Abstract
Proteus mirabilis commonly infects the complicated urinary tract and is associated with urolithiasis. Stone formation is caused by bacterial urease, which hydrolyzes urea to ammonia, causing local pH to rise, and leads to the subsequent precipitation of magnesium ammonium phosphate (struvite) and calcium phosphate (apatite) crystals. To prevent these infections, we vaccinated CBA mice with formalin-killed bacteria or purified mannose-resistant, Proteus-like (MR/P) fimbriae, a surface antigen expressed by P. mirabilis during experimental urinary tract infection, via four routes of immunization: subcutaneous, intranasal, transurethral, and oral. We assessed the efficacy of vaccination using the CBA mouse model of ascending urinary tract infection. Subcutaneous or intranasal immunization with formalin-killed bacteria and intranasal or transurethral immunization with purified MR/P fimbriae significantly protected CBA mice from ascending urinary tract infection by P. mirabilis (P < 0.05). To investigate the potential of MrpH, the MR/P fimbrial tip adhesin, as a vaccine, the mature MrpH peptide (residues 23 to 275, excluding the signal peptide), and the N-terminal receptor-binding domain of MrpH (residues 23 to 157) were overexpressed as C-terminal fusions to maltose-binding protein (MBP) and purified on amylose resins. Intranasal immunization of CBA mice with MBP-MrpH (residues 23 to 157) conferred effective protection against urinary tract infection by P. mirabilis (P < 0.002).
The urinary tract is a complicated epithelium-lined tube with an opening to the body surface. It is susceptible to infections by exogenous organisms that can colonize the peri-urethral area, enter the bladder via the urethra, and ascend the ureters to the kidneys. In some cases the organisms can enter the bloodstream and cause sepsis. Most urinary tract infections (UTIs) occur in otherwise healthy women with normal urinary tracts, but a significant proportion of UTIs develop in those with complicated urinary tracts, including those that are catheterized or otherwise instrumented or obstructed due to structural abnormalities that prevent the normal flow of urine (42).
Proteus mirabilis infects a high proportion of patients with complicated urinary tracts (29, 44). Importantly, once in the urinary tract, the bacterium appears to have a predilection for the kidney (10). Furthermore, this bacterium causes not only cystitis and acute pyelonephritis (9, 11, 13, 38, 41) but also urinary stones (14), further complicating already abnormal urinary tracts.
Stone formation is caused by the expression of bacterial urease, which hydrolyzes urea to ammonia, causing local pH to rise, and leads to the subsequent precipitation of magnesium ammonium phosphate (struvite) and calcium phosphate (apatite) crystals (14, 31, 32). The stones resulting from aggregation of such crystals begin forming at the surface of the epithelium (25), which complicates infection for three reasons. First, the P. mirabilis organisms caught within the interstices of the forming stones are very difficult to clear with only antibiotics. Second, the stone is a nidus for non-P. mirabilis bacteria to establish UTIs that also are difficult to eradicate. Third, the stone can obstruct urine flow; pelvic and renal stones are often associated with acute pyelonephritis, pyonephrosis, and/or chronic pyelonephritis. According to the most-recent U.S. statistics, patients made 1.3 million doctor visits for all causes of urinary stones (39) at a cost of $1.83 billion (7).
A urease-negative mutant of P. mirabilis was found to be much less virulent when assessed in a murine model of ascending UTI or this model modified by surgical placement of catheter material in the murine bladders (18, 19, 25). Urease is required for urolithiasis, because stones, in our experience, never form in mutants deficient in production of this enzyme (25). Although it would be logical to target urease to prevent stone formation, it may nevertheless not be a good vaccine target because it is cytoplasmic and thus not exposed to the bacterial surface.
Mannose-resistant, Proteus-like (MR/P) fimbriae, on the contrary, are expressed on the cell surface of P. mirabilis and should represent a candidate for the development of a vaccine to prevent P. mirabilis UTI and urolithiasis. We found that 69 of 71 isolates screened genotypically for mrp genes or phenotypically for mannose-resistant hemagglutination of erythrocytes, were positive (mrp+ or MR/P+) (30). Mutations abolishing MR/P fimbrial production attenuate the organism significantly as assessed by coinfections with the mutant and wild-type parent strains (5, 22-24). In experiments where mice were infected with P. mirabilis, 4 weeks after infection, a strong immunoglobulin response was mounted against MR/P fimbriae as assessed by Western blot (4, 17). In addition, when urine and bladder samples were assessed by PCR, we found that the mrp invertible element, which carries the promoter and controls transcription of mrp genes, is mostly (>90%) in the on position during infection (23, 45). This indicates that most of the bacterial population is synthesizing MR/P fimbriae during infection. Competition experiments between the phase-locked mutants (the locked-on and the locked-off mutants) and the wild-type strain revealed that MR/P fimbria is an important bladder colonization factor (23).
To prevent the serious consequences of infection with P. mirabilis, we have conducted studies with a goal of developing a vaccine that protects against infection by this species and thus the development of urolithiasis. We believe that several criteria make the development of a vaccine against P. mirabilis UTI both useful and feasible. First, a well-defined population that would benefit from immunization can be easily identified. Three categories of patients comprise this population: (i) those with known anatomically or functionally abnormal urinary tracts, including neurogenic bladders and urinary diversions; (ii) those early in the course of long-term catheterization (urethral, suprapubic, intermittent, and condom); and (iii) possibly, women with apparently normal urinary tracts but who are experiencing recurrent Escherichia coli UTIs (before they develop P. mirabilis infection). Although Proteus is only a small percentage, the denominator is so large that most patients with struvite stones and recurrent urinary infection are women who do not have abnormal or instrumented urinary tracts (12). This intimates that P. mirabilis, through the process of stone formation, can convert an uncomplicated UTI into a complicated one.
The second reason for preventing P. mirabilis infections is that they are often difficult to clear, as the bacteria reside within the urease-induced crystals, constituting a persistent reservoir for recurrent infection. Third, P. mirabilis infections can be particularly serious because the combination of stones and infection may result in renal damage, including acute pyelonephritis, bacteremia, and chronic pyelonephritis (38, 43). Fourth, P. mirabilis appears to have a large number of conserved surface antigens that do not differ significantly from strain to strain, regardless of whether the bacteria are from feces, urine of asymptomatic individuals, or urine of patients with catheter-associated bacteriuria or acute pyelonephritis (30). Fifth, P. mirabilis is present in the fecal flora of <5% of individuals (40), meaning that a vaccine that clears the organism from the stool would have little effect on the normal fecal flora.
The concept of an adhesin-based vaccine has earned credence from a report of a successful FimH (the adhesin of type 1 fimbriae) vaccine that has been shown to prevent experimental UTI by a uropathogenic strain of E. coli (20). The FimH adhesin was held in the native conformation by its chaperone FimC and used for systemic vaccination. Significant protection from transurethral (TU) challenge was reported (20).
In this report, we describe the use of a fimbrial component, MrpH, as a vaccine to prevent infection by P. mirabilis in a CBA mouse model of ascending UTI to test the efficacy of vaccination. We assessed protection after infection with live bacteria (17) and after vaccination with formalin-killed bacteria, purified MR/P fimbriae, the MrpH fimbrial adhesin, and a truncate of the MrpH fimbrial tip adhesin. We examined four routes of immunization: subcutaneous (s.c.), intranasal (i.n.), oral (OR), and transurethral (TU).
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth media.
P. mirabilis HI4320 (urease positive; produces MR/P fimbriae, P. mirabilis fimbriae [PMF], and ambient temperature fimbriae [ATF]; hemolysin positive; tetracycline resistant) was isolated from a woman with urinary catheter-associated bacteriuria (32, 44) and has been used extensively by our group for virulence studies. Ten pyelonephritis isolates of P. mirabilis were randomly selected from among 16 isolates that were cultured from the urine of adults (>105 CFU/ml) who had the clinical syndrome of acute pyelonephritis severe enough to require antibiotic therapy and hospitalization at the University of Maryland Medical Systems Hospital in Baltimore, Md. (30). Ten catheter isolates of P. mirabilis (each from a different patient) were randomly selected from a collection of bacterial isolates that were cultured from 35 patients with catheter-associated bacteriuria (30). Ten fecal isolates were randomly selected from a collection of 20 isolates that were cultured from routine stool cultures at the Hospital for Sick Children, Toronto, Canada; at the time of isolation, all patients were asymptomatic for P. mirabilis infection (30). E. coli DH5α [supE44lacU169(Δ80lacZM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1] was used in DNA cloning and overexpression of maltose-binding protein (MBP) fusions. Luria broth (containing, per liter, 10 g of tryptone, 5 g of yeast extract, and 10 g of NaCl) and nonswarming agar (containing, per liter, 10 g of tryptone, 5 g of yeast extract, 5 ml of glycerol, 0.4 g of NaCl, and 20 g of agar) were used to culture bacteria. Luria agar (containing, per liter, 10 g of tryptone, 5 g of yeast extract, 10 g of NaCl, and 20 g of agar) was used for Dienes test (8).
CBA mouse model of ascending UTI.
P. mirabilis HI4320 was cultured on nonswarming agar slants for 18 h at 37°C; the resulting bacterial lawns were suspended in phosphate-buffered saline (PBS) (containing, per liter, 8 g of NaCl, 0.2 g of KCl, 1.44 g of Na2HPO4, and 0.24 g of KH2PO4). Female CBA/J mice (18 to 22 g; 4 to 8 weeks old; Harlan Sprague-Dawley, Indianapolis, Ind.) were each inoculated TU with a 50-μl suspension containing 5 × 107 CFU of P. mirabilis strain HI4320 using a 0.28-mm-inner-diameter sterile polyethylene catheter connected to an infusion pump (Harvard Apparatus, Millis, Mass.) as described previously (15, 18). Seven days after challenge, surviving mice were sacrificed and bacteria in the bladder and kidneys were quantitatively cultured on nonswarming agar to measure the number of CFU per gram of tissue. The lower limit of detection in this assay was 102 CFU/g of tissue. Thus, this value (102 CFU/g of tissue) was assigned to samples with an undetectable level of colonization.
Preparation of formalin-killed bacterial cells.
P. mirabilis HI4320 bacteria were grown on nonswarming agar slants overnight at 37°C and then suspended in PBS. Formalin (10% formaldehyde in PBS) was added to the bacterial suspension to 1% and incubated with shaking overnight at 37°C. The bacterial suspension was then washed three times with PBS, and a sample was streaked on a Luria agar plate to validate total loss of viability.
Isolation of MR/P fimbriae.
P. mirabilis HI4320 was cultured in static broth, conditions favoring fimbriate bacteria (6). MR/P fimbriae were sheared from the bacterial surface by blending and purified by differential centrifugation as described previously (4).
Immunization of female CBA mice.
For systemic immunization, antigens were emulsified in Freund's complete adjuvant (for primary immunization) or Freund's incomplete adjuvant (for booster immunization) and injected s.c. into female CBA mice. For mucosal immunization, antigens were administered into female CBA mice i.n., TU, or OR. Formalin-killed P. mirabilis (109 CFU/mouse) were coadministered with cholera toxin (10 μg/mouse). Purified MR/P fimbriae were covalently coupled to cholera toxin at a ratio of 10:1 (wt/wt) (see below) and then injected into the mice at doses of 100 to 200 μg/mouse. Fifteen minutes prior to OR immunization, mice received 500 μl of sodium bicarbonate (0.1 M). The amount of antigen administered to each mouse was the same for mice immunized via different routes: 109 formalin-killed bacteria/mouse or 100 to 200 μg of MR/P fimbriae/mouse. The volume of antigen administered was different: 300 μl/mouse for s.c. immunization, 10 to 20 μl/mouse (half in each nostril) for i.n. immunization, 50 μl/mouse for TU immunization, and 500 μl/mouse for OR immunization. All mice were given a primary injection on day 0 and two booster injections, one on day 7 and the other on day 14. On day 21, sera (obtained by retro-orbital bleeding), urine, and vaginal washes (100 μl of PBS/mouse) were collected from anesthetized mice. Bile, bladder and kidney samples were collected from two mice of each group after extensive perfusion with PBS (30 ml/mouse). The remaining mice were TU inoculated with 5 × 107 CFU of wild-type P. mirabilis strain HI4320 as described above. Animal protocols were approved by the University of Maryland School of Medicine Institutional Review Board.
Construction, expression, and purification of MBP-MrpH (residues 23 to 275) and MBP-MrpH (residues 23 to 157) fusions.
An approximately 800-bp DNA fragment encoding the mature MrpH peptide (residues 23 to 275) was PCR amplified from an mrpH-encoding plasmid using primer 825 (5′-CTGCCATGGCCTCTATTTTTTCTTA-3′) and primer 824 (5′-CAAAATCAAAATTCATCATCATAAT-3′) and cloned into the EcoRI and HindIII sites of pMAL-c2 (New England Biolabs, Beverly, Mass.) to express MrpH (residues 23 to 275) as a C-terminal fusion to MBP; the resulting DNA construct was named pXL4605. A DNA fragment encoding residues 23 to 157 of MrpH was PCR amplified with primer 825 and primer 1445 (5′-GCGAAGCTTATGGTGGTGCGATACCGCATA-3′) and cloned into the EcoRI and HindIII sites of pMAL-c2 to express MrpH (residues 23 to 157) as a C-terminal fusion to MBP; the resulting DNA construct was named pLX4101. Expression and purification of MBP fusion proteins were carried out as described by Ausubel et al. (2). Briefly, E. coli DH5α cells containing various constructs were induced with isopropyl-β-d-thiogalactopyranoside (IPTG) (0.3 mM). Soluble protein derived from French-pressed (20,000 lb/in2) cell lysates of the induced cultures was passaged through an amylose column, and the fusion proteins were eluted from the column with 10 mM maltose.
SDS-PAGE and Western blot analysis.
Discontinuous one-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was carried out as described by Ausubel et al. (2) in a Hoefer SE200 Mighty Small Mini-Vertical unit (Pharmacia Biotech Inc.). After electrophoresis, gels were either stained with Coomassie blue or transferred to a polyvinylidene difluoride membrane (Immobilon-P; Millipore Corp., Bedford, Mass.). The blot was incubated with rabbit polyclonal antiserum against MrpH followed by goat anti-rabbit immunoglobulin G (IgG)-peroxidase conjugate and then developed using the enhanced chemiluminescent detection reagents (ECL; Amersham Pharmacia Biotech).
Covalent coupling of antigens to cholera toxin.
Purified MR/P fimbriae were covalently conjugated to cholera toxin at a ratio of 10 μg of MR/P fimbriae to 1 μg of cholera toxin (Sigma Chemical Co.). Purified MBP-MrpH (residues 23 to 275) and MBP-MrpH (residues 23 to 157) fusions were each covalently conjugated to cholera toxin at a molar ratio of 1:1. Covalent conjugation to cholera toxin was achieved using the heterobifunctional coupling reagent N-succinimidyl 3-(2-pyridyl dithio)propionate (SPDP) (Amersham Pharmacia Biotech) according to the instructions of the manufacturer.
ELISAs.
Enzyme-linked immunosorbent assay (ELISA) was performed in 96-well plates (Nunc-Immuno Plate PolySorp) according to the protocol described by Harlow and Lane (16). Briefly, each well was coated with 100 ng of antigen (formalin-killed P. mirabilis, MR/P fimbriae, or MBP-MrpH) in 60 mM carbonate buffer (pH 9.6). Twofold serial dilutions of sera, urine, vaginal washes, bladder extracts (in PBS containing 2% saponin [Sigma]), perfused kidney extracts, and bile from each mouse were added to the wells. Alternatively, urine or vaginal washes from all mice in a group were pooled and assayed in triplicate. Alkaline phosphatase conjugates of rabbit anti-mouse IgG and rabbit anti-mouse IgA were used as secondary antibodies to determine the titers of IgG and IgA in mouse sera, respectively. The substrate p-nitrophenylphosphate was used to measure the alkaline phosphatase activity.
PCR amplification and sequencing of the mrpH gene.
The mrpH gene was PCR-amplified from 10 P. mirabilis isolates (four pyelonephritis isolates, three catheter-associated bacteriuria isolates, and three fecal isolates) using primer 824 (see above) and primer 851 (5′-GAAGATTGAGGTTTTACATGTTTAT-3′). PCR products were electrophoresed on a 0.8% agarose gel, and DNA bands corresponding to the correct fragment size of mrpH (828 bp) were excised from the agarose gel and purified using the QIAquick Gel Extraction system (QIAGEN). DNA sequencing was performed by the Biopolymer Laboratory at the Department of Microbiology and Immunology, University of Maryland School of Medicine, using the dideoxy chain termination method with double-stranded DNA as the template. DNA sequencing reactions were processed by a 16-capillary automated DNA sequencer (model 3100; Applied Biosystems, Foster City, Calif.).
Statistical methods.
P values were determined by one-tailed Mann-Whitney test when comparing colonization levels and by one-tailed Fisher's exact test when comparing percentages of mice protected.
RESULTS
Protection against homologous challenge.
Immunity to reinfection with a homologous strain of P. mirabilis was previously assessed following experimental infection (17). CBA mice were inoculated TU with 5 × 107 CFU of P. mirabilis HI4320. After 4 weeks, this group of mice and a control group were treated with ampicillin for 3 days followed by a 4-day washout period. Both groups were then challenged with a Nalr mutant of the homologous strain. One week later, animals were sacrificed, and urine, bladder, and kidneys were quantitatively cultured. Only modest protection of the kidneys but not the bladder was noted. Thus, UTI with P. mirabilis does not protect against homologous urinary challenge; this is consistent with the clinical occurrence of recurrent infections with P. mirabilis. High serum antibody titers to specific antigens including MR/P fimbriae and flagella, however, were observed (4).
Vaccination using formalin-killed P. mirabilis.
To identify bacterial antigen preparations that were capable of protecting mice from infection with P. mirabilis by vaccination, we began our protection studies using crude preparations and then moved to more refined approaches using specific antigens. To test the efficacy of the whole-cell preparation, we immunized groups of 10 CBA mice on days 0, 7, and 14 with formalin-killed whole cells of P. mirabilis at a dose of 109 cells per mouse. Four routes of immunization were tested: s.c., i.n., TU, and OR. For s.c. immunization, the formalin-killed whole-cell preparation was emulsified in Freund's complete adjuvant for the primary injection on day 0 and in Freund's incomplete adjuvant for the two boosters on days 7 and 14. Groups of CBA mice were immunized s.c., i.n., TU, or OR with formalin-killed whole cells of P. mirabilis on days 0, 7, and 14 (see Materials and Methods for details). On day 21, immunized and naive mice were challenged TU with 5 × 107 CFU of P. mirabilis HI4320. Seven days after challenge, bacteria in bladder and kidneys of surviving mice were quantitatively cultured.
For immunization with whole killed P. mirabilis, significant decreases in colonization were achieved by s.c. (bladder, P = 0.005; kidney, P = 0.003) and i.n. (bladder, P = 0.004; kidney, P = 0.01) immunization (Table 1). After s.c. and i.n. immunization, 60 and 67% of mice, respectively, had undetectable numbers (limit of detection = 102 CFU/g) of bacteria in their bladder and kidneys 1 week after TU challenge; this compared to only 10% of unimmunized mice that were protected (P < 0.05 using Fisher's exact test). Mucosal immunization via the TU and the OR routes failed to induce significant protection of mice against UTI by P. mirabilis.
TABLE 1.
Immunization with whole killed P. mirabilis
| Group | % Protecteda | Log10 CFU/g of bladder
|
Log10 CFU/g of kidney
|
Mean antibody titer on day 21e in:
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | Pc | Mean | Median | Pc | Serumf
|
Urineg
|
Bladderf
|
Vaginal washg
|
Bilef
|
|||||||
| IgG | IgA | IgG | IgA | IgG | IgA | IgG | IgA | IgG | IgG | ||||||||
| Naive | 10 (1/10) | 6.59 | 7.05 | NAd | 5.04 | 5.72 | NA | <20 | <20 | <20 | <20 | <20 | <20 | <20 | <20 | <20 | <20 |
| s.c. | 60 (6/10)b | 3.44 | 2.00 | 0.005 | 3.19 | 2.00 | 0.003 | 40,960h | <20 | <20 | <20 | <20 | <20 | <20 | <20 | <20 | <20 |
| i.n. | 67 (6/9)b | 3.51 | 2.00 | 0.004 | 3.12 | 2.00 | 0.01 | 22,528h | 320h | 20 | 224 | 112h | <20 | <20 | 112 | 80h | 416h |
| TU | 33 (3/9) | 4.65 | 5.97 | 0.04 | 4.08 | 3.90 | 0.12 | 200h | <20 | <20 | <20 | <20 | <20 | <20 | <20 | <20 | <20 |
| OR | 29 (2/7) | 5.09 | 6.30 | 0.28 | 4.76 | 5.69 | 0.49 | 208h | <20 | <20 | <20 | <20 | <20 | <20 | <20 | <20 | 64h |
The percentage of mice with undetectable numbers (<102 CFU/g tissue) of bacteria in their bladder and kidneys (the number of mice with undetectable level of colonization in their bladder and kidneys/number of mice survived 7 days after transurethral challenge). The total number of mice for each group is 10.
The percentage of mice protected from infection is significantly higher in the vaccinated mice than in the naive mice (P < 0.05 using one-tailed Fisher's exact test).
P values are derived from one-tailed Mann-Whitney test comparing the colonization level of the vaccinated mice with that of the naive mice.
NA, not applicable.
Titers measured just prior to transurethral challenge; values expressed as the reciprocal of the highest detectable dilution.
Samples from individual mice were assayed by ELISA independently.
Samples from individual mice were pooled and assayed by ELISA in triplicate; pooled data not subjected to statistical analysis.
P < 0.001 compared to naive mice.
Antibody response to vaccination with formalin-killed P. mirabilis.
Groups of mice that displayed statistically significant levels of protection against homologous challenge (i.e., those vaccinated by s.c. and i.n. routes) mounted high titers in serum of IgG to whole killed bacteria (Table 1). However, mice vaccinated by the s.c. route did not produce detectable serum IgA or IgG and IgA in urine, bladder, vaginal wash, or bile. On the other hand, vaccination by the i.n. route elicited a broad antibody response yielding high titers of IgG and IgA in serum and detectable levels in urine, bladder, vaginal wash, and bile. Indeed, the i.n. route was the only group with immunoglobulin in the urine and bladder directed against P. mirabilis.
Vaccination using purified MR/P fimbriae.
We previously noted that infected mice mounted a strong serum antibody response against MR/P fimbriae, indicating that these cell surface structures were expressed in vivo (4). In vivo expression of MR/P fimbriae was further confirmed by the invertible element assay, a PCR-based assay that measures phase variation of MR/P fimbria, which indicated that in the heavily colonized mouse bladder, >90% of bacteria expressed MR/P fimbriae (23). We hypothesized that proteinaceous structures expressed on the surface of the bacterium could serve as effective antigens for vaccination. To test the efficacy of purified antigen preparations, MR/P fimbriae were sheared from the bacterial surface and then purified by differential centrifugation. Groups of CBA mice were immunized via i.n., TU, s.c., or OR routes on days 0, 7, and 14 at a dose of 100 to 200 μg of MR/P fimbriae per mouse (see Materials and Methods for details). On day 21, immunized and naive mice were challenged TU with 5 × 107 CFU of P. mirabilis HI4320. Seven days after challenge, bacteria in bladder and kidneys of surviving mice were quantitatively cultured.
For MR/P fimbria immunization, a significant reduction in colonization was observed with i.n. (bladder, P = 0.005; kidneys, P = 0.0004) and TU (bladder, P = 0.009; kidneys, P = 0.0005) immunization (Table 2). After i.n. and TU immunization, 60 and 63% of mice, respectively, had undetectable numbers (<102 CFU/g) of bacteria in their bladder and kidneys 1 week after TU challenge; this compared to only 10% of the naive mice that were protected (P < 0.05 using Fisher's exact test).
TABLE 2.
Immunization with purified MR/P fimbriae
| Group | % Protecteda | Log10 CFU/g of bladder
|
Log10 CFU/g of kidney
|
Mean antibody titer on day 21e in:
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | Pc | Mean | Median | Pc | Serumf
|
Urineg
|
Bladderf
|
Vaginal washg
|
Bilef
|
|||||||
| IgG | IgA | IgG | IgA | IgG | IgA | IgG | IgA | IgG | IgG | ||||||||
| Naive | 10 (1/10) | 6.09 | 7.01 | NAd | 4.80 | 4.67 | NA | <20 | <20 | <20 | <20 | <20 | <20 | <20 | <20 | <20 | <20 |
| s.c. | 25 (2/8) | 4.67 | 5.43 | 0.09 | 4.31 | 4.89 | 0.28 | 40,960h | <20 | <20 | <20 | <20 | <20 | 48 | <20 | <20 | <20 |
| i.n. | 60 (6/10)b | 3.24 | 2.00 | 0.005 | 2.86 | 2.00 | 0.0004 | 26,624h | 512h | 20 | <20 | <20 | <20 | <20 | 88 | 160h | 544h |
| TU | 63 (5/8)b | 3.11 | 2.00 | 0.009 | 2.92 | 2.00 | 0.0005 | 768h | <20 | <20 | <20 | <20 | <20 | <20 | <20 | <20 | <20 |
| OR | 33 (3/9) | 5.17 | 6.33 | 0.13 | 4.48 | 5.32 | 0.46 | <20 | <20 | <20 | <20 | <20 | <20 | <20 | <20 | <20 | <20 |
The percentage of mice with undetectable numbers (<102 CFU/g tissue) of bacteria in their bladder and kidneys (the number of mice with undetectable level of colonization in their bladder and kidneys/number of mice survived 7 days after transurethral challenge).
The percentage of mice protected from infection is significantly higher in the vaccinated mice than in the naive mice (P < 0.05 using one-tailed Fisher's exact test).
P values are derived from one-tailed Mann-Whitney test comparing the colonization level of the vaccinated mice with that of the naive mice.
NA, not applicable.
Titers measured just prior to transurethral challenge; values expressed as the reciprocal of the highest detectable dilution.
Samples from individual mice were assayed by ELISA independently.
Samples from individual mice were pooled and assayed by ELISA in triplicate; pooled data not subjected to statistical analysis.
P < 0.001 compared to naive mice.
Antibody response to vaccination with MR/P fimbriae.
In groups of mice protected from homologous challenge by i.n. and TU vaccination with MR/P fimbriae, the antibody response (Table 2) was not as vigorous as in mice vaccinated with whole killed cells (Table 1). Again the i.n.-vaccinated mice displayed the most broad response with detectable immunoglobulin measured in serum, urine, vaginal wash, and bile. Mice vaccinated by the TU route had detectable IgG only in the serum with no detectable response in any other compartment. The highest single titer was IgG in serum (1:40,960) in mice vaccinated by the s.c. route; high levels of immunoglobulin in serum, however, did not correlate with protection.
Selection of i.n. route for subsequent studies.
i.n. immunization with formalin-killed bacteria or MR/P fimbriae offered the most consistent protection. Furthermore, i.n. was the only route that elicited vaginal antibody production, which may be protective because the vagina may serve as a source of UTI organisms capable of causing UTI. Thus, the i.n. route of immunization was chosen for subsequent studies.
i.n. immunization with MrpH, the adhesin of MR/P fimbriae.
We have previously provided evidence that MrpH is the fimbrial tip adhesin component of MR/P fimbriae (22). These observations include that (i) MrpH shares 30% amino acid sequence identity with PapG, the Gal(α1-4)Gal-binding adhesin of E. coli P fimbriae; (ii) amino acid residue substitution of the N-terminal cysteine residues (C66S and C128S) of MrpH abolished receptor-binding activity (hemagglutinating ability) of MrpH but allowed normal fimbrial assembly; (iii) immunogold electron microscopy of P. mirabilis HI4320 revealed that MrpH was located at the tip of MR/P fimbriae, consistent with its role in receptor binding; and (iv) when aligned with other MR/P pilins (MrpA, -B, -E, -F, and -G), MrpH contains extra N-terminal amino acid sequence of 121 amino acid residues putatively associated with receptor binding (22).
MrpH, the MR/P fimbrial tip adhesin, represents a minor protein component in the MR/P fimbrial preparation (Fig. 1). Given the functional importance of MrpH, we hypothesized that increased specific immune response to MrpH would result in more effective protection of mice from urinary infection by P. mirabilis. To examine the effect of using the fimbrial tip adhesin as antigen, an MBP fusion of mature MrpH (amino acid residues 23 to 275) was constructed, overexpressed, and purified on an amylose column (Fig. 2, lanes 2 and 3). Purified MBP-MrpH (residues 23 to 275) was covalently conjugated to cholera toxin using SPDP and then used for i.n. immunization of mice on days 0, 7, and 14 at a dose of 10 μg of antigen per mouse. On day 21, 10 naive mice and 11 immunized mice were each challenged TU with 5 × 107 CFU of P. mirabilis strain HI4320. One week after challenge, mice were sacrificed and bacteria in bladder and kidneys were quantitatively cultured.
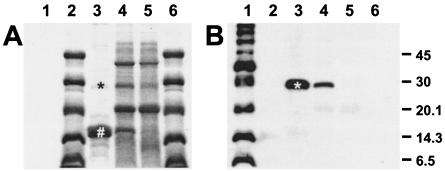
FIG. 1.
(A) SDS-15% PAGE and (B) Western blot analysis of the MR/P fimbrial preparation. Lane 1, biotinylated protein marker, broad range (New England Biolabs); lanes 2 and 6, Rainbow protein marker, low-molecular-mass range (Amersham Biosciences, Piscataway, N.J.); lane 3, the purified MR/P fimbrial preparation; lanes 4 and 5, the sheared-off surface protein preparations of P. mirabilis strain HI4320 (lane 4) and its isogenic MR/P fimbria-negative mutant (mutation in mrpH) (lane 5). The molecular masses of the bands shared by the biotinylated protein markers and the Rainbow protein markers are indicated on the right in kilodaltons. Western blot analysis using antibodies against MrpH indicated that the fimbrial tip adhesin (*) is a minor component in the MR/P fimbrial preparation, which consists of mostly the major structural subunit MrpA (#).
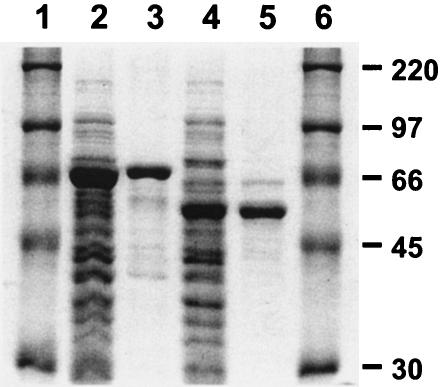
FIG. 2.
SDS-10% polyacrylamide gel showing the overexpression and purification of MBP-MrpH (residues 23 to 275) and MBP-MrpH (residues 23 to 157). Lanes 1 and 6, Rainbow protein markers, high-molecular-mass range (Amersham Biosciences); lane 2, crude cell lysate of E. coli DH5α overexpressing MBP-MrpH (residues 23 to 275); lane 3, MBP-MrpH (residues 23 to 275) fusion protein purified on an amylose resin; lane 4, crude cell lysate of E. coli DH5α overexpressing MBP-MrpH (residues 23 to 157); lane 5, MBP-MrpH (residues 23 to 157) fusion protein purified on an amylose resin. The molecular masses of proteins in the Rainbow marker are indicated on the right in kilodaltons.
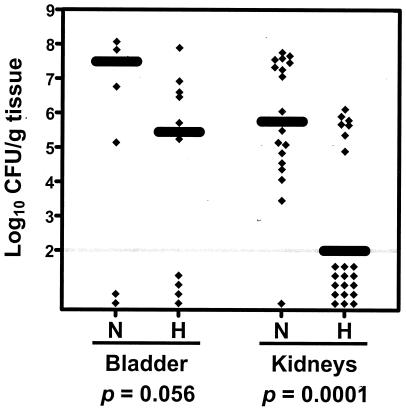
As shown in Fig. 3, the immunized mice were colonized by significantly fewer bacteria in kidneys (median log10 CFU/g of tissue: 5.76 for the naive mice versus 2.00 for the immunized mice; P = 0.0001) and they also tended to be colonized by fewer bacteria in the bladder (median log10 CFU/g of tissue: 7.49 for the naive mice versus 5.45 for the immunized mice; P = 0.056). 36% (4 of 11) of the immunized mice had undetectable levels of colonization (<102 CFU/g of tissue) in bladder and kidneys, compared to 0% (0 of 10) of the naive mice (P = 0.055).
FIG. 3.
P. mirabilis colonization in bladder and kidneys of the naive mice (N) and the mice immunized with MBP-MrpH (residues 23 to 275) (H). Each diamond represents log10 CFU per gram of tissue from an individual mouse. Samples with undetectable colonization were given a value of 2 log10 CFU/g of tissue (gray horizontal line). The black bars represent the median log10 CFU per gram of tissue. One-tailed P values were determined by Mann-Whitney test comparing the colonization levels in bladder and kidneys of the naive mice with those of the immunized mice.
Antibody response to i.n. vaccination with MrpH.
Highly significant IgG responses to MrpH vaccination were observed in the serum, bladder, and kidney and clearly measurable responses were quantified in urine and vaginal washes (Table 3). The latter responses rose in response to vaccination and primary and secondary boost. Significant IgA responses were also noted in serum, vaginal wash, and kidneys; the response in the bladder and urine was weak. This less robust response in the bladder correlated with less protection compared to the protection in the kidneys.
TABLE 3.
Antibody response in mice to i.n. vaccination with MBP-MrpH
| Mouse sample | Time of sampling | Antibody response
|
|||
|---|---|---|---|---|---|
| Naive
|
MrpH
|
||||
| IgGc | IgA | IgGc | IgAc | ||
| Serum (n = 4) | 5 ± 10 | 0 | >20,480b | 560 ± 160b | |
| Urine (pool of 10) | Post-primary immunization | 0a | 0 | 0 | 0 |
| Post-first booster | 0 | 0 | 40 | 0 | |
| Post-second booster (prechallenge) | 0 | 0 | 80 | 0 | |
| Vaginal wash (pool of 10) | Post-primary immunization | 0 | 0 | 20 | 20 |
| Post-first booster | 0 | 0 | 160 | 40 | |
| Post-second booster (prechallenge) | 0 | 0 | 640 | 80 | |
| Bladder (n = 4) | 0 | 0 | 2,240 ± 2,123b | 10 ± 12 | |
| Kidney (n = 4) | 0 | 0 | 7,040 ± 3,840b | 7,020 ± 3,840b | |
0 = no reading at 1:20 dilution.
P < 0.001 compared to naive control.
Some data are given as mean ± standard deviation.
i.n. immunization with MrpH truncate.
The amino-terminal receptor-binding domain (amino acid residues 23 to 157) of MrpH was also tested as a vaccine candidate for the following reasons. First, the advantage of using adhesin polypeptide as vaccine is that the stimulated immune response may specifically block the adherence of the adhesin to the host receptor and therefore effectively prevent bacterial attachment to host epithelia. The adhesive property of MrpH resides in its amino-terminal portion (residues 23 to 157). Therefore, using the receptor-binding domain of MrpH to immunize mice would induce more specific immune responses against this domain and therefore more effectively block bacterial adherence. Secondly, the carboxyl-terminal domain of MrpH shares strong sequence homology with other MR/P pilins (22), the building blocks of MR/P fimbriae, which require the periplasmic chaperone MrpD for proper folding. In the absence of MrpD, the carboxyl-terminal pilin domain of MrpH is not properly folded and could interfere with the folding of the receptor-binding domain. In our study, the fusion protein MBP-MrpH (residues 23 to 275) was >90% soluble when overexpressed in E. coli, but often precipitated out of solution at high concentrations, which complicated vaccine preparation. We reasoned that removing the carboxyl-terminal domain of MrpH would solve this problem.
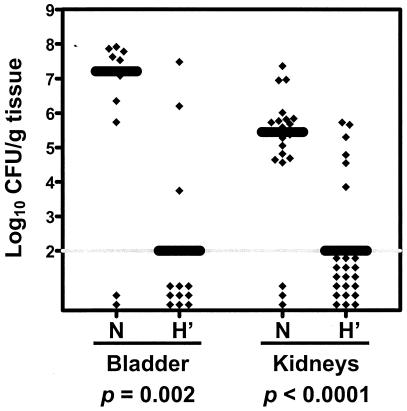
The truncate fusion, MBP-MrpH (residues 23 to 157) was constructed, overexpressed, and purified on an amylose column (Fig. 2, lanes 4 and 5). As we expected, the truncate MrpH fusion protein did not precipitate out of solution at high concentrations (5 mg/ml). i.n. immunization with MBP-MrpH (residues 23 to 157) was carried out according to the same procedure described above. Twelve naive mice and 12 immunized mice were used in this experiment; 1 of the 12 naive mice died before the day of challenge. We observed that, compared to the naive mice, mice immunized with the truncate form of MrpH were colonized by significantly fewer bacteria in the bladder (median log10 CFU/g of tissue: 7.21 for the naive mice versus 2.00 for the immunized mice; P = 0.002) and kidneys (median log10 CFU/g of tissue: 5.45 for the naive mice versus 2.00 for the immunized mice; P < 0.0001) (Fig. 4). The percentage of mice with undetectable levels of colonization in bladder and kidneys (≤2 log10 CFU/g of tissue) was significantly higher for the immunized mice than the naive mice (1 of 11 [9%] for the naive mice versus 9 of 12 [75%] for the immunized mice; P = 0.002).
FIG. 4.
P. mirabilis colonization in bladder and kidneys of the naive mice (N) and the mice immunized with MBP-MrpH (residues 23 to 157) (H'). Each diamond represents log10 CFU per gram of tissue from an individual mouse. Samples with undetectable colonization were given a value of 2 log10 CFU/g of tissue (gray horizontal line). The black bars represent the median log10 CFU per gram of tissue. One-tailed P values were determined by Mann-Whitney test comparing the colonization levels in bladder and kidneys of the naive mice with those of the immunized mice.
Conservation of mrpH and MrpH among strains.
For an MrpH vaccine to be effective against challenge with heterologous strains, MrpH must be conserved among diverse strains. Genetic variation of MR/P fimbriae was examined among different P. mirabilis strains, including 10 pyelonephritis isolates, 10 catheter-associated isolates, and 10 fecal isolates. Western blot analysis using antibodies against MrpA, the major pilin of MR/P fimbria, indicated that MR/P fimbriae were expressed at various levels in all 30 isolates (data not shown). Dot blot analysis using an mrpH probe revealed that all of the 30 isolates examined were mrpH positive (data not shown). Subsequently, 10 of the 30 isolates (four pyelonephritis, three catheter-associated, and three fecal strains) and the vaccine strain P. mirabilis HI4320 were subjected to the Dienes test, a mutual inhibition test for identifying unique strains with a discriminatory power of 0.98 (Fig. 5) (37). The mrpH genes from these 10 isolates were PCR amplified and sequenced. As shown in Table 4, among the 10 isolates, four belong to the same Dienes type (B), three each belong to a different Dienes type (C, D, and E), and the other three failed to swarm and thus were typed. Isolates belonging to the same Dienes type share 100% identity in the sequence of mrpH, but isolates that share 100% identity in the sequence of mrpH do not necessarily belong to the same Dienes type (Table 4). Overall, the mrpH gene sequences from the 10 isolates representing five different Dienes types share 99.4 to 100% identity with the mrpH gene sequence from P. mirabilis strain HI4320. Most significantly, none of these nucleotide changes in the mrpH gene resulted in any amino acid substitutions in the MrpH protein. These results demonstrated that MrpH is conserved among various P. mirabilis strains.
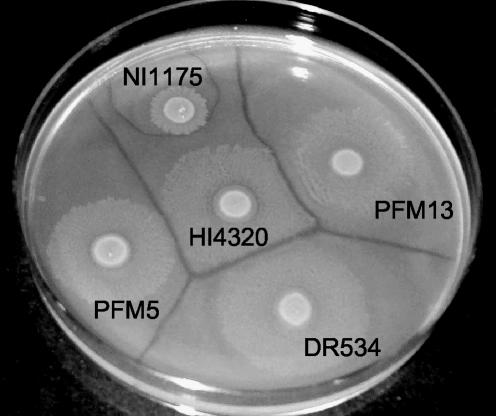
FIG. 5.
Representative Dienes types from among 10 P. mirabilis isolates for which mrpH was sequenced. Overnight cultures were standardized to an optical density at 600 nm of ∼0.8, and a 5-μl aliquot of each sample was spotted onto a Luria-Bertani agar plate. Plates were incubated overnight at 37°C and photographed the next day. Distinct lines of demarcation between isolates designate distinct Dienes types and thus distinct strains.
TABLE 4.
Conservation of mrpH and MrpH among various P. mirabilis strains
| Strain
|
Dienes type | Conservation of mrpH
|
% Identity in MrpHa | ||
|---|---|---|---|---|---|
| Source | Designation | % Identitya | Nucleotide change(s) | ||
| Catheter (vaccine strain) | HI4320 | A | 100 | ||
| Pyelonephritis | CPZ270 | B | 99.9 | T426A | 100 |
| Pyelonephritis | CFT36 | B | 99.9 | T426A | 100 |
| Pyelonephritis | CFT322 | NDb | 99.6 | C462A, T480G, G633A | 100 |
| Pyelonephritis | CFT403 | ND | 99.9 | A783T | 100 |
| Catheter | DR534 | B | 99.9 | T426A | 100 |
| Catheter | NI1175 | C | 99.4 | T549G, C640T, T666C, T717C, A783T | 100 |
| Catheter | TH3526 | B | 99.9 | T426A | 100 |
| Fecal | PFM2 | ND | 100 | 100 | |
| Fecal | PFM5 | D | 100 | 100 | |
| Fecal | PFM13 | E | 99.9 | T426A | 100 |
Calculated by comparison with the mrpH and MrpH sequences of P. mirabilis strain HI4320.
ND, not determined. The Dienes type of these strains was not determined because these strains failed to swarm on Luria agar.
DISCUSSION
A previous study by our group showed that experimental UTIs by P. mirabilis yield little protection to mice from reinfection by a homologous strain (17). In this study, we investigated the efficacy of vaccination via four different routes (s.c., i.n., TU, and OR) with a formalin-killed whole-cell preparation, a purified MR/P fimbrial preparation, and recombinant MBP fusions of MrpH, the tip adhesin of MR/P fimbria. Our results indicate that i.n. vaccination with recombinant MBP fusion of truncate MrpH (MBP-MrpH [residues 23 to 157]) is most effective in preventing UTI with P. mirabilis in mice.
Infection of the urinary tract by P. mirabilis is governed by the general principles of bacterial pathogenesis. Proteins produced by this species during infection may contribute to (i) colonization of the host, (ii) evasion of host defense, and (iii) damage of host tissue. Colonization requires movement of P. mirabilis to its niche, attachment to specific receptors, and survival in the urinary tract. At least four fimbriae have been identified in this species, including MR/P fimbriae, PMF, ATF, and the nonagglutinating fimbriae (1, 3, 4, 26, 33, 34). MR/P fimbriae and PMF appear to contribute to colonization by P. mirabilis in the CBA mouse model of ascending UTI. When separate groups of mice were TU inoculated with the parent strain and an MR/P-deficient isogenic mutant (constructed in our laboratory), the mutant was recovered in lower numbers than the parent strain from the bladder and kidneys of mice 1 week after experimental inoculation of the bladders (5). In similar experiments, the PMF fimbrial mutant was found to be present at a significantly lower concentration in the bladder only (27). An isogenic ATF-negative mutant of P. mirabilis was shown not to be attenuated in its ability to colonize mouse urinary tract (46). The contribution of nonagglutinating fimbria has not yet been tested but is of doubtful significance because of its lack of identifiable adherence property.
Observations from our laboratory and those of other investigators suggest that P. mirabilis vaccination may confer protection against infection with heterologous strains. At least four antigen preparations have been tested previously. Decades ago, Pazin and Braude immunized rats parenterally with purified flagella (35). They demonstrated that antiserum immobilized bacteria by binding to flagella and prevented the organisms from spreading from one kidney to the other kidney (i.e., down one ureter, into the bladder, and back up the other ureter). Moayeri et al. have shown that immunization with an outer membrane protein preparation protects BALB/c mice from homologous intravesicular challenge (28). Legagni-Fajardo et al. demonstrated that parenteral immunization with purified fimbrial preparation protects mice from TU challenge with homologous and heterologous strains (21). In an important follow-up to this study, Pellegrino and colleagues (36) purified recombinant fimbrial antigens of P. mirabilis including MrpA, PmfA, and UcaA and used these for vaccination and protection from hematogenous and TU challenge. They found that MrpA protected s.c. immunized mice in both models, demonstrating that, indeed, the MR/P fimbria is an important vaccine target. We had previously noted a strong serum immunoglobulin response to MR/P fimbriae in mice following experimental UTI, suggesting that this antigen is expressed in vivo and is immunogenic (4). In this report, we demonstrated that i.n. immunization with the adhesin molecule of the MR/P fimbria, MrpH, or with its N-terminal receptor-binding domain alone protected mice from experimental UTIs.
We tested four different immunization routes with the formalin-killed whole-cell preparation and purified MR/P fimbrial preparation. The immunization routes significantly affect the efficacy of the vaccine. The killed whole-cell vaccine is effective via s.c. or i.n. routes, whereas the MR/P fimbria vaccine is effective via i.n. or TU routes. Overall, the i.n. route of vaccination elicited the broadest antibody response, elevating titers in serum, urine, bladder, vaginal wash, and bile (Tables 1 and 2). Systemic immunization via the s.c. route induced the strongest serum IgG response, but it did not necessarily result in the most effective protection from infection. This is consistent with our previous finding that there is no significant correlation between serum IgG level and protection from infection (17).
Our data indicated that both the killed whole-cell vaccine and the MR/P fimbria vaccine were effective protecting mice from P. mirabilis infection, up to 67 and 63% protection, respectively. The recombinant MBP-MrpH (residues 23 to 157) vaccine resulted in a higher percentage of protection, 75%. However, the most significant advantage of the recombinant MBP-MrpH formulation over the first two is its reduced side effects. Compared to the naive mice, higher mortalities were observed in mice immunized with the killed whole-cell vaccine and the MR/P fimbria vaccine (Table 1 and 2). Such increases in mortality may due to possible side effects of vaccination; a few immunized mice suffered weight losses (data not shown).
The protection of mice by the truncate MrpH vaccine from P. mirabilis UTI is comparable to the protection by the FimH vaccine from E. coli UTI (20). For comparison to this study, if one uses a mean bladder weight of 0.5 g to convert the data of Langermann et al. (20), then protection of the bladder from colonization following challenge of vaccinated animals was nearly identical for E. coli and P. mirabilis. For the kidney, the P. mirabilis vaccine appeared to offer better protection (∼1.5 logs). This is especially important since P. mirabilis localizes to the kidney and can cause serious histological damage to these organs, even after a single infection. There are a few other differences as well. The protection from E. coli UTI was achieved by systemic immunization with the FimH vaccine. In our study, however, systemic immunization via the s.c. route with the MrpH vaccine, despite inducing a strong serum IgG response, yielded no protection from P. mirabilis UTI (data not shown). The FimH adhesin was coexpressed with and then copurified with the FimC chaperone based on the affinity binding of FimH to mannose. In our study, we tried coexpressing the MrpH adhesin with the MrpD chaperone, but >90% of overexpressed protein was composed of the MrpD-MrpD dimer. However, the lack of information about the receptor of MrpH made it difficult to separate the MrpH-MrpD dimer from the MrpD-MrpD dimer efficiently. We also tried overexpressing the mature MrpH polypeptide as a six-histidine- tagged recombinant protein, which resulted in formation of inclusion bodies. The addition of the ∼40-kDa MBP fusion to the N terminus of the mature MrpH polypeptide appeared to keep the recombinant protein in a soluble form. Thus, the MBP fusions of the MrpH adhesin were chosen for our vaccine study.
In our study, systemic immunization via the s.c. route did not yield protection from P. mirabilis infection (data not shown). Pellegrino et al. reported that s.c. immunization of mice with MrpA (the major structural subunit of the MR/P fimbriae) afforded great protection from intravenous challenge of P. mirabilis and a much smaller degree of protection (limited to kidneys only) from TU challenge of P. mirabilis (36). These results further suggest that systemic immunization yields better protection from systemic infections than from mucosal infections. Pellegrino et al. also found that there is no significant association between the degree of infection and IgG response in serum (36). In this study, we chose the i.n. route of mucosal immunization to test the efficacy of the MrpH vaccine because (i) it yielded consistent protection from P. mirabilis infection when the formalin-killed whole-cell preparation and purified MR/P fimbria preparation were tested as vaccine candidates, (ii) it induced broader antibody responses, and finally (iii) the route of vaccination is simple. Even though TU immunization with the formalin-killed whole-cell preparation and purified MR/P fimbria preparation did not yield consistent protection, it cannot be ruled out that TU immunization with MrpH may be protective.
For the vaccine to protect against heterologous challenge, it would be desirable for MrpH to be conserved among P. mirabilis strains. We have good evidence that virulence genes are conserved in P. mirabilis. For example, when the urease gene cluster was examined, we probed HindIII restriction digests of chromosomal preparations of 100 strains using the entire urease gene cluster (∼8 kb) as a probe. All 100 strains showed identical Southern blots without exception (30). To demonstrate that mrpH is conserved among strains, a fragment carrying the open reading frame was PCR-amplified from 10 P. mirabilis isolates representing five distinct Dienes types (8): four pyelonephritis isolates, three catheter-associated isolates, and three fecal isolates. Sequencing of these PCR-amplified fragments revealed that the nucleotide sequences of the mrpH genes are 99.4 to 100% identical and the predicted amino acid sequences of the MrpH proteins are 100% identical. Given the conservation of MrpH among various P. mirabilis strains, an MrpH-based vaccine would likely protect against heterologous infections.
The truncate MrpH vaccine protected 75% of mice from P. mirabilis UTI. To further improve the efficacy, other adhesin molecules may be added to the vaccine formulation. As discussed above, P. mirabilis produces at least four different types of fimbriae. Recent studies in our laboratory indicated that increased MR/P fimbrial expression in vivo might be a result of the outgrowth of (as a result of selection for) MR/P fimbriate bacteria (unpublished data). It is possible that immune responses to MrpH may allow an outgrowth of (i.e., selection of) bacteria expressing a different type of fimbriae in vaccinated mice. In this case, addition of other adhesin molecules in the vaccine formula may improve its efficacy.
In summary, UTI is the most frequently diagnosed kidney and urological disorder. While E. coli is the most common isolate in normal individuals, those with structural abnormalities of the urinary tract or catheterized individuals are very frequently infected with P. mirabilis. Because we are now beginning to understand the molecular mechanisms by which P. mirabilis establishes infection, evades the host defense, and damages host tissue; because we can identify a large and well defined group of patients who are affected by this species; and because there is evidence that vaccination would prevent infections and thus urolithiasis, we believe that the development of a vaccine that could be used to prevent these often devastating infections in a high-risk group of individuals is useful.
Acknowledgments
This work is supported by Public Health Service grant DK49720 from the National Institutes of Health.
Editor: J. N. Weiser
REFERENCES
- 1.Adegbola, R. A., D. C. Old, and B. W. Senior. 1983. The adhesins and fimbriae of Proteus mirabilis strains associated with high and low affinity for the urinary tract. J. Med. Microbiol. 16:427-431. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1995. Current protocols in molecular biology. John Wiley & Sons, Inc., New York, N.Y.
- 3.Bahrani, F. K., S. Cook, R. A. Hull, G. Massad, and H. L. Mobley. 1993. Proteus mirabilis fimbriae: N-terminal amino acid sequence of a major fimbrial subunit and nucleotide sequences of the genes from two strains. Infect. Immun. 61:884-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bahrani, F. K., D. E. Johnson, D. Robbins, and H. L. Mobley. 1991. Proteus mirabilis flagella and MR/P fimbriae: isolation, purification, N-terminal analysis, and serum antibody response following experimental urinary tract infection. Infect. Immun. 59:3574-3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bahrani, F. K., G. Massad, C. V. Lockatell, D. E. Johnson, R. G. Russell, J. W. Warren, and H. L. Mobley. 1994. Construction of an MR/P fimbrial mutant of Proteus mirabilis: role in virulence in a mouse model of ascending urinary tract infection. Infect. Immun. 62:3363-3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bahrani, F. K., and H. L. Mobley. 1994. Proteus mirabilis MR/P fimbrial operon: genetic organization, nucleotide sequence, and conditions for expression. J. Bacteriol. 176:3412-3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark, J. Y., I. M. Thompson, and S. A. Optenberg. 1995. Economic impact of urolithiasis in the United States. J. Urol. 154:2020-2024. [PubMed] [Google Scholar]
- 8.Dienes, L. 1946. Reproductive processes in Proteus culture. Proc. Soc. Exp. Biol. Med. 63:265-270. [DOI] [PubMed] [Google Scholar]
- 9.Eriksson, S., J. Zbornik, H. Dahnsjo, P. Erlanson, O. Kahlmeter, H. Fritz, and C. A. Bauer. 1986. The combination of pivampicillin and pivmecillinam versus pivampicillin alone in the treatment of acute pyelonephritis. Scand. J. Infect. Dis. 18:431-438. [DOI] [PubMed] [Google Scholar]
- 10.Fairley, K. F., N. E. Carson, R. C. Gutch, P. Leighton, A. D. Grounds, E. C. Laird, P. H. McCallum, R. L. Sleeman, and C. M. O'Keefe. 1971. Site of infection in acute urinary-tract infection in general practice. Lancet ii:615-618. [DOI] [PubMed] [Google Scholar]
- 11.File, T. M., Jr., J. S. Tan, S. J. Salstrom, and L. Johnson. 1985. Timentin versus piperacillin in the therapy of serious urinary tract infections. Am. J. Med. 79:91-95. [DOI] [PubMed] [Google Scholar]
- 12.Fowler, J. E., Jr. 1984. Bacteriology of branched renal calculi and accompanying urinary tract infection. J. Urol. 131:213-215. [DOI] [PubMed] [Google Scholar]
- 13.Gentry, L. O., B. A. Wood, M. D. Martin, and J. Smythe. 1980. Cefamandole alone and combined with gentamicin or tobramycin in the treatment of acute pyelonephritis. Scand. J. Infect. Dis. Suppl. 25:96-100. [PubMed] [Google Scholar]
- 14.Griffith, D. P., D. M. Musher, and C. Itin. 1976. Urease. The primary cause of infection-induced urinary stones. Investig. Urol. 13:346-350. [PubMed] [Google Scholar]
- 15.Hagberg, L., I. Engberg, R. Freter, J. Lam, S. Olling, and C. Svanborg Eden. 1983. Ascending, unobstructed urinary tract infection in mice caused by pyelonephritogenic Escherichia coli of human origin. Infect. Immun. 40:273-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harlow, E., and D. Lane. 1988. Antibodies: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 17.Johnson, D. E., F. K. Bahrani, C. V. Lockatell, C. B. Drachenberg, J. R. Hebel, R. Belas, J. W. Warren, and H. L. Mobley. 1999. Serum immunoglobulin response and protection from homologous challenge by Proteus mirabilis in a mouse model of ascending urinary tract infection. Infect. Immun. 67:6683-6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson, D. E., R. G. Russell, C. V. Lockatell, J. C. Zulty, J. W. Warren, and H. L. Mobley. 1993. Contribution of Proteus mirabilis urease to persistence, urolithiasis, and acute pyelonephritis in a mouse model of ascending urinary tract infection. Infect. Immun. 61:2748-2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones, B. D., C. V. Lockatell, D. E. Johnson, J. W. Warren, and H. L. Mobley. 1990. Construction of a urease-negative mutant of Proteus mirabilis: analysis of virulence in a mouse model of ascending urinary tract infection. Infect. Immun. 58:1120-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Langermann, S., S. Palaszynski, M. Barnhart, G. Auguste, J. S. Pinkner, J. Burlein, P. Barren, S. Koenig, S. Leath, C. H. Jones, and S. J. Hultgren. 1997. Prevention of mucosal Escherichia coli infection by FimH-adhesin-based systemic vaccination. Science 276:607-611. [DOI] [PubMed] [Google Scholar]
- 21.Legnani-Fajardo, C., P. Zunino, G. Algorta, and H. F. Laborde. 1991. Antigenic and immunogenic activity of flagella and fimbriae preparations from uropathogenic Proteus mirabilis. Can. J. Microbiol. 37:325-328. [DOI] [PubMed] [Google Scholar]
- 22.Li, X., D. E. Johnson, and H. L. Mobley. 1999. Requirement of MrpH for mannose-resistant Proteus-like fimbria-mediated hemagglutination by Proteus mirabilis. Infect. Immun. 67:2822-2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li, X., C. V. Lockatell, D. E. Johnson, and H. L. Mobley. 2002. Identification of MrpI as the sole recombinase that regulates the phase variation of MR/P fimbria, a bladder colonization factor of uropathogenic Proteus mirabilis. Mol. Microbiol. 45:865-874. [DOI] [PubMed] [Google Scholar]
- 24.Li, X., H. Zhao, L. Geymonat, F. Bahrani, D. E. Johnson, and H. L. Mobley. 1997. Proteus mirabilis mannose-resistant, Proteus-like fimbriae: MrpG is located at the fimbrial tip and is required for fimbrial assembly. Infect. Immun. 65:1327-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li, X., H. Zhao, C. V. Lockatell, C. B. Drachenberg, D. E. Johnson, and H. L. Mobley. 2002. Visualization of Proteus mirabilis within the matrix of urease-induced bladder stones during experimental urinary tract infection. Infect. Immun. 70:389-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Massad, G., F. K. Bahrani, and H. L. Mobley. 1994. Proteus mirabilis fimbriae: identification, isolation, and characterization of a new ambient-temperature fimbria. Infect. Immun. 62:1989-1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Massad, G., C. V. Lockatell, D. E. Johnson, and H. L. Mobley. 1994. Proteus mirabilis fimbriae: construction of an isogenic pmfA mutant and analysis of virulence in a CBA mouse model of ascending urinary tract infection. Infect. Immun. 62:536-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moayeri, N., C. M. Collins, and P. O'Hanley. 1991. Efficacy of a Proteus mirabilis outer membrane protein vaccine in preventing experimental Proteus pyelonephritis in a BALB/c mouse model. Infect. Immun. 59:3778-3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mobley, H. L., and R. Belas. 1995. Swarming and pathogenicity of Proteus mirabilis in the urinary tract. Trends Microbiol. 3:280-284. [DOI] [PubMed] [Google Scholar]
- 30.Mobley, H. L., and G. R. Chippendale. 1990. Hemagglutinin, urease, and hemolysin production by Proteus mirabilis from clinical sources. J. Infect. Dis. 161:525-530. [DOI] [PubMed] [Google Scholar]
- 31.Mobley, H. L., and R. P. Hausinger. 1989. Microbial ureases: significance, regulation, and molecular characterization. Microbiol. Rev. 53:85-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mobley, H. L., and J. W. Warren. 1987. Urease-positive bacteriuria and obstruction of long-term urinary catheters. J. Clin. Microbiol. 25:2216-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Old, D. C., and R. A. Adegbola. 1985. Antigenic relationships among type-3 fimbriae of Enterobacteriaceae revealed by immunoelectronmicroscopy. J. Med. Microbiol. 20:113-121. [DOI] [PubMed] [Google Scholar]
- 34.Old, D. C., and R. A. Adegbola. 1982. Haemagglutinins and fimbriae of Morganella, Proteus and Providencia. J. Med. Microbiol. 15:551-564. [DOI] [PubMed] [Google Scholar]
- 35.Pazin, G. J., and A. I. Braude. 1969. Immobilizing antibodies in pyelonephritis. J. Immunol. 102:1454-1465. [PubMed] [Google Scholar]
- 36.Pellegrino, R., U. Galvalisi, P. Scavone, V. Sosa, and P. Zunino. 2003. Evaluation of Proteus mirabilis structural fimbrial proteins as antigens against urinary tract infections. FEMS Immunol. Med. Microbiol. 36:103-110. [DOI] [PubMed] [Google Scholar]
- 37.Pfaller, M. A., I. Mujeeb, R. J. Hollis, R. N. Jones, and G. V. Doern. 2000. Evaluation of the discriminatory powers of the Dienes test and ribotyping as typing methods for Proteus mirabilis. J. Clin. Microbiol. 38:1077-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rubin, R. H., N. E. Tolkoff-Rubin, and R. S. Cotran. 1986. Urinary tract infection, pyelonephritis, and reflux nephropathy, p. 1085-1141. In F. C. Rector (ed.), The kidney. W. B. Saunders Co., Philadelphia, Pa.
- 39.Schappert, S. M. 1999. Ambulatory care visits to physician offices, hospital outpatient departments, and emergency departments: United States, 1997, p. 1-39. Vital health statistics series 13, no. 143. National Center for Health Statistics, Centers for Disease Control and Prevention, Atlanta, Ga. [PubMed]
- 40.Senior, B. W. 1983. Proteus morgani is less frequently associated with urinary tract infections than Proteus mirabilis—an explanation. J. Med. Microbiol. 16:317-322. [DOI] [PubMed] [Google Scholar]
- 41.Trollfors, B., M. Jertborn, J. Martinell, G. Norkrans, and G. Lidin-Janson. 1982. Mecillinam versus cephaloridine for the treatment of acute pyelonephritis. Infection 10:15-17. [DOI] [PubMed] [Google Scholar]
- 42.Warren, J. W. 1995. Clinical presentations and epidemiology of urinary tract infections, p. 3-27. In H. L. T. Mobley and J. W. Warren (ed.), Urinary tract infections: molecular pathogenesis and clinical management. ASM Press, Washington, D.C.
- 43.Warren, J. W., H. L. Muncie, Jr., J. R. Hebel, and M. Hall-Craggs. 1994. Long-term urethral catheterization increases risk of chronic pyelonephritis and renal inflammation. J. Am. Geriatr. Soc. 42:1286-1290. [DOI] [PubMed] [Google Scholar]
- 44.Warren, J. W., J. H. Tenney, J. M. Hoopes, H. L. Muncie, and W. C. Anthony. 1982. A prospective microbiologic study of bacteriuria in patients with chronic indwelling urethral catheters. J. Infect. Dis. 146:719-723. [DOI] [PubMed] [Google Scholar]
- 45.Zhao, H., X. Li, D. E. Johnson, I. Blomfield, and H. L. Mobley. 1997. In vivo phase variation of MR/P fimbrial gene expression in Proteus mirabilis infecting the urinary tract. Mol. Microbiol. 23:1009-1019. [DOI] [PubMed] [Google Scholar]
- 46.Zunino, P., L. Geymonat, A. G. Allen, C. Legnani-Fajardo, and D. J. Maskell. 2000. Virulence of a Proteus mirabilis ATF isogenic mutant is not impaired in a mouse model of ascending urinary tract infection. FEMS Immunol. Med. Microbiol. 29:137-143. [DOI] [PubMed] [Google Scholar]