Abstract
The melanin concentrating hormone (MCH) system is a new target to treat human disorders. Our aim was the preparation of the first PET-tracer for the MCHR1. [11C]SNAP-7941 is a carbon-11 labeled analog of the published MCHR1 antagonist SNAP-7941. The optimum reaction conditions were 2 min reaction time, ≤25 °C reaction temperature, and 2 mg/mL precursor (SNAP-acid) in acetonitrile, using [11C]CH3OTf as methylation agent. [11C]SNAP-7941 was prepared in a reliable and feasible manner with high radiochemical yields (2.9±1.6 GBq; 11.5±6.4% EOB, n=15).
Keywords: MCHR1, SNAP-7941, Carbon-11, Radioligand, PET
Graphical Abstract
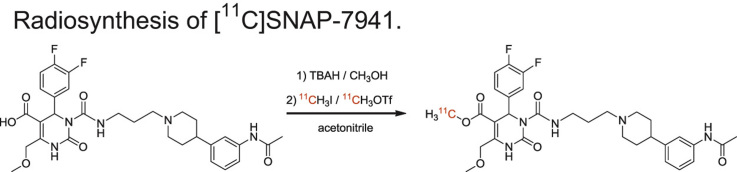
Radiosynthesis of [11C]SNAP-7941.
Highlights
► Synthesis of the first PET-tracer for the MCHR1 [11C]SNAP-7941. ► High radiochemical incorporation yields 2.9±1.6 GBq; 11.5±6.4% EOB. ► Preparation and characterization of a suitable labeling precursor; SNAP-acid.
1. Introduction
Melanin concentrating hormone (MCH) is a cyclic polypeptide, which was first isolated from the pituitary gland of the salmon as a hormone responsible for skin pigmentation (Kawauchi et al., 1983). In mammals, MCH is predominantly expressed in the lateral hypothalamus and zona incerta (Bittencourt et al., 1992; Casatti et al. 2002), and is also found in peripheral organs and tissues, such as the pancreas (Tadayyon et al., 2002), clonic epithelial cells (Kokkotou et al., 2008) or adipocytes (Bradley et al., 2000, 2002). It plays a key role in energy homeostasis, e.g. the control of food intake, body weight and metabolism (Ito et al., 2003; Marsh et al., 2002). Furthermore, it is involved in diabetes, gut inflammation and adiposity (Bradley et al., 2000, 2002; Kokkotou et al., 2008; Tadayyon et al., 2002). The biological function of MCH is mediated by two G-protein coupled receptors, MCH receptor 1 and 2 (MCHR1 (Chambers et al. 1999; Lembo et al.; 1999; Saito et al., 1999; Shimomura et al., 1999) and MCHR2 (An et al., 2001; Hill et al., 2001; Sailer et al. 2001; Wang et al., 2001)). The widespread distribution of MCH and its receptors and the involvement in a variety of pathologies make the MCH system interesting as a new target to treat human disorders. Several MCHR1 antagonists were presented in the last decade; some of them have entered clinical trials for the treatment of obesity (Luthin, 2007),while some are in the discussion of becoming anti-diabetic drugs (Gattrell et al., 2012). However, to enable confidence in preclinical to clinical translation of central MCHR1 pharmacology, a suitable Positron Emission Tomography (PET) tracer needs to be developed. Borowsky et al. (2002) presented the evaluation of a very potent MCHR1 antagonist, SNAP-7941 ((+)-methyl (4S)-3-{[(3-{4-[3-(acetylamino)phenyl]-1-piperidinyl}propyl)amino]carbonyl}-4-(3,4-difluorophenyl)-6-(methoxymethyl)-2-oxo-1,2,3,4-tetrahydro-5-pyrimidinecarboxylate hydrochloride, 1) as shown in Fig. 1. It is described to reduce food consumption and to decrease body weight in rats. The excellent binding affinity (Kd=0.18 nM) for the MCHR1 was one of the main reasons to choose this compound as target for radioactive labeling.
Fig. 1.
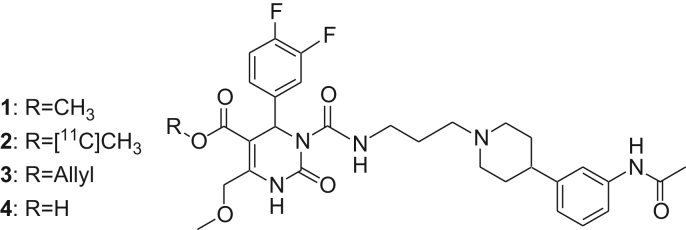
SNAP-7941 derivatives 1–4 (1: SNAP-7941; 2: [11C]SNAP-7941; 3: Allyl-SNAP; 4: SNAP-acid).
Hence, our aims were
-
1.
the preparation and characterization of a suitable labeling precursor (4, Fig. 1);
-
2.
the establishment of a radiosynthetic procedure for the preparation of the carbon-11 labeled analog, [11C]SNAP-7941 (2, Fig. 1) and its optimization;
-
3.
up-scaling and set-up of a fully automated preparation of [11C]SNAP-7941, including purification and formulation;
-
4.
set-up of a suitable quality control.
2. Experimental
2.1. Materials
All chemicals and solvents were obtained from commercial sources with analytical grade and used without further purification. Thin layer chromatography (TLC) was performed using TLC aluminum plates from Merck (silica gel 60 F254, no. 1.05554, 0.2 mm×20 cm×20 cm; reversed phase (RP)-18 F254s, no. 1.05559, 0.2 mm×20 cm×20 cm). Preparative TLC was performed using plates from the same company (silica gel 60 F254, no. 1.05717, 2 mm×20 cm×20 cm; RP-18 F254s, no. 1.05434, 0.2 mm×20 cm×20 cm). For column chromatography, silica gel 60 (70–230 mesh ASTM, no. 1.07734) or LiChroprep RP-18 silica gel (40–63 μm, 1.13900) from Merck was used. All chemicals and solvents for the synthesis of the precursor and reference compound were purchased from Sigma-Aldrich, Acros or VWR. Iodine (sublimated grade for analysis; ACS, Pharm.Eur.) was purchased from Merck (Darmstadt, Germany; product no. 1.04761.0100). Silver triflate impregnated carbon was prepared by dissolving 1 g of silver trifluoromethanesulfonate (Sigma-Aldrich, Vienna, Austria) in 20 mL acetonitrile (for DNA synthesis, ≤10 ppm H2O, Merck, Darmstadt, Germany). To this solution 3 g of Graphpac™-GC (80/100 mesh, Alltech, Deerfield, Illinois, USA) was added and the suspension was stirred in the dark for about 30 min. The solvent was removed under reduced pressure and the obtained powder was further dried in the dark for 2 h (rotary evaporator). Acetonitrile, ammonium acetate and ethanol (absolute) were purchased from Merck (Darmstadt, Germany). Tetrabutylammonium hydroxide 30-hydrate (≥98.0%) (TBAH), acetic acid, acetone, 2-butanone and methanol were obtained from Sigma-Aldrich (Vienna, Austria). Dimethylformamide (DMF) was obtained from Fluka (Buchs, Switzerland). 0.9% saline solution was purchased from B. Braun (Melsungen, Germany). 3% saline solution was obtained from a local pharmacy (Landesapotheke Salzburg, Austria). 125 mM phosphate buffer was prepared by dissolving 0.224 g sodium dihydrogenphosphate-monohydrate and 1.935 g disodiumhydrogenphosphate-dihydrate (both from Merck, Darmstadt, Germany) in 100 mL sterile water. Sterile water was purchased from Meditrade Medicare Medizinprodukte (Kufstein, Austria). Solid phase extraction (SPE) cartridges (SepPak® C18-plus) were purchased from Waters (Waters® Associates Milford, MA, USA). Semi-preparative high-performance liquid chromatography (HPLC) column (Chromolith® SemiPrep RP-18e; 100–10mm, precolumn: Chromolith® Guard Cartridge RP-18e; 5−4.6 mm) and analytical HPLC column (Chromolith® Performance RP-18e; 100−4.6 mm) were purchased from Merck (Darmstadt, Germany). Gas chromatography (GC) capillary column (forte GC Capillary Column ID-BP20; 12 m×0.22 mm×0.25 μm) was purchased from SGE Analytical Science Pty. Ltd. (Victoria, Australia).
2.2. Instrumentation
1H- and 13C-nuclear magnetic resonance (NMR) spectra were recorded on a Bruker Avance DPX-200 spectrometer at 27 °C (200.13 MHz for 1H, 50.32 MHz for 13C), a Varian UnityPlus 300 spectrometer at 28 °C (299.95 MHz for 1H, 75.43 MHz for 13C) or a Bruker Avance 500 spectrometer at 20 °C (500.13 MHz for 1H, 125.77 MHz for 13C, 470.59 MHz for 19F). Infrared spectroscopy (IR) spectra were recorded on a Perkin Elmer FT-IR spectrophotometer (Spectrum 1000). Mass spectra were obtained on a SHIMADZU GC/MS-Q95050A GC-17A instrument. High resolution mass spectra were recorded on a Finnigan MAT 8230 (EI 70 eV) or a Finnigan MAT 900 S (ESI, 4 kV, 3 μA, ACN/MeOH). Elemental analyses were performed at the Microanalytical Laboratory of the University of Vienna. [11C]CO2 was produced at a GE PET trace cyclotron (General Electric Medical System, Uppsala, Sweden) via the 14N(p,α)11C nuclear reaction by irradiation of a gas target (Aluminum) filled with N2 (+1% O2) (Air Liquide, Vienna, Austria). Typical beam currents were 48–50 μA and the irradiation was stopped as soon as the desired activity level was reached (approx. 50–65 GBq [11C]CO2, calculated by cyclotron operating software; corresponding to 30–40 min irradiation time). Generally, 7–12 GBq [11C]CH3 and 7–14 GBq [11C]CH3OTf, respectively, were obtained. The production of [11C]CH4, [11C]CH3I, [11C]CH3OTf and [11C]SNAP-7941 including semi-preparative HPLC were performed on a TRACERlab™ FX C Pro synthesis module (GE Healthcare, Uppsala, Sweden). Analytical HPLC was performed using a Merck-Hitachi LaChrom system consisting of a L-7100 pump, a Merck-Hitachi LaChrom L7400 UV-detector (operated at 254 nm) and a lead shielded NaI-radiodetector (Raytest Isotopenmessgeräte GmbH, Straubenhardt, Germany). Gas chromatography was performed using a 430-GC system (Burker Daltonik GmbH, Bremen, Germany). The osmolality was measured using a Wescor osmometer Vapro® 5600 (Sanova Medical Systems, Vienna, Austria) and pH was measured using a WTW inoLab 740 pH meter (WTW, Weilheim, Germany).
2.3. Organic chemistry
2.3.1. Precursor (SNAP-acid)
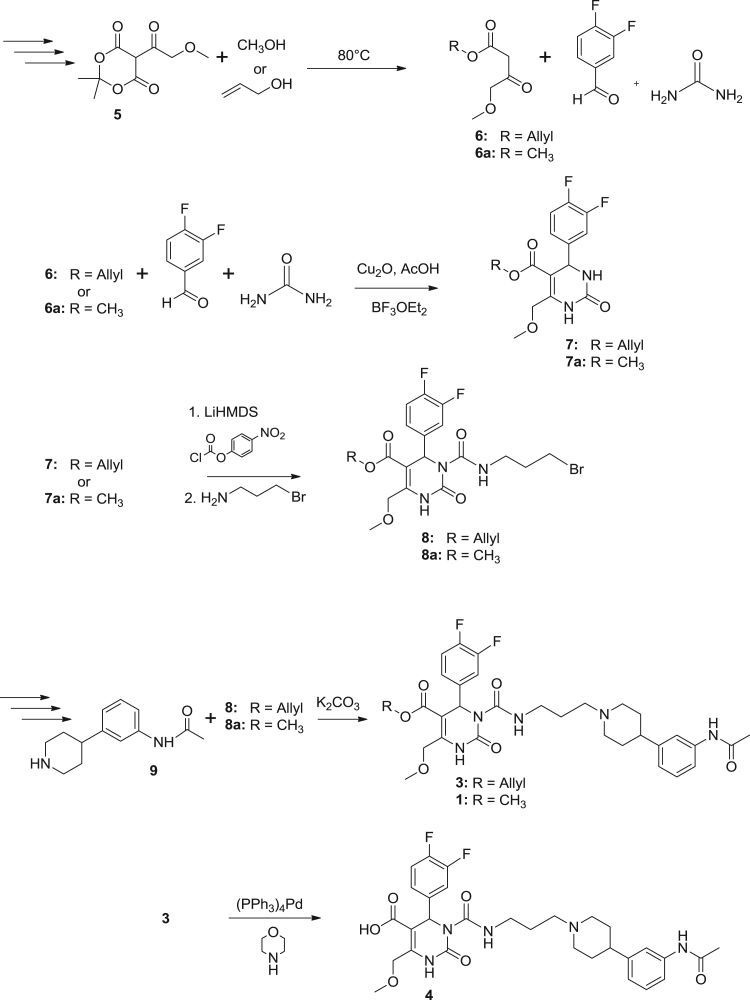
A reaction scheme is presented in Scheme 1. 6.40 g (29.60 mmol) of 5-(methoxyacetyl)-2,2-dimethyl-1,3-dioxane-4,6-dione (5; prepared in one step by condensation reaction of 2,2-dimethyl-1,3-dioxane-4,6-dione and methoxyacetyl chloride) and allyl alcohol (5.13 g, 88.33 mmol) were heated in toluene (90–100 mL) for 24 h at 80 °C. After distillation, the crude product was purified using column chromatography yielding 3.81 g (74.8%) prop-2-en-1-yl 4-methoxy-3-oxobutanoate (6) as yellowish oil. To a mixture of 3.70 g (21.50 mmol) of 6,3,4-difluorobenzaldehyde (3.15 g, 22.17 mmol), and urea (1.94 g, 32.30 mmol) in THF (18.4 ml) Cu2O (310 mg, 2.17 mmol) and acetic acid (130 μl) were added at room temperature, followed by dropwise addition of boron trifluoride diethyletherate (3.4 mL, 3.89 g, 24.43 mmol). The slurry was refluxed for 8 h, cooled, poured on a mixture of ice (30 g) and NaHCO3 (6 g) and filtered through Celite. After extraction with CH2Cl2 the solvent was evaporated and the purification of the residue was performed using column chromatography yielding 6.65 g (91.5%) prop-2-en-1-yl 4-(3,4-difluorophenyl)-6-(methoxymethyl)-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (7) as yellowish oil. To pyrimidinone 7 (6.54 g, 19.33 mmol) and 4-nitrophenyl chloroformate (13.65 g, 67.72 mmol) in THF (242.0 mL) LiHMDS (9.05 g, 54.06 mmol, 1 M in THF) was slowly added at −78 °C. After 10 min the reaction was quenched with H2O (6.1 mL) and warmed to 0 °C. K2CO3 (10.68 g, 77.27 mmol) and 3-aminopropylbromide hydrobromide (12.69 g, 57.96 mmol) were added and stirred overnight at room temperature. After washing with a solution of NaHCO3, extraction with Et2O, and subsequent column chromatography, prop-2-en-1-yl 3-[(3-bromopropyl)carbamoyl]-4-(3,4-difluorophenyl)-6-(methoxymethyl)-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (8) was obtained in good yield (8.50 g, 87.7%). 4.60 g (21.07 mmol) of N-[3-(piperidin-4-yl)phenyl]acetamide (9; prepared according to Schönberger (2006) in a 5-step-synthesis starting from N-Boc-piperidinone) was solved in acetonitrile and after addition of 8 (6.90 g, 13.74 mmol) and K2CO3 (21.08 g, 152.52 mmol) stirred under argon atmosphere for 37 h at 35 °C. After filtration and evaporation in vacuo of the solvent, the residue was washed twice with a solution of NaHCO3, extracted with ethyl acetate purified via column chromatography to obtain allyl-SNAP 3 (allyl-3-(3-(4-(3-acetamindoophenyl)piperidin-1-yl)propylcarbamoyl)-4-(3,4-difluorphenyl)-6-(methoxymethyl)-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylic ester)(3.70 g, 42.1%) as a pale yellow solid. After dissolving 3 (2.44 g, 3.81 mmol) in THF, (PPh3)4Pd (530 mg, 0.46 mmol) and morpholine (3.98 g, 44.38 mmol) were added under an argon atmosphere. After 17 h THF was evaporated and the residue was purified using column chromatography. 660 mg of SNAP-acid 4 (3-(3-(4-(3-acetamidophenyl)piperidin-1-yl)propylcarbamoyl)-4-(3,4-difluorophenyl)-6-(methoxymethyl)-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylic acid) (28.9%) was obtained after purification as an amorphic pale yellow solid.
Scheme 1.
Reaction scheme for the preparation of the precursor SNAP-acid (4) and the reference standard rac-SNAP-7941 (1).
All intermediates and products were analyzed spectroscopically via NMR, MS, and HRMS. For NMR analysis, the solvent signal was used as an internal standard which was related to TMS with δ=7.26 ppm (1H in CDCl3) and δ=77.0 ppm (13C in CDCl3).
6: 1H-NMR (200 MHz, CDCl3): δ (ppm) 3.39 (s, 3H, OCH3), 3.51 (s, 2H, 2-CH2), 4.05 (s, 2H, 4-OCH2), 4.61 (d, J=5.7 Hz, 2H, Allyl-OCH2), 5.20-5.35 (m, 2H, Allyl-CH2), 5.79-5.98 (m, 1H, Allyl-CH) 13C-NMR (50 MHz, CDCl3): δ (ppm) 45.6 (2-CH2), 59.3 (OCH3), 65.9 (Allyl-OCH2), 77.3 (4-OCH2), 118.7 (Allyl-CH2), 131.4 (Allyl-CH), 166.6 (1-COO), 201.4 (3-CO) MS: m/z (%) 173 (1), 172 (1), 115 (47), 84 (6), 69 (14), 55 (11), 45 (1 0 0), 43 (8), 42 (7), 41 (24) HRMS: m/z calculated for C8H12O4: 172.0736. Found: 172.0735.
7: 1H-NMR (500 MHz, CDCl3): δ (ppm) 3.44 (s, 3H, 7-OCH3), 4.48–4.56 (m, 2H, Allyl-OCH2), 4.64 (d, 2H, J=16.5 Hz, 6-OCH2), 5.17 (m, 2H, Allyl-CH2), 5.34 (d, 2H, J=3.0 Hz, 3-CH), 5.79 (m, 1H, Allyl-CH), 6.77 (s, 1H, 2a-NH), 7.02 (m, 1H, 15-CH), 7.06 (m, 1H, 14-CH), 7.11 (m, 1H, 11-CH), 7.66 (s, 1H, 1-NH) 13C-NMR (126 MHz, CDCl3): δ (ppm) 54.5 (53-CH), 59.1 (7-OCH3), 68.5 (6-OCH2), 98.2 (4-C), 115.7 (d, J=17.6 Hz, 11-CH), 117.4 (d, J=17.4 Hz, 14-CH), 118.4 (Allyl-CH2), 122.5 (15-CH), 131.8 (Allyl-CH), 140.4 (t, J=4.1 Hz, 10-C), 147.8 (5-C), 149.9 (12-CF), 150.3 (13-CF), 152.2 (2-CO), 164.3 (8-COO) 19F-NMR (471 MHz, CDCl3): δ (ppm) −136.7 (m, 12-CF), −138.5 (m, 13-CF) MS: m/z (%) 338 (11), 297 (47), 265 (53), 261 (34), 253 (32), 225 (1 0 0), 194 (28), 184 (51), 169 (35), 167 (33), 151 (32), 140 (27), 45 (33), 41 (98) HRMS: m/z calculated for C16H16F2N2O4: 339.1156. Found: 339.1150.
8: 1H-NMR (200 MHz, CDCl3): δ (ppm) 1.20–1.30 (m, 2H, 20-CH2Br), 2.02–2.15 (m, 2H, 19-CH2), 3.38 (t, J=6.4 Hz, 2H, 18-CH2), 3.46 (s, 3H, 7-OCH3), 4.59–4.62 (m, 2H, Allyl-OCH2), 4.67 (s, 2H, 6-CH2), 5.17–5.26 (m, 2H, Allyl-CH2), 5.74–5.94 (m, 1H, Allyl-CH), 6.66 (s, 1H, 3-CH), 7.02–7.21 (m, 3H, 11-CH, 14-CH, 15-CH), 7.88 (s, 1H, 1-NH), 9.08 (t, 1H, J=5.6 Hz, 17-NH) 13C-NMR (50 MHz, CDCl3): δ (ppm) 30.2 (20-CH2), 31.9 (19-CH2), 39.3 (18-CH2), 53.4 (3-CH), 59.1 (7-CH3), 65.6 (Allyl-OCH2), 68.0 (6-CH2), 101.4 (4-C), 115.9/116.2 (11-CH), 117.3/117.6 (14-CH), 118.8 (Allyl-CH2), 122.8/122.88/122.93/123.0 (15-CH), 131.3 (Allyl-CH), 141.0 (10-C), 146.3 (5-C), 152.4 (2-CO), 153.8 (16-CO), 163.8 (8-COO) MS: m/z (%) 502 (1), 463 (12), 420 (5), 337 (23), 279 (13), 261 (14), 168 (22), 142 (16), 56 (15), 45 (15), 43 (15), 41 (100) HRMS: m/z calculated for C20H22F2N3O5BrNa [M+Na]+: 524.0609. Found: 524.0611.
3: 1H-NMR (500 MHz, CDCl3): δ (ppm) 1.73–1.83 (m, 4H, 22,22′-(CH2)2), 1.74 (m, 2H, 19-CH2), 1.99 and 2.99 (m, 4H, 21,21′-(CH2)2), 2.16 (s, 3H, 32-CH3), 2.40 (t, 2H, J=7.0 Hz, 20-CH2), 2.44 (m, 1H, 23-CH), 3.32 and 3.40 (m, 2H, 18-CH2), 3.43 (s, 3H, 7-CH3), 4.54–4.64 (m, 2H, Allyl-CH2), 4.67 (s, 2H, 6-CH2), 5.20–5.21 (m, 2H, Allyl-CH2), 5.85 (m, 1H, Allyl-CH), 6.69 (s, 1H, 3-CH), 6.95 (d, 1H, J=7.6 Hz, 29-CH), 7.05 (m, 1H, 14-CH), 7.10 (m, 1H, 15-CH), 7.19 (m, 1H, 11-CH), 7.20 (m, 1H, 28-CH), 7.28 (d, 1H, J=8.0 Hz, 27-CH), 7.47 (s, 1H, 25-CH), 7.58 (s, 1H, 30-NH), 8.00 (s, 1H, 1-NH), 8.99 (t, 1H, J=5.4 Hz, 17-NH) 13C-NMR (126 MHz, CDCl3): δ (ppm) 24.5 (32-CH3), 26.4 (19-CH2), 33.0 (22,22′-(CH2)2), 39.7 (18-CH2), 42.7 (23-CH), 53.0 (3-CH), 54.33, 54.37 (21,21′-(CH2)2), 56.7 (20-CH2), 59.0 (7-CH3), 65.2 (Allyl-CH2), 68.0 (6-CH2), 101.6 (4-C), 116.2 (11-CH), 117.2 (14-CH), 117.5 (27-CH), 118.3 (25-CH), 118.6 (Allyl-CH2), 122.8 (29-CH), 123.1 (15-CH), 128.8 (28-CH), 131.6 (Allyl-CH), 137.7 (10-C), 138.0 (26-C), 146.5 (5-C), 147.4 (24-C), 149.9 (12- or 13-CF), 150.1 (12- or 13-CF), 152.2 (2-CO), 153.1 (16-CO), 163.8 (8-COO), 168.5 (31-CON) 19F-NMR (471 MHz, CDCl3): δ (ppm) −136.9 (m, 12- or 13-CF), −138.5 (m, 12- or 13-CF) MS: m/z (%) 641 (7), 640 (19), 628 (8), 346 (2), 345 (13), 324 (3), 303 (19), 302 (100) HRMS: m/z calculated C33H40F2N5O6 [M+H]+: 640.2947. Found: 640.2956. IR: (ν) (cm−1) 3311, 3147, 3087, 2937, 2809, 2771, 1718, 1674, 1647, 1610, 1593, 1541, 1517, 1490, 1468, 1437, 1393, 1371, 1306, 1280, 1211, 1115, 1077, 766.
4: 1H-NMR (200 MHz, d6-DMSO): δ (ppm) 1.52–1.66 (m, 6H, 19-CH2, 22,22′-(CH2)2), 1.89–2.01 (m, 5H, 21,21′–CH2, 32-CH3), 2.36 (m, 3H, 20-CH2, 23-CH), 2.98–2.99 (m, 2H, 21,21′–CH2), 3.22 (m, 5H, 7-OCH3, 18-CH2), 4.59 (dd, 2H, J=12.7 Hz, J=84.0 Hz, 6-CH2), 6.61 (s, 1H, 3-CH), 6.87 (d, 1H, J=6.4 Hz, 29-CH), 7.03–7.48 (m, 7H, 11-CH, 14-CH, 15-CH, 25-CH, 27-CH, 28-CH, 30-NH), 7.77–7.83 (m, 1H, 1-NH), 8.96 (m, 1H, 17-NH), 9.89 (s, 1H, 8-COOH) 13C-NMR (50 MHz, d6-DMSO): δ (ppm) 24.0 (32-CH3), 25.8 (19-CH2), 32.4 (22,22′-(CH2)2), 38.9 (18-CH2), 41.7 (23-CH), 52.5 (3-CH), 53.5 (21,21′-(CH2)2), 55.6 (20-CH2), 57.7 (7-OCH3), 66.3 (6-OCH2), 115.0/115.3 (11-CH), 116.9/117.2 (14-CH), 117.4 (27-CH), 117.7 (25-CH), 121.5 (29-CH), 122.6/122.8/122.8 (15-CH), 128.6 (28-CH), 139.1 (10-C), 139.4 (26-C), 146.1 (5-C), 146.6 (24-C), 152.9 (2-CO), 153.2 (16-CO), 168.1 (31-CON), 168.2 (8-COOH) MS: m/z (%) 601 (4), 600 (15), 579 (7), 345 (11), 324 (8), 303 (18), 302 (100), 301 (26), 279 (5) HRMS: m/z calculated for: C30H34F2N5O6 [M–H]-: 598.2477. Found: 598.2462 IR: (ν) (cm−1) 3416, 3256, 2961, 2925, 2853, 1711, 1685, 1651, 1610, 1556, 1514, 1489, 1424, 1375, 1261, 1223, 1096, 1021, 874, 799, 703.
2.3.2. Reference standard (rac-SNAP-7941)
Reference standard rac-SNAP-7941 (1, Fig. 1) was obtained according to Schönberger (2006). Preparation of all intermediates took place in analogy to the route described in Section 2.3 and is shown in Scheme 1. Purification was performed using column chromatography. 4.51 g (77%) rac-SNAP-7941 was obtained after purification as a yellow oil. NMR analysis (1H and 13C) of the final compound was in full accordance with the literature (Schönberger, 2006).
2.4. Radiochemistry
2.4.1. Synthesis of [11C]SNAP-7941
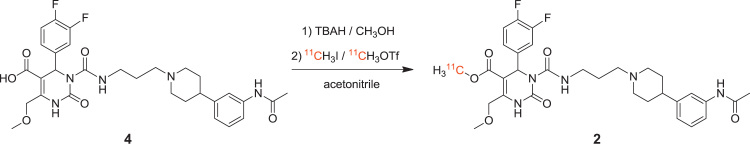
A reaction scheme is presented in Scheme 2. Using the TRACERlab™ FX C Pro synthesis module, [11C]CH3I and [11C]CH3OTf, respectively, were bubbled through a solution of SNAP-acid (0.01–4mg/mL, 0.02–6.67 mmol) in 500 μL solvent containing TBAH (1 equivalent). Different solvents (DMF (only for reactions with [11C]CH3I), acetone, 2-butanone and acetonitrile (≤10 ppm H2O; only for reactions with [11C]CH3OTf) and different reaction temperatures (DMF: 100–140 °C; acetone: 50 °C; 2-butanone: 20–75 °C and acetonitrile: 0–75 °C) were tested. After 1–10 min reaction time, the reaction mixture was quenched with water and the radiochemical yield was determined using analytical radio-HPLC (mobile phase: (water/acetic acid 97.5/2.5 v/v; 2.5 g/L ammonium acetate; pH 3.5)/acetonitrile 70/30 v/v; flow: 1 mL/min). Chromatograms were registered using an UV-detector (254 nm) and a NaI radioactivity detector in series.
Scheme 2.
Reaction scheme of the radiosynthesis of [11C]SNAP-7941 (2).
2.4.2. Purification of [11C]SNAP-7941
The crude reaction mixture was injected into the build-in HPLC system (mobile phase: (water/acetic acid 97.5/2.5 v/v; 2.5 g/L ammonium acetate; pH 3.5)/acetonitrile 75/25 v/v; flow: 8 mL/min, after 6.5 min: 10 mL/min). Chromatograms were registered using an UV-detector (254 nm) and a NaI radioactivity detector in series. The retention times were 2.8–3.6 min (k′=1.8–2.6) for SNAP-acid and 8.1–9.7 min (k′=7.1–8.7) for [11C]SNAP-7941. The [11C]SNAP-7941 fraction was cut and diluted with 100 mL water. The resulting solution was then pushed through a C18 SPE cartridge. After washing with 10 mL water the purified product was completely eluted with 1.5 mL of ethanol and 5 mL 0.9% saline solution. Formulation was done with a further 9 mL of physiological saline (0.9%), 1 mL of saline solution (3%) and 1 mL of phosphate buffer (125 nM). Hence, the final total volume was 17.5 mL.
2.4.3. Quality control of [11C]SNAP-7941
Chemical and radiochemical impurities were detected using an analytical radio-HPLC (for conditions see Radiosynthesis section). Retention times were 3.1–4.1 min (k′=0.6–1.1) for SNAP-acid and 5.3–7.9 min (k′=1.7–3.0) for [11C]SNAP-7941. The chemical identity of [11C]SNAP-7941 was determined by co-injection of the unlabeled reference compound, SNAP-7941. Residual solvents were analyzed by GC. Osmolality and pH were checked with dedicated equipment.
3. Results and discussion
3.1. Organic synthesis
A synthetic route to the precursor compound SNAP-acid (4) (660 mg, 28.9%) could be established via deallylation of allyl-SNAP (3). The use of other protecting groups (i.e. t-butyl, trimethylsilyl and p-methoxybenzyl) could not provide the desired precursor. The reference standard rac-SNAP-7941 (1) could be synthesized in good yields (4.51 g, 77%) and purity for the evaluation of the radiosynthesis.
3.2. Radiochemistry
The first preparation of [11C]SNAP-7941 followed by a fully-automated synthesis and purification was successful. Using [11C]CH3I as methylation agent, the radiochemical incorporation yield of [11C]SNAP-7941 was below 4% in all tested conditions (reaction solvent, reaction temperature, reaction time, precursor concentration.). Using [11C]CH3OTf as methylation agent, the radiochemical incorporation yield was still unsatisfactory low in acetone (< 2.5%). Reasonable yields could be achieved using 2-butanone (up to 45%) or acetonitrile (up to 50%) as reaction solvent. Acetonitrile evinced as the most suitable solvent regarding yield and avoidance of separation problems in preparative HPLC.
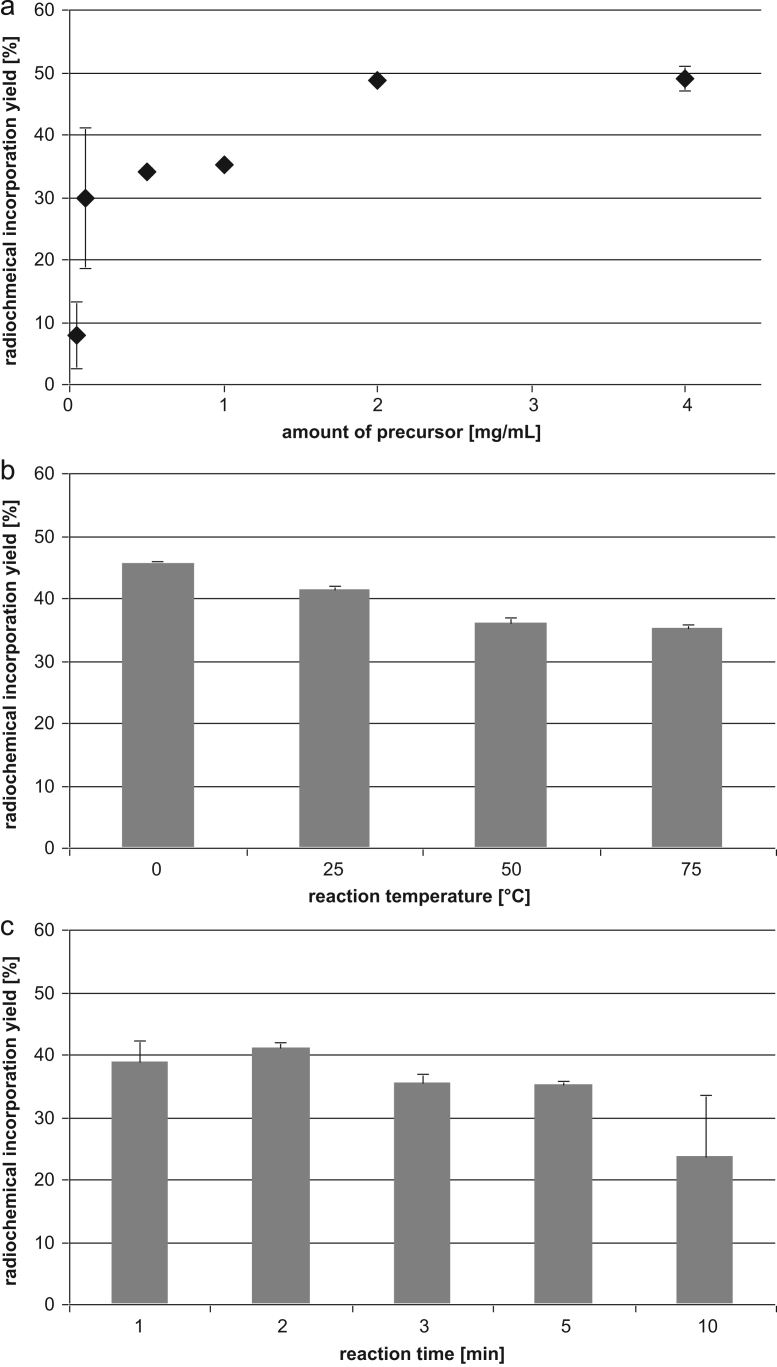
The evaluation of the precursor concentration, reaction temperature and reaction time using [11C]CH3OTf as methylation agent in acetonitrile is shown in Fig. 2. Highest radiochemical incorporation yields could be obtained using 4 mg/mL (49.0±2.0%) and 2 mg/mL (48.7±0.5%). No significant difference in the radiochemical incorporation yield was found between these two precursor concentrations. Significantly (P<0.01) lower yields were obtained with≤1 mg/mL of precursor. The radiochemical incorporation yields (n≥2) of [11C]SNAP-7941 (75 °C, 5 min) ranged from 8.8±6.4% using 0.05 mg/mL of precursor up to 49.0±2.0% using 4 mg/mL of precursor. No radiochemical conversion was observed using precursor concentrations <0.05 mg/mL (Fig. 2a).
Fig. 2.
Dependence of the radiochemical incorporation yield of [11C]SNAP-7941 (n≥2) on (a) amount of precursor (75 °C, 5 min), (b) reaction temperature (1 mg/mL, 5 min) (c) and reaction time (1 mg/mL, 75 °C). If not visible, error bars are within the margin of the symbols.
A clear inverse trend was found between temperatures versus radiochemical incorporation yields. At 1 mg/mL precursor for 5 min the radiochemical incorporation decreases with higher temperature: 45.7±0.3% at 0 °C, 41.4±0.6% at 25 °C, 36.2±0.6% at 50 °C and 35.3±0.6% at 75 °C (Fig. 2b). This trend was not due to the production of more side-products at higher temperatures, but due to the better conversion of [11C]CH3OTf at lower temperatures.
Regarding the reaction time (1 mg/mL precursor, 75 °C), the maximum radiochemical incorporation yield (41.1±1.0%) was achieved after 2 min (Fig. 2c). Although the maximum yield was achieved after 2 min reaction time, there was no significant difference within the tested conditions (1–10 min). Hence, the optimum reaction conditions were determined to be 2 min reaction time at ≤25 °C reaction temperature using 2 mg/mL precursor in 500 μL acetonitrile (Table 1).
Table 1.
Optimum reaction conditions and outcome for large scale preparation of [11C]SNAP-7941 (n=15).
| Reaction temperature (°C) | ≤25 |
|---|---|
| Reaction time (min) | 2 |
| Amount of precursor (mg/mL) | 2 |
| Yield (GBq)a | 2.9±1.6 |
| Yield (%)b | 17.5±3.6 |
| Specific activity (GBq/μmoL)a | 28.9±9.4 |
| Radiochemical purity (%) | >99 |
At end of synthesis (EOS).
Based on [11C]CH3OTf, corrected for decay.
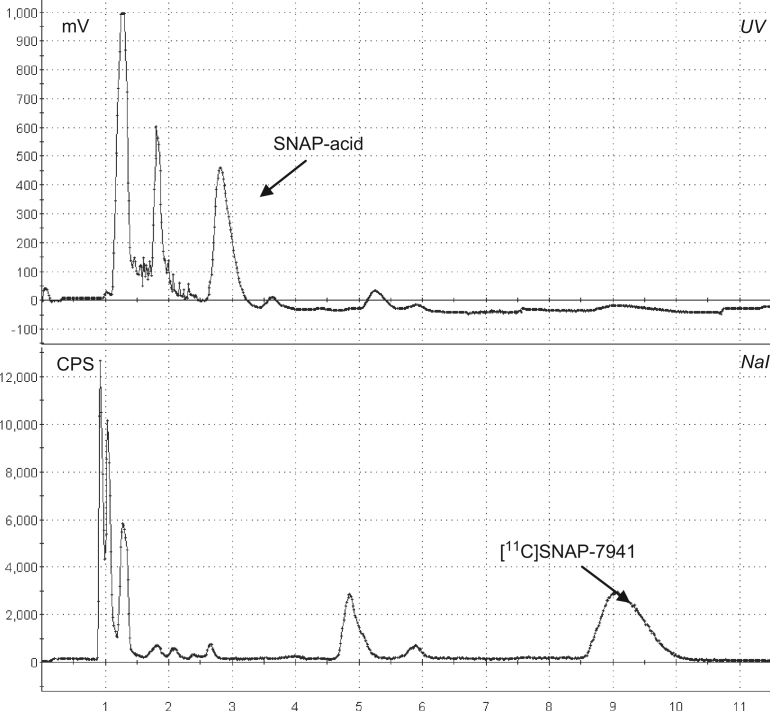
Purification using semi-preparative HPLC was straight forward using a monolithic reversed-phase column at moderate flow rates (8–10 mL/min). Sample chromatograms are shown in Fig. 3. Subsequent SPE purification resulted in a recovery of >95% of [11C]SNAP-7941. Until now, 15 complete high-scale radiosyntheses were performed (2 mg/mL precursor, 25 °C, 2 min). 2.9±1.6 GBq of formulated [11C]SNAP-7941 (17.5±3.6%, based on [11C]CH3OTf; 11.5±6.4% EOB) was produced within <40 min.
Fig. 3.
Semi-preparative HPLC chromatogram of the reaction solution of [11C]SNAP-7941.
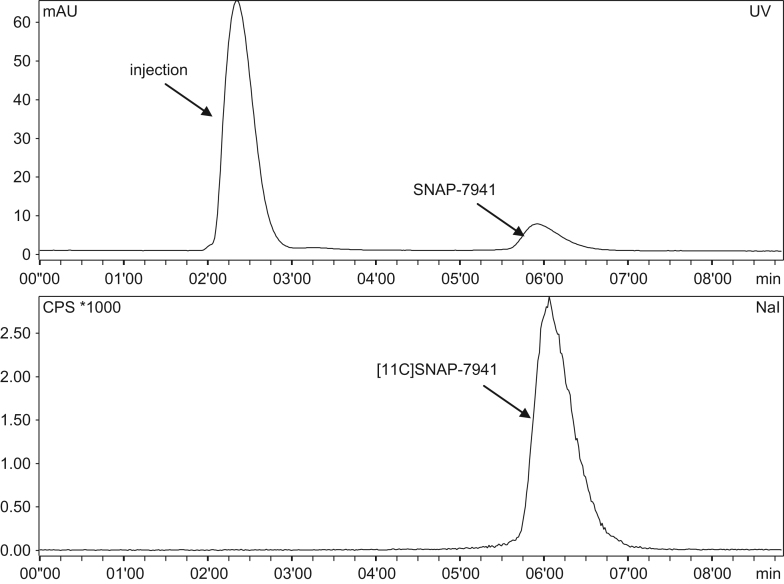
Full quality control was completed within 9 min. Radiochemical purity always exceeded 99% as determined by radio-HPLC. No residual precursor mass was detected but 9.6±6.4 μg of 12C-SNAP-7941 was found in the final product solution. Specific activity was determined via HPLC and found to be 28.9±9.4 GBq/μmol at the end of synthesis (EOS). Sample chromatograms are shown in Fig. 4. Residual solvent analysis revealed <5 ppm acetonitrile and no other impurities except ethanol (8.5%). Osmolality was 291±14 mosmol/kg and pH was 7.5±0.2.
Fig. 4.
Typical chromatogram of purified and formulated [11C]SNAP-7941 using analytical HPLC.
Achieved yields are sufficient to allow for preclinical and potentially clinical investigations. Due to general problems with the production of [11C]CO2 during the time frame of the presented study, specific activity was relatively low (28.9±9.4 GBq/μmol). Higher specific activities of [11C]SNAP-7941 are expected for future syntheses. Thus, the presented work is the basis for future applications that will further elucidate the role of MCHR1 in obesity, diabetes and several other pathologies.
4. Conclusion
[11C]SNAP-7941, the first PET-tracer for the MCHR1, was prepared in a reliable and feasible manner, starting from a suitable labeling precursor (SNAP-acid). The optimized reaction conditions evinced sufficient overall yields for subsequent preclinical and clinical investigations.
Acknowledgment
This research was part of an ongoing study, funded by the Austrian Science Fund (FWF P20977-B09; P.I.: M. Mitterhauser).
References
- An S., Cutler G., Zhao J.J., Huang S.G., Tian H., Li W., Liang L., Rich M., Bakleh A., Du J., Chen J.L., Dai K. Identification and characterization of a melanin-concentrating hormone receptor. Proc. Natl. Acad. Sci. USA. 2001;98:7576–7581. doi: 10.1073/pnas.131200698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittencourt J.C., Presse F., Arias C., Peto C., Vaughan J., Nahon J.L., Vale W., Sawchenko P.E. The melanin-concentrating hormone system of the rat brain: an immuno- and hybridization histochemical characterization. J. Comp. Neurol. 1992;319:218–245. doi: 10.1002/cne.903190204. [DOI] [PubMed] [Google Scholar]
- Borowsky B., Durkin M.M., Ogozalek K., Marzabadi M.R., DeLeon J., Lagu B., Heurich R., Lichtblau H., Shaposhnik Z., Daniewska I., Blackburn T.P., Branchek T.A., Gerald C., Vaysse P.J., Forray C. Antidepressant, anxiolytic and anorectic effects of a melanin-concentrating hormone-1 receptor antagonist. Nat. Med. 2002;8:825–830. doi: 10.1038/nm741. [DOI] [PubMed] [Google Scholar]
- Bradley R.L., Kokkotou E.G., Maratos-Flier E., Cheatham B. Melanin-concentrating hormone regulates leptin synthesis and secretion in rat adipocytes. Diabetes. 2000;49:1073–1077. doi: 10.2337/diabetes.49.7.1073. [DOI] [PubMed] [Google Scholar]
- Bradley R.L., Mansfield J.P., Maratos-Flier E., Cheatham B. Melanin-concentrating hormone activates signaling pathways in 3T3-L1 adipocytes. Am. J. Physiol. Endocrinol. Metab. 2002;283:E584–E592. doi: 10.1152/ajpendo.00161.2002. [DOI] [PubMed] [Google Scholar]
- Casatti C.A., Elias C.F., Sita L.V., Frigo L., Furlani V.C.G., Bauer J.A., Bittencourt J.C. Distribution of melanin-concentrating hormone neurons projecting to the medial mammillary nucleus. Neuroscience. 2002;115:899–915. doi: 10.1016/s0306-4522(02)00508-0. [DOI] [PubMed] [Google Scholar]
- Chambers J., Ames R.S., Bergsma D., Muir A., Fitzgerald L.R., Hervieu G., Dytko G.M., Foley J.J., Martin J., Liu W.S., Park J., Ellis C., Ganguly S., Konchar S., Cluderay J., Leslie R., Wilson S., Sarau H.M. Melanin-concentrating hormone is the cognate ligand for the orphan G-protein-coupled receptor SLC-1. Nature. 1999;400:261–265. doi: 10.1038/22313. [DOI] [PubMed] [Google Scholar]
- Gattrell W.T., Sambrook Smith C.P., Smith A.J. An example of designed multiple ligands spanning protein classes: dual MCH-1 R antagonists/DPPIV inhibitors. Bioorg. Med. Chem. Lett. 2012;22:2464–2469. doi: 10.1016/j.bmcl.2012.02.010. [DOI] [PubMed] [Google Scholar]
- Hill J., Duckworth M., Murdock P., Rennie G., Sabido-David C., Ames R.S., Szekeres P., Wilson S., Bergsma D.J., Gloger I.S., Levy D.S., Chamber J.K., Muir A.I. Molecular cloning and functional characterization of MCH2, a novel human MCH receptor. J. Biol. Chem. 2001;276:20125–20129. doi: 10.1074/jbc.M102068200. [DOI] [PubMed] [Google Scholar]
- Ito M., Gomori A., Ishihara A., Oda Z., Mashiko S., Matsushita H., Yumoto M., Ito M., Sano H., Tokita S., Moriya M., Iwaasa H., Kanatani A. Characterization of MCH-mediated obesity in mice. Am. J. Physiol. Endocrinol. Metab. 2003;284:E940–E945. doi: 10.1152/ajpendo.00529.2002. [DOI] [PubMed] [Google Scholar]
- Kawauchi H., Kawazoe I., Tsubokawa M., Kishida M., Baker B.I. Characterization of the melanin-concentrating hormone in chum salmon pituitaries. Nature. 1983;305:321–323. doi: 10.1038/305321a0. [DOI] [PubMed] [Google Scholar]
- Kokkotou E., Moss A.C., Torres D., Karagiannides I., Cheifetz A., Liu S., O'Brian M., Maratos-Flier E., Pothoulakis C. Melanin-concentrating hormone as a mediator of intestinal inflammation. Proc. Natl. Acad. Sci. USA. 2008;105:10613–10618. doi: 10.1073/pnas.0804536105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lembo P.M.C., Grazzini E., Cao J., Hubatsch D.A., Pelletier M., Hoffert C., St-Onge S., Pou C., Labreque J., Groblewski T., O'Donnell D., Payza K., Ahmad S., Walker P. The receptor for the orexigenic peptide melanin-concentrating hormone is a G-protein-coupled receptor. Nat. Cell. Biol. 1999;1:267–271. doi: 10.1038/12978. [DOI] [PubMed] [Google Scholar]
- Luthin D.R. Anti-obesity effects of small molecule melanin-concentrating hormone receptor1 (MCHR1) antagonists. Life Sci. 2007;81:423–440. doi: 10.1016/j.lfs.2007.05.029. [DOI] [PubMed] [Google Scholar]
- Marsh D.J., Weingarth D.T., Novi D.E., Chen H.Y., Turmbauer M.E., Chen A.S., Guan X.M., Jiang M.M., Feng Y., Camacho R.E., Shen Z., Frazier E.G., Yu H., Metzger J.M., Kuca S.J., Shearman L.P., Gopal-Truter S., MacNeil D.J., Strack A.M., MacIntyre D.E., Van der Ploeg L.H.T., Qian S. Melanin-concentrating hormone 1 receptor-deficient mice are lean, hyperactive, and hyperphagic and have altered metabolism. Proc. Natl. Acad. Sci. USA. 2002;99:3240–3245. doi: 10.1073/pnas.052706899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sailer A.W., Sano H., Zeng Z., McDonald T.P., Pan J., Pong S.S., Feighner S.D., Tan C.P., Fukami T., Iwaasa H., Hreniuk D.L., Morin N.R., Sadowski S.J., Ito M., Ito M., Bansal A., Ky B., Figueroa D.J., Jiang Q., Austin C.P., MacNeil D.J., Ishihara A., Ihara M., Kanatani A., Van der Ploeg L.H.T., Howard A.D., Liu Q. Identification and characterization of a second melanin-concentrating hormone receptor, MCH-2R. Proc. Natl. Acad. Sci. USA. 2001;98:7564–7569. doi: 10.1073/pnas.121170598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y., Nothacker H.P., Wang Z., Lin S.H., Leslie F., Civelli O. Molecular characterization of the melanin-concentrating-hormone receptor. Nature. 1999;400:265–269. doi: 10.1038/22321. [DOI] [PubMed] [Google Scholar]
- Schönberger, J., 2006. Studie zur Entwicklung neuer Leitstrukturen für die medikamentöse Adipositastherapie. Dissertation, Techn. Univ. Darmstadt.
- Shimomura Y., Mori M., Sugo T., Ishibashi Y., Abe M., Kurokawa T., Onda H., Nishimura O., Sumino Y., Fujino M. Isolation and identification of melanin-concentrating hormone as the endogenous ligand of the SLC-1 receptor. Biochem. Biophys. Res. Commun. 1999;261:622–626. doi: 10.1006/bbrc.1999.1104. [DOI] [PubMed] [Google Scholar]
- Tadayyon M., Welters H.J., Haynes A.C., Cluderay J.E., Hervieu G. Expression of melanin-concentrating hormone in insulin-producing cells: MCH stimulates insulin release in RINm5F and CRI-G1 cell-lines. Biochem. Biophys. Res. Commun. 2002;275:709–712. doi: 10.1006/bbrc.2000.3357. [DOI] [PubMed] [Google Scholar]
- Wang S., Behan J., O'Neill K., Weig B., Fried S., Laz T., Bayne M., Gustafson E., Hawes B.E. Identification and pharmacological characterization of a novel human melanin-concentrating hormone receptor, MCH-R2. J. Biol. Chem. 2001;276:34664–34670. doi: 10.1074/jbc.M102601200. [DOI] [PubMed] [Google Scholar]