Abstract
Objectives:
Determine the sero-prevalence of rift valley fever (RVF) among slaughterhouse personnel in Makkah during Hajj and define personal and work place correlates.
Materials and methods:
A sample of 294 participants were chosen randomly from slaughterhouse personnel in Makkah during Hajj 1419 (1999). Data were collected through personal interviews using a pre-designed questionnaire consisting of personal and work place variables, e.g. age, nationality, type and hours of work. A blood sample was collected from each participant and tested by enzyme immuno-assay for IgG antibody using killed antigen for rift valley virus.
Results:
Of the total sample, 17% was seropositive for RVF. The rate of infection varied with country of origin: Syria (10.6%), Egypt (21.2%), Bangladesh (22.6%), Mali (47.1%) and Niger (50%). The number of animals slaughtered per hour and daily hours of work were significantly associated with prevalence of RVF (p<0.05).Multivariate logistic regression analysis showed that nationality and daily hours of work predicted 84.1% of the occurrence of RVF.
Recommendations:
Sero-surveys should be done among slaughterhouse personnel in Saudi Arabia and other countries particularly in countries known to be free from RVF, e.g. Syria and Bangladesh to assess the situation of RVF. The importation of animals from endemic areas should be banned.
Keywords: Rift valley fever, slaughterhouses, Hajj
INTRODUCTION
Rift valley fever (RVF), a viral disease of animals as well as humans, causes periodic epizootics with heavy losses among domestic animals and in humans. The disease manifests from a febrile illness to fatal haemorrhagic fever, with probable late complications of encephalitis or ocular disease associated with considerable human morbidity and mortality.1,2
Since 1930, when the virus was first isolated during an investigation into an epidemic amongst sheep on a farm in the Rift Valley in Kenya, several outbreaks of RFV have occurred in some countries in Africa in subsequent decades with hundreds of deaths.1,3 Mediterranean and southwest Asian regions are receptive areas.4 Although mosquito bites constitute the main route of infection, contact with the blood of a sick animal causes transmission of the virus to humans via inoculation or inhalation.5
Animals slaughtered in Hajj are imported from different countries including the Rift Valley endemic areas. Persons working in slaughterhouses are at potentially high occupational risk of rift valley fever infections on account of their contact with animal blood. They represent a useful sentinel population for the surveillance of rift valley infection.6 No studies on the prevalence of RVF among this high-risk group have been conducted in Saudi Arabia. The aim of the present work was to determine the seroprevalence of RVF among slaughterhouse personnel in Makkah during Hajj and to define some personal and work place correlates.
MATERIALS AND METHODS
In Makkah, there are four permanent slaughterhouses, each with an area of 750-50000 square meters. In Mina there five slaughterhouses, much larger in area than those in Makkah, with areas ranging from 50000 – 320000 square meters.
A total sample of 294 slaughterhouse workers was chosen: 160 from Mina and 134 from Makkah. Since there was no information on the number of workers in each slaughterhouse in Mina, an equal number (32) was randomly chosen from each slaughterhouse. In Makkah, proportional allocation method was used to recruit the required sample. Within the slaughterhouse, they were selected randomly.
Data were collected from the morning of 10 Dhul-Hajjah through the evening of 13 Dhul-Hajjah (27-30 March 1999) using a pre-designed questionnaire that was completed through interviews with butchers and servants. The information collected consisted of personal data, e. g. age, nationality, and work-related factors, e.g. functions, hours of work, compensation and vaccination.
A blood sample of 10cc venous blood was collected from each participant. Samples were kept in ice containers for transfer to the laboratory where they were centrifuged and stored at –60°C. The refrigerated samples were transferred to the Center for Disease Control (CDC) in the USA where enzyme immunoassay for IgG antibody using killed antigen for rift valley virus was performed. The criteria for a positive result included both adjusted sum of absorbance for the four dilutions tested (1:100, 1:400, 1:600 & 1:6400) of serum >1.0 and dilution end point of ≥400. Sera that met one criterion were considered positive.
RESULTS
The total sample included 294 persons working in slaughterhouses. Their ages ranged from 21-60 years with a mean of 36.1± 7.9 years. Among them, 50 persons (17%) were seropositive for rift valley fever.
Table 1 shows that slaughterhouse personnel from Niger and Mali had the highest infection rate (50 and 47.1%, respectively), followed by those from Bangladesh (22.6%) and Egypt (21.2%). A round one tenth (10.6%) of the Syrians were infected. There was a significant statistical association between nationality and prevalence of RVF. None of the participants reported being vaccinated against RVF. The infection rate ranged from 12 to 26.1% in workers whose job was to remove the skin from the carcass. However, the association between occupation and prevalence of RVF was not statistically significant (p>0.05). Of those who reported history of fever, 35% tested positive to RVF compared to 15.7% among non-reporters. There was significant statistical association between the history of fever during the last year and the prevalence of RVF (p<0.05).
Table 1.
Personal factors and rift valley fever among slaughterhouse personnel in Makkah during Hajj 1419H
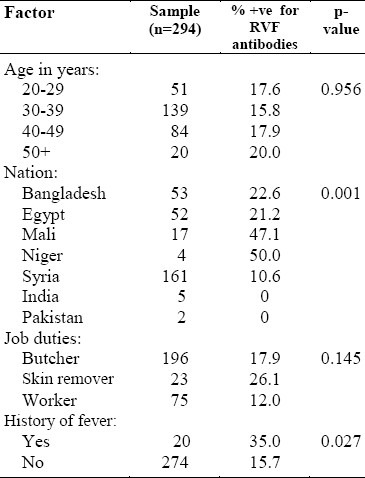
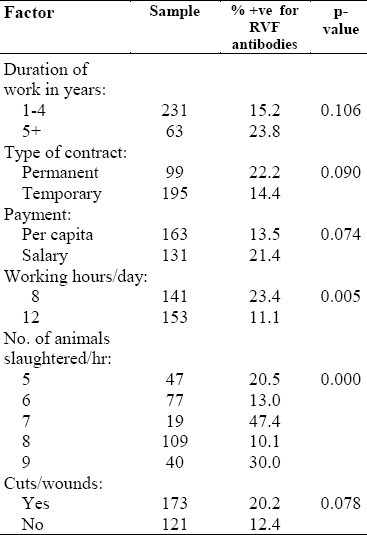
Table 2 reveals that the prevalence of RVF was 22.2% among permanent workers, 23.8% among those who had worked for more than five years in a slaughterhouse, 30% among those who slaughtered nine or more animals per hour, and 20.2% among those workers who had cuts or wounds on their hands. There was significant statistical association between both the number of animals slaughtered per hour and the daily number of hours of work with prevalence of RVF (p<0.05).
Table 2.
Work-related factors and Rift Valley Fever among slaughterhouse personnel in Makkah during Hajj 1419H
Of the infected persons, 12 (23.5%) were slaughtering animals for the first time during the 1419 Hajj season. Three of them were permanent butchers in Bangladesh. The other nine (5 Syrians, 3 Bangladeshis and 1 Egyptian) were temporary butchers. All of them reported a history of contact with animals in their own countries and none had ever been to Africa.
Results of multivariate logistic regression analysis showed that nationality and daily hours of work predicted 84.1% of the prevalence of RVF.
DISCUSSION
RVF virus is recognized as a highly infectious agent.7 Countries of the Mediterranean and Southwest Asia are advised to consider collecting sera for testing with RVF antigen. This will be done to exclude the presence of the RVF antibody and to familiarize hospital staff with virus diagnostic techniques.4 In September 2000, RVF was reported for the first time outside the African Continent. Cases were confirmed in Saudi Arabia and Yemen. It is not known whether the outbreak was a new introduction of the virus, or the pathogen had been present for some time and had only just come to the attention of public health authorities. This virgin-soil epidemic in the Arabian peninsula raised the threat of expansion into Asia and Europe.5,8
The present study revealed that 17% of the slaughterhouse personnel were infected with RVF. Their jobs involved high exposure to animal blood; the slaughter and handling of animal parts may explain this high prevalence rate. A similar study conducted in Egypt in 1993 revealed that the prevalence rate ranged from zero to 10%, with an average of 2%. It was much lower than the present study figure although Egypt experienced two epizootics and epidemics in 1977-1978 and 1993.6 The present higher rate may be attributed to the kind of animals slaughtered during Hajj. Mostly sheep, which are known to be more susceptible to RVF, are slaughtered at this time as opposed to the cattle slaughtered in Egypt.5
On the distribution of RVF by nationality, the present work revealed that Africans (from Mali, Niger and Egypt) had the highest infection rates. This finding is consistent with the endemicity of the disease in Africa.1 A study in Nigeria reported a higher sero-prevalence rate of RVF among livestock workers than in the general population.9 Another study in the Senegal River Basin revealed an overall sero-prevalence rate of 15.3%.2 In the same region, Wilson et al studied the risk factors of RVF and reported that persons dealing with sick animals had 3-6 times greater risk of infection than others.10
It is difficult to explain how Bangladeshis and Syrians who were slaughtering animals for the first time in Makkah were infected, as there was no evidence of the presence of the virus in Bangladesh or Syria, and none of the subjects had been to Africa. The slaughter of animals in Makkah in the 1419 Hajj season could not be the cause of sero-positivity, as finding antibodies in the blood of the subjects that soon was unlikely. However, the hypothesis is that vector mosquitoes of RVF are distributed almost worldwide, and the mild state of the disease may pass unnoticed as mild cases of influenza, while subclinical cases are detectable only by serology.11,12 No serological studies had been conducted before 1999 in Bangladesh, Syria or Saudi Arabia.
The present study revealed no association between the age of slaughterhouse personnel and the prevalence of RVF. The same finding was reported by the Egyptian study in which there was no significant difference in age between sero-positive and sero-negative reactors to RVF.6
Although the history of the fever was found to be statistically associated with the prevalence of RVF in crude analysis, it was not a predictor of infection in multivariate analysis. A study among Swedish UN soldiers in Egypt revealed that 4.7% of them were seropositive for RVF but none of them had reported a history of fever during their stay in Sinai.13 Furthermore, none of the seropositive abattoir workers in Egypt had had any episodes of fever during the two months prior to the study.6
RECOMMENDATIONS
(1) An RVF surveillance system with different approaches should be instituted, including serological surveys among animals and humans in Makkah. (2) Importation of animals from endemic areas should be banned. (3) Health education on the modes of transmission and methods of protection must be conducted in abattoirs. (4) Further studies should be done among Syrians and Bangladeshis in their countries to find the cause of their seropositivity.
ACKNOWLEDGMENTS
We thank Dr. James G. Olsen of the CDC in the USA, Dr. Mohamed Ameen Hashem of the Makkah slaughterhouses directorate, Dr. Sameer Saban of Makkah Health Affairs, and Dr. Haron Dywan, Mr.Adel Ashey, Mr. Mohamed Kedir, Mr. Ayman Shehata and Miss Heiam Aboresh for their assistance in the field and the laboratory work.
REFERENCES
- 1.WHO Technical quide for the diagnosis, prevention and control of rift valley fever. WHO/EMRO Technical Publication. 1983;8 [Google Scholar]
- 2.Jocelyn T, Michel P, Yaya T, Mustafa Lo, Roughy S, Josef V. Rift valley fever surveillance in the lower Senegal River basin: update 10 years after the epidemic. Tropical Medicine and International Health. 1999;4(8):580–5. doi: 10.1046/j.1365-3156.1999.00437.x. [DOI] [PubMed] [Google Scholar]
- 3.WHO. An outbreak of rift valley fever, Eastern Africa 1997-1998. Weekly Epid Record. 1998;73:105–12. [PubMed] [Google Scholar]
- 4.Shope RE, Peters CJ, Davies FG. The spread of rift valley fever and approaches to its control. Bulletin of the World Health Organization. 1982;60(3):299–304. [PMC free article] [PubMed] [Google Scholar]
- 5.WHO. fact sheets: Rift valley fever. [revised in September 2000]. http://www.who.int/
- 6.Abu-Elyazeed R, El-sharkawy, Olsen J, Bptros B, Soliman A, Salib A, et al. Prevalence of anti-rift valley fever IgM antibody in abattoir workers in the Nile delta during 1993 outbreak in Egypt. Bulletin of the world Health Organization. 1996;74(2):155–8. [PMC free article] [PubMed] [Google Scholar]
- 7.Alexander RA. Rift valley fever in The Union. J South African Vet Med Assoc. 1951;22:105–11. [Google Scholar]
- 8.WHO. Rift valley fever in Saudi Arabia- Update. 2000. Sep, http://www.who.int/ [PubMed]
- 9.Olalaye OD, Tomori O, Ladipo MA, Schmitz H. Rift valley fever in Nigeria: infections in humans. Rev Sci Tech. 1996;15(3):923–35. doi: 10.20506/rst.15.3.967. [DOI] [PubMed] [Google Scholar]
- 10.CDC. Diease information. Viral hemorrhagic fevers: fact sheets on rift valley fever. 2000. Sep, http://www.cdc.gov .
- 11.Morens DM. Rift valley fever in Africa: a disease of public health importance. Journal of Egyptian Public Health Association. 1981;LVL(5,6):444–53. [Google Scholar]
- 12.Madkour S. Epidemiological studies of rift valley fever in some governorates in Egypt. Journal of Egyptian Public Health Association. 1978;LIII(3,4):163–71. [PubMed] [Google Scholar]
- 13.Niklasson B, Meegan JM, Bengtsson E. Antibodies to rift valley fever in Swedish UN soldiers in Egypt and Sinai. Scand J Infect Dis. 1979;11(4):313–4. doi: 10.3109/inf.1979.11.issue-4.11. [DOI] [PubMed] [Google Scholar]


