Abstract
Feto-placental infections represent a major cause of pregnancy complications, and yet the underlying molecular and cellular mechanisms of vertical transmission are poorly understood. Listeria monocytogenes, a facultative intracellular pathogen, is one of a group of pathogens that are known to cause feto-placental infections in humans and other mammals. The purpose of this study was to evaluate possible mechanisms of vertical transmission of L. monocytogenes. Humans and guinea pigs have a hemochorial placenta, where a single layer of fetally derived trophoblasts separates maternal from fetal circulation. We characterized L. monocytogenes infection of the feto-placental unit in a pregnant guinea pig model and in primary human trophoblasts and trophoblast-derived cell lines. The clinical manifestations of listeriosis in the pregnant guinea pigs and the tropism of L. monocytogenes to the guinea pig placenta resembled those in humans. Trophoblast cell culture systems were permissive for listerial growth and cell-to-cell spread and revealed that L. monocytogenes deficient in internalin A, a virulence factor that mediates invasion of nonphagocytic cells, was 100-fold defective in invasion. However, crossing of the feto-placental barrier in the guinea pig model was independent of internalin A, suggesting a negligible role for internalin-mediated direct invasion of trophoblasts in vivo. Further understanding of vertical transmission of L. monocytogenes will help in designing more effective means of treatment and disease prevention.
Mammalian reproduction poses an immunological paradox because fetal alloantigens encoded by genes inherited from the father should provoke cell-mediated immune responses from the mother, potentially leading to fetal loss. There is evidence that pregnancy leads to suppression of cell-mediated immunity in the mother in order to prevent fetal rejection (34, 46). However, cell-mediated immunity is necessary for the defense against intracellular pathogens, and therefore suppression of this arm of the immune response might predispose the fetus to infection with intracellular pathogens. Indeed, organisms which commonly cause feto-placental infections are usually intracellular, including viruses such as cytomegalovirus (CMV) (51, 52) and human immunodeficiency virus (7, 37, 38), intracellular protozoan parasites such as Toxoplasma gondii (17), and intracellular bacterial pathogens such as Listeria monocytogenes (29, 30, 36). Among this group of pathogens, L. monocytogenes is the most tractable and has been used for decades as a model pathogen to dissect basic aspects of intracellular pathogenesis as well as the host's innate and acquired cellular immune responses in the murine model of infection (42). Acquired immunity to L. monocytogenes is entirely cell mediated and largely dependent on cytotoxic CD8+ T cells that recognize and lyse infected cells (23, 57).
L. monocytogenes is a ubiquitous gram-positive bacterium which has an unusually broad host range. Infection of humans and animals has been traced to contaminated foods. Ingestion of L. monocytogenes by humans occurs relatively frequently. On average, healthy adults in the United States ingest 105 L. monocytogenes organisms four times per year (39). However, listeriosis is a rare disease, with an incidence of ∼1,100 cases/year and high mortality of ∼250 cases/year in the United States (53). Most individuals who develop listeriosis have predisposing factors which render them more susceptible to infection (29, 30). About two-thirds of the cases occur in immunocompromised individuals. The most common clinical manifestation in this group of patients is central nervous system infection. Almost all of the remaining cases occur in pregnant women and carry the risk of feto-placental infection (29, 30). Pregnant women usually develop a nonspecific febrile illness, which can lead to feto-placental infection with serious consequences for the fetus or newborn child (36). In one study, L. monocytogenes was found to be the cause of 3.9% of second-trimester miscarriages in humans (22), and infection of the mother around term may lead to severe neonatal disease, with neonatal mortality ranging between 22 and 47% (10, 54).
We sought to develop a pregnant animal model to study feto-placental transmission of L. monocytogenes. We decided to focus on pregnant guinea pigs because of significant similarities between the structures of human and guinea pig placentas. Humans and rodents have a hemochorial placenta, which means that fetally derived cells called trophoblasts invade the uterus and are in direct contact with maternal blood. Of all the rodent placentas, guinea pig placenta resembles the human placenta most closely. Both species have a discoidal placenta, trophoblasts invade the uterus into the endometrium and inner third of the myometrium, and the maternal blood spaces are lined by one layer of trophoblasts during the later stages of pregnancy (19, 27). In addition to forming a physical barrier between maternal and fetal circulation, trophoblasts seem to play a role in the innate immune response to infection. Evidence comes from several studies which have shown that trophoblasts secrete cytokines, including interleukin-10 and the murine interleukin-8 analogs KC and macrophage inflammatory protein-2 (14, 45). Furthermore, trophoblasts have been shown to express the pattern recognition receptors Toll-like receptor-2 and Toll-like receptor-4 (15, 16).
We hypothesized that infection of trophoblasts is a critical step in the spread of infection from mother to fetus. In this study we focused on the importance of listerial virulence factors in crossing the physical barrier between maternal and fetal circulation. Trophoblasts might become infected with L. monocytogenes by either direct invasion or cell-to-cell spread. It has been shown previously that bacterial surface proteins, called internalins, mediate invasion of nonphagocytic cells by L. monocytogenes (3). The best characterized internalins are internalin A (InlA) (13) and internalin B (InlB) (3, 9), which bind to E-cadherin (35) and c-Met receptor tyrosine kinase (49), respectively. Similarities between human and guinea pig E-cadherins make the guinea pig a better animal model than the mouse to study the role of InlA in vivo (25, 26). Another possible mechanism by which trophoblasts can become infected is by cell-to-cell spread from infected maternal cells, specifically macrophages. Macrophages internalize L. monocytogenes by phagocytosis. Once L. monocytogenes has entered the host cell, it escapes into the cytoplasm, where the listerial ActA protein enables the bacterium to propel itself through the cytoplasm and spread from cell to cell without exposure to the extracellular environment (55).
In order to investigate mechanisms of vertical transmission, we developed and characterized a pregnant guinea pig model of listeriosis which mimicked aspects of human disease. Invasion of isolated human trophoblasts, which were permissive for listerial growth and cell-to-cell spread, was facilitated by InlA. Furthermore, we evaluated the role of InlA in crossing the feto-placental barrier in vivo.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The L. monocytogenes strains used in this study were derived from 10403S (2). All strains were propagated in brain heart infusion (BHI) (Becton Dickinson and Company, Sparks, Md.) agar and broth. For invasion assays and intracellular growth curves, L. monocytogenes strains were grown overnight in BHI broth to stationary phase. For animal infections, bacteria were grown in BHI broth to log phase, washed once with phosphate-buffered saline, and resuspended in fresh BHI broth. Aliquots were frozen and stored at −80°C. On the day of infection, aliquots were thawed, diluted 1:5 in fresh BHI broth, and grown to log phase.
Construction of deletion mutants. (i) Construction of ΔinlA exchange plasmid.
Both 5′ and 3′ fragments of the inlA gene (5′inlA and 3′inlA) were generated by PCR with L. monocytogenes genomic DNA as a template. Primers CATTTTAAAAGGTGGAATGACA and TCCGGATGTTACATTCGTTTTTCCTAAGA (the BspEI site is underlined) were used to generate 5′inlA. Primers TCCGGAATACCTGGAAGCGACACATCT (the BspEI site is underlined) and TCATTTTGTGTCACTGCATCTG were used to generate 3′inlA. Klenow fragment was used to generate blunt-end PCR products, which were ligated into the StuI site of pMTL20 (6) and then double digested with BamHI and BspEI, leading to release of 3′inlA. 3′inlA was ligated into the vector containing 5′inlA, generating an inlA fragment (ΔinlA) with deletion of bp 1734 to 3722 (inlA-inlB operon sequence from the National Center for Biotechnology Information; accession number AJ012346), which was confirmed by sequencing. ΔinlA was then subcloned into pMTL23 (6), digested with AatII, and inserted into pLMD1 (48), generating the ΔinlA allelic exchange plasmid.
(ii) Construction of ΔinlB exchange plasmid.
A 3′ fragment of the inlB gene (3′inlB) was generated by PCR with primers TCCGGAGGTACCTAACCTACGAAAAAGGCTA (the KpnI site is underlined) and TGGTCGTTATTAAAGTGACTTAAGGC (the EcoRI site is underlined) with L. monocytogenes genomic DNA as a template. 3′inlB was digested with KpnI and EcoRI and then cloned into pMTL23, which was then digested with XbaI. Klenow fragment was used to generate 3′inlB with blunt ends, which was subsequently digested with BamHI. The vector pMTL20 containing 3′inlA was digested with BglII, and blunt ends were created with Klenow fragment and digested with BamHI. The 3′inlB fragment was ligated into this vector, generating an inlB fragment (ΔinlB), with deletion of bp 4240 to 5910, which was confirmed by sequencing. A 2.8-kb cassette from pE194ts (48) was introduced at the AccI site of the ΔinlB-containing plasmid, generating the ΔinlB allelic exchange plasmid.
(iii) Construction of L. monocytogenes InlA and InlB deletion mutants (DP-L4405 and DP-L4406, respectively).
The ΔinlA and ΔinlB allelic exchange plasmids were transformed into L. monocytogenes 10403S and selected in the presence of erythromycin (5). In-frame deletion of inlA or inlB was confirmed by Southern blot analysis. Western blot analysis was used to confirm the lack of InlA protein expression in selected mutants.
(iv) Construction of L. monocytogenes InlA InlB double deletion mutant (DP-L4404).
A DNA fragment upstream of the 5′ end of inlA was generated by PCR with primers GGGCTGCAGAGAGTTTTGGCGGTAAGAGTG and AGGTTTTCCGCTTTAGTCCAGTTTTCCTAAGACCGTCTTCAT. A second DNA fragment of the 3′end of inlB was generated by PCR with primers CCCGAGCTCGCTGCTTTCGTCCAACCAATG and ATGAAGACGGTCTTAGGAAAACTGGACTAAAGCGGAAAACCT. These PCR products were then used as a template in a subsequent PCR with primers GGGCTGCAGAGAGTTTTGGCGGTAAGAGTG and CCCGAGCTCGCTGCTTTCGTCCAACCAATG to generate ΔinlAB, a DNA fragment with deletion of bp 1700 to 5389. ΔinlAB was cloned into the SacI and PstI sites of plasmid pKSV7 (50). Inserts were verified by sequencing analysis and integrated into the L. monocytogenes 10403S genome. Southern blots were performed to confirm the in-frame deletion of inlAB.
Construction of complementation strains.
DNA fragments containing the internalin A/B promoter and either inlAB or inlA were generated by PCR with wild-type L. monocytogenes strain 10403S chromosomal DNA as a template. Primer CGGGATCCAACGAGCCAACCGTGG (the BamHI site is underlined) annealed upstream of the internalin A/B promoter and was used in combination with primers CGGGATCCTTATTTCTGTGCCCTTAAATTAGC (the BamHI site is underlined) and CGGGATCCTCTCCGCTTGTACTTTCGCC (the BamHI site is underlined) to amplify DNA fragments that encode the internalin A/B promoter with either inlAB or inlA, respectively. Both PCR-generated DNA fragments were flanked by BamHI sites and were cloned into the BamHI site of the pPL2 site-specific shuttle integration vector (24). The inserts were verified by sequencing analysis. PPL2 containing the internalin A/B promoter with inlA or inlAB was integrated into DP-L4405 or DP-L4404, respectively, creating DP-L4454 and DP-L4455.
Primary human trophoblasts. Primary human trophoblasts were obtained from elective termination of pregnancy during the second trimester at the University of California, San Francisco (UCSF). Informed consent was obtained from all patients from whom tissue was collected. The UCSF Committee on Human Research approved the consent form. Placental and decidual tissues were collected within 1 h of isolation, washed in phosphate-buffered saline with antibiotics (penicillin, streptomycin, and gentamicin) (UCSF Cell Culture Facility), and placed on ice. Cells were isolated from human placentas by published methods (11, 28). Briefly, placentas were subjected to a series of enzymatic digests that detached cytotrophoblast stem cells from the underlying stromal core of the chorionic villus. Detached trophoblasts were purified over a Percoll gradient. To decrease residual macrophage contamination, the trophoblasts were further purified by incubation with anti-CD45-coated magnetic beads (Dynabeads M-450 CD 45; Dynal Biotech, Oslo, Norway). Trophoblasts were cultured overnight on Matrigel-coated 12-mm-diameter coverslips (BD Biosciences Discovery Labware, Bedford, Mass.) in Dulbecco's modified Eagle's medium-4.5 g of glucose per liter with 2% Nutridoma (Boehringer Mannheim Biochemicals, Indianapolis, Ind.). Immunofluorescence staining with monoclonal antibodies to CD45 (DakoCytomation, Carpinteria, Calif.) and cytokeratin confirmed that the cell population contained trophoblasts. Rat antitrophoblast monoclonal antibody to cytokeratin 7D3 was produced in the laboratory of S. J. Fisher (UCSF) according to previously described methods (8). Secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, Pa.) included rhodamine-conjugated goat anti-rat immunoglobulin G and fluorescein isothiocyanate-conjugated donkey anti-mouse immunoglobulin G.
Cell lines.
The human choriocarcinoma cell line BeWo was obtained from the American Type Culture Collection (Manassas, Va.) (ATCC CCL-98) (41) and grown in Ham's F12 medium with 2 mM l-glutamine, 1.5 g of sodium bicarbonate (Gibco, Grand Island, N.Y.) per liter and 10% fetal bovine serum (FBS) (Gemini, Woodland, Calif.). The guinea pig colorectal adenocarcinoma cell line GPC-16 was obtained from the American Type Culture Collection (ATCC CCL-242) (40) and grown in Eagle minimal essential medium with 2 mM l-glutamine and Earle's balanced salt solution adjusted to contain 1.5 g of sodium bicarbonate per liter, 0.1 mM nonessential amino acids, 1 mM sodium pyruvate (Gibco), and 10% FBS (Gemini). The J774 mouse macrophage-like cell line (43) was grown in suspension in a spinner flask in Dulbecco's modified Eagle's medium supplemented with 10% FBS and 2 mM l-glutamine.
Bacterial invasion and growth in primary cells and cell lines.
Invasion assays and growth curves were performed as previously described (18). Briefly, cells were plated onto 12-mm-diameter glass coverslips in their respective growth media without any antibiotics and grown overnight. The density of the plated cells was dependent on their size and rate of growth, because experiments were performed with a monolayer of cells. BeWo and J774 cells were plated at densities of 3 × 104 and 6 × 104 per coverslip, respectively, reflecting the larger size of BeWo cells in comparison to J774 cells. Primary human trophoblasts were plated at a density of 5 × 105 per coverslip, because not all of the primary cells survived the preparation and attached to the coverslip. Cells were infected with wild-type or mutant L. monocytogenes. The multiplicity of infection (MOI) was estimated based on the number of bacteria added to the culture medium and the estimated number of cells at the time of infection. For J774 and BeWo cells, we estimated doubling of the cell numbers overnight. For the primary human trophoblasts, we used the number of cells plated to calculate the MOI, which did not take into account that some of the cells did not survive and attach to the coverslips. At 1 h after infection, gentamicin (Sigma, St. Louis, Mo.) was added at a final concentration of 50 μg/ml. At specified times after infection (2 h for invasion assay and 2, 5, and 8 h for growth curves), coverslips were removed in triplicate and cells were lysed by vortexing each coverslip in 5 ml of sterile distilled water. Aliquots were plated onto Luria-Bertani (LB) agar plates (Becton Dickinson, Franklin Lakes, N.J.) and incubated overnight at 37°C. The average colony count was determined for each time point. A fourth coverslip was removed at each time point for fixation and staining of cells with Diff-Quik (Dade Behring, Dudingen, Switzerland).
Histology.
Tissue was fixed in 10% paraformaldehyde (Electron Microscopy Sciences, Ft. Washington, Pa.) and processed by routine methods to provide paraffin wax sections (4 μm), which were stained with hematoxylin and eosin (Histology Laboratory, Veterinary Medical Teaching Hospital, University of California, Davis). Immunohistochemistry was performed at the California Animal Health and Food Safety Laboratory (Davis), using a rabbit anti-Listeria primary antibody (Difco Laboratories, Detroit, Mich.) and a peroxidase detection kit (Vector Laboratories, Burlingame, Calif.).
Animal studies.
All animals were housed and handled in accordance with federal and institutional guidelines. The animal use committee at the University of California, Berkeley, approved the animal use protocol describing our studies. Pregnant female Hartley outbred guinea pigs between days 35 and 45 of gestation were purchased from Harlan Laboratories (Madison, Wis.) or Simonsen Laboratories (Gilroy, Calif.). Pregnant females were injected intravenously with L. monocytogenes into their ear veins. Animals were inoculated with 1 × 105, 1 × 106, or 2 × 107 bacteria. Inoculation was performed under general anesthesia with isofluorane (Baxter Healthcare Corporation, Deerfield, Ill.), and animals were premedicated with a subcutaneous injection of 0.05 mg of atropine (Phoenix Scientific, St. Joseph, Mo.) per kg. Animals were euthanatized at 24 or 48 h after inoculation, and organs were harvested and homogenized in 0.2% NP-40 (Biosciences, Inc., La Jolla, Calif.). Serial dilutions were plated on LB agar plates (Becton Dickinson) and incubated overnight at 37°C. Bacteria per organ were enumerated.
Competitive index analysis was performed as previously described (1). Briefly, the erythromycin-resistant wild-type 10403S strain was compared to the erythromycin-sensitive mutant strains. The two strains were mixed in a 1:1 ratio. Guinea pigs were injected with a total infectious dose of 1 × 105, 1 × 106 or 2 × 107. Animals were sacrificed at 24 or 48 h postinoculation, and organs were harvested and homogenized in 0.2% NP-40. Serial dilutions were plated on LB agar plates and BHI agar plates containing erythromycin (Sigma) at a concentration of 2 μg/ml. Agar plates were incubated overnight at 37°C, and erythromycin-sensitive and erythromycin-resistant colonies were enumerated. The competitive index was determined by calculation of the ratio between erythromycin-sensitive and erythromycin-resistant colonies.
Statistical analysis.
Statistical analysis was performed with the Mann-Whitney confidence interval test with the student edition of Minitab release 12.
RESULTS
Pregnant guinea pig model.
Pregnant guinea pigs were infected intravenously by injection into their ear veins with doses of 1 × 105, 1 × 106, or 2 × 107 L. monocytogenes organisms during the late second and early third trimester (days 42 to 52 of gestation). A dose of 105 did not lead to feto-placental infection and did not cause any significant infection in maternal spleen and liver (data not shown). The lowest possible dose causing maternal and feto-placental infection in all animals was 106 (data not shown). In all of our subsequent experiments we used either 1 × 106 or 2 × 107 bacteria per animal. The pregnant guinea pigs developed spontaneous abortions but appeared otherwise well after intravenous inoculation of L. monocytogenes. In one experiment, five out of seven guinea pigs had spontaneous abortions by 48 h postinoculation with 2 × 107 bacteria. The guinea pig mothers seemed to recover without further problems after miscarriage.
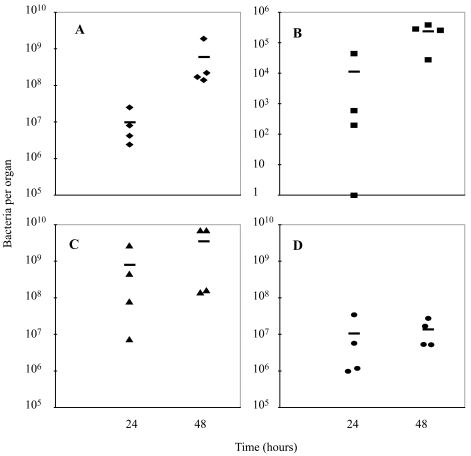
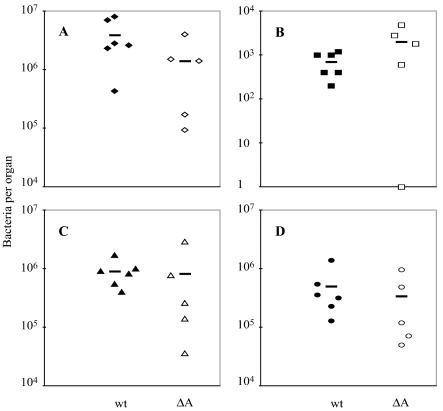
At 24 h after intravenous inoculation of 2 × 107 bacteria, all animals had placental infection, with bacterial numbers per placenta similar to those in maternal liver and spleen. (Fig. 1A, C, and D). At this time, three out of four fetuses were infected (Fig. 1B). At 48 h postinoculation, the numbers of L. monocytogenes had increased in all tested organs, but most of the increase was observed in the feto-placental unit. There was almost a 2-log-unit increase in bacteria per placenta (Fig. 1A). In contrast, the bacterial numbers in maternal liver and spleen had increased only slightly, by less than 1 log unit (Fig. 1C and D). All of the fetal livers were infected (Fig. 1B). Neither amniotic fluid nor fetal or maternal brain contained detectable bacteria (data not shown).
FIG. 1.
Listeriosis in pregnant guinea pigs between days 42 and 52 of gestation. Bacteria per placenta (A), fetal liver (B), maternal liver (C), and maternal spleen (D) were enumerated at 24 or 48 h after intravenous inoculation of animals with 2 × 107 wild-type L. monocytogenes organisms. The bars represent means.
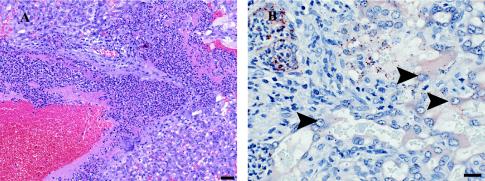
Histological examination was performed on placental sections taken from guinea pigs at 24 h postinoculation with 2 × 107 L. monocytogenes bacteria. All examined placentas contained multiple foci of inflammation, comprised mostly of neutrophils and small numbers of macrophages. Lesions were multifocally distributed within the labyrinth portion and along maternal arteries of the placenta (Fig. 2A). Immunohistochemistry for L. monocytogenes revealed large numbers of immunoreactive bacteria within the inflammatory infiltrate. The bacteria appeared to be mostly extracellular or inside macrophages. Small numbers of bacteria appeared to be within the cytoplasm of trophoblasts (Fig. 2B). Because a single trophoblast layer separates fetal from maternal circulation in the hemochorial placenta of humans and guinea pigs, this was of particular interest to us and suggested that trophoblast infection is a critical step in transmission of L. monocytogenes infection from mother to fetus.
FIG. 2.
Histological examination of guinea pig placental sections. Pregnant guinea pigs between days 42 and 52 of gestation were inoculated intravenously with 2 × 107 wild-type L. monocytogenes bacteria. (A) Hematoxylin-eosin staining of placental section, showing the labyrinthine region at 24 h postinoculation. Moderate numbers of neutrophils and very small numbers of macrophages infiltrate the central region of the lobe. There is fibrin deposition associated within this infiltrate. Bar, 50 μm. (B) Immunohistochemistry for L. monocytogenes reveals large numbers of immunoreactive bacteria within the inflammatory infiltrate. Some bacteria appear to be inside trophoblasts (arrowheads). Bar, 20 μm.
Infection of BeWo cells, a human trophoblast-derived cell line.
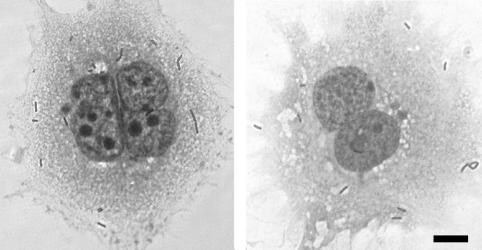
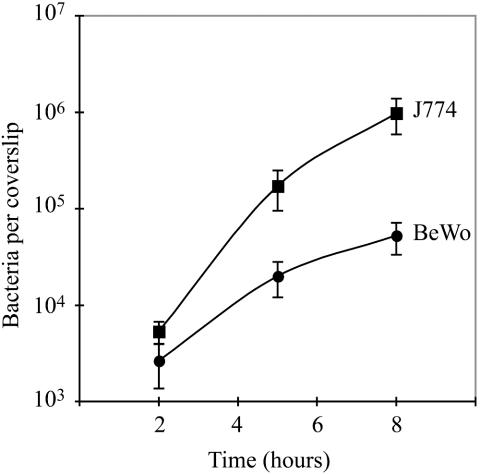
The BeWo cell line is a human choriocarcinoma cell line derived from trophoblasts. We found that BeWo cells were readily infected by wild-type L. monocytogenes (Fig. 3, right panel). At 1 h after infection we added gentamicin to the culture medium in order to observe the intracellular growth of L. monocytogenes without contamination by extracellular bacteria. Infection of BeWo cells at an MOI of 1:8 led to infection of 1 to 2% of the cells at 2 h postinoculation, which was followed by a 20-fold increase in bacterial numbers over the next 6 h (Fig. 4). The doubling time of L. monocytogenes in BeWo cells was 63 min between 2 and 5 h postinoculation. We compared the results for BeWo cells with L. monocytogenes infection of the macrophage-like cell line J774, which is easily infected by L. monocytogenes (Fig. 4) (18). We found that the rates of internalization in the two cell lines were comparable. The rate of growth in BeWo cells was ∼0.5-fold the rate of growth in J774 cells. L. monocytogenes appeared to spread normally from cell to cell in BeWo cells. BeWo cells grow in islet formations and at early time points of infection bacteria were observed in peripherally located cells, from where they seemed to have spread at later time points (data not shown).
FIG. 3.
Light micrographs of Diff-Quik stains of trophoblasts 2 h after infection with wild-type L. monocytogenes. (Left panel) Primary human trophoblasts. (Right panel) BeWo cells. In each case, gentamicin (50 μg/ml) was added 1 h after infection. Bar, 10 μm.
FIG. 4.
Growth of L. monocytogenes in BeWo cells and J774 murine macrophages. BeWo cells (3 × 104) and J774 cells (6 × 104) were plated on glass coverslips and grown overnight, leading to formation of monolayers. The difference in the number of plated BeWo and J774 cells reflects the difference in size between these two cell types. Cells were infected with a 1:20,000 dilution of an overnight culture of wild-type L. monocytogenes, resulting in MOIs of 1:8 for BeWo cells and 1:15 for J774 cells. At the specified times after infection, cells were lysed, and the number of bacteria per coverslip was determined in triplicate. In each case, gentamicin (50 μg/ml) was added 1 h after infection. Each growth curve represents the means and standard deviations from three separate experiments.
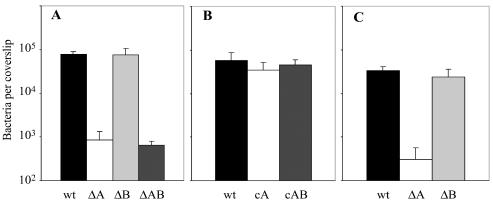
The L. monocytogenes virulence factors InlA and InlB interact with E-cadherin and c-Met-tyrosine kinase, respectively, to facilitate invasion of nonphagocytic cells (35, 49). InlA has been shown to be important for invasion of the human intestinal cell line Caco-2 and to facilitate crossing of the intestinal barrier in guinea pigs and transgenic mice expressing human E-cadherin under control of an intestinal specific promoter (26). We infected the guinea pig colorectal adenocarcinoma cell line GP-16 with an InlA deletion mutant of L. monocytogenes and found a 2-log-unit reduction in invasion (data not shown). We subsequently tested whether InlA or InlB facilitated invasion of trophoblasts, in particular because trophoblasts express E-cadherin and c-Met-tyrosine kinase (12, 21). Infection of BeWo cells with an InlA deletion mutant of L. monocytogenes led to a 2-log-unit reduction in invasion in comparison to wild type infection (Fig. 5A). Infection with an InlB deletion mutant had the same phenotype as wild-type L. monocytogenes infection (Fig. 5A). The InlA InlB double deletion mutant had the same phenotype in BeWo cells as the InlA single deletion mutant (Fig. 5A). Complementation of InlA single and InlA InlB double deletion mutants with the inlA gene or the inlA and inlB genes, respectively, led to complete restoration of the wild-type phenotype (Fig. 5B). These results indicated that L. monocytogenes directly infects BeWo cells in an InlA-dependant manner. However, even without InlA there was a low rate of infection, indicating that there might be other mechanisms by which L. monocytogenes can directly invade BeWo cells, although not very efficiently. The InlA deletion mutant had no defect in growth or cell-to-cell spread, despite the 100-fold decrease in entry (data not shown).
FIG. 5.
Invasion of trophoblasts by L. monocytogenes. Monolayers of cells were grown on glass coverslips. Trophoblasts were infected with L. monocytogenes 10403S (wild type [wt]) and the InlA deletion mutant (ΔA), the InlB deletion mutant (ΔB), the InlA InlB double deletion mutant (ΔAB), the InlA complementation mutant (cA), and the InlA InlB complementation mutant (cAB). BeWo cells were infected at an MOI of 1:1 (A and B), and primary human trophoblasts were infected at an estimated MOI of 20:1 (C). In each case, gentamicin (50 μg/ml) was added 1 h after infection. At 2 h after infection, monolayers were lysed and the number of bacteria per coverslip was determined in triplicate. All bacterial numbers are the averages and standard deviations from three separate experiments.
Infection of primary human trophoblasts.
We wanted to confirm our results for the BeWo cell line with primary cells. Primary human trophoblasts, derived from second-trimester placentas, resemble BeWo cells morphologically. We infected the primary trophoblasts with a higher MOI than that used for infection of BeWo cells, because we were able to assess L. monocytogenes infection at only one time point due to limited availability of primary trophoblasts. The MOI needed to be high enough not only for quantification of intracellular wild-type bacteria but also for quantification of the intracellular InlA deletion mutant at 2 h postinfection. We therefore chose to infect the primary trophoblasts at an estimated MOI of 20:1. Multiple bacteria per cell were observed 2 h after infection with wild-type L. monocytogenes (Fig. 3, left panel). Because of the presence of syncytium, it was difficult to estimate the percentage of infected cells. Like in BeWo cells, infection of primary human trophoblasts with the InlA deletion mutant led to 2-log-unit reduction in invasion (Fig. 5C). The InlB deletion mutant had the same phenotype as wild-type L. monocytogenes (Fig. 5C), and the InlA InlB double deletion mutant had the same phenotype as InlA single deletion mutant (data not shown).
Evaluation of the role of InlA in crossing of the placental barrier in vivo.
Based on in vitro results, we hypothesized that InlA-dependent invasion of trophoblasts plays a role in feto-placental transmission of L. monocytogenes. We infected pregnant guinea pigs with 106 L. monocytogenes wild-type or InlA deletion mutant bacteria. Bacteria per organ were enumerated at 48 h postinoculation. We found no statistically significant differences between wild-type and mutant bacterial numbers in placenta, fetal liver, or maternal organs (Fig. 6). Microscopic examination of placental sections showed no difference in the placental lesions caused by the InlA deletion mutant and those caused by the wild type. Immunohistochemical staining for L. monocytogenes showed small numbers of bacteria, which appeared to be in the cytoplasm of trophoblasts in both cases (Fig. 2B and data not shown).
FIG. 6.
Infection of pregnant guinea pigs with wild-type L. monocytogenes (wt) or the InlA deletion mutant (ΔA). Groups of one to three pregnant guinea pigs between days 42 and 52 of gestation were inoculated intravenously with 106 bacteria. Bacteria per placenta (A), fetal liver (B), maternal liver (C), and maternal spleen (D) were enumerated at 48 h postinoculation. Bars represent means.
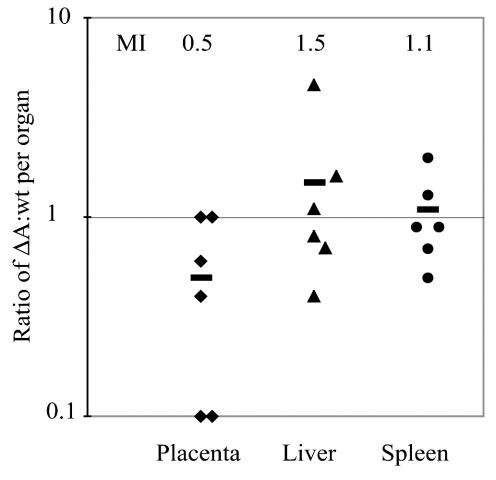
Competitive index analysis with a 1:1 ratio of erythromycin-sensitive InlA deletion mutant and erythromycin-resistant wild-type L. monocytogenes was performed in the pregnant guinea pig model. At 24 h after inoculation we found no statistically significant differences between the mean competitive indices in placenta and maternal organs, which were all close to 1 (Fig. 7). Furthermore, competitive index analysis with wild-type L. monocytogenes versus the InlA InlB double deletion mutant was performed under the same conditions except that the bacterial numbers per organ were enumerated at 48 h postinoculation. Again, the mean competitive indices were close to 1 and were not statistically different in fetal liver, placenta, and tested maternal organs (data not shown). These results indicate that InlA and InlA InlB deletion mutants and wild-type L. monocytogenes strains proliferate equally well in all tested organs.
FIG. 7.
Competitive index analysis of InlA deletion mutant (ΔA) and wild-type (wt) L. monocytogenes. Groups of three pregnant guinea pigs were inoculated intravenously with a 1:1 mixture of the two strains at a total dose of 2 × 107. Bacteria per organ were enumerated at 24 h postinoculation. Ratios between erythromycin-sensitive (ΔA) and erythromycin-resistant (wt) colonies were calculated for placenta, maternal liver, and spleen. Bars represent mean indices (MI).
DISCUSSION
L. monocytogenes is one of a group of pathogens that are known to be vertically transmitted in humans and other mammals. In this study we developed a pregnant guinea pig model of listeriosis. Like in humans, there seemed to be a tropism of L. monocytogenes to the guinea pig placenta, because the clinical manifestations in both species were similar. Subsequent to intravenous infection, guinea pigs developed spontaneous abortions without appearing to be systemically ill. Only 1 day after inoculation, the guinea pig placenta was consistently infected and comparable numbers of bacteria were found in placenta, maternal liver, and spleen. Infection of the fetus occurred slightly later than infection of the placenta, indicating that the infection spread from the placenta to the fetus. The ability of L. monocytogenes to proliferate in feto-placental tissues exceeded that in other organs, possibly due to impaired cell-mediated immune responses in the feto-placental unit.
A single trophoblast layer separates fetal from maternal circulation, suggesting that infection of trophoblasts might be a critical step in crossing the feto-placental barrier. Indeed, L. monocytogenes appeared to be in the cytosol of some trophoblasts. Trophoblast infection could occur by two possible mechanisms in vivo: direct invasion by extracellular bacteria or cell-to-cell spread from infected maternal cells, specifically macrophages. Internalins would play a role in direct invasion but not in cell-to-cell spread. Our in vitro results demonstrated that invasion of isolated primary human trophoblasts and BeWo cells was greatly enhanced by the presence of InlA. However, we found no difference in feto-placental infection with wild-type L. monocytogenes or the InlA deletion mutant in the pregnant guinea pig model of infection, indicating that InlA does not play a significant role in crossing the feto-placental barrier in vivo. One possible explanation for our findings is that trophoblast infection occurs via cell-to-cell spread in vivo. There are indications that cell-to-cell spread is an important mechanism of trophoblast infection by other pathogens. Studies on mechanisms of feto-placental transmission of CMV by using a coculture system of polarized uterine microvascular endothelial cells and trophoblasts showed that neutrophils transmitted CMV infection to uterine microvascular endothelial cells, which in turn infected trophoblasts (32). Comparison between the placental structures of different mammals also suggests that cell-to-cell spread might be an important mechanism for trophoblast infection. L. monocytogenes is the cause of spontaneous abortions in cows, sheep, and goats (20, 31, 56). Ruminants have a synepitheliochorial placenta, which means that the trophoblast layer is attached to an almost intact uterine mucosa (27). It seems that in these species, cell-to-cell spread from infected endothelial cells or migrating tissue macrophages would be more important than direct invasion for trophoblast infection.
Another explanation for our findings could be that direct invasion of trophoblasts in vivo is mediated by other internalins. Twenty proteins or open reading frames encoding for putative proteins with homology to InlA have been identified (4). Their roles in feto-placental transmission are unknown. We found a low level of infection with L. monocytogenes that was independent of InlA or InlB in primary human trophoblasts and BeWo cells. This suggests the presence of a mechanism of internalization not mediated by InlA or InlB, although this mechanism seems to be inefficient.
The nonpregnant mouse has been used extensively as a model host for listeriosis. There have been few studies of feto-placental infection in mice with L. monocytogenes. In contrast to our findings with the pregnant guinea pig model, only a small number of pregnant mice had feto-placental infection 18 or 24 h after intravenous inoculation, despite high bacterial burdens in maternal liver and spleen (reference 44 and our unpublished results). Interestingly, the pregnant guinea pig is also better than the mouse for studies of vertical transmission of CMV (33, 47). The reasons for the differences in susceptibility of the feto-placental unit to infection in the mouse and guinea pig are unknown. Structural differences in the feto-placental barrier could play a role. Both mice and guinea pigs have a hemochorial placenta, but mice have three layers of trophoblasts instead of one to separate maternal and fetal circulation. This might make the transmission of L. monocytogenes to the fetus less efficient. Another possible explanation is that the placental immunological environment might be more permissive for growth of L. monocytogenes in guinea pigs than in mice.
In our study we used intravenous inoculation of L. monocytogenes as the route of infection. In the setting of natural infection, L. monocytogenes is a food-borne disease and is thought to spread to the placenta from the gastrointestinal tract by hematogenous dissemination. Bypassing the gastrointestinal tract could lead to differences in dissemination, and we are in the process of developing an oral infection model in the pregnant guinea pig.
In summary, we demonstrate that human trophoblasts are permissive to listerial growth and we describe a pregnant guinea pig model to study feto-placental transmission of L. monocytogenes in vivo. Despite the importance of InlA for invasion of isolated human trophoblasts, we could not find a role for InlA in crossing of the feto-placental barrier in vivo. This underlines the importance of a good animal model for evaluating the significance of in vitro findings. Further studies will focus on the molecular and cellular mechanisms of listerial feto-placental transmission. This understanding is fundamental to the development of preventive measures against fetal infection.
Acknowledgments
This research was supported by NIH grants RO1 AI27655 and AI29619 (to D.A.P.) and by Pediatric Scientist Development Program NICHD grant award K-12 HD00850 (to A.I.B.).
We thank Shomyseh Sanjabi and Jeff Miller for construction of the InlA and InlB deletion mutants, Ramona Gonzales for construction of the InlA InlB double deletion mutant, Olga Genbacev and Eduardo Caceres for assistance with the primary human trophoblasts, John Parker for assistance with the guinea pig procedures, Mary O'Riordan for helpful discussions throughout this project, and Victoria Auerbuch-Stone for critical reading of the manuscript.
Editor: V. J. DiRita
REFERENCES
- 1.Auerbuch, V., L. L. Lenz, and D. A. Portnoy. 2001. Development of a competitive index assay to evaluate the virulence of Listeria monocytogenes actA mutants during primary and secondary infection of mice. Infect. Immun. 69:5953-5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bishop, D. K., and D. J. Hinrichs. 1987. Adoptive transfer of immunity to Listeria monocytogenes. The influence of in vitro stimulation on lymphocyte subset requirements. J. Immunol. 139:2005-2009. [PubMed] [Google Scholar]
- 3.Braun, L., and P. Cossart. 2000. Interactions between Listeria monocytogenes and host mammalian cells. Microbes Infect. 2:803-811. [DOI] [PubMed] [Google Scholar]
- 4.Cabanes, D., P. Dehoux, O. Dussurget, L. Frangeul, and P. Cossart. 2002. Surface proteins and the pathogenic potential of Listeria monocytogenes. Trends Microbiol. 10:238-245. [DOI] [PubMed] [Google Scholar]
- 5.Camilli, A., L. G. Tilney, and D. A. Portnoy. 1993. Dual roles of plcA in Listeria monocytogenes pathogenesis. Mol. Microbiol. 8:143-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chambers, S. P., S. E. Prior, D. A. Barstow, and N. P. Minton. 1988. The pMTL nic cloning vectors. I. Improved pUC polylinker regions to facilitate the use of sonicated DNA for nucleotide sequencing. Gene 68:139-149. [DOI] [PubMed] [Google Scholar]
- 7.Connor, E. M., R. S. Sperling, R. Gelber, P. Kiselev, G. Scott, M. J. O'Sullivan, R. VanDyke, M. Bey, W. Shearer, R. L. Jacobson, et al. 1994. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. N. Engl. J. Med. 331:1173-1180. [DOI] [PubMed] [Google Scholar]
- 8.Damsky, C. H., M. L. Fitzgerald, and S. J. Fisher. 1992. Distribution patterns of extracellular matrix components and adhesion receptors are intricately modulated during first trimester cytotrophoblast differentiation along the invasive pathway, in vivo. J. Clin. Investig. 89:210-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dramsi, S., I. Biswas, E. Maguin, L. Braun, P. Mastroeni, and P. Cossart. 1995. Entry of Listeria monocytogenes into hepatocytes requires expression of InIB, a surface protein of the internalin multigene family. Mol. Microbiol. 16:251-261. [DOI] [PubMed] [Google Scholar]
- 10.Evans, J. R., A. C. Allen, D. A. Stinson, R. Bortolussi, and L. J. Peddle. 1985. Perinatal listeriosis: report of an outbreak. Pediatr. Infect. Dis. 4:237-241. [PubMed] [Google Scholar]
- 11.Fisher, S. J., T. Y. Cui, L. Zhang, L. Hartman, K. Grahl, G. Y. Zhang, J. Tarpey, and C. H. Damsky. 1989. Adhesive and degradative properties of human placental cytotrophoblast cells in vitro. J. Cell Biol. 109:891-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Floridon, C., O. Nielsen, B. Holund, L. Sunde, J. G. Westergaard, S. G. Thomsen, and B. Teisner. 2000. Localization of E-cadherin in villous, extravillous and vascular trophoblasts during intrauterine, ectopic and molar pregnancy. Mol. Hum. Reprod. 6:943-950. [DOI] [PubMed] [Google Scholar]
- 13.Gaillard, J. L., P. Berche, C. Frehel, E. Gouin, and P. Cossart. 1991. Entry of L. monocytogenes into cells is mediated by internalin, a repeat protein reminiscent of surface antigens from gram-positive cocci. Cell 65:1127-1141. [DOI] [PubMed] [Google Scholar]
- 14.Guleria, I., and J. W. Pollard. 2000. The trophoblast is a component of the innate immune system during pregnancy. Nat. Med. 6:589-593. [DOI] [PubMed] [Google Scholar]
- 15.Harju, K., V. Glumoff, and M. Hallman. 2001. Ontogeny of Toll-like receptors Tlr2 and Tlr4 in mice. Pediatr. Res. 49:81-83. [DOI] [PubMed] [Google Scholar]
- 16.Holmlund, U., G. Cebers, A. R. Dahlfors, B. Sandstedt, K. Bremme, E. S. Ekstrom, and A. Scheynius. 2002. Expression and regulation of the pattern recognition receptors Toll-like receptor-2 and Toll-like receptor-4 in the human placenta. Immunology 107:145-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones, J. L., A. Lopez, M. Wilson, J. Schulkin, and R. Gibbs. 2001. Congenital toxoplasmosis: a review. Obstet. Gynecol. Surv. 56:296-305. [DOI] [PubMed] [Google Scholar]
- 18.Jones, S., and D. A. Portnoy. 1994. Intracellular growth of bacteria. Methods Enzymol. 236:463-467. [DOI] [PubMed] [Google Scholar]
- 19.Kaufmann, P., and M. Davidoff. 1977. The guinea-pig placenta. Adv. Anat. Embryol. Cell Biol. 53:5-91. [DOI] [PubMed] [Google Scholar]
- 20.Kirkbride, C. A. 1993. Bacterial agents detected in a 10-year study of bovine abortions and stillbirths. J. Vet. Diagn. Investig. 5:64-68. [DOI] [PubMed] [Google Scholar]
- 21.Kolatsi-Joannou, M., R. Moore, P. J. Winyard, and A. S. Woolf. 1997. Expression of hepatocyte growth factor/scatter factor and its receptor, MET, suggests roles in human embryonic organogenesis. Pediatr. Res. 41:657-665. [DOI] [PubMed] [Google Scholar]
- 22.Lallemand, A. V., D. A. Gaillard, P. H. Paradis, and C. G. Chippaux. 1992. Fetal listeriosis during the second trimester of gestation. Pediatr. Pathol. 12:665-671. [DOI] [PubMed] [Google Scholar]
- 23.Lane, F. C., and E. R. Unanue. 1972. Requirement of thymus (T) lymphocytes for resistance to listeriosis. J. Exp. Med. 135:1104-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lauer, P., M. Y. Chow, M. J. Loessner, D. A. Portnoy, and R. Calendar. 2002. Construction, characterization, and use of two Listeria monocytogenes site-specific phage integration vectors. J. Bacteriol. 184:4177-4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lecuit, M., S. Dramsi, C. Gottardi, M. Fedor-Chaiken, B. Gumbiner, and P. Cossart. 1999. A single amino acid in E-cadherin responsible for host specificity towards the human pathogen Listeria monocytogenes. EMBO J. 18:3956-3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lecuit, M., S. Vandormael-Pournin, J. Lefort, M. Huerre, P. Gounon, C. Dupuy, C. Babinet, and P. Cossart. 2001. A transgenic model for listeriosis: role of internalin in crossing the intestinal barrier. Science 292:1722-1725. [DOI] [PubMed] [Google Scholar]
- 27.Leiser, R., and P. Kaufmann. 1994. Placental structure: in a comparative aspect. Exp. Clin. Endocrinol. 102:122-134. [DOI] [PubMed] [Google Scholar]
- 28.Librach, C. L., Z. Werb, M. L. Fitzgerald, K. Chiu, N. M. Corwin, R. A. Esteves, D. Grobelny, R. Galardy, C. H. Damsky, and S. J. Fisher. 1991. 92-kD type IV collagenase mediates invasion of human cytotrophoblasts. J. Cell Biol. 113:437-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Linnan, M. J., L. Mascola, X. D. Lou, V. Goulet, S. May, C. Salminen, D. W. Hird, M. L. Yonekura, P. Hayes, R. Weaver, et al. 1988. Epidemic listeriosis associated with Mexican-style cheese. N. Engl. J. Med. 319:823-828. [DOI] [PubMed] [Google Scholar]
- 30.Lorber, B. 1997. Listeriosis. Clin. Infect. Dis. 24:1-9. [DOI] [PubMed] [Google Scholar]
- 31.Low, J. C., and C. P. Renton. 1985. Septicaemia, encephalitis and abortions in a housed flock of sheep caused by Listeria monocytogenes type 1/2. Vet. Rec. 116:147-150. [DOI] [PubMed] [Google Scholar]
- 32.Maidji, E., E. Percivalle, G. Gerna, S. Fisher, and L. Pereira. 2002. Transmission of human cytomegalovirus from infected uterine microvascular endothelial cells to differentiating/invasive placental cytotrophoblasts. Virology 304:53-69. [DOI] [PubMed] [Google Scholar]
- 33.Medearis, D. N. 1964. Mouse cytomegalovirus infection. III. Attempts to produce intrauterine infections. Am. J. Hyg. 80:113-120. [PubMed] [Google Scholar]
- 34.Mellor, A. L., and D. H. Munn. 2000. Immunology at the maternal-fetal interface: lessons for T cell tolerance and suppression. Annu. Rev. Immunol. 18:367-391. [DOI] [PubMed] [Google Scholar]
- 35.Mengaud, J., H. Ohayon, P. Gounon, R. M. Mege, and P. Cossart. 1996. E-cadherin is the receptor for internalin, a surface protein required for entry of L. monocytogenes into epithelial cells. Cell 84:923-932. [DOI] [PubMed] [Google Scholar]
- 36.Mylonakis, E., M. Paliou, E. L. Hohmann, S. B. Calderwood, and E. J. Wing. 2002. Listeriosis during pregnancy: a case series and review of 222 cases. Medicine (Baltimore). 81:260-269. [DOI] [PubMed] [Google Scholar]
- 37.Newell, M. L., and C. Peckham. 1993. Risk factors for vertical transmission of HIV-1 and early markers of HIV-1 infection in children. AIDS 7(Suppl. 1):S91-S97. [PubMed] [Google Scholar]
- 38.Newell, M. L., and C. Peckham. 1994. Vertical transmission of HIV infection. Acta Paediatr. Suppl. 400:43-45. [DOI] [PubMed] [Google Scholar]
- 39.Notermans, S., J. Dufrenne, P. Teunis, and T. Chakraborty. 1998. Studies on the risk assessment of Listeria monocytogenes. J. Food Prot. 61:244-248. [DOI] [PubMed] [Google Scholar]
- 40.O'Donell, R. W., and G. L. Cockerell. 1981. Establishment and biological properties of a guinea pig colonic adenocarcinoma cell line induced by N-methyl-N-nitrosourea. Cancer Res. 41:2372-2377. [PubMed] [Google Scholar]
- 41.Pattillo, R. A. 1968. Human hormone production in vitro. Science 159:1467-1469. [DOI] [PubMed] [Google Scholar]
- 42.Portnoy, D. A., V. Auerbuch, and I. J. Glomski. 2002. The cell biology of Listeria monocytogenes infection: the intersection of bacterial pathogenesis and cell-mediated immunity. J. Cell Biol. 158:409-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Portnoy, D. A., P. S. Jacks, and D. J. Hinrichs. 1988. Role of hemolysin for the intracellular growth of Listeria monocytogenes. J. Exp. Med. 167:1459-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Redline, R. W., and C. Y. Lu. 1988. Specific defects in the anti-listerial immune response in discrete regions of the murine uterus and placenta account for susceptibility to infection. J. Immunol. 140:3947-3955. [PubMed] [Google Scholar]
- 45.Roth, I., D. B. Corry, R. M. Locksley, J. S. Abrams, M. J. Litton, and S. J. Fisher. 1996. Human placental cytotrophoblasts produce the immunosuppressive cytokine interleukin 10. J. Exp. Med. 184:539-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sacks, G., I. Sargent, and C. Redman. 1999. An innate view of human pregnancy. Immunol. Today 20:114-118. [DOI] [PubMed] [Google Scholar]
- 47.Schleiss, M. R. 2002. Animal models of congenital cytomegalovirus infection: an overview of progress in the characterization of guinea pig cytomegalovirus (GPCMV). J. Clin. Virol. 25:S37-S49. [DOI] [PubMed] [Google Scholar]
- 48.Shen, H., M. K. Slifka, M. Matloubian, E. R. Jensen, R. Ahmed, and J. F. Miller. 1995. Recombinant Listeria monocytogenes as a live vaccine vehicle for the induction of protective anti-viral cell-mediated immunity. Proc. Natl. Acad. Sci. USA 92:3987-3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shen, Y., M. Naujokas, M. Park, and K. Ireton. 2000. InIB-dependent internalization of Listeria is mediated by the Met receptor tyrosine kinase. Cell 103:501-510. [DOI] [PubMed] [Google Scholar]
- 50.Smith, K., and P. Youngman. 1992. Use of a new integrational vector to investigate compartment-specific expression of the Bacillus subtilis spoIIM gene. Biochimie 74:705-711. [DOI] [PubMed] [Google Scholar]
- 51.Stagno, S., R. F. Pass, G. Cloud, W. J. Britt, R. E. Henderson, P. D. Walton, D. A. Veren, F. Page, and C. A. Alford. 1986. Primary cytomegalovirus infection in pregnancy. Incidence, transmission to fetus, and clinical outcome. JAMA 256:1904-1908. [PubMed] [Google Scholar]
- 52.Stagno, S., R. F. Pass, M. E. Dworsky, R. E. Henderson, E. G. Moore, P. D. Walton, and C. A. Alford. 1982. Congenital cytomegalovirus infection: the relative importance of primary and recurrent maternal infection. N. Engl. J. Med. 306:945-949. [DOI] [PubMed] [Google Scholar]
- 53.Tappero, J. W., A. Schuchat, K. A. Deaver, L. Mascola, J. D. Wenger, et al. 1995. Reduction in the incidence of human listeriosis in the United States. Effectiveness of prevention efforts? JAMA 273:1118-1122. [DOI] [PubMed] [Google Scholar]
- 54.Teberg, A. J., M. L. Yonekura, C. Salminen, and Z. Pavlova. 1987. Clinical manifestations of epidemic neonatal listeriosis. Pediatr. Infect. Dis. J. 6:817-820. [DOI] [PubMed] [Google Scholar]
- 55.Tilney, L. G., and D. A. Portnoy. 1989. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J. Cell Biol. 109:1597-1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wiedmann, M., S. Mobini, J. R. Cole, Jr., C. K. Watson, G. T. Jeffers, and K. J. Boor. 1999. Molecular investigation of a listeriosis outbreak in goats caused by an unusual strain of Listeria monocytogenes. J. Am. Vet. Med. Assoc. 215:369-371. [PubMed] [Google Scholar]
- 57.Zinkernagel, R. M. 1974. Restriction by H-2 gene complex of transfer of cell-mediated immunity to Listeria monocytogenes. Nature 251:230-233. [DOI] [PubMed] [Google Scholar]