Abstract
The mechanisms involved in the pathology of chronic chagasic cardiomyopathy are still debated, and the controversy has interfered with the development of new treatments and vaccines. Because of the potential of DNA vaccines for immunotherapy of chronic and infectious diseases, we tested if DNA vaccines could control an ongoing Trypanosoma cruzi infection. BALB/c mice were infected with a lethal dose (5 × 104 parasites) as a model of acute infection, and then they were treated with two injections of 100 μg of plasmid DNA 1 week apart, beginning on day 5 postinfection. Control mice had high levels of parasitemia and mortality and severe cardiac inflammation, while mice treated with plasmid DNA encoding trypomastigote surface antigen 1 or Tc24 had reduced parasitemia and mild cardiac inflammation and >70% survived the infection. The efficacy of the immunotherapy also was significant when it was delayed until days 10 and 15 after infection. Parasitological analysis of cardiac tissue of surviving mice indicated that most mice still contained detectable parasite kinetoplast DNA but fewer mice contained live parasites, suggesting that there was efficient but not complete parasite elimination. DNA vaccine immunotherapy was also evaluated in CD1 mice infected with a low dose (5 × 102 parasites) as a model of chronic infection. Immunotherapy was initiated on day 70 postinfection and resulted in improved survival and reduced cardiac tissue inflammation. These results suggest that DNA vaccines have strong potential for the immunotherapy of T. cruzi infection and may provide new alternatives for the control of Chagas' disease.
Chagas' disease, or American trypanosomiasis, is caused by the protozoan parasite Trypanosoma cruzi and has a widespread distribution in the Americas. An estimated 16 million to 18 million persons are infected, and close to 100 million people are at risk of infection. Transmission to humans occurs primarily through blood-sucking reduvid bugs, which deposit infective feces on the skin when they are feeding, but it may also occur through blood transfusion (30, 51).
The disease is characterized by an initial acute phase during which flagellated parasites (trypomastigotes) multiply in the blood, followed by an indeterminate phase with very low parasitemia and no apparent pathology. Susceptible hosts then enter a chronic phase with increasing tissue damage, mostly in cardiac and skeletal muscle tissues but sometimes also in the liver, spleen, colon, or esophagus, that progressively leads to cardiac failure and death (2, 5, 36). There is still considerable debate about the mechanisms involved in the pathology (14, 19, 44); some evidence indicates that damage is associated with the presence and replication of intracellular amastigotes in host tissues, while other studies have shown that autoimmunity induced by parasite antigens mimicking host proteins is responsible for tissue damage. Indeed, standard histopathological analysis of tissues from chronically infected hosts shows that there are abundant inflammatory cells but few or no amastigotes nests, suggesting that the pathology may develop in an almost complete absence of parasites. These observations have been associated with identification of several cross-reacting antibodies and T cells to support the autoimmune hypothesis (15, 24). However, more sensitive parasite detection techniques have confirmed that parasites persist in tissues and correlated this persistence with tissue damage (31, 32). Furthermore, it is well accepted that a T helper 1 (Th1) type of immune response is required for clearing the parasite from infected mice (18, 28, 33, 38, 42, 49), while such a response has also been associated with the intensity of the pathology during the chronic phase in humans (1, 16). All this controversy has interfered substantially with the development of immunotherapy and preventive vaccines as there is no clear agreement concerning whether the immune response should be stimulated (to eliminate the parasite) or inhibited (to avoid autoimmunity).
Nonetheless, several studies have focused on characterization of parasite antigens which may be used as vaccine candidates. Some recombinant or purified antigens, such as paraflagellar rod proteins, trans-sialidase, trypomastigote excretory-secretory antigens (including Tc24), glycoprotein 82, and trypomastigote surface antigen 1 (TSA-1), have shown promise because they induce significant protection in some mouse models (6, 28, 38, 52). More recently, DNA vaccines encoding some of these antigens have also been tested. Because DNA vaccines have a strong Th1 bias in the immune response which they induce (39), they appear to be particularly promising for use against parasites such as T. cruzi. Indeed, immunization of mice with a DNA vaccine encoding trans-sialidase (8, 34, 37), TSA-1 (13, 50), ASP-1 and ASP-2 (13), or KMP11-HSP70 fusion protein (35) leads to a Th1-type immune response that is protective in the case of TSA-1, ASP-1, ASP-2, or KMP11-HSP70. Similarly, immunization with a plasmid encoding the complement regulatory protein, but not the recombinant protein, can protect against challenge (40). These data support the idea that using DNA vaccines may be a better strategy than using recombinant vaccines against T. cruzi.
A very attractive feature of DNA vaccines is that they may also be used for immunotherapy once an infection has been initiated. This therapeutic potential has been observed against various pathogens, including human immunodeficiency virus (7), rabies virus (26), Mycoplasma pulmonis (23), Mycobacterium tuberculosis (27), Leishmania major (17), and Schistosoma mansoni (11). These studies demonstrated that administration of a DNA vaccine (alone or in combination with additional drug therapy in the case of rabies virus and Schistosoma) could clear or greatly reduce an ongoing infection by one of these pathogens.
In the case of T. cruzi, drug therapy relies on nitrofurans (such as nifurtimox, now discontinued) or nitroimidazoles (such as benznidazole). However, these drugs have little efficacy (limited to early stages of the infection), and they have serious side effects, so that efforts are needed to identify new treatments (20, 41, 47). Indeed, Chagas' disease is one of the most neglected diseases in terms of development of new drugs (45). Thus, we investigated whether DNA vaccine immunotherapy could be effective in controlling an ongoing T. cruzi infection in mice. We tested DNA plasmids encoding T. cruzi antigens TSA-1 and Tc24, which had previously been shown to be highly immunogenic and to provide good protection as prophylactic DNA and recombinant protein vaccines, respectively (43, 50). We report here the first data showing that DNA vaccine immunotherapy may be a promising strategy for control of T. cruzi infection.
MATERIALS AND METHODS
Experimental infection of mice.
T. cruzi strains H1 (low virulence) and H4 (high virulence), previously isolated from human cases in Yucatán, Mexico (4), and maintained in the laboratory by monthly passages in CD1 mice, were used for infection. Two distinct experimental models of infection were used in this study. First, we used BALB/c mice as a model of acute lethal infection. Four- to six-week-old BALB/c mice were infected by intraperitoneal injection of a lethal dose, 5 × 104 blood trypomastigotes (strain H4). The development of infection was monitored by counting peripheral blood parasites every 3 days with a Neubauer cell, and mortality was recorded daily. Surviving mice were sacrificed on days 45 to 50 postinfection (end of the acute phase) or on day 140 postinfection (chronic phase) to evaluate short- and long-term effects. Spleen, liver, heart, and skeletal muscle tissues were removed and processed for histopathological analysis and T. cruzi parasite detection (see below).
Second, as a model of chronically infected mice, we used 4- to 6-week-old CD1 mice that were infected intraperitoneally with a low dose, 5 × 102 blood trypomastigotes (strain H1). The infection was monitored as described above for up to 140 days, when surviving mice were sacrificed. Spleen, liver, heart, and skeletal muscle tissues were removed and processed for histopathological analysis and T. cruzi parasite detection.
The experimental protocol was approved by the Bioethics Committee of the Universidad Autónoma de Yucatán, and all animal experiments were carried out according to the established guidelines. All mice were bred and cared for at the animal facilities of our research center.
Plasmid construction.
The cDNA encoding T. cruzi TSA-1 (kindly provided by J. Manning, Molecular Biology and Biochemistry, University of California, Irvine) was digested with BamHI and EcoRI to generate a 1,900-bp fragment corresponding to the 5′ region (nucleotides 1 to 1851) (50) and was subcloned into pcDNA3 (Invitrogen, Carlsbad, Calif.) by using standard molecular biology protocols, yielding pcDNA3-TSA1. Tc24 DNA was amplified by PCR from T. cruzi DNA by using forward primer 5′-CTATGGTACCGAGTCAAGGAGAGCAACGAG-3′ and reverse primer 5′-GGCGAATTCACGTCACTCTGCGGCCGCGAGCT-3′. PCR products were digested with BamHI and KpnI and also cloned into pcDNA3, yielding pcDNA3-Tc24. Plasmids were checked by extensive restriction digestion and sequencing prior to injection. Plasmid DNA was prepared by using a Bio-Rad Maxi-Prep kit (Bio-Rad, Hercules, Calif.) and was stored at −20°C until it was used.
Immunotherapy of infected mice.
Immunotherapy with DNA vaccines was tested in both the acute and chronic infection models described above. For treatment of an acute lethal infection, BALB/c mice were anesthetized with penthobarbital on day 5 postinfection and treated by intramuscular injection in the tibialis anterioris of 100 μg of plasmid DNA encoding TSA-1 or Tc24 in a saline solution (0.9% NaCl) (23, 47, 48). One week later (day 12 postinfection), a second dose of plasmid DNA was administered similarly. Control groups of mice received 100 μg of empty pcDNA3 plasmid or 100 μl of the saline solution. In additional experiments, initiation of plasmid DNA treatment was delayed until 10 and 15 days postinfection.
For treatment during the chronic phase, CD1 mice also received two doses of plasmid DNA, 1 week apart, as described above. However, plasmid DNA treatment was initiated on day 70 postinfection, about 30 days after the end of the acute phase, as assessed by monitoring the parasitemia.
Histopathological analysis.
Spleen, liver, heart, and skeletal muscle tissues were removed from sacrificed animals for histopathological analysis. Samples were fixed in 10% formaldehyde and included in paraffin. Five-micrometer sections were then stained with hematoxylin and eosin, and at least five sections per tissue per mouse were examined for the presence of acute and/or chronic inflammation and parasites. Inflammation was identified as mild, moderate, or severe, and amastigote nests were identified as undetectable, rare, scattered, or abundant.
T. cruzi detection and recovery.
We tested for the presence of T. cruzi kinetoplast DNA (kDNA) in blood and cardiac tissue by PCR by using the TC1 and TC2 primers (9). Recovery of live T. cruzi parasites from cardiac tissue of treated mice was also examined by inoculating naïve BALB/c mice with 200-μl portions of tissue homogenates and monitoring the appearance of circulating parasites in peripheral blood for up to 1 month.
RESULTS
Efficacy of DNA vaccine immunotherapy administered during the acute phase.
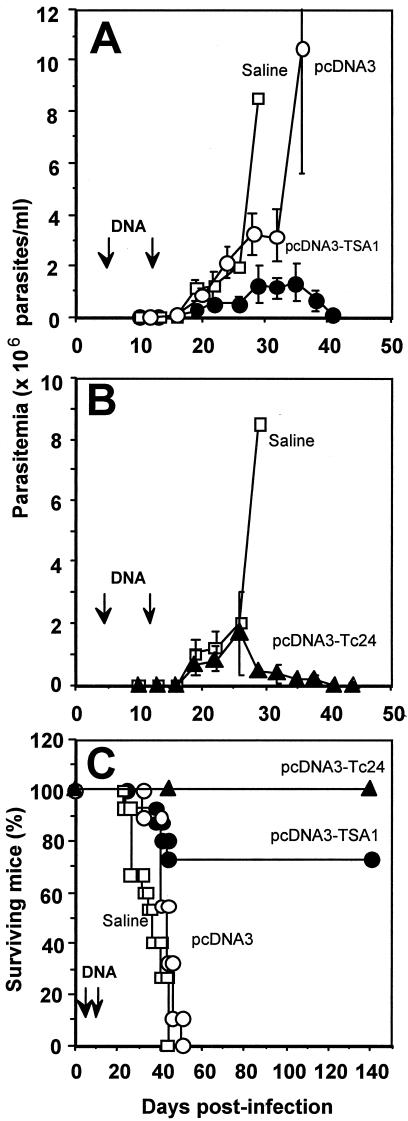
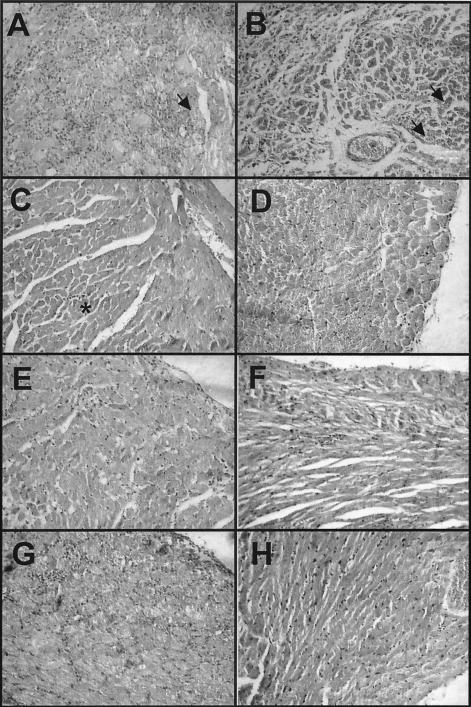
We initially administered DNA vaccines encoding TSA-1 and Tc24 antigens during the acute phase of infection in highly susceptible BALB/c mice and evaluated the efficacy this immunotherapy by monitoring the parasitemia and mortality of infected mice. As expected, mice that were inoculated with a lethal dose, 5 × 104 trypomastigotes, and received only the saline solution rapidly developed a high level of parasitemia (Fig. 1A), and all died by day 45 postinfection (Fig. 1C). Similarly, mice that were treated with the empty plasmid pcDNA3 also developed a high level of parasitemia, albeit slightly delayed, and all died by day 50 (Fig. 1A and C). In contrast, mice that were treated with two injections of plasmid DNA encoding TSA-1, on days 5 and 12 postinfection, had a lower level of parasitemia, and circulating parasites became undetectable by direct microscopic examination by day 42 (Fig. 1A). In addition, more than 70% of the pcDNA3-TSA1-treated mice survived the acute phase of the infection (Fig. 1C). Immunotherapy with the plasmid encoding Tc24 also controlled the infection, as indicated by the lower level of parasitemia and the survival of 100% of the mice treated with this plasmid (Fig. 1B and C). Histopathological analysis of cardiac tissue at the end of the acute phase (days 40 to 50 postinfection) indicated that control mice that received the saline solution or the empty plasmid had extensive inflammation with diffuse infiltration of mononuclear cells (Fig. 2A and B). Some necrosis and fibrosis were observed, and scattered to abundant T. cruzi amastigote nests were detected. On the other hand, mice treated with plasmid DNA encoding TSA-1 had only mild focal inflammation and tissue damage (Fig. 2C), and only rare amastigote nests were detected.
FIG. 1.
Parasitemia and survival of BALB/c mice infected with a lethal dose of T. cruzi and treated with DNA vaccines. Mice were infected with 5 × 104 T. cruzi parasites (strain H4) and treated on days 5 and 12 (arrows) with intramuscular injections of 100 μg of plasmid DNA encoding TSA-1 (•) or Tc24 (▴), control pcDNA3 plasmid (○), or a saline solution (□). (A and B) Infection monitored by measuring parasitemia. The values are means ± standard errors of the means for 3 to 15 mice per group. (C) Survival of infected and treated BALB/c mice.
FIG. 2.
Histopathological analysis of cardiac tissue from BALB/c mice infected with a lethal dose of T. cruzi and treated with DNA vaccines. Cardiac tissue was examined on days 40 to 50 postinfection (A, B, C, E, and G) or on day 140 postinfection (D, F, and H). Infected mice treated with a saline solution (A) or the control pcDNA3 plasmid (B) died by days 40 to 45 and had extensive inflammatory infiltrates, scattered to abundant amastigote nests (arrows), and some fibrosis and necrosis. Mice treated with 100 μg of DNA vaccine encoding TSA-1 on days 5 and 12 (C) had only mild focal inflammation (asterisk). When treatment was delayed and administered on days 10 and 17 postinfection (E) or on days 15 and 22 postinfection (G), the inflammation level appeared to be intermediate between that of treated mice and that of untreated mice. On day 140 after infection, BALB/c mice treated with TSA-1 DNA on days 5 and 12 (D) or on days 10 and 17 (F), as well as mice treated with Tc24 DNA (H), all had very mild and diffuse inflammatory infiltrates, and amastigote nests were undetectable except in one mouse.
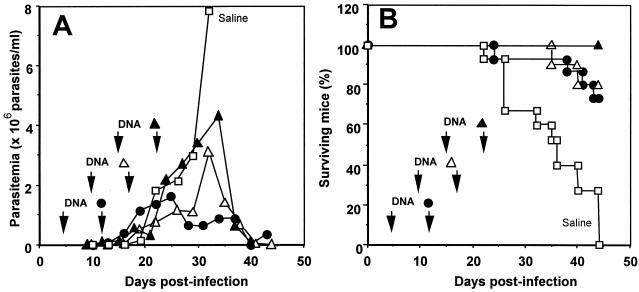
We then tested whether the timing of plasmid DNA administration was crucial for efficacy of the treatment. We thus delayed initiation of the immunotherapy until 10 and 15 days postinfection (Fig. 3). This delay in injection of plasmid pcDNA3-TSA1 resulted in progressively higher levels of parasitemia (Fig. 3A). Nonetheless, the parasitemia remained controlled and eventually became undetectable, as it did when treatment was initiated on day 5 postinfection. Accordingly, 70 to 100% of the treated mice survived the acute phase of infection (Fig. 3B). Histopathological analysis also showed that there was a reduction in tissue damage on day 50 postinfection even when treatment was delayed, although the infiltrates of inflammatory cells appeared to be more abundant and diffuse than those observed in mice treated on day 5 postinfection (Fig. 2E and G). Taken together, these data indicate that immunotherapy with a DNA vaccine encoding TSA-1 or Tc24 can induce short-term (up to 50 days) control of a lethal T. cruzi infection in BALB/c mice.
FIG. 3.
Parasitemia and survival of BALB/c mice infected with a lethal dose of T. cruzi and treated with DNA vaccines at different times. Mice were infected with 5 × 104 T. cruzi parasites (strain H4), and then they were treated on days 5 and 12 (•), on days 10 and 17 (▵), or on days 15 and 22 (▴) postinfection with intramuscular injections of 100 μg of plasmid DNA encoding TSA-1 or they were treated with a saline solution (□). (A) Infection monitored by measuring parasitemia. The values are means for 5 to 15 mice per group. (B) Survival of infected and treated BALB/c mice.
Because of the complex nature of T. cruzi pathology, such short-term control of the acute phase may not be sufficient to prevent development of the more damaging chronic phase of the disease. We thus investigated the long-term effects of DNA vaccine immunotherapy by evaluating cardiac tissue damage and T. cruzi persistence in treated mice at 140 days postinfection. As circulating blood parasites are difficult to detect by microscopic examination at this stage, we used PCR to test for the presence of the parasite. We did not detect T. cruzi kDNA in the blood of treated mice at this stage. Histopathological analysis of cardiac tissue revealed mild and diffuse inflammatory infiltrates even when treatment had been delayed, and rare amastigote nests were present in only one mouse (Fig. 2D, F, and H). However, PCR analyses of the cardiac biopsies revealed that all mice treated with plasmid DNA encoding TSA-1 (on days 5 and 12 or on days 10 and 17) were positive for T. cruzi kDNA, whereas only one-third of the mice treated with Tc24 showed evidence of T. cruzi kDNA (χ2 = 6.19; P = 0.04) (Table 1). Finally, we evaluated the presence of live infective parasites in the hearts of the treated mice by inoculating heart tissue homogenates from these animals into naïve BALB/c mice and monitoring the parasitemia. When mice were treated with the TSA-1 plasmid on days 5 and 12 postinfection, only one-third of the animals still had live parasites in the cardiac tissues on day 140. When treatment was initiated 10 days postinfection, three-quarters of the mice had live parasites (Table 1). All of the mice treated with plasmid DNA encoding Tc24 appeared to be free of live T. cruzi parasites, as naïve mice injected with heart homogenates from these mice remained uninfected (Table 1). However, the differences were not statistically significant (χ2 = 5.14; P = 0.07). Taken together, these data indicate that DNA vaccine immunotherapy during the acute phase had long-term effects that reduced the development of chronic chagasic cardiomyopathy in treated mice.
TABLE 1.
Parasitological study on day 140 after infection of BALB/c mice treated during the acute phase of the infection
| Treatment | Time of treatment (days postinfection) | Parasite DNA in blooda | Inflammationb | Amastigote nestsc | Parasite DNA in heart tissued | Live parasites isolated from hearte |
|---|---|---|---|---|---|---|
| pcDNA3-TSA1 | 5 | 0/3 | + | − to + | 3/3 | 1/3 |
| 10 | 0/4 | ++ | − to + | 4/4 | 3/4 | |
| pcDNA3-Tc24 | 5 | 0/3 | + | − | 1/3 | 0/3 |
| pcDNA3f | 5 | NDg | +++ | + to +++ | ND | ND |
| Salinef | 5 | ND | +++ | + to +++ | ND | ND |
Number of mice with blood positive for T. cruzi as determined by PCR/number of mice analyzed.
+, mild; ++, moderate; +++, severe.
Histological detection of amastigote nests in heart tissue sections. −, undetectable; +, rare; ++, scattered; +++, abundant.
Number of mice with T. cruzi kDNA in heart tissue as determined by PCR/number of mice analyzed (χ2 = 6.19; P = 0.04).
Number of mice from which live T. cruzi parasites were isolated from heart tissue/number of mice analyzed (χ2 = 5.14; P = 0.07).
Tissues were obtained from the mice on day 45 postinfection.
ND, not done.
Efficacy of DNA vaccine immunotherapy administered during the chronic phase.
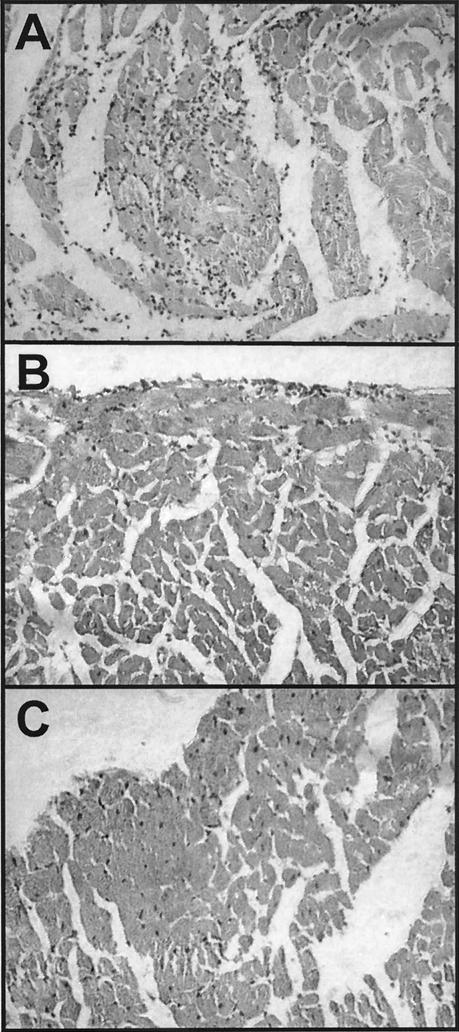
We then tested the efficacy of DNA vaccine immunotherapy during the chronic phase of infection. We used CD1 mice that were infected with a low dose of parasites, which allowed more than 75% of these mice to survive the acute phase of the infection (data not shown). Surviving mice then received plasmid DNA encoding TSA-1 or a saline treatment on day 70 postinfection and were monitored up to day 140 postinfection. Both groups of mice had no circulating parasites in their blood at this stage, as assessed by PCR (Table 2). All seven mice that received the pcDNA3-TSA1 vaccine treatment survived up to day 140, whereas only four (66%) of the six pcDNA3-treated control mice and three (42%) of the seven saline solution-treated control mice survived (χ2 = 7.23; P = 0.027). In addition, histopathological analysis indicated that control mice that received the saline solution or pcDNA3 had extensive diffuse inflammation and chronic myocarditis (Table 2 and Fig. 4A). On the other hand, mice treated with the DNA vaccine encoding TSA-1 had mild focal inflammation (Table 2 and Fig. 4B). As expected, we did not observe amastigote nests in cardiac tissue sections from either group, confirming the low sensitivity of the standard technique for histopathologic detection of parasites. However, we detected T. cruzi kDNA and live parasites in cardiac biopsies of all treated and control mice (Table 2). Taken together, these data suggest that DNA vaccine immunotherapy during the chronic phase may reduce but not eliminate T. cruzi parasites and thus limit progression of chronic chagasic cardiomyopathy.
TABLE 2.
Parasitological study on day 140 postinfection of CD1 mice treated during the chronic phase of the infection
| Treatment | Time of treatment (days postinfection) | Survivala | Parasite DNA in bloodb | Inflammationc | Amastigote nestsd | Parasite DNA in heart tissuee | Live parasites isolated from heartf |
|---|---|---|---|---|---|---|---|
| pcDNA3-TSA1 | 70 | 7/7 | 0/7 | + | − | 7/7 | 7/7 |
| pcDNA3 | 70 | 4/6 | 0/4 | ++ | − | 4/4 | NDg |
| Saline | 70 | 3/7 | 0/3 | +++ | − | 3/3 | 3/3 |
Number of mice surviving on day 140/number of mice studied (χ2 = 7.23; P = 0.027).
Number of mice with blood positive for T. cruzi as determined by PCR/number of mice analyzed.
+, mild; ++, moderate; +++, severe.
Histopathological detection of amastigote nests in heart tissue sections. −, undetectable.
Number of mice with detectable T. cruzi DNA in heart tissue/number of mice analyzed.
Number of mice from which live T. cruzi parasites were isolated from heart tissue/number of mice analyzed.
ND, not done.
FIG. 4.
Histopathological analysis of cardiac tissue from CD1 mice infected with a low dose of T. cruzi and treated with DNA vaccines. (A and B) Mice were infected with 5 × 102 T. cruzi parasites, treated with an intramuscular injection of a saline solution (A) or with 100 μg of DNA vaccine encoding TSA-1 (B) during the chronic phase on days 70 and 77 postinfection, and sacrificed on day 140 postinfection. The cardiac tissue of control mice had extensive inflammatory infiltrates, while that of DNA-treated mice had only mild diffuse inflammation. (C) Uninfected control CD1 mice.
DISCUSSION
DNA vaccines have been described as a powerful alternative for development of preventive vaccines against a wide variety of infectious and chronic diseases (25). Interestingly, they also have been shown to have potential for immunotherapy against several pathogens (7, 11, 17, 23, 26, 27). Given the lack of effective therapeutic drugs for use against T. cruzi, we investigated the efficacy of DNA vaccine immunotherapy against T. cruzi infection.
We used DNA vaccines encoding T. cruzi antigens TSA-1 and Tc24, because both of these antigens were reported to be highly immunogenic and protective in previous vaccine studies (43, 50). In addition, TSA-1 has been shown to induce significant protection as a preventive DNA vaccine (13, 50). To our knowledge, this is the first report which clearly demonstrates that intramuscular injection of only two doses of plasmid DNA encoding TSA-1 or Tc24 during the acute phase of infection is sufficient to induce control of an otherwise lethal T. cruzi infection. Indeed, such treatments dramatically reduced the parasitemia and cardiac tissue damage and resulted in increased survival of the infected animals. Most noticeable was the ability of DNA vaccine immunotherapy to reduce the levels of parasites to levels below PCR- or infection-detectable levels in some of the treated mice in which we were unable to detect parasite DNA or live parasites in cardiac biopsies. The efficacy of the treatment remained significant when its administration was delayed. However, the mice treated later during the acute phase had slightly higher levels of parasitemia and more extensive inflammatory damage than the mice treated soon after infection. Thus, when the immunotherapy was administered to chronically infected mice (70 days postinfection), we found that it was too late to eliminate the parasites. Nonetheless, treatment during the chronic phase remained sufficiently efficient to reduce tissue damage and chronic disease progression, and it significantly improved survival. Thus, the DNA vaccine immunotherapy was effective and beneficial when it was administered at any stage of infection.
The mechanisms of action of our DNA vaccine immunotherapy are not known, but our results are consistent with possible reorientation and/or potentiation of the immune response from a nonprotective Th2 response to a protective Th1 response (21). Indeed, it is accepted that DNA vaccines have a significant Th1 bias (10, 22, 39), and a DNA vaccine encoding TSA-1 has been shown to induce strong CD8+ responses (50). Also, reorientation of the immune response has been observed in the case of DNA vaccine therapy against Leishmania or Mycobacterium infections (17, 27). A shift in the immune response may have to occur early enough in the infection to eliminate T. cruzi and avoid dissemination or establishment in host target tissues, which may explain the lower efficacy of the treatment when it is delayed. Understanding these immune mechanisms and their kinetics should provide important clues for improving and optimizing the efficacy of this therapy, and studies to do this are under way.
An additional important observation is that our results support the idea that parasite persistence (3) rather that autoimmunity (12) may cause chronic inflammation and tissue damage and thus disease. Thus, stimulation of the immune response, and probably stimulation of a Th1-type response, did not lead to exacerbation of the disease. This contradicts the results of previous studies of chagasic patients which suggested that chronic chagasic cardiomyopathy is associated with a Th1 response (1, 16), but it is in agreement with other suggestions that control of T. cruzi infection should be aimed at parasite elimination through drug therapy or stimulation of the immune response (46). Quantification of parasites following immunotherapy should clarify this point and establish a possible correlation between parasite load and inflammatory damage.
In conclusion, we showed here that DNA vaccine immunotherapy can effectively control T. cruzi infection and significantly reduce the progression of chronic chagasic cardiomyopathy in mice. The efficacy of this strategy appears to be superior to or possibly comparable to the efficacy of chemotherapy, and the strategy has the major advantage of requiring only two doses, whereas drug treatments rely on daily doses for a prolonged time (29, 47). The treatment described here may thus represent a promising alternative for Chagas' disease therapy, alone or in combination with other drug treatments.
Acknowledgments
This work was funded by grant 30621-M from the Consejo Nacional de Ciencia y Tecnología (CONACYT).
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Abel, L. C., L. V. Rizzo, B. Ianni, F. Albuquerque, F. Bacal, D. Carrara, E. A. Bocchi, H. C. Teixeira, C. Mady, J. Kalil, and E. Cunha-Neto. 2001. Chronic Chagas' disease cardiomyopathy patients display an increased IFN-gamma response to Trypanosoma cruzi infection. J. Autoimmun. 17:99-107. [DOI] [PubMed] [Google Scholar]
- 2.Andrade, Z. A. 1991. Pathogenesis of Chagas' disease. Res. Immunol. 142:126-129. [DOI] [PubMed] [Google Scholar]
- 3.Anez, N., H. Carrasco, H. Parada, G. Crisante, A. Rojas, C. Fuenmayor, N. Gonzalez, G. Percoco, R. Borges, P. Guevara, and J. L. Ramirez. 1999. Myocardial parasite persistence in chronic chagasic patients. Am. J. Trop. Med. Hyg. 60:726-732. [DOI] [PubMed] [Google Scholar]
- 4.Barrera-Perez, M. A., M. E. Rodriguez-Felix, E. Guzman-Marin, J. Zavala-Velázquez, and E. Dumonteil. 2001. Biological behaviour of three strains of Trypanosoma cruzi from Yucatan, Mexico. Rev. Biomed. 12:224-231. [Google Scholar]
- 5.Bestetti, R. B., and G. Muccillo. 1997. Clinical course of Chagas' heart disease: a comparison with dilated cardiomyopathy. Int. J. Cardiol. 60:187-193. [DOI] [PubMed] [Google Scholar]
- 6.Biragyn, A., K. Tani, M. C. Grimm, S. Weeks, and L. W. Kwak. 1999. Genetic fusion of chemokines to a self tumor antigen induces protective, T-cell dependent antitumor immunity. Nat. Biotechnol. 17:253-258. [DOI] [PubMed] [Google Scholar]
- 7.Boyer, J. D., K. E. Ugen, M. Chattergoon, B. Wang, A. Shah, M. Agadjanyan, M. L. Bagarazzi, A. Javadian, R. Carrano, L. Coney, W. V. Williams, and D. B. Weiner. 1997. DNA vaccination as anti-human immunodeficiency virus immunotherapy in infected chimpanzees. J. Infect. Dis. 176:1501-1509. [DOI] [PubMed] [Google Scholar]
- 8.Costa, F., G. Franchin, V. L. Pereira-Chioccola, M. Ribeirao, S. Schenkman, and M. M. Rodrigues. 1998. Immunization with a plasmid DNA containing the gene of trans-sialidase reduces Trypanosoma cruzi infection in mice. Vaccine 16:768-774. [DOI] [PubMed] [Google Scholar]
- 9.Dorn, P. L., D. Engelke, A. Rodas, R. Rosales, S. Melgar, B. Brahney, J. Flores, and C. Monroy. 1999. Utility of the polymerase chain reaction in detection of Trypanosoma cruzi in Guatemalan Chagas' disease vectors. Am. J. Trop. Med. Hyg. 60:740-745. [DOI] [PubMed] [Google Scholar]
- 10.Dumonteil, E. 2001. Vacunas de DNA: el presente y el futuro. Rev. Biomed. 11(Suppl. 1):S7-S12. [Google Scholar]
- 11.Dupre, M. Herv, A. M. Schacht, A. Capron, and G. Riveau. 1999. Control of schistosomiasis pathology by combination of Sm28GST DNA immunization and praziquantel treatment. J. Infect. Dis. 180:454-463. [DOI] [PubMed] [Google Scholar]
- 12.Engman, D. M., and J. S. Leon. 2002. Pathogenesis of Chagas heart disease: role of autoimmunity. Acta Trop. 81:123-132. [DOI] [PubMed] [Google Scholar]
- 13.Garg, N., and R. L. Tarleton. 2002. Genetic immunization elicits antigen-specific protective immune responses and decreases disease severity in Trypanosoma cruzi infection. Infect. Immun. 70:5547-5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Girones, N., and M. Fresno. 2003. Etiology of Chagas disease myocarditis: autoimmunity, parasite persistence, or both? Trends Parasitol. 19:19-22. [DOI] [PubMed] [Google Scholar]
- 15.Girones, N., C. I. Rodriguez, E. Carrasco-Marin, R. F. Hernaez, J. L. de Rego, and M. Fresno. 2001. Dominant T- and B-cell epitopes in an autoantigen linked to Chagas' disease. J. Clin. Investig. 107:985-993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gomes, J. A., L. M. Bahia-Oliveira, M. O. Rocha, O. A. Martins-Filho, G. Gazzinelli, and R. Correa-Oliveira. 2003. Evidence that development of severe cardiomyopathy in human Chagas' disease is due to a Th1-specific immune response. Infect. Immun. 71:1185-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Handman, E., A. H. Noormohammadi, J. M. Curtis, T. Baldwin, and A. Sjolander. 2000. Therapy of murine cutaneous leishmaniasis by DNA vaccination. Vaccine 18:3011-3017. [DOI] [PubMed] [Google Scholar]
- 18.Hoft, D. F., and C. S. Eickhoff. 2002. Type 1 immunity provides optimal protection against both mucosal and systemic Trypanosoma cruzi challenges. Infect. Immun. 70:6715-6725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kierszenbaum, F. 1999. Chagas' disease and the autoimmunity hypothesis. Clin. Microbiol. Rev. 12:210-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kinnamon, K. E., B. T. Poon, W. H. Hanson, and V. B. Waits. 1998. Activity of anticancer compounds against Trypanosoma cruzi-infected mice. Am. J. Trop. Med. Hyg. 58:804-806. [DOI] [PubMed] [Google Scholar]
- 21.Kumar, S., and R. L. Tarleton. 2001. Antigen-specific Th1 but not Th2 cells provide protection from lethal Trypanosoma cruzi infection in mice. J. Immunol. 166:4596-4603. [DOI] [PubMed] [Google Scholar]
- 22.Lai, W. C., and M. Bennett. 1998. DNA vaccines. Crit. Rev. Immunol. 18:449-484. [DOI] [PubMed] [Google Scholar]
- 23.Lai, W. C., S. P. Pakes, K. Ren, Y.-S. Lu, and M. Bennett. 1996. Therapeutic effect of DNA immunization of genetically susceptible mice infected with virulent Mycoplasma pulmonis. J. Immunol. 158:2513-2516. [PubMed] [Google Scholar]
- 24.Leon, J. S., L. M. Godsel, K. Wang, and D. M. Engman. 2001. Cardiac myosin autoimmunity in acute Chagas' heart disease. Infect. Immun. 69:5643-5649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu, M. A. 2003. DNA vaccines: a review. J. Int. Med. 253:402-410. [DOI] [PubMed] [Google Scholar]
- 26.Lodmell, D. L., and L. C. Ewalt. 2001. Post-exposure DNA vaccination protects mice against rabies virus. Vaccine 19:2468-2473. [DOI] [PubMed] [Google Scholar]
- 27.Lowrie, D. B., R. E. Tascon, V. L. D. Bonato, V. M. F. Lima, L. H. Faccioli, E. Stravropoulos, M. J. Colston, R. G. Hewinson, K. Moelling, and C. L. Silva. 1999. Therapy of tuberculosis in mice by DNA vaccination. Nature 400:269-271. [DOI] [PubMed] [Google Scholar]
- 28.Miller, M. J., R. A. Wrightsman, and J. E. Manning. 1996. Trypanosoma cruzi: protective immunity in mice immunized with paraflagellar rod proteins is associated with a T-helper type 1 response. Exp. Parasitol. 84:156-167. [DOI] [PubMed] [Google Scholar]
- 29.Molina, J., J. Urbina, R. Gref, Z. Brener, and J. M. Rodrigues, Jr. 2001. Cure of experimental Chagas' disease by the bis-triazole DO870 incorporated into ‘stealth' polyethyleneglycol-polylactide nanospheres. J. Antimicrob. Chemother. 47:101-104. [DOI] [PubMed] [Google Scholar]
- 30.Moncayo, A. 1997. Progress towards the elimination of transmission of Chagas disease in Latin America. World Health Stat. Q. 50:195-198. [PubMed] [Google Scholar]
- 31.Monteon-Padilla, V., N. Hernandez-Becerril, M. A. Ballinas-Verdugo, A. Aranda-Fraustro, and P. A. Reyes. 2001. Persistence of Trypanosoma cruzi in chronic chagasic cardiopathy patients. Arch. Med. Res. 32:39-43. [DOI] [PubMed] [Google Scholar]
- 32.Mortara, R. A., S. Silva, and N. N. Taniwaki. 2000. Confocal fluorescence microscopy: a powerful tool in the study of Chagas' disease. Rev. Soc. Bras. Med. Trop. 33:79-82. [DOI] [PubMed] [Google Scholar]
- 33.Paiva, C. N., M. T. Castelo-Branco, J. Lannes-Vieira, and C. R. Gattass. 1999. Trypanosoma cruzi: protective response of vaccinated mice is mediated by CD8+ cells, prevents signs of polyclonal T lymphocyte activation, and allows restoration of a resting immune state after challenge. Exp. Parasitol. 91:7-19. [DOI] [PubMed] [Google Scholar]
- 34.Pereira-Chioccola, V. L., F. Costa, M. Ribeirao, I. S. Soares, F. Arena, S. Schenkman, and M. M. Rodrigues. 1999. Comparison of antibody and protective immune responses against Trypanosoma cruzi infection elicited by immunization with a parasite antigen delivered as naked DNA or recombinant protein. Parasite Immunol. 21:103-110. [DOI] [PubMed] [Google Scholar]
- 35.Planelles, L., M. C. Thomas, C. Alonso, and M. C. Lopez. 2001. DNA immunization with Trypanosoma cruzi HSP70 fused to the KMP11 protein elicits a cytotoxic and humoral immune response against the antigen and leads to protection. Infect. Immun. 69:6558-6563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rassi, A., Jr., A. Rassi, and W. C. Little. 2000. Chagas' heart disease. Clin. Cardiol. 23:883-889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodrigues, M. M., M. Ribeirao, V. Pereira-Chioccola, L. Renia, and F. Costa. 1999. Predominance of CD4 Th1 and CD8 Tc1 cells revealed by characterization of the cellular immune response generated by immunization with a DNA vaccine containing a Trypanosoma cruzi gene. Infect. Immun. 67:3855-3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Santori, F. R., G. S. Paranhos Bacalla, D. A. S. J. Franco, L. M. Yamauchi, J. E. Araya, and N. Yoshida. 1996. A recombinant protein based on the Trypanosoma cruzi metacyclic trypomastigote 82-kilodalton antigen that induces an effective immune response to acute infection. Infect. Immun. 64:1093-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seder, R. A., and A. V. S. Hill. 2000. Vaccine against intracellular infections requiring cellular immunity. Nature 406:793-798. [DOI] [PubMed] [Google Scholar]
- 40.Sepulveda, P., M. Hontebeyrie, P. Liegeard, A. Mascilli, and K. A. Norris. 2000. DNA-based immunization with Trypanosoma cruzi complement regulatory protein elicits complement lytic antibodies and confers protection against Trypanosoma cruzi infection. Infect. Immun. 68:4986-4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sepulveda-Boza, S., and B. K. Cassels. 1996. Plant metabolites active against Trypanosoma cruzi. Planta Med. 62:98-105. [DOI] [PubMed] [Google Scholar]
- 42.Silva, J. S., G. N. Vespa, M. A. Cardoso, J. C. Aliberti, and F. Q. Cunha. 1995. Tumor necrosis factor alpha mediates resistance to Trypanosoma cruzi infection in mice by inducing nitric oxide production in infected gamma interferon-activated macrophages. Infect. Immun. 63:4862-4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taibi, A., B. Plumas-Marty, A. Guevara-Espinoza, R. Schöneck, H. Pessoa, M. Loyens, R. Piras, T. Aguirre, H. Gras-Masse, M. Bossus, A. Tartar, A. Capron, and A. Ouassi. 1993. Trypanosoma cruzi: immunity induced in mice and rats by trypomastigote excretory-secretory antigens and identification of a peptide sequence containing a T cell epitope with protective activity. J. Immunol. 151:2676-2689. [PubMed] [Google Scholar]
- 44.Tarleton, R. L., and L. Zhang. 1999. Chagas disease etiology: autoimmunity or parasite persistence? Parasitol. Today 15:94-99. [DOI] [PubMed] [Google Scholar]
- 45.Trouiller, P., P. Olliaro, E. Torreele, J. Orbinski, R. Laing, and N. Ford. 2002. Drug development for neglected diseases: a deficient market and a public health failure. Lancet 359:2188-2191. [DOI] [PubMed] [Google Scholar]
- 46.Urbina, J. A. 1999. Parasitological cure of Chagas disease: is it possible? Is it relevant? Mem. Inst. Oswaldo Cruz Rio de J. 94:349-355. [DOI] [PubMed] [Google Scholar]
- 47.Urbina, J. A., G. Payares, J. Molina, C. Sanoja, A. Liando, K. Lazardi, M. M. Piras, R. Piras, N. Perz, P. Wincker, and J. F. Ryley. 1996. Cure of short- and long-term experimental Chagas' disease using D0870. Science 273:969-971. [DOI] [PubMed] [Google Scholar]
- 48.Urbina, J. A., G. Payares, C. Sanoja, J. Molina, R. Lira, Z. Brener, and A. J. Romanha. 2003. Parasitological cure of acute and chronic experimental Chagas disease using the long-acting experimental triazole TAK-187. Activity against drug-resistant Trypanosoma cruzi strains. Int. J. Antimicrob. Agents 21:39-48. [DOI] [PubMed] [Google Scholar]
- 49.Vespa, G. N., F. Q. Cunha, and J. S. Silva. 1994. Nitric oxide is involved in control of Trypanosoma cruzi-induced parasitemia and directly kills the parasite in vitro. Infect. Immun. 62:5177-5182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wizel, B., N. Garg, and R. L. Tarleton. 1998. Vaccination with trypomastigote surface antigen 1-encoding plasmid DNA confers protection against lethal Trypanosoma cruzi infection. Infect. Immun. 66:5073-5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.World Health Organization. 1998. Chagas disease. Interruption of transmission Wkly. Epidemiol. Rec. 73:1-4. [PubMed] [Google Scholar]
- 52.Wrightsman, R. A., B. D. Dawson, D. L. Fouts, and J. E. Manning. 1994. Identification of immunodominant epitopes in Trypanosoma cruzi trypomastigote surface antigen-1 protein that mask protective epitopes. J. Immunol. 153:3148-3154. [PubMed] [Google Scholar]