Abstract
Cryptococcus neoformans is an opportunistic pathogen invading the immunocompromised host. Infection starts with the inhalation of acapsular or sparsely encapsulated cells, after which capsule synthesis is initiated. The capsule is the main virulence factor of this yeast-like fungus. Pulmonary surfactant protein D (SP-D) is an important component of the local innate defense system. In the present study, interactions of SP-D with intact C. neoformans cells and their isolated capsular components were investigated. Although encapsulated cryptococci were bound, SP-D showed the highest affinity for acapsular C. neoformans. Only acapsular cryptococci were aggregated by SP-D. Furthermore, the cryptococcal capsular components glucuronoxylomannan (GXM) and mannoprotein 1 (MP1) were bound with relatively high affinity, in contrast to GalXM and MP2. Binding as well as aggregation of acapsular C. neoformans by SP-D could be inhibited by GXM in concentrations that are likely to be present in the lung after infection, suggesting that not only the capsule hampers SP-D function within the innate defense system of the lung but also the secreted capsular component GXM.
Cryptococcus neoformans is a basidiomycete yeast-like fungus with a predilection for the respiratory and nervous systems. In healthy individuals, infection is mild, self-limiting, and restricted to the lung. However, in immunocompromised individuals, particularly those with AIDS, this opportunistic fungus can cause life-threatening infections (14, 33). Although meningitis is the predominant clinical presentation, the primary site of infection is the lung. As only particles smaller than 2 μm in diameter can reach the alveoli, it is believed that infection starts with inhalation of basidiospores or desiccated forms of C. neoformans, which are small and weakly encapsulated (20, 33). Without treatment, cryptococcosis easily disseminates throughout the body in the immunocompromised host, causing the most severe problems in the central nervous system.
An important contributor to the virulence of C. neoformans is the ability to produce a large capsule, which functions as an antiphagocytic agent (19, 20, 41). Furthermore, it has been shown that cell wall components of C. neoformans, which can be released in great amounts into the body fluids, modulate immune responses both in vivo and in vitro (20, 41). Eighty-eight percent of the cryptococcal capsule is made up of the high-molecular-weight polysaccharide glucuronoxylomannan (GXM), which consists of a linear mannan backbone containing single d-xylose, d-glucuronic acid, and O-acetyl moiety substitutions. Another, minor, polysaccharide capsule constituent is GalXM, consisting of a galactan backbone with oligosaccharide side chains composed of galactose, mannose, and xylose residues (37). Other components of the cryptococcal capsule include the mannoproteins (MPs) (17), which are made up of a large carbohydrate part linked to a relatively small peptide part (7 to 17% of the total mass) (42).
Current antifungal therapies have limited success. Although the success rate of the initial therapy is 75%, rates of relapse within 1 year are as high as 60%, and many patients require lifelong suppressive therapy (34). These facts clearly stress the need for a better understanding of the host's immune response towards C. neoformans and its cell wall components.
As infection with C. neoformans starts in the lung, the pulmonary innate immune system is the first defense mechanism of the host that is encountered by these pathogens. Surfactant protein D (SP-D), present in the alveolar lining fluid, is part of this local innate immune system (3, 9, 22, 25, 27). SP-D belongs to the family of collectins, together with SP-A, which is also present in the lung, and the serum proteins mannose binding lectin and bovine conglutinin. Collectins are multimeric proteins consisting of a short cysteine-rich, N-terminal cross-linking domain followed by a collagen domain, an α-helical neck domain, and finally a C-type lectin domain, or carbohydrate recognition domain (CRD) (8, 26). They interact with a wide variety of microorganisms and can modulate the function of immune effector cells.
It has been shown that many pathogenic microorganisms, such as viruses, bacteria, and fungi, can be bound by SP-D in vitro through interactions of the CRD of SP-D with glycoconjugates present on the outsides of the pathogens (10, 22). Binding of microorganisms by SP-D can result in their aggregation (22, 25, 27), which is thought to stimulate their removal by enhancing their mucociliary clearance (9). Furthermore, binding of SP-D to microorganisms can stimulate (4, 24, 29) as well as inhibit (13, 39) their subsequent uptake and killing by phagocytes, depending on the specific pathogen involved.
Among the fungi which are known to be bound by SP-D are Aspergillus fumigatus, Saccharomyces cerevisiae, and Pneumocystis carinii (2, 30). Furthermore, two papers have reported that SP-D binds to the acapsular, but not to the encapsulated, form of C. neoformans (35, 43). Interestingly, it was also found that SP-D binding resulted in the aggregation of acapsular but not encapsulated C. neoformans (35).
Serological reactivity of the capsular polysaccharide of C. neoformans is used to define five serotypes (A, B, C, D, and AD) (16), showing that capsule structures differ among various isolates. In addition, it was shown that strains within a particular serotype produce structurally different capsular GXM molecules (7, 36). Therefore, we hypothesized that besides the presence or absence of a capsule, capsule structure may also influence the ability of SP-D to bind to C. neoformans. The first aim of the present study was to investigate the binding of SP-D to four different C. neoformans strains: two of the tested strains were of the A serotype, one with a capsule of normal size (NIH 37) and the other producing a thicker capsule (NCPF 3168). The other two cryptococcal strains tested were NIH B3501, belonging to serotype D, and its acapsular, non-GXM-producing mutant NIH B4131, also known as Cap67. Our second aim was to identify possible carbohydrate ligands for SP-D present on the cell wall of C. neoformans. Therefore, purified GXM, GalXM, MP1, and MP2 preparations were tested in a solid-phase binding assay. The third aim was to test whether GXM, in concentrations that may be encountered in the epithelial lining fluid (ELF), can interfere with the ability of SP-D to aggregate acapsular C. neoformans.
MATERIALS AND METHODS
Reagents.
(Bis)acrylamide for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was purchased from Bio-Rad (Hercules, Calif.). Unless otherwise indicated, all other chemicals came from Sigma (St. Louis, Mo.) or Merck (Darmstadt, Germany). Protran blot membranes for Western blotting were obtained from Schleicher and Schuell (Dassel, Germany).
Purification and fluorescein isothiocyanate (FITC) labeling of human SP-D.
Human SP-D was isolated from bronchoalveolar lavage (BAL) fluid of patients suffering from alveolar proteinosis, as previously described (11). Briefly, BAL fluid was centrifuged at 28,000 × g to collect the SP-D-containing surfactant pellet. SP-D was eluted from the surfactant pellet by stirring the pellet in 50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 0.05% Tween 20, and 100 mM maltose. After centrifugation at 28,000 × g, the SP-D in the supernatant was further purified by gel filtration chromatography on a Bio-prep1000/17 column (Bio-Rad) in 50 mM Tris-HCl (pH 7.4) containing 150 mM NaCl and 5 mM EDTA (32). Purity of the SP-D preparation was tested by SDS-PAGE and subsequent Coomassie blue staining and Western blotting. Samples were stored at 4°C with sodium azide added to a final concentration of 0.02% or at −20°C.
For FITC labeling, SP-D (1 mg/ml) was dialyzed against 0.1 M sodium carbonate buffer (pH 9.0), after which 25 μg of FITC/ml was added and the mixture was incubated overnight at 4°C in the dark. Coupling of FITC was stopped by addition of excess NH4Cl, and unincorporated label was removed by gel filtration chromatography by using Bio-Gel P-10 (Bio-Rad) in a spin column.
Preparation of antibodies.
The SP-D antibody was raised in rabbit against purified human SP-D, as described previously (38). The immunoglobulin G (IgG) fraction was purified by affinity chromatography on protein A-Sepharose CL 4B. Western blot analysis using total BAL fluid of alveolar proteinosis patients showed only one band at the predicted position of human SP-D (43 kDa), demonstrating its specificity. Purified antibodies were stored at −20°C in 50% glycerol.
C. neoformans strains.
SP-D binding to four different strains of C. neoformans was studied. The thickness of the polysaccharide capsule was determined for each strain by use of Indian ink staining and subsequent light microscopic examination. The C. neoformans strains tested were NIH B3501 (serotype D; capsule thickness, ≤0.5 μm) and its acapsular, GXM-lacking mutant NIH B4131 (Cap67), NIH 37 (serotype A; capsule thickness, ≤0.5 μm), and NCPF 3168 (serotype A; capsule thickness, ∼2 μm). Strain NIH 37 was used for GXM isolation procedures (see below).
Determination of SP-D binding to C. neoformans.
For the SP-D binding experiments, a small amount of C. neoformans culture grown on Sabouraud agar was harvested and suspended in TBS-B (150 mM NaCl, 20 mM Tris-HCl [pH 7.4], and 0.2% bovine serum albumin [BSA]). Yeast cells were washed in two cycles of centrifugation (1,500 × g for 7 min at 22°C) and resuspended in TBS-B. For each binding experiment, 106 cells were used. In between incubation steps, cell pellets were washed three times by using 400 μl of TBS-B containing 5 mM CaCl2. Yeast cells were incubated with or without 10 μg of SP-D/ml in a total reaction volume of 300 μl for 1 h at 22°C with constant rotation. Afterwards, bound SP-D was detected using anti SP-D IgG (370 ng/ml) and subsequent incubation with Alexa-488-conjugated goat anti-rabbit IgG (diluted 1:200) for 1 h at 22°C. To assess divalent cation requirement, parallel experiments were performed in the presence of 5 mM EDTA. Finally, SP-D binding to yeast cells was analyzed by flow cytometry on a FACScan (Becton Dickinson, San Jose, Calif.).
To assess the influence of GXM (for isolation procedures, see below), mannan, and maltose on the binding of SP-D to acapsular C. neoformans, 106 yeast cells were incubated with FITC-conjugated SP-D (10 μg/ml) in TBS-B containing 5 mM CaCl2, to which increasing concentrations of GXM, mannan, or maltose were added. Mean fluorescence per cell was determined on a FACScan flow cytometer, and inhibition of binding was expressed as percentage of maximal binding as determined in the absence of a competitor. Inhibition of SP-D-induced aggregation by carbohydrate inhibitors was evaluated by analysis of 5,000 events in scatter plots. To quantify aggregation, the number of events in the upper right and lower right quadrants of the scatter plots was determined and inhibition was expressed as percentage of maximal aggregation as determined in the presence of SP-D but in the absence of a saccharide inhibitor.
Purification of GXM.
GXM of strain NIH 37 was isolated and purified as described previously (6). In short, 50 ml of autoclaved C. neoformans culture was centrifuged to remove cells. Polysaccharides were precipitated by addition of 3 volumes of 95% ethanol at 4°C and subsequent centrifugation. The polysaccharide pellet was washed once with 95% ethanol. GXM, which has a negative charge, was precipitated using cetyltrimethylammonium bromide (CTAB). The CTAB-GXM complex was washed once with 10% ethanol, and after centrifugation, the GXM-containing pellet was dissolved in 1 M NaCl. After ultrasonic irradiation, the GXM preparation was dialyzed to remove the CTAB. Finally, the GXM preparation was lyophilized and stored at −20°C until use.
Purification of GalXM, MP1, and MP2.
To facilitate the purification of GalXM and MPs, the culture supernatant of the acapsular mutant of C. neoformans was used. GalXM, MP1, and MP2 were isolated as described previously (5). Briefly, the culture supernatant was concentrated by ultrafiltration, and after dialysis against water, the retentate was recovered by lyophilization. This preparation was passed through a ConA-agarose column, and the effluent was preserved for GalXM purification via anion chromatography on a DEAE-cellulose column (Whatman Chemical Separations Ltd., Kent, United Kingdom). MPs were eluted from the ConA affinity column by using methyl-α-d-mannopyranoside. MP1 and MP2 were separated by anion chromatography on a DEAE-cellulose column, dialyzed against water, lyophilized, and stored at −20°C until use. GalXM, MP1, and MP2 fractions were found to be negative for ConA contamination as tested by SDS-PAGE, amino acid analysis, and molecular mass analysis.
Endotoxin contamination.
All cell wall components were tested with the QCL-1000 Limulus amebocyte lysate assay (Bio Whittaker, Walkersville, Md.) for lipopolysaccharide contamination. The lipopolysaccharide contamination levels for the GXM, GalXM, MP1, and MP2 fractions were 0.47, 2.4, 5.4, and 52 ng/mg of cell wall component, respectively.
Binding of SP-D to purified capsule components of C. neoformans.
Binding of SP-D to GXM, GalXM, MP1, and MP2 was tested using a solid-phase binding assay. Costar polystyrene 96-well plates (Corning, Acton, Mass.) were coated with the indicated cell wall components in 0.1 M sodium carbonate buffer (pH 9.6). The desired amount of cell wall component (0 to 2,000 ng/well) contained in 50 μl of 0.1 M carbonate buffer was placed in each microtiter well and allowed to adhere to the plastic overnight at 4°C. Unoccupied binding places were blocked with 200 μl of blocking buffer (5 mM Tris-HCl [pH 7.4], 150 mM NaCl, 2% BSA, 0.05% Tween 20) at 37°C for 1 h. Afterwards, wells were extensively flushed with washing buffer (5 mM Tris-HCl [pH 7.4], 150 mM NaCl, 0.05% Tween 20). All subsequent incubations were performed at 37°C for 1 h, and after every incubation, wells were extensively rinsed with washing buffer containing 5 mM CaCl2. First, wells were incubated with various concentrations of SP-D (0 to 2,000 ng/ml) in 50 μl of incubation buffer (5 mM Tris-HCl [pH 7.4], 150 mM NaCl, 0.1% BSA, 0.05% Tween 20) containing 5 mM CaCl2. Binding was detected using anti-human SP-D IgG in a concentration of 740 ng/ml (diluted in incubation buffer containing 5 mM CaCl2). The bound rabbit antibodies were detected using 50 μl of horseradish peroxidase-conjugated goat anti-rabbit antibody (GαRIgG-HRP; Nordic, Tilburg, The Netherlands) in a concentration of 2.5 μg/ml (in incubation buffer containing 5 mM CaCl2). After incubation with GαRIgG-HRP, wells were washed twice with washing buffer and once with H2O, both containing 5 mM CaCl2. The peroxidase reaction was performed using 150 μl of substrate buffer (100 μg of tetramethylbenzidine/ml and 1 mM H2O2 in 0.1 M citric acid buffer [pH 4.0]). The color development was stopped by adding 50 μl of 4 N H2SO4. Absorbance was measured at 450 nm with a Benchmark microtiter plate reader (Bio-Rad).
To examine the divalent cation requirement of SP-D binding, wells were coated with 500 ng of the indicated cryptococcal capsular component and incubated with SP-D (1 μg/ml) in incubation buffer in the presence of either 5 mM CaCl2, 5 mM EDTA, or both 5 mM CaCl2 and 10 mM EDTA or in the absence of divalent cations. Furthermore, binding was also studied in the presence of 5 mM MnCl2 or 5 mM MgCl2. Bound SP-D was detected as described above. Other experiments were used to study whether specific sugars could competitively inhibit SP-D binding to the indicated cell wall components. For that purpose, microtiter plates coated with 500 ng of the indicated cell wall component per well were incubated with SP-D (1 μg/ml) in incubation buffer containing 5 mM CaCl2 supplemented with 20 mM maltose or mannose. Binding of SP-D was detected as described above.
Cryptococcal aggregation assay.
The same four strains that were tested for SP-D binding were used to study whether they could be aggregated by SP-D. Aggregation was determined as described previously (35). Briefly, C. neoformans was grown on Sabouraud dextrose agar at 22°C. A small amount was harvested and washed two times in phosphate-buffered saline (PBS; Invitrogen, Breda, The Netherlands) containing 0.2% BSA (PBS-B). Afterwards, cells were pelleted at 1,500 × g and resuspended in PBS-B plus 1.5 mM CaCl2 at a concentration of 107 cells per ml. One hundred microliters of the yeast suspension was incubated with SP-D (10 μg/ml) in a Costar flat-bottom tissue culture plate (Corning) at 37°C for 1 h and observed by light microscopy (Leica DM IRBE; Leica GmbH, Heidelberg, Germany).
In order to quantify SP-D-induced aggregation of nonencapsulated C. neoformans (strain NIH B4131) in the presence of different concentrations of soluble GXM, aggregation was also determined using fluorescence-activated cell sorter analysis as described in “Determination of SP-D binding to C. neoformans.”
RESULTS
SP-D binds encapsulated as well as acapsular C. neoformans.
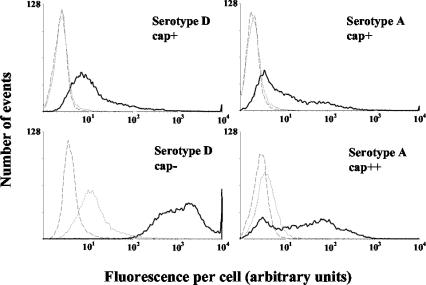
The initial experiments were used to study whether SP-D binds to four different C. neoformans strains (Fig. 1). Based on previous studies (35, 43), the finding that SP-D had the highest affinity for the acapsular mutant of C. neoformans (strain B4131) was as expected. On the other hand, SP-D did also bind to the encapsulated strains but with much lower affinity. In the absence of SP-D, but in the presence of anti-SP-D antibodies and subsequent Alexa-488-conjugated goat anti-rabbit IgG, no signal higher than background was detected, indicating that the shift in fluorescence was specific for SP-D. Furthermore, incubation of C. neoformans with SP-D in the presence of 5 mM EDTA highly reduced SP-D binding to the acapsular form of C. neoformans and totally inhibited SP-D attachment to all encapsulated strains tested, which shows that SP-D binding depended on the presence of divalent cations. Interestingly, judged on the SP-D-induced shift of mean fluorescence per cell of the encapsulated C. neoformans strains tested, SP-D had the highest affinity for strain NCPF 3168, which has the thickest capsule (Fig. 1).
FIG. 1.
SP-D binds to different strains of C. neoformans. C. neoformans cells (106) were incubated in buffer containing 5 mM CaCl2 in the presence (bold lines) or absence (dashed lines) of 10 μg of SP-D/ml or in buffer containing 5 mM EDTA and 10 μg of SP-D/ml (dotted lines). SP-D binding was detected using rabbit anti-SP-D IgG and Alexa-488-conjugated goat anti-rabbit IgG, after which cells were analyzed by flow cytometry. Results of FACScan analysis of 10,000 cells are shown. Data are from an experiment representative of three independent experiments. Serotype D, cap+, strain NIH B3501; serotype D, cap−, strain NIH B4131; serotype A, cap+, strain NIH 37; serotype A, cap++, strain NCPF 3168.
For all strains tested, the shift in mean fluorescence per cell, measured using the above-described detection system (Fig. 1), was comparable to the shift seen when FITC-labeled SP-D was used, indicating that SP-D clearly bound to the various strains of C. neoformans irrespectively of the method of detection (results not shown).
SP-D aggregates only acapsular C. neoformans.
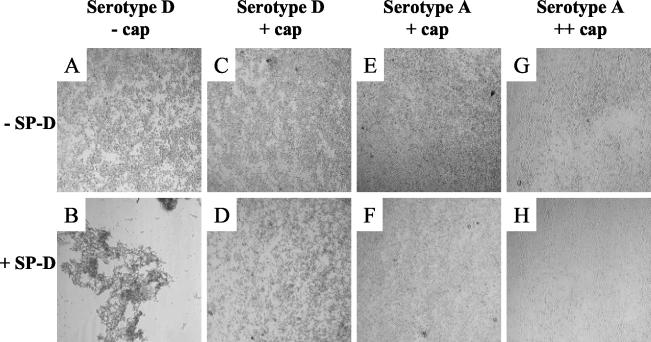
As reviewed earlier (9), an important function of SP-D is thought to be aggregation of microorganisms. Therefore, we investigated whether binding correlated with aggregation of the different strains tested. Confirming the results of an earlier study by Schelenz et al. (35), we found that only acapsular C. neoformans cells were aggregated by SP-D. Although binding of SP-D to encapsulated C. neoformans could be detected (Fig. 1)—in contrast to the findings of Schelenz et al.—this binding did not result in aggregation (Fig. 2). In Fig. 2, aggregation induced by an SP-D concentration of 10 μg/ml is shown. However, for the encapsulated C. neoformans strain NCPF 3168, even SP-D concentrations as high as 160 μg/ml were found not to induce aggregation (data not shown).
FIG. 2.
SP-D aggregates only acapsular C. neoformans. Cells (106) in 100 μl were incubated in PBS containing 1.5 mM calcium for 60 min at 37°C in the presence (+ SP-D) or absence (− SP-D) of 10 μg of SP-D/ml as indicated and analyzed by light microscopy (magnification, ×10). Results from an experiment representative of three independent experiments are shown.
SP-D binds GXM and MP1 but not GalXM and MP2.
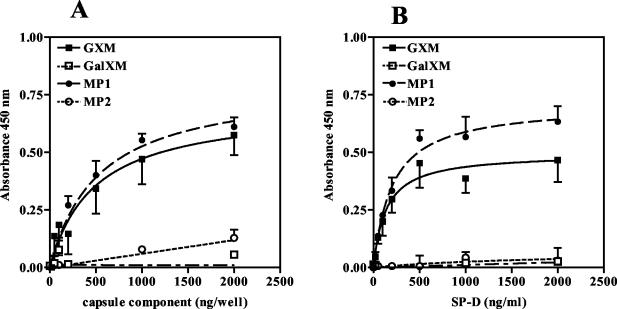
In order to identify possible ligands of SP-D present on the outside of acapsular as well as encapsulated C. neoformans, solid-phase binding assays were performed using isolated cell wall components. The cell wall consists largely of carbohydrates, which are therefore likely candidates to interact with SP-D. The difference between acapsular C. neoformans and encapsulated C. neoformans is the latter's ability to produce GXM. Cell wall components that are present on both acapsular and encapsulated forms include GalXM and the MPs. As shown in Fig. 3, of the four components tested, SP-D bound to only GXM and MP1 with high affinity while hardly any binding to GalXM or MP2 was detected. As expected, SP-D association was found to be dependent on the amount of GXM or MP1 used to coat each well (Fig. 3A). For GXM and MP1, SP-D binding was shown to be saturable, and saturation was reached between 500 and 1,000 ng of SP-D (Fig. 3B)/ml. Furthermore, when equal amounts of GXM and MP1 were used per well, more SP-D tended to bind to MP1-coated wells (Fig. 3 and 4).
FIG. 3.
SP-D binds to capsule components of C. neoformans. (A) Microtiter wells were coated with increasing amounts of the indicated capsular components and incubated with SP-D (1 μg/ml). Nonspecific binding, measured in wells coated with coating buffer only, was subtracted from total binding for each data point. (B) Binding of increasing concentrations of SP-D (0 to 2,000 ng/ml) to wells coated with 500 ng of the indicated capsular component. Binding was corrected for background by subtracting nonspecific binding, as measured for each SP-D concentration in wells coated with coating buffer only, from total binding. SP-D binding was detected as described in Materials and Methods. Results shown are for an experiment representative of three independent experiments each performed in triplicate. Values are means ± standard deviations (SD) (n = 3).
FIG. 4.
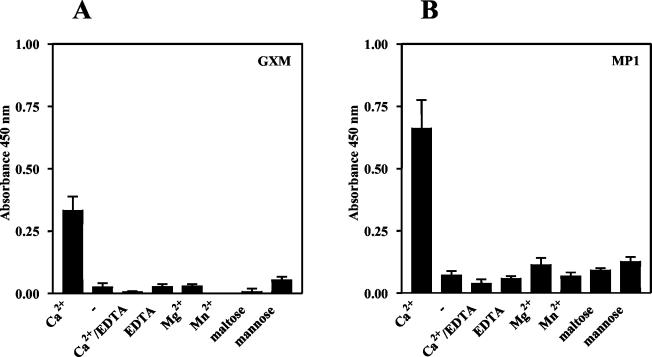
SP-D binding to GXM (A) and MP1 (B) is calcium dependent and can be inhibited by saccharides. Microtiter plate wells were coated with 500 ng of the indicated capsular component per well and incubated with SP-D (1 μg/ml) in the presence of 5 mM CaCl2 (Ca2+), 5 mM MgCl2 (Mg2+), 5 mM MnCl2 (Mn2+), 5 mM EDTA, 5 mM CaCl2 and 10 mM EDTA (Ca2+/EDTA), 5 mM CaCl2 and 20 mM mannose (mannose), or 5 mM CaCl2 and 20 mM maltose (maltose) or in the absence of divalent cations (−). Presented data were corrected for nonspecific binding. Results shown are for an experiment representative of three independent experiments each performed in triplicate. Values are means ± SD (n = 3).
Since SP-D normally binds to glycoconjugates via its C-type lectin domain (CRD), we studied whether the presence of calcium was required for binding to the cell wall components of C. neoformans. As shown in Fig. 4, in the absence of calcium, no SP-D binding to any of the tested glycoconjugates was detected. Also, the presence of EDTA besides calcium resulted in total loss of SP-D binding. Furthermore, other divalent cations like manganese and magnesium could not substitute for calcium. These results indicate that the interaction of SP-D with C. neoformans depends totally on the presence of calcium. We also investigated the involvement of the CRD by testing whether carbohydrates could competitively inhibit SP-D binding. For that purpose, 20 mM maltose or 20 mM mannose was added to the incubation buffer. Both saccharides strongly inhibited SP-D binding (Fig. 4). Taken collectively, these observations show that SP-D binds to GXM and MP1 via its CRD.
GXM inhibits SP-D binding and aggregation of acapsular C. neoformans.
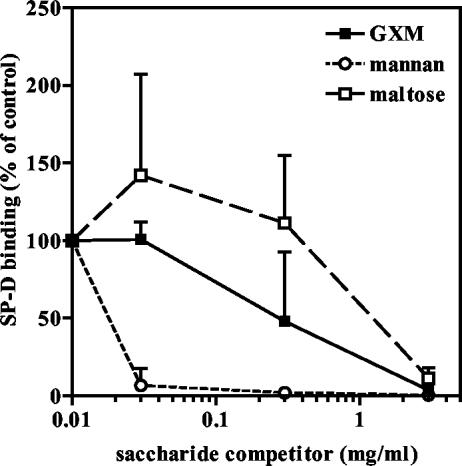
It is known that C. neoformans sheds large amounts of capsular material into its environment (41). Therefore, we investigated whether the main cryptococcal capsular component, GXM, could competitively inhibit SP-D binding and subsequent aggregation of acapsular C. neoformans. In these experiments, mannan and maltose were included as positive controls, as it is known that SP-D has high affinity for the polymannose glycan mannan and maltose is one of the most widely used inhibitors for SP-D binding. All three saccharides were found to inhibit SP-D binding to acapsular C. neoformans (Fig. 5). The inhibitory potency of mannan was greater than that of GXM, which was greater than that of maltose. GXM inhibited SP-D binding to acapsular yeast cells totally at a concentration of 3 mg/ml but a reduction of more than 60% was already seen at a concentration of 0.3 mg/ml. Maltose was inhibitory only at the highest of the concentrations tested, which was 3 mg/ml (8 mM).
FIG. 5.
SP-D binding to acapsular C. neoformans can be inhibited by GXM, mannan, and maltose. Results of flow cytometric analysis of binding of FITC-labeled SP-D (10 μg/ml) to acapsular C. neoformans (strain B4131) in the presence of increasing concentrations of the indicated saccharides are shown. Data are expressed as percentages of maximal SP-D binding as measured in the absence of a (poly)saccharide inhibitor. Data represent the means ± SD of results from three independent experiments (n = 3).
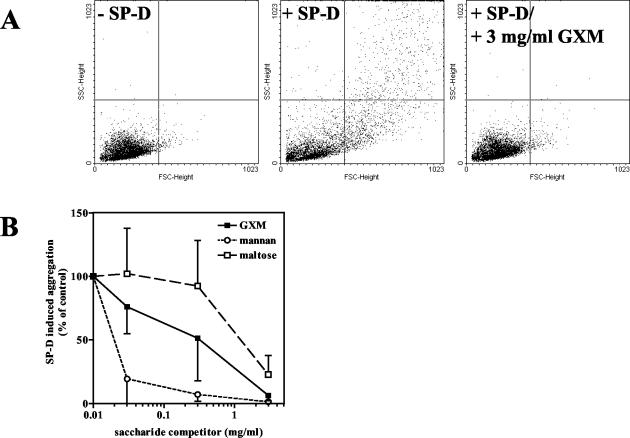
As it is thought that an important function of SP-D is to aggregate microorganisms (9), we analyzed whether addition of the above-mentioned carbohydrates also inhibited the SP-D-induced aggregation of acapsular C. neoformans. In Fig. 6A, scatter plots are shown for acapsular yeast cells incubated with or without SP-D (10 μg/ml). An increase in the number of larger particles was detected when SP-D (10 μg/ml) was added, representative of SP-D-induced aggregation. Moreover, this aggregation was inhibited when, together with SP-D, GXM was added at a concentration of 3 mg/ml (Fig. 6A). Aggregation was quantified by counting the number of events in the upper right and lower right quadrants of the scatter plots shown in Fig. 6A (Fig. 6B). The results of the carbohydrate-dependent inhibition of SP-D-induced aggregation of acapsular C. neoformans (Fig. 6A and B) were correlated with those of the carbohydrate-dependent inhibition of SP-D binding (Fig. 5); mannan was found to be the most powerful inhibitor of aggregation but GXM also inhibited SP-D-induced aggregation while maltose was a less powerful antiagglutinin (Fig. 6A and B).
FIG. 6.
SP-D-induced aggregation of acapsular C. neoformans can be inhibited by GXM, mannan, and maltose. Shown are the results of flow cytometric analysis of SP-D-induced aggregation of acapsular C. neoformans (strain B4131) incubated with SP-D (10 μg/ml) in the presence of increasing concentrations of the indicated saccharides. Scatter plots are shown in panel A, whereas for panel B aggregation was quantified by counting the number of events detected in the upper right and lower right quadrants of the scatter plots. Data are presented as percentages of maximal aggregation as measured in the absence of a saccharide inhibitor. Data represent the means ± SD of results from three independent experiments. SSC, side scatter; FSC, forward scatter.
DISCUSSION
SP-D plays a crucial role in the innate defense system of the lung. It is thought that an important function of this protein is to aggregate microorganisms, thereby facilitating their mucociliary clearance (9). SP-D can also enhance uptake and killing by phagocytic cells (4, 24, 29). However, depending on the pathogen involved, in some cases SP-D binding to microorganisms impairs their subsequent uptake by phagocytes (13, 39). C. neoformans is an opportunistic pathogenic fungus that enters the body via the lungs, causing life-threatening infections in the immunocompromised host (20). Here we report that SP-D interacts with intact crypotococcal fungal cells and their purified capsular components GXM and MP1. GXM, which is shed into the environment in great amounts, can inhibit SP-D-induced aggregation of acapsular C. neoformans.
In addition to the previously reported binding to acapsular C. neoformans, in the present study we found that SP-D also bound to encapsulated strains of C. neoformans, although the amount of SP-D bound per cell was much lower in the case of these encapsulated strains. These results were unexpected as it has previously been reported that SP-D does not bind to capsular C. neoformans (35, 43). The exact cause of the differences between the data obtained in the present study and those in previous reports is as yet unclear but may be related to differences in sensitivities of the antibodies used (35) or the different experimental approaches to the detection of bound protein (43). The ability to detect SP-D binding to three different strains of encapsulated cryptococci by using an antibody-based detection system as well as by using FITC-labeled SP-D strengthens our conclusion that SP-D can bind to encapsulated cryptococci. The binding of SP-D to both encapsulated and acapsular cells was found to be dependent on calcium and is most probably due to binding of SP-D via its CRD. However, only the acapsular mutant of C. neoformans was aggregated by SP-D, in line with results published by Schelenz et al. (35). In other words, despite the observation that SP-D binds to encapsulated cryptococci, it did not cause aggregation of these strains. This is most probably caused by the low number of SP-D molecules bound per fungal cell compared to that of molecules bound per acapsular C. neoformans cell. Another factor that may contribute to the inability of SP-D to aggregate encapsulated cryptococci is the negative charge of the predominant component of the cryptococcal capsule, GXM, which results in a net repulsive force between separate cryptococci (21, 28), thereby making it more difficult for SP-D to aggregate these encapsulated cryptococci. Importantly, the observation that encapsulated cryptococci are not aggregated supports the generally accepted idea that capsule production is an important virulence factor for C. neoformans (20).
C. neoformans initiates infection in the lungs in an acapsular or hardly encapsulated form. Although not much is known about the composition of the cell wall in these infectious stages of C. neoformans, apart from the fact that it lacks GXM, the finding that SP-D binds to and aggregates the nonencapsulated mutant strain NIH B4131 suggests that SP-D may represent an effective first line of defense against nonencapsulated stages of C. neoformans by facilitating its mucociliary clearance or by restricting the spread of C. neoformans to localized areas within the lung. Furthermore, SP-D may have a direct inhibitory effect on C. neoformans growth, similar to its effect on Candida albicans growth (39). Although many studies have shown that SP-D binding to specific microorganisms stimulates their subsequent uptake by phagocytic cells (9), binding of SP-D to C. albicans decreased its subsequent uptake by alveolar macrophages. This was attributed to the large size of the aggregated C. albicans complexes (39). For the same reason, we expect that phagocytosis will not substantially contribute to the removal of SP-D-aggregated C. neoformans complexes from the lung. On the other hand, SP-D binding to encapsulated C. neoformans cells did not result in aggregation. It may be that in the absence of aggregation SP-D contributes to the pulmonary defense against C. neoformans by enhancing the uptake of the pathogen by phagocytic cells, as SP-D has been shown to promote the phagocytosis of many microorganisms via opsonization (15, 24, 29). The validity of this hypothesis will be tested in future experiments.
To determine potential ligands for SP-D on the surface of C. neoformans, solid-phase binding assays were performed with some of its purified capsule components, GXM, GalXM, MP1, and MP2. It was found that SP-D bound with highest affinity to GXM and to MP1 (Fig. 3 and 4). The finding that SP-D bound with high affinity to GXM was surprising, because GXM is a major constituent (88% of capsular mass) only of encapsulated cryptococci and is not present in acapsular strains. So despite the fact that SP-D did bind with much lower affinity to encapsulated cryptococci than to acapsular cryptococci, the main capsule component itself was recognized quite well. These results suggest that the assembly of GXM in the capsule may be important in lowering the affinity for SP-D and thereby preventing aggregation. It may be that GXM is packed in such a way that sugar residues for which SP-D has affinity are oriented towards the inside of the capsule. GalXM and MPs are present on the outside of acapsular C. neoformans and are considered to be the remnants of the cryptococcal capsule in this mutant strain (B4131). Therefore, these carbohydrate structures were thought to represent likely ligands for SP-D. Our finding that SP-D bound to MP1 via its CRD and hardly to GalXM and MP2 suggests that MP1 may indeed represent an important ligand on acapsular yeast cells. As the cryptococcal cell wall underneath the capsule also contains β(1-6) glucans (18), which have been shown to be bound by SP-D with high affinity (1), these structures may represent other important ligands for SP-D on the cell wall of not yet fully encapsulated yeast cells. Especially because a thick GXM-containing capsule is not present in the acapsular mutant (strain B4131), these underlying glucan structures may be more surface exposed and thereby accessible for the attachment of SP-D.
Binding of SP-D to encapsulated C. neoformans cells can be explained by assuming that it interacts to some extent with GXM. Furthermore, because encapsulated cryptococci also produce MPs, one could expect that these MPs can also function as ligands for SP-D binding on encapsulated cells. Although it was shown that in encapsulated cells MPs are mainly localized at the inner part of the capsule and are presumably not accessible to the relatively large SP-D multimers, MPs tend to diffuse from the inner to the outer parts of the cell wall, from which they are shed into the environment (40). This means that at the outer surface of encapsulated cryptococci, there is also a small amount of MPs present, which may serve as an SP-D ligand.
For all capsular components tested, binding of SP-D was found to be dependent on calcium and could be inhibited using saccharide inhibitors (Fig. 4). This indicates that the CRD of SP-D was involved in these interactions, as was already described for many other carbohydrate ligands on diverse microorganisms (9).
In general, it is thought that SP-D attachment to (microbial) glycoconjugates cannot be predicted solely by analyzing their monosaccharide compositions, as the manner in which the separate saccharides are assembled within these structures seems to be very important (1). The carbohydrate moieties of both MPs have very similar monosaccharide compositions, containing high percentages of residues for which SP-D has high affinity when these residues are presented as monosaccharides (42). However, only MP1 is a ligand for SP-D, which further emphasizes the great importance of the actual bonding and orientation of the monosaccharide residues.
In contrast to those of the MPs, the structures of GXM and GalXM have been elucidated. GXM is composed of a linear α(1-3)-linked mannan backbone containing single d-xylose, d-glucuronic acid, and O-acetyl residue substitutions (6), whereas GalXM has an α(1-6)-linked galactan backbone which is branched at C-3 of alternate galactose units with oligosaccharides composed of α-d-Man-(1-3)-α-d-Man-(1-4)-β-d-Gal, with zero to three terminal d-xylose residue substitutions (37). As mannose and glucuronic acid residues have been shown to be ligands for SP-D when presented as monosaccharides (31), these residues may contribute to the binding of SP-D to GXM. The fact that GalXM consists in large part of galactose, for which SP-D has low affinity (23), is in line with the observed weak affinity of SP-D for GalXM. Moreover, the presence of mannose residues, for which SP-D has relatively high affinity, as 30% of the total composition of GalXM suggests that these mannose residues are not presented in a manner that allows SP-D binding.
The capsule is thought to be one of the main virulence factors of C. neoformans, as it functions as an antiphagocytic agent. The main cryptococcal capsule component, GXM, is shed into the environment in large amounts, and high levels can be detected in the diverse body fluids. GXM has been shown to have all kinds of immunomodulatory effects (41). As aggregation of microorganisms is one of the main functions of SP-D, we examined whether GXM is capable of inhibiting the SP-D-induced aggregation of acapsular C. neoformans. GXM inhibited SP-D-induced aggregation of acapsular C. neoformans in a concentration-dependent manner and was found to be more potent than the disaccharide maltose but less potent than the glycan mannan. These findings are in agreement with the fact that SP-D binds with higher affinity to polysaccharides than to mono- or disaccharides. GXM concentrations found to inhibit SP-D-induced aggregation ranged from 3 to 0.03 mg/ml. Although GXM levels in the ELF have never been determined, there are good reasons to assume that concentrations found to inhibit SP-D-induced aggregation of acapsular C. neoformans can be encountered in the ELF. Firstly, after deposition in the lungs, C. neoformans readily starts producing a capsule, thereby also releasing soluble capsule components. Furthermore, GXM concentrations in sera of patients with disseminated cryptococcosis are in the range of several hundred micrograms per milliliter. In exceptional cases, GXM levels of up to 20 mg/ml in serum have been found (12). It seems reasonable to assume that levels similar to those found in plasma can be present in the lung, as this organ is sometimes heavily affected. In addition, it is also possible that C. neoformans, after entering the lungs, gives rise to high pulmonary concentrations of GXM in localized areas within the lungs.
The presence of high GXM levels in the ELF may result in the protection of not yet fully encapsulated and thereby unprotected C. neoformans cells from SP-D-induced aggregation and subsequent removal by mucociliary clearance. This may lead to the exacerbation of the infection with a greater risk of dissemination throughout the body. In addition to impaired aggregation of acapsular forms of C. neoformans, the aggregation of a wide variety of microorganisms by SP-D will most probably be inhibited by GXM as well.
In summary, the present paper describes SP-D binding to acapsular as well as to encapsulated C. neoformans and identifies as possible ligands for these interactions GXM and MP1. Furthermore, the finding that the secreted capsule component GXM can inhibit the aggregation of acapsular C. neoformans may be extended to other microorganisms, as it is known that, for instance, bacteria also shed cell wall material into their environments. The secretion of cell wall material may therefore represent a general mechanism by which pathogenic microorganisms protect themselves or each other from collectin-based pulmonary defense mechanisms.
Acknowledgments
We received financial support from the European Commission (contract QLK2-CT-2000-00325).
We thank our colleagues of the Department of Pulmonology (AMC, University of Amsterdam, The Netherlands) for providing lavage fluids from alveolar proteinosis patients.
Editor: T. R. Kozel
REFERENCES
- 1.Allen, M. J., A. Laederach, P. J. Reilly, and R. J. Mason. 2001. Polysaccharide recognition by surfactant protein D: novel interactions of a C-type lectin with nonterminal glucosyl residues. Biochemistry 40:7789-7798. [DOI] [PubMed] [Google Scholar]
- 2.Allen, M. J., D. R. Voelker, and R. J. Mason. 2001. Interactions of surfactant proteins A and D with Saccharomyces cerevisiae and Aspergillus fumigatus. Infect. Immun. 69:2037-2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Awasthi, S., J. J. Coalson, B. A. Yoder, E. C. Crouch, and R. J. King. 2001. Deficiencies in lung surfactant proteins A and D are associated with lung infection in very premature neonatal baboons. Am. J. Respir. Crit. Care Med. 163:389-397. [DOI] [PubMed] [Google Scholar]
- 4.Bufler, P., B. Schmidt, D. Schikor, A. Bauernfeind, E. C. Crouch, and M. Griese. 2003. Surfactant protein A and D differently regulate the immune response to nonmucoid Pseudomonas aeruginosa and its lipopolysaccharide. Am. J. Respir. Cell Mol. Biol. 28:249-256. [DOI] [PubMed] [Google Scholar]
- 5.Chaka, W., A. F. Verheul, V. V. Vaishnav, R. Cherniak, J. Scharringa, J. Verhoef, H. Snippe, and I. M. Hoepelman. 1997. Cryptococcus neoformans and cryptococcal glucuronoxylomannan, galactoxylomannan, and mannoprotein induce different levels of tumor necrosis factor alpha in human peripheral blood mononuclear cells. Infect. Immun. 65:272-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cherniak, R., L. C. Morris, B. C. Anderson, and S. A. Meyer. 1991. Facilitated isolation, purification, and analysis of glucuronoxylomannan of Cryptococcus neoformans. Infect. Immun. 59:59-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cherniak, R., L. C. Morris, and S. H. Turner. 1992. Glucuronoxylomannan of Cryptococcus neoformans serotype D: structural analysis by gas-liquid chromatography-mass spectrometry and by 13C-nuclear magnetic resonance spectroscopy. Carbohydr. Res. 223:263-269. [DOI] [PubMed] [Google Scholar]
- 8.Crouch, E., A. Persson, D. Chang, and J. Heuser. 1994. Molecular structure of pulmonary surfactant protein D (SP-D). J. Biol. Chem. 269:17311-17319. [PubMed] [Google Scholar]
- 9.Crouch, E., and J. R. Wright. 2001. Surfactant proteins A and D and pulmonary host defense. Annu. Rev. Physiol. 63:521-554. [DOI] [PubMed] [Google Scholar]
- 10.Crouch, E. C., K. Hartshorn, and I. Ofek. 2000. Collectins and pulmonary innate immunity. Immunol. Rev. 173:52-65. [DOI] [PubMed] [Google Scholar]
- 11.Crouch, E. C., A. Persson, and D. Chang. 1993. Accumulation of surfactant protein D in human pulmonary alveolar proteinosis. Am. J. Pathol. 142:241-248. [PMC free article] [PubMed] [Google Scholar]
- 12.Eng, R. H., E. Bishburg, S. M. Smith, and R. Kapila. 1986. Cryptococcal infections in patients with acquired immune deficiency syndrome. Am. J. Med. 81:19-23. [DOI] [PubMed] [Google Scholar]
- 13.Ferguson, J. S., D. R. Voelker, F. X. McCormack, and L. S. Schlesinger. 1999. Surfactant protein D binds to Mycobacterium tuberculosis bacilli and lipoarabinomannan via carbohydrate-lectin interactions resulting in reduced phagocytosis of the bacteria by macrophages. J. Immunol. 163:312-321. [PubMed] [Google Scholar]
- 14.Fries, B. C., D. L. Goldman, R. Cherniak, R. Ju, and A. Casadevall. 1999. Phenotypic switching in Cryptococcus neoformans results in changes in cellular morphology and glucuronoxylomannan structure. Infect. Immun. 67:6076-6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hartshorn, K. L., K. B. M. Reid, M. R. White, J. C. Jensenius, S. M. Morris, A. I. Tauber, and E. C. Crouch. 1996. Neutrophil deactivation by influenza A viruses: mechanisms of protection after viral opsonization with collectins and hemagglutination-inhibiting antibodies. Blood 87:3450-3461. [PubMed] [Google Scholar]
- 16.Ikeda, R., T. Shinoda, Y. Fukazawa, and L. Kaufman. 1982. Antigenic characterization of Cryptococcus neoformans serotypes and its application to serotyping of clinical isolates. J. Clin. Microbiol. 16:22-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.James, P. G., and R. Cherniak. 1992. Galactoxylomannans of Cryptococcus neoformans. Infect. Immun. 60:1084-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.James, P. G., R. Cherniak, R. G. Jones, C. A. Stortz, and E. Reiss. 1990. Cell-wall glucans of Cryptococcus neoformans Cap 67. Carbohydr. Res. 198:23-38. [DOI] [PubMed] [Google Scholar]
- 19.Kozel, T. R. 1977. Non-encapsulated variant of Cryptococcus neoformans. II. Surface receptors for cryptococcal polysaccharide and their role in inhibition of phagocytosis by polysaccharide. Infect. Immun. 16:99-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kozel, T. R. 1995. Virulence factors of Cryptococcus neoformans. Trends Microbiol. 3:295-299. [DOI] [PubMed] [Google Scholar]
- 21.Kozel, T. R., E. Reiss, and R. Cherniak. 1980. Concomitant but not causal association between surface charge and inhibition of phagocytosis by cryptococcal polysaccharide. Infect. Immun. 29:295-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lawson, P. R., and K. B. M. Reid. 2000. The roles of surfactant proteins A and D in innate immunity. Immunol. Rev. 173:66-78. [DOI] [PubMed] [Google Scholar]
- 23.Lu, J., C. Teh, U. Kishore, and K. B. M. Reid. 2002. Collectins and ficolins: sugar pattern recognition molecules of the mammalian innate immune system. Biochim. Biophys. Acta 1572:387-400. [DOI] [PubMed] [Google Scholar]
- 24.Madan, T., P. Eggleton, U. Kishore, P. Strong, S. S. Aggrawal, P. U. Sarma, and K. B. M. Reid. 1997. Binding of pulmonary surfactant proteins A and D to Aspergillus fumigatus conidia enhances phagocytosis and killing by human neutrophils and alveolar macrophages. Infect. Immun. 65:3171-3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mason, R. J., K. Greene, and D. R. Voelker. 1998. Surfactant protein A and surfactant protein D in health and disease. Am. J. Physiol. 275:L1-L13. [DOI] [PubMed] [Google Scholar]
- 26.McCormack, F. X. 1998. Structure, processing and properties of surfactant protein A. Biochim. Biophys. Acta 1408:109-131. [DOI] [PubMed] [Google Scholar]
- 27.McCormack, F. X., and J. A. Whitsett. 2002. The pulmonary collectins, SP-A and SP-D, orchestrate innate immunity in the lung. J. Clin. Investig. 109:707-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nosanchuk, J. D., and A. Casadevall. 1997. Cellular charge of Cryptococcus neoformans: contributions from the capsular polysaccharide, melanin, and monoclonal antibody binding. Infect. Immun. 65:1836-1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ofek, I., A. Mesika, M. Kalina, Y. Keisari, R. Podschun, H. Sahly, D. Chang, D. McGregor, and E. C. Crouch. 2001. Surfactant protein D enhances phagocytosis and killing of unencapsulated phase variants of Klebsiella pneumoniae. Infect. Immun. 69:24-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Riordan, D. M., J. E. Standing, K. Y. Kwon, D. Chang, E. C. Crouch, and A. H. Limper. 1995. Surfactant protein D interacts with Pneumocystis carinii and mediates organism adherence to alveolar macrophages. J. Clin. Investig. 95:2699-2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Persson, A., D. Chang, and E. C. Crouch. 1990. Surfactant protein D is a divalent cation-dependent carbohydrate-binding protein. J. Biol. Chem. 265:5755-5760. [PubMed] [Google Scholar]
- 32.Persson, A., D. Chang, K. Rust, M. Moxley, W. Longmore, and E. C. Crouch. 1989. Purification and biochemical characterization of CP4 (SP-D), a collagenous surfactant-associated protein. Biochemistry 28:6361-6367. [DOI] [PubMed] [Google Scholar]
- 33.Rodrigues, M. L., C. S. Alviano, and L. R. Travassos. 1999. Pathogenicity of Cryptococcus neoformans: virulence factors and immunological mechanisms. Microbes Infect. 1:293-301. [DOI] [PubMed] [Google Scholar]
- 34.Saag, M. S., W. G. Powderly, G. A. Cloud, P. Robinson, M. H. Grieco, P. K. Sharkey, S. E. Thompson, A. M. Sugar, C. U. Tuazon, J. F. Fisher, et al. 1992. Comparison of amphotericin B with fluconazole in the treatment of acute AIDS-associated cryptococcal meningitis. N. Engl. J. Med. 326:83-89. [DOI] [PubMed] [Google Scholar]
- 35.Schelenz, S., R. Malhotra, R. B. Sim, U. Holmskov, and G. J. Bancroft. 1995. Binding of host collectins to the pathogenic yeast Cryptococcus neoformans: human surfactant protein D acts as an agglutinin for acapsular yeast cells. Infect. Immun. 63:3360-3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turner, S. H., R. Cherniak, E. Reiss, and K. J. Kwon-Chung. 1992. Structural variability in the glucuronoxylomannan of Cryptococcus neoformans serotype A isolates determined by 13C NMR spectroscopy. Carbohydr. Res. 233:205-218. [DOI] [PubMed] [Google Scholar]
- 37.Vaishnav, V. V., B. E. Bacon, M. O'Neill, and R. Cherniak. 1998. Structural characterization of the galactoxylomannan of Cryptococcus neoformans Cap67. Carbohydr. Res. 306:315-330. [DOI] [PubMed] [Google Scholar]
- 38.van de Wetering, J. K., M. van Eijk, L. M. G. van Golde, T. Hartung, J. A. G. van Strijp, and J. J. Batenburg. 2001. Characteristics of surfactant protein A and D binding to lipoteichoic acid and peptidoglycan, 2 major cell wall components of gram-positive bacteria. J. Infect. Dis. 184:1143-1151. [DOI] [PubMed] [Google Scholar]
- 39.van Rozendaal, B. A. W. M., A. B. van Spriel, J. G. J. van de Winkel, and H. P. Haagsman. 2000. Role of pulmonary surfactant protein D in innate defense against Candida albicans. J. Infect. Dis. 182:917-922. [DOI] [PubMed] [Google Scholar]
- 40.Vartivarian, S. E., G. H. Reyes, E. S. Jacobson, P. G. James, R. Cherniak, V. R. Mumaw, and M. J. Tingler. 1989. Localization of mannoprotein in Cryptococcus neoformans. J. Bacteriol. 171:6850-6852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vecchiarelli, A. 2000. Immunoregulation by capsular components of Cryptococcus neoformans. Med. Mycol. 38:407-417. [DOI] [PubMed] [Google Scholar]
- 42.Walenkamp, A. M., W. S. Chaka, A. F. Verheul, V. V. Vaishnav, R. Cherniak, F. E. J. Coenjaerts, and I. M. Hoepelman. 1999. Cryptococcus neoformans and its cell wall components induce similar cytokine profiles in human peripheral blood mononuclear cells despite differences in structure. FEMS Immunol. Med. Microbiol. 26:309-318. [DOI] [PubMed] [Google Scholar]
- 43.Walenkamp, A. M., A. F. Verheul, J. Scharringa, and I. M. Hoepelman. 1999. Pulmonary surfactant protein A binds to Cryptococcus neoformans without promoting phagocytosis. Eur. J. Clin. Investig. 29:83-92. [DOI] [PubMed] [Google Scholar]