Abstract
Successful vaccines against serogroup A and C meningococcal strains have been developed, but current serogroup B vaccines provide protection against only a limited range of strains. The ideal meningococcal vaccine would provide cross-reactive immunity against the variety of strains that may be encountered in any community, but it is unclear whether the meningococcus possesses immune targets that have the necessary level of cross-reactivity. We have generated a phoP mutant of the meningococcus by allele exchange. PhoP is a component of a two-component regulatory system which in other bacteria is an important regulator of virulence gene expression. Inactivation of the PhoP-PhoQ system in Salmonella leads to avirulence, and phoP mutants have been shown to confer protection against virulent challenge. These mutants have been examined as potential live attenuated vaccines. We here show that a phoP mutant of the meningococcus is avirulent in a mouse model of infection. Moreover, infection of mice with the phoP mutant stimulated a bactericidal immune response that not only killed the infecting strain but also showed cross-reactive bactericidal activity against a range of strains with different serogroup, serotype, and serosubtyping antigens. Sera from the mutant-infected mice contained immunoglobulin G that bound to the surface of a range of meningococcal strains and mediated opsonophagocytosis of meningococci by human phagocytic cells. The meningococcal phoP mutant is thus a candidate live, attenuated vaccine strain and may also be used to identify cross-reactive protective antigens in the meningococcus.
Neisseria meningitidis is the major cause of epidemic meningitis worldwide. Vaccines based on capsular polysaccharide of the meningococcus are effective for protecting against serogroup A and C disease but provide only short-lived immunity (40). A conjugated serogroup C polysaccharide vaccine has recently been introduced in the United Kingdom, and its introduction has been associated with a dramatic reduction of serogroup C meningococcal disease (5). However, the serogroup B polysaccharide is poorly immunogenic in humans, and no vaccine is currently available for this serogroup. The development of a vaccine for serogroup B disease is thus a priority for many areas of the world.
Antibody is thought to provide protective immunity to meningococcal disease, and a number of targets have been identified, such as capsular polysaccharide and the major outer membrane proteins (OMPs). Since the serogroup B polysaccharide is poorly immunogenic in humans, attention has focused on the meningococcal OMPs. However, many OMPs are subject to antigenic variation, and although OMP-based vaccines have been developed, they give only limited immunity against genetically diverse meningococcal strains (11, 40).
Several lines of evidence suggest that important and pan-reactive determinants of immunity remain to be discovered: (i) in immunocompetent individuals, a single episode of meningococcemia confers permanent immunity to all types of meningococci (2, 16, 17), and (ii) carriage of commensal species such as Neisseria lactamica provides immunity to meningococcal disease (2, 15-17, 29). Both of these findings indicate that natural infection (with either N. meningitidis or N. lactamica) provides long-term, cross-reactive protection, but the identity of the antigens involved is unknown. They are unlikely to be characterized OMPs, since these antigens cannot induce this type of immunity and bactericidal immune responses in immunized volunteers do not correlate with antibodies to the class 1, 2, or 5 OMPs (34, 52). Polysaccharide and lipooligosaccharide antigens are similarly variable and are unlikely to provide long-lived immunity, since they are T-cell-independent antigens incapable of stimulating immunological memory.
The protective antigens recognized during natural infection have so far escaped detection, possibly because they are expressed in vivo only during infection. Successful pathogens such as Salmonella, Shigella, and Listeria are known to express virulence genes in response to the host environment (35). Environmentally determined gene regulation is also seen in the meningococcus, leading, for example, to the expression in vivo of iron-regulated proteins (48) and to modulation of capsule and pilus expression altering epithelial adherence and invasion (24, 44, 49), but the regulators are unknown.
Two-component regulatory systems control gene expression in many bacteria in response to environmental signals. These systems comprise a membrane-associated sensor kinase protein and a cytoplasmic transcriptional regulator. In response to external stimuli, the membrane-located sensor undergoes a conformational change resulting in phosphorylation of the regulator. This in turn affects its ability to bind to DNA at specific promoter sites and thus regulates its activity as a transcriptional regulator. Many two-component systems are involved in controlling virulence gene expression in response to changes in magnesium levels and pH. For example, the Salmonella PhoP-PhoQ system regulates more than 40 different genes, termed PhoP-activated (pag) and PhoP-repressed (prg) genes (19-21, 36). In Salmonella enterica serovar Typhimurium these genes affect survival within macrophages, resistance to host antimicrobial peptides and acid pH, invasion of epithelial cells, and antigen presentation. Inactivation of the PhoP-PhoQ system attenuates virulence by more than 10,000-fold (36, 37). PhoP-PhoQ homologues have been shown to be involved in control of virulence in a range of bacteria (25, 42, 50), and detection of genes regulated by PhoP has been shown to be powerful route towards identification of genes involved in virulence (1, 8, 22).
Two-component regulatory systems have also been reported for N. meningitidis, such as the PilA-PilB (3, 4) and NtrX-NtrY (The Institute for Genomic Research N. meningitidis genome database locus NMB0115/NMB0114) systems. Although PhoP-PhoQ homologues have been reported for many gram-negative bacteria (20, 33), including a possible PhoP homologue in Neisseria gonorrhoeae (20), they were unknown in the meningococcal genome until we recently identified a PhoP-PhoQ-like system in this organism (28). The PhoP system was inactivated by allele replacement, yielding a phoP knockout mutant, and its phenotype was compared to that of the wild type. There were many differences in polypeptide profiles between the wild type and the mutant, supporting an effect on gene regulation. The mutant also displayed many growth characteristics that were similar to those of phoP mutants of Salmonella: it showed poor growth at low concentrations of magnesium and was sensitive to defensins and other environmental stresses. Magnesium-regulated differences in protein expression were absent in the mutant, indicating that the meningococcal PhoP-PhoQ system may, as in Salmonella, respond to changes in environmental magnesium levels. These results are consistent with the PhoP homologue playing a role in the meningococcus similar to that played by PhoP itself in Salmonella and with involvement of the meningococcal PhoP in the regulation of virulence genes. In support of this conclusion, we found that the mutant was unable to grow in mouse serum and was attenuated in its ability to traverse through a layer of human epithelial cells. However, we could not examine the virulence of this mutant in the mouse because the strain used was already avirulent in this disease model.
In this study we describe the generation of a new phoP meningococcal mutant derived from a serogroup C strain previously demonstrated to be virulent in the mouse (45), and we show that the phoP mutant is completely avirulent in the mouse model of infection. These results confirm that the PhoP-PhoQ system is involved in controlling virulence genes in the meningococcus. We also demonstrate that mice infected with the mutant generate immunity to the meningococcus that is cross-reactive against diverse meningococci.
MATERIALS AND METHODS
Bacterial strains and plasmid.
The N. meningitidis strains used in this study were supplied by the Health Protection Agency, Manchester, United Kingdom, and Escherichia coli (DH5α) was supplied by Invitrogen. Growth was routinely on Columbia agar base (Oxoid) plates supplemented with 6% (vol/vol) defibrinated horse blood (CB agar) (TC supplies) and in Mueller-Hinton (MH) broth (Oxoid). Peptone broth, used for growth in some experiments, contained 5 g of neutralized bacteriological peptone (Oxoid), 1 g of Na2HPO4, and 1% (vol/vol) glucose per liter (pH 7.35). Plasmid pUS 2130 (28) is a pUC19-based plasmid with the ampicillin gene replaced by the aph gene. Regions flanking either side of the phoP gene were inserted on either side of the aph gene.
Allele exchange in N. meningitidis.
Linearized plasmid pUS2130 was transformed into the meningococcus and confirmed by PCR and Southern blot analysis as previously described by Johnson et al. (28).
Recombinant DNA techniques.
Standard PCR, cloning, and recombinant DNA techniques were used (47). Restriction and modification enzymes were purchased from Roche or Gibco-BRL and used according to the manufacturer's recommendations. All PCRs were performed on a Perkin-Elmer 2400 PCR machine.
Mouse infection studies.
A murine intraperitoneal challenge model of infection (18, 51) was utilized. Meningococcal strains were grown overnight at 37°C in 5% CO2 on blood agar plates, and then single colonies were inoculated into 200 ml of MH broth. The broth cultures were incubated with orbital agitation at 37°C for 4 h and then adjusted on the basis of optical density (OD) to the required concentration for inoculation. In parallel, CFU determinations of inocula were carried out by serial dilution and replicate colony counting. Various doses of inocula were administered intraperitoneally (i.p.) with human holotransferrin (10 mg) in a total volume of 500 μl to groups of five 6- to 8-week-old Harlan-NIH inbred female mice. The day after infection, mice that had not already been humanely killed were boosted i.p. with human holotransferrin (10 mg). A health scoring system based on guidelines (41) and experience (12) was used to assess the health of individual mice. Healthy mice were given an arbitrary health score of 5. The following observable symptoms resulted in deductions in health score: eyes shut, −1; ruffled fur, −1; ruffled fur and eyes shut, −2; and immobility, −4. Health was regularly scored in this manner, and as soon as immobile mice were detected they were humanely killed. Blood was taken from all of the surviving mice at 4 weeks postinfection. For the purpose of clarity, this is referred to as phoP serum.
Bacterial antibody surface binding assay.
The ability of murine antisera to bind to the surface of whole bacteria was assessed by using an adaptation of the methods of Rioux et al. (46) and Moe et al. (39). Bacteria were grown shaking for 4 h at 35°C in MH broth, killed by the addition of sodium azide 0.2% (wt/vol) and 100 μM phenylmethylsulfonyl fluoride, followed by a further 48 h of incubation at 35°C. The bacteria were spun to remove all signs of the preservative before being resuspended in blocking buffer (2% [wt/vol] bovine serum albumin [BSA] in phosphate-buffered saline [PBS]) at an OD at 650 nm (OD650) of 0.5. Test serum (5 μl) was added to 500 μl of bacteria and incubated at room temperature for 1 h. The bacteria were washed twice in blocking buffer, and the pellet was resuspended in 500 μl of goat anti-mouse-fluorescein isothiocyanate (FITC) conjugate (Sigma) (1:500 in PBS). This was incubated at room temperature in the dark for 1 h. The bacteria were then analyzed by flow cytometry with a Becton Dickinson FACScan and Cellquest software. Quality assurance procedures using standard operating practice and Rainbow beads (Becton Dickinson) ensured reproducibility.
Opsonophagocytosis.
N. meningitidis strain H44/76 was grown in batch culture for 4 h at 37°C in a 5% CO2 atmosphere in Frantz medium containing 14 μM ethylenediamine di(o-hydroxyphenyl acetic acid) and 10 μM 2′,7′-bis-(2-carboxyethyl)-5-(and 6)-carboxyfluorescein acetoxymethyl ester (molecular probes). The culture was killed by using a combination of 0.2% (wt/vol) sodium azide and 100 μM phenylmethylsulfonyl fluoride at 37°C. After cultures were confirmed dead by CFU determination, they were stored at 4°C. Human peripheral blood mononuclear leukocytes (PBMLs) from volunteers were separated from 20 ml of heparinized blood by two treatments with a red blood cell lysis buffer (150 mM NH4Cl, 9.5 mM NaHCO3, 2.4 mM EDTA Na2 · 2H2O). PBMLs were subsequently washed and resuspended in Dulbecco's PBS containing 0.5% (wt/vol) BSA and 0.1% (wt/vol) glucose (DPBSGA). HL60 cells were obtained from European Collection of Cell Cultures (Health Protection Agency, Porton Down, United Kingdom) and used between passages 15 and 35 (1/5 to 1/10 dilution at each passage). The cells were differentiated with 10.7 μM dimethyl formamide (DMF) and left for 5 days before utilization in the assay. For the assay, 20 μl of mouse or human serum diluted 1/10 in assay buffer (DPBSGA plus 0.9 mM CaCl2 · 2H2O and 0.5 mM MgSO4 · 7H2O) was added to 20 μl of a bacterial suspension at 6.25 × 108 bacteria/ml in assay buffer and then incubated with shaking at 37°C for 30 min. This mixture was further incubated for 15 min after the addition of 10 μl of baby rabbit complement. (Pel freeze) After the addition of 40 μl HL60 cells or human PBMLs at 2.5 × 107 cells/ml, the reaction mixture was further incubated for 30 min. The reaction was terminated by addition of ice-cold DPBS to the reaction mixture up to a total volume of 0.9 ml, and samples were refrigerated until immediately before analysis. The reaction mixture was analyzed with an EPICS XL flow cytometer. Fluorescence analysis was conducted by using a gated region set to select effector cells. The percentage of effector cells that were fluorescent above an arbitrary limit was determined along with the average fluorescence intensity of each analyzed effector cell by using EXPO32 ADC V1.2 analysis software. These two values were multiplied to generate a phagocytosis product (32) for each sample. The mean and standard deviation (SD) for duplicate analysis were calculated.
Bactericidal assay.
MH broth was inoculated with the test strains at an OD595 of 0.07 to 0.1 and incubated at 35°C until an OD595 of 0.4 was reached. The bacteria were diluted 1:10,000 in Gey's balanced salt solution-0.5% (wt/vol) BSA (Gibco). The assay was set up in a microtiter plate such that each well contained 20 μl of twofold serially diluted heat-inactivated phoP serum in Gey's balanced salt solution-0.5% (wt/vol) BSA, 10 μl of diluted bacteria, and 10 μl of 1:5-diluted baby rabbit complement (Serotec). Control wells were set up to contain either heat-inactivated complement, complement, or serum with heat-inactivated complement. The assay also included negative mouse serum from naive mice and the monoclonal antibody to the meningococcal Pl.7 epitope (National Institute of Biological Standards and Control, Potters Bar, United Kingdom), which acted as a positive control against strains H44/76 and MC58. The microtiter plates were incubated for 1 h at 35°C. Ten microliters from each well was spotted onto warm CB plates and incubated at 35°C. The bactericidal titer was determined as the reciprocal dilution of serum showing ≥50% killing at T60 compared to the heat-inactivated complement control well (7). The assay was performed in duplicate on at least three occasions.
Polymyxin B sensitivity.
Parent and mutant meningococci were inoculated into peptone broth at an OD595 of 0.2. A 50-μl aliquot was used to inoculate 5 ml of peptone broth containing different concentrations of MgCl2 and polymyxin B. The broths were incubated at 35°C with orbital agitation for 18 h. OD595 readings were taken as a measure of survival. The mean and SD for triplicate analysis were calculated.
RESULTS
Generation of a phoP mutation in a mouse-virulent strain.
A new phoP mutant was generated in a mouse-virulent strain, L91543 (C2a P1.2, ST-11, ET-37 complex; United Kingdom), by using the same allele exchange strategy as before (28). The allele exchange event was confirmed by PCR and Southern blotting (data not shown).
Phenotypic characterization of the phoP mutant.
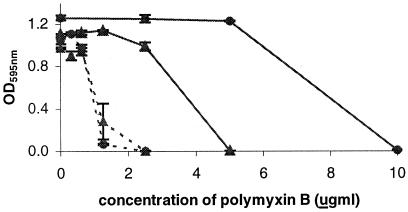
The new phoP mutant demonstrated growth characteristics similar to those of the previous mutant in that it grew to a lower OD after a given time period than the wild-type strain, showed poor growth at low levels of MgCl2 (e.g., 1 mM), and had a smaller colony morphology after 18 h of incubation on CB agar (data not shown). The new mutant was tested for its sensitivity to the polycationic antibiotic polymyxin B at various concentrations of MgCl2. The mutant was clearly more sensitive to the polymyxin B than the parent strain (Fig. 1). The phoP mutant also retained expression of C capsular polysaccharide, as determined by dot blotting with anti-C polysaccharide (data not shown).
FIG. 1.
Sensitivity of wild-type and phoP mutant strains to polymyxin. Wild-type (solid line) and phoP mutant (dashed lines) meningococci were grown in peptone broth containing 10 mM (circles) or 5 mM (triangles) MgCl2 and the indicated concentrations of polymyxin B. Bacteria were grown overnight at 35°C, and OD595 readings were taken as a measure of survival. The data are the averages of triplicate readings with SDs.
Virulence.
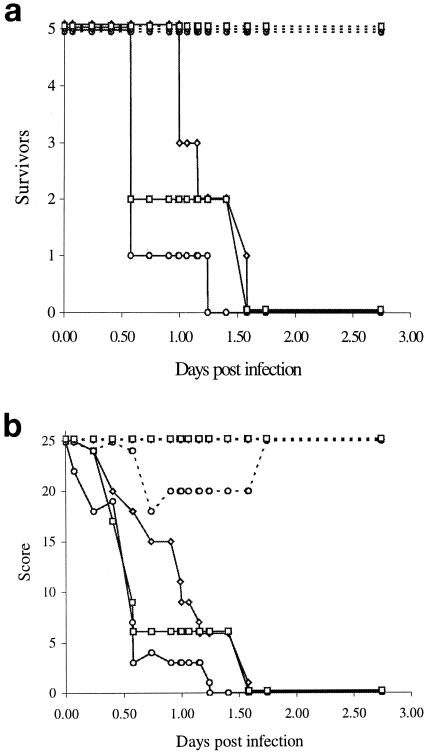
We utilized a mouse i.p. challenge model of infection (18, 51) in which meningococci were administered by i.p. injection to groups of five mice at three different challenge doses. Mice challenged with wild-type meningococci at all three doses became sick and had died or were humanely killed by 2 days (Fig. 2). However, mice inoculated with the phoP mutant bacteria showed no ill health at any of the doses tested. The surviving mice were sacrificed at 4 weeks postinoculation, and serum samples (phoP serum) were obtained.
FIG. 2.
Virulence of wild-type and phoP mutant strains. Various doses of meningococci were administered i.p. with human holotransferrin (10 mg) to groups of five Harlan-NIH inbred female mice. The day after infection, mice that had not already been humanely killed were boosted i.p. with human holotransferrin (10 mg). (a) Number of surviving mice at different times postinfection. (b) Health scores for surviving mice. Healthy mice were given an arbitrary health score of 5. The following observable symptoms resulted in cumulative deductions in health score: ruffled fur, −1, eyes shut, −1; ruffled fur and eyes shut, −2; immobile, −4. Health was regularly monitored, with any signs of ill health being indicative of lethal infection and scored as above; the mice were humanely killed when immobile. Solid lines, wild type; dashed lines, phoP mutant. The number of bacterial cells inoculated into the mice was 6 × 107 (circles), 6 × 106 (diamonds), or 6 × 105 (squares).
Immunogenicity.
phoP serum from each group of five mice inoculated with the phoP mutant meningococci were pooled and assessed for the presence of antibodies against the meningococcus.
(i) Surface-binding antibody.
Flow cytometry was used to measure antibody binding to the surface of intact meningococci. Standard meningococcal strains were grown, and the bacteria were killed before being incubated with murine sera. The bacteria were then incubated with one of three antibody-FITC conjugates, namely, goat anti-mouse immunoglobulin G (IgG) (either whole molecule or Fc specific) or goat anti-mouse IgM (μ chain specific), to measure the level and class of surface-binding antibody. To assess cross-reactivity, we used a range of bacterial strains as targets in the binding assay (Table 1).
TABLE 1.
Complement-mediated SBA with pooled phoP mouse serum against different meningococcal strains
| Meningococcal strain | Typing antigen
|
SBA titer (≥50% killing) | Maximum observed killing (%) | ||
|---|---|---|---|---|---|
| Group | Type | Subtype | |||
| L91543 (wild type) | C | 2a | 2 | 10,240 | 100 |
| phoP mutant | C | 2a | 2 | 10,240 | 100 |
| M97 250613 | C | 2a | 2,5 | 1,024 | 98 |
| M97 250611 | C | 2b | 2,5 | 512 | 69 |
| M97 250631 | C | 4 | 4 | 64 | 83 |
| M97 250609 | B | 4 | 4 | 64 | 66 |
| H44/76 | B | 15 | 7,16 | 16 | 70 |
| MC58 | B | 15 | 7,16 | 256 | 66 |
| L92/231 | W135 | NTa | 6 | 512 | 74 |
| L92/85 | Y | NT | 2 | 1,024 | 100 |
NT, nontypeable.
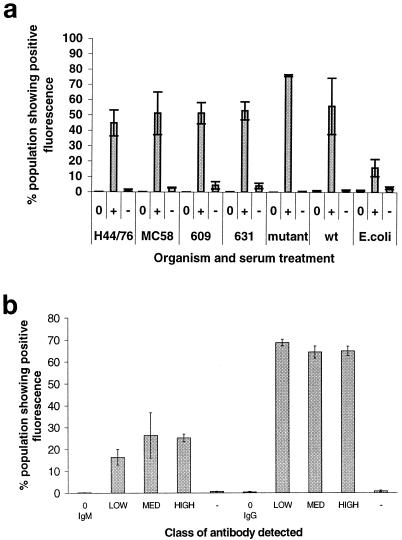
The phoP mutant was incubated with each of the three phoP antisera and anti-mouse IgG (whole molecule), which detects all mouse Igs. When the phoP serum from the mice inoculated with the high dose was incubated with a range of meningococcal target strains again with anti-mouse IgG (whole molecule), surface binding to each strain examined was detected (Fig. 3a). Flow cytometry clearly demonstrated that antiserum raised against the phoP mutant recognizes other meningococci but not the unrelated E. coli (P = 0.0003 by analysis of variance [ANOVA]). When the results obtained with the various meningococcal strains were compared against each other, analysis of variance just reached significance (P = 0.05), showing significant variation in the binding of the antiserum to the different strains. However, when strains excluding those obtained with the phoP mutant were analyzed, there was no significant difference in binding of the antibody to the different strains (P = 0.82 by ANOVA). Thus, the antibody raised in mice shows a higher level of recognition of the inoculating phoP mutant strain, but it also recognizes other serogroup C strains as well as serogroup B strains, suggesting that it is recognizing conserved epitopes on the surface of the cells.
FIG. 3.
Surface antibody binding of sera to meningococcal cells. Serum was incubated with killed bacteria. The bacteria were subsequently washed and incubated with fluorescein-labeled anti-mouse Ig antibody to label surface-binding antibody, and the percentage of labeled cells and intensity of fluorescence were measured by flow cytometry. The sera used included phoP serum (+) and naive mouse serum (−). As a further control, cells were also incubated with the secondary antibody alone (0). (a) phoP serum from the high-dose-inoculated animals was incubated with a range of killed meningococcal cells and with E. coli cells, using anti-mouse IgG (whole molecule) as labeled secondary antibody. The neisserial strains tested included H44/76, MC58, and 609 (all serogroup B) and 631, the mutant, and the wild type (wt) (all serogroup C). Results represent the mean ± SD for three replicate experiments. (b) The pooled serum from each group of mice (low, medium, and high doses, as described in the text) was incubated with phoP mutant bacteria and either IgM μ chain-specific or IgG Fc-specific secondary antibodies. The percentage of organisms showing fluorescence was determined. Results represent the mean ± SD for three replicate experiments. In all of these experiments the bacteria were analyzed with a Becton Dickinson FACScan and Cellquest software.
In order to further characterize the humoral response, the phoP mutant was incubated with phoP serum and the class of surface-bound antibody was determined by using secondary antibodies specific for either the μ chain of IgM or the Fc region of IgG (Fig. 3b). Significant increases in the percentage of bacteria staining with either IgG- or IgM-specific antibody compared to bacteria incubated with the second antibody alone were observed (P < 0.001 by ANOVA for IgM and IgG). In addition, the percentage of bacteria staining for meningococcus-specific IgG was significantly greater than that for IgM (low-dose IgM versus low-dose IgG, P < 0.0001; medium-dose IgM versus medium-dose IgG, P < 0.004; high-dose IgM versus high-dose IgG, P < 0.0001 [t test results]). When the mean fluorescence intensities of the populations were compared, the values with the anti-IgG second antibody showed about 10 times the fluorescence intensity than the values obtained with the anti-IgM secondary antibody (despite the secondary antibodies having similar levels of FITC substitution [data not shown]). This predominance of IgG in the response to the phoP mutant suggests that the organism has persisted long enough to stimulate a T-cell-dependent antibody class switch, but this will need to be confirmed.
(ii) Bactericidal antibody.
Complement-mediated killing of the homologous and heterologous meningococcal strains was determined with the phoP serum. The serum showed various levels of bactericidal activity against all of the strains tested (Table 1). The highest activity was found against the wild-type strain and strains that were of the same serogroup (serogroup C) or serosubtype (P1.2) as the parent strain. However, bactericidal activity was also detected against all of the tested strains, including serogroup B, serogroup Y, and serogroup W135 strains. The cross-reactivity could not be fully accounted for by presence of any one, or any combination, of the known serogrouping, serotyping, or serosubtyping antigens (Table 1). Interestingly, the homologous strain (and the wild type) gave by far the highest levels of bactericidal activity (10 times the titer of any other strain), despite the fact that many of the strains shared the same serogroup, serotype, or serosubtype antigens. This result appears to indicate that the principal target of the bactericidal activity in the phoP serum is unlikely to be the polysaccharide capsule, PorA, or PorB alone (the antigenic targets for the serogrouping, serotyping, and serosubtyping assays). Also noteworthy is the percent killing of the input cells in the bactericidal assay. Although the phoP serum efficiently killed 100% of the homologous bacteria (at the titer that gave maximum killing), the maximum killing observed with heterologous strains was often significantly less than 100%. This might indicate that the target antigens were not constitutively expressed on all bacterial cells.
(iii) Opsonophagocytosis.
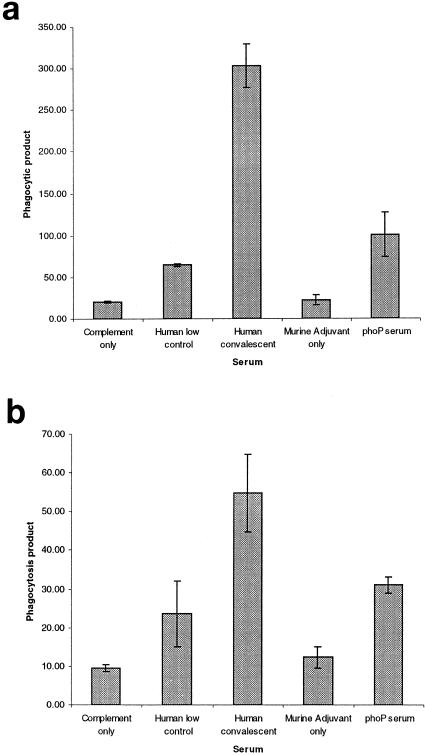
The opsonophagocytic activity of the phoP serum was measured by using freshly prepared PBMLs from a donor and DMF-differentiated HL60 cells (Fig. 4). The target for phagocytosis was fluorescently labeled N. meningitidis strain H44/76 (B15:P1.7,16). The level of opsonophagocytosis was compared to those of a negative control (alum-only-immunized) mouse serum, a convalescent-phase human serum that had previously demonstrated high levels of opsonophagocytic activity, a human volunteer serum that had previously demonstrated low levels of opsonophagocytic activity, and a no-antibody control. In both assays the phoP serum mediated opsonophagocytosis of this serogroup B strain.
FIG. 4.
Opsonophagocytosis activity of phoP serum. Fluorescently labeled meningococcal cells (H44/76) were incubated with a range of sera, together with complement and either human PBMLs (a) or HL60 cells differentiated with DMF (b). Serum samples included a human serum from a patient with low levels of opsonophagocytic activity (human low control), a human serum from a convalescent patient with high levels of opsonophagocytic activity (human convalescent serum), serum from mice injected with alum adjuvant alone (murine adjuvant only), and phoP serum. A further control was meningococcal cells incubated with complement alone (complement only). Uptake of meningococci was measured by flow cytometry. The phagocytosis product was determined by multiplying the percentage of fluorescent cells by the mean fluorescence of the gated phagocytes. Bars indicate means of duplicate determinations ± SD.
DISCUSSION
Our previous study showed that a meningococcal phoP mutant demonstrated a distinct phenotype (increased sensitivity to stresses such as osmotic stress and alkaline pH, altered protein profile, and inability to penetrate an epithelial cell layer) that suggested that PhoP regulates virulence genes in the meningococcus. The present study confirms and extends this work. A new phoP mutant of a mouse-virulent strain has been generated. The mutant showed a phenotype similar to that of the previously generated strain, and we were able to demonstrate an additional in vitro phenotype: increased sensitivity to the antibiotic polymyxin B. In addition, the use of a mouse-virulent strain allowed us to clearly demonstrate that the phoP mutation attenuates virulence in the meningococcus. The phoP mutant was completely avirulent in the mouse model. This result confirms our previous conjecture that, similar to the case for Salmonella, meningococcal PhoP is likely to be a key regulator of virulence genes in the meningococcus. Our previous studies demonstrated numerous differences in protein profile between the wild type and the mutant, so it is likely that many genes are involved. In Salmonella, the PhoP-PhoQ system modulates expression of more than 40 proteins in response to the host environment (13, 19, 38). The identity of the genes regulated by the meningococcal PhoP is currently unknown, but it is interesting that the meningococcal phoP mutant was, like Salmonella phoP mutants, sensitive to the antimicrobial peptide polymyxin. In Salmonella, polymyxin resistance is controlled by the pmrAB locus, which is activated by PhoP when cells are grown with low levels of magnesium (23, 30). We have identified a pmrA-like gene in the meningococcal genome (data not shown), indicating that a similar system may operate in the meningococcus.
Natural infection with the meningococcus involves colonization of the human nasopharynx, where it establishes an asymptomatic infection in most individuals. Nonpathogenic strains remain in the nasopharynx, but pathogenic strains may penetrate the epithelial cell layer to gain access to the submucosa, where they may then enter the lymph and thereby gain access to the systemic circulation. Once in the blood, the pathogen may be neutralized by nonspecific and specific immune mechanisms, but it may also establish an infection leading to septicemia and/or meningitis. It is clear that natural infection and disease involve a number of steps, including infection of epithelial, endothelial, and immune cells; passage through a series of cell layers (the nasopharyngeal epithelium and the endothelial cells lining the blood vessels); penetration of extracellular barriers (the epithelial and endothelial basement membranes); and survival in tissue fluid (lymph and serum) and resistance to phagocytosis. A number of genetic switches are likely to operate to regulate the expression of capsular and outer membrane protein genes in response to the changing environment. Identification of the PhoP-regulated genes and further dissection of the translocation process in the wild type and the mutant will help to elucidate meningococcal pathogenicity and may identify novel targets for vaccine development.
However, the most exciting result of this study is the demonstration that infection of mice with the phoP mutant meningococcus stimulates an immune response, which indicates that it may produce cross-reactive protection, as is seen following meningococcal disease (2). The arm of immunity that confers cross-reactive protection in meningococcal disease is unclear. Classic studies performed in the 1960s demonstrated the importance of bactericidal antibody for protection of humans (2); however, recent work indicates that opsonizing antibody activity may also be important, particularly for serogroup B disease (31, 43). A key aim of vaccination is to generate bactericidal and/or opsonizing antibody capable of neutralizing meningococcal strains from the range of serogroups, serotypes, and serosubtypes that are involved in disease. Current meningococcal vaccines incorporate either the polysaccharide serogroup antigen (either alone or conjugated to a protein), which provides serogroup-specific protection, or OMPs, which provide no or very limited protection against heterologous strains.
Our results indicate that mice infected with the group C serogroup phoP mutant generate antibody that binds to the surface of the meningococcus and has both bactericidal and opsonophagocytic activity against a range of strains, including group B strains. The serum bactericidal assay (SBA) titers vary greatly (from 16 to 10,240) depending on the target strain. However, studies on postvaccination sera and in animal models of infection indicate that protection may be associated with relatively small increases in SBA titers (as low as fourfold) (7). Infection of mice with the meningococcal phoP mutant therefore appears to generate an immune response that has the hallmarks of natural meningococcal infection in humans. It will clearly be of interest to determine whether the mice are indeed protected from disease in a challenge model, but they at least have these correlates of protection. The identity of the target cross-reactive antigens that are recognized by the mouse antibody is also of great interest. They are unlikely to be any of the currently known antigens alone, as the cross-reactivity did not correlate with any of the group-, type-, or subtype-specific protein antigens. Although we have not yet measured reactivity against lipopolysaccharide (LPS), we do not consider it likely that LPS is the target for the cross-reactive response, since LPS tends to be highly variable between strains. Also, at least part of the cross-reactive response that we detected was IgG mediated, but both LPS and other polysaccharides are T-cell-independent antigens that do not stimulate class switching to IgG. The availability of sera from infected mice should allow us to identify the target antigens.
Another aspect of these experiments is the indication that the meningococcal phoP mutant is a potential live rationally attenuated vaccine candidate. Mice infected with the phoP mutant were entirely healthy but produced antibodies with bacterial and opsonophagocytic activity. Salmonella phoP mutants are similarly avirulent (10, 37, 38), and infection confers protection in mice. Salmonella vaccine strains with phoP mutations and mutations in PhoP-regulated genes have been constructed and are currently being evaluated as candidate rationally attenuated live typhoid vaccines (14, 26, 27) and also as a vaccine vector (6, 9). Although there are many problems with developing and testing a live meningococcal vaccine, the possibility of generating long-lived cross-reactive protection indicates that the approach should at least be explored.
Acknowledgments
This work was supported by the Meningitis Trust. Work at the Health Protection Agency, Porton Down, was supported by the United Kingdom Department of Health.
We thank Ray Borrow (Vaccine Evaluation Department, Manchester Medical Microbiology Partnership, Manchester, United Kingdom) for helpful discussions on the bactericidal assay, and we thank Sarah Morley and Simon Kroll (Department of Paediatrics, Faculty of Medicine, Imperial College of Science and Technology, London, United Kingdom) for provision of human serum samples.
Editor: J. N. Weiser
REFERENCES
- 1.Adams, P., R. Fowler, N. Kinsella, G. Howell, M. Farris, P. Coote, and C. D. Connor. 2001. Proteomic detection of PhoPQ- and acid-mediated repression of Salmonella motility. Proteomics 1:597-607. [DOI] [PubMed] [Google Scholar]
- 2.Ala'Aldeen, D. A. A., and K. Cartwright. 1996. Neisseria meningitidis: vaccines and vaccine candidates. J. Infect. 33:153-157. [DOI] [PubMed] [Google Scholar]
- 3.Arvidson, C. G., and M. So. 1995. The Neisseria transcriptional regulator PilA has a GTPase activity. J. Biol. Chem. 270:26045-26048. [DOI] [PubMed] [Google Scholar]
- 4.Arvidson, C. G., and M. So. 1997. Isolation and biochemical characterization of the PilA protein of Neisseria meningitidis. Arch. Biochem. Biophys. 348:357-362. [DOI] [PubMed] [Google Scholar]
- 5.Balmer, P., R. Borrow, and E. Miller. 2002. Impact of meningococcal C conjugate vaccine in the UK. J. Med. Microbiol. 51:717-722. [DOI] [PubMed] [Google Scholar]
- 6.Benyacoub, J., S. Hopkins, A. Potts, S. Kelly, J. P. Kraehenbuhl, R. Curtiss, G. De, and H. Nardelli. 1999. The nature of the attenuation of Salmonella typhimurium strains expressing human papillomavirus type 16 virus-like particles determines the systemic and mucosal antibody responses in nasally immunized mice. Infect. Immun. 67:3674-3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borrow, R., and G. M. Carlone. 2001. Serogroup B and C serum bactericidal assays, p. 289-304. In A. J. Pollard and M. C. Maiden (ed.), Meningococcal vaccines: methods in molecular medicine. Humana Press, Totawa, N.J. [DOI] [PubMed]
- 8.Detweiler, C. S., D. B. Cunanan, and S. Falkow. 2001. Host microarray analysis reveals a role for the Salmonella response regulator phoP in human macrophage cell death. Proc. Natl. Acad. Sci. USA 98:5850-5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DiPetrillo, M. D., T. Tibbetts, H. Kleanthous, K. P. Killeen, and E. L. Hohmann. 1999. Safety and immunogenicity of phoP/phoQ-deleted Salmonella typhi expressing Helicobacter pylori urease in adult volunteers. Vaccine 18:449-459. [DOI] [PubMed] [Google Scholar]
- 10.Eisenstein, T. K., J. J. Meissler, S. I. Miller, and B. A. Stocker. 1998. Immunosuppression and nitric oxide production induced by parenteral live Salmonella vaccines do not correlate with protective capacity: a phoP::Tn10 mutant does not suppress but does protect. Vaccine 16:24-32. [DOI] [PubMed] [Google Scholar]
- 11.Fredriksen, J. H., E. Rosenqvist, E. Wedege, K. Bryn, G. Bjune, L. O. Froholm, A. K. Lindbak, B. Mogster, E. Namork, and U. Rye. 1991. Production, characterization and control of MenB-vaccine “Folkehelsa”: an outer membrane vesicle vaccine against group B meningococcal disease. NIPH Ann. 14:67-79. [PubMed] [Google Scholar]
- 12.Funnell, S., K. Reddin, and A. Robinson. 1999. Refinement in vaccine research, p. 118-121. In C. Hendriksen and D. Morton (ed.), Humane endpoints in animal experiments for biomedical research. Royal Society of Medicine, London, United Kingdom.
- 13.Garcia, V., F. C. Soncini, and E. A. Groisman. 1996. Mg2+ as an extracellular signal: environmental regulation of Salmonella virulence. Cell 84:165-174. [DOI] [PubMed] [Google Scholar]
- 14.Garmory, H. S., K. A. Brown, and R. W. Titball. 2002. Salmonella vaccines for use in humans: present and future perspectives. FEMS Microbiol. Rev. 26:339-353. [DOI] [PubMed] [Google Scholar]
- 15.Gold, R., I. Goldschneider, M. L. Lepow, T. F. Draper, and M. Randolph. 1978. Carriage of Neisseria meningitidis and Neisseria lactamica in infants and children. J. Infect. Dis. 137:112-121. [DOI] [PubMed] [Google Scholar]
- 16.Goldschneider, I., E. C. Gotschlich, and M. S. Artenstein. 1969. Human immunity to the meningococcus. I. The role of humoral antibodies. J. Exp. Med. 129:1307-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldschneider, I., E. C. Gotschlich, and M. S. Artenstein. 1969. Human immunity to the meningococcus. II. Development of natural immunity. J. Exp. Med. 129:1327-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gorringe, A. R., K. M. Reddin, P. Voet, and J. T. Poolman. 2001. Animal models for meningococcal disease, p. 241-254. In A. J. Pollard and M. C. J. Maiden (ed.), Meningococcal vaccines: methods and protocols. Humana Press, Totawa, N.J. [DOI] [PubMed]
- 19.Groisman, E. A. 1998. The ins and outs of virulence gene expression: Mg2+ as a regulatory signal. Bioessays 20:96-101. [DOI] [PubMed] [Google Scholar]
- 20.Groisman, E. A., E. Chiao, C. J. Lipps, and F. Heffron. 1989. Salmonella typhimurium phoP virulence gene is a transcriptional regulator. Proc. Natl. Acad. Sci. USA 86:7077-7081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Groisman, E. A., and M. H. Saier. 1990. Salmonella virulence: new clues to intramacrophage survival. Trends Biochem. Sci. 15:30-33. [DOI] [PubMed] [Google Scholar]
- 22.Guina, T., E. C. Yi, H. Wang, M. Hackett, and S. I. Miller. 2000. A PhoP-regulated outer membrane protease of Salmonella enterica serovar Typhimurium promotes resistance to alpha-helical antimicrobial peptides. J. Bacteriol. 182:4077-4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gunn, J. S., and S. I. Miller. 1996. PhoP-PhoQ activates transcription of pmrAB, encoding a two-component regulatory system involved in Salmonella typhimurium antimicrobial peptide resistance. J. Bacteriol. 178:6857-6864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hammerschmidt, S., R. Hilse, J. P. van Putten, R. Gerardy Schahn, A. Unkmeir, and M. Frosch. 1996. Modulation of cell surface sialic acid expression in Neisseria meningitidis via a transposable genetic element. EMBO J. 15:192-198. [PMC free article] [PubMed] [Google Scholar]
- 25.Hitchen, P. G., J. L. Prior, P. C. Oyston, M. Panico, B. W. Wren, R. W. Titball, H. R. Morris, and A. Dell. 2002. Structural characterization of lipo-oligosaccharide (LOS) from Yersinia pestis: regulation of LOS structure by the PhoPQ system. Mol Microbiol. 44:1637-1650. [DOI] [PubMed] [Google Scholar]
- 26.Hohmann, E. L., C. A. Oletta, K. P. Killeen, and S. I. Miller. 1996. phoP/phoQ-deleted Salmonella typhi (Ty800) is a safe and immunogenic single-dose typhoid fever vaccine in volunteers. J Infect. Dis. 173:1408-1414. [DOI] [PubMed] [Google Scholar]
- 27.Hohmann, E. L., C. A. Oletta, and S. I. Miller. 1996. Evaluation of a phoP/phoQ-deleted, aroA-deleted live oral Salmonella typhi vaccine strain in human volunteers. Vaccine 14:19-24. [DOI] [PubMed] [Google Scholar]
- 28.Johnson, C. R., J. Newcombe, H. A. Borde, A. R. Gorringe, S. G. P. Funnell, and J. McFadden. 2001. Generation and characterisation of a phop homologue mutant of Neisseria meningitidis. Mol. Microbiol. 39:1345-1355. [DOI] [PubMed] [Google Scholar]
- 29.Kim, J. M., R. E. Mandrell, and J. M. Griffiss. 1989. Neisseria lactamica and Neisseria meningitidis share lipooligosaccharide epitopes but lack common capsular and class 1, 2 and 3 protein epitopes. Infect. Immun. 57:602-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kox, L. F., M. M. Wosten, and E. A. Groisman. 2000. A small protein that mediates the activation of a two-component system by another two-component system. EMBO J. 19:1861-1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lehmann, A. K., A. Halstensen, I. S. Aaberge, J. Holst, T. E. Michaelsen, S. Sornes, L. M. Wetzler, and H. Guttormsen. 1999. Human opsonins induced during meningococcal disease recognize outer membrane proteins PorA and PorB. Infect. Immun. 67:2552-2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lehmann, A. K., S. Sornes, and A. Halstensen. 2000. Phagocytosis: measurement by flow cytometry. J. Immunol. Methods 243:229-242. [DOI] [PubMed] [Google Scholar]
- 33.Macfarlane, E. L., A. Kwasnicka, M. M. Ochs, and R. E. Hancock. 1999. PhoP-PhoQ homologues in Pseudomonas aeruginosa regulate expression of the outer-membrane protein OprH and polymyxin B resistance. Mol. Microbiol. 34:305-316. [DOI] [PubMed] [Google Scholar]
- 34.Mandrell, R. E., and W. D. Zollinger. 1989. Human immune response to meningococcal outer membrane protein epitopes after natural infection or vaccination. Infect. Immun. 57:1590-1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mekalanos, J. J. 1992. Environmental signals controlling expression of virulence determinants in bacteria. J. Bacteriol. 174:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller, S. I., A. M. Kukral, and J. J. Mekalanos. 1989. A 2-component regulatory system (Phop-Phoq) controls Salmonella typhimurium virulence. Proc. Natl. Acad. Sci. USA 86:5054-5058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller, S. I., W. P. Loomis, C. Alpuche Aranda, I. Behlau, and E. Hohmann. 1993. The PhoP virulence regulon and live oral Salmonella vaccines. Vaccine 11:122-125. [DOI] [PubMed] [Google Scholar]
- 38.Miller, S. I., and J. J. Mekalanos. 1990. Constitutive expression of the phoP regulon attenuates Salmonella virulence and survival within macrophages. J. Bacteriol. 172:2485-2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moe, G. R., M. Zuno, S. S. Lee, A. H. Lucas, and D. M. Granoff. 2001. Functional activity of anti-neisserial surface protein A monoclonal antibodies against strains of Neisseria meningitidis serogroup B. Infect. Immun. 69:3762-3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morley, S. L., and A. J. Pollard. 2001. Vaccine prevention of meningococcal disease, coming soon? Vaccine 20:666-687. [DOI] [PubMed] [Google Scholar]
- 41.Morton, D. B. 1999. Refinement of in vivo tests. Dev. Biol. Stand. 101:187-193. [PubMed] [Google Scholar]
- 42.Moss, J. E., P. E. Fisher, B. Vick, E. A. Groisman, and A. Zychlinsky. 2000. The regulatory protein PhoP controls susceptibility to the host inflammatory response in Shigella flexneri. Cell Microbiol. 2:443-452. [DOI] [PubMed] [Google Scholar]
- 43.Naess, L. M., T. Aarvak, A. Aase, F. Oftung, E. A. Hoiby, R. Sandin, and T. E. Michaelsen. 1999. Human IgG subclass responses in relation to serum bactericidal and opsonic activities after immunization with three doses of the Norwegian serogroup B meningococcal outer membrane vesicle vaccine. Vaccine 17:754-764. [DOI] [PubMed] [Google Scholar]
- 44.Pettersson, A., A. Maas, D. van Wassenaar, P. van der Ley, and J. Tommassen. 1995. Molecular characterization of FrpB, the 70-kilodalton iron-regulated outer membrane protein of Neisseria meningitidis. Infect. Immun. 63:4181-4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reddin, K. M., L. Crowley, S. O. Clark, P. J. Vincent, A. R. Gorringe, M. J. Hudson, and A. Robinson. 2001. Bordetella pertussis fimbriae are effective carrier proteins in Neisseria meningitidis serogroup C conjugate vaccines. FEMS Immunol. Med. Microbiol. 31:153-162. [DOI] [PubMed] [Google Scholar]
- 46.Rioux, S., D. Martin, H. W. Ackermann, J. Dumont, J. Hamel, and B. R. Brodeur. 2001. Localization of surface immunogenic protein on group B streptococcus. Infect. Immun. 69:5162-5165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 48.Schryvers, A. B., and I. Stojiljkovic. 1999. Iron acquisition systems in the pathogenic Neisseria. Mol. Microbiol. 32:1117-1123. [DOI] [PubMed] [Google Scholar]
- 49.Stojiljkovic, I., V. Hwa, L. de Saint Martin, P. O'Gaora, X. Nassif, F. Heffron, and M. So. 1995. The Neisseria meningitidis haemoglobin receptor: its role in iron utilization and virulence. Mol. Microbiol. 15:531-541. [DOI] [PubMed] [Google Scholar]
- 50.Teng, F., L. Wang, K. V. Singh, B. E. Murray, and G. M. Weinstock. 2002. Involvement of PhoP-PhoS homologs in Enterococcus faecalis virulence. Infect. Immun. 70:1991-1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilks, K. E., K. L. Dunn, J. L. Farrant, K. M. Reddin, A. R. Gorringe, P. R. Langford, and J. S. Kroll. 1998. Periplasmic superoxide dismutase in meningococcal pathogenicity. Infect. Immun. 66:213-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zollinger, W. D., and R. E. Mandrell. 1983. Studies of the human antibody response to specific meningococcal outer membrane proteins of serotypes 2 and 15. Med. Trop. 43:143-147. [Google Scholar]