed on ice during the surgery. After surgery, cell viability was assessed using the trypan blue assay.
Surgical Procedure
All animal experiments were conducted under a protocol approved by Drexel University College of Medicine’s Institutional Animal Care and Use Committee in accordance with NIH policy on the humane care and use of laboratory animals.
Two locations were selected in the normal rats for cell transplantation: spinal cord white matter at the third cervical level at the right side of the cord and the right corpus callosum. Adult female Sprague-Dawley rats (225-250 g) received an intraperitoneal injection of an anesthetic cocktail (ketamine 95 mg/kg; xylazine 10 mg/kg; and acepromazine 0.7 mg/kg). For spinal cord white matter transplantation (n = 4), the back musculature was excised, and a laminectomy was performed at cervical level C3-4. Two microliters of NPC suspension were injected at 1.5 mm from the midline, 1 mm from the dura using a microsyringe connected with a nanoliter controller at 20 nl/sec. After injection, the needle was kept in place for 2 min and then slowly withdrawn. For a corpus callosum transplant (n = 4), the same rats were placed in the stereotactic frame after the fur over the head was shaved; the skin was cleaned with povidone-iodine followed by 70% ethanol. NPCs in 2 μl of cell suspension were injected into the corpus callosum at 2.8 mm from the bregma and 2.8 mm from the dura at the right hemisphere. The rats who received transplants at the spinal cord and the corpus callosum survived for 2 weeks. An additional 5 rats that had NPCs grafted at the corpus callosum survived for 5 weeks (Table I). All rats received an injection of cyclosporine A (10 mg/kg; Sandoz Pharmaceuticals, East Hanover, NJ) 2 days before transplantation and daily thereafter until the end of the experiment.
TABLE I.
Animal Numbers and Survival Times
| Groups | Number of animals | Survival time, weeks |
|---|---|---|
| NPC-CC | 4 | 2 |
| NPC-CC | 5 | 5 |
| NPC-SC | 4a | 2 |
| NPC-CC-TB | 4 | 3 |
| CC-TB | 2 | 1 |
Same rats as used in NPC-CC
CC = corpus callosum; NPC = neural precursor cells; SC = spinal cord; TB = True Blue
Retrograde Tracing
The retrograde tracer True Blue (2%, 1 μl) was injected at the corpus callosum 1 mm away from the midline contralateral to the NPC graft (n = 4) 2 weeks after transplantation of NPCs. For the control group, True Blue was injected at the same location without NPCs grafted into the corpus callosum (n = 2). The animals were sacrificed 1 week after injection.
Tissue Preparation
The rats were perfused transcardially with cold saline (0.9% NaCl) followed by 4% paraformaldehyde. Samples of brain and cervical spinal cord with transplanted cells were dissected and postfixed in 4% paraformaldehyde overnight, then transferred to a 30% sucrose solution for 2 to 3 days before cryosectioning. Coronal sections (30 μm) from brain and horizontal sections from spinal cord were cut and collected in 6 serial sets. All sections were kept at -20°C in cryoprotectant medium (11.5 mM NaH2PO4.H2O, 38.5 mM Na2HPO4, 0.25 mM PVP-40, 876 mM sucrose, 5.38 M ethylene glycol).
Histochemical Staining with Alkaline Phosphatase
One set of sections was stained with AP to assess the presence and location of graft-derived cells in the spinal white matter and the corpus callosum. Sections were washed with phosphate-buffered saline (PBS) and heated at 60°C for 1 h to inactivate endogenous enzyme activity. Sections were then wash briefly in AP buffer (100 mM Tris, 100 mM NaCl, 50 mM MgCl2 at pH 9.5) and incubated at room temperature in the dark with an AP-staining solution containing 1.0 mg/ml nitroblue tetrazolium (Sigma-Aldrich), 0.1 mg/ml 5-bromo-4-chloro-3-indolyl phosphate (Sigma-Aldrich), and 5 mM levamisole (Sigma-Aldrich) in AP buffer for 2 h. Slides were coverslipped in Vectashield (Vector Laboratories, Burlingame, CA) and visualized with light microscopy.
Immunohistochemical Analyses
Tissue sections were washed with PBS, blocked with 10% goat serum for 1 h at room temperature, and then incubated in primary antibodies at room temperature overnight. Polyclonal antibody against AP (1:200, Accurate Chemical, Westbury, NY) was used to identify graft-derived cells. Several antibodies were used to assess the phenotype of the cells. NeuN (1:100, monoclonal, Chemicon, Millipore, Billerica, MA) was used to identify neurons. Glial fibrillary acidic protein (GFAP) (1:1000, monoclonal, Chemicon) was used to identify astrocytes. Oligodendrocytes were identified with anti-oligodendrocytes (RIP, 1:1000, monoclonal, Chemicon). Nestin (1:1000, monoclonal, Chemicon) was used for undifferentiated NPCs. Tuj1 (1:1000, monoclonal, Chemicon) was used to identify neuronal growth. To identify the phenotype of the neurons derived from the NPC graft, the following antibodies, together with the AP antibody, were used for double staining: 5-HT (1:20000, Immunostar, Hudson, WI) for serotonergic neurons; anti-tyrosine hydroxylase (1:150, Millipore) for dopaminergic neurons; glutamate transporter vesicular ½ (1:10000/1:2500, Chemicon) for glutamatergic neurons; GAD 65/67 (1:2000, Chemicon) for GABAergic neurons; and ChAT (1:100, Chemicon) for cholinergic neurons for the sections from NPCs at the corpus callosum. Sections were incubated with goat anti-mouse and goat anti-rabbit secondary antibodies (1:400, Jackson ImmunoResearch Laboratories, West Grove, PA) conjugated to either rhodamine or fluorescein isothiocyanate for 2 h at room temperature. Tissue was coverslipped with anti-fade mounting medium (Vectashield, Vector) with 4′,6-diamidino-2-phenylindole (DAPI) to identify nuclei. For AP diaminobenzidine (DAB) staining, sections were blocked with 10% goat serum in PBS for 2 h, incubated with polyclonal antibody against AP (1:200, Accurate) and 2% goat serum in PBS containing 0.3% Triton X-100 at 4°C overnight, and reacted with goat anti-rabbit biotinylated secondary antibody and ABC reagent (Vector), each for 2 h at room temperature. Staining was visualized with Sigmafast DAB (Sigma-Aldrich). Sections were mounted on gelatin-coated slides, dehydrated in graded ethanol, cleaned in CitriSolv (Fisher Scientific), and overlaid with a coverslip.
Quantification of NPC cell survival
Double staining of AP and DAPI was used to selectively identify and count graft-derived cells at 2 weeks post-transplantation (n = 3 animals/group). Images of AP-positive immunofluorescence from the entire graft of the horizontally sectioned spinal cord or the corpus callosum were obtained using an Olympus FluoView 1000 confocal laser scanning microscope (Olympus America, Center Valley, PA) at 20× magnification and analyzed with Olympus FluoView software as previously described (Jin et al., 2011). Total numbers of AP/DAPI-positive cells were counted in every sixth section. The spinal cord or corpus callosum was subdivided into the rostral–caudal axis and the medial–lateral axis in 1-mm blocks around the transplant epicenter. The numbers of AP/DAPI-positive cells in every 6th section were obtained for all 1-mm blocks, and the total number of surviving cells in the graft was then calculated as the sum of the values for all of the sections.
Quantification of Neurons and Axons Derived from the NPC Graft
Neurons derived from the NPC graft were quantified using the same procedure described for NPC cell survival. AP and NeuN were used to selectively identify graft-derived neurons at 2 weeks post-transplantation (n = 3 animals/group). The number of AP-positive axons growing along the corpus callosum was analyzed using the Leica DMRB fluorescent microscope (Leica Microsystems, Wetzlar, German) with 20× magnification at 2 and 5 weeks post-transplantation (n = 3 animals/group). Axon numbers were analyzed at three points along the corpus callosum (three sections/animal): midline; 1.5 mm from the midline on the transplant side (ipsilateral); and 1.5 mm from the midline on the contralateral side.
Statistical Analysis
Student t test was used for quantification of transplant survival and graft-derived neurons derived from spinal white matter and corpus callosum, with significance set at P < 0.05. One-way analysis of variance was used to compare axonal growth at 2 and 5 weeks at different points. Post hoc analysis was performed using the Bonferroni test with a significance level of 0.05.
RESULTS
NPC Grafts Survived and Differentiated in Both Locations
Two weeks after transplantation into spinal white matter or corpus callosum, grafted NPCs were detected by histological or immunochemical staining with AP, in both regions in all animals (Fig. 1). About 58% of transplanted cells survived in spinal white matter, while about 51% survived in the corpus callosum, with no significant difference in survival between the two regions (P = 0.46). Since our previous studies showed cell survival at 5 weeks after NPC transplantation into the spinal cord (Lepore and Fischer, 2005), we also examined NPC survival in the corpus callosum 5 weeks after transplantation. The NPC graft showed survival at 5 weeks in all animals (data not shown). We assessed the phenotypes of the NPCs 2 weeks following transplantation using double staining with AP and NeuN and found that the grafted cells differentiated into mature neurons in both locations (Fig. 2). All graft-derived neurons were localized at the graft region. There were no significant differences in the numbers of NeuN-positive cells between the two regions, with 906 ± 236 NeuN-positive neurons in the corpus callosum and 1040 ± 316 in the spinal white matter (mean ± SEM, P = 0.54), representing 3.6% and 4.2% of neurons, respectively.
Fig. 1.
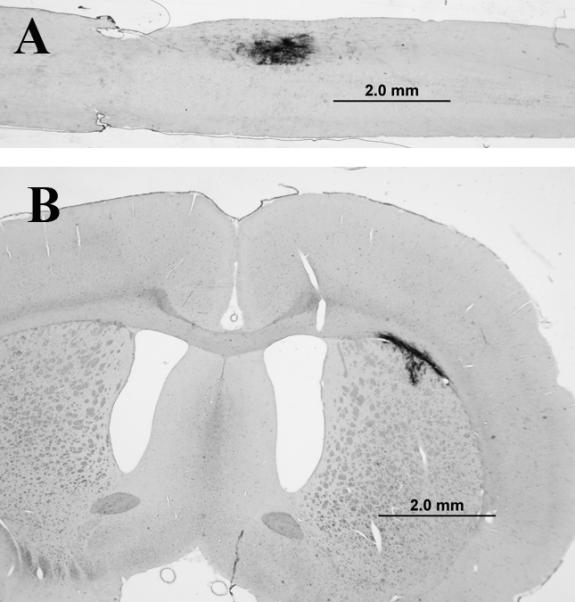
NPC survival in spinal cord and corpus callosum. Alkaline phosphatase (AP) histostaining shows that grafted NPCs survived in both spinal cord white matter (panel A) and corpus callosum (CC, panel B). Scale bar = 2 mm.
Fig. 2.
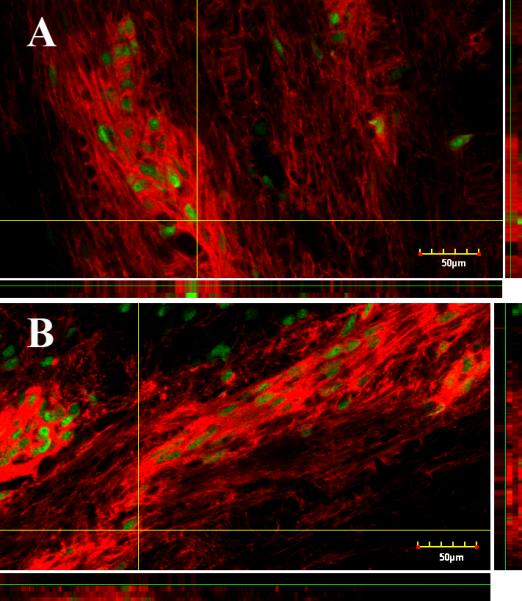
Grafts of NPCs generate neurons in spinal white matter and corpus callosum. NPCs grafted into intact spinal white matter (A) and corpus callosum (B) differentiated into mature neurons. Double staining of AP (red) and NeuN (green) shows NPC-derived neurons in spinal white matter (A) and corpus callosum (B). Scale bar = 50 μm.
Our previous studies showed that NPCs grafted into the spinal cord differentiated into glutamatergic and GABAergic neurons. With grafting of NRPs alone, there were also a small number of cholinergic neurons but no serotonergic neurons (Han et al., 2002; Bonner et al., 2010). We examined the phenotypes of neurons derived from NPCs at the corpus callosum. Besides glutamatergic and GABAergic neurons, we did not see any positive staining for serotonergic, cholinergic, or dopaminergic neurons in the corpus callosum (data not shown). The grafted cells also generated astrocytes and oligodendrocytes in both spinal white matter and corpus callosum, as determined by double staining of AP and GFAP and RIP, respectively (Fig. 3). There were no differences in cell survival rates or in the differentiation profile in spinal cord compared to our previous studies (Han et al., 2002; Lepore et al., 2004) or in the corpus callosum at 2 or 5 weeks after transplantation.
Fig. 3.

Grafts of NPCs generate astrocytes and oligodendrocytes in spinal white matter and corpus callosum. Grafted NPCs also differentiated into astrocytes and oligodendrocytes in the intact spinal white matter (panels A and C) and corpus callosum (panels B and D). Double staining of AP (green, panels a and d) and GFAP (red, panels b and e) is visible in the grafted NPC-derived astrocytes. Double staining of AP (green, panels g and j) and RIP (red, panels h and k) shows NPC-derived oligodendrocytes (merged, panels i and l). Figures a–l show images at higher magnification taken from highlighted areas surrounded by white boxes. Scale bar = 100 μm in A–D; scale bar = 10 μm in a–l. AP, alkaline phosphatase; GFAP, glial fibrillary acidic protein; CC, corpus callosum; SC, spinal cord. Rostral at the left side in Figure 3A and C.
NPC Grafts Showed Widespread Cell Migration in Spinal White Matter But Not in the Corpus Callosum
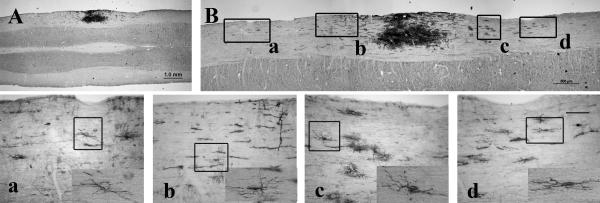
Although grafted NPCs showed similar survival and differentiation patterns in both spinal white matter and corpus callosum, dramatic differences were observed in cell migration. In the spinal cord, NPCs migrated from the grafted site in both rostral and caudal directions along the white matter 2 weeks after transplant, which was similar at 5 weeks (Lepore et al., 2004). Figure 4 shows the NPCs grafted into spinal white matter at low and high magnification (panels A and B, respectively) with the boxes corresponding to cell migration at a distance of 2.9 mm (inset a) and 1.3 mm (inset b) rostrally, and 1.5 mm (inset c) and 2.1 mm (inset d) caudally from the center of the graft. The migrating cells showed several phenotypes similar to those we observed previously (Han et al., 2004), such as astrocyte-, oligodendrocyte-, and undifferentiated NPC-like morphology, whose identity was verified by double staining with AP and GFAP, RIP, and nestin (data not shown). No AP-positive neurons derived from transplants were detected in the migration stream (data not shown). In contrast, grafted NPCs in the corpus callosum remained localized in the transplantation site, and few cells migrated out of the transplant area (Fig. 5A, B). No NPC-derived neurons migrated out of the transplants in the corpus callosum (data not shown).
Fig. 4.
Migration of NPCs from transplantation site along the spinal white matter. AP staining shows NPCs grafted into spinal white matter (A) migrating rostrally and caudally. Panels A and B show the entire NPC graft together with the migrating cells. Panels a–d show higher magnification of images from different regions along the migration path of the AP-positive cells corresponding to the labeled boxes in B. The insets shown in panels a–d demonstrate the morphology of the migrating AP cells. Scale bar = 1 mm in A; 500 μm in B; and 100 μm in a–d. Rostral at the left side in Figure 4A and B.
Fig. 5.
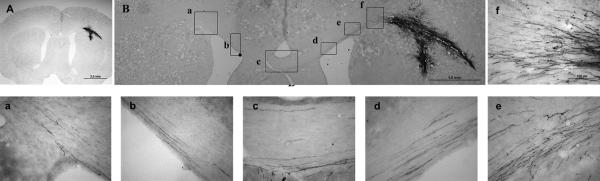
Extensive axonal growth from grafted NPCs in the corpus callosum. Although grafted NPCs did not migrate from the transplant site at the corpus callosum, AP-positive axons from grafted NPCs showed extensive growth out of NPC-derived neurons along the corpus callosum. These axons not only reached to the midline of the corpus callosum but also entered the contralateral side of the corpus callosum (panels A and B). Panels A and B are images of the entire transplant. Unlike grafted NPCs in the spinal white matter, few NPCs migrated from the transplant. However, clear AP-positive fibers extended out of the transplant along the corpus callosum (panels d , e and f), reached to the midline (panel c), and crossed the midline toward the contralateral corpus callosum (panels a and b). Images in boxes a–f in panel B are shown at a high magnification. Scale bar = 2 mm in A; 1 mm in B; and 100 μm in a–f.
NPC Grafts Showed Robust Axonal Growth in the Corpus Callosum
Despite the extensive cell migration in the spinal white matter, there was no evidence of axons growing from graft-derived neurons and no AP-positive axons were detected growing out of the transplant in the spinal white matter (Fig. 4A, B). In contrast, in the corpus callosum, there was robust growth of AP-positive axons out of the transplants along the corpus callosum, which not only reached to the midline of the corpus callosum but also entered into the contralateral side of the corpus callosum (Fig. 5A, B). The boxes in Figure 5B show axonal growth in different locations of the corpus callosum from near the graft site (e-f) to the midline (c-d) and the contralateral side (a-b). Counting graft-derived AP-positive axons along the corpus callosum (Fig. 6) showed that there were about 334 ± 11 AP-positive axons (mean ± SEM) near the transplant at 1.5 mm away from the midline (ipsilateral); 154 ± 23 AP-positive fibers at the midline; and 92 ± 5 AP-positive fibers at 1.5 mm from the midline on the contralateral side at 2 weeks post-transplantation. A similar pattern of AP-positive fibers along the corpus callosum was observed in the animals at 5 weeks post-transplantation, with 334 ± 32 AP-positive fibers on the ipsilateral side; 188 ± 31 AP-positive fibers at the midline; and 106 ± 19 AP-positive fibers on the contralateral side. There were no significant differences in the numbers of AP-positive axons between 2 and 5 weeks post-transplantation. However, there were significant differences in the number of AP-positive axons along the corpus callosum in both time groups (ipsilateral vs midline, P < 0.001; ipsilateral vs contralateral, P < 0.001), indicating that the number of graft-derived axons growing along the corpus callosum decreases with the distance. Double staining with AP and GFAP verified that these AP-positive fibers were not astrocytic processes (Fig 7, top panel) but rather axonal processes stained with Tuj1, a neuron-specific marker (Fig 7, middle panel). To further verify the identity and reach of the AP-positive axons along the corpus callosum, the retrograde tracer True Blue was injected into the corpus callosum contralateral to the NPC graft side. True Blue-labeled neurons were then detected within AP-positive cells at the corpus callosum grafting site (Fig 7, bottom panel), supporting the identity of the AP fibers along the corpus callosum as axons originating from graft-derived neurons. True Blue injected into the corpus callosum in control animals without the NPC graft did not show any labeled cells in the corpus callosum (data not shown).
Fig. 6.

The growth of axons derived from graft along the corpus callosum. There were more AP-positive fibers along the corpus callosum at the ipsilateral side near the graft and there was no significant difference between 2 and 5 weeks post-transplantation. The number of AP-positive fibers decreased with distance. At the midline and contralateral of the corpus callosum, there was a significant decrease in AP-positive fibers in animals at both 2 and 5 weeks, *P < 0.001, ipsilateral vs midline, and ipsilateral vs contralateral at 2 and 5 weeks. There was no difference in AP-positive fibers comparing midline with contralateral at both 2 and 5 weeks (P > 0.05).
Fig. 7.

Confirmation of axonal growth from NPC transplant along the corpus callosum. In the top panel, double staining of AP and GFAP demonstrates that AP-positive fibers did not colocalize with astrocyte processes (identified by GFAP). In the middle panel, double staining of AP and Tuj1 shows many AP-positive fibers also stained by Tuj1, a specific neuronal marker. In the bottom panel, True Blue retrograde labeling of the corpus callosum colocalized with AP-positive cells in the graft, confirming that AP-positive fibers along the corpus callosum are derived from transplanted NPC neurons. Scale bar =100 μm. AP, alkaline phosphatase; GFAP, glial fibrillary acidic protein.
DISCUSSION
We demonstrated the influence of the white matter in different regions of the CNS on specific properties of NPC transplants. Although NPCs survive and differentiate into mature neural phenotypes in both spinal white matter and corpus callosum, their response with respect to cell migration and axonal growth was distinctly region-specific. NPCs grafted in spinal white matter migrated from the graft site along the white matter in rostral and caudal directions, but no axonal growth was detected from the grafted cells into spinal white matter. In contrast, NPCs grafted in the corpus callosum did not migrate out of the graft but remained in the graft area, whereas axons from graft-derived neurons extended along the corpus callosum, across the midline toward the contralateral side of the corpus callosum.
Unlike multipotent NSCs, lineage-restricted precursors are less dependent on instructive signals from the grafting environment for specification of their phenotype. When pluripotent stem cells were grafted into the spinal cord, the majority of the cells differentiated into astrocytes but not into neurons (Cao et al., 2001). In contrast, lineage-restricted NPCs can generate neurons from NRPs even if grafted into a nonneurogenic environment such as the spinal cord or striatum (Lepore et al., 2004). The combination of GRPs and NRPs also creates a microenvironment at the grafting site that is protective for cell survival and conducive for neuronal differentiation at the injury site (Lepore and Fischer, 2005), suggesting that this strategy can be used for cell replacement in the adult CNS. For example, neurons derived from NPCs grafted into the CNS may serve as a source of replacement neurons or relays that reconnect disrupted axons following spinal cord injury (Bonner et al., 2010, 2011). The generation of NeuN-positive neurons derived from an NPC graft at the corpus callosum is similar to what we observed in the spinal cord, confirming that the neuronal phenotype can be obtained in different CNS regions.
Importantly, we found that NPCs grafted into the corpus callosum remained near the transplant region and did not migrate along the white matter. In contrast, NPCs grafted into the lateral funiculus in the spinal cord migrated along the white matter in both rostral and caudal directions. Cell migration has been shown to vary in different regions of the CNS. When human NSCs were grafted into the mouse cerebral cortex, cells migrated in all directions from the injection site. Migration was lower in the hippocampus; in the thalamus, the transplanted cells remained at the injection site (Watson et al., 2006). NRPs from the spinal cord, when grafted into the neonatal anterior forebrain subventricular zone, migrated to distinct regions throughout the forebrain including the olfactory bulb, frontal cortex, and occipital cortex but not to the hippocampus (Yang et al., 2000). Mixed GRPs/NRPs grafted into the adult striatum also migrated along white matter bundles in the striatum and appeared to extend axons along the corpus callosum (Lepore et al., 2006). All of these data indicate that different regions have differential effects on cell migration. In the spinal cord, migration of grafted mixed NRPs and GRPs occurred in both the intact and the injured spinal cord. Previous studies have shown that graft-derived cells can be found up to 15 mm from the transplantation site by 3 weeks, with the NRPs remaining at the grafting site and the GRPs migrating along the spinal white matter (Lepore et al., 2004). Similarly, GRPs grafted into the adult spinal cord showed continuous migration along the orientation of longitudinal white matter tracts both caudally and rostrally away from the injection site (Han et al., 2004). Cell migration occurred more frequently in the white matter than in the gray matter (Enomoto et al., 2003; Han et al., 2004). The migrating cells exhibited simple migratory morphology, with elongated cell bodies and nuclei parallel to the direction of migration. At later time points, the morphology of the cells became more complex, and the cells were identified as astrocytes and oligodendrocytes but not as neurons. Consistent with these results, we found that the migrating cells were either astrocytes, stained with GFAP, or oligodendrocytes, stained with RIP. Many of the migrating cells were also identified as undifferentiated, nestin-positive precursors, but no neurons were found in the migrating stream. Taken together, these results likely indicate that the grafted GRPs (but not NRPs) initially migrate along the white matter and later differentiate into mature glial phenotypes of astrocytes and oligodendrocytes. This model is also supported by results from our previous studies with human GRPs that showed extensive migration of GRPs, with graft-derived astrocytes present a long distance from the grafting area (Jin et al., 2011). Neurons derived from grafted NPCs did not migrate from transplanted sites in either grafting system. However, previous studies have shown that grafting of NRPs alone resulted in the dispersion of neurons along the spinal white matter (Han et al., 2002). NRPs grafted into spinal white matter migrated in both rostral and caudal directions along the tracts, whereas the migration of NRPs grafted into the gray matter was limited (Han et al., 2002). This finding may be caused by the condition of the grafted cells. NPCs are mixed with NRPs and GRPs. When NRPs and GRPs are integrated, GRPs may restrict the migration of neurons from NRPs. The difference in the migration patterns to the spinal white matter and the corpus callosum might be due to differences in the signals and extracellular matrix in these two specific regions.
Another important result from this study is the difference in axonal growth derived from NPCs grafted at different locations. In the spinal cord, no clear axonal growth was apparent from grafted NPCs when they were transplanted into the lateral funiculus of spinal white matter, although migration occurred along the white matter. Bonner et al. (2010) showed that minimal axonal extension occurred when GRPs/NRPs were grafted into the dorsal column lesion model. However, injecting a lentiviral vector expressing brain-derived neurotrophic factor rostrally into the injured area generated a neurotrophin gradient and promoted directional growth of axons for up to 9 mm, which indicated that a specific neurotrophic factor can direct axonal growth from graft-derived neurons. Interestingly, when we acutely grafted a small segment of embryonic day 14 fetal spinal cord tissue into a cervical lateral funiculus injury, long graft-derived fibers could be seen projecting from the transplant into the surrounding intact spinal cord (Lepore and Fischer, 2005), which indicates that the properties of NRPs/GRPs and embryonic day 14 fetal spinal cord tissue are different on axonal growth in an injury environment. In this study, in the corpus callosum, axonal growth from graft-derived neurons traced from the transplant site along the corpus callosum not only reached the midline of the corpus callosum but also entered the contralateral corpus callosum without any neurotrophic factor guidance. The corpus callosum is a good model to examine axonal growth from different types of neurons in the white matter, because it contains the potent inhibitory molecules (Caroni and Schwab, 1988). When dorsal root ganglia (DRG, postnatal day 1-2) were grafted into one side of the corpus callosum, only a few axons from the grafted DRG grew along the corpus callosum, crossed the midline, and entered the contralateral side. However, specific factors such as fibroblast growth factor and nerve growth factor provided as gradients by multiple injections of adenovirus encoding these factors along the corpus callosum caused more axons to grow along the pathway, toward the contralateral side of the corpus callosum (Ziemba et al., 2008). Dopaminergic neurons from embryonic day 14 mesencephalon tissue grafted into the corpus callosum did not show any dopaminergic axons growing out of the transplant site along the corpus callosum (Jin et al., 2011). However, creating a growth-supporting pathway with glial–derived neurotrophic factor (GDNF) or GDNF/GFRα1 along the corpus callosum can attract dopaminergic axons growing out of the transplant along the corpus callosum. A recent study showed that callosal axons from the medial and lateral regions of the mouse cerebral cortex pass through the dorsal and ventral parts, respectively, of the corpus callosum. These results suggest the role of axonal segregation in the corpus callosum, mediated at least partly by EphA3, in correct pathfinding of callosal neurons (Nishikimi et al., 2011). There is also evidence that ephrins and Eph receptors play a significant role in the development of the corpus callosum (Mendes et al., 2006). The corpus callosum model can therefore be used to study how specific molecules present in the corpus callosum can affect axonal growth of different types of neurons, underscoring the importance of environmental cues for the fate of neurons. In this context, our study has shown that signals from corpus callosum and spinal white matter have different effects on transplants of neural stem cells with respect to cell migration and axonal growth.
In summary, we compared NPCs grafted in different regions of the CNS: the corpus callosum (brain) and spinal white matter. NPCs survived and differentiated into neurons and glia in both regions. However, each region affected cell migration and axonal growth differently. This study indicates that the properties of different CNS environments are important when developing a cell transplant strategy.
Acknowledgments
We thank Dr. Angelo Lepore (Thomas Jefferson University) for his assistance with using the confocal microscope.
Grant information: The present study was funded by NIH (SP01NS055976), Shriners Hospital for Children #85100, and the CHN Foundation #160746.
REFERENCES
- Bonner JF, Blesch A, Neuhuber B, Fischer I. Promoting directional axon growth from neural progenitors grafted into the injured spinal cord. J Neurosci Res. 2010;88:1182–1192. doi: 10.1002/jnr.22288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner JF, Connors TM, Silverman WF, Kowalski DP, Lemay MA, Fischer I. Grafted neural progenitors integrate and restore synaptic connectivity across the injured spinal cord. J Neurosci. 2011;31:4675–4686. doi: 10.1523/JNEUROSCI.4130-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretzner F, Plemel JR, Liu J, Richter M, Roskams AJ, Tetzlaff W. Combination of olfactory ensheathing cells with local versus systemic cAMP treatment after a cervical rubrospinal tract injury. J Neurosci Res. 2010;88:2833–2846. doi: 10.1002/jnr.22440. [DOI] [PubMed] [Google Scholar]
- Cao QL, Zhang YP, Howard RM, Walters WM, Tsoulfas P, Whittemore SR. Pluripotent stem cells engrafted into the normal or lesioned adult rat spinal cord are restricted to a glial lineage. Exp Neurol. 2001;167:48–58. doi: 10.1006/exnr.2000.7536. [DOI] [PubMed] [Google Scholar]
- Caroni P, Schwab ME. Two membrane protein fractions from rat central myelin with inhibitory properties for neurite growth and fibroblast spreading. J Cell Biol. 1988;106:1281–1288. doi: 10.1083/jcb.106.4.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto M, Shinomiya K, Okabe S. Migration and differentiation of neural progenitor cells from two different regions of embryonic central nervous system after transplantation into the intact spinal cord. Eur J Neurosci. 2003;17:1223–1232. doi: 10.1046/j.1460-9568.2003.02555.x. [DOI] [PubMed] [Google Scholar]
- Han SS, Kang DY, Mujtaba T, Rao MS, Fischer I. Grafted lineage-restricted precursors differentiate exclusively into neurons in the adult spinal cord. Exp Neurol. 2002;177:360–375. doi: 10.1006/exnr.2002.7995. [DOI] [PubMed] [Google Scholar]
- Han SS, Liu Y, Tyler-Polsz C, Rao MS, Fischer I. Transplantation of glial-restricted precursor cells into the adult spinal cord: survival, glial-specific differentiation, and preferential migration in white matter. Glia. 2004;45:1–16. doi: 10.1002/glia.10282. [DOI] [PubMed] [Google Scholar]
- Hill CE, Moon LD, Wood PM, Bunge MB. Labeled Schwann cell transplantation: cell loss, host Schwann cell replacement, and strategies to enhance survival. Glia. 2006;53:338–343. doi: 10.1002/glia.20287. [DOI] [PubMed] [Google Scholar]
- Jin Y, Fischer I, Tessler A, Houle JD. Transplants of fibroblasts genetically modified to express BDNF promote axonal regeneration from supraspinal neurons following chronic spinal cord injury. Exp Neurol. 2002;177:265–275. doi: 10.1006/exnr.2002.7980. [DOI] [PubMed] [Google Scholar]
- Jin Y, Neuhuber B, Singh A, Bouyer J, Lepore A, Bonner J, Himes T, Campanelli JT, Fischer I. Transplantation of human glial restricted progenitors and derived astrocytes into a contusion model of spinal cord injury. J Neurotrauma. 2011;28:579–594. doi: 10.1089/neu.2010.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Zhang C, Ziemba KS, Goldstein GA, Sullivan PG, Smith GM. Directing dopaminergic fiber growth along a preformed molecular pathway from embryonic ventral mesencephalon transplants in the rat brain. J Neurosci Res. 2011;89:619–627. doi: 10.1002/jnr.22575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi-Abdolrezaee S, Eftekharpour E, Wang J, Schut D, Fehlings MG. Synergistic effects of transplanted adult neural stem/progenitor cells, chondroitinase, and growth factors promote functional repair and plasticity of the chronically injured spinal cord. J Neurosci. 2010;30:1657–1676. doi: 10.1523/JNEUROSCI.3111-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepore AC, Fischer I. Lineage-restricted neural precursors survive, migrate, and differentiate following transplantation into the injured adult spinal cord. Exp Neurol. 2005;194:230–242. doi: 10.1016/j.expneurol.2005.02.020. [DOI] [PubMed] [Google Scholar]
- Lepore AC, Han SS, Tyler-Polsz CJ, Cai J, Rao MS, Fischer I. Differential fate of multipotent and lineage-restricted neural precursors following transplantation into the adult CNS. Neuron Glia Biol. 2004;1:113–126. doi: 10.1017/s1740925x04000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepore AC, Neuhuber B, Connors TM, Han SS, Liu Y, Daniels MP, Rao MS, Fischer I. Long-term fate of neural precursor cells following transplantation into developing and adult CNS. Neuroscience. 2006;142:287–304. doi: 10.1016/j.neuroscience.2005.12.067. [DOI] [PubMed] [Google Scholar]
- Liu Y, Kim D, Himes BT, Chow SY, Schallert T, Murray M, Tessler A, Fischer I. Transplants of fibroblasts genetically modified to express BDNF promote regeneration of adult rat rubrospinal axons and recovery of forelimb function. J Neurosci. 1999;19:4370–4387. doi: 10.1523/JNEUROSCI.19-11-04370.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P, Jones LL, Snyder EY, Tuszynski MH. Neural stem cells constitutively secrete neurotrophic factors and promote extensive host axonal growth after spinal cord injury. Exp Neurol. 2003;181:115–129. doi: 10.1016/s0014-4886(03)00037-2. [DOI] [PubMed] [Google Scholar]
- Mendes SW, Henkemeyer M, Liebl DJ. Multiple Eph receptors and B-class ephrins regulate midline crossing of corpus callosum fibers in the developing mouse forebrain. J Neurosci. 2006;26:882–892. doi: 10.1523/JNEUROSCI.3162-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikimi M, Oishi K, Tabata H, Torii K, Nakajima K. Segregation and pathfinding of callosal axons through EphA3 signaling. J Neurosci. 2011;31:16251–16260. doi: 10.1523/JNEUROSCI.3303-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao MS. Stem sense: a proposal for the classification of stem cells. Stem Cells Dev. 2004;13:452–455. doi: 10.1089/scd.2004.13.452. [DOI] [PubMed] [Google Scholar]
- Ruff CA, Fehlings MG. Neural stem cells in regenerative medicine: bridging the gap. Panminerva Med. 2010;52:125–147. [PubMed] [Google Scholar]
- Shihabuddin LS, Horner PJ, Ray J, Gage FH. Adult spinal cord stem cells generate neurons after transplantation in the adult dentate gyrus. J Neurosci. 2000;20:8727–8735. doi: 10.1523/JNEUROSCI.20-23-08727.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singec I, Jandial R, Crain A, Nikkhah G, Snyder EY. The leading edge of stem cell therapeutics. Annu Rev Med. 2007;58:313–328. doi: 10.1146/annurev.med.58.070605.115252. [DOI] [PubMed] [Google Scholar]
- Watson DJ, Walton RM, Magnitsky SG, Bulte JW, Poptani H, Wolfe JH. Structure-specific patterns of neural stem cell engraftment after transplantation in the adult mouse brain. Hum Gene Ther. 2006;17:693–704. doi: 10.1089/hum.2006.17.693. [DOI] [PubMed] [Google Scholar]
- Xu XM, Chen A, Guénard V, Kleitman N, Bunge MB. Bridging Schwann cell transplants promote axonal regeneration from both the rostral and caudal stumps of transected adult rat spinal cord. J Neurocytol. 1997;26:1–16. doi: 10.1023/a:1018557923309. [DOI] [PubMed] [Google Scholar]
- Yang H, Mujtaba T, Venkatraman G, Wu YY, Rao MS, Luskin MB. Region-specific differentiation of neural tube-derived neuronal restricted progenitor cells after heterotopic transplantation. Proc Natl Acad Sci U S A. 2000;97:13366–13371. doi: 10.1073/pnas.97.24.13366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemba KS, Chaudhry N, Rabchevsky AG, Jin Y, Smith GM. Targeting axon growth from neuronal transplants along preformed guidance pathways in the adult CNS. J Neurosci. 2008;28:340–348. doi: 10.1523/JNEUROSCI.3819-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]