Abstract
Extracellular nucleotides induce cellular responses in the central nervous system (CNS) through the activation of ionotropic P2X and metabotropic P2Y nucleotide receptors. Activation of these receptors regulates a wide range of physiological and pathological processes. In this review, we present an overview of the current literature regarding P2X and P2Y receptors in the CNS with a focus on the contribution of P2X7 and P2Y2 receptor-mediated responses to neuroinflammatory and neuroprotective mechanisms.
Keywords: P2Y2 Receptor, P2X7 receptor, neuroprotection, neuroinflammation
Introduction
Extracellular nucleotides, such as adenosine 5-triphosphate (ATP) and uridine 5’-triphosphate (UTP), are released from cells under a variety of physiological and pathological conditions whereupon they activate P2 nucleotide receptors on the surface of neighboring cells (Burnstock et al., 1997; Heilbronn et al., 1997). P2 receptors are a diverse family of plasma membrane proteins that can be segregated into two subtypes: the P2X receptors that are ATP-selective cation channels and the P2Y receptors for ATP, UTP or their metabolites that are coupled to heterotrimeric G proteins (Abbracchio and Burnstock, 1994; von Kügelgen and Wetter, 2000). To date, genes for 7 P2X receptors and 8 P2Y receptors have been cloned and their protein products have been extensively characterized in a variety of cell and tissue types (Burnstock and Kennedy, 1985; Sak and Webb, 2002). In the central nervous system (CNS), multiple P2X and P2Y receptor subtypes are expressed in neurons, glial cells, oligodendrocytes, macrophages and endothelium where they regulate physiological responses, including neurotransmission, pain perception, phagocytosis, and maintenance of the blood-brain barrier (Weisman et al., 2005; Gonzalez et al., 2005; Peterson et al., 2010). Pathophysiological responses are also regulated by P2X and P2Y receptors, including the propagation of inflammation due to the release of nucleotide agonists from damaged or diseased cells (Bours et al., 2011; Ferrero, 2011; Fumagalli et al., 2011). This review describes the contributions of both P2X and P2Y receptors to cell specific functions in the CNS and focuses on the dual roles of the ionotropic P2X7 receptor (P2X7R) for ATP and the G protein-coupled P2Y2 receptor (P2Y2R) for ATP and UTP in the regulation of proinflammatory responses in the brain. Recent studies have found that the release of extracellular ATP from stressed or damaged cells of the CNS can activate microglial cell P2X7Rs, which increases cytokine release, e.g., interleukin-1β (IL-1β), and the phagocytic activity of microglial cells (Bianco et al., 2005b). Additionally, IL-1β has been shown to upregulate P2Y2R expression in neurons to promote neuroprotective responses (Kong et al., 2009). These findings are reviewed in this paper and suggest that both P2X7 and P2Y2 receptors are promising targets for the treatment of neurodegenerative and other inflammatory diseases.
P2X receptors in the Central Nervous System
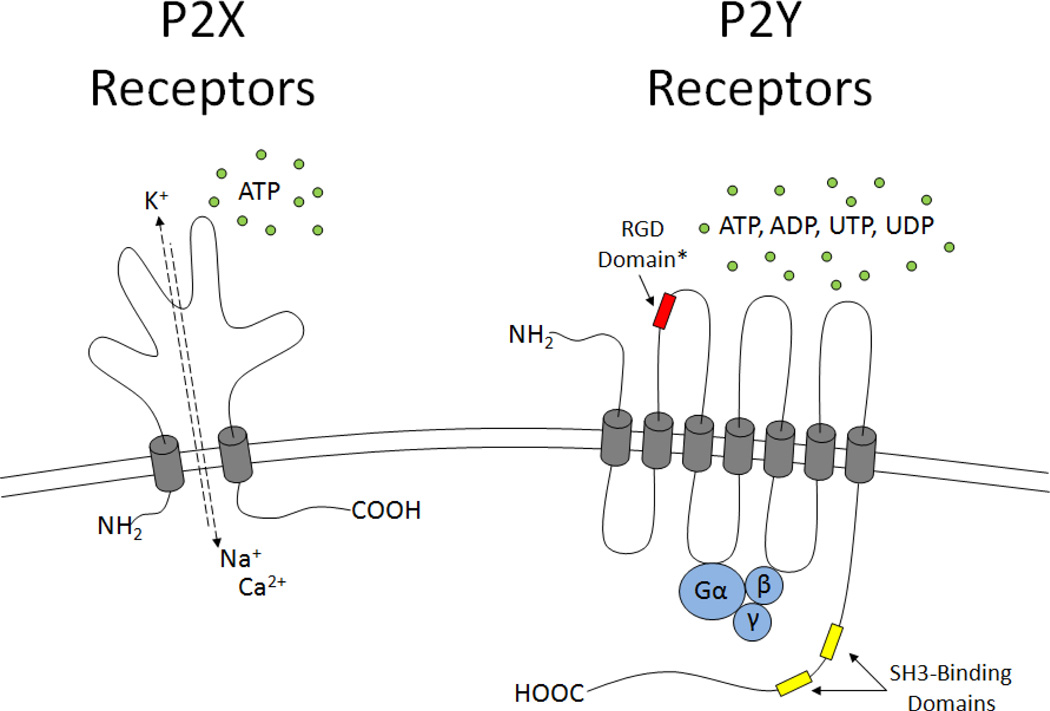
P2X receptors (P2XRs) are ligand-gated, nonselective cation channels activated by extracellular ATP. Seven pharmacologically distinct P2XR subtypes have been identified, i.e., P2X1–P2X7, and shown to be activated by ATP and its analogues (MacKenzie et al., 1999; North and Surprenant, 2000). P2XRs range from 379–595 amino acids in length and, as shown in Figure 1, consist of two transmembrane domains, a large extracellular loop and intracellular N- and C-termini (Valera et al., 1994). P2XRs share ~ 50% sequence homology with one third of the extracellular loop conserved, suggesting an ATP binding site (Vial et al., 2004). P2X1 and P2X3 receptors rapidly desensitize (within milliseconds), whereas P2X2, P2X5, P2X6 and P2X7 receptors desensitize slowly (within seconds) upon activation by ATP (Ralevic and Burnstock, 1998). Depending on the subtype, P2XR subunits interact in a variety of homo- or heteromeric forms to regulate a wide range of cellular responses in the CNS under physiological and pathological conditions (Khakh and North, 2006; Koles et al., 2007). The distribution of P2XR subtypes in the CNS is dependent upon species, brain region and cell type (Collo et al., 1996; Vulchanova et al., 1996; Kanjhan et al., 1999; Vulchanova et al., 1997; Collo et al., 1997; Atkinson et al., 2000; Deuchars et al., 2001; Rubio and Soto, 2001; Sim et al., 2004). The P2X2R, P2X4R and P2X6R appear to be abundantly expressed throughout the brain, whereas the remaining subtypes are expressed in distinct regions (Collo et al., 1996; Tanaka et al., 1996; Kanjhan et al., 1999). In neurons, activation of P2XRs by extracellular ATP has been reported to have both presynaptic and postsynaptic effects including the modulation of transmembrane currents and neurotransmitter release (Nakatsuka and Gu, 2001; Watano et al., 2004; Rodrigues et al., 2005; Patti et al., 2006; Jameson et al., 2008; Illes et al., 2011). All types of glia, e.g., astrocytes, oligodendrocytes, Schwann cells and microglial cells express P2XRs where they can regulate inflammatory, neurodegenerative and neuroprotective responses (Illes et al., 2011). Most notably, the P2X7R has gained recognition for its possible role in neurodegenerative disorders (Duan and Neary, 2006; Takenouchi et al., 2010). The function of the CNS is dependent on neuronal-glial interactions and the complexity of P2XR signaling in both cell types adds to the difficulty in interpreting ATP-mediated events in vivo. The cell and tissue distribution and functional relevance of homomeric and heteromeric P2X receptors in the nervous system are summarized in Table 1.
Fig. 1. Structural Features of P2X and P2Y Receptors.
Based on structure and function, P2 nucleotide receptors can be divided into two classes. The P2X receptors are nonselective ligand-gated cation channels featuring two transmembrane domains and a large extracellular loop. P2X receptors interact in a wide variety of homo- and heteromeric forms depending on tissue-specific expression and receptor subtype (i.e., P2X1–7) and they are activated by extracellular ATP. The P2X7R has received much attention due to its capacity for intracellular signaling via a large C-terminal tail and its participation in inflammatory processes. The P2YRs are classical G protein-coupled receptors featuring an extracellular N-terminus, 7 transmembrane domains, and an intracellular C-terminus that is structurally diverse between P2Y receptor subtypes. The Gq-coupled P2Y1,2,4,6,11 and the Gi-coupled P2Y12,13,14 receptors are activated by adenine and uridine tri- and dinucleotides with pharmacologically distinct efficacies and potencies. The P2Y2R subtype has been shown to associate with integrins via an extracellular RGD domain and to transactivate growth factor receptors via the binding of Src to Src-homology-3 (SH3) domains located within the C-terminus.
*- only present in the P2Y2 receptor
Table 1. P2X Receptors in the Nervous System.
The expression and function of P2X receptor subtypes in the nervous system are summarized in Table 1. This table highlights the information presented in this review article and is not considered to be comprehensive.
| Receptor Subtype | Expression | Function | |
|---|---|---|---|
| Homomeric | Cell Type | Region | |
| P2X1 | Neurons, astrocytes, microglia | Cerebral cortex, superior cervical ganglia | Neurogenic smooth muscle contraction, platelet activation, neuron and glial responses |
| P2X2 | Neurons, astrocytes | Cerebral cortex, cerebellum, hippocampus, striatum, habenula, substantia nigra, dorsal root ganglia, mesenteric ganglia | Nociceptive transmission, hyperalgesia, allodynia, pre- and postnatal neurogenesis |
| P2X3 | Neurons | Dorsal root ganglia, spinal cord | Enhance glutamate and substance P release, neuropathic pain sensation |
| P2X4 | Neurons, astrocytes | Cerebellum, hippocampus, brainstem, spinal cord | Release of brain-derived neurotrophic factor, induce neuropathic pain, prostaglandin E2 release, synaptic strengthening, hypersensitivity to sensory stimuli |
| P2X5 | Neurons | Cerebral cortex, cerebellum, hippocampus, hypothalamus, thalamus, olfactory bulb, globus pallidum, midbrain and hindbrain | Interconnection of cortical areas, post-synaptic purinergic transmission |
| P2X6 | Neurons, astrocytes | Cerebellum, hippocampus, purkinje neurons, pyramidal neurons, sensory ganglia | Rarely forms functional homomeric receptors |
| P2X7 | Neurons, astrocytes, microglia | Cerebral cortex, hippocampus, brainstem, nucleus accumbens, spinal cord | Release of proinflammatory cytokines, apoptosis, membrane pore formation, glutamate release, ATP release, induction of synaptic plasticity |
| Heteromeric | |||
| P2X1/P2X2 | Neurons | Superior cervical ganglia | ATP-mediated physiological responses |
| P2X1/P2X5 | Astrocytes | Cerebral cortex | ATP-evoked membrane currents |
| P2X2/P2X3 | Neurons | Dorsal root ganglia | Similar to P2X3, but with reduced desensitization |
| P2X2/P2X6 | Neurons | Dogiel type II neurons in myenteric plexus | ATP-mediated physiological responses |
P2X1Rs have been shown to cause contraction of neurogenic smooth muscle (Mulryan et al., 2000; Vial and Evans, 2000), platelet activation (Hechler et al., 2003; Mahaut-Smith et al., 2004), and neuronal (Calvert and Evans, 2004; Watano et al., 2004) and glial cell responses (Lalo et al., 2008). Among the P2XRs, the P2X1R has the highest affinity for ATP (EC50 ~ 1 M) (Rettinger and Schmalzing, 2003). The P2X1R is often observed in a heteromeric complex with the P2X2R and P2X5R resulting in biophysical and pharmacological properties distinct from those observed when each of these receptor subtypes is expressed separately (Le et al., 1997; Torres et al., 1998; Haines et al., 1999; Le et al., 1999; Duckwitz et al., 2006; Ase et al., 2010). In superior cervical ganglia neurons, the P2X1R contributes to ATP-mediated responses by forming a heteromeric unit with the P2X2R (Calvert and Evans, 2004), whereas ATP-evoked biphasic membrane currents in mouse cortical astrocytes are regulated by P2X1R/P2X5R heteromeric channels (Lalo et al., 2008).
P2X2Rs are widely expressed in the CNS with predominant expression in the cerebral cortex, cerebellum, striatum, hippocampus, habenula, substantia nigra, dorsal ganglia neurons, mesenteric ganglia neurons and glial cells (Kidd et al., 1995; Vulchanova et al., 1996; Pankratov et al., 1998; Xiang et al., 1998; Kanjhan et al., 1999; Xiang and Burnstock, 2004; Brass et al., 2011). The P2X2R is distinguished from other members of the P2XR family, since multiple splice variants exist in different mammalian species with diverse functional properties (Coddou et al., 2011). Many studies have shown that P2X2Rs play a role in nociceptive transmission, hyperalgesia and allodynia, particularly when present as functional heterotrimers with P2X3Rs (Honore et al., 2002; Jarvis, 2003; Xiang and Burnstock, 2004). The pharmacological properties of P2X2R/P2X3R are similar to the P2X3R, but the desensitization rate of the P2X3R is reduced by its interaction with the P2X2R (Koshimizu et al., 2002). P2X2Rs and P2X2R/P2X3R have been implicated in pain processing (Wirkner et al., 2007); however with chronic pain their functions are altered by the action of other P2XRs, especially those expressed in immune cells, such as microglia (Trang et al., 2006). In addition to interactions with P2X2Rs, P2X3Rs also form homotrimeric receptors that are prominently expressed in primary sensory neurons where they enhance the release of glutamate and substance P (Gu and MacDermott, 1997; Vulchanova et al., 1998; Nakatsuka et al., 2001; Nakatsuka and Gu, 2001), which contribute to both acute and chronic pain sensation (Jarvis, 2003). In vivo studies using P2X2R−/−, P2X3R−/− and P2X2R−/−/P2X3R−/− mice have contributed significantly to our understanding of neuropathic and inflammatory pain sensation and have lead to the development of therapeutic antagonists to these receptors (Ford, 2011; Gum et al., 2011). The P2X2R also has been suggested to play a role in pre- and postnatal neurogenesis (Cheung et al., 2005).
The P2X4R is expressed throughout the central and peripheral nervous systems (Buell et al., 1996; Rubio and Soto, 2001; Bo et al., 2003; Calvert and Evans, 2004; Tsuda et al., 2009). The P2X4R is upregulated in activated microglial cells after spinal cord or peripheral nerve injury where it appears to mediate the release of brain-derived neurotrophic factor and induce neuropathic pain (Ulmann et al., 2008). Recent studies provide evidence that the functional expression of P2X4Rs in tissue-resident macrophages regulates inflammation-dependent prostaglandin E2 release (Ulmann et al., 2010). Activation of homomeric P2X4Rs in hippocampal neurons has been suggested to contribute to synaptic strengthening and hypersensitivity to sensory stimuli (Tsuda et al., 2009; Baxter et al., 2011). In addition, hippocampal synaptic transmission and long-term potentiation were abolished in P2X4R−/− mice (Sim et al., 2006). A unique characteristic of the P2X4R is its modulation by trace metals; copper inhibits whereas zinc and cobalt potentiate P2X4R activity (Coddou et al., 2011). P2X4R activity has been shown to be modulated by the allosteric effector ivermectin (Coddou et al., 2011). Heteromeric assembly of the P2X4R with the P2X1, P2X6 and P2X7 receptor subtypes has been described (Le et al., 1998; Nicke et al., 2005; Guo et al., 2007), although the functional relevance of these complexes in vivo is currently unknown.
The expression of the P2X5R subtype in the mouse CNS is most abundant in the olfactory bulb, cerebral cortex, globus pallidum, hippocampus, thalamus, hypothalamus, cerebellar cortex, and mid- and hindbrain nuclei (Guo et al., 2008). Although in vitro data have demonstrated ATP-evoked currents coupled to P2X5R activation, little is known about the physiological relevance of P2X5Rs in the CNS. Guo et al. speculate that P2X5R expression in the molecular layer of the cerebral cortex could play a role in interconnection of local cortical areas and P2X5R expression in the olfactory bulb suggests a role in fast excitatory post-synaptic purinergic transmission (Guo et al., 2009). In vitro studies have shown that activation of the homomeric P2X5R results in small, nondesensitizing currents, whereas activation of frequently observed heteromeric P2X5/P2X1 receptors results in slowly desensitizing ATP-evoked currents (Torres et al., 1998; Le et al., 1999). A P2X5R−/− mouse has not yet been developed; however, it will be critical for evaluating the role of the P2X5R in vivo. Interestingly, a recent study indicates that most humans express only a nonfunctional isoform of the P2X5R (Kotnis et al., 2010).
In the CNS, the P2X6R is expressed in Purkinje cells in the cerebellum, pyramidal cells in the hippocampus and sensory ganglia (Collo et al., 1996; Rubio and Soto, 2001; Robertson et al., 2001; Burnstock and Knight, 2004; da Silva et al., 2007). The ability of the P2X6R to form functional homomeric receptors is very low due to inefficient glycosylation of the N-terminus (Jones et al., 2004; Ormond et al., 2006). P2X6Rs readily form functional heteromers with P2X2 and P2X4 receptors, where activation of one subtype potentiates the activity of the other (Egan et al., 2004). In the myenteric plexus, the P2X6R is expressed in Dogiel type II neurons where it likely regulates physiological responses to ATP as a heteromeric complex with P2X2Rs (Yu et al., 2010).
Among the P2X receptor subtypes, the P2X7 receptor has gained prominent recognition as a regulator of inflammatory responses (Lister et al., 2007). P2X7Rs are expressed in many types of cells, notably in immune cells where activation by ATP increases the release of proinflammatory cytokines and apoptotic cell death (Pelegrin and Surprenant, 2006; Ferrari et al., 2006). The P2X7R was first cloned from rat brain (Surprenant et al., 1996), and subsequently has been found to be expressed in microglia, neurons and astrocytes (Ballerini et al., 1996; Brandle et al., 1998; Ferrari et al., 2006; Takenouchi et al., 2010). The P2X7R requires high concentrations of ATP (> 0.1 mM) for activation, although the photoaffinity ligand BzATP is a more potent agonist (Gonzalez et al., 1989; Erb et al., 1990). Stimulation of the P2X7R regulates the gating of non-selective cation channels, mitochondrial and plasma membrane depolarization, the formation of plasma membrane pores, plasma membrane blebbing, and the production of reactive oxygen species (ROS), responses ultimately leading to cell death (Erb et al., 1990; Schulze-Lohoff et al., 1998; Morelli et al., 2003; Verhoef et al., 2003; Wang et al., 2004; Adinolfi et al., 2005; Lister et al., 2007; Roger et al., 2008; Bours et al., 2011). P2X7R activity is dramatically potentiated by decreasing the divalent cation concentration, indicating that ATP4− may be the active ligand (Steinberg and Silverstein, 1987; Weisman et al., 1989; Hickman et al., 1996). P2X7Rs have been shown to mediate the release of neurotransmitters, e.g., glutamate, GABA, and ATP, and may be required for the induction of synaptic plasticity (Atkinson et al., 2004; Gordon et al., 2005; Duan and Neary, 2006). It also has been shown that P2X7R activation induces hypoxia- and caspase-dependent neuronal cell death (Kong et al., 2005; Sugiyama et al., 2010). Activation of P2X7Rs in glial cells results in the release of the proinflammatory cytokines TNFα, IL-1β and leukotrienes, thereby triggering or potentiating neuroinflammation (Hide et al., 2000; Suzuki et al., 2004; Ballerini et al., 2005; Kataoka et al., 2009), as described below. The P2X7R is upregulated in damaged nerves (Cavaliere et al., 2004; Franke et al., 2004a) and in nerves obtained from neuropathic pain patients (Chessell et al., 2005). In a mouse model of neuropathic pain, hypersensitivity to pain stimuli was completely absent upon deletion of the P2X7R (Chessell et al. 2005). The P2X7R is also upregulated in microglia around β-amyloid plaques in a mouse model of Alzheimer’s disease (AD) where it mediates superoxide production (Parvathenani et al., 2003). Enhanced expression of P2X7Rs also was observed in microglia derived from postmortem AD brains compared with glia obtained from non-demented brains (McLarnon et al., 2006). Furthermore, studies with a mouse model of Huntington’s disease suggest that P2X7Rs may play a role in disease pathogenesis (Diaz-Hernandez et al., 2009). Therefore, the P2X7R receptor could represent a therapeutic target for treating neurodegenerative diseases.
P2Y Receptors in the Central Nervous System
P2Y receptors (P2YRs) are classical heterotrimeric G protein-coupled seven-pass transmembrane receptors, as shown in Fig. 1. The extracellular N-terminus contains several potential glycosylation sites and the C-terminus contains consensus phosphorylation sites for protein kinases (Nguyen et al., 1995; Erb et al., 1995; Brinson and Harden, 2001; Flores et al., 2005). The intracellular loops and C-terminus have structural diversity among P2YR subtypes, thereby influencing the degree of coupling with Gq/11, Gs, and Gi proteins (Abbracchio et al., 2006). The length of human P2YRs varies from 328 (P2Y6R) to 377 (P2Y2R) amino acids and the composition reveals two structurally distinct subgroups within the P2YR family, the Gq-coupled P2Y1, P2Y2, P2Y4, P2Y6,and P2Y11 receptors and the Gi-coupled P2Y12, P2Y13 and P2Y14 receptors (Erb et al., 2006; Abbracchio et al., 2006). The degree of sequence homology among members of the human P2YR family ranges from 20 to 50%, suggesting a relatively high functional diversity (Shaver, 2001). It has been demonstrated that positively charged amino acids within transmembrane domains of P2YRs contribute to agonist binding (Erb et al., 1995; Jiang et al., 1997; Jacobson et al., 1999). P2YRs, whose agonists are adenine and/or uridine nucleotides, are expressed in many cell types comprising the CNS and have been shown to regulate neurotransmission, inflammation, cell growth, and apoptosis (Burnstock et al., 1972; Burnstock, 2009; Neary and Zimmermann, 2009; Burnstock and Verkhratsky, 2010). P2Y1, P2Y12, P2Y13 and P2Y14 receptors are activated by adenine nucleotides only, whereas the P2Y2R and rodent P2Y4R can be activated by either adenine or uridine nucleotides and the human P2Y4 and P2Y6 receptors are selective for uridine nucleotides (Abbracchio et al., 2006). The P2Y14R subtype is activated by UDP-glucose (Sak and Webb, 2002; Burnstock and Knight, 2004; Abbracchio et al., 2006). All eight P2Y receptor subtypes are expressed in primary rat astrocytes or astrocytoma cells (Fumagalli et al., 2003; Abbracchio et al., 2006; Kreda et al., 2008; Brandenburg et al., 2010), although the expression patterns vary with age (Lenz et al., 2000; Jacques-Silva et al., 2004). Rodent neurons express P2Y1,2,4,6,12,13 receptors (Kong et al., 2009; Espada et al., 2010; Köles et al., 2011). P2YRs are expressed at postsynaptic terminals where P2Y1, P2Y2 and P2Y4 receptors are neuromodulators that have inhibitory roles in synaptic transmission (Rodrigues et al., 2005; Rodrigues et al., 2006; Sperlágh and Illes, 2007; Fischer and Krügel, 2007). The cell and tissue distribution, agonist specificities and functional relevance of P2Y receptors in the nervous system are summarized in Table 2.
Table 2. P2Y Receptors in the Nervous System.
The agonists, expression and function of P2Y receptor subtypes in the nervous system are summarized in Table 2. This table highlights the information presented in this review article and is not considered to be comprehensive.
| Receptor Subtype |
Agonist | Expression | Function | |
|---|---|---|---|---|
| Cell Type | Region | |||
| P2Y1 | ADP, ATP | Neurons, astrocytes, microglia, oligodendrocytes | Cerebral cortex, cerebellum, hippocampus, midbrain, caudate nucleus, putamen, globus pallidus, habenula, subthalamic nucleus, dorsal root ganglia, dorsal horn | Synaptic transmission modulation, provides neuroprotection by stimulating IL-6 release from astrocytes, brain development and repair, sensory reception |
| P2Y2 | ATP, UTP | Neurons, astrocytes, microglia | Cerebral cortex, cerebellum, hippocampus, nucleus accumbens, spinal cord | Promote neurite outgrowth, stimulate α-secretase-dependent processing of amyloid precursor protein, increase phagocytosis of Aβ peptide, regulation of intracellular calcium waves, stimulate proliferation, modulate pain sensation, increase cell motility |
| P2Y4 | ATP, UTP | Neurons, astrocytes, microglia | Cerebral cortex, hippocampus | Synaptic transmission modulation, regulation of blood-brain barrier function, blood flow, metabolic trafficking, water homeostasis |
| P2Y6 | UDP, UTP | Neurons, astrocytes, microglia | Cerebral cortex, cerebellum, hippocampus, amygdala, cingulate gyrus, putamen, nucleus accumbens, superior cervical ganglia, dorsal root ganglia | Stimulate phagocytic activity, neuroinflammatory responses |
| P2Y11 | ATP, ADP | Neurons | Cerebellum, hippocampus, parahippocampal gyrus, putamen, striatum, nucleus accumbens | Neuroinflammatory responses |
| P2Y12 | ADP | Neurons, astrocytes, microglia, oligodendrocytes | Cerebral cortex, cerebellum, hippocampus, nucleus accumbens | Regulation of migration and chemotaxis |
| P2Y13 | ADP | Neurons, astrocytes | Brainstem | Modulation of synaptic transmission, modulates expression of cell survival genes |
| P2Y14 | UDP-glucose | Astrocytes, microglia | Cerebral cortex, cerebellum | Modulation of immune system's anti-tumor response |
The P2Y1R has a widespread distribution in mammalian brain, including the cerebral cortex, hippocampus, caudate nucleus, putamen, globus pallidus, habenula, subthalamic nucleus, midbrain and cerebellum, as demonstrated in autoradiographic and immunohistochemical studies (Simon et al., 1997; Moore et al., 2000; Morán-Jiménez and Matute, 2000). The P2Y1R is intensely expressed in Purkinje cells, in deep layers of the cerebral cortex and in areas of the hippocampus sensitive to ischemia (Morán-Jiménez and Matute, 2000). P2Y1R immunoreactivity has also been observed in oligodendrocytes and astrocytes in brain white matter tracts and optic nerves (Simon et al., 1995; Fumagalli et al., 2003). P2Y1Rs have been suggested to play important roles in glial cell functions (Morán-Jiménez and Matute, 2000). P2Y1R activation in astrocytes of hippocampal cultures has been suggested to provide neuroprotection from oxidative stress by increasing IL-6 release (Fujita et al., 2009). P2Y1Rs are also expressed in microglial cells (Gotsch et al., 1997; Simon et al., 1997; Fumagalli et al., 2003; Franke et al., 2004a; Bianco et al., 2005a), rat neuroprogenitor cells (Mishra et al., 2006), and various sensory neurons such as dorsal root ganglia and dorsal horn neurons (Sanada et al., 2002; Ruan and Burnstock, 2003; Kobayashi et al., 2006). Studies have suggested potential roles for P2Y1Rs in brain development and repair (Mishra et al., 2006) and sensory reception (Ruan and Burnstock, 2003; Gerevich et al., 2005).
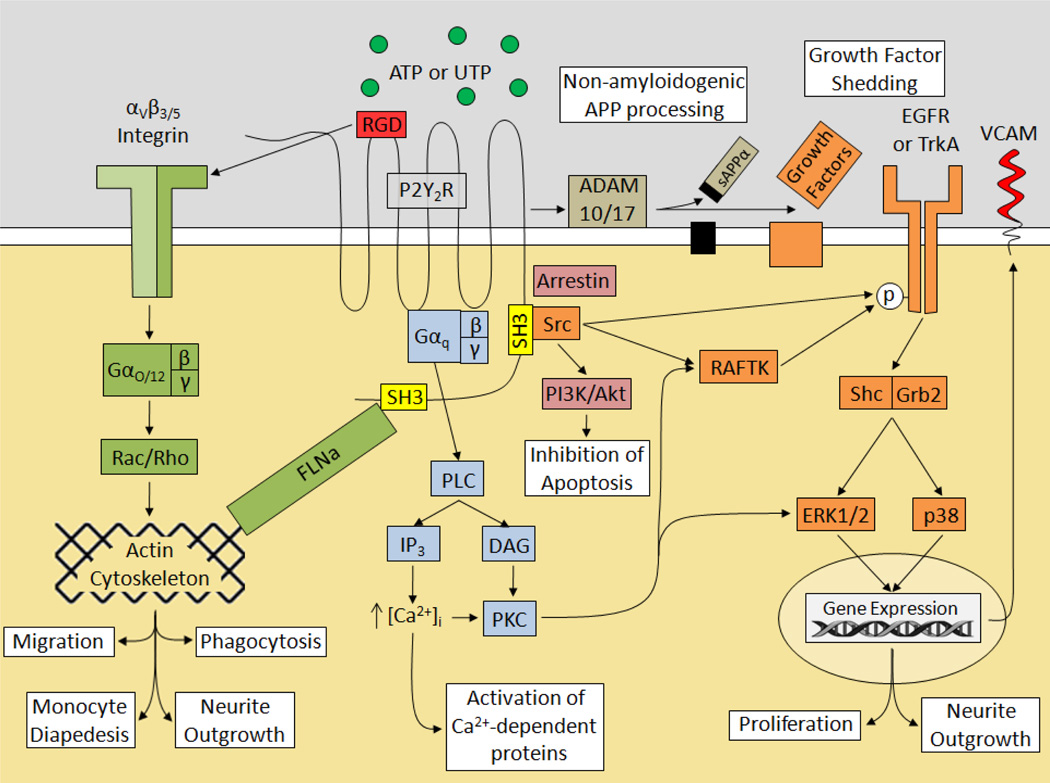
The contribution of the P2Y2R subtype to CNS functions is becoming better understood (Franke and Illes, 2006; Inoue, 2008; Peterson et al., 2010) and appears to be most relevant under pathophysiological conditions, such as inflammation and bacterial infection (Peterson et al., 2010; Chen et al., 2010). The mammalian P2Y2R is equipotently activated by ATP or UTP (Weisman et al., 2005; Abbracchio et al., 2006) and is upregulated in many cell and animal models of inflammation or injury (Turner et al., 1997; Koshiba et al., 1997; Seye et al., 2002; Shen et al., 2004; Schrader et al., 2005; Kong et al., 2009), including acute and chronic stages of spinal cord injury (Rodríguez-Zayas et al., 2010), brain ischemia, mechanical injury to the nucleus accumbens and brain trauma (Franke et al., 2004a; Franke et al., 2004b), suggesting that P2Y2R upregulation represents a cellular response to tissue damage and inflammation. P2Y2R expression under proinflammatory conditions is regulated by NF-κB binding to the P2Y2R promoter (Degagne et al., 2009), consistent with the established role of NF-κB activation in the induction of inflammation (Wullaert et al., 2011). In addition to the typical Gq-coupled activation of the PLC/IP3/PKC pathway, the P2Y2R has a variety of structural motifs that enable it to activate integrin and growth factor receptor signaling cascades, as shown in Fig. 2. For example, P2Y2R activation by ATP or UTP has been shown to induce phosphorylation of growth factor receptors which increases the activities of the MAP kinases ERK1/2 and the related adhesion focal tyrosine kinase (RAFTK) via a pathway dependent upon Src and Shc/Grb2 (Soltoff, 1998; Soltoff et al., 1998; Seye et al., 2004). However, other studies show that P2Y2R-mediated EGFR phosphorylation is Src-independent, but requires the release of growth factors via P2Y2R-dependent activation of the matrix metalloproteases ADAM10 and ADAM17 (Ratchford et al., 2010). These differences appear to be due to cell type, e.g., endothelial vs. epithelial. The P2Y2R is unique among GPCRs in that it contains the consensus integrin-binding motif Arg-Gly-Asp (RGD) in a putative extracellular domain that enables the association of the P2Y2R with αvβ3/5 integrins allowing activation of heterotrimeric Go and G12 proteins that regulate the activities of the small GTPases Rho and Rac (Erb et al., 2001; Bagchi et al., 2005; Liao et al., 2007), regulators of actin polymerization and cytoskleletal rearrangements required for cell migration (Xu et al., 2003; Jang et al., 2005). In addition, the P2Y2R contains SH3-binding motifs in its C-terminal domain that can interact with the actin-binding protein filamin A (FLNa), another known regulator of cytoskeletal rearrangements, suggesting that the P2Y2R may regulate migration of cells by both RGD-dependent interactions with integrins and C-terminal-dependent association with actin-binding proteins (Yu et al., 2008). Moreover, the P2Y2R has been reported to regulate migration of some cell types via transactivation of growth factor receptors (Bagchi et al., 2005; Norambuena et al., 2010), suggesting that a complex array of signaling events uniquely coupled to P2Y2R activation may be required to optimize the cytoskeletal rearrangements whereby the P2Y2R regulates cell-specific functions, e.g., neurite outgrowth, phagocytosis, synapse formation and chemokinesis.
Fig. 2. P2Y2R Signaling Pathways.
The P2Y2R modulates a variety of cellular processes through classical G protein-coupled receptor pathways and unique receptor motifs. Activation of the P2Y2R by ATP or UTP stimulates the Gq-dependent activation of PLC leading to the generation of IP3 and DAG. IP3 triggers a release of Ca2+ from intracellular stores leading to an increase in [Ca2+]i and the activation of Ca2+-dependent proteins, whereas DAG serves to activate PKC leading to activation of a variety of downstream proteins including RAFTK; also known as Pyk2) and the MAP kinases ERK1/2. The Src-homology-3 (SH3) domains in the C-terminus allow the P2Y2R to stimulate Src-dependent transactivation of growth factor receptors and their downstream signaling molecules Shc and Grb2 leading to ERK1/2 and p38 activation of cell proliferation and neurite outgrowth. The P2Y2R also has been shown to upregulate VCAM-1 through a pathway involving VEGFR-2. Furthermore, P2Y2R activation has been shown to stimulate the PI3K/Akt pathway to inhibit apoptosis in neurons, a response that was dependent on Src activation. SH3 domains also allow the P2Y2R to interact with the actin cytoskeleton via the actin-binding protein filamin A (FLNa). Alternatively the P2Y2R can access the actin cytoskeleton through an extracellular RGD domain that interacts with αvβ3/5 integrins to enable activation of Go and G12 proteins allowing the P2Y2R to stimulate cell migration, phagocytosis, neurite outgrowth, and diapedesis by activating the cytoskeletal regulators Rac and Rho. Lastly, the P2Y2R is able to activate the matrix metalloproteases ADAM10 and ADAM17 to induce non-amyloidogenic APP processing and shedding of growth factors.
P2Y2R expression in rat primary cortical neurons is upregulated in response to IL-1β (Kong et al., 2009), a cytokine whose levels are elevated in the brains of AD patients (Xie et al., 2004; Lee et al., 2010). Subsequent activation of these upregulated P2Y2Rs in neurons promotes neurite outgrowth (Pooler et al., 2005) and generates the non-amyloidogenic soluble APPα peptide, rather than neurotoxic Aβ1–42 peptide aggregates associated with AD (Kong et al., 2009). In mouse primary microglial cells, the P2Y2R is upregulated in the presence of Aβ1–42 and when activated can increase the phagocytosis and degradation of neurotoxic forms of Aβ (Boucsein et al., 2003; Peterson et al., 2010; Kim et al., 2012). In astrocytic cells, the P2Y2R has been suggested to contribute to synaptic transmission through the regulation of intracellular calcium waves (Halassa et al., 2009) and upregulates anti-apoptotic protein expression to promote cell survival (Chorna et al., 2004). Thus, P2Y2R upregulation in response to proinflammatory conditions likely serves a neuroprotective role in the CNS that requires contributions from both glial and neuronal P2Y2Rs, as described in more detail below.
The human P2Y4R is preferentially activated by uridine nucleotides, whereas the rat and mouse P2Y4Rs are stimulated equipotently by ATP and UTP (Webb et al., 1998; Brunschweiger and Muller, 2006; Jacobson et al., 2009). P2Y4R mRNA is highly expressed in human brain (Moore et al., 2001). Single cell RT-PCR demonstrated the expression of P2Y4Rs in rat hippocampal pyramidal neurons (Rodrigues et al., 2005). The expression of P2Y4Rs in astrocytes and microglial cells has been extensively documented (Webb et al., 1998; Lenz et al., 2000; Fumagalli et al., 2003; Bianco et al., 2005a). P2Y4Rs, as well as P2Y2Rs, are strongly expressed in glial endfeet in proximity to blood vessel walls (Simard et al., 2003) where their activation by ATP has been postulated to regulate blood–brain barrier function, blood flow, metabolic trafficking and water homeostasis (Simard et al., 2003; Zonta et al., 2003).
The P2Y6R is activated by uridine 5’-diphosphate (UDP), and to a lesser extent UTP (Communi et al., 1996). In 18 areas of the human brain, the level of P2Y6R mRNA expression was highest in the amygdala, cingulate gyrus, nucleus accumbens and putamen (Moore et al., 2001). Single cell RT-PCR revealed P2Y6R mRNA in 2 of 12 pyramidal neurons of rat hippocampus (Rodrigues et al., 2005). In addition, P2Y6R mRNA has been demonstrated in superior cervical ganglion (Calvert and Evans, 2004; Calvert et al., 2004) and dorsal-root ganglion neurons (Sanada et al., 2002; Ruan and Burnstock, 2003). Functional studies have revealed the presence of P2Y6R activity in cerebellar and cortical astrocytes (Fumagalli et al., 2003; Bennett et al., 2003). P2Y6R activation has been shown to increase phagocytotic activity of microglia, postulated to occur in vivo in response to UTP released from damaged cells (Koizumi et al., 2007; Liu et al., 2009). Consistent with this hypothesis, injury has been shown to induce increased P2Y6R expression in astroglial cells (Franke et al., 2004a; Franke et al., 2004b). In microglial cells stimulated overnight with bacterial lipopolysaccharide, P2Y6R-mediated increases in the intracellular calcium concentration were observed, suggesting a role for the P2Y6R in neuroinflammation (Bianco et al., 2005a).
The P2Y11R can couple to multiple G proteins to regulate the activity of two second messenger systems: adenylate cyclase-mediated cAMP production, and PLC-dependent production of IP3 and DAG that modulate calcium release from intracellular storage sites and protein kinase C activation, respectively (Communi et al., 1999). The P2Y11R is activated by ATP or ADP, but not by uridine nucleotides (Communi et al., 1999). P2Y11R mRNA expression is prominent in nucleus accumbens, parahippocampal gyrus, putamen and striatum (Burnstock and Knight, 2004). The P2Y11R has been localized to single rat hippocampal pyramidal neurons and to Purkinje cells in adult rat cerebellum (Rodrigues et al., 2005; Volonté et al., 2006). Inhibition of the P2Y11R has been shown to delay ATP-induced neutrophil apoptosis, suggesting a role for the P2Y11R in the regulation of neuroinflammatory responses (Vaughan et al., 2007).
The P2Y12R is widely distributed in the brain with a pattern consistent with expression in astrocytes (Hollopeter et al., 2001; Kunapuli et al., 2003). RT-PCR has demonstrated the presence of P2Y12R mRNA in single rat hippocampal pyramidal neurons (Rodrigues et al., 2005). Cortical and cerebellar astrocytes and astrocytes in the rat nucleus accumbens also express P2Y12Rs (Fumagalli et al., 2003; Franke et al., 2004b; Carrasquero et al., 2005). P2Y12Rs have been suggested to regulate the migration of microglial cells towards damaged neurons (Sasaki et al., 2003). P2Y12R expression in microglia is robust in the 'resting' state, but dramatically reduced in activated microglia, and P2Y12R−/− mice have significantly diminished directional branch extension toward sites of cortical damage in vivo (Haynes et al., 2006). In contrast, a recent study concludes that the expression of the P2Y12R in the CNS is restricted to oligodendrocytes (Amadio et al., 2006). It also has been suggested P2Y12Rs contribute to the migration and adhesion of glial cell processes to axons during pre-myelination (Amadio et al., 2006).
The P2Y13R is activated by ADP (Marteau et al., 2003) and 2-methylthio ADP is a potent synthetic agonist (Burnstock, 2006b), similar to the P2Y12R; however ATP and ATP analogues are inactive at the P2Y13R (Communi et al., 1997). P2Y13R expression has been localized to brainstem astrocytes and glutamatergic neurons (Moore et al., 2000; Moore et al., 2001; Jiménez et al., 2011). P2Y13Rs, along with P2Y1 and P2Y12 receptors, have been shown to regulate Na+ and Cl−-dependent synaptic glycinergic neurotransmitter transporters to increase transport of glycine from the synaptic cleft, thereby maintaining quantal glycine levels in inhibitory synaptic vesicles (Gomeza et al., 2003; Jiménez et al., 2011). The P2Y13R can also activate the glycogen synthase kinase-3 (GSK-3)-dependent phosphatidylinositoI 3-kinase (PI3K)/Akt survival pathway to increase translocation of the GSK-3 substrate β-catenin to the nucleus, where it modulates expression of cell survival genes (Ortega et al., 2008).
The P2Y14R is expressed in astrocytes (Moore et al., 2001), and RT-PCR and single cell Ca2+ imaging has documented the functional expression of P2Y14Rs in rat cortical and cerebellar astrocytes (Fumagalli et al., 2003; Carrasquero et al., 2005). Agonists of the P2Y14R include UDP-glucose, UDP-galactose, UDP-glucuronic acid and UDP-N-acetylglucosamine, but not adenine or uridine nucleotides (Chambers et al., 2000; Abbracchio et al., 2003; Burnstock, 2006a). UDP-glucose has been shown to be released from a variety of cell lines, and UDP-glucose levels can exceed those of ATP under various conditions (Lazarowski et al., 2003). Functionally, P2Y14Rs in primary microglial cells from rat brain have been shown to modulate the calcium response to bacterial lipopolysaccharide (Bianco et al., 2005a). P2Y14Rs expressed in immature dendritic cells have been suggested to play a role in the immune system’s anti-tumor response (Skelton et al., 2003; Fischer and Krügel, 2007).
Neuroinflammatory P2X7Rs Regulate Neuroprotective P2Y2R Expression
P2X7R activation contributes to neuroinflammation by promoting mitochondrial and plasma membrane depolarization, the formation of plasma membrane pores, plasma membrane blebbing, and the production of reactive oxygen species (ROS) (Schulze-Lohoff et al., 1998; Morelli et al., 2003; Verhoef et al., 2003; Wang et al., 2004; Adinolfi et al., 2005; Lister et al., 2007; Roger et al., 2008; Bours et al., 2011). In addition, P2X7R activation promotes neuroinflammation by causing the release of proinflammatory cytokines, such as IL-1β and TNF-α (Di Virgilio, 2007; Lister et al., 2007; Tschopp and Schroder, 2010) and activation of MAP kinases and NF-κB, resulting in upregulation of proinflammatory gene products, including COX-2, chemokines and cell adhesion molecules (Pfeiffer et al., 2004; Potucek et al., 2006; Lister et al., 2007; Lenertz et al., 2009; Skaper et al., 2009; Shiratori et al., 2010) and the P2Y2R (Degagne et al., 2009). Importantly, P2X7R-mediated pore formation initially increases ATP release through P2X7R interactions with a pannexin hemi-channel in cells (Pelegrin and Surprenant, 2006). P2X7R-mediated IL-1β and ATP release is a mechanism whereby the P2X7R regulates functional P2Y2R expression in neurons and provides agonist for the activation of the upregulated P2Y2R and other P2 receptors (Peterson et al., 2010). ATP release also can occur from activated microglia and astrocytes in response to oxidative stress (Peterson et al., 2010), following neuronal excitation (Bodin and Burnstock, 2001; Fields, 2011), via volume-activated anion channels (Fields, 2011), or upon exposure of cells to fibrillar or oligomeric forms of amyloidogenic A 1–42 peptides (Inoue, 2008; Sanz et al., 2009; El Khoury and Luster, 2008; Kim et al., 2012). Thus, P2X7 and P2Y2 receptors may represent promising targets to control inflammatory responses associated with neurodegenerative diseases. Indeed, mice deficient in the P2X7R (P2X7R−/− mice) exhibit decreased inflammatory responses (Labasi et al., 2002; Chessell et al., 2005; McGaraughty et al., 2007; Lucattelli et al., 2011), including a reduction in pulmonary fibrosis in a mouse model of lung inflammation (Lucattelli et al., 2011) and the absence of pain hypersensitivity in mouse models of chronic inflammation and neuropathic pain (Chessell et al., 2005). Phase I and II clinical trials for selective P2X7R antagonists are presently underway for the treatment of rheumatoid arthritis and other inflammatory diseases (Friedle et al. 2010; Arulkumaran et al. 2011).
Upregulation of the P2Y2R in response to P2X7R activation appears to promote neuroprotective responses. The ability of the P2Y2R to stimulate neuroprotective responses depends upon the coupling of the receptor to intracellular signaling pathways that are distinct among the P2YR family (see Fig. 2). These responses associated with P2Y2R upregulation include the outgrowth and stabilization of dendritic spines (Jang et al., 2005; Bamburg and Bloom, 2009; Peterson et al., 2010), which requires RGD-dependent P2Y2R/αv integrin interaction to stimulate Rac and Rho and induce cytoskeletal rearrangements (Bagchi et al., 2005; Liao et al., 2007) and upregulation of neurofilament M and neurofilaments that promote neurite outgrowth (Pooler et al., 2005). P2Y2Rs also require Src to co-localize with the tyrosine receptor kinase A (TrkA) in the presence of nerve growth factor, a pathway that regulates neurite outgrowth and cell division via the activation of p38 and ERK1/2 MAP kinases (Arthur et al., 2005, 2006a). In neural progenitor cells isolated from the subventricular zone of adult mouse brain, P2Y2R activation was shown to induce proliferative responses such as the transient activation of the epidermal growth factor receptor (EGFR), the MAP kinases ERK1/2 and the transcription factor CREB (Grimm et al., 2009). Other studies indicate that the P2Y2R mediates the activation of PI3-kinase/Akt and MAP kinases to inhibit apoptosis of PC12 pheochromocytoma cells and dorsal root ganglion neurons (Arthur et al., 2006a; Arthur et al., 2006b). P2Y2R upregulation by IL-β1 and subsequent activation in primary cortical neurons increases amyloid precursor protein (APP) processing via activation of matrix metalloproteases (i.e., α-secretases), a neuroprotective response that produces a non-amyloidogenic soluble APP peptide (i.e., sAPPα) rather than neurotoxic amyloidogenic Aβ peptide (Kong et al., 2009). IL-1β is known to stimulate neuronal synthesis of APP and increase the release of neurotoxic Aβ, which further enhances IL-1β production (Walker et al., 1995). We postulate that upregulation of the P2Y2R induced by IL-1β in vivo counteracts the potential neurotoxic effects of IL-1β-dependent elevations in APP levels by promoting generation of non-toxic sAPPα instead of Aβ. Thus, P2Y2R upregulation in the CNS may delay the progression of neurodegeneration associated with reactive gliosis and chronic inflammation in AD and other neurological disorders.
Glial cells, including astrocytes and microglia, play important neuroprotective roles. Astrocytes contribute to the maintenance of the blood-brain barrier (BBB) (Sudo et al., 1998; Shlosberg et al., 2010; Barreto et al., 2011), which prevents invasion of pathogenic, neurotoxic substances into the brain from the circulation (Takenouchi et al., 2009; Sugama et al., 2009). Astrocytes also release neurotrophic factors that regulate neuronal survival and sprouting and supply energy substrates to neurons (Giaume et al., 2010). Astrocytes have been shown to release ATP under a variety of pathological conditions (Butt, 2011; Hamilton et al., 2008; Hamilton et al., 2010) and ATP levels are elevated sufficiently by inflammation in vivo to activate P2 nucleotide receptors (Butt, 2011). P2Y2Rs are upregulated in reactive astrocytes of the rat cortex and nucleus accumbens in response to mechanical injury (Franke et al., 2004a; Franke et al., 2004b) and have been suggested to enhance astrocyte survival (Chorna et al., 2004; Burgos et al., 2007). In addition, interactions between the P2Y2R and integrins have been demonstrated to regulate the migration of astrocytes (Wang et al., 2005; Bagchi et al., 2005; Liao et al., 2007). It also has been shown that P2X7R activation increases the expression of P2Y2Rs in rat astrocytes (D'Alimonte et al., 2007) likely via P2X7R-mediated IL-1β release (Chakfe et al., 2002; Suzuki et al., 2004; Choi et al., 2007; Mingam et al., 2008; Sanz et al., 2009; Takenouchi et al., 2009).
Microglia have important immunoregulatory functions in the CNS. Injury or other insults to the CNS trigger transformation of quiescent microglia into activated phenotypes, i.e., phagocytic macrophages (Kreutzberg, 1996; Streit, 2002). Activated microglia have neuroprotective functions (Streit, 2002; Butovsky et al., 2005; Turrin and Rivest, 2006; Lalancette-Hébert et al., 2007; El Khoury et al., 2007), although sustained activation can be neurotoxic (Butovsky et al., 2005; Boillée et al., 2006; Streit, 2006; Neumann and Takahashi, 2007). Microglial cell activation by proinflammatory cytokines has been shown to increase cell motility and proliferation (Brown and Neher, 2010), responses associated with reactive gliosis in neurodegenerative diseases. Adenine and uridine nucleotides have been shown to increase the motility of microglial cells (Honda et al., 2001; Haynes et al., 2006; Koizumi et al., 2007) via activation of P2Y2 and P2Y12 receptors (Haynes et al., 2006; Chen et al., 2006) and ATP release can significantly increase microglial process extension towards a site of injury (Davalos et al., 2005). The endogenous expression of P2Y2Rs has been reported in mouse microglia (Shigemoto-Mogami et al., 2001; Crain et al., 2009) where they have been shown to regulate responses associated with reactive gliosis (Chorna et al., 2004; Franke et al., 2004b; Wang et al., 2005; Weisman et al., 2005; Burgos et al., 2007; Peterson et al., 2010). For example, the P2Y2R agonists UTP and ATP released from apoptotic cells have been shown to induce migration of phagocytic cells (Elliott et al., 2009), which presumably serves to enhance the clearance of cellular debris. Microglial cells exposed to Aβ also have been shown to release ATP (Rogers and Lue, 2001; Sanz et al., 2009). Studies using peritoneal macrophages in mice have shown that stimulation of P2Y2 and P2Y12 receptors induces the formation of lamellipodia in membrane protrusions which is required for cell motility (Kronlage et al., 2010). Co-activation of P2Y2 and P2Y6 receptors in human monocytes enhances migration, a response shown to involve toll-like receptor-induced IL-8 release (Ben Yebdri et al., 2009; Kukulski et al., 2010). We have found that P2Y2R activation increases mouse microglial cell migration and phagocytic activity, such as the uptake of neurotoxic oligomeric A 1–42, responses that are absent in microglia from P2Y2R−/− mice (Kim et al., 2012). Both activated astrocytes and microglia internalize and degrade A (Chung et al., 1999; Wyss-Coray et al., 2001; Pihlaja et al., 2008; Mandrekar et al., 2009; Kong et al., 2010), a pathway that reduces Aβ toxicity in neurons that is postulated to play a role in the progression of AD. We speculate that P2Y2Rs in glial cells contribute to the phagocytosis and degradation of neurotoxic forms of Aβ in vivo under conditions where elevated levels of ATP release and IL-1β generation occur (Ferrari et al., 1997; Di Virgilio et al., 1998).
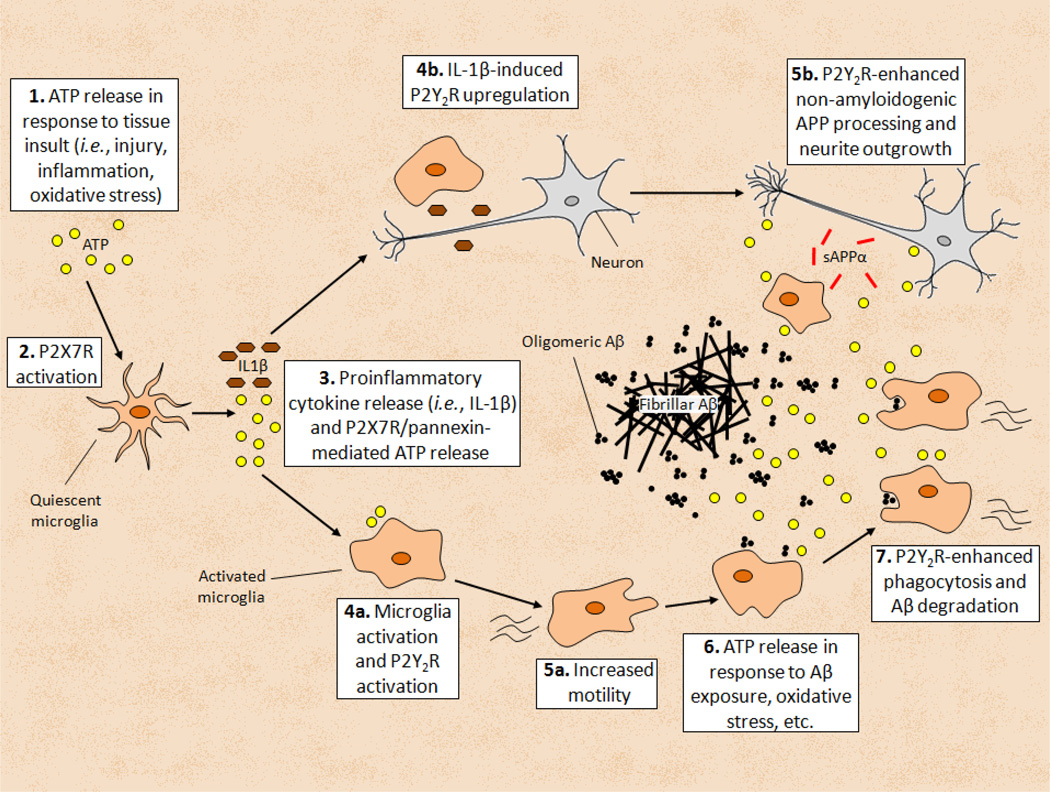
Recent studies suggest that peripheral leukocytes and hematopoietic cells that differentiate into microglia have important functions in the CNS (Gate et al., 2010), particularly in response to tissue injury (Hawkes and McLaurin, 2009). P2Y2Rs in endothelial cells that form the BBB may also regulate the migration of leukocytes across the BBB towards sites of injury or disease in the brain. Activation of the endothelial P2Y2R has been shown to enhance the diapedesis of neutrophils towards the chemoattactant lipopolysaccharide of gram-negative bacteria (Kukulski et al., 2010) through a mechanism involving Rho kinase activation, suggesting that P2Y2R associations with integrins may be involved (Bagchi et al., 2005; Liao et al., 2007). Microglia derived from bone marrow have been shown to phagocytose Aβ deposits in the brain of AD mice to a greater extent than resident brain microglia (Simard et al., 2006). Thus, diapedesis of microglia across the BBB, in addition to neurite outgrowth, non-amyloidogenic APP processing and phagocytosis of neurotoxic forms of Aβ may comprise a neuroprotective phenotype linked to P2Y2R activation in several cell types that comprise the brain (i.e., neurons, glial cells and endothelium). The neuroprotective pathways by which P2X7R-mediated upregulation and activation of P2Y2Rs are suggested to contribute to neuroprotection in the brain are shown in Fig. 3.
Fig. 3. P2X7R-mediated Neuroinflammation Stimulates P2Y2R-mediated Neuroprotective Responses.
(1) ATP released under neuroinflammatory conditions can (2) activate the P2X7R to stimulate (3) the release of proinflammatory cytokines, including interleukin-1β (IL-1β), and further ATP release via interaction of the P2X7R with pannexin hemi-channels. In response to the proinflammatory environment, quiescent microglia take on an (4a) activated phenotype and P2Y2R activation by extracellular ATP increases (5a) cell motility. In addition, (4b) IL-1β upregulates P2Y2R expression in neurons and glia through NF-κB activation. (6) ATP release (from Aβ exposure, cytokine exposure, oxidative stress, etc.) provides agonist for the (5b) P2Y2R to stimulate non-amyloidogenic APP processing and neurite outgrowth through P2Y2R interactions with matrix metalloproteases and the actin cytoskeleton, respectively. Another neuroprotective response to (7) P2Y2R activation in microglial cells is increased phagocytosis and degradation of neurotoxic oligomeric Aβ1–42.
Conclusion
This review summarizes data indicating that seven ionotropic P2X and eight G protein-coupled P2Y receptors for extracellular nucleotides are expressed in cell types comprising the CNS and these P2X and P2Y receptor subtypes have been shown to regulate diverse physiological and pathological responses under a variety of conditions. Recent studies indicate that activation of the P2X7R rsubtype during inflammation causes upregulation and activation of P2Y2Rs to promote neuroprotective responses. These findings suggest that ATP released from injured or stressed cells in the CNS can activate P2X7Rs in microglial cells to increase the release of proinflammatory cytokines, such as IL-1β, that increase the expression of the P2Y2R, particularly in neurons. Other studies indicate that both P2X7R and P2Y2R activation can increase phagocytosis of neurotoxic forms of Aβ and that activation of the P2Y2R increases non-amyloidogenic APP processing, neuroprotective responses that are postulated to delay the onset or retard the progression of neurodegenerative diseases, such as Alzheimer’s disease. In addition, P2Y2R activation in neurons has been shown to increase neurite outgrowth. The P2Y2R contains multiple motifs that enable its activation to directly couple to integrin and growth factor receptor signaling pathways that play a role in cell proliferation and differentiation and cytoskeletal rearrangements that are critical for tissue repair. Thus, the studies described in this review suggest that the P2X7R and P2Y2R are promising targets for the treatment of neurodegenerative diseases.
References Cited
- Abbracchio MP, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Miras-Portugal MT, King BF, Gachet C, Jacobson KA, Weisman GA, Burnstock G. Characterization of the UDP-glucose receptor (re-named here the P2Y14 receptor) adds diversity to the P2Y receptor family. Trends Pharmacol Sci. 2003;24(2):52–55. doi: 10.1016/S0165-6147(02)00038-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbracchio MP, Burnstock G. Purinoceptors: are there families of P2X and P2Y purinoceptors? Pharmacol Ther. 1994;64(3):445–475. doi: 10.1016/0163-7258(94)00048-4. [pii] [DOI] [PubMed] [Google Scholar]
- Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, Weisman GA. International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev. 2006;58(3):281–341. doi: 10.1124/pr.58.3.3. [pii] 10.1124/pr.58.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adinolfi E, Pizzirani C, Idzko M, Panther E, Norgauer J, Di Virgilio F, Ferrari D. P2X7 receptor: Death or life? Purinergic Signal. 2005;1(3):219–227. doi: 10.1007/s11302-005-6322-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amadio S, Tramini G, Martorana A, Viscomi MT, Sancesario G, Bernardi G, Volonté C. Oligodendrocytes express P2Y12 metabotropic receptor in adult rat brain. Neuroscience. 2006;141(3):1171–1180. doi: 10.1016/j.neuroscience.2006.05.058. [pii] 10.1016/j.neuroscience.2006.05.058. [DOI] [PubMed] [Google Scholar]
- Arthur DB, Akassoglou K, Insel PA. P2Y2 receptor activates nerve growth factor/TrkA signaling to enhance neuronal differentiation. Proc Natl Acad Sci U S A. 2005;102(52):19138–19143. doi: 10.1073/pnas.0505913102. [pii] 10.1073/pnas.0505913102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur DB, Akassoglou K, Insel PA. P2Y2 and TrkA receptors interact with Src family kinase for neuronal differentiation. Biochem Biophys Res Commun. 2006a;347(3):678–682. doi: 10.1016/j.bbrc.2006.06.141. [pii] 10.1016/j.bbrc.2006.06.141. [DOI] [PubMed] [Google Scholar]
- Arthur DB, Georgi S, Akassoglou K, Insel PA. Inhibition of apoptosis by P2Y2 receptor activation: novel pathways for neuronal survival. J Neurosci. 2006b;26(14):3798–3804. doi: 10.1523/JNEUROSCI.5338-05.2006. [pii] 10.1523/JNEUROSCI.5338-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arulkumaran N, Unwin RJ, Tam FW. A potential therapeutic role for P2X7 receptor (P2X7R) antagonists in the treatment of inflammatory diseases. Expert Opin Investig Drugs. 2011;20(7):897–915. doi: 10.1517/13543784.2011.578068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ase AR, Bernier LP, Blais D, Pankratov Y, Seguela P. Modulation of heteromeric P2X1/5 receptors by phosphoinositides in astrocytes depends on the P2X1 subunit. J Neurochem. 2010;113(6):1676–1684. doi: 10.1111/j.1471-4159.2010.06734.x. [pii] 10.1111/j.1471-4159.2010.06734.x. [DOI] [PubMed] [Google Scholar]
- Atkinson L, Batten TF, Deuchars J. P2X(2) receptor immunoreactivity in the dorsal vagal complex and area postrema of the rat. Neuroscience. 2000;99(4):683–696. doi: 10.1016/s0306-4522(00)00233-5. [pii] [DOI] [PubMed] [Google Scholar]
- Atkinson L, Batten TF, Moores TS, Varoqui H, Erickson JD, Deuchars J. Differential co-localisation of the P2X7 receptor subunit with vesicular glutamate transporters VGLUT1 and VGLUT2 in rat CNS. Neuroscience. 2004;123(3):761–768. doi: 10.1016/j.neuroscience.2003.08.065. [DOI] [PubMed] [Google Scholar]
- Bagchi S, Liao Z, Gonzalez FA, Chorna NE, Seye CI, Weisman GA, Erb L. The P2Y2 nucleotide receptor interacts with αv integrins to activate Go and induce cell migration. J Biol Chem. 2005;280(47):39050–39057. doi: 10.1074/jbc.M504819200. [pii] 10.1074/jbc.M504819200. [DOI] [PubMed] [Google Scholar]
- Ballerini P, Ciccarelli R, Caciagli F, Rathbone MP, Werstiuk ES, Traversa U, Buccella S, Giuliani P, Jang S, Nargi E, Visini D, Santavenere C, Di Iorio P. P2X7 receptor activation in rat brain cultured astrocytes increases the biosynthetic release of cysteinyl leukotrienes. Int J Immunopathol Pharmacol. 2005;18(3):417–430. doi: 10.1177/039463200501800303. [DOI] [PubMed] [Google Scholar]
- Ballerini P, Rathbone MP, Di Iorio P, Renzetti A, Giuliani P, D'Alimonte I, Trubiani O, Caciagli F, Ciccarelli R. Rat astroglial P2Z (P2X7) receptors regulate intracellular calcium and purine release. Neuroreport. 1996;7(15–17):2533–2537. doi: 10.1097/00001756-199611040-00026. [DOI] [PubMed] [Google Scholar]
- Bamburg JR, Bloom GS. Cytoskeletal pathologies of Alzheimer disease. Cell Motil Cytoskeleton. 2009;66(8):635–649. doi: 10.1002/cm.20388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreto GE, Gonzalez J, Torres Y, Morales L. Astrocytic-neuronal crosstalk: implications for neuroprotection from brain injury. Neurosci Res. 2011;71(2):107–113. doi: 10.1016/j.neures.2011.06.004. [pii] 10.1016/j.neures.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Baxter AW, Choi SJ, Sim JA, North RA. Role of P2X4 receptors in synaptic strengthening in mouse CA1 hippocampal neurons. Eur J Neurosci. 2011;34(2):213–220. doi: 10.1111/j.1460-9568.2011.07763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Yebdri F, Kukulski F, Tremblay A, Sévigny J. Concomitant activation of P2Y2 and P2Y6 receptors on monocytes is required for TLR1/2-induced neutrophil migration by regulating IL-8 secretion. Eur J Immunol. 2009;39(10):2885–2894. doi: 10.1002/eji.200939347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett GC, Ford AP, Smith JA, Emmett CJ, Webb TE, Boarder MR. P2Y receptor regulation of cultured rat cerebral cortical cells: calcium responses and mRNA expression in neurons and glia. Br J Pharmacol. 2003;139(2):279–288. doi: 10.1038/sj.bjp.0705242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco F, Fumagalli M, Pravettoni E, D'Ambrosi N, Volonte C, Matteoli M, Abbracchio MP, Verderio C. Pathophysiological roles of extracellular nucleotides in glial cells: differential expression of purinergic receptors in resting and activated microglia. Brain Res Brain Res Rev. 2005a;48(2):144–156. doi: 10.1016/j.brainresrev.2004.12.004. [pii] 10.1016/j.brainresrev.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Bianco F, Pravettoni E, Colombo A, Schenk U, Moller T, Matteoli M, Verderio C. Astrocyte-derived ATP induces vesicle shedding and IL-1 beta release from microglia. J Immunol. 2005b;174(11):7268–7277. doi: 10.4049/jimmunol.174.11.7268. [pii] [DOI] [PubMed] [Google Scholar]
- Bo X, Kim M, Nori SL, Schoepfer R, Burnstock G, North RA. Tissue distribution of P2X4 receptors studied with an ectodomain antibody. Cell Tissue Res. 2003;313(2):159–165. doi: 10.1007/s00441-003-0758-5. [DOI] [PubMed] [Google Scholar]
- Bodin P, Burnstock G. Purinergic signalling: ATP release. Neurochem Res. 2001;26(8–9):959–969. doi: 10.1023/a:1012388618693. [DOI] [PubMed] [Google Scholar]
- Boillée S, Yamanaka K, Lobsiger CS, Copeland NG, Jenkins NA, Kassiotis G, Kollias G, Cleveland DW. Onset and progression in inherited ALS determined by motor neurons and microglia. Science. 2006;312(5778):1389–1392. doi: 10.1126/science.1123511. [pii]10.1126/science.1123511. [DOI] [PubMed] [Google Scholar]
- Boucsein C, Zacharias R, Färber K, Pavlovic S, Hanisch UK, Kettenmann H. Purinergic receptors on microglial cells: functional expression in acute brain slices and modulation of microglial activation in vitro. Eur J Neurosci. 2003;17(11):2267–2276. doi: 10.1046/j.1460-9568.2003.02663.x. [pii] [DOI] [PubMed] [Google Scholar]
- Bours MJ, Dagnelie PC, Giuliani AL, Wesselius A, Di Virgilio F. P2 receptors and extracellular ATP: a novel homeostatic pathway in inflammation. Front Biosci (Schol Ed) 2011;3:1443–1456. doi: 10.2741/235. [pii] [DOI] [PubMed] [Google Scholar]
- Brandenburg LO, Jansen S, Wruck CJ, Lucius R, Pufe T. Antimicrobial peptide rCRAMP induced glial cell activation through P2Y receptor signalling pathways. Mol Immunol. 2010;47(10):1905–1913. doi: 10.1016/j.molimm.2010.03.012. [pii]10.1016/j.molimm.2010.03.012. [DOI] [PubMed] [Google Scholar]
- Brandle U, Guenther E, Irrle C, Wheeler-Schilling TH. Gene expression of the P2X receptors in the rat retina. Brain Res Mol Brain Res. 1998;59(2):269–272. doi: 10.1016/s0169-328x(98)00159-4. [DOI] [PubMed] [Google Scholar]
- Brass D, Grably MR, Bronstein-Sitton N, Gohar O, Meir A. Using antibodies against P2Y and P2X receptors in purinergic signaling research. Purinergic Signal. 2011 doi: 10.1007/s11302-011-9278-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinson AE, Harden TK. Differential regulation of the uridine nucleotide-activated P2Y4 and P2Y6 receptors. SER-333 and SER-334 in the carboxyl terminus are involved in agonist-dependent phosphorylation desensitization and internalization of the P2Y4 receptor. J Biol Chem. 2001;276(15):11939–11948. doi: 10.1074/jbc.M009909200. [pii]10.1074/jbc.M009909200. [DOI] [PubMed] [Google Scholar]
- Brown GC, Neher JJ. Inflammatory neurodegeneration and mechanisms of microglial killing of neurons. Mol Neurobiol. 2010;41(2–3):242–247. doi: 10.1007/s12035-010-8105-9. [doi] [DOI] [PubMed] [Google Scholar]
- Brunschweiger A, Muller CE. P2 receptors activated by uracil nucleotides--an update. Curr Med Chem. 2006;13(3):289–312. doi: 10.2174/092986706775476052. [DOI] [PubMed] [Google Scholar]
- Buell G, Collo G, Rassendren F. P2X receptors: an emerging channel family. Eur J Neurosci. 1996;8(10):2221–2228. doi: 10.1111/j.1460-9568.1996.tb00745.x. [DOI] [PubMed] [Google Scholar]
- Burgos M, Neary JT, González FA. P2Y2 nucleotide receptors inhibit trauma-induced death of astrocytic cells. J Neurochem. 2007;103(5):1785–1800. doi: 10.1111/j.1471-4159.2007.04872.x. [pii]10.1111/j.1471-4159.2007.04872.x. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Historical review: ATP as a neurotransmitter. Trends Pharmacol Sci. 2006a;27(3):166–176. doi: 10.1016/j.tips.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Purinergic signalling. Br J Pharmacol. 2006b;147(Suppl 1):S172–S181. doi: 10.1038/sj.bjp.0706429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Purinergic signalling: past, present and future. Braz J Med Biol Res. 2009;42(1):3–8. doi: 10.1590/s0100-879x2008005000037. [pii] [DOI] [PubMed] [Google Scholar]
- Burnstock G, Campbell G, Satchell D, Smythe A. Evidence that adenosine triphosphate or a related nucleotide is the transmitter substance released by non-adrenergic inhibitory nerves in the gut. 1970. Br J Pharmacol. 1997;120(4 Suppl):337–357. doi: 10.1111/j.1476-5381.1997.tb06815.x. discussion 334–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G, Dumsday B, Smythe A. Atropine resistant excitation of the urinary bladder: the possibility of transmission via nerves releasing a purine nucleotide. Br J Pharmacol. 1972;44(3):451–461. doi: 10.1111/j.1476-5381.1972.tb07283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G, Kennedy C. Is there a basis for distinguishing two types of P2-purinoceptor? Gen Pharmacol. 1985;16(5):433–440. doi: 10.1016/0306-3623(85)90001-1. [DOI] [PubMed] [Google Scholar]
- Burnstock G, Knight GE. Cellular distribution and functions of P2 receptor subtypes in different systems. Int Rev Cytol. 2004;240:31–304. doi: 10.1016/S0074-7696(04)40002-3. [DOI] [PubMed] [Google Scholar]
- Burnstock G, Verkhratsky A. Long-term (trophic) purinergic signalling: purinoceptors control cell proliferation, differentiation and death. Cell Death Dis. 2010;1:e9. doi: 10.1038/cddis.2009.11. [pii]10.1038/cddis.2009.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovsky O, Talpalar AE, Ben-Yaakov K, Schwartz M. Activation of microglia by aggregated beta-amyloid or lipopolysaccharide impairs MHC-II expression and renders them cytotoxic whereas IFN-gamma and IL-4 render them protective. Mol Cell Neurosci. 2005;29(3):381–393. doi: 10.1016/j.mcn.2005.03.005. [pii] 10.1016/j.mcn.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Butt AM. ATP: a ubiquitous gliotransmitter integrating neuron-glial networks. Semin Cell Dev Biol. 2011;22(2):205–213. doi: 10.1016/j.semcdb.2011.02.023. [pii]10.1016/j.semcdb.2011.02.023. [DOI] [PubMed] [Google Scholar]
- Calvert JA, Atterbury-Thomas AE, Leon C, Forsythe ID, Gachet C, Evans RJ. Evidence for P2Y1, P2Y2, P2Y6 and atypical UTP-sensitive receptors coupled to rises in intracellular calcium in mouse cultured superior cervical ganglion neurons and glia. Br J Pharmacol. 2004;143(5):525–532. doi: 10.1038/sj.bjp.0705959. [pii] 10.1038/sj.bjp.0705959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvert JA, Evans RJ. Heterogeneity of P2X receptors in sympathetic neurons: contribution of neuronal P2X1 receptors revealed using knockout mice. Mol Pharmacol. 2004;65(1):139–148. doi: 10.1124/mol.65.1.139. [pii] [DOI] [PubMed] [Google Scholar]
- Carrasquero LM, Delicado EG, Jiménez AI, Pérez-Sen R, Miras-Portugal MT. Cerebellar astrocytes co-express several ADP receptors. Presence of functional P2Y13-like receptors. Purinergic Signal. 2005;1(2):153–159. doi: 10.1007/s11302-005-6211-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaliere F, Amadio S, Sancesario G, Bernardi G, Volonte C. Synaptic P2X7 and oxygen/glucose deprivation in organotypic hippocampal cultures. J Cereb Blood Flow Metab. 2004;24(4):392–398. doi: 10.1097/00004647-200404000-00004. [DOI] [PubMed] [Google Scholar]
- Chakfe Y, Seguin R, Antel JP, Morissette C, Malo D, Henderson D, Seguela P. ADP and AMP induce interleukin-1beta release from microglial cells through activation of ATP-primed P2X7 receptor channels. J Neurosci. 2002;22(8):3061–3069. doi: 10.1523/JNEUROSCI.22-08-03061.2002. 22/8/3061 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers JK, Macdonald LE, Sarau HM, Ames RS, Freeman K, Foley JJ, Zhu Y, McLaughlin MM, Murdock P, McMillan L, Trill J, Swift A, Aiyar N, Taylor P, Vawter L, Naheed S, Szekeres P, Hervieu G, Scott C, Watson JM, Murphy AJ, Duzic E, Klein C, Bergsma DJ, Wilson S, Livi GP. A G protein-coupled receptor for UDP-glucose. J Biol Chem. 2000;275(15):10767–10771. doi: 10.1074/jbc.275.15.10767. [DOI] [PubMed] [Google Scholar]
- Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, Nizet V, Insel PA, Junger WG. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006;314(5806):1792–1795. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- Chen Y, Yao Y, Sumi Y, Li A, To UK, Elkhal A, Inoue Y, Woehrle T, Zhang Q, Hauser C, Junger WG. Purinergic signaling: a fundamental mechanism in neutrophil activation. Sci Signal. 2010;3(125):ra45. doi: 10.1126/scisignal.2000549. [pii] 10.1126/scisignal.2000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chessell IP, Hatcher JP, Bountra C, Michel AD, Hughes JP, Green P, Egerton J, Murfin M, Richardson J, Peck WL, Grahames CB, Casula MA, Yiangou Y, Birch R, Anand P, Buell GN. Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain. 2005;114(3):386–396. doi: 10.1016/j.pain.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Cheung KK, Chan WY, Burnstock G. Expression of P2X purinoceptors during rat brain development and their inhibitory role on motor axon outgrowth in neural tube explant cultures. Neuroscience. 2005;133(4):937–945. doi: 10.1016/j.neuroscience.2005.03.032. [DOI] [PubMed] [Google Scholar]
- Choi HB, Ryu JK, Kim SU, McLarnon JG. Modulation of the purinergic P2X7 receptor attenuates lipopolysaccharide-mediated microglial activation and neuronal damage in inflamed brain. J Neurosci. 2007;27(18):4957–4968. doi: 10.1523/JNEUROSCI.5417-06.2007. [pii]10.1523/JNEUROSCI.5417-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chorna NE, Santiago-Perez LI, Erb L, Seye CI, Neary JT, Sun GY, Weisman GA, Gonzalez FA. P2Y receptors activate neuroprotective mechanisms in astrocytic cells. J Neurochem. 2004;91(1):119–132. doi: 10.1111/j.1471-4159.2004.02699.x. [DOI] [PubMed] [Google Scholar]
- Chung H, Brazil MI, Soe TT, Maxfield FR. Uptake, degradation, and release of fibrillar and soluble forms of Alzheimer's amyloid beta-peptide by microglial cells. J Biol Chem. 1999;274(45):32301–32308. doi: 10.1074/jbc.274.45.32301. [DOI] [PubMed] [Google Scholar]
- Coddou C, Yan Z, Obsil T, Huidobro-Toro JP, Stojilkovic SS. Activation and regulation of purinergic P2X receptor channels. Pharmacol Rev. 2011;63(3):641–683. doi: 10.1124/pr.110.003129. [pii] 10.1124/pr.110.003129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collo G, Neidhart S, Kawashima E, Kosco-Vilbois M, North RA, Buell G. Tissue distribution of the P2X7 receptor. Neuropharmacology. 1997;36(9):1277–1283. doi: 10.1016/s0028-3908(97)00140-8. [pii] [DOI] [PubMed] [Google Scholar]
- Collo G, North RA, Kawashima E, Merlo-Pich E, Neidhart S, Surprenant A, Buell G. Cloning OF P2X5 and P2X6 receptors and the distribution and properties of an extended family of ATP-gated ion channels. J Neurosci. 1996;16(8):2495–2507. doi: 10.1523/JNEUROSCI.16-08-02495.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Communi D, Govaerts C, Parmentier M, Boeynaems JM. Cloning of a human purinergic P2Y receptor coupled to phospholipase C and adenylyl cyclase. J Biol Chem. 1997;272(51):31969–31973. doi: 10.1074/jbc.272.51.31969. [DOI] [PubMed] [Google Scholar]
- Communi D, Parmentier M, Boeynaems JM. Cloning, functional expression and tissue distribution of the human P2Y6 receptor. Biochem Biophys Res Commun. 1996;222(2):303–308. doi: 10.1006/bbrc.1996.0739. [DOI] [PubMed] [Google Scholar]
- Communi D, Robaye B, Boeynaems JM. Pharmacological characterization of the human P2Y11 receptor. Br J Pharmacol. 1999;128(6):1199–1206. doi: 10.1038/sj.bjp.0702909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crain JM, Nikodemova M, Watters JJ. Expression of P2 nucleotide receptors varies with age and sex in murine brain microglia. J Neuroinflammation. 2009;6:24. doi: 10.1186/1742-2094-6-24. [pii] 10.1186/1742-2094-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Alimonte I, Ciccarelli R, Di Iorio P, Nargi E, Buccella S, Giuliani P, Rathbone MP, Jiang S, Caciagli F, Ballerini P. Activation of P2X7 receptors stimulates the expression of P2Y2 receptor mRNA in astrocytes cultured from rat brain. Int J Immunopathol Pharmacol. 2007;20(2):301–316. doi: 10.1177/039463200702000210. [pii] [DOI] [PubMed] [Google Scholar]
- da Silva RL, Resende RR, Ulrich H. Alternative splicing of P2X6 receptors in developing mouse brain and during in vitro neuronal differentiation. Exp Physiol. 2007;92(1):139–145. doi: 10.1113/expphysiol.2006.921304. [DOI] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8(6):752–758. doi: 10.1038/nn1472. [pii] 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- Degagne E, Grbic DM, Dupuis AA, Lavoie EG, Langlois C, Jain N, Weisman GA, Sevigny J, Gendron FP. P2Y2 receptor transcription is increased by NF-κB and stimulates cyclooxygenase-2 expression and PGE2 released by intestinal epithelial cells. J Immunol. 2009;183(7):4521–4529. doi: 10.4049/jimmunol.0803977. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuchars SA, Atkinson L, Brooke RE, Musa H, Milligan CJ, Batten TF, Buckley NJ, Parson SH, Deuchars J. Neuronal P2X7 receptors are targeted to presynaptic terminals in the central and peripheral nervous systems. J Neurosci. 2001;21(18):7143–7152. doi: 10.1523/JNEUROSCI.21-18-07143.2001. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Virgilio F. Liaisons dangereuses: P2X7 and the inflammasome. Trends Pharmacol Sci. 2007;28(9):465–472. doi: 10.1016/j.tips.2007.07.002. [pii] 10.1016/j.tips.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Di Virgilio F, Chiozzi P, Falzoni S, Ferrari D, Sanz JM, Venketaraman V, Baricordi OR. Cytolytic P2X purinoceptors. Cell Death Differ. 1998;5(3):191–199. doi: 10.1038/sj.cdd.4400341. [DOI] [PubMed] [Google Scholar]
- Diaz-Hernandez M, Diez-Zaera M, Sanchez-Nogueiro J, Gomez-Villafuertes R, Canals JM, Alberch J, Miras-Portugal MT, Lucas JJ. Altered P2X7-receptor level and function in mouse models of Huntington's disease and therapeutic efficacy of antagonist administration. FASEB J. 2009;23(6):1893–1906. doi: 10.1096/fj.08-122275. [DOI] [PubMed] [Google Scholar]
- Duan S, Neary JT. P2X7 receptors: properties and relevance to CNS function. Glia. 2006;54(7):738–746. doi: 10.1002/glia.20397. [DOI] [PubMed] [Google Scholar]
- Duckwitz W, Hausmann R, Aschrafi A, Schmalzing G. P2X5 subunit assembly requires scaffolding by the second transmembrane domain and a conserved aspartate. J Biol Chem. 2006;281(51):39561–39572. doi: 10.1074/jbc.M606113200. [pii] 10.1074/jbc.M606113200. [DOI] [PubMed] [Google Scholar]
- Egan TM, Cox JA, Voigt MM. Molecular structure of P2X receptors. Curr Top Med Chem. 2004;4(8):821–829. doi: 10.2174/1568026043451005. [DOI] [PubMed] [Google Scholar]
- El Khoury J, Luster AD. Mechanisms of microglia accumulation in Alzheimer's disease: therapeutic implications. Trends Pharmacol Sci. 2008;29(12):626–632. doi: 10.1016/j.tips.2008.08.004. [pii] 10.1016/j.tips.2008.08.004. [DOI] [PubMed] [Google Scholar]
- El Khoury J, Toft M, Hickman SE, Means TK, Terada K, Geula C, Luster AD. Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat Med. 2007;13(4):432–438. doi: 10.1038/nm1555. [pii]10.1038/nm1555. [DOI] [PubMed] [Google Scholar]
- Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, Walk SF, Park D, Woodson RI, Ostankovich M, Sharma P, Lysiak JJ, Harden TK, Leitinger N, Ravichandran KS. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature. 2009;461(7261):282–286. doi: 10.1038/nature08296. [pii] 10.1038/nature08296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb L, Garrad R, Wang Y, Quinn T, Turner JT, Weisman GA. Site-directed mutagenesis of P2U purinoceptors. Positively charged amino acids in transmembrane helices 6 and 7 affect agonist potency and specificity. J Biol Chem. 1995;270(9):4185–4188. doi: 10.1074/jbc.270.9.4185. [DOI] [PubMed] [Google Scholar]
- Erb L, Liao Z, Seye CI, Weisman GA. P2 receptors: intracellular signaling. Pflugers Arch. 2006;452(5):552–562. doi: 10.1007/s00424-006-0069-2. [DOI] [PubMed] [Google Scholar]
- Erb L, Liu J, Ockerhausen J, Kong Q, Garrad RC, Griffin K, Neal C, Krugh B, Santiago-Perez LI, Gonzalez FA, Gresham HD, Turner JT, Weisman GA. An RGD sequence in the P2Y2 receptor interacts with αVβ3 integrins and is required for Go-mediated signal transduction. J Cell Biol. 2001;153(3):491–501. doi: 10.1083/jcb.153.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb L, Lustig KD, Ahmed AH, Gonzalez FA, Weisman GA. Covalent incorporation of 3'-O-(4-benzoyl)benzoyl-ATP into a P2 purinoceptor in transformed mouse fibroblasts. J Biol Chem. 1990;265(13):7424–7431. [PubMed] [Google Scholar]
- Espada S, Ortega F, Molina-Jijón E, Rojo AI, Pérez-Sen R, Pedraza-Chaverri J, Miras-Portugal MT, Cuadrado A. The purinergic P2Y13 receptor activates the Nrf2/HO-1 axis and protects against oxidative stress-induced neuronal death. Free Radic Biol Med. 2010;49(3):416–426. doi: 10.1016/j.freeradbiomed.2010.04.031. [pii] 10.1016/j.freeradbiomed.2010.04.031. [DOI] [PubMed] [Google Scholar]
- Ferrari D, Chiozzi P, Falzoni S, Dal Susino M, Collo G, Buell G, Di Virgilio F. ATP-mediated cytotoxicity in microglial cells. Neuropharmacology. 1997;36(9):1295–1301. doi: 10.1016/s0028-3908(97)00137-8. [pii] [DOI] [PubMed] [Google Scholar]
- Ferrari D, Pizzirani C, Adinolfi E, Lemoli RM, Curti A, Idzko M, Panther E, Di Virgilio F. The P2X7 receptor: a key player in IL-1 processing and release. J Immunol. 2006;176(7):3877–3883. doi: 10.4049/jimmunol.176.7.3877. [DOI] [PubMed] [Google Scholar]
- Ferrero ME. Purinoceptors in inflammation: potential as anti-inflammatory therapeutic targets. Front Biosci. 2011;17:2172–2186. doi: 10.2741/3846. [pii] [DOI] [PubMed] [Google Scholar]
- Fields RD. Nonsynaptic and nonvesicular ATP release from neurons and relevance to neuron-glia signaling. Semin Cell Dev Biol. 2011;22(2):214–219. doi: 10.1016/j.semcdb.2011.02.009. [pii]10.1016/j.semcdb.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer W, Krügel U. P2Y receptors: focus on structural, pharmacological and functional aspects in the brain. Curr Med Chem. 2007;14(23):2429–2455. doi: 10.2174/092986707782023695. [DOI] [PubMed] [Google Scholar]
- Flores RV, Hernandez-Perez MG, Aquino E, Garrad RC, Weisman GA, Gonzalez FA. Agonist-induced phosphorylation and desensitization of the P2Y2 nucleotide receptor. Mol Cell Biochem. 2005;280(1–2):35–45. doi: 10.1007/s11010-005-8050-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford AP. In pursuit of P2X3 antagonists: novel therapeutics for chronic pain and afferent sensitization. Purinergic Signal. 2011 doi: 10.1007/s11302-011-9271-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke H, Gunther A, Grosche J, Schmidt R, Rossner S, Reinhardt R, Faber-Zuschratter H, Schneider D, Illes P. P2X7 receptor expression after ischemia in the cerebral cortex of rats. J Neuropathol Exp Neurol. 2004a;63(7):686–699. doi: 10.1093/jnen/63.7.686. [DOI] [PubMed] [Google Scholar]
- Franke H, Illes P. Involvement of P2 receptors in the growth and survival of neurons in the CNS. Pharmacol Ther. 2006;109(3):297–324. doi: 10.1016/j.pharmthera.2005.06.002. [pii]10.1016/j.pharmthera.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Franke H, Krügel U, Grosche J, Heine C, Härtig W, Allgaier C, Illes P. P2Y receptor expression on astrocytes in the nucleus accumbens of rats. Neuroscience. 2004b;127(2):431–441. doi: 10.1016/j.neuroscience.2004.05.003. [pii]10.1016/j.neuroscience.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Friedle SA, Curet MA, Watters JJ. Recent patents on novel P2X7 receptor antagonists and their potential for reducing central nervous system inflammation. Recent Pat CNS Drug Discov. 2010;5(1):35–45. doi: 10.2174/157488910789753530. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita T, Tozaki-Saitoh H, Inoue K. P2Y1 receptor signaling enhances neuroprotection by astrocytes against oxidative stress via IL-6 release in hippocampal cultures. Glia. 2009;57(3):244–257. doi: 10.1002/glia.20749. [DOI] [PubMed] [Google Scholar]
- Fumagalli M, Brambilla R, D'Ambrosi N, Volonté C, Matteoli M, Verderio C, Abbracchio MP. Nucleotide-mediated calcium signaling in rat cortical astrocytes: Role of P2X and P2Y receptors. Glia. 2003;43(3):218–203. doi: 10.1002/glia.10248. [DOI] [PubMed] [Google Scholar]
- Fumagalli M, Lecca D, Abbracchio MP. Role of purinergic signalling in neuro-immune cells and adult neural progenitors. Front Biosci. 2011;17:2326–2341. doi: 10.2741/3856. [pii] [DOI] [PubMed] [Google Scholar]
- Gate D, Rezai-Zadeh K, Jodry D, Rentsendorj A, Town T. Macrophages in Alzheimer's disease: the blood-borne identity. J Neural Transm. 2010;117(8):961–970. doi: 10.1007/s00702-010-0422-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerevich Z, Müller C, Illes P. Metabotropic P2Y1 receptors inhibit P2X3 receptor-channels in rat dorsal root ganglion neurons. Eur J Pharmacol. 2005;521(1–3):34–38. doi: 10.1016/j.ejphar.2005.08.001. [pii] 10.1016/j.ejphar.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Giaume C, Koulakoff A, Roux L, Holcman D, Rouach N. Astroglial networks: a step further in neuroglial and gliovascular interactions. Nat Rev Neurosci. 2010;11(2):87–99. doi: 10.1038/nrn2757. [pii]10.1038/nrn2757. [DOI] [PubMed] [Google Scholar]
- Gomeza J, Ohno K, Hülsmann S, Armsen W, Eulenburg V, Richter DW, Laube B, Betz H. Deletion of the mouse glycine transporter 2 results in a hyperekplexia phenotype and postnatal lethality. Neuron. 2003;40(4):797–806. doi: 10.1016/s0896-6273(03)00673-1. [pii] [DOI] [PubMed] [Google Scholar]
- Gonzalez FA, Ahmed AH, Lustig KD, Erb L, Weisman GA. Permeabilization of transformed mouse fibroblasts by 3'-O-(4-benzoyl)benzoyl adenosine 5'-triphosphate and the desensitization of the process. J Cell Physiol. 1989;139(1):109–115. doi: 10.1002/jcp.1041390116. [DOI] [PubMed] [Google Scholar]
- Gonzalez FA, Weisman GA, Erb L, Seye CI, Sun GY, Velazquez B, Hernandez-Perez M, Chorna NE. Mechanisms for inhibition of P2 receptors signaling in neural cells. Mol Neurobiol. 2005;31(1–3):65–79. doi: 10.1385/MN:31:1-3:065. [pii] 10.1385/MN:31:1-3-065. [DOI] [PubMed] [Google Scholar]
- Gordon GR, Baimoukhametova DV, Hewitt SA, Rajapaksha WR, Fisher TE, Bains JS. Norepinephrine triggers release of glial ATP to increase postsynaptic efficacy. Nat Neurosci. 2005;8(8):1078–1086. doi: 10.1038/nn1498. [DOI] [PubMed] [Google Scholar]
- Gotsch U, Borges E, Bosse R, Boggemeyer E, Simon M, Mossmann H, Vestweber D. VE-cadherin antibody accelerates neutrophil recruitment in vivo. J Cell Sci. 1997;110(Pt 5):583–588. doi: 10.1242/jcs.110.5.583. [DOI] [PubMed] [Google Scholar]
- Grimm I, Messemer N, Stanke M, Gachet C, Zimmermann H. Coordinate pathways for nucleotide and EGF signaling in cultured adult neural progenitor cells. J Cell Sci. 2009;122(Pt 14):2524–2533. doi: 10.1242/jcs.044891. [pii] 10.1242/jcs.044891. [DOI] [PubMed] [Google Scholar]
- Gu JG, MacDermott AB. Activation of ATP P2X receptors elicits glutamate release from sensory neuron synapses. Nature. 1997;389(6652):749–753. doi: 10.1038/39639. [DOI] [PubMed] [Google Scholar]
- Gum RJ, Wakefield B, Jarvis MF. P2X receptor antagonists for pain management: examination of binding and physicochemical properties. Purinergic Signal. 2011 doi: 10.1007/s11302-011-9272-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C, Masin M, Qureshi OS, Murrell-Lagnado RD. Evidence for functional P2X4/P2X7 heteromeric receptors. Mol Pharmacol. 2007;72(6):1447–1456. doi: 10.1124/mol.107.035980. [DOI] [PubMed] [Google Scholar]
- Guo W, Xu X, Gao X, Burnstock G, He C, Xiang Z. Expression of P2X5 receptors in the mouse CNS. Neuroscience. 2008;156(3):673–692. doi: 10.1016/j.neuroscience.2008.07.062. [DOI] [PubMed] [Google Scholar]
- Guo W, Sun J, Xu X, Bunstock G, He C, Xiang Z. P2X receptors are differentially expressed on vasopressin-and oxytocin-containing neurons in the supraoptic and paraventricular nuclei of rat hypothalamus. Histochem Cell Biol. 2009;131(1):29–41. doi: 10.1007/s00418-008-0493-9. [DOI] [PubMed] [Google Scholar]
- Haines WR, Torres GE, Voigt MM, Egan TM. Properties of the novel ATP-gated ionotropic receptor composed of the P2X1 and P2X5 isoforms. Mol Pharmacol. 1999;56(4):720–727. [PubMed] [Google Scholar]
- Halassa MM, Fellin T, Haydon PG. Tripartite synapses: roles for astrocytic purines in the control of synaptic physiology and behavior. Neuropharmacology. 2009;57(4):343–346. doi: 10.1016/j.neuropharm.2009.06.031. [pii] 10.1016/j.neuropharm.2009.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton N, Vayro S, Kirchhoff F, Verkhratsky A, Robbins J, Gorecki DC, Butt AM. Mechanisms of ATP- and glutamate-mediated calcium signaling in white matter astrocytes. Glia. 2008;56(7):734–749. doi: 10.1002/glia.20649. [DOI] [PubMed] [Google Scholar]
- Hamilton N, Vayro S, Wigley R, Butt AM. Axons and astrocytes release ATP and glutamate to evoke calcium signals in NG2-glia. Glia. 2010;58(1):66–79. doi: 10.1002/glia.20902. [DOI] [PubMed] [Google Scholar]
- Hawkes CA, McLaurin J. Selective targeting of perivascular macrophages for clearance of beta-amyloid in cerebral amyloid angiopathy. Proc Natl Acad Sci U S A. 2009;106(4):1261–1266. doi: 10.1073/pnas.0805453106. [pii] 10.1073/pnas.0805453106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes SE, Hollopeter G, Yang G, Kurpius D, Dailey ME, Gan WB, Julius D. The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat Neurosci. 2006;9(12):1512–1519. doi: 10.1038/nn1805. [pii] 10.1038/nn1805. [DOI] [PubMed] [Google Scholar]
- Hechler B, Lenain N, Marchese P, Vial C, Heim V, Freund M, Cazenave JP, Cattaneo M, Ruggeri ZM, Evans R, Gachet C. A role of the fast ATP-gated P2X1 cation channel in thrombosis of small arteries in vivo. J Exp Med. 2003;198(4):661–667. doi: 10.1084/jem.20030144. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbronn E, Knoblauch BH, Müller CE. Uridine nucleotide receptors and their ligands: structural, physiological, and pathophysiological aspects, with special emphasis on the nervous system. Neurochem Res. 1997;22(8):1041–1050. doi: 10.1023/a:1022487128766. [DOI] [PubMed] [Google Scholar]
- Hickman SE, Semrad CE, Silverstein SC. P2Z purinoceptors. Ciba Found Symp. 1996;198:71–83. doi: 10.1002/9780470514900.ch4. discussion 83–90. [DOI] [PubMed] [Google Scholar]
- Hide I, Tanaka M, Inoue A, Nakajima K, Kohsaka S, Inoue K, Nakata Y. Extracellular ATP triggers tumor necrosis factor-alpha release from rat microglia. J Neurochem. 2000;75(3):965–972. doi: 10.1046/j.1471-4159.2000.0750965.x. [DOI] [PubMed] [Google Scholar]
- Hollopeter G, Jantzen HM, Vincent D, Li G, England L, Ramakrishnan V, Yang RB, Nurden P, Nurden A, Julius D, Conley PB. Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature. 2001;409(6817):202–207. doi: 10.1038/35051599. [DOI] [PubMed] [Google Scholar]
- Honda S, Sasaki Y, Ohsawa K, Imai Y, Nakamura Y, Inoue K, Kohsaka S. Extracellular ATP or ADP induce chemotaxis of cultured microglia through Gi/o-coupled P2Y receptors. J Neurosci. 2001;21(6):1975–1982. doi: 10.1523/JNEUROSCI.21-06-01975.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honore P, Kage K, Mikusa J, Watt AT, Johnston JF, Wyatt JR, Faltynek CR, Jarvis MF, Lynch K. Analgesic profile of intrathecal P2X3 antisense oligonucleotide treatment in chronic inflammatory and neuropathic pain states in rats. Pain. 2002;99(1–2):11–19. doi: 10.1016/s0304-3959(02)00032-5. [pii] [DOI] [PubMed] [Google Scholar]
- Illes P, Verkhratsky A, Burnstock G, Franke H. P2X Receptors and Their Roles in Astroglia in the Central and Peripheral Nervous System. Neuroscientist. 2011 doi: 10.1177/1073858411418524. [pii] 10.1177/1073858411418524. [DOI] [PubMed] [Google Scholar]
- Inoue K. Purinergic systems in microglia. Cell Mol Life Sci. 2008;65(19):3074–3080. doi: 10.1007/s00018-008-8210-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, Hoffmann C, Kim YC, Camaioni E, Nandanan E, Jang SY, Guo DP, Ji XD, von Kugelgen I, Moro S, Ziganshin AU, Rychkov A, King BF, Brown SG, Wildman SS, Burnstock G, Boyer JL, Mohanram A, Harden TK. Molecular recognition in P2 receptors: ligand development aided by molecular modeling and mutagenesis. Prog Brain Res. 1999;120:119–132. doi: 10.1016/s0079-6123(08)63550-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, Ivanov AA, de Castro S, Harden TK, Ko H. Development of selective agonists and antagonists of P2Y receptors. Purinergic Signal. 2009;5(1):75–89. doi: 10.1007/s11302-008-9106-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques-Silva MC, Rodnight R, Lenz G, Liao Z, Kong Q, Tran M, Kang Y, Gonzalez FA, Weisman GA, Neary JT. P2X7 receptors stimulate AKT phosphorylation in astrocytes. Br J Pharmacol. 2004;141(7):1106–1117. doi: 10.1038/sj.bjp.0705685. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameson HS, Pinol RA, Mendelowitz D. Purinergic P2X receptors facilitate inhibitory GABAergic and glycinergic neurotransmission to cardiac vagal neurons in the nucleus ambiguus. Brain Res. 2008;1224:53–62. doi: 10.1016/j.brainres.2008.06.012. [pii] 10.1016/j.brainres.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang DH, Han JH, Lee SH, Lee YS, Park H, Kim H, Kaang BK. Cofilin expression induces cofilin-actin rod formation and disrupts synaptic structure and function in Aplysia synapses. Proc Natl Acad Sci U S A. 2005;102(44):16072–16077. doi: 10.1073/pnas.0507675102. [pii] 10.1073/pnas.0507675102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis MF. Contributions of P2X3 homomeric and heteromeric channels to acute and chronic pain. Expert Opin Ther Targets. 2003;7(4):513–522. doi: 10.1517/14728222.7.4.513. [DOI] [PubMed] [Google Scholar]
- Jiang Q, Guo D, Lee BX, Van Rhee AM, Kim YC, Nicholas RA, Schachter JB, Harden TK, Jacobson KA. A mutational analysis of residues essential for ligand recognition at the human P2Y1 receptor. Mol Pharmacol. 1997;52(3):499–507. doi: 10.1124/mol.52.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez E, Zafra F, Pérez-Sen R, Delicado EG, Miras-Portugal MT, Aragón C, López-Corcuera B. P2Y purinergic regulation of the glycine neurotransmitter transporters. J Biol Chem. 2011;286(12):10712–10724. doi: 10.1074/jbc.M110.167056. [pii]10.1074/jbc.M110.167056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CA, Vial C, Sellers LA, Humphrey PP, Evans RJ, Chessell IP. Functional regulation of P2X6 receptors by N-linked glycosylation: identification of a novel alpha beta-methylene ATP-sensitive phenotype. Mol Pharmacol. 2004;65(4):979–985. doi: 10.1124/mol.65.4.979. [DOI] [PubMed] [Google Scholar]
- Kanjhan R, Housley GD, Burton LD, Christie DL, Kippenberger A, Thorne PR, Luo L, Ryan AF. Distribution of the P2X2 receptor subunit of the ATP-gated ion channels in the rat central nervous system. J Comp Neurol. 1999;407(1):11–32. [pii] [PubMed] [Google Scholar]
- Kataoka A, Tozaki-Saitoh H, Koga Y, Tsuda M, Inoue K. Activation of P2X7 receptors induces CCL3. production in microglial cells through transcription factor NFAT. J Neurochem. 2009;108(1):115–125. doi: 10.1111/j.1471-4159.2008.05744.x. [DOI] [PubMed] [Google Scholar]
- Khakh BS, North RA. P2X receptors as cell-surface ATP sensors in health and disease. Nature. 2006;442(7102):527–532. doi: 10.1038/nature04886. [pii] 10.1038/nature04886. [DOI] [PubMed] [Google Scholar]
- Kidd EJ, Grahames CB, Simon J, Michel AD, Barnard EA, Humphrey PP. Localization of P2X purinoceptor transcripts in the rat nervous system. Mol Pharmacol. 1995;48(4):569–573. [PubMed] [Google Scholar]
- Kim HJ, Ajit D, Peterson TS, Wang Y, Camden JM, Wood WG, Sun GY, Erb LE, Petris M, Weisman GA. Nucleotides released from fibrillar Aβ1–42-treated microglial cells increase cell migration and fibrillar Aβ1–42 uptake through P2Y2 receptor activation. J Neurochem. doi: 10.1111/j.1471-4159.2012.07700.x. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Fukuoka T, Yamanaka H, Iyamanaka H, Dai Y, Obata K, Tokunaga A, Noguchi K. Neurons and glial cells differentially express P2Y receptor mRNAs in the rat dorsal root ganglion and spinal cord. J Comp Neurol. 2006;498(4):443–454. doi: 10.1002/cne.21066. [DOI] [PubMed] [Google Scholar]
- Koizumi S, Shigemoto-Mogami Y, Nasu-Tada K, Shinozaki Y, Ohsawa K, Tsuda M, Joshi BV, Jacobson KA, Kohsaka S, Inoue K. UDP acting at P2Y6 receptors is a mediator of microglial phagocytosis. Nature. 2007;446(7139):1091–1095. doi: 10.1038/nature05704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koles L, Furst S, Illes P. Purine ionotropic (P2X) receptors. Curr Pharm Des. 2007;13(23):2368–2384. doi: 10.2174/138161207781368747. [DOI] [PubMed] [Google Scholar]
- Köles L, Leichsenring A, Rubini P, Illes P. P2 receptor signaling in neurons and glial cells of the central nervous system. Adv Pharmacol. 2011;61:441–493. doi: 10.1016/B978-0-12-385526-8.00014-X. [pii] 10.1016/B978-0-12-385526-8.00014-X. [DOI] [PubMed] [Google Scholar]
- Kong Q, Peterson TS, Baker O, Stanley E, Camden J, Seye CI, Erb L, Simonyi A, Wood WG, Sun GY, Weisman GA. Interleukin-1β enhances nucleotide-induced and α-secretase-dependent amyloid precursor protein processing in rat primary cortical neurons via up-regulation of the P2Y2 receptor. J Neurochem. 2009;109(5):1300–1310. doi: 10.1111/j.1471-4159.2009.06048.x. [pii] 10.1111/j.1471-4159.2009.06048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Q, Wang M, Liao Z, Camden JM, Yu S, Simonyi A, Sun GY, Gonzalez FA, Erb L, Seye CI, Weisman GA. P2X7 nucleotide receptors mediate caspase-8/9/3-dependent apoptosis in rat primary cortical neurons. Purinergic Signal. 2005;1(4):337–347. doi: 10.1007/s11302-005-7145-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Y, Ruan L, Qian L, Liu X, Le Y. Norepinephrine promotes microglia to uptake and degrade amyloid beta peptide through upregulation of mouse formyl peptide receptor 2 and induction of insulin-degrading enzyme. J Neurosci. 2010;30(35):11848–11857. doi: 10.1523/JNEUROSCI.2985-10.2010. [pii]10.1523/JNEUROSCI.2985-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshiba M, Apasov S, Sverdlov V, Chen P, Erb L, Turner JT, Weisman GA, Sitkovsky MV. Transient up-regulation of P2Y2 nucleotide receptor mRNA expression is an immediate early gene response in activated thymocytes. Proc Natl Acad Sci U S A. 1997;94(3):831–836. doi: 10.1073/pnas.94.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshimizu TA, Ueno S, Tanoue A, Yanagihara N, Stojilkovic SS, Tsujimoto G. Heteromultimerization modulates P2X receptor functions through participating extracellular and C-terminal subdomains. J Biol Chem. 2002;277(49):46891–46899. doi: 10.1074/jbc.M205274200. [pii] [DOI] [PubMed] [Google Scholar]
- Kotnis S, Bingham B, Vasilyev DV, Miller SW, Bai Y, Yeola S, Chanda PK, Bowlby MR, Kaftan EJ, Samad TA, Whiteside GT. Genetic and functional analysis of human P2X5 reveals a distinct pattern of exon 10 polymorphism with predominant expression of the nonfunctional receptor isoform. Mol Pharmacol. 2010;77(6):953–960. doi: 10.1124/mol.110.063636. [DOI] [PubMed] [Google Scholar]
- Kreda SM, Seminario-Vidal L, Heusden C, Lazarowski ER. Thrombin-promoted release of UDP-glucose from human astrocytoma cells. Br J Pharmacol. 2008;153(7):1528–1537. doi: 10.1038/sj.bjp.0707692. [pii]10.1038/sj.bjp.0707692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19(8):312–318. doi: 10.1016/0166-2236(96)10049-7. [pii] [DOI] [PubMed] [Google Scholar]
- Kronlage M, Song J, Sorokin L, Isfort K, Schwerdtle T, Leipziger J, Robaye B, Conley PB, Kim HC, Sargin S, Schön P, Schwab A, Hanley PJ. Autocrine purinergic receptor signaling is essential for macrophage chemotaxis. Sci Signal. 2010;3(132):ra55. doi: 10.1126/scisignal.2000588. [pii]10.1126/scisignal.2000588. [DOI] [PubMed] [Google Scholar]
- Kukulski F, Ben Yebdri F, Bahrami F, Fausther M, Tremblay A, Sevigny J. Endothelial P2Y2 receptor regulates LPS-induced neutrophil transendothelial migration in vitro. Mol Immunol. 2010;47(5):991–999. doi: 10.1016/j.molimm.2009.11.020. [pii] 10.1016/j.molimm.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunapuli SP, Ding Z, Dorsam RT, Kim S, Murugappan S, Quinton TM. ADP receptors--targets for developing antithrombotic agents. Curr Pharm Des. 2003;9(28):2303–2316. doi: 10.2174/1381612033453947. [DOI] [PubMed] [Google Scholar]
- Labasi JM, Petrushova N, Donovan C, McCurdy S, Lira P, Payette MM, Brissette W, Wicks JR, Audoly L, Gabel CA. Absence of the P2X7 receptor alters leukocyte function and attenuates an inflammatory response. J Immunol. 2002;168(12):6436–6445. doi: 10.4049/jimmunol.168.12.6436. [DOI] [PubMed] [Google Scholar]
- Lalancette-Hébert M, Gowing G, Simard A, Weng YC, Kriz J. Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain. J Neurosci. 2007;27(10):2596–2605. doi: 10.1523/JNEUROSCI.5360-06.2007. [pii] 10.1523/JNEUROSCI.5360-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalo U, Pankratov Y, Wichert SP, Rossner MJ, North RA, Kirchhoff F, Verkhratsky A. P2X1 and P2X5 subunits form the functional P2X receptor in mouse cortical astrocytes. J Neurosci. 2008;28(21):5473–5480. doi: 10.1523/JNEUROSCI.1149-08.2008. [pii] 10.1523/JNEUROSCI.1149-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarowski ER, Shea DA, Boucher RC, Harden TK. Release of cellular UDP-glucose as a potential extracellular signaling molecule. Mol Pharmacol. 2003;63(5):1190–1197. doi: 10.1124/mol.63.5.1190. [DOI] [PubMed] [Google Scholar]
- Le KT, Babinski K, Seguela P. Central P2X4 and P2X6 channel subunits coassemble into a novel heteromeric ATP receptor. J Neurosci. 1998;18(18):7152–7159. doi: 10.1523/JNEUROSCI.18-18-07152.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le KT, Boue-Grabot E, Archambault V, Seguela P. Functional and biochemical evidence for heteromeric ATP-gated channels composed of P2X1 and P2X5 subunits. J Biol Chem. 1999;274(22):15415–15419. doi: 10.1074/jbc.274.22.15415. [DOI] [PubMed] [Google Scholar]
- Le KT, Paquet M, Nouel D, Babinski K, Seguela P. Primary structure and expression of a naturally truncated human P2X ATP receptor subunit from brain and immune system. FEBS Lett. 1997;418(1–2):195–199. doi: 10.1016/s0014-5793(97)01380-x. [pii] [DOI] [PubMed] [Google Scholar]
- Lee YJ, Han SB, Nam SY, Oh KW, Hong JT. Inflammation and Alzheimer's disease. Arch Pharm Res. 2010;33(10):1539–1556. doi: 10.1007/s12272-010-1006-7. [DOI] [PubMed] [Google Scholar]
- Lenertz LY, Gavala ML, Hill LM, Bertics PJ. Cell signaling via the P2X7 nucleotide receptor: linkage to ROS production, gene transcription, and receptor trafficking. Purinergic Signal. 2009;5(2):175–187. doi: 10.1007/s11302-009-9133-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz G, Gottfried C, Luo Z, Avruch J, Rodnight R, Nie WJ, Kang Y, Neary JT. P2Y purinoceptor subtypes recruit different mek activators in astrocytes. Br J Pharmacol. 2000;129(5):927–936. doi: 10.1038/sj.bjp.0703138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Z, Seye CI, Weisman GA, Erb L. The P2Y2 nucleotide receptor requires interaction with αV integrins to access and activate G12. J Cell Sci. 2007;120(Pt 9):1654–1662. doi: 10.1242/jcs.03441. [pii] 10.1242/jcs.03441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister MF, Sharkey J, Sawatzky DA, Hodgkiss JP, Davidson DJ, Rossi AG, Finlayson K. The role of the purinergic P2X7 receptor in inflammation. J Inflamm (Lond) 2007;4:5. doi: 10.1186/1476-9255-4-5. [pii] 10.1186/1476-9255-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu GD, Ding JQ, Xiao Q, Chen SD. P2Y6 receptor and immunoinflammation. Neurosci Bull. 2009;25(3):161–164. doi: 10.1007/s12264-009-0120-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucattelli M, Cicko S, Muller T, Lommatzsch M, De Cunto G, Cardini S, Sundas W, Grimm M, Zeiser R, Durk T, Zissel G, Sorichter S, Ferrari D, Di Virgilio F, Virchow JC, Lungarella G, Idzko M. P2X7 receptor signaling in the pathogenesis of smoke-induced lung inflammation and emphysema. Am J Respir Cell Mol Biol. 2011;44(3):423–429. doi: 10.1165/rcmb.2010-0038OC. [pii] 10.1165/rcmb.2010-0038OC. [DOI] [PubMed] [Google Scholar]
- MacKenzie AB, Surprenant A, North RA. Functional and molecular diversity of purinergic ion channel receptors. Ann N Y Acad Sci. 1999;868:716–729. doi: 10.1111/j.1749-6632.1999.tb11351.x. [DOI] [PubMed] [Google Scholar]
- Mahaut-Smith MP, Tolhurst G, Evans RJ. Emerging roles for P2X1 receptors in platelet activation. Platelets. 2004;15(3):131–144. doi: 10.1080/09537100410001682788. Y1ADLQQNP2K67HYW [pii] [DOI] [PubMed] [Google Scholar]
- Mandrekar S, Jiang Q, Lee CY, Koenigsknecht-Talboo J, Holtzman DM, Landreth GE. Microglia mediate the clearance of soluble Aβ through fluid phase macropinocytosis. J Neurosci. 2009;29(13):4252–4262. doi: 10.1523/JNEUROSCI.5572-08.2009. [pii]10.1523/JNEUROSCI.5572-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marteau F, Le Poul E, Communi D, Labouret C, Savi P, Boeynaems JM, Gonzalez NS. Pharmacological characterization of the human P2Y13 receptor. Mol Pharmacol. 2003;64(1):104–112. doi: 10.1124/mol.64.1.104. [pii] 10.1124/mol.64.1.104. [DOI] [PubMed] [Google Scholar]
- McGaraughty S, Chu KL, Namovic MT, Donnelly-Roberts DL, Harris RR, Zhang XF, Shieh CC, Wismer CT, Zhu CZ, Gauvin DM, Fabiyi AC, Honore P, Gregg RJ, Kort ME, Nelson DW, Carroll WA, Marsh K, Faltynek CR, Jarvis MF. P2X7-related modulation of pathological nociception in rats. Neuroscience. 2007;146(4):1817–1828. doi: 10.1016/j.neuroscience.2007.03.035. [pii] 10.1016/j.neuroscience.2007.03.035. [DOI] [PubMed] [Google Scholar]
- McLarnon JG, Ryu JK, Walker DG, Choi HB. Upregulated expression of purinergic P2X7 receptor in Alzheimer disease and amyloid-beta peptide-treated microglia and in peptide-injected rat hippocampus. J Neuropathol Exp Neurol. 2006;65(11):1090–1097. doi: 10.1097/01.jnen.0000240470.97295.d3. [DOI] [PubMed] [Google Scholar]
- Mingam R, De Smedt V, Amédée T, Bluthé RM, Kelley KW, Dantzer R, Layé S. In vitro and in vivo evidence for a role of the P2X7 receptor in the release of IL-1 β in the murine brain. Brain Behav Immun. 2008;22(2):234–244. doi: 10.1016/j.bbi.2007.08.007. [pii]10.1016/j.bbi.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra SK, Braun N, Shukla V, Füllgrabe M, Schomerus C, Korf HW, Gachet C, Ikehara Y, Sévigny J, Robson SC, Zimmermann H. Extracellular nucleotide signaling in adult neural stem cells: synergism with growth factor-mediated cellular proliferation. Development. 2006;133(4):675–684. doi: 10.1242/dev.02233. [pii] 10.1242/dev.02233. [DOI] [PubMed] [Google Scholar]
- Moore D, Chambers J, Waldvogel H, Faull R, Emson P. Regional and cellular distribution of the P2Y1 purinergic receptor in the human brain: striking neuronal localisation. J Comp Neurol. 2000;421(3):374–384. doi: 10.1002/(sici)1096-9861(20000605)421:3<374::aid-cne6>3.0.co;2-z. [pii] [DOI] [PubMed] [Google Scholar]
- Moore DJ, Chambers JK, Wahlin JP, Tan KB, Moore GB, Jenkins O, Emson PC, Murdock PR. Expression pattern of human P2Y receptor subtypes: a quantitative reverse transcription-polymerase chain reaction study. Biochim Biophys Acta. 2001;1521(1–3):107–119. doi: 10.1016/s0167-4781(01)00291-3. [DOI] [PubMed] [Google Scholar]
- Morán-Jiménez MJ, Matute C. Immunohistochemical localization of the P2Y1 purinergic receptor in neurons and glial cells of the central nervous system. Brain Res Mol Brain Res. 2000;78(1–2):50–58. doi: 10.1016/s0169-328x(00)00067-x. [pii] [DOI] [PubMed] [Google Scholar]
- Morelli A, Chiozzi P, Chiesa A, Ferrari D, Sanz JM, Falzoni S, Pinton P, Rizzuto R, Olson MF, Di Virgilio F. Extracellular ATP causes ROCK I-dependent bleb formation in P2X7-transfected HEK293 cells. Mol Biol Cell. 2003;14(7):2655–2664. doi: 10.1091/mbc.02-04-0061. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulryan K, Gitterman DP, Lewis CJ, Vial C, Leckie BJ, Cobb AL, Brown JE, Conley EC, Buell G, Pritchard CA, Evans RJ. Reduced vas deferens contraction and male infertility in mice lacking P2X1 receptors. Nature. 2000;403(6765):86–89. doi: 10.1038/47495. [DOI] [PubMed] [Google Scholar]
- Nakatsuka T, Gu JG. ATP P2X receptor-mediated enhancement of glutamate release and evoked EPSCs in dorsal horn neurons of the rat spinal cord. J Neurosci. 2001;21(17):6522–6531. doi: 10.1523/JNEUROSCI.21-17-06522.2001. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsuka T, Mena N, Ling J, Gu JG. Depletion of substance P from rat primary sensory neurons by ATP, an implication of P2X receptor-mediated release of substance P. Neuroscience. 2001;107(2):293–300. doi: 10.1016/s0306-4522(01)00342-6. [pii] [DOI] [PubMed] [Google Scholar]
- Neary JT, Zimmermann H. Trophic functions of nucleotides in the central nervous system. Trends Neurosci. 2009;32(4):189–198. doi: 10.1016/j.tins.2009.01.002. [pii]10.1016/j.tins.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Neumann H, Takahashi K. Essential role of the microglial triggering receptor expressed on myeloid cells-2(TREM2) for central nervous tissue immune homeostasis. J Neuroimmunol. 2007;184(1–2):92–99. doi: 10.1016/j.jneuroim.2006.11.032. [pii]10.1016/j.jneuroim.2006.11.032. [DOI] [PubMed] [Google Scholar]
- Nguyen T, Erb L, Weisman GA, Marchese A, Heng HH, Garrad RC, George SR, Turner JT, O'Dowd BF. Cloning, expression, and chromosomal localization of the human uridine nucleotide receptor gene. J Biol Chem. 1995;270(52):30845–30848. doi: 10.1074/jbc.270.52.30845. [DOI] [PubMed] [Google Scholar]
- Nicke A, Kerschensteiner D, Soto F. Biochemical and functional evidence for heteromeric assembly of P2X1 and P2X4 subunits. J Neurochem. 2005;92(4):925–933. doi: 10.1111/j.1471-4159.2004.02939.x. [DOI] [PubMed] [Google Scholar]
- Norambuena A, Palma F, Poblete MI, Donoso MV, Pardo E, González A, Huidobro-Toro JP. UTP controls cell surface distribution and vasomotor activity of the human P2Y2 receptor through an epidermal growth factor receptor-transregulated mechanism. J Biol Chem. 2010;285(5):2940–2950. doi: 10.1074/jbc.M109.081166. [pii]10.1074/jbc.M109.081166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North RA, Surprenant A. Pharmacology of cloned P2X receptors. Annu Rev Pharmacol Toxicol. 2000;40:563–580. doi: 10.1146/annurev.pharmtox.40.1.563. [DOI] [PubMed] [Google Scholar]
- Ormond SJ, Barrera NP, Qureshi OS, Henderson RM, Edwardson JM, Murrell-Lagnado RD. An uncharged region within the N terminus of the P2X6 receptor inhibits its assembly and exit from the endoplasmic reticulum. Mol Pharmacol. 2006;69(5):1692–1700. doi: 10.1124/mol.105.020404. [DOI] [PubMed] [Google Scholar]
- Ortega F, Pérez-Sen R, Miras-Portugal MT. Gi-coupled P2Y-ADP receptor mediates GSK-3 phosphorylation and β-catenin nuclear translocation in granule neurons. J Neurochem. 2008;104(1):62–73. doi: 10.1111/j.1471-4159.2007.05021.x. [pii] 10.1111/j.1471-4159.2007.05021.x. [DOI] [PubMed] [Google Scholar]
- Pankratov Y, Castro E, Miras-Portugal MT, Krishtal O. A purinergic component of the excitatory postsynaptic current mediated by P2X receptors in the CA1 neurons of the rat hippocampus. Eur J Neurosci. 1998;10(12):3898–3902. doi: 10.1046/j.1460-9568.1998.00419.x. [DOI] [PubMed] [Google Scholar]
- Parvathenani LK, Tertyshnikova S, Greco CR, Roberts SB, Robertson B, Posmantur R. P2X7 mediates superoxide production in primary microglia and is up-regulated in a transgenic mouse model of Alzheimer's disease. J Biol Chem. 2003;278(15):13309–13317. doi: 10.1074/jbc.M209478200. [DOI] [PubMed] [Google Scholar]
- Patti L, Raiteri L, Grilli M, Parodi M, Raiteri M, Marchi M. P2X7 receptors exert a permissive role on the activation of release-enhancing presynaptic alpha7 nicotinic receptors co-existing on rat neocortex glutamatergic terminals. Neuropharmacology. 2006;50(6):705–713. doi: 10.1016/j.neuropharm.2005.11.016. [pii] 10.1016/j.neuropharm.2005.11.016. [DOI] [PubMed] [Google Scholar]
- Pelegrin P, Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1β release by the ATP-gated P2X7 receptor. EMBO J. 2006;25(21):5071–5082. doi: 10.1038/sj.emboj.7601378. [pii] 10.1038/sj.emboj.7601378 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson TS, Camden JM, Wang Y, Seye CI, Wood WG, Sun GY, Erb L, Petris MJ, Weisman GA. P2Y2 nucleotide receptor-mediated responses in brain cells. Mol Neurobiol. 2010;41(2–3):356–366. doi: 10.1007/s12035-010-8115-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer ZA, Aga M, Prabhu U, Watters JJ, Hall DJ, Bertics PJ. The nucleotide receptor P2X7 mediates actin reorganization and membrane blebbing in RAW 264.7 macrophages via p38 MAP kinase and Rho. J Leukoc Biol. 2004;75(6):1173–1182. doi: 10.1189/jlb.1203648. [pii] [DOI] [PubMed] [Google Scholar]
- Pihlaja R, Koistinaho J, Malm T, Sikkila H, Vainio S, Koistinaho M. Transplanted astrocytes internalize deposited beta-amyloid peptides in a transgenic mouse model of Alzheimer's disease. Glia. 2008;56(2):154–163. doi: 10.1002/glia.20599. [DOI] [PubMed] [Google Scholar]
- Pooler AM, Guez DH, Benedictus R, Wurtman RJ. Uridine enhances neurite outgrowth in nerve growth factor-differentiated PC12 [corrected] Neuroscience. 2005;134(1):207–214. doi: 10.1016/j.neuroscience.2005.03.050. [DOI] [PubMed] [Google Scholar]
- Potucek YD, Crain JM, Watters JJ. Purinergic receptors modulate MAP kinases and transcription factors that control microglial inflammatory gene expression. Neurochem Int. 2006;49(2):204–214. doi: 10.1016/j.neuint.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50(3):413–492. [PubMed] [Google Scholar]
- Ratchford AM, Baker OJ, Camden JM, Rikka S, Petris MJ, Seye CI, Erb L, Weisman GA. P2Y2 nucleotide receptors mediate metalloprotease-dependent phosphorylation of epidermal growth factor receptor and ErbB3 in human salivary gland cells. J Biol Chem. 2010;285(10):7545–7555. doi: 10.1074/jbc.M109.078170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettinger J, Schmalzing G. Activation and desensitization of the recombinant P2X1 receptor at nanomolar ATP concentrations. J Gen Physiol. 2003;121(5):451–461. doi: 10.1085/jgp.200208730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson SJ, Ennion SJ, Evans RJ, Edwards FA. Synaptic P2X receptors. Curr Opin Neurobiol. 2001;11(3):378–386. doi: 10.1016/s0959-4388(00)00222-1. [DOI] [PubMed] [Google Scholar]
- Rodrigues RJ, Almeida T, de Mendonca A, Cunha RA. Interaction between P2X and nicotinic acetylcholine receptors in glutamate nerve terminals of the rat hippocampus. J Mol Neurosci. 2006;30(1–2):173–176. doi: 10.1385/JMN:30:1:173. [DOI] [PubMed] [Google Scholar]
- Rodrigues RJ, Almeida T, Richardson PJ, Oliveira CR, Cunha RA. Dual presynaptic control by ATP of glutamate release via facilitatory P2X1, P2X2/3, and P2X3 and inhibitory P2Y1, P2Y2, and/or P2Y4. receptors in the rat hippocampus. J Neurosci. 2005;25(27):6286–6295. doi: 10.1523/JNEUROSCI.0628-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Zayas AE, Torrado AI, Miranda JD. P2Y2 receptor expression is altered in rats after spinal cord injury. Int J Dev Neurosci. 2010;28(6):413–421. doi: 10.1016/j.ijdevneu.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger S, Pelegrin P, Surprenant A. Facilitation of P2X7 receptor currents and membrane blebbing via constitutive and dynamic calmodulin binding. J Neurosci. 2008;28(25):6393–6401. doi: 10.1523/JNEUROSCI.0696-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J, Lue LF. Microglial chemotaxis, activation, and phagocytosis of amyloid β-peptide as linked phenomena in Alzheimer's disease. Neurochem Int. 2001;39(5–6):333–340. doi: 10.1016/s0197-0186(01)00040-7. [DOI] [PubMed] [Google Scholar]
- Ruan HZ, Burnstock G. Localisation of P2Y1 and P2Y4 receptors in dorsal root, nodose and trigeminal ganglia of the rat. Histochem Cell Biol. 2003;120(5):415–426. doi: 10.1007/s00418-003-0579-3. [DOI] [PubMed] [Google Scholar]
- Rubio ME, Soto F. Distinct Localization of P2X receptors at excitatory postsynaptic specializations. J Neurosci. 2001;21(2):641–653. doi: 10.1523/JNEUROSCI.21-02-00641.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sak K, Webb TE. A retrospective of recombinant P2Y receptor subtypes and their pharmacology. Arch Biochem Biophys. 2002;397(1):131–136. doi: 10.1006/abbi.2001.2616. [DOI] [PubMed] [Google Scholar]
- Sanada M, Yasuda H, Omatsu-Kanbe M, Sango K, Isono T, Matsuura H, Kikkawa R. Increase in intracellular Ca2+and calcitonin gene-related peptide release through metabotropic P2Y receptors in rat dorsal root ganglion neurons. Neuroscience. 2002;111(2):413–422. doi: 10.1016/s0306-4522(02)00005-2. [DOI] [PubMed] [Google Scholar]
- Sanz JM, Chiozzi P, Ferrari D, Colaianna M, Idzko M, Falzoni S, Fellin R, Trabace L, Di Virgilio F. Activation of microglia by amyloid β requires P2X7 receptor expression. J Immunol. 2009;182(7):4378–4385. doi: 10.4049/jimmunol.0803612. [DOI] [PubMed] [Google Scholar]
- Sasaki Y, Hoshi M, Akazawa C, Nakamura Y, Tsuzuki H, Inoue K, Kohsaka S. Selective expression of Gi/o-coupled ATP receptor P2Y12 in microglia in rat brain. Glia. 2003;44(3):242–250. doi: 10.1002/glia.10293. [DOI] [PubMed] [Google Scholar]
- Schrader AM, Camden JM, Weisman GA. P2Y2 nucleotide receptor up-regulation in submandibular gland cells from the NOD.B10 mouse model of Sjogren's syndrome. Archives of Oral Biology. 2005;50(6):533–540. doi: 10.1016/j.archoralbio.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Schulze-Lohoff E, Hugo C, Rost S, Arnold S, Gruber A, Brune B, Sterzel RB. Extracellular ATP causes apoptosis and necrosis of cultured mesangial cells via P2Z/P2X7 receptors. Am J Physiol. 1998;275(6 Pt 2):F962–F971. doi: 10.1152/ajprenal.1998.275.6.F962. [DOI] [PubMed] [Google Scholar]
- Seye CI, Kong Q, Erb L, Garrad RC, Krugh B, Wang M, Turner JT, Sturek M, Gonzalez FA, Weisman GA. Functional P2Y2 nucleotide receptors mediate uridine 5'-triphosphate-induced intimal hyperplasia in collared rabbit carotid arteries. Circulation. 2002;106(21):2720–2726. doi: 10.1161/01.cir.0000038111.00518.35. [DOI] [PubMed] [Google Scholar]
- Seye CI, Yu N, Gonzalez FA, Erb L, Weisman GA. The P2Y2 nucleotide receptor mediates vascular cell adhesion molecule-1 expression through interaction with VEGF receptor-2(KDR/Flk-1) J Biol Chem. 2004;279(34):35679–35686. doi: 10.1074/jbc.M401799200. [DOI] [PubMed] [Google Scholar]
- Shaver SR. P2Y receptors: biological advances and therapeutic opportunities. Curr Opin Drug Discov Devel. 2001;4(5):665–670. [PubMed] [Google Scholar]
- Shen J, Seye CI, Wang M, Weisman GA, Wilden PA, Sturek M. Cloning, up-regulation, and mitogenic role of porcine P2Y2 receptor in coronary artery smooth muscle cells. Mol Pharmacol. 2004;66(5):1265–1274. doi: 10.1124/mol.104.002642. [DOI] [PubMed] [Google Scholar]
- Shigemoto-Mogami Y, Koizumi S, Tsuda M, Ohsawa K, Kohsaka S, Inoue K. Mechanisms underlying extracellular ATP-evoked interleukin-6 release in mouse microglial cell line, MG-5. J Neurochem. 2001;78(6):1339–1349. doi: 10.1046/j.1471-4159.2001.00514.x. [DOI] [PubMed] [Google Scholar]
- Shiratori M, Tozaki-Saitoh H, Yoshitake M, Tsuda M, Inoue K. P2X7 receptor activation induces CXCL2 production in microglia through NFAT and PKC/MAPK pathways. J Neurochem. 2010;114(3):810–819. doi: 10.1111/j.1471-4159.2010.06809.x. [DOI] [PubMed] [Google Scholar]
- Shlosberg D, Benifla M, Kaufer D, Friedman A. Blood-brain barrier breakdown as a therapeutic target in traumatic brain injury. Nat Rev Neurol. 2010;6(7):393–403. doi: 10.1038/nrneurol.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim JA, Chaumont S, Jo J, Ulmann L, Young MT, Cho K, Buell G, North RA, Rassendren F. Altered hippocampal synaptic potentiation in P2X4 knock-out mice. J Neurosci. 2006;26(35):9006–9009. doi: 10.1523/JNEUROSCI.2370-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim JA, Young MT, Sung HY, North RA, Surprenant A. Reanalysis of P2X7 receptor expression in rodent brain. J Neurosci. 2004;24(28):6307–6314. doi: 10.1523/JNEUROSCI.1469-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard AR, Soulet D, Gowing G, Julien JP, Rivest S. Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer's disease. Neuron. 2006;49(4):489–502. doi: 10.1016/j.neuron.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Simard M, Arcuino G, Takano T, Liu QS, Nedergaard M. Signaling at the gliovascular interface. J Neurosci. 2003;23(27):9254–9262. doi: 10.1523/JNEUROSCI.23-27-09254.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon J, Webb TE, Barnard EA. Distribution of [35S]dATP α S binding sites in the adult rat neuraxis. Neuropharmacology. 1997;36(9):1243–1251. doi: 10.1016/s0028-3908(97)00124-x. [DOI] [PubMed] [Google Scholar]
- Simon J, Webb TE, King BF, Burnstock G, Barnard EA. Characterisation of a recombinant P2Y purinoceptor. Eur J Pharmacol. 1995;291(3):281–289. doi: 10.1016/0922-4106(95)90068-3. [DOI] [PubMed] [Google Scholar]
- Skaper SD, Debetto P, Giusti P. The P2X7 purinergic receptor: from physiology to neurological disorders. FASEB J. 2009;24(2):337–345. doi: 10.1096/fj.09-138883. [DOI] [PubMed] [Google Scholar]
- Skelton L, Cooper M, Murphy M, Platt A. Human immature monocyte-derived dendritic cells express the G protein-coupled receptor GPR105(KIAA0001, P2Y14) and increase intracellular calcium in response to its agonist, uridine diphosphoglucose. J Immunol. 2003;171(4):1941–1949. doi: 10.4049/jimmunol.171.4.1941. [DOI] [PubMed] [Google Scholar]
- Soltoff SP. Related adhesion focal tyrosine kinase and the epidermal growth factor receptor mediate the stimulation of mitogen-activated protein kinase by the G-protein-coupled P2Y2 receptor. Phorbol ester or [Ca2+]i elevation can substitute for receptor activation. J Biol Chem. 1998;273(36):23110–23117. doi: 10.1074/jbc.273.36.23110. [DOI] [PubMed] [Google Scholar]
- Soltoff SP, Avraham H, Avraham S, Cantley LC. Activation of P2Y2 receptors by UTP and ATP stimulates mitogen-activated kinase activity through a pathway that involves related adhesion focal tyrosine kinase and protein kinase C. J Biol Chem. 1998;273(5):2653–2660. doi: 10.1074/jbc.273.5.2653. [DOI] [PubMed] [Google Scholar]
- Sperlágh B, Illes P. Purinergic modulation of microglial cell activation. Purinergic Signal. 2007;3(1–2):117–127. doi: 10.1007/s11302-006-9043-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg TH, Silverstein SC. Extracellular ATP4− promotes cation fluxes in the J774 mouse macrophage cell line. J Biol Chem. 1987;262(7):3118–3122. [PubMed] [Google Scholar]
- Streit WJ. Microglia as neuroprotective, immunocompetent cells of the CNS. Glia. 2002;40(2):133–139. doi: 10.1002/glia.10154. [DOI] [PubMed] [Google Scholar]
- Streit WJ. Microglial senescence: does the brain's immune system have an expiration date? Trends Neurosci. 2006;29(9):506–510. doi: 10.1016/j.tins.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Sudo S, Tanaka J, Toku K, Desaki J, Matsuda S, Arai T, Sakanaka M, Maeda N. Neurons induce the activation of microglial cells in vitro. Exp Neurol. 1998;154(2):499–510. doi: 10.1006/exnr.1998.6911. [DOI] [PubMed] [Google Scholar]
- Sugama S, Takenouchi T, Cho BP, Joh TH, Hashimoto M, Kitani H. Possible roles of microglial cells for neurotoxicity in clinical neurodegenerative diseases and experimental animal models. Inflamm Allergy Drug Targets. 2009;8(4):277–284. doi: 10.2174/187152809789352249. [DOI] [PubMed] [Google Scholar]
- Sugiyama T, Oku H, Shibata M, Fukuhara M, Yoshida H, Ikeda T. Involvement of P2X7 receptors in the hypoxia-induced death of rat retinal neurons. Invest Ophthalmol Vis Sci. 2010;51(6):3236–3243. doi: 10.1167/iovs.09-4192. [DOI] [PubMed] [Google Scholar]
- Surprenant A, Rassendren F, Kawashima E, North RA, Buell G. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor(P2X7) Science. 1996;272(5262):735–738. doi: 10.1126/science.272.5262.735. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Hide I, Ido K, Kohsaka S, Inoue K, Nakata Y. Production and release of neuroprotective tumor necrosis factor by P2X7 receptor-activated microglia. J Neurosci. 2004;24(1):1–7. doi: 10.1523/JNEUROSCI.3792-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenouchi T, Sekiyama K, Sekigawa A, Fujita M, Waragai M, Sugama S, Iwamaru Y, Kitani H, Hashimoto M. P2X7 receptor signaling pathway as a therapeutic target for neurodegenerative diseases. Arch Immunol Ther Exp(Warsz) 2010;58(2):91–96. doi: 10.1007/s00005-010-0069-y. [DOI] [PubMed] [Google Scholar]
- Takenouchi T, Sugama S, Iwamaru Y, Hashimoto M, Kitani H. Modulation of the ATP-lnduced release and processing of IL-1β in microglial cells. Crit Rev Immunol. 2009;29(4):335–345. doi: 10.1615/critrevimmunol.v29.i4.40. [DOI] [PubMed] [Google Scholar]
- Tanaka J, Murate M, Wang CZ, Seino S, Iwanaga T. Cellular distribution of the P2X4 ATP receptor mRNA in the brain and non-neuronal organs of rats. Arch Histol Cytol. 1996;59(5):485–490. doi: 10.1679/aohc.59.485. [DOI] [PubMed] [Google Scholar]
- Torres GE, Haines WR, Egan TM, Voigt MM. Co-expression of P2X1 and P2X5 receptor subunits reveals a novel ATP-gated ion channel. Mol Pharmacol. 1998;54(6):989–993. doi: 10.1124/mol.54.6.989. [DOI] [PubMed] [Google Scholar]
- Trang T, Beggs S, Salter MW. Purinoceptors in microglia and neuropathic pain. Pflugers Arch. 2006;452(5):645–652. doi: 10.1007/s00424-006-0074-5. [DOI] [PubMed] [Google Scholar]
- Tschopp J, Schroder K. NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nat Rev Immunol. 2010;10(3):210–215. doi: 10.1038/nri2725. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Kuboyama K, Inoue T, Nagata K, Tozaki-Saitoh H, Inoue K. Behavioral phenotypes of mice lacking purinergic P2X4 receptors in acute and chronic pain assays. Mol Pain. 2009;5:28. doi: 10.1186/1744-8069-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JT, Weisman GA, Camden JM. Upregulation of P2Y2 nucleotide receptors in rat salivary gland cells during short-term culture. Am J Physiol. 1997;273(3 Pt 1):C1100–C1107. doi: 10.1152/ajpcell.1997.273.3.C1100. [DOI] [PubMed] [Google Scholar]
- Turrin NP, Rivest S. Tumor necrosis factor a but not interleukin 1 β mediates neuroprotection in response to acute nitric oxide excitotoxicity. J Neurosci. 2006;26(1):143–151. doi: 10.1523/JNEUROSCI.4032-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmann L, Hatcher JP, Hughes JP, Chaumont S, Green PJ, Conquet F, Buell GN, Reeve AJ, Chessell IP, Rassendren F. Up-regulation of P2X4 receptors in spinal microglia after peripheral nerve injury mediates BDNF release and neuropathic pain. J Neurosci. 2008;28(44):11263–11268. doi: 10.1523/JNEUROSCI.2308-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmann L, Hirbec H, Rassendren F. P2X4 receptors mediate PGE2 release by tissue-resident macrophages and initiate inflammatory pain. EMBO J. 2010;29(14):2290–2300. doi: 10.1038/emboj.2010.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valera S, Hussy N, Evans RJ, Adami N, North RA, Surprenant A, Buell G. A new class of ligand-gated ion channel defined by P2X receptor for extracellular ATP. Nature. 1994;371(6497):516–519. doi: 10.1038/371516a0. [DOI] [PubMed] [Google Scholar]
- Vaughan KR, Stokes L, Prince LR, Marriott HM, Meis S, Kassack MU, Bingle CD, Sabroe I, Surprenant A, Whyte MK. Inhibition of neutrophil apoptosis by ATP is mediated by the P2Y11 receptor. J Immunol. 2007;179(12):8544–8553. doi: 10.4049/jimmunol.179.12.8544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoef PA, Estacion M, Schilling W, Dubyak GR. P2X7 receptor-dependent blebbing and the activation of Rho-effector kinases, caspases, and IL-1 beta release. J Immunol. 2003;170(11):5728–5738. doi: 10.4049/jimmunol.170.11.5728. [DOI] [PubMed] [Google Scholar]
- Vial C, Evans RJ. P2X receptor expression in mouse urinary bladder and the requirement of P2X1 receptors for functional P2X receptor responses in the mouse urinary bladder smooth muscle. Br J Pharmacol. 2000;131(7):1489–1495. doi: 10.1038/sj.bjp.0703720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vial C, Roberts JA, Evans RJ. Molecular properties of ATP-gated P2X receptor ion channels. Trends Pharmacol Sci. 2004;25(9):487–493. doi: 10.1016/j.tips.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Volonté C, Amadio S, D'Ambrosi N, Colpi M, Burnstock G. P2 receptor web: complexity and fine-tuning. Pharmacol Ther. 2006;112(1):264–280. doi: 10.1016/j.pharmthera.2005.04.012. [DOI] [PubMed] [Google Scholar]
- von Kügelgen I, Wetter A. Molecular pharmacology of P2Y-receptors. Naunyn Schmiedebergs Arch Pharmacol. 2000;362(4–5):310–323. doi: 10.1007/s002100000310. [DOI] [PubMed] [Google Scholar]
- Vulchanova L, Arvidsson U, Riedl M, Wang J, Buell G, Surprenant A, North RA, Elde R. Differential distribution of two ATP-gated channels(P2X receptors) determined by immunocytochemistry. Proc Natl Acad Sci U S A. 1996;93(15):8063–8067. doi: 10.1073/pnas.93.15.8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vulchanova L, Riedl MS, Shuster SJ, Buell G, Surprenant A, North RA, Elde R. Immunohistochemical study of the P2X2 and P2X3 receptor subunits in rat and monkey sensory neurons and their central terminals. Neuropharmacology. 1997;36(9):1229–1242. doi: 10.1016/s0028-3908(97)00126-3. [DOI] [PubMed] [Google Scholar]
- Vulchanova L, Riedl MS, Shuster SJ, Stone LS, Hargreaves KM, Buell G, Surprenant A, North RA, Elde R. P2X3 is expressed by DRG neurons that terminate in inner lamina II. Eur J Neurosci. 1998;10(11):3470–3478. doi: 10.1046/j.1460-9568.1998.00355.x. [DOI] [PubMed] [Google Scholar]
- Walker DG, Kim SU, McGeer PL. Complement and cytokine gene expression in cultured microglial derived from postmortem human brains. J Neurosci Res. 1995;40(4):478–493. doi: 10.1002/jnr.490400407. [DOI] [PubMed] [Google Scholar]
- Wang M, Kong Q, Gonzalez FA, Sun G, Erb L, Seye C, Weisman GA. P2Y nucleotide receptor interaction with αV integrin mediates astrocyte migration. J Neurochem. 2005;95(3):630–640. doi: 10.1111/j.1471-4159.2005.03408.x. [DOI] [PubMed] [Google Scholar]
- Wang Q, Wang L, Feng YH, Li X, Zeng R, Gorodeski GI. P2X7 receptor-mediated apoptosis of human cervical epithelial cells. Am J Physiol Cell Physiol. 2004;287(5):C1349–C1358. doi: 10.1152/ajpcell.00256.2004. [DOI] [PubMed] [Google Scholar]
- Watano T, Calvert JA, Vial C, Forsythe ID, Evans RJ. P2X receptor subtype-specific modulation of excitatory and inhibitory synaptic inputs in the rat brainstem. J Physiol. 2004;558(Pt 3):745–757. doi: 10.1113/jphysiol.2004.066845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb TE, Henderson DJ, Roberts JA, Barnard EA. Molecular cloning and characterization of the rat P2Y4 receptor. J Neurochem. 1998;71(4):1348–1357. doi: 10.1046/j.1471-4159.1998.71041348.x. [DOI] [PubMed] [Google Scholar]
- Weisman GA, De BK, Pritchard RS. Ionic dependence of the extracellular ATP-induced permeabilization of transformed mouse fibroblasts: role of plasma membrane activities that regulate cell volume. J Cell Physiol. 1989;138(2):375–383. doi: 10.1002/jcp.1041380221. [DOI] [PubMed] [Google Scholar]
- Weisman GA, Wang M, Kong Q, Chorna NE, Neary JT, Sun GY, Gonzalez FA, Seye CI, Erb L. Molecular determinants of P2Y2 nucleotide receptor function: implications for proliferative and inflammatory pathways in astrocytes. Molecular Neurobiology. 2005;31(1–3):169–183. doi: 10.1385/MN:31:1-3:169. [DOI] [PubMed] [Google Scholar]
- Wirkner K, Sperlagh B, Illes P. P2X3 receptor involvement in pain states. Mol Neurobiol. 2007;36(2):165–183. doi: 10.1007/s12035-007-0033-y. [DOI] [PubMed] [Google Scholar]
- Wullaert A, Bonnet MC, Pasparakis M. NF-κB in the regulation of epithelial homeostasis and inflammation. Cell Res. 2011;21(1):146–158. doi: 10.1038/cr.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss-Coray T, Lin C, Yan F, Yu GQ, Rohde M, McConlogue L, Masliah E, Mucke L. TGF-β1 promotes microglial amyloid-beta clearance and reduces plaque burden in transgenic mice. Nat Med. 2001;7(5):612–618. doi: 10.1038/87945. [DOI] [PubMed] [Google Scholar]
- Xiang Z, Bo X, Oglesby I, Ford A, Burnstock G. Localization of ATP-gated P2X2 receptor immunoreactivity in the rat hypothalamus. Brain Res. 1998;813(2):390–397. doi: 10.1016/s0006-8993(98)01073-7. [DOI] [PubMed] [Google Scholar]
- Xiang Z, Burnstock G. P2X2 and P2X3 purinoceptors in the rat enteric nervous system. Histochem Cell Biol. 2004;121(3):169–179. doi: 10.1007/s00418-004-0620-1. [DOI] [PubMed] [Google Scholar]
- Xie Z, Smith CJ, Van Eldik LJ. Activated glia induce neuron death via MAP kinase signaling pathways involving JNK and p38. Glia. 2004;45(2):170–179. doi: 10.1002/glia.10314. [DOI] [PubMed] [Google Scholar]
- Xu J, Wang F, Van Keymeulen A, Herzmark P, Straight A, Kelly K, Takuwa Y, Sugimoto N, Mitchison T, Bourne HR. Divergent signals and cytoskeletal assemblies regulate self-organizing polarity in neutrophils. Cell. 2003;114(2):201–214. doi: 10.1016/s0092-8674(03)00555-5. [DOI] [PubMed] [Google Scholar]
- Yu N, Erb L, Shivaji R, Weisman GA, Seye CI. Binding of the P2Y2 nucleotide receptor to filamin A regulates migration of vascular smooth muscle cells. Circ Res. 2008;102(5):581–588. doi: 10.1161/CIRCRESAHA.107.162271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Zhao Z, Sun J, Guo W, Fu J, Burnstock G, He C, Xiang Z. Expression of P2X6 receptors in the enteric nervous system of the rat gastrointestinal tract. Histochem Cell Biol. 2010;133(2):177–188. doi: 10.1007/s00418-009-0659-0. [DOI] [PubMed] [Google Scholar]
- Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T, Carmignoto G. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci. 2003;6(1):43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]