Abstract
Mechanisms of brain injury in intraventricular hemorrhage (IVH) of premature infants are elusive; and no therapeutic strategy exists to prevent the brain damage in these infants. Therefore, there is a need to develop an in vitro organotypic forebrain slice-culture model to advance mechanistic studies and therapeutic developments for this disorder. We cultured forebrain slices from E29 rabbit pups and treated the cultured slices (CS) with moderate (50 μl) or large (100 μl) amount of autologous blood to mimic moderate and severe IVH. Blood-induced damage to CS was evaluated for propidium-iodide staining, LDH levels, microglial density, neuronal degeneration, myelination, and gliosis over 2 weeks after the initiation of culture. CS were viable for at least 14 days in vitro (DIV). The application of blood induced significant neural cell degeneration. Degenerating cells were more abundant and LDH levels were elevated in a dose-dependent manner in CS treated with 50 or 100 μl of blood compared to untreated controls. Microglial density was higher in blood-treated CS compared to controls at both 7 and 14 days post-treatment. Myelination was reduced and gliosis enhanced in blood-treated CS. Selective application of blood fractions revealed that CS treated with plasma displayed more hypomyelination and gliosis compared to RBC-treated slices. This study developed and characterized a novel rabbit forebrain-slice culture model of IVH that exhibits neuropatholgical changes similar to human infants with IVH. Importantly, plasma appears to induce greater white matter damage than erythrocytes in IVH, indicating plasma a source of neurotoxic components.
Keywords: Intraventricular hemorrhage, Slice culture, Gliosis, forebrain, Myelin, Microglia
INTRODUCTION
Intraventricular hemorrhage (IVH) is the most common neurological disorder of premature infants that results in neurodevelopmental delay, cerebral palsy and mental retardation in the survivors (Ballabh et al. 2004). The hemorrhage typically initiates in the germinal matrix and progresses to IVH with the rupture of ventricular ependyma and subsequent leaking of blood into the lateral ventricle. The germinal matrix is located on the head of the caudate nucleus and beneath the ventricular ependyma, consisting of a highly vascularized collection of neuronal-glial precursor cells (Ballabh 2010) The development of IVH results in ependymal loss, destruction of germinal matrix, and subsequent periventricular hypomyelination and gliosis. IVH-induced brain injury is attributed to the products of erythrocyte lysis, plasma components, and pressure effect resulting from the hemorrhage (Aronowski and Zhao 2011). Erythrocyte lysis releases heme and iron, inducing oxidative stress and activating complement cascade (Hua et al. 2007), while plasma contains thrombin and complement proteins, which are neurotoxic (Fujimoto et al. 2006). No therapeutic or preventive strategy is currently available against the brain injury produced by IVH. Therefore, it would be extraordinarily useful to develop and characterize an organotypic slice culture (CS) model, in which tissue obtained from forebrain of prematurely born animal can be exposed to autologous blood to model in vitro IVH in preterm infants. Such an in vitro model system can be employed to study mechanisms of brain injury in isolation from vascular alterations, and to evaluate neuroprotective strategies as a treatment for IVH.
Use of organotypic brain CS as a tool to answer fundamental questions has become an accepted experimental model in molecular biological, immunohistochemical, electrophysiological and electron microscopic studies (Lossi et al. 2009). After about a week in culture, during which time dead cells and debris are lost as slices thin, the remaining cells are viable, receiving and sending impulses through intact axons and functional synaptic connections. By this time, slices have recovered from alterations in metabolism produced by release of enzymes, cations, and neurotransmitters during the sectioning procedure. Recent studies have shown that organotypic slices can be viable for up to 6–10 weeks in the culture medium, offering avenues to study a number of pathophysiological processes—neurogenesis, synaptogenesis, hypoxia-ischemia, plasticity and regeneration and bacterial or viral insults—and the influence of pharmacological and genetic interventions on them (Gahwiler et al. 1997; Gogolla et al. 2006; Pena). However, a slice culture model of IVH has yet to be developed and characterized.
We selected premature rabbit pups (E29, term=32d) to obtain organotypic slices because of their close resemblance to humans in a number of key aspects. First, rabbit pups have a gyrencephalic brain with abundant germinal matrix and perinatal brain growth, like humans but unlike rodents. Second, premature rabbit pups develop spontaneous germinal matrix hemorrhage at a similar frequency to humans (5–10%). Third, in our glycerol-induced rabbit model of IVH, premature pups (E29, term = 32d) with hemorrhage exhibit periventricular inflammatory changes, hypomyelination, gliosis and motor impairment, all similar to human survivors of IVH.(Ballabh et al. 2007; Chua et al. 2009; Georgiadis et al. 2008) We hypothesized that CS exposed to autologous blood would develop more extensive inflammatory changes, neuronal degeneration, hypomyelination and gliosis compared with control slices not exposed to blood. We also postulated that plasma and RBC treatment might have different effects on myelination and gliosis in CS. We found that blood treatment of CS resulted in greater cell death, microglial infiltration, hypomyelination and gliosis compared with untreated control slices. In addition, plasma-treated CS exhibited more gliosis and hypmyelination than RBC-treated CS. Hence, the present study highlights a novel in vitro organotypic CS model of IVH in premature infants.
Materials and Methods
Animals
The Institutional Animal Care and Use Committee of New York Medical College approved all experimental protocols. We obtained timed pregnant New Zealand rabbits from Charles River Laboratories, Inc (Wilmington, MA, USA). We delivered premature pups by cesarean section at 29 days of gestational age (full-term, 32 days). Pups were dried and kept in an infant incubator prewarmed to a temperature of 35°C. Pups were fed 1 mL puppy milk (Esbilac; PETAG Inc) at 2 hours of age and then approximately 2 mL every 12 hours (100 ml/kg per day). We waited for pups to stabilize for 24 hours in the extrauterine environment before preparing slice cultures, as premature infants typically develop IVH in the first 72 hours of life, but not immediately after birth.
Organotypic forebrain slice culture
Premature rabbit pups (E29) of 18–24 hours of postnatal age were briefly anesthetized using ketamine (35 mg/kg I.M.) and xylazine (5mg/kg I.M.). About 1 ml of blood was drawn from each pup by cardiac puncture, collected in an EDTA tube, and stored at 4°C. The pup was immediately decapitated; and brain was rapidly dissected and immersed in ice-cold artificial cerebrospinal fluid (NaCl 118, KCl 2.5, MgSO4 3, NaH2PO4 1.1, NaHCO3 26, CaCl2 0.5, and glucose 11 mM), bubbled with 95% oxygen and 5% carbon dioxide. Forebrain was detached from mid brain and then two cerebral hemispheres were separated using a scalpel (Supplemental Fig. 1). We next cut 300 μm coronal slices from each cerebral hemisphere at the level of mid-septal nucleus using a McIlwain chopper. We chose this level because the germinal matrix is conspicuous at this location. Slices were carefully separated in cold oxygenated aCSF and transferred onto culture inserts (Millipore, Catelog # PICM01250, 0.4 um), which were placed into six-well plates containing our slice culture medium (50% MEM, 25% HBSS, 25% heat-inactivated horse serum, 13mM HEPES, 2.5 mg/ml glucose, 320 mOsm). Slices were cultured at 33 °C in 95% air/5% CO2 for 14 days in vitro (DIV). We added either 50 (moderate IVH) or 100 μl (severe IVH) of blood (drawn from the pup before sacrifice) in the culture medium at days 1 (1–2 h post-slice cutting) and 3. The culture medium was changed at 3, 7, 9, 11, and 13 DIV.
Viability studies
Propidium iodide (PI) uptake assay
Damage to the viability of neural cells in the cultured slice was assessed by PI uptake based on a prior study (Su et al. 2011). PI penetrates only into the cells with damaged plasma and nuclear membrane and binds to nucleic acids rendering them fluorescent. Slices were incubated with 5 μg/ml PI (Sigma, USA) for 45 min. After several washes in 0.01M PBS, unfixed slices were frozen in optimum cutting compound (Sakura Finetechnical, Tokyo, Japan) and cut into 10 μm sections using a cryostat. The sections were visualized under a Confocal microscope (Nikon Instruments, Japan).
LDH assay
LDH released in the culture media was measured by a cytotoxicity assay kit (cat # G1782, Promega Corp., Madison, WI) at postnatal days 3, 7, 9, 11 and 14 as previously described (Singer et al. 1999). Briefly, the color generated in the samples upon treatment with substrate and 30 minutes of incubation was quantified by measuring wavelength absorbance at 490 nm. The values were normalized to the protein concentration of the sample.
Immunohistochemistry
The slices were fixed in 4% paraformaldehyde in PBS (0.01 M, Ph 7.4) for an hour and then were cryoprotected by immersing into 30% sucrose in PBS for 48h. The slices were then frozen into optimum cutting compound (Sakura Finetechnical, Tokyo, Japan) and coronal sections of 10 μm thickness were cut on a cryostat. The primary antibodies used in experiments included rabbit monoclonal Iba1 (Wako chemicals, Japan), mouse monoclonal GFAP (catalog #G6171, St Louis, MO, USA), rat monoclonal myelin basic protein (catalog # AB7439, Abcam, Cambridge, MA, USA ). The secondary antibodies used were Cy-3 conjugate donkey anti-mouse, cy-3 conjugate donkey anti-goat and FITC conjugate donkey anti-rat (Jackson Immunoresearch, West Grove, PA, USA). The Immunostaining was performed as in our prior study (Ballabh et al. 2007). Briefly, we hydrated the fixed sections in 0.01M PBS and incubated with the primary antibodies diluted in PBS at 4°C overnight. After washing in PBS, the sections were incubated with secondary antibody diluted in 1% normal goat serum in PBS at room temperature for 60 minutes. Finally, after washes in PBS, sections were mounted with Slow Fade Light Antifade reagent (Molecular Probes, Invitrogen, CA, USA) and were visualized under a Confocal microscope (Nikon Instruments, Japan). Cells were counted in random images by a blinded investigator. We counted objects in 25 images (3–5 slices) per brain (n = 4–5) for every parameter. To evaluate neuronal degeneration in cryosections from fixed culture slices, we performed Fluoro-Jade B (Chemicon) staining according to the manufacturer’s instruction.
Quantification of propidium iodide (+) cells, degenerating neurons and microglia
Unbiased counting was performed on sections imaged with a confocal microscope by an investigator blinded as to experimental group. We counted propidium iodide (+) cells, Fluoro Jade (+) degenerating neurons, and Iba 1(+) microglia in 4–5 cryosections by examining every other section taken from a slice culture. We counted objects in 5 images per coronal section for each parameter (4–5 images × 5 coronal section × 5–6 pups=125–150 images).
Western Blot analyses
We homogenized the frozen slice in sample buffer (3% SDS, 10% glycerol, 62.5mMol TRIS-HCL, and 100 mMDTT) using a mechanical homogenizer and boiled the samples immediately for 5 minutes. We next determined protein concentration in the sample using RC DC protein assay kit (Biorad, CA, USA) and used dilutions of BSA as the standard. Total protein samples were separated by SDS-PAGE according to the previously described method.(Braun et al. 2007) Equal amounts of protein (10–20 mcg) were loaded into 4–15% gradient precast gel (Biorad, CA, USA). The separated proteins were transferred onto polyvinylidene difluoride (PVDF) membrane by electro-transfer. The membranes were then incubated with primary antibodies--mouse monoclonal GFAP (catalog # G6171, St Louis, MO, USA) and rat monoclonal myelin basic protein (catalog # AB7439, Abcam, Cambridge, MA, USA). We detected target proteins with chemiluminescence ECL system (Amersham) by using secondary antibodies conjugated with horseradish peroxidase (Jackson immunoresearch, PA, USA). We next stripped the blots with stripping buffer (Pierce) and incubated with β actin primary antibody followed by secondary antibody and detection with chemiluminescence ECL system. As described previously, (Ballabh et al. 2007) the blots from each experiment were densitometrically analyzed using J-image. The optical density values were normalized by taking ratio the target protein and β actin.
Statistical analyses
Data are expressed as means ± standard error of the mean (S.E.M.). Two-way analysis of variance was used to compare differences in the neural cell viablity (propidium iodide staining) and LDH levels between slices treated with blood (50 or 100ul) and without blood at days 3, 7 and 14. The independent factors were postnatal age (d3 vs. d7 vs. 14) and presence of blood. To compare GFAP and MBP levels, measured by Western blot analyses, between groups, we used one way analysis of variance. All post-hoc comparisons to test for differences between means were done using Tukey multiple comparison test at the P<0.05 significance level.
RESULTS
Blood treatment reduces viability of neural cells in organotypic slice cultures of rabbit forebrain
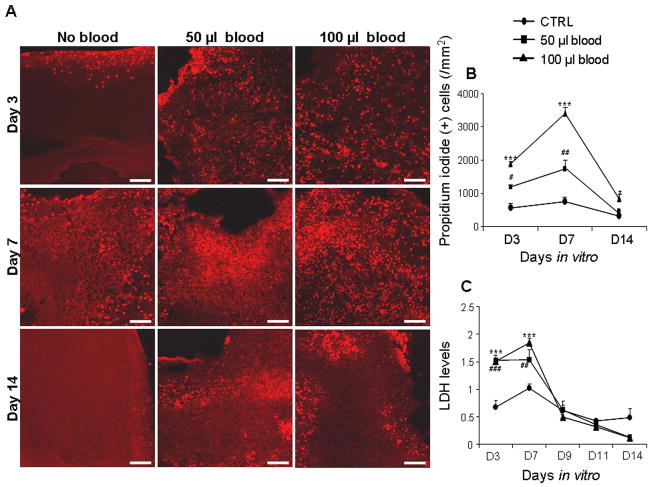
To assess the viability of untreated versus blood-treated cultured-slices (CS), we quantified cell death by counting number of propidium iodide positive cells at 3, 7, and 14 DIV, and measured LDH levels at 3, 7, 9, 11, and 14 DIV (n = 5 pups). CS treated with 50 or 100 μl blood mimicked moderate and severe IVH respectively (Fig. 1). The PI (+) cells in the CS treated with 50 or 100 μl blood were significantly larger in number at day 7 compared to both days 3 and 14 (P <0.01 both, Fig. 1A, B). The PI (+) cells in the control CS (untreated) also showed a trend for higher number at day 7 compared to days 3 and 14. This suggests that cells in the CS start to die within the first 3 days of slicing, peak at 7 DIV, and subsequently stabilize and cease to degenerate. We next compared PI (+) cells between blood-treated CS and untreated controls. We found that cell degeneration was significantly greater in CS treated with 50 or 100 μl volumes of blood compared with untreated controls at days 3 (P=0.02, 0.001) and 7 (P=0.001 both). At day 14, CS exposed to 100 μl blood exhibited more numerous PI+ cells than controls (P=0.046), but there was no difference in the cell death between 50 μl blood treated CS and controls at day 14. A comparison between 50 and 100 μl blood treated slice depicted that the density of PI (+) cells were significantly greater in CS treated with 100 μl blood compared with 50 μl blood at 3 and 7 DIV (P<0.01 and 0.001), but not at 14 DIV.
Figure 1. Blood exposure reduces the viability of neural cells in the CS.
A) Representative labeling of cryosections from CS with propidium iodide (PI) at 3, 7 and 14 DIV. Note greater abundance of PI (+) cells in CS treated with blood compared to untreated controls for 3 and 7 DIV; and higher density of PI (+) cells are at d 7 than at d 3 and 14 in blood exposed slices. Scale bar=50 μm. B) Data are mean ± s.e.m (n=4–5 pups). PI (+) cells were significantly more in CS treated with 50 and 100 μl blood compared with controls at d 3 and 7. PI (+) cells were higher in number in CS treated with 100 μl blood compared with 50 μl at 3 and 7 DIV. C) Data are mean ± s.e.m (n=6 pups each group). LDH levels were the highest at day 7 for untreated and blood treated CS. LDH levels were less in untreated CS compared to both 50 and 100 μl blood-treated CS at 3 and 7 DIV. LDH levels were higher in 100 μl blood-treated CS compared to 50 μl treated CS at day 7. *P<0.05, ***P<0.001 for the comparison between 100 μl blood exposure and controls. #P<0.05, ###P< 0.01, ###P<0.001 for the comparison between 50 μl blood exposure and controls.
Given that rabbit pups were anesthetized with ketamine before euthanization, that CS were treated with autologus blood, and that ketamine can trigger neuroapoptosis (Brambrink et al. 2012), we compared the viability of neural cells between untreated and ketamine-treated CS. We treated CS with ketamine at a concentration of 200ng/ml, based on the pharmacokinetics of ketamine evaluated in rats (Edwards and Mather 2003; Palenicek et al. 2011) and the dose of anesthetic we employed. We found no significant difference in the density of PI (+) cells in ketamine-treated slices compared to untreated controls at either 3 days (500±154vs. 573±124/mm2; Ketamine-treated and untreated slice respectively) or 7 days (768±174 vs. 660.4±151/mm2) in culture.
We next measured LDH released in the culture media of blood-treated and untreated CS as a second measure of cellular damage. Consistent with PI labeling, LDH concentration peaked at day 7 and then consistently decreased from day 9–14 in both untreated and blood-treated CS. LDH levels were higher in CS treated with either 50 or 100 μl blood compared to untreated controls at both days 3 (P=0.001 both) and 7 (P=0.01 and 0.001), but not at days 9, 11 or 14 (n=5 pups each group; Fig. 1C). LDH concentration was higher in 100 μl blood-treated CS relative to 50 μl blood treated slices at day 7, but not on other days. Taken together, blood-exposure of CS produced dose-dependent neural cell degeneration consistent with in vivo experiments in both rabbits and humans (Georgiadis et al. 2008).
Blood induces inflammation and neuronal degeneration in organotypic slice cultures of rabbit forebrain
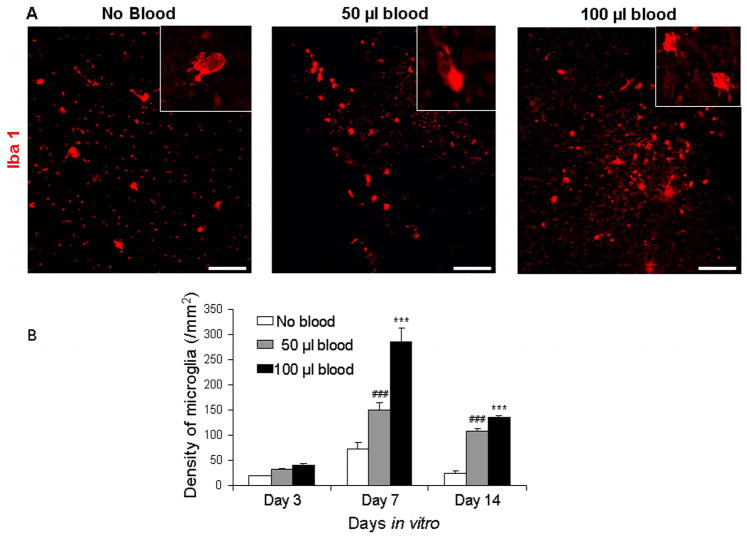
Since IVH is known to induce inflammation and neuronal degeneration in both rabbits and humans (Georgiadis et al. 2008), we assessed microglial density in immunolabeled (Iba-1) sections and neuronal degeneration in Fluoro-Jade B stained sections from the CS (n=5 pups each group). In untreated slices, the density of microglial cells was greater at 7 DIV relative to 3 and 14 DIV (P=0.01, 0.025; Fig. 2). Likewise, CS treated with 50 or 100 μl blood displayed higher microglial density at day 7 compared with day 3 (P=0.001 both) and 14 (0.029 and 0.001). We then compared between blood-treated and untreated slices. Microglial number was significantly higher in CS treated with 50 or 100 μl blood compared with untreated controls at days 7 and 14 (P=0.001 all), but not at day 3. A comparison between 50 and 100 μl blood treated slice revealed that microglia were more abundant in CS treated with 100 μl than with 50 μl blood at day 7 (P<0.01), but not at days 3 or 14. The majority of the microglial cells displayed ameboid morphology, indicating they were activated microglia.
Figure 2. Blood induces microgliosis in the CS.
A) Representative immunolabeling of cryosections from CS (day 3) with Iba1 antibody. Note abundant microglia in blood-treated CS and few in untreated slice. Inset shows microglia under high magnification. Scale bar= 50 μm. B) Data are mean ± s.e.m (n=5 pups). The density of microglial cells was more at day 7 compared with 3 and 14 DIV for 50 and 100 μl blood treated as well as untreated slices. Microglia are more abundant in CS treated with 50 and 100 μl blood compared with untreated controls at 7 and 14 DIV. ***P<0.001 for the comparison between 100 μl blood exposure and controls. ###P<0.001 for the comparison between 50 μl blood exposure and controls.
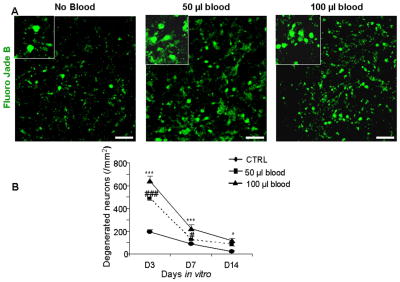
Neuronal degeneration, assessed by Fluoro Jade B labeling, peaked at 3 DIV and subsequently, declined as a function of postnatal age in both control and blood-treated slices. Fluoro Jade B positive cells were more abundant at day 3 compared with either day 7 or 14 in control (P < 0.1 and 0.001), 50 μl (P<0.001 both) or 100 μl blood treated CS (P<0.001 both, Fig. 3). Comparison between control and blood-treated CS showed that the density of degenerated neurons was higher in CS exposed to either blood dose compared with untreated controls at days 3 (P< 0.001 both) and 7 (P< 0.001, 0.02). By day 14, degenerating neurons were fewer in untreated CS compared with CS treated with 100 μl blood (P=0.023), but not in slices exposed to 50 μl. These data indicate that exposure of the CS to blood induced microgliosis and neuronal degeneration.
Figure 3. Blood treatment enhances neuronal degeneration.
A) Representative Fluoro-Jade B labeling of fixed cryosections from CS at day 3. Note abundance of degenerating neurons in CS exposed to blood and relatively few in untreated cultured slices. Scale bar, 20 μm. B) Data are mean ± s.e.m (n=5 pups). The density of degenerating neurons was higher in CS treated with 50 or 100 μl blood compared to controls at 3 and 7 DIV. At day 14, degenerating neurons were greater in number in CS exposed to 100 μl blood than controls. ***P<0.001, *P<0.05 for the comparison between 100 μl blood exposure and controls. ###P<0.001, #P<0.01 for the comparison between 50 μl blood exposure and controls.
Blood treatment of organotypic forebrain slice cultures suppresses myelination and enhances gliosis
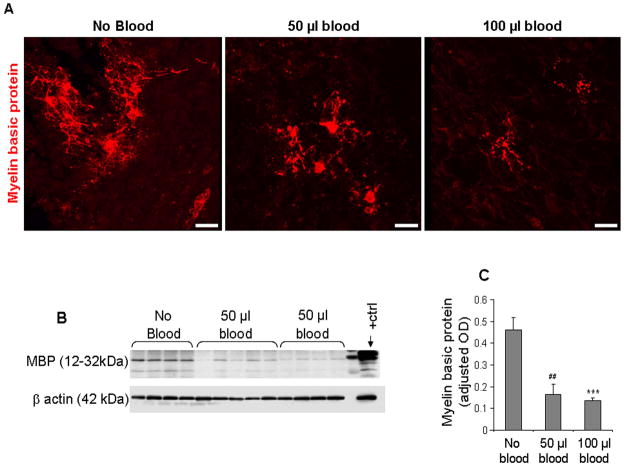
Myelination in term rabbit pups commences at day 5 (Drobyshevsky et al. 2005); and moderate-to-severe IVH induces hypomyelination of the white matter in E29 rabbit pups, which is noticeable at postnatal day 14.(Chua et al. 2009) We assessed myelination in immunolabeled sections and quantified myelin basic protein by Western blot analyses at 14 DIV in CS (n=5 pups each). Immunolabeling with myelin basic protein antibody revealed that MBP+ oligodendrocytes and focal areas of myelin were more abundant in untreated controls compared with blood-treated CS (Fig. 4A). Western blot analyses confirmed that myelination was less in CS treated with 50 μl or 100 μl blood compared to controls (P<0.01 and 0.001, Fig. 4B). However, the difference in MBP expression between 50 and 100 μl blood-treated CS was not significant.
Figure 4. Blood exposure suppresses myelination in CS.
A) Cryosections from CS were stained with myelin basic protein (MBP) antibody. Note fewer MBP (+) oligodendrocytes and less myelin in blood-treated slices compared with untreated controls at 14 DIV. Scale bar, 20 μm. B) Representative Western blot analysis of MBP in untreated and blood-treated (50 or 100 μl ) CS. Rabbit pup brain (postnatal d 14) was used as positive controls. C) The bar graph shows mean ± s.e.m. (n=4–5 pups each group). The values were normalized to β-actin levels. MBP level was higher in untreated CS compared with 50 or 100 μl blood-treated slices. ***P<0.001 for the comparison between 100 μl blood exposure and controls. ##P<0.001 for the comparison between 50 μl blood exposure and controls.
IVH, similar to other perinatal insults, induces gliosis (Chua et al. 2009). Therefore, we compared gliosis between blood-treated and untreated CS using GFAP immunolabeling and Western blot analyses. Immunohistochemistry revealed more extensive gliosis, both hypertrophic astrocytes and many processes, in blood-treated CS compared to controls (Fig. 5A). Western blot analyses confirmed higher GFAP levels in CS treated with 50 or 100 μl blood relative to controls (P=0.003, 0.02, Fig. 5B,C). The comparison between 50 and 100 μl blood treated slices was not significant. Collectively, presence of blood suppressed myelination and enhanced reactive gliosis in the CS, consistent with in vivo IVH (Chua et al. 2009).
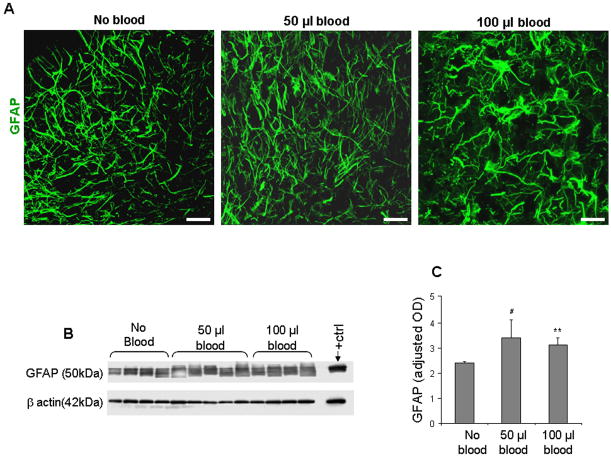
Figure 5. Blood treatment enhances gliosis in CS.
A) Representative GFAP immuno-labeling of fixed cryosections from CS at 14 DIV. Note abundant hypertrophic astrocytes with many processes in blood-treated CS while relatively few in untreated controls. Scale bar, 20 μm. B) Representative Western blot analysis of GFAP in untreated and blood-treated (50 or 100 μl) CS. Rabbit pup brain (postnatal d 14) with IVH was used as positive controls. C) The bar graph shows mean ± s.e.m. (n=4–5 pups each group). The values were normalized to β-actin levels. GFAP levels were higher in 50 or 100 μl blood-treated CS compared with untreated controls. **P<0.01 for the comparison between 100 μl blood exposure and controls. #P<0.05 for the comparison between 50 μl blood exposure and controls.
Plasma-treated CS exhibits more gliosis and less myelination than RBC-treated CS
Finally, we compared the effects of plasma to RBC on myelination and gliosis in CS. To this end, we separated plasma and RBC, and treated subsets of CS with either plasma or RBC in two volumes--50 or 100 μl. The CS were harvested at 14 DIV and myelination and gliosis were evaluated by myelin basic protein and GFAP immunohistochemistry and Western blot analyses (n=5 pups ea.). Immunolabeling showed that areas of myelin and MBP (+) oligodendrocytes were more abundant in RBC-treated relative to plasma-treated CS. Western blot analyses confirmed that MBP levels were more reduced in plasma-treated CS compared with RBC-treated slices for both 50 and 100 μl volumes (P=0.03, 0.02, Fig. 6). However, there was no significant difference in the myelination between CS-treated with 50 and 100 μl of plasma, or between CS-treated with 50 and 100 μl of RBC. GFAP immunostainining revealed more abundant reactive gliosis in plasma-treated slices than in RBC treated slices. Western blot analyses confirmed that GFAP levels were higher in CS-treated with 50 μl plasma compared to same volume of RBC. Overall, plasma treatment resulted in less myelination and more gliosis than RBC.
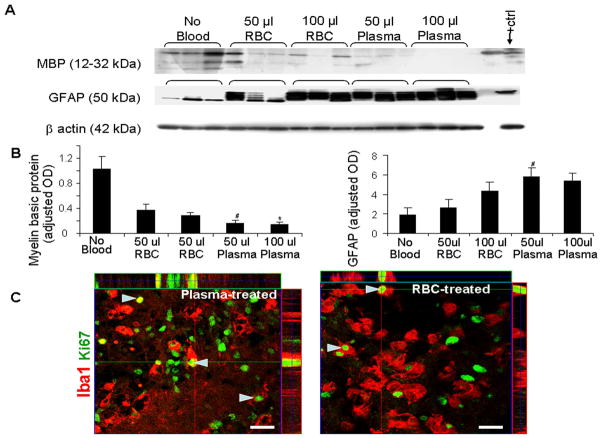
Figure 6.
Plasma induces greater hypomyelination and gliosis than RBCs. A) Representative MBP and GFAP Western blot analysis in CS treated with 50 or 100 μl blood, plasma, and no treatment. Each lane represents lysate from whole coronal slice taken at the level of midseptal nucleus of one brain. Rabbit pup brain (postnatal d 14) was used as positive control. B) The bar graph shows mean ± s.e.m. (n=5 pups each group). The values were normalized to β-actin levels. MBP levels were less in plasma-treated CS compared with RBC-treated slices for both 50 and 100 μl volumes. GFAP levels were higher in CS-treated with 50 μl plasma than CS exposed to 50 μl RBC. *P<0.05 for the comparison between 100 μl blood and 100 μl plasma exposure. #P<0.05 for the comparison between 50 μl blood and 50 μl plasma exposure. C) Representative immunofluorescence of fixed cryosections from CS at 7 DIV stained with Ki67 and Iba1 antibodies. Note Iba1 staining microglia with Ki67 signals indicating microglial proliferation (arrowhead) in both plasma and RBC treated CS. Above and right to the image are orthogonal views in x–z and y–z planes of a composite of z-stack of a series of confocal images taken 0.6 μm apart. Scale bar, 20 μm.
To determine whether the elevation in microglial density in blood-treated CS resulted from migration of macophages from the blood and/or proliferation of resident microglia, we compared the microglial density and their proliferation between plasma and RBC treated (50 μl each) CS at 7 DIV. Double immunolabeling of CS with Iba1 and Ki67 antibodies showed no significant difference in density of Iba1 (+) microglia (156 ± 3.2 vs. 148 ± 13/mm2; plasma and RBCtreated CS respectively), or percent proliferating microglial cells (9.4 ± 2.3 vs. 12.3 ± 1.4%), between plasma- and RBC-treated CS at 7 DIV. These data indicate that elevation in the density of microglia in blood-treated slices was predominantly due to their prolferation.
Since RBC induces toxicity by release of heme and subsequently, iron, we evaluated the timing of RBC hemolysis in our culture medium. Hemolysis was 5.4 ± 0.5% at 48 h and 72.5 ± 1.2% at 72 h when RBC was incubated in the culture media. Given that blood was added to the media on days 1 and 3, and that culture media was changed at days 3, 7, 9, 11 and 13, the iron was probably released predominantly after 2–3 DIV, suggesting that plasma-induced slice injury perhaps preceded the RBC-induced damage.
Discussion
The present study highlights a novel organotypic forebrain slice culture model that mimics IVH in premature infants. This preparation can be used as a tool to answer fundamental questions related to the mechanisms of brain injury and therapies to protect the developing brain of premature infants with IVH. Specifically, our experiments revealed that forebrain CS thrived well for 2 weeks, and that CS exposed to blood exhibited more extensive inflammation, cell death, gliosis and hypomyelination compared with untreated controls. In addition, plasma treatment of CS induced greater gliosis and hypomyelination than RBC.
There are a number of unique features of our culture system: 1) use of prematurely delivered rabbit pups, 2) use of the forebrain to obtain slices for culturing, and 3) addition of autologous blood to CS to mimic IVH in premature infants. We chose rabbits because of their close similarities to humans in perinatal brain growth, abundant germinal matrix, cerebral blood supply from the internal carotid and vertebral arteries, and gyrencephalic brain. To our knowledge, this is the first demonstration of an organotypic slice culture system using preterm rabbit forebrain. Conventional slice culture models use slices of hippocampus or cerebellum of postnatal rodents (Gahwiler et al. 1997; Lossi et al. 2009; Su et al. 2011). We selected the forebrain coronal slice at the level of mid-septal nucleus to culture because the germinal matrix exhibits maximum thickness at this level. IVH initiates in the germinal matrix resulting in damage to both neuronal and glial precursor cells. We anticipate that including the germinal region in the forebrain slice will allow investigators to perform developmental neuropathology studies. We added autologus blood to the slices in 50μl and 100 μl volumes to model moderate and severe IVH respectively, and the blood treatments produced similar effects in a dose dependent manner as in in vivo IVH.
There are a few limitations of the slice culture model of IVH. Injury in IVH is predominantly periventricular as brain regions around the ventricles are exposed to the adverse effects of blood and alterations of ventricular pressure. However, in intraparenchymal hemorrhage (grade 4 IVH) of premature infants, damage is observed predominantly around the site of blood accumulation. The present model system exhibited more diffuse injury because the entire CS was exposed to blood. IVH results in increased intracranial pressure, exerting a pressure effect around the blood-filled ventricle and potentially causing arterial vasospasm and cerebral ischemia (Verhagen et al. 2010). These events cannot be replicated in the present culture system, but direct effects of blood, its components and exogenous agents can be systemically evaluated in this model system to determine their neurotoxicity and test strategies of neuroprotection.
infiltration and apoptosis predominantly in the first 7 days after the initiation of hemorrhage. Subsequently, there is hypomyelination and reactive gliosis in the periventricular white matter. Similarly, our slice culture model of IVH displayed microglial activation most abundance at day 7 relative to 3 and 14 DIV. Blood treatment of CS induced neuronal degeneration, hypomyelination, and gliosis, similar to preterm rabbit pups and premature infants with IVH (Chua et al. 2009; Niwa et al. 2011). Hence, the histology of blood-exposed CS is likely a good reflection of neuropathology of IVH survivors.
that have shown to induce glutamate excitotoxicity and stimulate production of pro-inflammatory cytokines (Ramos-Mandujano et al. 2007; Veerhuis et al. 2011). RBCs release heme and iron, triggering Fenton reaction that induces oxidative damage of neurons and glia (Wu et al. 2011). In our cultures, it took about 48–72 h for erythrocytes to hemolyze and release iron. Since we added erythrocytes to the culture medium at 1 and 3 DIV, iron induced oxidative damage was likely to occur at 2–3 DIV. This suggests that thrombin and complement in plasma are likely to be the early causes of brain injury, while iron released from erythrocyte is expected to induce later oxidative damage. This indicates that iron chelation therapy for IVH can wait probably until day 3, but therapy to antagonize the effects of thrombin and complement should be initiated immediately after the onset of IVH.
In conclusion, this study describes a novel forebrain-slice culture model of IVH of premature infants, which exihibits neuropatholgical changes similar to those of human infants and rabbit pups with IVH. Our experiments also found that plasma induced greater white matter damage than erythrocytes. This model can be used to study the mechanisms of brain injury and faciliate development of therapeutic strategies to minimize brain damage after IVH.
Supplementary Material
Forebrain was detached from mid brain (1) and two cerebral hemisphers were seperated using a scalpel (2). Coronal slices of 300 μm thickness was cut from each cerebral hemisphere at the level of mid-septal nucleus using a McIlwain chopper (3).
Acknowledgments
Source of funding: NIH/NINDS grant RO1 NS071263 (PB), Scientist development grant from American Heart Association (GV).
Authors thank Joanne Abrahams for the assistance with images.
References
- Aronowski J, Zhao X. Molecular pathophysiology of cerebral hemorrhage: secondary brain injury. Stroke. 2011;42(6):1781–1786. doi: 10.1161/STROKEAHA.110.596718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballabh P. Intraventricular hemorrhage in premature infants: mechanism of disease. Pediatr Res. 2010;67(1):1–8. doi: 10.1203/PDR.0b013e3181c1b176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004;16(1):1–13. doi: 10.1016/j.nbd.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Ballabh P, Xu H, Hu F, Braun A, Smith K, Rivera A, Lou N, Ungvari Z, Goldman SA, Csiszar A, Nedergaard M. Angiogenic inhibition reduces germinal matrix hemorrhage. Nat Med. 2007;13(4):477–485. doi: 10.1038/nm1558. [DOI] [PubMed] [Google Scholar]
- Brambrink AM, Evers AS, Avidan MS, Farber NB, Smith DJ, Martin LD, Dissen GA, Creeley CE, Olney JW. Ketamine-induced neuroapoptosis in the fetal and neonatal rhesus macaque brain. Anesthesiology. 2012;116(2):372–384. doi: 10.1097/ALN.0b013e318242b2cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun A, Xu H, Hu F, Kocherlakota P, Siegel D, Chander P, Ungvari Z, Csiszar A, Nedergaard M, Ballabh P. Paucity of pericytes in germinal matrix vasculature of premature infants. J Neurosci. 2007;27(44):12012–12024. doi: 10.1523/JNEUROSCI.3281-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua CO, Chahboune H, Braun A, Dummula K, Chua CE, Yu J, Ungvari Z, Sherbany AA, Hyder F, Ballabh P. Consequences of intraventricular hemorrhage in a rabbit pup model. Stroke. 2009;40(10):3369–3377. doi: 10.1161/STROKEAHA.109.549212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drobyshevsky A, Song SK, Gamkrelidze G, Wyrwicz AM, Derrick M, Meng F, Li L, Ji X, Trommer B, Beardsley DJ, Luo NL, Back SA, Tan S. Developmental changes in diffusion anisotropy coincide with immature oligodendrocyte progression and maturation of compound action potential. J Neurosci. 2005;25(25):5988–5997. doi: 10.1523/JNEUROSCI.4983-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards SR, Mather LE. Alfentanil potentiates anaesthetic and electroencephalographic responses to ketamine in the rat. Eur J Pharmacol. 2003;460(1):27–35. doi: 10.1016/s0014-2999(02)02827-3. [DOI] [PubMed] [Google Scholar]
- Fujimoto S, Katsuki H, Kume T, Akaike A. Thrombin-induced delayed injury involves multiple and distinct signaling pathways in the cerebral cortex and the striatum in organotypic slice cultures. Neurobiol Dis. 2006;22(1):130–142. doi: 10.1016/j.nbd.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Gahwiler BH, Capogna M, Debanne D, McKinney RA, Thompson SM. Organotypic slice cultures: a technique has come of age. Trends Neurosci. 1997;20(10):471–477. doi: 10.1016/s0166-2236(97)01122-3. [DOI] [PubMed] [Google Scholar]
- Georgiadis P, Xu H, Chua C, Hu F, Collins L, Huynh C, Lagamma EF, Ballabh P. Characterization of acute brain injuries and neurobehavioral profiles in a rabbit model of germinal matrix hemorrhage. Stroke. 2008;39(12):3378–3388. doi: 10.1161/STROKEAHA.107.510883. [DOI] [PubMed] [Google Scholar]
- Gogolla N, Galimberti I, DePaola V, Caroni P. Preparation of organotypic hippocampal slice cultures for long-term live imaging. Nat Protoc. 2006;1(3):1165–1171. doi: 10.1038/nprot.2006.168. [DOI] [PubMed] [Google Scholar]
- Hua Y, Keep RF, Hoff JT, Xi G. Brain injury after intracerebral hemorrhage: the role of thrombin and iron. Stroke. 2007;38(2 Suppl):759–762. doi: 10.1161/01.STR.0000247868.97078.10. [DOI] [PubMed] [Google Scholar]
- Lossi L, Alasia S, Salio C, Merighi A. Cell death and proliferation in acute slices and organotypic cultures of mammalian CNS. Prog Neurobiol. 2009;88(4):221–245. doi: 10.1016/j.pneurobio.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Niwa T, de Vries LS, Benders MJ, Takahara T, Nikkels PG, Groenendaal F. Punctate white matter lesions in infants: new insights using susceptibility-weighted imaging. Neuroradiology. 2011;53(9):669–679. doi: 10.1007/s00234-011-0872-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palenicek T, Fujakova M, Brunovsky M, Balikova M, Horacek J, Gorman I, Tyls F, Tislerova B, Sos P, Bubenikova-Valesova V, Hoschl C, Krajca V. Electroencephalographic spectral and coherence analysis of ketamine in rats: correlation with behavioral effects and pharmacokinetics. Neuropsychobiology. 2011;63(4):202–218. doi: 10.1159/000321803. [DOI] [PubMed] [Google Scholar]
- Pena F. Organotypic cultures as tool to test long-term effects of chemicals on the nervous system. Curr Med Chem. 2010;17(10):987–1001. doi: 10.2174/092986710790820679. [DOI] [PubMed] [Google Scholar]
- Ramos-Mandujano G, Vazquez-Juarez E, Hernandez-Benitez R, Pasantes-Morales H. Thrombin potently enhances swelling-sensitive glutamate efflux from cultured astrocytes. Glia. 2007;55(9):917–925. doi: 10.1002/glia.20513. [DOI] [PubMed] [Google Scholar]
- Singer CA, Figueroa-Masot XA, Batchelor RH, Dorsa DM. The mitogen-activated protein kinase pathway mediates estrogen neuroprotection after glutamate toxicity in primary cortical neurons. J Neurosci. 1999;19(7):2455–2463. doi: 10.1523/JNEUROSCI.19-07-02455.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su T, Paradiso B, Long YS, Liao WP, Simonato M. Evaluation of cell damage in organotypic hippocampal slice culture from adult mouse: a potential model system to study neuroprotection. Brain Res. 2011;1385:68–76. doi: 10.1016/j.brainres.2011.01.115. [DOI] [PubMed] [Google Scholar]
- Veerhuis R, Nielsen HM, Tenner AJ. Complement in the brain. Mol Immunol. 2011;48(14):1592–1603. doi: 10.1016/j.molimm.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhagen EA, Ter Horst HJ, Keating P, Martijn A, Van Braeckel KN, Bos AF. Cerebral oxygenation in preterm infants with germinal matrix-intraventricular hemorrhages. Stroke. 2010;41(12):2901–2907. doi: 10.1161/STROKEAHA.110.597229. [DOI] [PubMed] [Google Scholar]
- Wu H, Wu T, Xu X, Wang J. Iron toxicity in mice with collagenase-induced intracerebral hemorrhage. J Cereb Blood Flow Metab. 2011;31(5):1243–1250. doi: 10.1038/jcbfm.2010.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Forebrain was detached from mid brain (1) and two cerebral hemisphers were seperated using a scalpel (2). Coronal slices of 300 μm thickness was cut from each cerebral hemisphere at the level of mid-septal nucleus using a McIlwain chopper (3).