Abstract
Campylobacter jejuni is a microaerophilic, asaccharolytic bacterium. The identity of the carbon and energy sources used by C. jejuni in vivo is unknown, but the genome sequence of strain NCTC11168 indicates the presence of genes for catabolism of a limited range of amino acids, including serine. Specific omission of l-serine from a defined medium containing a mixture of amino acids led to a dramatic decrease in cell yields. As C. jejuni does not have a biosynthetic serine requirement, this supports earlier suggestions that l-serine is a preferentially catabolized amino acid. Serine transport was found to be mediated by at least two systems in strain 11168; a high-capacity, low-affinity l-serine-specific system encoded by Cj1625c (sdaC) and a higher-affinity l-serine/l-threonine system responsible for residual l-serine transport in an sdaC mutant. Catabolism of l-serine to pyruvate and ammonia is carried out by SdaA (encoded by Cj1624c), which was overexpressed, purified, and shown to be an oxygen-labile iron-sulfur enzyme. l-Serine dehydratase activity in an sdaA mutant was reduced 10-fold compared to that in the wild type, but the residual activity (due to the anabolic l-threonine dehydratase) could not support either growth on or utilization of l-serine in defined media. However, although sdaA mutants showed no obvious growth defect in complex media, they completely failed to colonize 3-week-old chickens as assayed both by cloacal swabs taken over a 6-week period and by cecal colony counts postmortem. In contrast, the isogenic parent strain colonized chickens to high levels within 1 week of inoculation. The results show that an active SdaA is essential for colonization of the avian gut by C. jejuni and imply that catabolism of l-serine is crucially important for the growth of this bacterium in vivo.
Campylobacter jejuni, a gram-negative, microaerophilic bacterium, is a leading cause of human enteric disease worldwide and is a major public health and economic burden (7). Although pathogenic in humans, C. jejuni is part of the normal commensal flora of many bird species, and ingestion of contaminated poultry is a common route for infection. The pathogenic mechanisms of C. jejuni after infection of the human intestinal tract are relatively poorly understood and are generally considered to involve mucosal adherence, host cell invasion, and toxin production (15). The ability to compete for nutrients, particularly carbon and nitrogen sources, is an aspect of pathogen physiology that has received relatively little attention, and it is often assumed that sugars such as glucose are preferred carbon sources in vivo. However, as C. jejuni is asaccharolytic (29, 32), alternative carbon sources for growth in the host must be used. Amino acids are likely candidates, as they could potentially be deaminated to a small number of intermediates that can directly feed into the central metabolism, including pyruvate (from serine and alanine), oxaloacetate (from aspartate), and 2-oxoglutarate (from glutamate). Deamination will also generate ammonia, which can be used as a nitrogen source. Nevertheless, there are few data on which amino acids might be important in vivo. C. jejuni is known to grow at the expense of amino acids in vitro and has been shown to utilize serine, aspartate, glutamate, and proline (17). Consistent with these findings, the complete genome sequence of C. jejuni NCTC 11168 has revealed the presence of a number of homologues of amino acid-catabolizing enzymes (24).
The study by Leach et al. (17) suggested that, during growth in either complex or defined media, l-serine is preferentially utilized compared to other amino acids. This is not due to a simple biosynthetic requirement for serine, as C. jejuni 11168 contains all three genes (serABC) necessary for serine biosynthesis from 3-phosphoglycerate (24). An open reading frame (Cj1624c) displaying sequence similarity to sdaA, which in Escherichia coli encodes an l-serine dehydratase, is present in C. jejuni. SdaA catalyzes the deamination of serine to pyruvate and ammonia, both of which can be readily assimilated. Although many serine dehydratases also deaminate threonine, the bacterial l-serine dehydratases are much more specific for serine (9). Serine and threonine dehydratases have been purified from many organisms and are usually found to be pyridoxal-5′-phosphate (PLP)-containing enzymes. However oxygen-labile l-serine dehydratases devoid of PLP have been discovered in some strictly anaerobic bacteria, e.g., Peptostreptococcus asaccharolyticus (8) and Clostridium propionicum (12). These enzymes are inactivated upon exposure to air and can be specifically reactivated by Fe2+ under anaerobic conditions. The P. asaccharolyticus enzyme was found to contain stoichiometric amounts of Fe and inorganic sulfur, sufficient to form one [4Fe-4S] cluster per heterodimer (8). l-Serine dehydratases from several other bacteria, including Clostridium acidiurici (5) and E. coli (22), have been recognized to require activation by Fe2+, thus making it likely that these enzymes also contain Fe-S centers.
The ability to utilize serine as a carbon or nitrogen source necessitates its efficient transport into the cell. In C. jejuni 11168, sdaA is the downstream gene of a two-gene operon of which the first gene (Cj1625c) encodes a putative serine transporter, homologous to E. coli SdaC, an H+:l-serine symporter (28). In this study, we have constructed and characterized sdaA and sdaC mutants of C. jejuni, in order to determine the importance of serine as a carbon and nitrogen source in this bacterium. We present evidence that SdaC encodes a high-capacity, low-affinity l-serine-specific uptake system, but an additional high-affinity system still allows residual serine transport in an sdaC mutant. The kinetic and biochemical properties of the purified serine dehydratase (SdaA) show that it is an oxygen-labile iron-sulfur protein specific for l-serine deamination. Although the expression of sdaA was not necessary for growth in complex media in vitro, SdaA was found to be essential for C. jejuni to grow on or utilize l-serine in defined media, as well as for colonization of the ceca of 3-week-old chickens, suggesting a specific requirement for l-serine catabolism for growth in vivo.
MATERIALS AND METHODS
Bacterial strains, media and culture conditions.
C. jejuni strains NCTC 11168 and 11168H, a hypermotile variant (13), were routinely cultured at 37°C under microaerobic conditions (10% [vol/vol] O2, 5% [vol/vol] CO2, and 85% [vol/vol] N2) in a MACS growth cabinet (Don Whitley Scientific Ltd., Shipley, United Kingdom) on Columbia agar containing 5% (vol/vol) lysed horse blood and 10 μg each of amphotericin B and vancomycin ml−1. Liquid cultures of C. jejuni were grown microaerobically with gentle agitation, either in brain heart infusion supplemented with 5% (vol/vol) fetal calf serum (BHI-FCS) or in the defined medium MEM-α (Gibco Ltd.), both containing the above antibiotics and 50 μM FeSO4. To select for C. jejuni mutants carrying antibiotic-resistant determinants, either kanamycin or chloramphenicol was added to a final concentration of 30 μg ml−1 each. E. coli DH5α was cultured in Luria-Bertani (LB) medium supplemented with appropriate antibiotics at 37°C. For growth assays, C. jejuni overnight starter cultures were prepared in BHI before inoculation into fresh BHI or MEM-α to which either 20 mM pyruate or 20 mM l-serine was added. Growth was monitored at 600 nm in an Amersham Pharmacia Biotech Ultrospec 2000 spectrophotometer. For amino acid utilization studies, MEM-α containing 20 mM pyruvate and either 100 mg of each amino acid liter−1 or with one specific amino acid omitted was inoculated with C. jejuni and was incubated microaerobically for 48 h. Final cell densities were measured at 600 nm.
DNA isolation and manipulation.
Plasmid DNA was isolated by using the Qiagen Miniprep kit (Qiagen Ltd., Crawley, United Kingdom). C. jejuni chromosomal DNA was extracted by using a modified sodium dodecyl sulfate (SDS) lysis procedure (20). Standard techniques were employed for the cloning, transformation, preparation, and restriction analysis of plasmid DNA from E. coli (27).
Inactivation of the sdaA and sdaC genes.
For inactivation of the sdaA gene, a fragment of Cj1624c was PCR amplified (forward primer, 5′-ATGCTGTTGGCCCTTCTTCTTCTCATAC-3′; and reverse primer, 5′-ATGCAGTGATGGGAGTAATCAATCTTGG-3′) from C. jejuni 11168 chromosomal DNA and was cloned into pGEM-T Easy (Promega Ltd., United Kingdom). A chloramphenicol acetyltransferase cassette originating from Campylobacter coli (33) was then cloned into a unique NheI site in Cj1624c to generate pJV10. Primers (forward primer, 5′-AGTCTCCTAAATGGACTAGTCAGGATAC-3′; and reverse primer, 5′-AGTCTACGAAACCTTGCCAAAATATCAA-3′) were designed to amplify a fragment of Cj1625c by PCR that was subsequently cloned into pGEM-T Easy. The aphA-3-containing SmaI fragment of pJMK30 (31), which confers kanamycin resistance, was then cloned into the BglII site within the cloned gene to generate pJV11. All antibiotic-resistant constructs were introduced into C. jejuni NCTC 11168 or 11168H by electroporation (6). Transformants were selected on Columbia blood agar plates supplemented with the appropriate antibiotic. Single colonies were then restreaked, and chromosomal DNA was extracted by the microLYSIS kit (Web Scientific Ltd., Crewe, United Kingdom) for screening by PCR, to verify that allelic exchange by double homologous recombination had occurred.
Preparation of cell extracts and enzyme assays.
Cell extracts of C. jejuni were prepared from 300-ml cultures grown for 16 h to the early stationary phase. Cells were harvested by centrifugation (20 min, 4°C, 8,000 × g), washed with 0.1 M Tris-HCl pH 8.0, and then resuspended in 1 ml of the same buffer before sonication as above. The crude extract was clarified by centrifugation (14,000 × g for 30 min), and the supernatant was retained for use on the same day. Serine dehydratase activities were assayed spectrophotometrically in a coupled reaction with l-lactate dehydrogenase by monitoring the decrease in NADH concentration at 340 nm (8). For purified enzyme, assays were performed anaerobically in stoppered glass cuvettes. In these assays, oxygen was removed from all the assay reagents by repeated flushing with nitrogen. Kinetic parameters were determined by an s/v-against-s plot (11). Cystathionine β-lyase was assayed as described previously (4).
[14C]serine transport assays.
C. jejuni cells grown for 16 h in 200-ml BHI-FCS batch cultures were harvested by centrifugation, washed once, and resuspended in 5 ml of 50 mM phosphate buffer, pH 7.4, and kept under microaerobic conditions at 37°C for no longer than 4 h. An aliquot (100 μl) of the cell suspension was added to 4 ml of 50 mM phosphate buffer (pH 7.4) containing 2 mM MgSO4, 50 μg of chloramphenicol ml−1, and 0.5% (wt/vol) sodium lactate and was allowed to equilibrate by gently stirring at 37°C for 5 min. The assay was initiated by the addition of the specified concentration of 14C-l-serine (1.8 to 2.2 GBq mmol−1). Samples (0.4 ml) were withdrawn at the indicated time points, collected by vacuum filtration through membrane filters (0.45-μm pore size), and washed twice with 0.1 M LiCl. Sample filters were then immersed in FluoroSafe 2 scintillation cocktail (BDH Ltd., United Kingdom) and were counted in a Beckman LS 1801 scintillation counter.
For competition experiments, stock solutions of amino acids were diluted into the assay mixture to the specified final concentration, immediately before the addition of [14C]l-serine. In some experiments, the uncoupler, carbonylcyanide-p-trifluoromethoxyphenylhydrazone (FCCP), was added 10 min before the labeled substrate to a final concentration of 10 μM.
1H NMR spectroscopy.
Culture samples (1.5 ml) were centrifuged to remove cells (13,800 × g, 5 min) and the supernatants were used for nuclear magnetic resonance (NMR) analysis. 1H NMR was carried out under similar conditions as described previously (18) by using a Bruker AMX500 spectrometer operating at 500 MHz. Spectra were acquired into 8,000 complex points over a spectral width of 12.5 KHz, and the solvent (H2O) signal was reduced by presaturation for 2 s. Samples (0.45 ml of culture supernatant plus 0.05 ml of D2O) were run in 5-mm-diameter tubes at 30°C. Chemical shifts were established by reference to a standard (trimethylsilylpropionate, TSP; 0 ppm) added to the samples.
Overexpression and purification of serine dehydratase.
Primers (forward, 5′-GTACCATATGAGTAATTTAAGCATTTTT-3′; and reverse, 5′-GTACGGATCCTTAGCATACTGTTTTTAA-3′, NdeI and BamHI restriction sites underlined) were designed to amplify the complete sdaA gene from C. jejuni 11168 chromosomal DNA by PCR. The resulting amplicon was digested with BamHI and NdeI and was cloned into similarly restricted pET-14b to give pJV12. This was then transformed into E. coli BL21(DE3 pLysS) cells, which were grown aerobically at 30°C in LB medium containing kanamycin (30 μg ml−1) and chloramphenicol (30 μg ml−1) to an optical density at 600 nm of 0.6, before induction by 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG). Induced cells were then grown for a further 5 h before harvesting by centrifugation (20 min, 4°C, 8,000 × g). Cell pellets were resuspended in 20 mM potassium phosphate buffer, pH 7.4, containing 0.5 M NaCl, and were sonicated (6 × 10-s bursts, 12-μm amplitude, Soniprep 150; MSE), and the sonicate was centrifuged at 14,000 × g for 30 min at 4°C. The His6 affinity tag permitted the purification of the enzyme from the supernatant by affinity chromatography on nickel-nitrilotriacetic acid resin according to the protocol of the HisTrap kit (Amersham Pharmacia Biotech Ltd., Bucks, United Kingdom). The protein was eluted from the resin by a step gradient from 100 to 300 mM imidazole in 20 mM potassium phosphate buffer, pH 7.0, containing 0.5 M NaCl, before desalting on an Econo-Pac 10DG column (Bio-Rad Ltd., United Kingdom) according to the manufacturer's instructions. Molecular mass determinations of water-dialyzed SdaA by electrospray mass spectrometry were performed by S. Thorpe, Department of Chemistry, University of Sheffield, with a VG Bio Q spectrometer. Native molecular mass was determined by gel filtration chromatography in 10 mM Tris-HCl, pH 8.0, containing 200 mM NaCl on a calibrated Superdex-200 column attached to a Pharmacia fast protein liquid chromatography system.
Chicken colonization studies.
Day-of-hatching specific-pathogen-free (SPF) Rhode Island Red chicks were obtained from the Poultry Production Unit, Institute for Animal Health, Compton, United Kingdom. All the birds were inoculated orally with 0.1 ml of Campylobacter-free adult gut flora preparations. This was produced by static culture of 1 g of cecal contents in 10 ml of LB broth for 23 h at 37°C, taken from a 50-week-old SPF chicken immediately after the bird had been killed. The birds thus inoculated were housed under appropriate SPF conditions until 3 weeks of age when they were used in colonization trials. Birds were fed ad libitum on a vegetable-based diet (Special Diet Services, Manea, Cambridgeshire, United Kingdom). Groups of 20 3-week-old birds with the developed gut flora were inoculated orally with 0.1 ml of culture containing 107 CFU of the desired Campylobacter strain. Sterile cloacal swabs were taken from birds unidirectionally, and fecal secretion was assessed by both direct counts and enrichment counts as follows: cloacal swabs were taken at weekly intervals and were mixed into 1 ml of modified Exeter broth (2). Swabs were streaked in a standard manner onto Campylobacter blood-free selective agar (CCDA and CM739 [Oxoid Ltd., United Kingdom] containing CCDA selective supplement SR155; Oxoid Ltd.), and colony counts were scored positive (>1 CFU, direct counts) or negative after 2 days of incubation. Swabs were returned to the enrichment medium and were incubated under microaerobic conditions at 37°C. After 2 days the enrichment cultures were checked for Campylobacter by plating on Campylobacter blood-free agar. These were scored as positive or negative results and are referred to as enrichment counts. At 6 weeks postinfection, the birds were killed humanely and postmortem bacterial counts were determined from the cecal contents of five birds from each group. Samples were diluted in phosphate-buffered saline) and were immediately plated on Campylobacter blood-free agar. All postmortem counts are expressed as number of CFU per gram.
RESULTS
Effect of omission of l-serine from growth media.
Specific amino acids that enhance the growth of C. jejuni 11168 in vitro were identified by measuring final cell densities after 48 h growth in MEM-α containing 20 mM pyruvate and either a mixture of all 20 amino acids (control) or with individual amino acids omitted. In a typical experiment, single omission of Ala, Asp, Glu, Gly, His, Ile, Lys, Phe, Pro, Thr, Trp, or Tyr resulted in a <25% decrease in final yields, whereas omission of methionine or cysteine resulted in moderate growth inhibition (to 39 or 32% of the final optical density of the control, respectively). However, the specific absence of l-serine in the medium was most detrimental to growth, resulting in a 54%-lower final cell yield compared to the result for the complete amino acid control.
Construction of sdaA and sdaC mutants: sdaC encodes a low-affinity l-serine specific transporter.
C. jejuni sdaA and sdaC mutants were generated by the insertion of nonpolar antibiotic resistance cassettes (containing an internal promoter but lacking transcriptional terminators) into unique restriction sites within the cloned genes. The resulting constructs were electroporated into C. jejuni 11168, generating chromosomal null mutants of sdaA and sdaC. The correct construction of the mutants was verified by PCR amplification of the target genes, yielding fragment sizes consistent with the insertion of the 0.75- and 1.5-kb chloramphenicol and kanamycin resistance cassettes, respectively (data not shown).
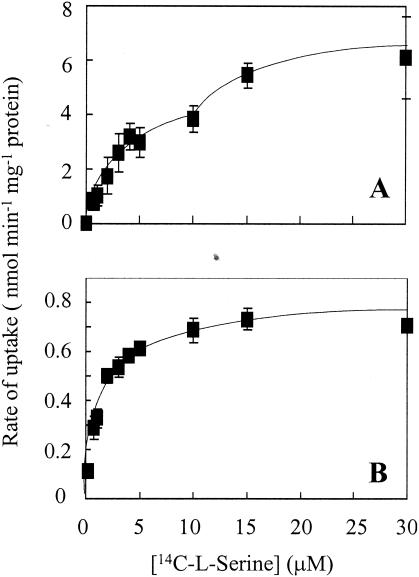
The wild type and sdaA and sdaC mutants were assayed for [14C]l-serine uptake. At an external concentration of 10 μM, the sdaC mutant exhibited a significantly lower rate of [14C]l-serine uptake (143 ± 2 pmol min−1 mg of protein−1) than did the wild type (1,073 ± 168 pmol min−1 mg of protein−1). In contrast, the sdaA mutant did not display any deficiency in [14C]l-serine transport (1,287 ± 250 pmol min−1 mg of protein−1). The concentration dependence of [14C]l-serine transport was examined in the wild type and the sdaC mutants. In the wild type (Fig. 1A), over a [14C]l-serine concentration range of 0.25 to 30 μM, the rate of [14C]l-serine uptake was biphasic, a pattern indicative of the presence of at least two transport systems with different affinities for serine. The higher-affinity system had a Km value of about 2.5 μM, but an accurate estimate of the affinity of the lower-affinity system could not be obtained. In the sdaC mutant (Fig. 1B), the [14C]serine uptake rates, at all concentrations tested, were significantly lower than those measured in the wild type and the sdaA mutant. The data could be fitted to a monophasic hyperbolic saturation curve with a single Km of 1.4 μM. Thus, the data indicate that sdaC encodes the low-affinity serine transporter and that the remaining transport activity in the sdaC mutant is due to a separate high-affinity system.
FIG. 1.
Concentration dependence of [14C]l-serine uptake by C. jejuni 11168 wild-type (A) and sdaC (B) strains. Each data point represents the mean of assays on two different batches of cells, and the error bars represent the standard deviation.
Substrate specificity of serine transport.
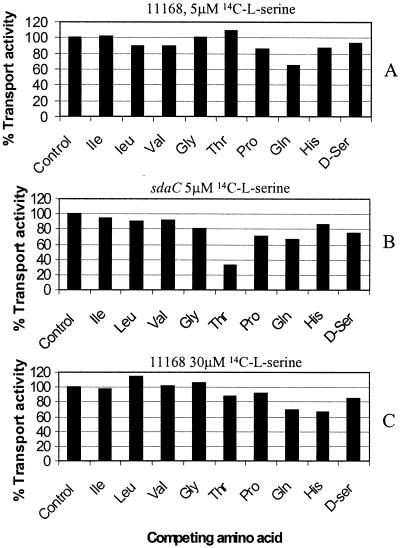
In wild-type cells transport of low (5 μM) or high (30 μM) concentrations of l-serine was strongly inhibited by the protonophore FCCP, which provides a route for the dissipation of the proton electrochemical gradient. The specificity of [14C]l-serine transport was investigated by examining the ability of other amino acids, including l-isoleucine, l-leucine, l-valine, l-glycine, l-threonine, l-proline, l-glutamine, l-histidine, and d-serine, to inhibit [14C]l-serine accumulation. To examine high-affinity transport, the amino acids were added at a concentration of 50 μM, and [14C]l-serine was present at a concentration of 5 μM. In wild-type cells, [14C]l-serine transport was not significantly inhibited by any of the amino acids tested, with the exception (as expected) of unlabeled l-serine, which resulted in 84% inhibition (Fig. 2A). However, in the sdaC mutant, strong competition was observed with 50 μM l-threonine (Fig. 2B) with a 68% inhibition of [14C]serine transport. The substrate specificity of low-affinity serine transport in the wild type was also investigated, by performing competition experiments at a [14C]l-serine concentration of 30 μM. When present at a concentration of 300 μM, none of the amino acids tested significantly inhibited [14C]serine transport (Fig. 2C). Thus, low-affinity serine transport (encoded by sdaC) is specific for l-serine, while the additional high-affinity transporter revealed in the sdaC mutant may also transport l-threonine. The presence of both transporters probably masks threonine inhibition of transport of the high-affinity system in wild-type cells. As neither l-isoleucine, l-leucine, or l-valine inhibited the low-affinity transport of [14C]l-serine into the wild type (Fig. 2C), it is unlikely that the branched-chain amino acid (LIV) transport system contributes to uptake of serine at the concentrations used.
FIG. 2.
Effect of competing amino acids on [14C]l-serine uptake in the wild type (A and C) and sdaC mutant (B). High-affinity uptake was examined by using 5 μM [14C]l-serine, with the addition of 50 μM competing unlabeled amino acid as indicated, while low-affinity uptake was examined with 30 μM [14C]l-serine and 300 μM competing amino acid as indicated. Inhibition is shown as the percentage of the uptake rate observed in the control incubation, where no competing amino acid was added. The data shown are from a single representative experiment; other experiments gave similar results.
Requirement of serine dehydratase activity for growth on and utilization of l-serine.
The ability of MEM-α, supplemented with 20 mM l-serine or 20 mM pyruvate, to support the growth of the wild type and the sdaA mutant is illustrated in Fig. 3. The wild type grew well with either l-serine or pyruvate as the primary carbon source in MEM-α. However, although the final cell yield of the sdaA mutant on pyruvate was comparable to that of the wild type, the sdaA mutant did not grow on l-serine (Fig. 3A). This indicates that serine dehydratase is essential for growth with serine but not pyruvate as the major carbon source. 1H NMR spectroscopy of culture supernatants was employed to directly measure the utilization of serine in wild-type and sdaA mutant cells grown with 20 mM l-serine in MEM. The NMR spectra presented in Fig. 3B and C show that, in contrast to the wild type, the sdaA mutant was unable to metabolize serine to any significant extent. In contrast to the obvious phenotype in MEM, no differences in either growth rate or final cell yields were observed between the wild type and the sdaA mutant when grown in the complex BHI-FCS medium (data not shown).
FIG. 3.
(A) Growth of the C. jejuni wild type and sdaA mutant in MEM-α, in the presence and absence of an added carbon source. Solid bars (c), no added carbon source; open bars (pyr), 20 mM sodium pyruvate; and hatched bars (ser), 20 mM l-serine. Strains were pregrown overnight in BHI-FCS, harvested, washed, and resuspended in MEM-α before being inoculated into prewarmed MEM-α. It should be noted that, in MEM-α alone (solid bars), a basal level of growth occurs owing to the presence of amino acids in the medium (starting optical density at 600 nm [OD 600 nm] was 0.28). The data represent the mean of two separate experiments ± standard deviation. (B and C) Analysis of l-serine utilization by C. jejuni wild-type (B) and sdaA mutant (C) strains conducted by using proton NMR spectroscopy. Cells were grown with 20 mM l-serine in MEM-α. Culture supernatants for each strain were collected before (T0) and after 24 h (T24) of inoculation. The serine proton resonances at a chemical shift of 3.7 to 3.9 ppm are indicated with arrows, as determined from the spectrum for MEM-α alone. The chemical shift reference used was 1 mM trimethylsilylpropionate (0 ppm), which was included in all the samples. The identity of the resonance at 1.2 ppm in the sdaA samples was not determined.
sdaA encodes an l-serine specific dehydratase that is PLP independent.
Serine dehydratase enzyme activity was decreased 10-fold in cell extracts of the sdaA mutant compared to the wild-type parent strain (Table 1). The residual activity observed in the sdaA mutant could be due to the metC product, cystathionine β-lyase, which has been reported to contribute to serine deamination in vivo (4). However, C. jejuni contains only two pseudogene fragments encoding a putative cystathionine β-lyase (Cj1392/1393), and no activity could be detected when wild-type or sdaA mutant cell extracts were assayed for this enzyme. Another potential serine-deaminating enzyme is threonine dehydratase, of which there are two types, catabolic and biosynthetic. These two enzymes are readily distinguished by the fact that the biosynthetic enzyme is sensitive to inhibition by isoleucine. A gene encoding a biosynthetic threonine dehydratase, ilvA (Cj0828c), was identified from the genome sequence of strain 11168 (24). Threonine dehydratases are PLP-dependent enzymes, which can be inactivated by carbonyl reagents, including phenylhydrazine and hydroxylamine, that specifically interact with the Schiff base to which the prosthetic group is bound (9). The residual serine dehydratase activity detected in the sdaA mutant was completely inhibited by 10 mM phenylhydrazine or 1 mM hydroxylamine (Table 1), thus confirming that a PLP-dependent enzyme is responsible for this activity. Moreover, the activity was completely abolished in the presence of 1 mM isoleucine. Thus, the low residual activity in the sdaA mutant can be ascribed to the biosynthetic threonine dehydratase. Importantly, wild-type l-serine dehydratase activity was not significantly changed in the presence of either phenylhydrazine or hydroxylamine (Table 1), suggesting that the C. jejuni SdaA enzyme is devoid of PLP. The wild-type activity was weakly inhibited by d-serine and l-threonine but was more strongly inhibited by l-cysteine (Table 1). We attempted to complement the 11168 sdaA mutant by using a “knock-in” approach, i.e., by integrating a plasmid containing a wild-type copy of the sdaA gene at the rdxA locus that, when disrupted, gives rise to metronidazole resistance, thus allowing positive selection for integrants. However, while such integrants were obtained, as proved by PCR analysis, none was complemented for SdaA enzyme activity.
TABLE 1.
Effect of phenylhydrazine, hydroxylamine, and amino acids on serine dehydratase activity in cell extracts of the C. jejuni 11168 wild type and sdaA mutanta
| Addition |
l-Serine dehydratase activity (nmol min−1 mg of protein−1) for:
|
|
|---|---|---|
| 11168 wild-type | 11168 sdaA mutant | |
| None | 434 ± 42 | 40 ± 9 |
| 10 mM phenylhydrazine | 402 ± 81 | 0 |
| 1 mM hydroxylamine | 430 ± 16 | 0 |
| 1 mM isoleucine | 431 ± 62 | 0 |
| 100 mM d-serine | 260 ± 93 | ND |
| 100 mM l-threonine | 194 ± 11 | ND |
| 50 mM l-cysteine | 22 ± 9 | ND |
The mean serine dehydratase activity ± standard deviation is indicated for experiments performed in duplicate. ND, not determined.
Unexpectedly, serine dehydratase activity due to SdaA (as assayed in the wild type in the presence of 1 mM hydroxylamine) was found to be completely undetectable in the sdaC mutant (data not shown). This indicates a polar effect of the sdaC mutation on sdaA, although the antibiotic resistance cartridge within the sdaC gene is devoid of known transcriptional terminators and was present in the same transcriptional orientation as the sdaC gene.
Purification and catalytic properties of l-serine dehydratase.
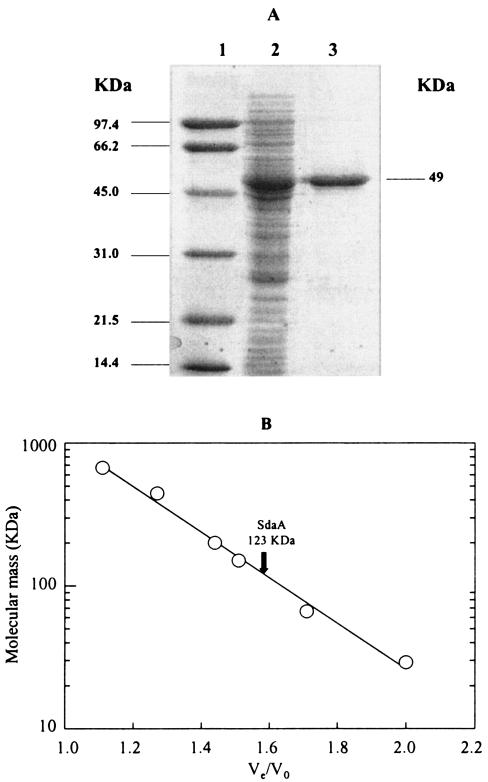
In order to determine the biochemical properties of the C. jejuni serine dehydratase, Cj1624 was overexpressed in E. coli BL21(DE3) as a histidine-tagged protein, followed by purification using Ni-affinity chromatography. Although serine dehydratase in C. jejuni cell extracts was stable in the presence of air, the purified enzyme was inactivated upon exposure to air (see below), which necessitated purification to be carried out under strictly anaerobic conditions. The enzyme was purified ninefold to apparent homogeneity in a single step, to a specific activity of 115 U mg of protein−1, with the yield being almost 100%. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of the purified enzyme gave a single band of approximately 49 kDa (Fig. 4A). The predicted molecular mass of the overexpressed SdaA plus the His-tag residues is 52,032 Da; electrospray mass spectroscopy gave a molecular mass of 51,758 Da. The apparent molecular mass of the native enzyme was determined as 123 ± 3 kDa by gel filtration on a calibrated Superdex-200 column (Fig. 4B), which suggests that the native enzyme is probably a homodimer.
FIG. 4.
(A) Purification of recombinant C. jejuni serine dehydratase. Lane 1, molecular mass markers (phosphorylase B, 97 kDa; bovine serum albumin, 66 kDa; ovalbumin, 45 kDa; carbonic anhydrase, 31 kDa; soybean trypsin inhibitor, 21.5 kDa; and lysozyme, 14.4 kDa). Lane 2, 15 μg of cell extract protein from BL21(DE3 pLysS pJV12) after IPTG induction for 5 h. Lane 3, purified l-serine dehydratase (3 μg) following chromatography on nickel-nitrilotriacetic acid resin. (B) Determination of the native molecular weight of purified SdaA by gel filtration on Superdex-200. The column was calibrated by using thyroglobulin (669 kDa), horse spleen apoferritin (443 kDa), sweet potato amylase (200 kDa), yeast alcohol dehydrogenase (150 kDa), bovine serum albumin (66 kDa), and bovine erythrocyte carbonic anhydrase (29 kDa). Ve, protein elution volume; Vo, column void volume (as determined with blue dextran).
The catalytic properties of SdaA were determined under anaerobic conditions immediately following the purification procedure. The enzyme was highly specific for l-serine, with a Km of 14.6 ± 3.4 mM and a Vmax of 403 ± 35 μmol min−1 mg of protein−1. Neither l-threonine, d-serine, nor l-cysteine was detectably deaminated (data not shown). d-Serine was slightly inhibitory to l-serine dehydratase activity (in one set of experiments, 52.1 ± 6 U of l-serine dehydratase activity mg of protein−1 was observed in the presence of 100 mM d-serine, compared to 73.5 ± 4.3 U mg of protein−1 in its absence), while no l-serine dehydratase activity at all was detected in the presence of 50 mM l-cysteine. This is consistent with the properties of the activity observed in cell extracts (Table 1).
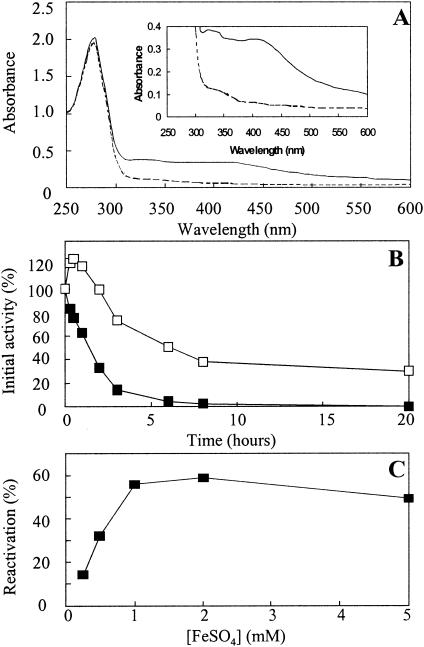
SdaA is an iron-sulfur enzyme: reversible inactivation of the purified enzyme in air.
The pure enzyme was yellow-brown. Its UV spectrum revealed, in addition to the protein peak at 280 nm, two broad shoulders at 300 to 350 nm and at 420 nm (Fig. 5A and inset). These spectral features are characteristic of enzymes containing iron-sulfur clusters. Purified l-serine dehydratase was inactivated, with a half-life of approximately 1.5 h, upon exposure to air (Fig. 5B). This was associated with the loss of the 300- to 350-nm and 420-nm shoulders in the UV spectrum (Fig. 5A). The inactivated enzyme could be reactivated somewhat with Fe2+ and dithiothreitol (DTT) under strict anaerobic conditions (Fig. 5C), with activity being restored to about 60% of the activity of enzyme stored under anaerobic conditions. The reactivation was highly specific for Fe2+, as other metal ions such as Fe3+, Cu2+, Mg2+, Mn2+, and Ni2+ had no effect (data not shown). Similarly, serine dehydratase activity could not be restored by DTT alone, in the absence of Fe2+ (data not shown).
FIG. 5.
SdaA is an oxygen-labile iron-sulfur enzyme. (A) UV/visible spectra of active pure l-serine dehydratase (solid line) measured under anaerobic conditions and air-inactivated l-serine dehydratase (broken line), showing loss of absorbance at 420 nm (characteristic of iron-sulfur enzymes) upon exposure to air. (B) Inactivation kinetics of l-serine dehydratase in air. SdaA in 20 mM potassium phosphate buffer, pH 7.0, was incubated at 4°C under anaerobic conditions (□) or in the presence of air (▪). Samples were withdrawn at the indicated time points and were assayed under anaerobic conditions for l-serine dehydratase activity as described in Materials and Methods. The data are from a single assay but are representative of two independent experiments. (C) Reactivation of l-serine dehydratase with Fe2+ and DTT. Active l-serine dehydratase in 50 mM potassium phosphate buffer, pH 7.0, was exposed to air for 24 h at 4°C until no activity could be detected. The enzyme was then transferred to an anaerobic chamber and was incubated for 1 h at 4°C with FeSO4 at the specified concentrations and with 10 mM DTT in each case. The resulting l-serine dehydratase activities were measured under anaerobic conditions. Reactivation is expressed as a percentage of the activity of the enzyme stored under anaerobic conditions for the duration of the inactivation in air and subsequent reactivation with Fe2+ and DTT.
C. jejuni sdaA mutants do not colonize the avian intestinal tract.
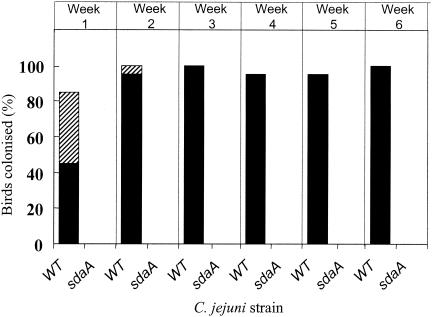
An sdaA mutant was employed to test the importance of serine dehydratase in the in vivo colonization of C. jejuni in a chicken model. Due to the lack of SdaA activity in the sdaC mutant, we could not test the in vivo role of SdaC separate from SdaA. The sdaA mutation was introduced into C. jejuni strain 11168H, a hypermotile variant previously described by Karlyshev et al. (13). This strain has been found to be colonization proficient to high levels in the chick intestinal tract (M. A. Jones, K. Marston, B. Wren, and P. A. Barrow, unpublished data). C. jejuni 11168, in contrast, has a persistent low-level infection and undergoes adaptation to be a high-level colonizer of chickens only over a period of weeks (Jones, Marston, and Barrow, unpublished). PCR was used to verify the correct construction of the 11168H sdaA mutant (data not shown), and we confirmed that this mutant had the expected growth defect on l-serine and was devoid of SdaA-mediated serine dehydratase enzyme activity. At weekly intervals after inoculation, the colonization data from cloacal swabs were expressed as a dual percentage of the groups of birds colonized as assessed by both direct counts and growth after incubation in an enrichment medium (Fig. 6). This semiquantitative method has been previously described in genetic mapping studies for colonization of chickens by Salmonella enterica serovar Typhimurium (1, 30) and has recently been used in studies on avian resistance to Campylobacter colonization (Y. Boyd, Jones, and Barrow, unpublished). Figure 6 clearly shows that the 11168H sdaA mutant failed to colonize the avian host to a detectable level when compared to the parent strain, which within 1 week had colonized 90 to 100% of the birds inoculated. In addition to the cloacal swab data, cecal content counts of sacrificed birds at 6 weeks postinfection showed that strain 11168H colonized to a level of 2.81 × 108 ± 1.92 × 108 CFU g−1, but the counts for the birds inoculated with the 11168H sdaA mutant were below detectable limits (<100 CFU g−1).
FIG. 6.
Colonization of 3-week-old chickens by C. jejuni 11168H (WT) and 11168H sdaA. Groups of 20 chickens with Campylobacter-free adult gut flora were inoculated with 107 CFU of either strain. At weekly intervals, cloacal swabs were taken and the extent of colonization was determined. The data are expressed as a dual percentage of the number of chickens colonized, as assessed by both direct counts (solid black bars) and enrichment counts (hatched bars). See Materials and Methods for further details.
DISCUSSION
In this study, the specific omission of l-serine from a defined growth medium resulted in the largest decrease in cell yields of all the amino acids tested, despite the presence of all three genes (serA, serB, and serC) required for serine biosynthesis (24). In the absence of serine, both the growth rate and final cell density were lowered. Previous studies had suggested that serine is preferentially utilized when C. jejuni is grown with a mixture of amino acids (17). Thus, serine catabolism appears to be especially important in the normal physiology of C. jejuni, presumably because the two products of serine deamination (pyruvate and ammonia) provide forms of carbon and nitrogen that can feed directly into the central metabolism. Pyruvate has been shown to be one of the best carbon sources for C. jejuni, as well as being the starting point for gluconeogenesis (32), and ammonia can be incorporated via the glutamine synthetase/glutamate:2-oxoglutarate aminotransferase route (24).
Serine transport in C. jejuni is carried out by at least two systems, a low-affinity l-serine-specific transporter encoded by sdaC and a high-affinity transporter that was revealed by analysis of the residual transport kinetics of the sdaC mutant. The hydropathy profile (16) of SdaC shows that it contains 10 or 11 potential membrane-spanning segments and that its overall topology is similar to the E. coli SdaC protein and other members of the hydroxy/aromatic amino acid permease family (26). The identity of the high-affinity transporter is unknown, but the competition studies suggest that it could be a dual serine-threonine transporter. This type of system is known in other bacteria, for example, the Na+-coupled threonine-serine uptake system in E. coli (10).
Several lines of evidence show that the sdaA gene encodes an l-serine dehydratase that is devoid of PLP. The enzyme lacks the consensus sequence centered around a lysyl residue to which PLP binds via a Schiff base (23), in addition to lacking the glycine-rich region reported for all PLP-dependent enzymes (19). Moreover, the carbonyl reagents phenylhydrazine and hydroxylamine did not significantly affect enzyme activity. The absorption spectrum of the purified protein was, however, typical of that for an iron-sulfur protein, with broad peaks at 300 to 350 and 420 nm. Consistent with this, the C. jejuni SdaA sequence contains four conserved cysteine residues, which could coordinate a [4Fe-4S] cluster. Moreover, both the spectral features and enzyme activity were lost upon exposure to air, but activity could be restored by treatment with ferrous iron and a reducing agent. These properties are similar to those exhibited by the l-serine dehydratases of many anaerobes, e.g., P. asaccharolyticus, which are PLP-independent, iron-sulfur-containing enzymes (8, 9). This family of enzymes share mechanistic similarities with the dehydration of citrate by aconitase (9), but they are generally highly specific for l-serine deamination. Although the purified C. jejuni enzyme did not detectably deaminate l-threonine, this amino acid and d-serine were weak inhibitors of both the purified enzyme and the activity in cell extracts. l-Cysteine inhibition was much more potent, and studies of the SdaA enzymes from several anaerobes have shown both d-serine and particularly l-cysteine to be competitive inhibitors (8, 12). The employment of an oxygen-labile dehydratase for serine catabolism is another example of the use by C. jejuni of a type of enzyme more commonly found in anaerobic bacteria than aerobes. Other examples include the pyruvate and 2-oxoglutarate:acceptor oxidoreductases, which are used instead of the 2-oxoacid dehydrogenase multienzyme complexes as found in most aerobes (14).
Mutation of sdaA resulted in a ∼10-fold reduction in the activity of serine dehydratase in cell extracts, and the residual activity was fully sensitive to inhibition by the carbonyl reagents phenylhydrazine and hydroxylamine. This, coupled with the inhibition of the residual activity by isoleucine, strongly indicates that the PLP-dependent biosynthetic l-threonine dehydratase (IlvA; Cj0828c) is responsible for the low but detectable rate of serine deamination in the mutant. However, this residual activity was unable to support growth of the mutant on l-serine in a minimal medium and there was no evidence from 1H NMR spectroscopy for significant utilization of l-serine by intact cells. Despite this clear phenotype and the apparent preference of C. jejuni for serine catabolism demonstrated by the study of Leach et al. (17) and in this study by the results of amino acid omission, growth of the sdaA mutant in a complex medium (BHI-FCS) was indistinguishable from that of the parent strain. This indicates that an inability to deaminate serine does not result in a growth disadvantage when a larger array of carbon sources in addition to amino acids are available. It was therefore considered important to determine if the ability of this mutant to colonize an animal model was compromised, in order to obtain information on whether serine is available and is preferentially utilized in vivo.
A variety of avian hosts are important natural reservoirs of C. jejuni, but the chicken is the most relevant in terms of the human food chain. Various chick infection models have been used very successfully in studies comparing colonization of different strains of wild-type or mutant bacteria (3, 21, 34), but in the field, birds are naturally colonized at 2 or 3 weeks of age. In this study, we have used 3-week-old chickens for colonization trials with a hypermotile variant of strain 11168 (13), which resulted in high-level colonization within 1 week, and this level of colonization was maintained for the duration of the experiments. The complete lack of colonization of the isogenic sdaA mutant, evidenced by the repeated lack of recovery of viable bacteria by either enrichment from cloacal swabs or by cecal content counts at the end of the experiment, clearly shows that serine dehydratase encoded by sdaA is an essential colonization factor for C. jejuni. The sdaA mutant shows only one phenotype relating to l-serine utilization; it has a growth rate identical to that of the parent strain in complex media and we have detected no increased sensitivity to oxidative stress, for example, that might lead to decreased survival in the host. Thus, the data suggest that a source of serine is readily available to the bacteria and that its catabolism via SdaA is a crucial determinant of the ability of C. jejuni to grow in the cecum. These results are surprising in view of the potentially diverse types of alternative carbon, nitrogen, or energy sources that would be present in this environment and indicate a high degree of selectivity in the types of amino acid utilized. An explanation is provided by the finding that aspartate, glutamate, serine, and proline are in fact the most abundant amino acids present in chicken excreta (25) and that these are the only amino acids (along with asparagine and glutamine) for which C. jejuni has the necessary catabolic enzymes.
Acknowledgments
This work was funded by a grant from the United Kingdom Biotechnology and Biological Sciences Research Council to D.J.K.
Editor: J. T. Barbieri
REFERENCES
- 1.Barrow, P. A., N. Bumstead, K. Marston, M. A. Lovell, and P. Wigley. Faecal shedding and intestinal colonisation of Salmonella enterica in in-bred chickens; the effect of host-genetic background. Epidemiol. Infect., in press. [DOI] [PMC free article] [PubMed]
- 2.Bayliss, C. L., S. McFee, K. W. Martin, T. J. Humphrey, and R. P. Betts. 2000. Comparison of three enrichment media for the isolation of Campylobacter spp. from foods. J. Appl. Microbiol. 89:884-891. [DOI] [PubMed] [Google Scholar]
- 3.Bras, A. M., S. Chatterjee, B. W. Wren, D. G. Newell, and J. M. Ketley. 1999. A novel Campylobacter jejuni two-component system important for temperature-dependent growth and colonization. J. Bacteriol. 181:3298-3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown, E. A., R. D'Ari, and E. B. Newman. 1990. A relationship between l-serine degradation and methionine biosynthesis in Escherichia coli K-12. J. Gen. Microbiol. 136:1017-1023. [DOI] [PubMed] [Google Scholar]
- 5.Carter, J. E., and R. D. Sagers. 1972. Ferrous ion-dependent l-serine dehydratase from Clostridium acidiurici. J. Bacteriol. 109:757-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrero, R. L., V. Cussac, P. Courcoux, and A. Labigne-Roussel. 1992. Construction of isogenic urease-negative mutants of Helicobacter pylori by allelic exchange. J. Bacteriol. 174:4212-4217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedman, C. R., J. Neiman, H. C. Wegener, and R. V. Tauxe. 2000. Epidemiology of Campylobacter jejuni in the United States and other industrialized nations, p. 121-138. In I. Nachamkin and M. J. Blaser (ed.), Campylobacter, 2nd ed. ASM Press, Washington D.C.
- 8.Grabowski, R., and W. Buckel. 1991. Purification and properties of an iron-sulfur-containing and pyridoxal-phosphate-independent l-serine dehydratase from Peptostreptococcus assacharolyticus. Eur. J. Biochem. 199:89-94. [DOI] [PubMed] [Google Scholar]
- 9.Grabowski, R., A. E. M. Hofmeister, and W. Buckel. 1993. Bacterial l-serine dehydratases: a new family of enzymes containing iron-sulfur clusters. Trends Biochem. Sci. 18:297-300. [DOI] [PubMed] [Google Scholar]
- 10.Hama, H., T. Shimamoto, M. Tsuda, and T. Tsuchiya. 1987. Properties of a Na+-coupled serine-threonine transport system in Escherichia coli. Biochim. Biophys. Acta 905:231-239. [DOI] [PubMed] [Google Scholar]
- 11.Henderson, P. J. F. 1992. Statistical analysis of enzyme kinetic data, p. 277-316. In R. Eisenthal and M. J. Danson (ed.), Enzyme assays. A practical approach. IRL Press, Oxford, United Kingdom.
- 12.Hofmeister, A. E. M., R. Grabowski, D. Linder, and W. Buckel. 1993. l-Serine and l-threonine dehydratase from Clostridium propionicum: two enzymes with different prosthetic groups. Eur. J. Biochem. 215:341-349. [DOI] [PubMed] [Google Scholar]
- 13.Karlyshev, A. V., D. Linton, N. A. Gregson, and B. W. Wren. 2002. A novel paralogous gene family involved in phase-variable flagella-mediated motility in Campylobacter jejuni. Microbiology 148:473-480. [DOI] [PubMed] [Google Scholar]
- 14.Kelly, D. J. 2001. The physiology and metabolism of Campylobacter jejuni and Helicobacter pylori. J. Appl. Microbiol. 90:16S-24S. [DOI] [PubMed] [Google Scholar]
- 15.Ketley, J. M. 1997. Pathogenesis of enteric infection by Campylobacter. Microbiology 143:5-21. [DOI] [PubMed] [Google Scholar]
- 16.Kyte, J., and R. F. Doolittle. 1982. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 157:105-132. [DOI] [PubMed] [Google Scholar]
- 17.Leach, S., P. Harvey, and R. Wait. 1997. Changes with growth rate in the membrane lipid composition of and amino acid utilization by continuous cultures of Campylobacter jejuni. J. Appl. Microbiol. 82:631-640. [DOI] [PubMed] [Google Scholar]
- 18.Leighton, M. P., D. J. Kelly, M. P. Williamson, and J. G. Shaw. 2001. An NMR and enzyme study of carbon metabolism in Neisseria meningitidis. Microbiology 147:1473-1482. [DOI] [PubMed] [Google Scholar]
- 19.Marceau, M., S. D. Lewis, and J. A. Shafer. 1988. The glycine-rich region of Escherichia coli d-serine dehydratase. Altered interaction with pyridoxal 5′-phosphate produced by substitution of aspartic acid for glycine. J. Biol. Chem. 263:16934-16941. [PubMed] [Google Scholar]
- 20.Marmur, J. 1961. A procedure for the isolation of deoxyribonucleic acid from microorganisms. J. Mol. Biol. 3:208-218. [Google Scholar]
- 21.Newell, D. G. 2001. Animal models of Campylobacter jejuni colonisation and disease and the lessons to be learned from similar Helicobacter pylori models. J. Appl. Microbiol. 90:57S-67S. [DOI] [PubMed] [Google Scholar]
- 22.Newman, E. B., D. Dumont, and C. Walker. 1985. In vitro and in vivo activation of l-serine deaminase in Escherichia coli K-12. J. Bacteriol. 162:1270-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ogawa, H., K. Konishi, and M. Fujioka. 1989. The peptide sequences near the bound pyridoxal phosphate are conserved in serine dehydratase from rat liver and threonine dehydratases from yeast and Escherichia coli. Biochim. Biophys. Acta 996:139-141. [DOI] [PubMed] [Google Scholar]
- 24.Parkhill, J., B. W. Wren, K. Mungall, J. M Ketley, C. Churcher, D. Basham, T. Chillingworth, R. M. Davies, T. Feltwell, S. Holroyd, K. Jagels, A. V. Karlyshev, S. Moule, M. J. Pallen, C. W. Penn, M. A. Quail, M.-A. Rajandream, K. M. Rutherford, A. H. M. van Vliet, S. Whitehead, and B. G. Barrell. 2000. The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature 403:665-668. [DOI] [PubMed] [Google Scholar]
- 25.Parsons, C. M., L. M. Potter, and R. D. Brown, Jr. 1983. Effects of dietary carbohydrate and of intestinal microflora on excretion of endogenous amino-acids by poultry. Poult. Sci. 62:483-489. [DOI] [PubMed] [Google Scholar]
- 26.Saier, M. 2000. A functional-phylogenetic classification system for transmembrane solute transporters. Microbiol. Mol. Biol. Rev. 64:354-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sambrook, H., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 28.Shao, Z. Q., R. T. Lin, and E. B. Newman. 1994. Sequencing and characterisation of the sdaC gene and identification of the sdaBC operon in Escherichia coli K-12. Eur. J. Biochem. 222:901-907. [DOI] [PubMed] [Google Scholar]
- 29.Smibert, R. M. 1984. Genus Campylobacter Sebald and Véron 1963, 907AL, p. 111-117. In N. R. Krieg and J. G. Holt (ed.), Bergey's manual of systematic bacteriology, vol. 1. Williams & Wilkins, Baltimore, Md.
- 30.Smith, H. W., and J. F. Tucker. 1975. The effect of antibiotic therapy on the fecal excretion of Salmonella typhimurium by experimentally infected chickens. J. Hyg. Camb. 75:275-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Vliet, A. H. M., K. G. Wooldridge, and J. M. Ketley. 1998. Iron-responsive gene regulation in a Campylobacter jejuni fur mutant. J. Bacteriol. 180:5291-5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Velayudhan, J., and D. J. Kelly. 2002. Analysis of gluconeogenic and anaplerotic enzymes in Campylobacter jejuni: an essential role for phosphoenolpyruvate carboxykinase. Microbiology 148:685-694. [DOI] [PubMed] [Google Scholar]
- 33.Wang, Y., and D. E. Taylor. 1990. Chloramphenicol resistance in Campylobacter coli: nucleotide sequence, expression, and cloning vector construction. Gene 94:23-28. [DOI] [PubMed] [Google Scholar]
- 34.Wassenaar, T. M., B. A. M. van der Zeijst, R. Ayling, and D. G. Newell. 1993. Colonisation of chicks by motility mutants of Campylobacter jejuni demonstrates the importance of flagellin A expression. J. Gen. Microbiol. 139:1171-1175. [DOI] [PubMed] [Google Scholar]