Abstract
Urinary levels of tissue inhibitor of metalloproteinase 1 (TIMP-1) higher than those of matrix metalloproteinase 9 (MMP-9) during acute pyelonephritis have previously been associated with a higher degree of acute inflammation and of postinfective renal scarring. The aim of the present study was to evaluate possible mechanisms by which TIMP-1 could affect the scarring process already during the acute phase of inflammation. The growth of Escherichia coli, bactericidal activity of fresh human blood, and respiratory burst, spontaneous apoptosis, and trans-basement membrane migration of normal human granulocytes were studied in vitro in the presence of different concentrations of recombinant human TIMP-1. To imitate the “normal” environment during inflammation in the kidney, granulocytes were also incubated with a conditioned medium from E. coli-stimulated renal epithelial cells. In order to compare our data with the in vivo situation, blood and urinary leukocyte levels were analyzed for 40 children with acute pyelonephritis, together with urinary MMP-9 and TIMP-1 levels. TIMP-1 at a concentration of 500 ng/ml increased the bactericidal activity of blood, increased the respiratory burst of granulocytes, decreased phosphatidylserine exposure and caspase 3 activity, which are features of spontaneous apoptosis, and inhibited granulocyte transmigration. Moreover, in the patients with pyelonephritis, MMP-9/TIMP-1 ratios in urine correlated with the degree of leukocyte transmigration. Thus, our data suggest that TIMP-1 specifically blocks the transmigration of granulocytes into urine. Entrapped and activated granulocytes, protected from apoptosis, might excessively destroy renal parenchyma and thus contribute to the pathogenesis of renal scarring following acute pyelonephritis.
The accumulation of granulocytes is an early event in the pathogenesis of bacterial infections. Pyelonephritis, or bacterial infection of the renal parenchyma, is caused in the majority of cases by bacteria ascending from the perineum, urethra, and urinary bladder (12). In response to bacteria, renal epithelial cells produce a number of cytokines (20) and chemokines (15). The chemokine concentration gradient results in an influx of granulocytes, which are activated by cytokines. To reach the bacteria, granulocytes need to traverse the endothelium, subendothelial basement membrane, epithelial cell basement membrane, and renal epithelial cells. Extensive data have been reported about the role of adhesion molecules in the process of granulocyte binding to and traversing the endothelium and renal epithelium (1, 9). Transmigration of professional immune cells across the basement membrane is thought to depend on specific degradation of membrane constituents. This is why matrix metalloproteinase 9 (MMP-9), a collagen IV-specific protease, and its main inhibitor, tissue inhibitor of metalloproteinase 1 (TIMP-1), have been implicated in this process. However, there are data both supporting (23, 29) and opposing (7) the hypothesis about the role of MMP-9 and TIMP-1 in trans-basement membrane migration of leukocytes. At the site of inflammation, activated granulocytes phagocytose and kill bacteria via production and secretion of enzymes and reactive oxygen and nitrogen molecules. After killing bacteria, granulocytes die by programmed cell death, or apoptosis (2). Local destruction of the tissue is a side effect of the normal inflammatory reaction and renal scarring might be a result of vast kidney destruction due to an excessive inflammatory response. Similarly, contraction and collapse of the tubulointerstitial parenchyma after direct inoculation of Escherichia coli into the kidneys of rats led to the development of postinfective renal fibrosis (16).
TIMP-1 has been suggested to be a mediator of renal fibrosis by inhibiting proteolytic activity and thereby promoting an accumulation of collagen in the kidney (19). In accordance with this, we have previously shown that children with urinary levels of TIMP-1 higher than those of MMP-9 during acute pyelonephritis had a higher degree of acute inflammation, as measured by renal static scintigraphy, and more severe changes on scans performed after 1 year, indicating renal scarring (4).
An increase in collagen content seems to take place mainly later during inflammation, not during the acute phase (16). Therefore, the aim of the present study was to elucidate other potential mechanisms by which TIMP-1 affects the process of postinfective renal fibrosis during the acute phase of inflammation. The in vitro effect of different concentrations of TIMP-1 on bacterial growth and on the respiratory burst, apoptosis, and transmigration of normal human granulocytes was studied. Clinical data from children with pyelonephritis were used to compare our transmigration results with the in vivo situation.
MATERIALS AND METHODS
Patients.
This study has been approved by the ethics committee of the Karolinska Hospital, and informed consent was obtained from parents of the children. Urine and blood were collected from 40 infants and children with acute first-time pyelonephritis without symptoms or signs of any other infection (4). The number of leukocytes in the blood was measured by use of a hematologic counter, and leukocyturia was tested semiquantitatively, with Ecur-Test (Boehringer Mannheim, Mannheim, Germany), and recorded as grades 0 to 3, corresponding to 0, 10 to 25, 75, and 500 × 106 leukocytes/ml. The urine/blood leukocyte ratio was calculated.
99mTc-dimercaptosuccinic acid scintigraphy (DMSA) was performed within 10 days after admission and 1 year after the acute infection. From the size and degree of a defect in DMSA uptake, a DMSA score was calculated (32).
Isolation of normal human granulocytes.
The use of normal human granulocytes for this study has been approved by the ethics committee of the Karolinska Hospital, and informed consent was obtained from healthy volunteers. Blood was collected in 10-ml citrate tubes (Vacutainer; Becton Dickinson, San Jose, Calif.) and purified by Percoll (Amersham Pharmacia Biotech, Uppsala, Sweden) centrifugation. The erythrocytes in the granulocyte pellet were hemolysed with 4°C isotonic NH4Cl-EDTA lysing solution (154 mM NH4Cl, 10 mM KHCO3, 0.1 mM EDTA, pH 7.2) and incubated for 5 min at 15°C. Cells were washed with phosphate-buffered saline (PBS) before being counted in an EPICS XL flow cytometer (Beckman Coulter, Inc., Fullerton, Calif.). Both flow cytometric characteristics, forward scatter and side scatter, and the morphology of cells after May-Grünwald-Giemsa staining showed that >95% of isolated cells were granulocytes.
Human renal epithelial cells.
Human renal epithelial cells (A498) (American Type Culture Collection, Rockville, Md.) were grown in Eagle's minimal essential medium (Sigma, St. Louis, Mo.) supplemented with 200 mM glutamine (Sigma), 1.0 mM sodium pyruvate (Invitrogen Life Technologies), 0.1 mM nonessential amino acids (Life Technologies), and 10% fetal bovine serum (Life Technologies).
Bacteria.
E. coli CFT O73 was isolated from a patient with pyelonephritis. It expresses type 1, P, and S fimbriae and hemolysin and induces pyelonephritis in experimentally challenged normal mice (20). The bacteria were grown overnight on cystine-lactose electrolyte-deficient agar at 37°C and in Luria-Bertani (LB) broth for 5 h, to reach the logarithmic phase of growth, and then were washed twice in PBS. The bacterial concentration was measured by spectrophotometry at a wavelength of 600 nm and confirmed by viable counting on blood agar plates.
Conditioned medium.
Human renal epithelial cells have been shown to produce a number of chemokines (15) and cytokines (3, 13) after stimulation with bacteria. To imitate the “normal” environment during inflammation and to stimulate the migration of granulocytes, a conditioned medium from E. coli-stimulated renal epithelial cells was prepared. Human renal epithelial cells (A498) were used when they formed a confluent layer in 75-cm2 culture flasks. The experiments were performed with 0.5 ml of serum-free medium per cm2 of growth area. The medium was supplemented with 40 μg of gentamicin per ml to suppress the growth of bacteria and thus protect the epithelial cells during stimulation. Bacteria (108) were added to 1 ml of medium. With these concentrations of gentamicin and bacteria, we and others were able to maintain the viability of bacteria during the experiment (13, 15). After incubation at 37°C in 5% CO2-95% O2 with 80% humidity for 24 h, the medium was gradually centrifuged (350 × g for 10 min followed by 2,500 × g for 10 min) to get rid of cells, larger particles, and bacteria, and supernatants were frozen at −20°C until they were used.
Measurement of MMP-9 and TIMP-1 levels.
Before enzyme-linked immunosorbent assay analysis, urine was centrifuged at 350 × g for 10 min to remove cells and larger particles. Enzyme-linked immunosorbent assay kits for total human MMP-9 and TIMP-1 were obtained from R&D Systems (Abingdon, United Kingdom). MMP-9 and TIMP-1 levels were determined according to the manufacturer's instructions. The detection limits were 0.36 ng/ml for MMP-9 and 0.12 ng/ml for TIMP-1. The urinary levels of creatinine were analyzed by Jaffé's method. Urinary levels of measured proteins were expressed as protein/creatinine ratios. MMP-9/TIMP-1 ratios were calculated.
TIMP-1.
The 184-amino-acid recombinant human TIMP-1 (R&D Systems) was used for all of our experiments.
Bacterial growth in blood and LB broth.
Bacterial growth in fresh blood, or the Lancefield assay (22), was used to assess the effect of TIMP-1 on the bactericidal activity of blood. A solution from overnight culture of E. coli CFT O73 on cystine-lactose electrolyte-deficient agar and a 5-h culture in LB broth was diluted in PBS to a concentration of 40,000 CFU/ml. Freshly drawn human blood (250 μl) in citrate was mixed with 25 μl of the bacterial suspension together with different concentrations of TIMP-1. Under similar conditions, the effect of TIMP-1 on the growth of bacteria was evaluated by use of 250 μl of LB broth with 25 μl of the same bacterial suspension. In both cases, the tubes were slowly rotated at 37°C for 3 h, and the number of bacteria was analyzed by plating serial dilutions of samples on blood agar plates.
Measurement of the respiratory burst of granulocytes.
Peripheral blood from healthy volunteers was collected in citrate tubes (Vacutainer). One hundred microliters of whole blood was hemolysed with 2 ml of 4°C isotonic NH4Cl-EDTA lysing solution and incubated for 5 min at 15°C. After centrifugation at 300 × g for 6 min at 4°C, the supernatant was aspirated and the leukocyte suspension was washed twice in PBS. Leukocyte pellets were incubated for 15 min at 37°C with 5 μM dichlorofluorescein diacetate (DCFH) (Eastman Kodak Company, Rochester, N.Y.) in PBS-glucose. DCFH enters the cells, where it is trapped after deacetylation by intracellular esterases. For evaluation of the effect of TIMP-1 on the metabolic activation of granulocytes, the cells were incubated for 30 min with PBS-glucose or conditioned medium, together with 20, 100, or 500 ng of TIMP-1 per ml. DCFH is oxidized by hydrogen peroxide to highly fluorescent 2′,7′-dichlorofluorescein. After stoppage of activation by the addition of ice-cold PBS, the cells were analyzed in the FL-1 channel of a flow cytometer. The median fluorescence intensity of a cell was used as a measure of hydrogen peroxide production and thus respiratory burst of granulocytes. The fluorescence increase (percentage) of samples stimulated with conditioned medium and/or TIMP-1 versus nonstimulated (negative-control) samples was calculated.
Spontaneous apoptosis of granulocytes.
Granulocytes, isolated as described above, were incubated in RPMI 1640 (Sigma) with 4% human serum albumin (Pharmacia AB, Stockholm, Sweden) and 40 μg of gentamicin per ml at 37°C in 5% CO2-95% O2 with 80% humidity. Alternatively, conditioned medium, also supplemented with 4% human serum albumin and 40 μg of gentamicin per ml, was used. Human recombinant TIMP-1 at concentrations of 20, 100, and 500 ng/ml was added to the cell suspension in both RPMI and conditioned media. After 24 h, cells were analyzed for morphology, phosphatidylserine exposure, and caspase 3 activity.
Morphological analysis of granulocytes.
Slides for morphological analysis were prepared by cytocentrifugation at 50 × g for 3 min in a Cytospin 3 cytocentrifuge (Shandon Scientific Ltd., Astmoor, United Kingdom). The cells were stained with May-Grünwald-Giemsa stain and the morphology was analyzed by light microscopy.
Apoptosis assays (i) AV-PI staining.
Granulocytes were analyzed for phosphatidylserine exposure by annexin V (AV) staining and for necrosis by propidium iodide (PI) staining. Double staining with AV and PI allows us to distinguish early apoptotic (AV+ PI−), late apoptotic-necrotic (AV+ PI+), and necrotic (AV− PI+) cells. A minimum of 100,000 granulocytes were incubated for 10 min on ice with cold AV-binding buffer, PI, and AV (Beckman Coulter, Inc., Fullerton, Calif.) according to the manufacturer's instructions. The cells were then analyzed in the FL-1 and FL-3 channels of a flow cytometer. An unstained sample corresponding to each experiment (stimulated cells) was used to set the background staining. The stained population was then divided into four populations according to AV and PI positivity and negativity and the proportions for the entire cell population were calculated.
(ii) Caspase 3 activity measurement.
Caspase 3 activity in granulocytes was measured by the cell permeative substrate PhiPhiLux (OncoImmunin, Inc., Gaithersburg, Md.). A minimum of 100,000 cells were incubated with PhiPhiLux (10 μM) and 4% human serum albumin at 37°C for 1 h according to the manufacturer's instructions. Fluorescence was measured in the FL-2 channel of a flow cytometer. Median fluorescence intensity was used as a measure of caspase 3 activity in a cell, and the decrease in intensity (percentage) versus that of the control was calculated.
Transmigration assay.
The migration of granulocytes was investigated by use of BD BioCoat (BD Biosciences, Bedford, Mass.) cell culture inserts, with a membrane pore size of 3 μm, coated with type IV collagen. Alternatively, inserts with a pore size of 3 μm were used after coating with Matrigel (BD Biosciences), diluted 1:3 in PBS, at a concentration of 15 μl/0.3 cm2. Matrigel is a solubilized basement membrane preparation containing laminin, collagen IV, heparan sulfate proteoglycans, entactin, and nidogen. For induction of the migration of granulocytes, 500 μl of conditioned medium from human renal epithelial cells stimulated with E. coli CFT O73 was added to the lower chamber of a 24-well plate. The medium was enriched with 4% human serum albumin. RPMI 1640 (Sigma) with 4% albumin served as a negative control. Subsequently, 500 μl of cell suspension of 2 × 106 granulocytes/ml of RPMI 1640 with 4% albumin was added to the upper chamber. For assessment of the effect of TIMP-1 on the transmigration of granulocytes, 20, 100, or 500 ng of TIMP-1 per ml was added to the cell suspension in the upper chamber. The plates were incubated at 37°C in 5% CO2-95% O2 with 80% humidity. After 0, 4, and 24 h, the remaining cells in the upper chamber were aspirated and the upper sides of the membranes were wiped with cotton swabs. The transmigrated cells were harvested from the lower chamber. In order to remove adherent granulocytes, 500 μl of ice-cold 2% EDTA in PBS was placed into the wells and shaken for 10 min. The number of transmigrated granulocytes was measured by flow cytometry and expressed as a percentage of the cell number added to the upper chamber at the beginning of the experiment. The viability and apoptosis of cells during the transmigration assay were determined by the AV-PI staining method followed by flow cytometry analysis.
Data analysis.
The data are presented as medians (columns in figures) and ranges (bars in figures), excluding outliers, for at least three experiments. Outliers were not excluded from statistical analysis. Each set of experiments using granulocytes or blood was repeated with cells from at least three donors. Kruskal-Wallis analysis of variance and Spearman's rank correlation test were used for statistical analysis.
RESULTS
TIMP-1 increases bactericidal activity of normal blood but does not influence the growth of bacteria.
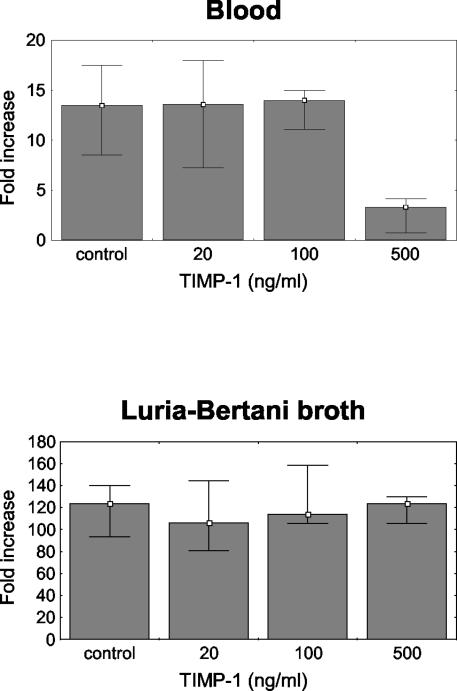
The growth of E. coli CFT O73 was suppressed in normal fresh human blood compared to growth in LB broth (124-fold increase in LB broth [range, 94- to 140-fold]; 14-fold increase in blood [range, 9- to 18-fold]; P < 0.05). TIMP-1 did not significantly influence the growth of E. coli in LB broth at any of the concentrations used (20, 100, and 500 ng/ml). On the other hand, at a concentration of 500 ng/ml, TIMP-1 significantly decreased the growth of E. coli in blood (threefold increase; range, one- to fourfold; P < 0.05), indicating an increase in the bactericidal activity of blood (Fig. 1).
FIG. 1.
Growth of E. coli in fresh human blood (Lancefield assay) (top) and in LB broth (bottom). Growth is expressed as the fold increase in bacterial number compared to the number at 0 h. At a concentration of 500 ng/ml, TIMP-1 significantly inhibited the growth of E. coli in blood (P < 0.05), indicating a stimulating effect on bactericidal activity. TIMP-1 did not significantly influence the growth of E. coli in LB broth at any of the concentrations used. Data are presented as medians and ranges, excluding outliers, from four experiments.
TIMP-1 activates normal human granulocytes.
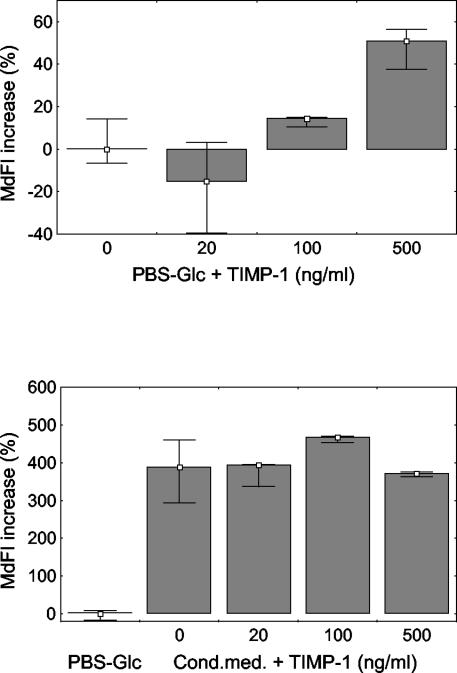
The respiratory burst of granulocytes was studied by measuring the production of hydrogen peroxide. TIMP-1 at a concentration of 500 ng/ml significantly increased the hydrogen peroxide production by granulocytes versus that by control cells (median fluorescence intensity increase of 51%, with a range of 38 to 57%; P < 0.05). When conditioned medium without TIMP-1 was added, the respiratory burst increased. No additive effect was observed by further addition of TIMP-1 (Fig. 2).
FIG. 2.
Respiratory burst of normal human granulocytes in PBS-glucose (PBS-Glc) and conditioned medium (Cond.med.). Median fluorescence intensity (MdFI) was used as a measure of the respiratory burst of a cell. The median respiratory burst of a control group of samples served as a reference value, and percentages of increase versus this value were calculated. TIMP-1 at a concentration of 500 ng/ml significantly increased the metabolic activity of granulocytes (P < 0.05). The addition of conditioned medium significantly increased the metabolic activity of the cells (P < 0.05). Further addition of TIMP-1 did not have a significant additive effect. Data are presented as medians and ranges, excluding outliers, from four experiments.
TIMP-1 protects normal human granulocytes from spontaneous apoptosis.
To study the effect of TIMP-1 on spontaneous apoptosis, we incubated normal human granulocytes with RPMI medium supplemented with 4% human serum albumin or with conditioned medium, together with 20, 100, and 500 ng of TIMP-1 per ml. Several features of apoptosis were studied, namely morphological changes, phosphatidylserine exposure, and caspase 3 activity.
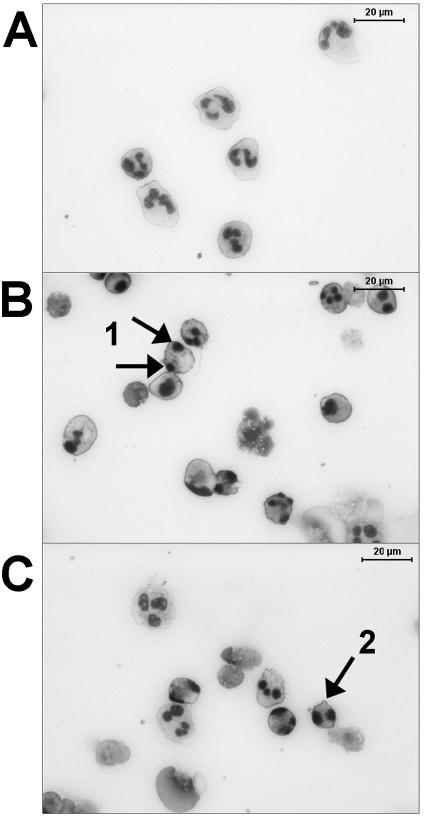
We were able to recover 19% (range, 11 to 31%) of the cells incubated for 24 h in RPMI and 22% (range, 20 to 35%) of the cells incubated in RPMI with 500 ng of TIMP-1 per ml. The cell recovery rate from the conditioned medium was 58% (range, 41 to 92%) without TIMP-1 and 62% (range, 42 to 86%) after the addition of 500 ng of TIMP-1 per ml. Although the cell recovery rate was significantly higher in cells incubated in conditioned medium than in those incubated in RPMI (P < 0.05), there was no difference between cells that were treated or not treated with TIMP-1, either in RPMI or conditioned medium. After 24 h, we observed typical apoptotic features on granulocytes, such as nuclear compaction and segregation, condensation of cytoplasm, nuclear fragmentation, and cytoplasmic blebbing (Fig. 3).
FIG. 3.
Morphology of normal human granulocytes stained with May-Grünwald-Giemsa stain immediately after isolation (A) and after 24 h in RPMI with 4% human serum albumin (B) and together with 500 ng of TIMP-1 per ml (C). After 24 h, typical apoptotic features, such as nuclear compaction and segregation (arrows 1), condensation of cytoplasm, nuclear fragmentation (arrows 1), and cytoplasmic blebbing (arrow 2), were seen. Defects in the cytoplasmic membrane and the presence of “cell shadows,” a consequence of cell lysis, indicated cell necrosis.
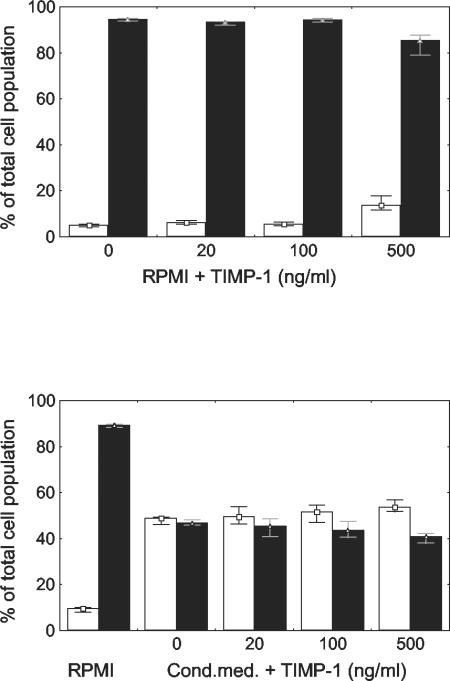
To quantify the apoptotic changes, we measured phosphatidylserine exposure in the outer leaflet of the cytoplasmic membrane by AV-PI staining. After 24 h, only 5% (range, 4 to 5%) of the cells were both AV and PI negative and 95% (range, 94 to 95%) were AV positive, indicating a high level of spontaneous apoptosis. In the cells treated with 500 ng of TIMP-1 per ml, 14% of the cells (range, 12 to 18%) were both AV and PI negative and 85% (range, 79 to 88%) were AV positive, which was a significant difference versus the cells without TIMP-1 treatment (P < 0.05) (Fig. 4, top).
FIG. 4.
Phosphatidylserine exposure in the outer leaflet of the cytoplasmic membrane of granulocytes after 24 h in RPMI with 4% human serum albumin and in conditioned medium (Cond.med.), as measured by AV staining. Empty bars indicate the percentages of cells that were both AV and PI negative and nonapoptotic, and filled bars indicate the percentages of apoptotic, AV-positive cells, both PI positive and negative. TIMP-1 at a concentration of 500 ng/ml decreased the number of AV-positive cells (P < 0.05). In the presence of conditioned medium, the number of AV-positive cells decreased (P < 0.05). The addition of TIMP-1 further decreased the degree of phosphatidylserine exposure, with a statistically significant effect at a concentration of 500 ng/ml (P < 0.05). Data are presented as medians and ranges, excluding outliers, from four experiments.
The degree of apoptosis had decreased already in the presence of the conditioned medium without TIMP-1 (Fig. 4, bottom). For conditioned medium, we found that 49% (range, 46 to 49%) of the cells were both AV and PI negative and 47% (range, 46 to 48%) were AV positive. The addition of TIMP-1 further decreased the degree of phosphatidylserine exposure, with a statistically significant effect at a concentration of 500 ng/ml, in which 54% of cells were both AV and PI negative (range, 52 to 57%) and 41% of cells were AV positive (range, 38 to 42%) (P < 0.05) (Fig. 4).
The caspase 3 activity of granulocytes, as measured by median fluorescence intensity of a cell, was also assessed. At a concentration of 500 ng/ml, TIMP-1 reduced caspase 3 activity 13% (range, 12 to 15%) in RPMI and 7% (range, 6 to 7%) in conditioned medium (P < 0.05).
TIMP-1 inhibits the transmigration of normal human granulocytes.
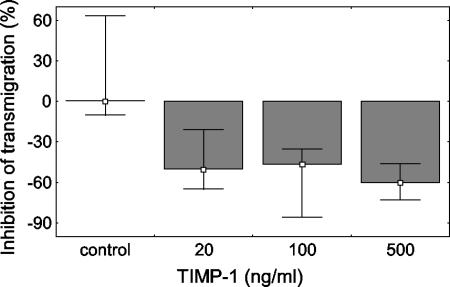
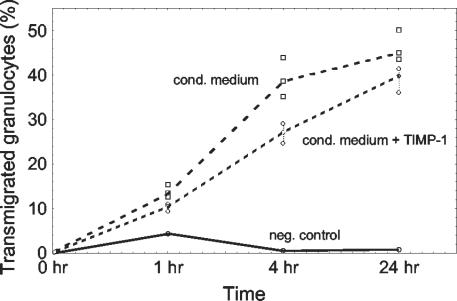
In the in vitro transwell model, TIMP-1 significantly inhibited the transmigration of granulocytes at 4 h by 50% at a concentration of 20 ng/ml, by 46% at a concentration of 100 ng/ml, and by 60% at a concentration of 500 ng/ml, with ranges of 21 to 65%, 35 to 86%, and 46 to 73%, respectively (P < 0.05) (Fig. 5). However, we did not find any statistically significant difference between the effects of the different concentrations of TIMP-1 used. Furthermore, we studied the effect of TIMP-1 on granulocyte transmigration at 1, 4, and 24 h. TIMP-1 (100 ng/ml) inhibited the transmigration of granulocytes at both 4 and 24 h (P < 0.05), with the highest observed difference at 4 h (Fig. 6). Similar results were obtained with membranes that were precoated with either collagen IV or Matrigel.
FIG. 5.
Percentages of transmigrated granulocytes after 4 h of attraction by a conditioned medium in an in vitro transwell model. The number of transmigrated granulocytes is expressed as a percentage of the cell number added to the upper chamber at the beginning of the experiment. The transmigration of granulocytes was significantly inhibited by 20, 100, and 500 ng of TIMP-1 per ml (P < 0.05). There was no statistical difference between the effects of the different TIMP-1 concentrations used. Data are presented as medians and ranges, excluding outliers, from three experiments.
FIG. 6.
Time response of TIMP-1 effect on granulocyte transmigration. TIMP-1 (100 ng/ml) significantly inhibited the transmigration of granulocytes at both 4 and 24 h, with the largest observed difference at 4 h (P < 0.05). The negative control shows the number of transmigrated granulocytes stimulated with RPMI and human serum albumin. Data are presented as medians and ranges, excluding outliers, from three experiments.
MMP-9/TIMP-1 urinary ratios correlate with the degree of leukocyte transmigration during acute pyelonephritis.
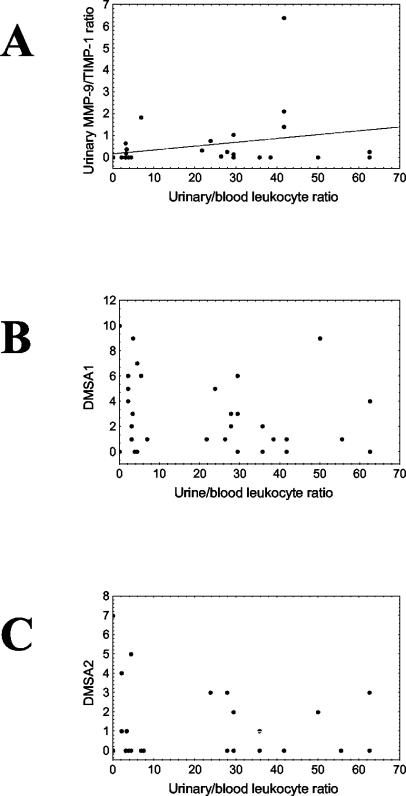
To compare our results with the in vivo situation, we examined urinary and blood leukocyte levels for 40 children with pyelonephritis, together with urinary MMP-9 and TIMP-1 levels. The median blood leukocyte level was 17 × 106/ml, with a range of 8 × 106 to 25 × 106/ml of blood, and the median leukocyturia was of level 3, which corresponds to 500 × 106 cells/ml of urine. We calculated urinary leukocyte/blood leukocyte ratios as a measurement of urine-blood leukocyte transmigration (median, 22 [range, 0 to 63]) and urinary MMP-9/TIMP-1 ratios (median, 0 [range, 0 to 6]) as a possible measurement of the ability of leukocytes to digest collagen IV. We found a correlation between urinary leukocyte/blood leukocyte ratios and urinary MMP-9/TIMP-1 ratios (R = 0.40; P < 0.05). For the group of children studied, there was a reverse tendency for correlation between urinary leukocyte/blood leukocyte ratios and both acute and follow-up DMSA scores (Fig. 7).
FIG. 7.
Correlation between urinary leukocyte/blood leukocyte ratios (x axis) and MMP-9/TIMP-1 ratios (A), DMSA scores during the acute phase of inflammation (B), and DMSA scores 1 year after infection (C). We found a correlation between urinary leukocyte/blood leukocyte ratios and urinary MMP-9/TIMP-1 ratios (R = 0.40; P < 0.05). For the group of 40 children studied, there was a reverse tendency for a correlation between urinary leukocyte/blood leukocyte ratios and both acute and follow-up DMSA scores.
DISCUSSION
In the present study, we show that TIMP-1 activates normal human granulocytes, protects them from apoptosis, and inhibits their transmigration through basement membranes.
TIMP-1 is a well-known profibrotic agent that is important in the development of scarring in different organs. Accordingly, many authors have proposed a link between transforming growth factor beta 1 and TIMP-1 in the pathogenesis of renal fibrosis (6). An inhibition of proteolysis in the tissue, which might promote an accumulation of collagen, has been suggested as the most probable mechanism of the profibrotic action of TIMP-1 (19). In line with these findings, we have previously shown that children with higher urinary TIMP-1 than MMP-9 levels had significantly more severe changes in both acute and follow-up DMSA scans, indicating a higher degree of acute inflammation and also a higher degree of postinfective renal fibrosis (4). Since the accumulation of collagen does not seem to take place during the acute phase of inflammation (16), we sought to identify additional mechanisms of action of TIMP-1.
We studied three different concentrations of TIMP-1, namely 20, 100, and 500 ng/ml, which correspond to the highest urinary levels during pyelonephritis (20 ng/ml) (4) and normal serum levels (approximately 100 ng/ml) (17). Tissue levels of TIMP-1 during bacterial infections have not been determined yet, but from other types of inflammation (14) we can estimate them to be around 500 ng/ml or higher.
TIMP-1 at a concentration of 500 ng/ml stimulated the bactericidal activity of fresh human blood. One of the potential mechanisms involved in the process of bacterial killing is the oxidative burst of granulocytes, with the production of hydrogen peroxide. This measure is of particular importance during the acute inflammation and scarring process because of the bactericidal and cytotoxic properties of hydrogen peroxide (31). We have shown that TIMP-1 at a concentration of 500 ng/ml significantly activates normal human granulocytes. The addition of conditioned medium, containing a mixture of different inflammatory products of renal epithelial cells, to the granulocyte suspension also strongly increased the respiratory burst. The addition of TIMP-1 to conditioned medium did not have any further influence. The cells might already have been stimulated to their upper limit with conditioned medium alone, with no capacity left for a further increase with additional TIMP-1. However, we cannot rule out that the result might depend on a dual action of TIMP-1 in conditioned medium. TIMP-1 might activate cells directly, as was shown in PBS-glucose. At the same time, TIMP-1, by inhibiting MMP-9, might modulate the activity of another signal that is present in conditioned medium. Accordingly, some cytokines and chemokines, such as interleukin 1β (IL-1β), tumor necrosis factor alpha, and IL-8, are known to be degraded or activated by MMP-9 (28).
The majority of TIMP-1 effects, such as inhibition of cell invasion, remodeling of tissues (28), ovulation, pregnancy, and parturition (35), are considered to be determined by the ability of TIMP-1 to inhibit MMPs by forming specific complexes. However, TIMP-1 has been shown to exhibit a variety of unexpected cellular functions which are independent of MMP inhibitory activity, such as cell growth-promoting activity (33), gonadal steroidogenesis (30), and modulation of apoptosis (27). Our findings broaden the spectrum of the effects of TIMP-1 with granulocyte-activating properties. To our knowledge, this is the first evidence of cytokine-like properties of TIMP-1 during an acute inflammation.
Changes in the apoptotic process during inflammation have been suggested as a mechanism for the pathogenesis of different diseases (26). In the present study, we evaluated morphological changes, phosphatidylserine exposure, and caspase 3 activity of normal human granulocytes as features of their spontaneous apoptosis. Although the differences were not large, our results imply that TIMP-1 at a concentration of 500 ng/ml might protect granulocytes from apoptosis in both RPMI and conditioned medium. Previously, TIMPs have been shown to influence the cell proliferation-apoptosis balance of different cells (27). TIMP-1 suppressed apoptosis in rat mesangial cells (25), mouse hepatic stellate cells (34), human breast epithelial cells (24), B cells (10), and erythroid cells (21). Our data are in line with these findings and suggest that TIMP-1 might also have a similar effect on normal human granulocytes.
Granulocyte migration is a crucial step during inflammation. Failure of migration of granulocytes can cause their entrapment in the renal tissue, which has been suggested as a mechanism initiating a process of excessive tissue damage and scarring (8, 11). In our study, TIMP-1, irrespective of the concentration used, inhibited the transmigration of normal human granulocytes through a membrane that was precoated either with colllagen IV or with a mixture of different extracellular matrix proteins (Matrigel). The largest effect on transmigration was observed at 4 h. At 24 h, the number of transmigrated granulocytes was also influenced by a high degree of apoptosis, and therefore the difference between the measured groups was smaller than at 4 h.
Since collagen IV is one of the most important basement membrane constituents, MMP-9, a collagen IV-specific protease, and its main inhibitor, TIMP-1, have been proposed to be involved in the process of trans-basement membrane migration of different cells, and several studies have confirmed this hypothesis (5, 23, 29). However, conflicting data have been obtained, questioning the role of MMP-9 and TIMP-1 in the process (7, 18). Various chemoattractants (IL-8, N-formyl-methionyl-leucyl-phenylalanine) have been used to induce the migration of cells (7), and this fact could be one of the reasons for the different results obtained. Our result with conditioned medium, however, supports the hypothesis about the role of TIMP-1 in granulocyte migration through basement membranes.
To compare in vivo data with the situation during acute pyelonephritis, we calculated urinary leukocyte/blood leukocyte ratios and urinary MMP-9/TIMP-1 ratios as measurements of leukocyte transmigration into the urine and leukocyte proteolytic activity on collagen IV. A positive correlation supported the data from in vivo experiments. Interestingly, for our group of children, there was a reverse tendency for correlation between urinary leukocyte/blood leukocyte ratios and both acute and follow-up DMSA scores, suggesting the significance of tissue but not urinary levels of leukocytes for both acute pyelonephritic changes and the development of renal scarring.
We conclude that TIMP-1 activates normal human granulocytes. Moreover, TIMP-1 prolongs the life span of granulocytes in the tissue by specific blockage of their transmigration through basement membranes into the urine and by hindering their spontaneous apoptosis. Entrapped activated granulocytes might excessively destroy renal parenchyma and thereby contribute to the pathogenesis of renal scarring following acute pyelonephritis.
Acknowledgments
This work was supported by funds from the Karolinska Institute, Magnus Bergvalls Foundation, and Stiftelsen Frimurare Barnhuset. Milan Chromek received financial support from the Swedish Institute.
We thank Ali Moshfegh, Elham Dadfar, Anna Nopp, and Denis Stygar for advice concerning laboratory techniques.
Editor: F. C. Fang
REFERENCES
- 1.Agace, W. W., M. Patarroyo, M. Svensson, E. Carlemalm, and C. Svanborg. 1995. Escherichia coli induces transuroepithelial neutrophil migration by an intercellular adhesion molecule 1-dependent mechanism. Infect. Immun. 63:4054-4062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akgul, C., D. A. Moulding, and S. W. Edwards. 2001. Molecular control of neutrophil apoptosis. FEBS Lett. 487:318-322. [DOI] [PubMed] [Google Scholar]
- 3.Brauner, A., M. Soderhall, S. H. Jacobson, J. Lundahl, U. Andersson, and J. Andersson. 2001. Escherichia coli-induced expression of IL-1 alpha, IL-1 beta, IL-6 and IL-8 in normal human renal tubular epithelial cells. Clin. Exp. Immunol. 124:423-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chromek, M., K. Tullus, O. Hertting, G. Jaremko, A. Khalil, Y. H. Li, and A. Brauner. 2003. Matrix metalloproteinase-9 and tissue inhibitor of metalloproteinases-1 in acute pyelonephritis and renal scarring. Pediatr. Res. 53:698-705. [DOI] [PubMed] [Google Scholar]
- 5.Delclaux, C., C. Delacourt, M. P. D'Ortho, V. Boyer, C. Lafuma, and A. Harf. 1996. Role of gelatinase B and elastase in human polymorphonuclear neutrophil migration across basement membrane. Am. J. Respir. Cell Mol. Biol. 14:288-295. [DOI] [PubMed] [Google Scholar]
- 6.Eddy, A. 2001. Role of cellular infiltrates in response to proteinuria. Am. J. Kidney Dis. 37:S25-S29. [DOI] [PubMed] [Google Scholar]
- 7.Felkel, C., U. Scholl, M. Mader, P. Schwartz, K. Felgenhauer, R. Hardeland, W. Beuche, and F. Weber. 2001. Migration of human granulocytes through reconstituted basement membrane is not dependent on matrix metalloproteinase-9 (MMP-9). J. Neuroimmunol. 116:49-55. [DOI] [PubMed] [Google Scholar]
- 8.Frendeus, B., G. Godaly, L. Hang, D. Karpman, A. C. Lundstedt, and C. Svanborg. 2000. Interleukin 8 receptor deficiency confers susceptibility to acute experimental pyelonephritis and may have a human counterpart. J. Exp. Med. 192:881-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Godaly, G., L. Hang, B. Frendeus, and C. Svanborg. 2000. Transepithelial neutrophil migration is CXCR1 dependent in vitro and is defective in IL-8 receptor knockout mice. J. Immunol. 165:5287-5294. [DOI] [PubMed] [Google Scholar]
- 10.Guedez, L., W. G. Stetler-Stevenson, L. Wolff, J. Wang, P. Fukushima, A. Mansoor, and M. Stetler-Stevenson. 1998. In vitro suppression of programmed cell death of B cells by tissue inhibitor of metalloproteinases-1. J. Clin. Investig. 102:2002-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hang, L., B. Frendeus, G. Godaly, and C. Svanborg. 2000. Interleukin-8 receptor knockout mice have subepithelial neutrophil entrapment and renal scarring following acute pyelonephritis. J. Infect. Dis. 182:1738-1748. [DOI] [PubMed] [Google Scholar]
- 12.Hansson, S., and U. Jodal. 1999. Urinary tract infection, p. 835-850. In T. M. Barratt, E. D. Avner, and W. E. Harmon (ed.), Pediatric nephrology. Lippincott Williams & Wilkins, Baltimore, Md.
- 13.Hedges, S. R., M. Bjarnadottir, W. Agace, L. Hang, and C. Svanborg. 1996. Immunoregulatory cytokines modify Escherichia coli induced uroepithelial cell IL-6 and IL-8 responses. Cytokine 8:686-697. [DOI] [PubMed] [Google Scholar]
- 14.Hegemann, N., B. Kohn, L. Brunnberg, and M. F. Schmidt. 2002. Biomarkers of joint tissue metabolism in canine osteoarthritic and arthritic joint disorders. Osteoarthritis Cartilage 10:714-721. [DOI] [PubMed] [Google Scholar]
- 15.Hertting, O., A. Khalil, G. Jaremko, M. Chromek, Y. H. Li, M. Bakhiet, T. Bartfai, K. Tullus, and A. Brauner. 2003. Enhanced chemokine response in experimental acute Escherichia coli pyelonephritis in IL-1beta-deficient mice. Clin. Exp. Immunol. 131:225-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hewitson, T. D., I. A. Darby, T. Bisucci, C. L. Jones, and G. J. Becker. 1998. Evolution of tubulointerstitial fibrosis in experimental renal infection and scarring. J. Am. Soc. Nephrol. 9:632-642. [DOI] [PubMed] [Google Scholar]
- 17.Holten-Andersen, M. N., I. J. Christensen, H. J. Nielsen, H. Lilja, G. Murphy, V. Jensen, N. Brunner, and T. Piironen. 2002. Measurement of the noncomplexed free fraction of tissue inhibitor of metalloproteinases 1 in plasma by immunoassay. Clin. Chem. 48:1305-1313. [PubMed] [Google Scholar]
- 18.Huber, A. R., and S. J. Weiss. 1989. Disruption of the subendothelial basement membrane during neutrophil diapedesis in an in vitro construct of a blood vessel wall. J. Clin. Investig. 83:1122-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson, T. S., J. L. Haylor, G. L. Thomas, M. Fisher, and A. M. El Nahas. 2002. Matrix metalloproteinases and their inhibitions in experimental renal scarring. Exp. Nephrol. 10:182-195. [DOI] [PubMed] [Google Scholar]
- 20.Khalil, A., A. Brauner, M. Bakhiet, L. G. Burman, G. Jaremko, B. Wretlind, and K. Tullus. 1997. Cytokine gene expression during experimental Escherichia coli pyelonephritis in mice. J. Urol. 158:1576-1580. [PubMed] [Google Scholar]
- 21.Lambert, E., C. Boudot, Z. Kadri, M. Soula-Rothhut, M. L. Sowa, P. Mayeux, W. Hornebeck, B. Haye, and E. Petitfrere. 2003. Tissue inhibitor of metalloproteinases-1 signalling pathway leading to erythroid cell survival. Biochem. J. 372:767-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lancefield, R. C. 1957. Differentiation of group A streptococci with a common R antigen into three serological types, with special reference to the bactericidal test. J. Exp. Med. 106:525-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leppert, D., E. Waubant, R. Galardy, N. W. Bunnett, and S. L. Hauser. 1995. T cell gelatinases mediate basement membrane transmigration in vitro. J. Immunol. 154:4379-4389. [PubMed] [Google Scholar]
- 24.Li, G., R. Fridman, and H. R. Kim. 1999. Tissue inhibitor of metalloproteinase-1 inhibits apoptosis of human breast epithelial cells. Cancer Res. 59:6267-6275. [PubMed] [Google Scholar]
- 25.Lin, H., X. Chen, J. Wang, and Z. Yu. 2002. Inhibition of apoptosis in rat mesangial cells by tissue inhibitor of metalloproteinase-1. Kidney Int. 62:60-69. [DOI] [PubMed] [Google Scholar]
- 26.Mak, T. W., and W. C. Yeh. 2002. Signaling for survival and apoptosis in the immune system. Arthritis Res. 4:S243-S252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mannello, F., and G. Gazzanelli. 2001. Tissue inhibitors of metalloproteinases and programmed cell death: conundrums, controversies and potential implications. Apoptosis 6:479-482. [DOI] [PubMed] [Google Scholar]
- 28.Opdenakker, G., P. E. Van den Steen, B. Dubois, I. Nelissen, E. Van Coillie, S. Masure, P. Proost, and J. Van Damme. 2001. Gelatinase B functions as regulator and effector in leukocyte biology. J. Leukoc. Biol. 69:851-859. [PubMed] [Google Scholar]
- 29.Osman, M., M. Tortorella, M. Londei, and S. Quaratino. 2002. Expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases define the migratory characteristics of human monocyte-derived dendritic cells. Immunology 105:73-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shores, E. M., and M. G. Hunter. 2000. Production of tissue inhibitors of metalloproteinases (TIMPs) by pig ovarian cells in vivo and the effect of TIMP-1 on steroidogenesis in vitro. J. Reprod. Fertil. 120:73-81. [PubMed] [Google Scholar]
- 31.Sies, H., and H. de Groot. 1992. Role of reactive oxygen species in cell toxicity. Toxicol. Lett. 64-65:547-551. [DOI] [PubMed] [Google Scholar]
- 32.Tullus, K., O. Fituri, T. Linne, R. Escobar-Billing, I. Wikstad, A. Karlsson, L. G. Burman, B. Wretlind, and A. Brauner. 1994. Urine interleukin-6 and interleukin-8 in children with acute pyelonephritis, in relation to DMSA scintigraphy in the acute phase and at 1-year follow-up. Pediatr. Radiol. 24:513-515. [DOI] [PubMed] [Google Scholar]
- 33.Wang, T., K. Yamashita, K. Iwata, and T. Hayakawa. 2002. Both tissue inhibitors of metalloproteinases-1 (TIMP-1) and TIMP-2 activate Ras but through different pathways. Biochem. Biophys. Res. Commun. 296:201-205. [DOI] [PubMed] [Google Scholar]
- 34.Yoshiji, H., S. Kuriyama, J. Yoshii, Y. Ikenaka, R. Noguchi, T. Nakatani, H. Tsujinoue, K. Yanase, T. Namisaki, H. Imazu, and H. Fukui. 2002. Tissue inhibitor of metalloproteinases-1 attenuates spontaneous liver fibrosis resolution in the transgenic mouse. Hepatology 36:850-860. [DOI] [PubMed] [Google Scholar]
- 35.Zhao, Y. G., A. Z. Xiao, X. M. Cao, and C. Zhu. 2002. Expression of matrix metalloproteinase-2, -9 and tissue inhibitors of metalloproteinase-1, -2, -3 mRNAs in rat uterus during early pregnancy. Mol. Reprod. Dev. 62:149-158. [DOI] [PubMed] [Google Scholar]