Abstract
We reported previously studies in an in situ perfused swine preparation demonstrating that endotoxemia induced lung injury required the presence of the liver and that the response was accompanied by oxidative stress. To determine whether lung and liver mitochondrial oxidative stress was important to the response, we compared the effects of equimolar amounts of two antioxidants, n-acetylcysteine, which does not replenish mitochondrial glutathione, and procysteine which does, on endotoxemia induced lung injury in the swine preparation. In a swine perfused liver-lung preparation, we measured physiologic, biochemical and cellular responses of liver and lung to endotoxemia with and without the drugs. Endotoxemia caused oxidation of the mitochondria-specific protein, thioredoxin-2, in both the lungs and the liver. Procysteine reduced thioredoxin-2 oxidation, attenuated hemodynamic, gas exchange, hepatocellular dysfunction, and cytokine responses and prevented lung edema. n-acetylcysteine had more modest effects and did not prevent lung edema. Conclusions: We conclude that mitochondrial oxidation may be critical to the pathogenesis of endotoxemia-induced liver-dependent lung injury and that choices of antioxidant therapy for such conditions must consider the desired subcellular target in order to be optimally effective.
Keywords: sepsis, oxidant stress, mitochondria
1. INTRODUCTION
Several clinical and experimental studies implicate oxidative stress in the pathophysiology of acute lung injury [1,2,3]. In some experimental preparations, antioxidants attenuate lung injury, but clinical trials of antioxidants as treatment for acute lung injury severe enough to meet criteria for the diagnosis of the acute respiratory distress syndrome (ARDS) have had mixed results [4,5].
As a pathologic mechanism of disease, oxidative stress has most often been considered a global phenomenon, disrupting all cellular targets equally and indiscriminately. However, there is increasing evidence that such a concept is an oversimplification [6,7,8]. It appears that redox signaling is important in many physiologic processes [7], and that there is both physiologic and anatomic compartmentalization of redox phenomena [6,7,8]. Understanding the consequences of compartmentalized alterations in redox state and rationales for antioxidant therapy need to take these factors into account.
We reported previously studies in an in situ perfused swine preparation demonstrating that endotoxemia induced lung injury was a consequence of interacting responses of the liver and the lungs and that the response was accompanied by evidence of global oxidant stress [9]. Several experimental forms of endotoxin induced tissue injury appear to involve mitochondrial injury as a basic pathogenetic event [10,11,12].
We hypothesized that mitochondrial oxidative stress in the lungs and the liver was critical in the pathogenesis of liver dependent endotoxin induced acute lung injury. To address that hypothesis, we compared the effects of equimolar amounts of two antioxidants, n-acetylcysteine (NAC), which does not replenish mitochondrial glutathione, and procysteine (PRO; L-2-oxothiazolidine-4-carboxylate) which does [13], on endotoxemia induced lung injury in the swine preparation.
2. METHODS
2.1 Experimental model
We used an in-situ perfused piglet preparation similar to the model used in our previous study [9] which permits perfusion of either the lung alone or the lung and the liver in series. A detailed description of the preparation and a diagram are in the earlier publication [9]. We used healthy young pigs (Palmetto Farm, Reevesville, SC) of either sex weighing 8-12 kg. Our experiments adhered to the guidelines of National Institute of Health for use of experimental animals and were approved by the animal care and use committee of Emory University. Animals were maintained in the Emory University Division of Animal Resources, an AAALAC approved facility.
Piglets were anesthetized, a tracheostomy was performed, and cannulae were placed in a carotid artery and a jugular vein. After heparinization, the animals were exsanguinated by drawing blood through the carotid artery with simultaneous infusion of equal volume of normal saline through the jugular vein. The blood was used to fill the warm water-jacketed blood reservoir and the perfusion circuit. The chest and the abdomen were then opened and the hepatic artery was isolated and ligated. The in-flow and the out-flow cannulae to the liver were placed in portal vein and inferior vena cava respectively. The in-flow and out-flow perfusion cannulae to the lungs were placed in the pulmonary artery and left atrium respectively. The cannulae were then connected to the extracorporeal perfusion circuit containing blood at 40°C. The lungs were perfused at a constant flow rate of 40ml/kg/min (up to 400ml/min), while the liver was perfused at 200ml/minute. Lung in-flow pressure was not allowed to rise above 40cmH20 by including an overflow circuit that reduced flow to the lung when in-flow pressure exceeded that level. The lungs were ventilated using a piston ventilator (Harvard Apparatus, Dover, MA) with room air and 5% CO2 . The minute ventilation was adjusted to maintain perfusate pH between 7.35 and 7.45. Throughout the experiments, pressures in the pulmonary artery, left atrium and portal vein and flow in and out of both organs was monitored continuously and the data were stored in a computer for later analysis.
2.2 Experimental Protocols and Data Collection
The experimental groups were as follows: lung-liver perfusion with endotoxin and procysteine (PRO+endo), lung-liver perfusion with endotoxin and n-acetylcysteine (NAC+endo), lung-liver perfusion with endotoxin but without either drug (endo), and lung-liver perfusion without either endotoxin or drugs (control).
For endotoxin groups, 5μg/kg body weight of Escherichia coli endotoxin (Serotype O55:B5 Difco, Detroit, MI), dissolved in normal saline was introduced into the blood reservoir after a stable baseline period. For the groups that received PRO (L-2-oxothiazolidine-4-carboxylate; MW=147.5, Clintec Technologies Inc, Chicago, IL) a loading dose of 50 mg/kg was given an hour before endotoxin followed by continuous infusion of 50mg/kg/hour for three hours. Groups receiving NAC, followed the same protocol as the PRO group except that equimolar NAC (MW=163.9, Sigma-Aldrich, St. Louis, MO), loading dose 55mg/kg followed by a 55mg/kg infusion for 3 hours was substituted for PRO.
Perfusate blood samples were collected twice before endotoxin administration (thirty minutes before and immediately before), and at three time points thereafter (thirty minutes, one hour, and two hours after endotoxin). Automated cell count and differential counts were performed using a device which is specifically designed for use in laboratory animals (Hemevet, CDC Technologies, Oxford, CT). Blood gas analysis was performed using a portable blood gas analyzer (Rapidlab 248, Diamond Diagnostics, East Walpole, MA). For pH values outside the range of 7.35-7.45, adjustment was made either by changing the ventilation rate or by administration of sodium bicarbonate. Serum samples were separated and stored at −80° for measurement of TNF-α, and interleukin-6 (IL-6).
At each time point, we recorded pressures and flows in and out of the lungs and flow to the liver. Pulmonary vascular resistance (PVR) was calculated as in-flow pressure (pulmonary artery pressure) minus out-flow pressures (left atrial pressure) divided by total blood flow through the lungs, and expressed in cmH2O/ml/min. Perfusate temperature was measured continuously and kept between 38 and 40°C by adjusting the temperature of the water in the water-jacket surrounding the reservoir.
2.3 Cytokine Measurements
Plasma concentrations of TNFα and IL-6 were measured separately using Quantikine® ELISA kits (R&D Systems, Minneapolis, MN), according to the manufacturer’s instructions. Briefly, samples were dispensed into 96-well micro-titer plates that are precoated with porcine monoclonal or polyclonal antibodies specific to each of the above cytokines. After washing away any unbound substances, an enzyme (horseradish peroxidase)-linked monoclonal (TNF-α) or polyclonal (IL-6) antibody specific to the above cytokines was added to the wells. Following a wash to remove any unbound antibody-enzyme reagent, a substrate solution (hydrogen peroxide/ tetramethylbenzidine) was added to the wells. The reaction was terminated with a stop buffer and absorbance read at 450 nm using an MRX Revelation (Dynex Technologies, Chantilly, VA) multi-well plate reader. Values of IL-6 and TNF-α were determined by reference to a standard curve constructed using the porcine proteins and computer software capable of generating a four parameter logistic curve-fit.
2.4 Measurement of Mitochondrial Thioredoxin-2 (Trx2)
Analysis of Trx2 is slightly modified from the original description of the method as described by Damdimopolous et al. [14]. Tissue proteins were collected by acid precipitation using 10% trichloric acetic acid. Samples were incubated for 60 min at 4°C, centrifuged, washed with acetone for 30 min at 4°C, centrifuged, resuspended in 20 mM Tris, pH 8.0, containing 15 mM 4-acetoamido-4-maleimidylstilbene-2,2-disulfonic acid (AMS; Molecular Probes) and incubated for 3 h at room temperature. Separation of the oxidized (lower band) and reduced (upper band) thioredoxin-2 (Trx2) within a single sample was performed by nonreducing, SDS polyacrylamide (15%) gel electrophoresis. Proteins were electroblotted onto nitrocellulose membranes. Trx2 was detected with a rabbit Trx2 primary antibody and an IR 800 goat anti-rabbit secondary antibody (Rockland Immunochemicals, Inc., Gilbertsville, PA). Membranes were scanned using the Odyssey Scanning System (Li-Cor). Desitometric analysis of membranes was performed with the Odyssey Scanning Software 2.0. Band densitometric values were then applied to the Nernst equation, where Eo=-330 mV at the mitochondrial pH of 8.0.
2.5 Lung Wet to Dry Weight Ratios
At the completion of each experiment, both lungs were harvested and weighed (wet weight). The lungs were then homogenized and the homogenate dried to constant weight in an oven (dry weight). The ratio of wet/dry lung weight was calculated as a measure of the amount of lung water (edema) that was present.
2.6 Statistical Analysis
Statistically significant differences were determined by a statistical program (GraphPad InStat) using repeated measure analysis of variance (ANOVA)and Tukey-Kramer for post hoc test. P<0.05 was considered significant. In most cases the data are presented as change from baseline, with the baseline calculated as the mean of values at thirty minutes prior and immediately prior to administering endotoxin.
3. RESULTS
3.1 Pulmonary Vascular Resistance (PVR) and Perfusate Oxygenation
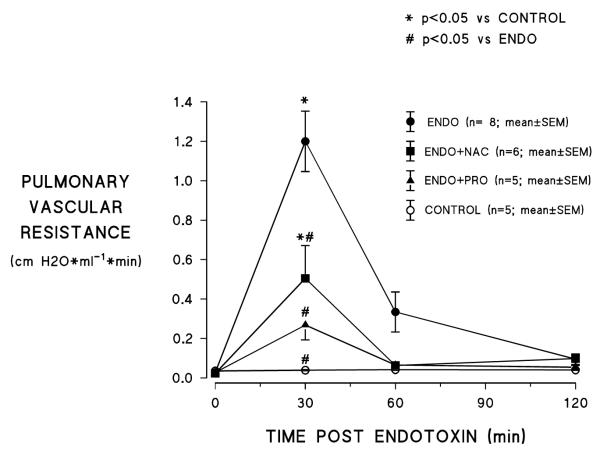
As shown in figure 1, endotoxin treatment caused an increase in PVR which peaked at 30 min, then declined toward baseline over the following 90 min; PVR did not change over the 2h observation period in control experiments when no endotoxin was administered. Treatment with either PRO or NAC significantly attenuated endotoxin induced increased PVR. However, PVR still increased to a level significantly above control values at 30 min after endotoxin with NAC treatment whereas the increase in PRO treated animals was not significantly greater than control values at the same time point.
Figure 1.
Effects of n-acetylcysteine (NAC) and procysteine (PRO) on endotoxin (ENDO) induced increases in pulmonary vascular resistance (ENDO=endotoxin alone; ENDO+NAC=endotoxin+n-acetylcysteine;
ENDO+PRO=endotoxin+procysteine; control=no treatment) .
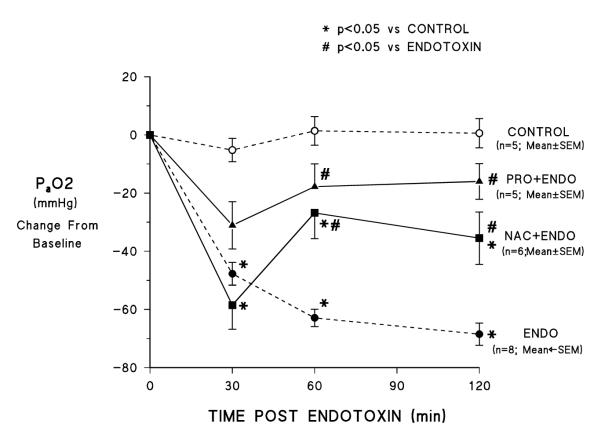
Figure 2 shows PO2 in perfusate blood over the course of the experiments. Typical of the endotoxin response in this preparation [9], endotoxemia resulted in an early marked fall in perfusate PO2 that continued to decline over the rest of the observation period; perfusate PO2 did not change over the course of the experiment in control preparations that received no endotoxin. NAC treatment did not prevent the early hypoxemia (the 30 min PO2 value is significantly lower than control and not different from the endotoxin alone experiments). However, unlike in the endotoxin alone studies, PO2 increased from the 30 min value at 60 and 120 min and at both those later time points the value was significantly higher than in the endotoxin alone experiments (although still significantly lower than in control experiments where no endotoxin was administered). PRO attenuated the early endotoxin-induced hypoxemia (mean 30 min values were lower than baseline but the difference was not significant) and at 60 and 120 min the values were significantly higher than in endotoxin alone experiments and not significantly lower than in control studies.
Figure 2.
Effects of NAC and PRO on endotoxin induced hypoxemia. Labels are the same as for figure 1.
3.2 Biochemical Indices of Liver Function
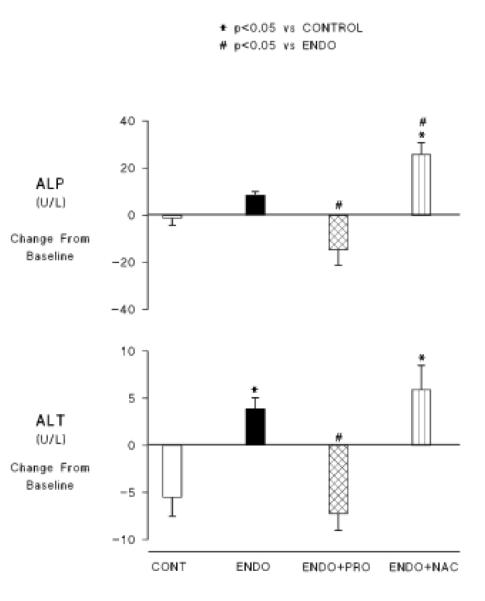
At the end of the 2 h observation period, perfusate concentrations of two enzymes that reflect liver function, alkaline phosphatase (ALP) and alanine aminotransferase (ALT), were measured using the mammalian liver profile kit with the VetScan device from Abaxis, Inc. Those data are shown in figure 3. Endotoxemia resulted in increases in average circulating concentrations of both enzymes (the increase in ALT reached statistical significance) reflecting some degree of hepatic dysfunction. Both enzymes increased in the group receiving NAC to levels significantly higher than in the control group. ALP levels were also significantly higher than in the group receiving endotoxin alone. PRO treatment prevented any increase in either enzyme.
Figure 3.
Effects of NAC and PRO on perfusate concentrations of the liver enzymnes, alkaline phosphatase (ALP) and alanine aminotransferase (ALT). Abbreviations are the same as in previous figures.
3.3 Perfusate Cytokine Concentrations
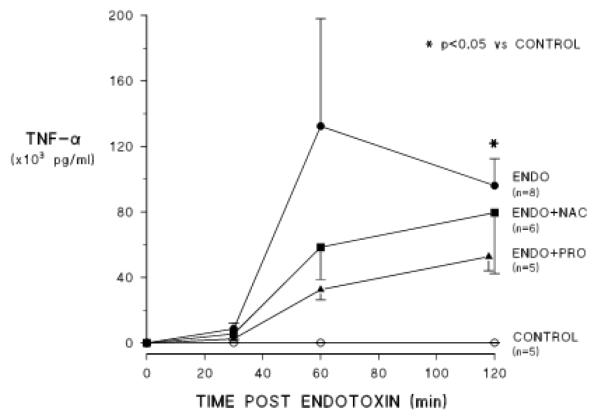
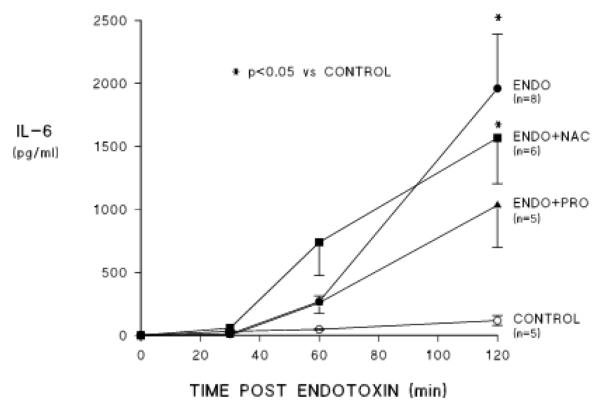
Figures 4 and 5 show the effects of the two treatments on endotoxin induced release of proinflammatory cytokines into the circulation. Endotoxemia causes release of both TNF-α and IL-6 over the course of the two hours of observation. When treated with either NAC or PRO, perfusate TNF-α concentrations did not increase significantly after endotoxin administration. On average, the response was less with either drug and there was greater suppression of this response with PRO than with NAC (figure 4), but these differences were not statistically significant. IL-6 concentrations increased on average in the procysteine treated group but the values at 2 h were not statistically different from those in the control group. The IL-6 response was not affected by NAC (figure 5).
Figure 4.
Effects of NAC and PRO on endotoxin induced changes in perfusate concentrations of tumor necrosis alpha (TNF). Labels are the same as in previous figures.
Figure 5.
Effects of NAC and PRO on endotoxin induced changes in perfusate concentrations of interleukin (IL) 6. Labels are the same as in previous figures.
3.4 Pulmonary Edema as Measured by Lung Wet to Dry Weight Ratio
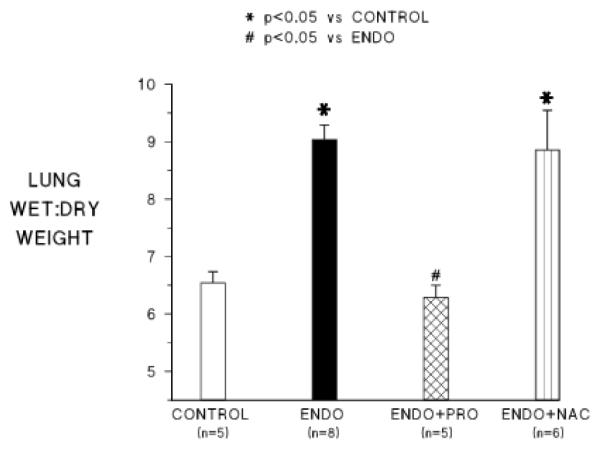
Figure 6 shows lung wet to dry weight ratios at the end of the experiment (2 h after endotoxin) for each of the groups. Endotoxemia in this preparation causes increased lung water (pulmonary edema) as we observed in this series of experiments. Wet:dry lung weights were significantly higher than control in animals receiving endo alone and in animals receiving NAC+endo. However, in the group receiving PRO+endo, lung wet:dry weights were not significantly different than control and were significantly lower than the endo alone treated group.
Figure 6.
Pulmonary edema as measured by lung wet to dry weight ratio at the end of the experiments in the four groups. Abbreviations are the same as in the other figures.
3.5 Mitochondrial Thioredoxin (Thioredoxin-2) Redox Status
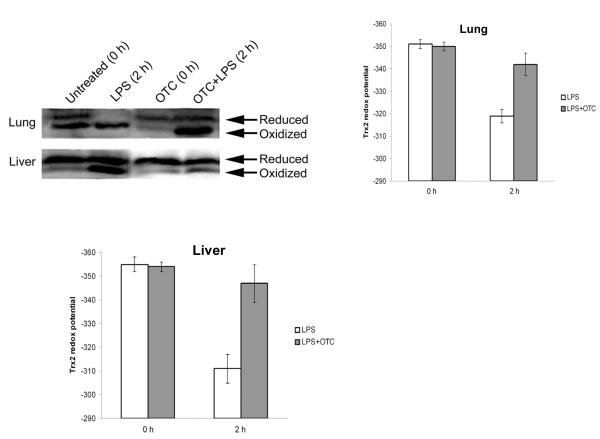
To determine effects of endotoxemia with and without procysteine treatment on mitochondrial oxidant stress, we determined the amount and redox state of the mitochondrial specific protein, thioredoxin-2 in liver and lung at baseline and at the 2 h time point. Figure 7 shows those data. Endotoxemia resulted in a marked oxidation of thioredoxin-2, reflected in a 30-40 mV decrease in redox potential in both the liver and the lungs. Treatment with PRO prevented endotoxin-induced thioredoxin-2 oxidation.
Figure 7.
Effect of procysteine (abbreviated here as OTC) on endotoxin (abbreviated here as LPS) induced oxidation of the mitochondrial protein thioredoxin-2 (Trx-2) in liver and lung. A redox electrophoretic gel (see methods) is shown as well as the calculated redox potentials (see methods for the calculation).
4. DISCUSSION
In spite of overwhelming evidence that oxidative stress is an integral part of the pathogenesis of acute lung injury, clinical trials of antioxidant therapy have been less than convincing [4,5]. But, until recently, the phenomenon of oxidant stress has been viewed as a global response that is reflected in measurements made in a single extracellular compartment [15,16,17] and manipulated by systemic administration of antioxidants, usually antioxidant vitamins [e.g. 18,19] or the glutathione precursor, n-acetylcysteine [e.g. 4,20]. Both this concept and the rationale are probably too simplistic in light of more recent developments in redox biology [7].
Redox signaling is a critical part of normal physiology that fine tunes homeostatic processes. These reactions are compartmentalized both biochemically and anatomically [7]. Critical metabolic functions in mitochondria are subserved by redox reactions but are also vulnerable to oxidative stress [6,7]. In various experimental animal preparations, endotoxemia causes mitochondrial dysfunction in several organs including the liver [10,12,21,22]. Some studies attribute experimental injury to excessive oxidation of critical mitochondrial proteins [11,23,24]. Our findings that endotoxemia in the in situ perfused swine lung-liver preparation caused lung and liver dysfunction and coincident oxidation of the mitochondrial antioxidant protein, thioredoxin-2, indicating mitochondrial oxidative stress in both organs is consistent with those earlier observations.
Many studies of the effects of chemical antioxidants on endotoxin responses in animal preparations have used n-acetylcysteine, which repletes glutathione, as the antioxidant molecule of choice [20,21,22]. Such studies, including an earlier study of ours done in a chronically instrumented sheep preparation [20], found that several physiologic and biochemical responses to endotoxin were moderated by NAC but the magnitude of the effects was less than ideal for a therapeutic agent; available clinical studies with NAC have been generally disappointing [4]. As in those earlier reports, we found in the present study that NAC does moderate some endotoxin responses in the swine preparation, but the effects are generally not large and endotoxin induced pulmonary edema (increased lung water) was not prevented.
Guidot and Brown compared effects of NAC and PRO on mitochondrial and cytosolic glutathione concentrations and surfactant synthesis in alveolar type II cells harvested from ethanol fed rats [13]. They found that NAC increased cytosolic but not mitochondrial glutathione while PRO increased glutathione in both compartments. In addition, PRO but not NAC restored surfactant synthesis to normal levels from their initial ethanol depressed levels. Those studies showing differential effects on repletion of mitochondrial redox balance and others showing that endotoxin causes mitochondrial oxidative stress [10,11,22,23], prompted us to compare effects of the same two antioxidants on responses to endotoxemia in the swine liver lung preparation. We found that OTC prevented endotoxemia induced oxidation of the mitochondrial protein, thioredoxin-2 and had greater effects than NAC on several physiologic and biochemical responses. Most striking was the difference in effects of the two drugs on endotoxin induced pulmonary edema. PRO completely prevented endotoxin induced pulmonary edema while NAC had no effect.
We measured thioredoxin-2 and its redox state as an index of mitochondrial oxidant stress rather than to implicate OTC protection of that specific protein from oxidation as the mechanism of OTC’s effect. However, thioredoxin is reported to have anti-inflammatory effects and administration of exogenous thioredoxin has been reported to prevent several kinds of experimental lung injury [25]. Our data do not permit a specific conclusion about the pathogenetic role or therapeutic potential of thioredoxin, but the area is worthy of further study.
It is obvious that endotoxemia in the in situ perfused swine lung-liver preparation is different than intact humans with acute lung injury. Species differences in responses to endotoxin and both species and individual differences in responses to drugs are notorious. In addition, a perfusion circuit that includes only the liver and lungs is without the contributions of the other organs and their interactions to the response. However, two clinical studies are worth citing in this context. Bernard and associates in 1997 reported the results of a small clinical study (N=14-17 in each of 3 groups) in which effects of NAC or PRO were compared with usual care in patients with the clinical diagnosis of ARDS [3]. They found that either agent increased total erythrocyte glutathione and mortality was about 40% for all 3 groups. However, the median number of “failure free days” was significantly greater in patients receiving PRO. On average, the NAC group had more failure free days than controls but fewer than in the PRO treated group. A more recent prospective double blind placebo controlled trial of PRO in 215 patients with ARDS (sharing some of the same authors with the earlier study) was stopped early because of greater mortality and fewer ventilator free days in the PRO treated group [5]. However, mortality in the placebo group in that trial was 15.8%, much lower than that reported in other studies in ARDS patients and less than half the mortality in the earlier study of Bernard et al. It seems possible that differences in results in this and the earlier Bernard et al. study [4] were due to differences in characteristics of the populations of patients studied. In view of these results it may be unlikely that additional studies of PRO as therapy for ARDS will be done. Exploration of other agents that have an excellent safety profile in humans and protect mitochondrial redox status could be fruitful.
5. CONCLUSIONS
The results in the present study demonstrate that two chemical antioxidants, both aimed at increasing levels of glutathione, have different effects on responses of the lungs and liver to endotoxemia. PRO was more effective at suppressing physiologic and biochemical responses than NAC and PRO prevented pulmonary edema while NAC did not. The fact that the greater effect of PRO was accompanied by attenuation of endotoxin induced mitochondrial oxidative stress and the data of others implicating mitochondrial dysfunction in the pathogenesis of the endotoxin response suggest that these subcellular organelles may be a critical target for antioxidant therapy. Compartmentalization of drug effects should be considered when identifying and testing antioxidant therapies that might have clinical utility.
ACKNOWLEDGEMENTS
This work is funded by Grants #HL083019-04 and #HL07089-01 from the National Heart Lung and Blood Institute
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Brigham KL. Oxidant stress and adult respiratory distress syndrome. Eur Respir J. 1999;3:482s–484s. [PubMed] [Google Scholar]
- [2].Chow CW, Abreu MTH, Suzuki T, Downey GP. Oxidative stress and acute lung injury. Am J Respir Cell Mol Biol. 2003;29:427–32. doi: 10.1165/rcmb.F278. [DOI] [PubMed] [Google Scholar]
- [3].Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- [4].Bernard GR, Wheeler AP, Arons MM, Morris PE, Paz HL, Russell JA, Wright PE. A trial of antioxidants N-acetylcysteine and procysteine in ARDS. The Antioxidant in ARDS Study Group. Chest. 1997;112:164–172. doi: 10.1378/chest.112.1.164. [DOI] [PubMed] [Google Scholar]
- [5].Morris PE, Papadakos P, Russell JA, Wunderink R, Schuster DP, Truwit JD, Vincent J-LV, Bernard GR. A double-blind placebo-controlled study to evaluate the safety and efficacy of L-2-oxothiazolidine-4-carboxylic acid in treatment of patients with acute respiratory distress syndrome. Crit Care Med. 2008;36:782–788. doi: 10.1097/CCM.0B013E318164E7E4. [DOI] [PubMed] [Google Scholar]
- [6].Go YM, Park H, Koval M, Orr M, Reed M, Liang Y, Smith D, Pohl J, Jones DP. A key role for mitochondria in endothelial signaling by plasma cysteine/cystine redox potential. Free Radical Biology & Medicine. 2010;48:275–283. doi: 10.1016/j.freeradbiomed.2009.10.050. 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Jones DP. Radical-free biology of oxidative stress. Am J Physiol Cell Physiol. 2008;295:C849–868. doi: 10.1152/ajpcell.00283.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Jones DP, Go YM, Anderson CL, Ziegler TR, Kinkade JM, Kirlin WG. Cysteine/cystine couple is a newly recognized node in the circuitry for biologic redox signaling and control. FASEB J. 2004;18:1246–1248. doi: 10.1096/fj.03-0971fje. [DOI] [PubMed] [Google Scholar]
- [9].Siore AM, Parker RE, Stecenko AA, McKean M, Christman BW, Cruz-Gervis R, Brigham KL. Endotoxin-induced acute lung injury requires interaction with the liver. Am J Physiol Lung Cell Mol Physiol. 2005;289:L769–L776. doi: 10.1152/ajplung.00137.2005. [DOI] [PubMed] [Google Scholar]
- [10].Suliman HB, Carraway MS, Piantadosi CA. Postlipopolysaccharide oxidative damage of mitochondrial DNA. Am J Resp Crit Care Med. 2003;167:570–579. doi: 10.1164/rccm.200206-518OC. [DOI] [PubMed] [Google Scholar]
- [11].Suliman HB, Welty-Wolf KE, Carraway M, Tatro L, Piantadosi CA. Lipopolysaccharide induces oxidative cardiac mitochondrial damage and biogenesis. Cardiovasc Res. 2004;64:279–288. doi: 10.1016/j.cardiores.2004.07.005. [DOI] [PubMed] [Google Scholar]
- [12].Uedono Y, Takeyama N, Yamagami K, Tanaka T. Lipopolysaccharide-mediated hepatic glutathione depletion and progressive mitochondrial damage in mice: protective effect of glutathione monoethyl ester. J Surg Res. 1997;70:49–54. doi: 10.1006/jsre.1997.5068. [DOI] [PubMed] [Google Scholar]
- [13].Guidot DM, Brown LAS. Mitochondrial glutathione replacement restores surfactant synthesis and secretion in alveolar epithelial cells of ethanol fed rats. Alcohol Clin Exp Res. 2000;24:1070–1076. [PubMed] [Google Scholar]
- [14].Damdimopolous AE, Miranda-Vizuete A, Pelto-Huikko M, Gustafsson JA, Spyrou G. Human mitochondrial thioredoxin. Involvement in mitochondrial membrane potential and cell death. J Biol Chem. 2002;277:33249–33257. doi: 10.1074/jbc.M203036200. [DOI] [PubMed] [Google Scholar]
- [15].Pacht ER, Kindt GC, Lykens MG. Increased antioxidant activity in bronchoalveolar lavage fluid after acute lung injury in sheep. Crit Care Med. 1992;20:1441–1447. doi: 10.1097/00003246-199210000-00013. [DOI] [PubMed] [Google Scholar]
- [16].Popov I, Lewin G. Photochemiluminescent detection of antiradical activity, VI. Antioxidant characteristics of human blood plasma, low density lipoprotein, serum albumin and amino acids during in vitro oxidation. Luminescence. 1999;14:169–174. doi: 10.1002/(SICI)1522-7243(199905/06)14:3<169::AID-BIO539>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- [17].Rao SK, Palazzo RS, Metz HN, Wilson DW, Nikolic SD. Redox potential measurements of plasma in patients undergoing coronary artery bypass graft and its clinical sigtnificance. J Pharmacol Toxicol Methods. 1997;38:151–156. doi: 10.1016/s1056-8719(97)00080-4. [DOI] [PubMed] [Google Scholar]
- [18].Garcia-Bailo B, El-Sohemy A, Haddad PS, Arora P, Benzaied F, Karmali F, Badawi A, Vitamins D. C and E in the prevention of type 2 diabetes mellitus: modulation of inflammation and oxidative stress. Biologics. 2011;5:7–19. doi: 10.2147/BTT.S14417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ozkaya D, Naziroglu M, Armagan A, Koroglu BK, Colakoglu N, Kukner A, Sonmez TT. Dietary vitamin C and E modulates oxidative stress induced-kidney and lens injury in diabetic aged male rats through modulating glucose homeostasis and antioxidant systems. Cell Biochem Funct. 2011;29:287–293. doi: 10.1002/cbf.1749. [DOI] [PubMed] [Google Scholar]
- [20].Bernard GR, Lucht WD, Niedermeyer ME, Snapper JR, Ogletree ML. Effect of N-acetylcysteine on the pulmonary response to endotoxin in awake sheep and upon in vitro granulocyte function. J Clin Invest. 1984;73:1772–1784. doi: 10.1172/JCI111386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Nakano H, Fujiwara Y, Kitamura K, Matsumiya A, Sakai H, Hatakeyama T, Yamaguchi M, Jacek D. Susceptibility to lipolysaccharide of cholestatic rat liver produced with bile duct ligation: assessments of the mitochondrial glutathione pool and the effects of N-acetylcysteine. Eur Surg Res. 2000;32:148–154. doi: 10.1159/000008756. [DOI] [PubMed] [Google Scholar]
- [22].Ritter C, da Cunha AA, Echer JC, Andrades M, Reinke A, Lucchiari N, Rocha J, Streck EL, Menna-Barreto S, Moreira JC, Dal-Pizzol F. Effects of N-acetylcysteine plus deferoxamine in lipopolysaccharide-induced acute lung injury in rat. Crit Care Med. 2006;34:471–477. doi: 10.1097/01.ccm.0000199069.19193.89. [DOI] [PubMed] [Google Scholar]
- [23].Alvarez S, Boveris A. Mitochondrial nitric oxide metabolism in rat muscle during endotoxemia. Free Radic Biol Med. 2004;37:1472–1478. doi: 10.1016/j.freeradbiomed.2004.06.034. [DOI] [PubMed] [Google Scholar]
- [24].Gray KD, MacMillan-Crow LA, Somovic MO, Stain SC, May AK. Pulmonary MnSOD is nitrated following hepatic ischemia-reperfusion. Surg Infect (Larchmt) 2004;5:166–173. doi: 10.1089/sur.2004.5.166. [DOI] [PubMed] [Google Scholar]
- [25].Nakamura T, Kakamura H, Hoshino T, Ueda S, Wada H, Yodo J. Redox regulation of lung inflammation by thioredoxin. Antioxidants & Redox Signaling. 2005;7:60–71. doi: 10.1089/ars.2005.7.60. [DOI] [PubMed] [Google Scholar]