Abstract
Dipyridamole (Dip) is the most common vasodilator employed with positron emission tomography (PET) for the evaluation of individuals with hypertrophic cardiomyopathy (HC). The aim of this study was to evaluate whether PET quantification of regional myocardial perfusion (rMP), myocardial blood flow (MBF) and coronary flow reserve (CFR) are comparable between Dip and the newer vasodilator agent, Regadenoson (Reg) in HC. An additional aim was to evaluate the association between vasodilator-induced ST segment depression on ECG and myocardial flow in HC. N-13 ammonia PET was performed in 57 symptomatic HC patients at rest and during vasodilator stress (peak) with either Dip (0.56 mg/kg during 4-min infusion) or Reg (0.4 mg fixed bolus dose) for assessment of ECG, rMP (17 AHA-summed difference score [SDS]), MBF and CFR. The Dip and Reg groups consisted of 28 and 29 patients respectively. Baseline characteristics, including resting MBF (0.92 ± 0.22 vs. 0.89 ± 0.23 ml/min/g; P = 0.6) were similar between the Dip and Reg groups. During stress, the presence and severity of abnormal rMP (SDS 5.5 ± 5.5 vs. 5.8 ± 6.7, P=0.8), peak MBF (1.81 ± 0.44 vs. 1.82 ± 0.50 ml/min/g; P = 0.9) and CFR (2.02 ± 0.53 vs. 2.12 ± 0.12; P = 0.5) were comparable between Dip and Reg. Fewer patients exhibited side effects with Reg (2 vs.7; p=0.06). Vasodilator-induced ST segment depression showed a high specificity (~92%) but low sensitivity (~34%) to predict abnormal rMP (SDS ≥ 2). In conclusion, measurement of rMP and quantitative flow with PET is similar between Regadenoson and Dipyridamole in patients with symptomatic HC. Regadenoson is tolerated better than Dipyridamole and is easier to administer. Vasodilator-induced ST segment depression is a specific but non-sensitive marker for prediction of abnormal rMP in HC.
Keywords: PET, hypertrophic cardiomyopathy, Regadenoson, ECG
Introduction
Regadenoson is a newer FDA-approved vasodilator agent administered as a single fixed dose (0.4 mg) that binds more selectively to the coronary A2a adenosine receptors than Dipyridamole or Adenosine. At present, there are no reported studies investigating the clinical utility of Regadenoson as a vasodilator stress agent for cardiac single photon emission computed tomography (SPECT) or positron emission tomography (PET) imaging in patients with hypertrophic cardiomyopathy (HC). The main aim of the present study was to compare Regadenoson to the standard agent Dipyridamole for regional myocardial perfusion (rMP) and quantification of myocardial blood flow (MBF) with PET in HC patients. In addition, we evaluated the association between vasodilator-induced electrocardiographic (ECG) changes and myocardial perfusion in HC.
Methods
We performed a retrospective analysis of consecutive patients with a history of HC diagnosed by echocardiography, who were referred for cardiac PET for clinical indications between January of 2009 and February of 2012. Subjects with history of coronary artery disease (CAD), prior surgical myectomy or alcohol septal ablation were excluded. The diagnosis of HC was based on echocardiographic criteria by demonstrating left ventricular (LV) hypertrophy with wall thickness ≥ 15 mm1. Left ventricular outflow tract gradients were identified by Doppler echocardiography under basal conditions and after the Valsalva maneuver and amyl nitrite (inhaled) challenge to elicit latent obstruction during provocation.
All patients were imaged using a GE Discovery VCT PET/CT system. Individuals were positioned with the assistance of a CT topogram and a low-dose CT scan was performed for attenuation correction of PET emission data. Subsequently, PET images were acquired using a same day rest/stress protocol as following: 13N-NH3 (~10 mCi) was injected intravenously as a bolus and a 2-dimensional list-mode PET scan was obtained over 20 minutes. Approximately 60 minutes after injection of the resting dose, Dipyridamole or Regadenoson was administered for vasodilator stress. Dipyridamole was the drug of choice at the beginning of our PET protocol for HC in 2009; however, as of May of 2011, all HC patients were stressed with Regadenoson. Dipyridamole (0.56 mg/kg) was infused over a period of 4 min, followed by a second dose of 13N-NH3 (~10 mCi) 4 min after the end of dipyridamole infusion. Regadenoson (0.4 mg) was injected as a bolus (~ 15 – 20 s), followed by a 5 ml saline flush, and 13N-NH3 was administered 30 seconds later. Stress acquisition began concomitantly with the second 13N-NH3 injection, and all other parameters were the same as during rest. Heart rate, blood pressure and a 12-lead ECG were recorded before, during and after the completion of the stress protocol. List-mode data were re-sampled to create various images including static (4-min pre-scan delay), ECG-gated (8 bins per cardiac cycle), and 36-frame-dynamic (20 × 6sec, 5 × 12sec, 4 × 30sec, 5 × 60sec, 2 × 300sec).
For the ECG analysis, patients with left bundle branch block and electronically paced ventricular rhythms were excluded. All ECGs were evaluated at baseline for the presence of LV hypertrophy (by Sokolow– Lyon criterion or Cornell product) and ST-T abnormalities. Vasodilator-induced ST-segment changes were evaluated in all leads. Any ST segment deviation from the J point at baseline was subtracted from that observed during pharmacologic stress to determine the overall ST segment shift in each lead. Negative shift values signified depression and positive represented elevation of the ST-segment. ST changes were summed and averaged out in leads II, III, aVF to assess mean ST-segment depression in the inferior leads, and this was also performed in V3, V4 and V5 to estimate mean ST-segment depression in the precordial leads. For each patient the final ST-segment depression reported consisted of the largest (maximum) ST shift between the mean inferior and mean precordial leads. Reciprocal ST-segment elevation in aVR, and V1 was also evaluated.
The CardIQ Physio package (GE Healthcare) was used for analysis of the LV ejection fraction at rest and during stress. PET rMP was semi-quantitatively assessed for each set of rest/stress images using the Summed Difference Score (SDS) and the standard 17-segment 5-point scale, which is the American Heart Association model. An SDS equal to or greater than 2 was considered abnormal in this study. Quantitative Gated SPECT software (Cedars-Sinai Medical Center) was employed for regional wall motion assessment using a 5-point, 17-segment LV model scoring system. This method derived a resting and stress wall motion score. Pharmacologic-induced wall motion segmental abnormalities were considered present when the stress-rest wall motion score difference was > 1. The Munich Heart package was used for absolute flow quantification. Software computation of MBF is based on a well-established 2 tissue-compartment tracer kinetic model as previously described2. Global MBF during vasodilator-stress and rest was measured in milliliters/minute/gram (ml/min/g). Coronary flow reserve (CFR) was determined as the ratio of stress MBF to rest MBF (unitless). Resting MBF and CFR were also corrected for the rate pressure product (RPP), a product of the resting heart rate and systolic blood pressure, using the following equation:3 Corrected resting MBF = observed resting MBF × 8500 (mean RPP of the study cohort) / RPP of each subject. Corrected CFR = observed stress MBF/corrected resting MBF.
Statistical analyses were performed using SPSS (version 19.0). Continuous variables are presented as mean ± SD. An independent-measures t-test was used to assess the differences between the parametric subgroups and the Mann-Whitney U test was used for the non-parametric groups. Categorical variables were compared between groups using chi-square tests and presented as percentages. Receiver operator characteristics curves were used to determine the optimal maximum ST-segment depression cut point that identifies abnormal rMP by PET. A P value < 0.05 was considered statistically significant.
Results
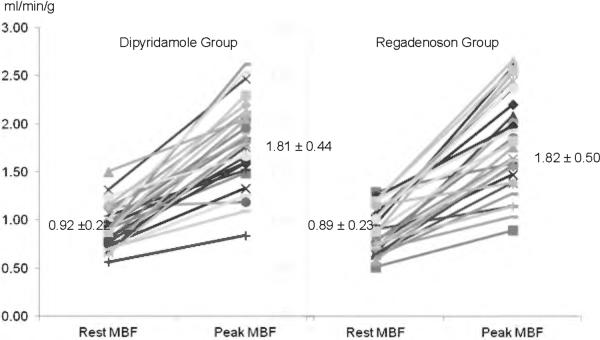
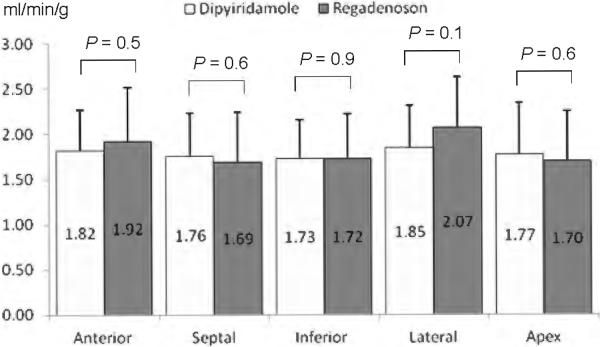
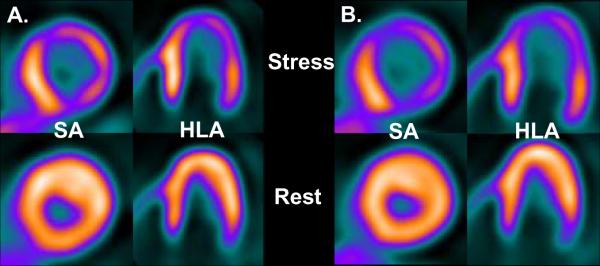
A total of 57 individuals with HC were included in this cohort: 28 were stressed with Dipyridamole (group 1) and 29 patients with Regadenoson (group 2). Baseline and echocardiographic characteristics, including maximal wall thickness, were highly comparable between both groups as shown in Table 1. Heart rate and mean arterial blood pressure were statistically similar between the groups prior to the administration of either stress agent, although there was a trend for higher RPP in the Regadenoson group (Table 2). During vasodilator stress, peak heart rate showed a trend for higher values with Regadenoson compared to Dipyridamole, but mean blood pressure dropped to a similar nadir with both agents (Table 2). From a safety profile perspective, 7 patients (26%) experienced side effects with Dipyridamole including chest tightness/pain (n=4), nausea (n=3), and hypotension (n=3) requiring Aminophylline in all occasions. In contrast, only 2 individuals (7%, p = 0.06) experienced side effects after Regadenoson administration (headache and chest pain). Aminophylline was given in one case (chest pain). The prevalence of abnormal rMP (71 vs. 83%, P = 0.3) and severity of reversible perfusion defects (SDS of 5.5 ± 5.5 vs. 5.8 ± 6.7, P = 0.8) were similar in patients undergoing Dipyridamole and Regadenoson vasodilator stress. At baseline, global MBF was similar between the Dipyridamole and Regadenoson group (Figure 1). After pharmacologic administration, the hyperemic-MBF achieved in the entire LV with Dipyridamole was similar to that obtained with Regadenoson (Figure 1). As a result, global CFR was not significantly different between HC patients stressed with Dipyridamole or Regadenoson (2.02 ± 0.53 vs. 2.12 ± 0.12; P = 0.5). After correction for baseline differences in heart rate and systolic blood pressure, the resting MBF (0.99 ± 0.26 vs. 0.88 ± 0.22 ml/min/g; P = 0.1) and CFR (1.90 ± 0.52 vs. 2.19 ± 0.74; P = 0.1) remained comparable between the Dipyridamole and Regadenoson group. Regionally, no significant differences between Dipyridamole and Regadenoson in hyperemic-MBF were observed in any myocardial wall. In the lateral wall there was a slight trend for higher peak flow values with Regadenoson (Figure 2). Figure 3 shows PET images of one HC individual who underwent pharmacologic stress with both Dipyridamole and Regadenoson 9 months apart.
Table 1.
Similar baseline characteristics of patients with hypertrophic cardiomyopathy
| Characteristic | Dipyridamole Group (n = 28) | Regadenoson Group (n = 29) | p Value |
|---|---|---|---|
| Age (years) | 51 ± 16 | 51 ± 12 | 0.9 |
| Men | 16 (57%) | 17 (59%) | 0.9 |
| Chest pain and/or dyspnea | 25 (89%) | 27 (93%) | 0.6 |
| Syncope | 4 (14%) | 4 (14%) | 0.9 |
| Hypertension | 12 (43%) | 13 (45%) | 0.9 |
| Diabetes mellitus | 3 (11%) | 2 (7%) | 0.6 |
| Beta-blockers | 25 (89%) | 26 (90%) | 0.9 |
| Calcium channel blockers | 5 (18%) | 4 (14%) | 0.7 |
| Family history of sudden cardiac death | 7 (25%) | 8 (28%) | 0.8 |
| Family history of hypertrophic cardiomyopathy | 5 (18%) | 6 (21%) | 0.8 |
| Maximal myocardial wall thickness (cm) | 2.16 ± 0.57 | 2.17 ± 0.49 | 0.9 |
| Left ventricular posterior wall (cm) | 1.20 ± 0.24 | 1.19 ± 0.30 | 0.9 |
| Left ventricular ejection fraction (%) | 60 ± 7 | 58 ± 9 | 0.4 |
| Resting outflow tract gradient (mm Hg) | 27 ± 24 | 25 ± 31 | 0.7 |
| Provoked outflow tract gradient (mm Hg) | 69 ± 53 | 47 ± 47 | 0.1 |
| Nonobstructive outflow tract gradients | 9 (32%) | 17 (58%) | |
| Obstructive outflow tract gradients | 10 (36%) | 6 (21%) | 0.1 |
| Latent outflow tract obstruction | 9 (32%) | 6 (21%) |
Data are expressed as mean ± SD or as number (percentage)
Table 2.
Baseline and stress-induced hemodynamics in patients with hypertrophic cardiomyopathy
| Characteristics | Dipyridamole Group (n = 28) | Regadenoson Group (n = 29) | p Value |
|---|---|---|---|
| Baseline heart rate (beat/minute) | 61 ± 10 | 64 ± 10 | 0.3 |
| Peak heart rate (beat/minute) | 86 ± 15 | 92 ± 14 | 0.1 |
| Heart rate difference (beat/minute) | 25 ± 13 | 28 ± 10 | 0.3 |
| Baseline systolic blood pressure (mm Hg) | 133 ± 16 | 138 ± 23 | 0.4 |
| Baseline diastolic blood pressure (mm Hg) | 67 ± 12 | 70 ± 11 | 0.4 |
| Baseline mean blood pressure (mm Hg) | 89 ± 12 | 92 ± 14 | 0.4 |
| Mean blood pressure nadir (mm Hg) | 82 ± 14 | 86 ± 14 | 0.3 |
| Mean blood pressure difference (mm Hg) | −8 ± 8 | −7 ± 7 | 0.7 |
| Baseline rate pressure product (bpm * mm Hg) | 8114 ± 1576 | 8818 ± 2019 | 0.1 |
Values are expressed as mean ± SD
Figure 1.
Baseline and vasodilator-induced hyperemic (peak) myocardial blood flow (MBF) of the entire left ventricle with Dipyridamole and Regadenoson in patients with hypertrophic cardiomyopathy were very similar
Figure 2.
Regional differences of hyperemic myocardial blood flow by wall distribution with Dipyridamole and Regadenoson in patients with hypertrophic cardiomyopathy. Only the lateral left ventricular wall showed a slight difference between the two vasodilators
Figure 3.
PET images of same individual with hypertrophic cardiomyopathy undergoing pharmacologic stress with Dipyridamole (A.) at baseline and with Regadenoson (B.) 9 months later. Please note similar distribution of regional myocardial perfusion abnormalities in the anterior, lateral and inferior walls on the two stress studies (Top row). Impaired vasodilator-induced global myocardial blood flow with Dipyridamole (1.09 ml/min/g) and Regadenoson (1.00 ml/min/g) was nearly identical on both occasions. SA = short axis, HLA = horizontal long axis
A total of 53 patients had evaluable ECGs at baseline (26 with Dipyridamole and 27 in the Regadenoson group). Patients were divided into quartiles based on the maximum vasodilator-induced ST shift as shown in Table 3. Significant ST segment depression (≥1 mm) occurred in 14 patients in a diffuse pattern (II,III, aVF and V3–5), and was observed equally with Dipyridamole and Regadenoson. Reciprocal ST segment elevation (≥ 1mm) was observed in aVR, aVL and V1. This was seen in 3 patients (11%) who received Dipyridamole and 1 subject (4%, P=0.3) stressed with Regadenoson. Using the standard cut-point of ≥ 1 mm shift, vasodilator-induced ST segment depression showed a high specificity (~92%) but low sensitivity (~34%) to predict abnormal rMP. When maximal ST segment shift during stress ECG was plotted versus abnormal rMP on a receiver operator characteristics curve, an ST segment deviation greater than 0.67 mm from baseline showed a somewhat better cut point to predict abnormal rMP (sensitivity ~ 51%, specificity ~ 92). Figure 4 illustrates the high specificity and Figure 5, the low sensitivity of ST segment shifts to predict abnormal rMP. Patients with greater ST segment deviations had greater LV outflow tract gradients, LV ejection fraction and ST-T abnormalities on ECG at baseline, and higher incidence of vasodilator-induced tachycardia, chest pain, LV systolic dysfunction and regional wall motion abnormalities, compared to patients with lesser ST segment shifts (Table 3). Maximal wall thickness, corrected MBF, stress MBF and CFR were similar among patients with varying degrees of ST shifts. Abnormal rMP showed a trend for higher incidence in patients with greater ST shifts (Table 3). Patients with vasodilator-induced chest pain (n=5) had significantly greater wall thickness (2.54 ± 1.00 vs. 2.08 ± 0.40 cm; P = 0.045) and lower corrected CFR (1.50 ± 0.44 vs. 2.13 ± 0.65; P = 0.04). All patients with vasodilator induced chest pain had abnormal rMP and wall motion abnormalities on PET; 4 out 5 patients had ST depression ≥ 1 mm on stress ECG.
Table 3.
Characteristics of patients with hypertrophic cardiomyopathy according to electrocardiographic changes in response to vasodilator stress
| Characteristic | Vasodilator-induced ST-segment deviation groups (n=53) |
p Value | |||
|---|---|---|---|---|---|
| <0.1 mm (n=12) | 0.1 – 0.4 mm (n=14) | 0.5 – 0.9 mm (n=13) | ≥ 1.0 mm (n=14) | ||
| Echocardiography | |||||
| Maximal myocardial wall thickness (cm) | 2.28 ± 0.44 | 2.09 ± 0.56 | 1.97 ± 0.36 | 2.15 ± 0.57 | 0.5 |
| Left ventricular posterior wall (cm) | 1.18 ± 0.25 | 1.16 ± 0.28 | 1.17 ± .21 | 1.18 ± .22 | 0.9 |
| Resting outflow tract gradient (mm Hg) | 11 ± 9 | 21 ± 26 | 23 ± 18 | 38 ± 32 | 0.04 |
| Provoked outflow tract gradient (mm Hg) | 52 ± 50 | 43 ± 42 | 44 ± 27 | 88 ± 67 | 0.06 |
| Nonobstructive outflow tract gradients | 6 (50%) | 8 (57%) | 5 (38.5%) | 5 (36%) | |
| Obstructive outflow tract gradients | 1 (8%) | 3 (21.5%) | 3 (23%) | 7 (50%) | 0.2 |
| Latent outflow tract obstruction | 5 (42%) | 3 (21.5%) | 5 (38.5%) | 2 (14%) | |
| Baseline Electrocardiography | |||||
| Strain pattern and/or T wave inversion | 3 (25%) | 7 (50%) | 11 (85%) | 11 (79%) | 0.007 |
| Early-repolarization changes | 3 (25%) | 3 (21%) | 1 (8%) | 1 (7%) | 0.5 |
| Any ST-T abnormality | 6 (50%) | 10 (71%) | 12 (92%) | 12 (86%) | 0.07 |
| Left ventricular hypertrophy | 3 (25%) | 7 (50%) | 7 (54%) | 8 (57%) | 0.4 |
| Hemodynamics | |||||
| Resting heart rate (beat/minute) | 58 ± 10 | 66 ± 11 | 61 ± 8 | 67 ± 8 | 0.053 |
| Resting systolic blood pressure (mm Hg) | 139 ± 25 | 130 ± 18 | 133 ± 18 | 136 ± 19 | 0.7 |
| Rate pressure product (bpm*mm Hg) | 8106 ± 2368 | 8666 ± 1907 | 8123 ± 1507 | 9045 ± 1741 | 0.5 |
| Peak heart rate (beat/minute) | 84 ± 12 | 90 ± 9 | 85 ± 11 | 101 ± 16 | 0.002 |
| Heart rate difference (beat/minute) | 26 ± 10 | 24 ± 11 | 24 ± 7 | 35 ± 13 | 0.03 |
| Regadenoson as vasodilator stress agent | 9 (75%) | 6 (43%) | 5 (38.5%) | 7 (50%) | 0.3 |
| Vasodilator-induced chest pain | 0 | 1 (7%) | 0 | 4 (29%) | 0.03 |
| Stress Electrocardiography | |||||
| Mean II-III-aVF ST segment shift (mm) | −0.00 ± 0.03 | −0.18 ± 0.13 | −0.59 ± 0.20 | −1.05 ± 0.66 | <0.0001 |
| Mean V3–V5 ST segment shift (mm) | −0.00 ± 0.02 | −0.15 ± 0.15 | −0.63 ± 0.20 | −1.58 ± 0.63 | <0.0001 |
| Maximum ST segment shift (mm) | −0.01 ± 0.01 | −0.21 ± 0.12 | −0.68 ± 0.16 | −1.60 ± 0.62 | <0.0001 |
| Positron Emission Tomography | |||||
| Summed difference score (unitless) | 3.4 ± 3.2 | 7.6 ± 9.4 | 5.0 ± 3.1 | 7.4 ± 6.1 | 0.3 |
| Abnormal regional myocardial perfusion | 8 (67%) | 9 (64%) | 12 (92%) | 13 (93%) | 0.1 |
| Rest left ventricular ejection fraction (%) | 53 ± 8 | 59 ± 9 | 61 ± 8 | 62 ± 7 | 0.04 |
| Stress ejection fraction (%) | 48 ± 12 | 51 ± 13 | 44 ± 11 | 43 ± 11 | 0.3 |
| Ejection fraction reserve (%) | −5 ± 12 | −8 ± 11 | −17 ± 12 | −19 ± 13 | 0.01 |
| Negative LVEF reserve | 7 (58%) | 10 (71%) | 11 (85%) | 14 (100%) | 0.055 |
| Stress-induced wall motion abnormalities | 3 (25%) | 8 (57%) | 10 (77%) | 12 (86%) | 0.008 |
| Rest myocardial blood flow (ml/min/g) | 0.75 ± 0.14 | 0.95 ± 0.22 | 0.98 ± 0.24 | 0.95 ± 0.22 | 0.04 |
| Stress myocardial blood flow (ml/min/g) | 1.75 ± 0.41 | 1.81 ± 0.56 | 1.92 ± 0.44 | 1.88 ± 0.42 | 0.8 |
| Coronary flow reserve (unitless) | 2.37 ± 0.49 | 1.97 ± 0.60 | 2.04 ± 0.60 | 2.02 ± 0.44 | 0.2 |
| Corrected rest myocardial flow (ml/min/g) | 0.83 ± 0.22 | 0.96 ± 0.27 | 1.05 ± 0.28 | 0.91 ± 0.20 | 0.2 |
| Corrected coronary flow reserve | 2.22 ± 0.66 | 1.99 ± 0.75 | 1.95 ± 0.65 | 2.15 ± 0.60 | 0.7 |
Data are expressed as mean ± SD or as number (percentage)
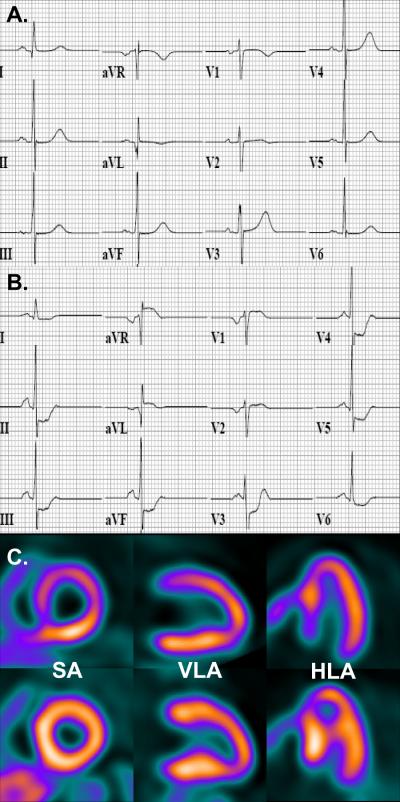
Figure 4.
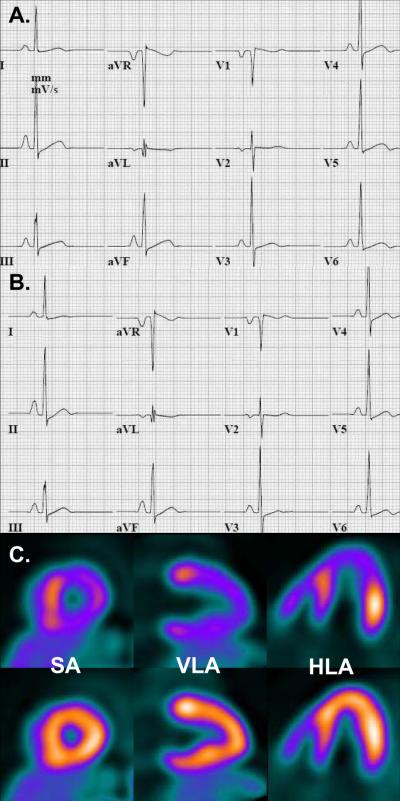
Representative ECG at baseline (A), during Dipyridamole stress (B) and accompanying PET images (C, stress images top row, rest images bottom row) in an individual with hypertrophic cardiomyopathy demonstrating Dipyridamole-induced diffuse ≥ 1 mm ST segment depression in leads II, III, aVF, V3-6, and reciprocal ST segment elevation in aVR, and less prominent in aVL and V1 (B). PET images show abnormal regional myocardial perfusion in the septum and anterior wall during pharmacologic stress (C, top row images) but normal perfusion at rest (C, bottom row images). SA = short axis, VLA = vertical long axis, HLA = horizontal long axis
Figure 5.
ECG at baseline (A), during Regadenoson stress (B) and accompanying PET images (C, stress images top row, rest images bottom row) in an individual with hypertrophic cardiomyopathy who showed extensive and severe reversible myocardial perfusion abnormalities involving the entire anterior and inferior walls (C, top row images) despite minimal ST segment shift (<0.5 mm from baseline) during pharmacologic stress (B). SA = short axis, VLA = vertical long axis, HLA = horizontal long axis
Discussion
The main results of this study are the following: 1) Regadenoson and Dipyridamole are comparable as vasodilators for stress testing in HC patients, 2) Regadenoson is better tolerated than Dipyridamole, 3) vasodilator-induced ST segment depression is specific but non-sensitive to predict abnormal rMP, and 4) chest pain elicited during vasodilator stress is likely due to myocardial ischemia in patients with HC.
We observed similar values of peak MBF and CFR following infusion of Dipyridamole or Regadenoson in a cohort of HC patients with similar baseline clinical and imaging characteristics. Predictors that could potentially affect peak MBF, such as age, maximal LV thickness, as well as co-morbidities and medications (beta-blockers and calcium channel antagonists), were similar in these 2 groups. These results are in agreement with Gourdazi et al4, who compared 82Rb-PET-derived flow measurements with Dipyridamole and Regadenoson in a group of 104 matched-patients (for clinical and hemodynamic characteristics) with normal rMP undergoing evaluation to exclude CAD4. In this study, peak MBF (2.09 ± 0.57 vs. 2.19 ± 0.64 ml/min/g; P=0.39) as well as CFR (2.75 ± 0.66 vs. 2.89 ± 0.76; p=0.31) were not significantly different between patients stressed with Dipyridamole and Regadenoson.4 In another study, Lie et al compared the coronary vasodilator effect of Adenosine and Regadenoson in 34 patients undergoing a clinically-indicated cardiac catheterization. They measured the intracoronary peak blood flow velocity with continuous Doppler ultrasonography in coronary vessels with < 50% stenosis. They reported a peak increase in coronary blood flow velocity of 3.1 ± 0.44 and 3.1 ± 0.52-fold above baseline using Adenosine (18 mcg) and Regadenoson (400 mcg) respectively.5 The presence of abnormal stress rMP on SPECT is common in patients with HC (up to 62% of patients in one series), and has been associated with an increased risk of cardiovascular death compared to patients with normal stress rMP6. We observed a high prevalence of abnormal rMP (n=44/57) in our HC cohort. The presence and severity (evaluated by SDS) of myocardial perfusion abnormalities elicited by Regadenoson and Dipyridamole were similar in our study, which is also in agreement with previous studies conducted in patients with known or suspected CAD undergoing comparative SPECT evaluations with Regadenoson and other vasodilator agents7, 8.
Regadenoson was better tolerated as reflected by fewer side effects during pharmacologic stress and elicited a higher HR response with a similar BP nadir compared to Dipyridamole, observations that have been consistently reported in other studies4,7–9. Regadenoson offers an important advantage over Adenosine and Dipyridamole whose administration as a continuous infusion not only activates adenosine A2a receptors on arteriolar smooth muscle cells (coronary vasodilatation), but also stimulates adenosine A1, A2b, and A3 receptors, which may result in undesirable side effects. In contrast, Regadenoson is a potent, low affinity A2a receptor agonist, with a significantly lower affinity for A1 adenosine receptors and very low (if any) affinity for the A2b and A3 receptors, which probably explains the lower frequency of side effects from Regadenoson10.
Vasodilator-induced ST segment depression appears to be a specific but non-sensitive tool for prediction of abnormal rMP in HC. Only 1 patient with ST depression ≥ 1 mm showed normal rMP (~92% specificity), whereas 29 patients with ST depression < 1 mm exhibited abnormal rMP (~34 % sensitivity). This is in clear agreement with a previous study by Marshall et al who also reported poor sensitivity (24%) and high specificity (91%) of adenosine-induced ST segment depression on stress ECG to predict reversible myocardial perfusion abnormalities on radionuclide scintigraphic in patients undergoing evaluation for CAD11. We found that an ST segment shift > 0.67 mm appears to improve the sensitivity of ECG changes, without affecting its specificity, to predict abnormal rMP (sensitivity ~ 51%, specificity ~ 92) in HC patients. Lazzeroti et al observed that HC individuals demonstrating ST segment depression during Dipyridamole were associated with a higher incidence of adverse cardiovascular events compared to subjects without significant ST changes during a mean follow-up of 6 years12. We found that global parameters such as maximal wall thickness, stress MBF, and CFR were similar across the different vasodilator-induced ST shift groups, although, patients with ST depression ≥ 1 mm complained of chest pain to a greater extent, showed a higher incidence of segmental wall motion abnormalities, and were more likely to have abnormal rMP compared to patients with lesser ST shifts, suggesting that myocardial ischemia elicited by pharmacologic stress can be the underlying explanation for the ST shifts seen in some of these patients. In this first regard, chest pain appears to represent true ischemic angina as evidenced by the significant lower CFR, and presence of abnormal rMP and wall motion abnormalities in all 5 patients who experienced chest pain during pharmacologic stress.
Patients with greater ST deviation demonstrated significantly higher heart rates during vasodilator stress, an observation that has been previously reported by many authors in patients undergoing pharmacologic stress with Adenosine or Dipyridamole for evaluation of CAD11, 13–15. Diastolic filling time is an important determinant of the coronary perfusion pressure and heart rate is a major determinant affecting diastole duration. It is conceivable that in HC patients, vasodilator-induced tachycardia would significantly shorten the diastolic filling time and consequently the coronary perfusion pressure especially in susceptible myocardial segments demonstrating impaired coronary vasodilator reserve (which ultimately translates into abnormal rMP), leading to flow heterogeneity between impaired and non-impaired myocardium and the development of subendocardial hypoperfusion and ST segment deviation in vulnerable segments, similar or equivalent to the “coronary steal” phenomenon observed in patients with obstructive CAD13, 16.
The major limitation of the present study is its retrospective design. We divided patients into 2 groups according to the vasodilator employed and compared their baseline characteristics, before proceeding with the study analysis. While a prospective intra-individual cross-over comparison would have been ideal for establishing the final conclusions, such a design is unlikely to be carried out in HC individuals in order to address the specific aim of our study given the high cost involved and minimal outcome gain. Additionally, despite the relatively small sample size (when compared to the CAD literature), we would like to point out that this is one of the largest series of HC patients studied with PET imaging.
Acknowledgements
The authors would like to thank Mrs. Judy Buchanan, Division of Nuclear Medicine, Johns Hopkins University, for her helpful editorial assistance. This work was partially supported by a grant from the National Institutes of Health (HL098046). Dr. Pozios is supported in part by grants from the Hellenic Cardiology Society.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Maron BJ, McKenna WJ, Danielson GK, Kappenberger LJ, Kuhn HJ, Seidman CE, Shah PM, Spencer WH, 3rd, Spirito P, Ten Cate FJ, Wigle ED. American College of Cardiology/European Society of Cardiology clinical expert consensus document on hypertrophic cardiomyopathy. A report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents and the European Society of Cardiology Committee for Practice Guidelines. J Am Coll Cardiol. 2003;42:1687–1713. doi: 10.1016/s0735-1097(03)00941-0. [DOI] [PubMed] [Google Scholar]
- 2.Hutchins GD, Schwaiger M, Rosenspire KC, Krivokapich J, Schelbert H, Kuhl DE. Noninvasive quantification of regional blood flow in the human heart using N-13 ammonia and dynamic positron emission tomographic imaging. J Am Coll Cardiol. 1990;15:1032–1042. doi: 10.1016/0735-1097(90)90237-j. [DOI] [PubMed] [Google Scholar]
- 3.Bravo PE, Pinheiro A, Higuchi T, Rischpler C, Merrill J, Santaularia-Tomas M, Abraham MR, Wahl RL, Abraham TP, Bengel FM. PET/CT assessment of symptomatic individuals with obstructive and nonobstructive hypertrophic cardiomyopathy. J Nucl Med. 2012;53:407–414. doi: 10.2967/jnumed.111.096156. [DOI] [PubMed] [Google Scholar]
- 4.Goudarzi B, Fukushima K, Bravo P, Merrill J, Bengel FM. Comparison of the myocardial blood flow response to regadenoson and dipyridamole: a quantitative analysis in patients referred for clinical 82Rb myocardial perfusion PET. Eur J Nucl Med Mol Imaging. 2011;38:1908–1916. doi: 10.1007/s00259-011-1853-6. [DOI] [PubMed] [Google Scholar]
- 5.Lieu HD, Shryock JC, von Mering GO, Gordi T, Blackburn B, Olmsted AW, Belardinelli L, Kerensky RA. Regadenoson, a selective A2A adenosine receptor agonist, causes dosedependent increases in coronary blood flow velocity in humans. J Nucl Cardiol. 2007;14:514–520. doi: 10.1016/j.nuclcard.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 6.Sorajja P, Chareonthaitawee P, Ommen SR, Miller TD, Hodge DO, Gibbons RJ. Prognostic utility of single-photon emission computed tomography in adult patients with hypertrophic cardiomyopathy. Am Heart J. 2006;151:426–435. doi: 10.1016/j.ahj.2005.02.050. [DOI] [PubMed] [Google Scholar]
- 7.Hendel RC, Bateman TM, Cerqueira MD, Iskandrian AE, Leppo JA, Blackburn B, Mahmarian JJ. Initial clinical experience with regadenoson, a novel selective A2A agonist for pharmacologic stress single-photon emission computed tomography myocardial perfusion imaging. J Am Coll Cardiol. 2005;46:2069–2075. doi: 10.1016/j.jacc.2005.05.097. [DOI] [PubMed] [Google Scholar]
- 8.Iskandrian AE, Bateman TM, Belardinelli L, Blackburn B, Cerqueira MD, Hendel RC, Lieu H, Mahmarian JJ, Olmsted A, Underwood SR, Vitola J, Wang W. Adenosine versus regadenoson comparative evaluation in myocardial perfusion imaging: results of the ADVANCE phase 3 multicenter international trial. J Nucl Cardiol. 2007;14:645–658. doi: 10.1016/j.nuclcard.2007.06.114. [DOI] [PubMed] [Google Scholar]
- 9.Cerqueira MD, Nguyen P, Staehr P, Underwood SR, Iskandrian AE. Effects of age, gender, obesity, and diabetes on the efficacy and safety of the selective A2A agonist regadenoson versus adenosine in myocardial perfusion imaging integrated ADVANCE-MPI trial results. JACC Cardiovasc Imaging. 2008;1:307–316. doi: 10.1016/j.jcmg.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Gao Z, Li Z, Baker SP, Lasley RD, Meyer S, Elzein E, Palle V, Zablocki JA, Blackburn B, Belardinelli L. Novel short-acting A2A adenosine receptor agonists for coronary vasodilation: inverse relationship between affinity and duration of action of A2A agonists. J Pharmacol Exp Ther. 2001;298:209–218. [PubMed] [Google Scholar]
- 11.Marshall ES, Raichlen JS, Tighe DA, Paul JJ, Breuninger KM, Chung EK. ST-segment depression during adenosine infusion as a predictor of myocardial ischemia. Am Heart J. 1994;127:305–311. doi: 10.1016/0002-8703(94)90117-1. [DOI] [PubMed] [Google Scholar]
- 12.Lazzeroni E, Picano E, Morozzi L, Maurizio AR, Palma G, Ceriati R, Iori E, Barilli A. Dipyridamole-induced ischemia as a prognostic marker of future adverse cardiac events in adult patients with hypertrophic cardiomyopathy. Echo Persantine Italian Cooperative (EPIC) Study Group, Subproject Hypertrophic Cardiomyopathy. Circulation. 1997;96:4268–4272. doi: 10.1161/01.cir.96.12.4268. [DOI] [PubMed] [Google Scholar]
- 13.Chambers CE, Brown KA. Dipyridamole-induced ST segment depression during thallium-201 imaging in patients with coronary artery disease: angiographic and hemodynamic determinants. J Am Coll Cardiol. 1988;12:37–41. doi: 10.1016/0735-1097(88)90353-1. [DOI] [PubMed] [Google Scholar]
- 14.Abbott BG, Afshar M, Berger AK, Wackers FJ. Prognostic significance of ischemic electrocardiographic changes during adenosine infusion in patients with normal myocardial perfusion imaging. J Nucl Cardiol. 2003;10:9–16. doi: 10.1067/mnc.2002.127625. [DOI] [PubMed] [Google Scholar]
- 15.Chow BJ, Wong JW, Yoshinaga K, Ruddy TD, Williams K, deKemp RA, DaSilva J, Beanlands RS. Prognostic significance of dipyridamole-induced ST depression in patients with normal 82Rb PET myocardial perfusion imaging. J Nucl Med. 2005;46:1095–1101. [PubMed] [Google Scholar]
- 16.Becker LC. Conditions for vasodilator-induced coronary steal in experimental myocardial ischemia. Circulation. 1978;57:1103–1110. doi: 10.1161/01.cir.57.6.1103. [DOI] [PubMed] [Google Scholar]