Abstract
The induction of interleukin-8 (IL-8) in vitro has been suggested to correlate with the reactogenicity of Vibrio cholerae vaccine candidates. V. cholerae vaccine candidate 638, a hemagglutinin protease/hap-defective strain, was recently reported to be well tolerated in human volunteers, suggesting a role for Hap in reactogenicity. We examined the role of hap in the induction of IL-8 in intestinal epithelial T84 cells. Wild-type V. cholerae strains 3038 and C7258 and a vaccine candidate strain, JBK70, induced levels of IL-8 similar to those of their isogenic hap mutants. Supernatant containing Hap did not stimulate IL-8 production at a variety of concentrations tested, suggesting that Hap itself does not induce IL-8 production. Furthermore, supernatant from CVD115, which had deletions of hap and rtxA (encoding repeats in toxin) and was derived from a reactogenic strain, CVD110, induced IL-8 production in T84 cells in a dose-dependent manner. The IL-8-stimulating activity of CVD115 culture supernatants was growth phase dependent and was strongest in stationary phase cultures. This IL-8 stimulator(s) was resistant to heat treatment but sensitive to proteinase. Protease activity in vitro did not correlate with the reactogenicity of V. cholerae vaccine candidates. Our data suggest that Hap is not an IL-8 inducer in T84 cells and that the IL-8 stimulator in the supernatant of V. cholerae culture may play a role in reactogenicity.
The acute diarrheal disease cholera remains a significant public health problem, causing more than five million cases and 200,000 deaths annually in the world (47). Vibrio cholerae, the etiologic agent of cholera, is transmitted by contaminated water and food. It colonizes the surface of the small intestine, where it secretes cholera toxin (CT), which is largely responsible for the acute diarrhea characteristic of cholera. Although there are more than 200 O-antigen serogroups of V. cholerae, only the O1 and O139 serogroups are associated with epidemic cholera (22). The O1 serogroup can be divided further into two biotypes, classical and El Tor, based on various biochemical and phenotypic differences (for a review, see reference 22). One important difference in particular is that V. cholerae strains of the classical biotype do not have rtx genes, encoding repeats in toxin (27), and the majority of classical strains do not produce hemagglutinin/protease (Hap) or hemolysin (22). Several groups of investigators have constructed a variety of live attenuated V. cholerae O1 and O139 vaccine candidates by deleting the ctx genes encoding cholera toxin (21, 26, 30, 46, 48). However, in clinical trials most V. cholerae vaccine candidates still exhibit reactogenicity, which includes mild diarrhea, malaise, nausea, vomiting, abdominal cramps, low-grade fever, and headache (8, 21, 45, 46, 48). The only licensed oral live cholera vaccine, CVD103-HgR, is well tolerated and highly protective in North American volunteers (26, 43, 46); however, it was poorly protective in a field trial involving 67,500 people in Indonesia (37).
Although cholera is commonly considered to be a noninflammatory secretory disease, there are numerous indications that there is an inflammatory component to the disease. Qadri et al. recently reported the presence of congested blood vessels and the infiltration of polymorphonuclear leukocytes (PMN) in the lamina propria at the acute stage of cholera in both adults and children infected with V. cholerae O1 and O139 (36). In addition, high levels of lactoferrin were found in stool samples of volunteers who received the El Tor vaccine candidate CVD110, strongly suggesting intestinal inflammation (41). The levels of fecal lactoferrin found with CVD110 were nearly as high as those found in stool samples from volunteers who ingested Shigella, the classic etiologic agent of inflammatory diarrhea (41). Interestingly, the fecal lactoferrin levels in human volunteers infected with wild-type V. cholerae strains were much lower than the levels in those infected with Shigella and Clostridium difficile (33), and the difference was probably due to the dilution effect of the voluminous rice water stools seen with CT-positive V. cholerae strains during V. cholerae infection. Results from several animal studies have also indicated an inflammatory component to the disease. In a mouse pulmonary model of infection, Fullner et al. (14) saw evidence of inflammation including infiltration of PMN and tissue damage. Elevated mRNA levels of interleukin-8 (IL-8) and IL-1 were also observed in a rabbit ileum model after rabbits were infected with reactogenic V. cholerae strains in the ileum loop model (E. Boedeker and J. B. Kaper, unpublished data).
Although the mechanisms of reactogenicity caused by V. cholerae vaccine candidates are still not known, reducing or eliminating the reactogenicity has been one of the top priorities for live V. cholerae vaccine development. Levine et al. (26) suggested two potential hypotheses to explain the diarrhea and other symptoms seen with the attenuated strains. The first hypothesis was that there was a previously unidentified enterotoxin that could cause these symptoms in the absence of CT but whose existence had been masked by the presence of CT. Two novel toxins, the accessory cholera enterotoxin (Ace) (49) and the zonula occludens toxin (Zot) (10), were hypothesized to be responsible for these symptoms and subsequently characterized. However, CVD110, which had specifically deletions of the ace, zot, hly (hemolysin), and ctxA genes, was still reactogenic (46). The second hypothesis was that mere colonization of the proximal small bowel by a strongly adhering organism could somehow lead to reactogenicity. In support of this hypothesis, the nonreactogenic CVD103-HgR colonizes the intestine at a greatly reduced level relative to CVD110 and other reactogenic strains. The observation that nonmotile mutants, Peru-15 (7, 23) and Bengal-15 (8) derived from reactogenic V. cholerae vaccine candidates, were nonreactogenic in volunteers led Waldor and Mekalanos to propose the mucus penetration model (52), in which motility allows V. cholerae strains to penetrate the mucus layer, resulting in reactogenicity. However, the molecular and cellular mechanisms of reactogenicity are still not known.
V. cholerae secretes hemagglutinin/protease (Hap), which is encoded by the hap gene (12). Hap can perturb the paracellular barrier function in epithelial cells by degrading occludin in tight junctions (31, 55). Hap can also hydrolyze mucin to enhance the detachment of V. cholerae from cultured epithelial cells (11). The hap gene is positively regulated by HapR at the transcription level (20). It was reported that a CTXφ and hap-defective vaccine strain, 638, was not reactogenic in human volunteers and this strain induced lower levels of IL-8 than its parent wild-type strain in HT29 cells (38). The protease activity in V. cholerae vaccine strains has been associated with decreases in the transcellular epithelial resistance of polarized T84 intestinal epithelial cells (31). These results suggested a role of Hap in reactogenicity including inflammatory diarrhea. However, Fullner et al. recently reported that the deletion of hap in V. cholerae did not affect the production of IL-6, or macrophage inflammatory protein 2 in a murine pulmonary model, and the hap mutant was more virulent than its wild-type parent strain although the mechanism was not clear (14).
In the present study, we investigated the role of Hap in the induction of IL-8 in T84 cells and correlated the protease activity and motility of V. cholerae vaccine candidates with the reactogenicity seen in human volunteer trials. Our data suggest that V. cholerae hap mutants stimulate amounts of IL-8 similar to those stimulated by their parental wild-type strains in T84 cells and that Hap itself was not able to stimulate IL-8 production. Interestingly, the supernatant of CVD115 contains an unidentified inducer that stimulated IL-8 in a dose-dependent manner. The inducer seemed to be heat resistant and to be secreted abundantly during stationary phase. Finally, there is no correlation between reactogenicity and protease activity in the V. cholerae vaccine candidates tested. Our results indicate that a stimulator of IL-8 other than Hap may be responsible for inflammation contributing to the reactogenicity of attenuated V. cholerae vaccine strains.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
Bacterial strains and plasmids used in this study are shown in Table 1. Bacteria were grown overnight at 37°C with shaking at 200 rpm in 5 ml of Luria-Bertani (LB) broth. All V. cholerae strains were then subcultured (at a dilution of 1:1,000) into 5 ml of LB broth and grown with shaking for 18 h at 30°C in tryptic soy broth (TSB) to optimize the expression of hap (4) unless otherwise indicated. Selective antibiotics were added at the following concentrations: ampicillin, 100 μg/ml; kanamycin, 25 μg/ml; tetracycline 2.5 μg/ml; and polymyxin, 50 μg/ml.
TABLE 1.
Strains and plasmids used in this studya
| Strain or plasmid | Biotype and serotype | Relevant characteristic(s) | Reference or source |
|---|---|---|---|
| V. cholerae strains | |||
| 3038 | El Tor, Ogawa | Wild type | 15 |
| HAP-1 | El Tor, Ogawa | hap mutant of 3038 | 15 |
| C7258 | El Tor, Ogawa | Wild type | 3 |
| 638 | El Tor, Ogawa | ΔctxAB Δzot Δace ΔorfU Δcep Δhap::celA mutant of C7258 | 3 |
| N16961 | El Tor, Inaba | Wild type | 17 |
| JBK70 | El Tor, Inaba | ΔctxAB mutant of N16961 | 21 |
| HLZ1 | El Tor, Inaba | Δhap mutant of JBK70 | This study |
| CVD110 | El Tor, Ogawa | ΔctxA Δzot Δace hlyA::mer ΔctxB of E7946 | 46 |
| CVD114 | El Tor, Ogawa | ΔrtxA mutant of CVD110 | This study |
| CVD115 | El Tor, Ogawa | Δhap mutant of CVD114 | This study |
| 395 | Classical, Ogawa | Wild type | 25 |
| CVD101 | Classical, Ogawa | ΔctxA mutant of 395 | 26a |
| CVD111 | El Tor, Ogawa | ΔctxA Δzot Δace hlyA::mer from N16117 | 44 |
| CVD103-HgR | Classic, Inaba | ΔctxA mutant of 569B | 26 |
| E. coli strains | |||
| DH5α | K-12 cloning host strain | Stratagene | |
| SM10λpir | thiL thrL leuB6 supE44 tonA21 lacY1 recA::RP4-2-Tc::Mu Kmr | 39 | |
| Plasmids | |||
| pHM5 | Suicide vector | 35 | |
| pCR-Blunt II-Topo | PCR blunt cloning vector with topoisomerase | Invitrogen | |
| pXZ5 | pHM5 containing hap1 fragment, 1.2 kb upstream of hap | This study | |
| pXZ6 | pCR-Blunt II-TOPO containing hap2 fragment, 1 kb downstream of hap | This study | |
| pXZ7 | pHM5 containing hap1 and tetracycline cassette | This study | |
| pXZ8 | pHM5 containing hap1, Tet, and hap2 | This study | |
| pDQG1 | pHM5 containing 1 kb upstream of rtxA | This study | |
| pDQG2 | pHM5 containing 1 kb upstream and downstream of rtxA | This study |
All V. cholerae strains belong to the O1 serotype.
Eukaryotic cell lines and culture conditions.
T84 human colonic carcinoma epithelial cells were obtained from the American Type Culture Collection. T84 cells were grown in Dulbecco modified Eagle medium and Ham's F-12 medium (DMEM/F-12) (Gibco, Grand Island, N.Y.) supplemented with 10% heat-inactivated fetal calf serum, 100 U of penicillin/ml, and 100 μg of streptomycin/ml and incubated at 37°C with 5% CO2. One million cells were seeded in 24-well plates and grown until they reached 90 to 95% confluence. Prior to infection, cells were washed twice with DMEM/F-12 medium without serum and antibiotics and maintained in serum-free medium for at least 2 h before infection.
Recombinant DNA techniques.
Plasmid purification, PCR, restriction digestion, ligation, transformation, and DNA gel electrophoresis were performed by standard methods (39). DNA sequence analysis was performed at the University of Maryland Biopolymer Facility on an ABI automated sequencer with DNA purified by QIAGEN midi-columns. All oligonucleotide primers used are listed in Table 2.
TABLE 2.
Primers used for this study
| Primer | Sequence |
|---|---|
| K2573 | 5′-CCAATGCATCTCATGTTTGACAGCTTATCA-3′ |
| K2574 | 5′-GCTCTAGACTTCCATTCAGGTCGAGGTG-3′ |
| K2576 | 5′-GAAGATCT ACGTCATGCTCGCACTAGAA-3′ |
| K2577 | 5′-GCTCTAGACCAATGCATGCGAAATACCGCGTTCAATC-3′ |
| K2578 | 5′-GCTCTAGAACCTCTTCTTGGGATTGTCG-3′ |
| K2579 | 5′-CGAGCTCCACCATCGCCTGTTCTGCTT-3′ |
| K2993 | 5′-CGAGCTCAAAGCTCACCGCAATAACT-3′ |
| K2994 | 5′-GCTCTAGACCAATGCATTACAATCTCATCAGCCTTAG-3′ |
| K2995 | 5′-GAAGATCTGTGGCGATGGCAATGTGTCA-3′ |
| K2996 | 5′-GCTCTAGATGGCGACGACTACACTGG-3′ |
Construction of hap mutant.
To construct a hap mutant, DNA fragments upstream (hap1) and downstream (hap2) of hap were amplified by PCR with Pfu polymerase by using primer K2576 with K2577 and primer K2578 with K2579, respectively. The 1.2-kb hap1 PCR fragment was digested with BglII/XbaI and linked into the BglII/XbaI site of the λpir-dependent sacB suicide vector pHM5, yielding pXZ5. The tetracycline (tet) cassette from pBR322 was amplified with the primer pair K2573/K2574 and then inserted into the NsiI/XbaI site of plasmid pXZ5, creating pXZ7. A 1-kb PCR product, hap2, was cloned into plasmid pCRII-Topo (Invitrogen), yielding pXZ6, then into the XbaI/SacI-digested plasmid pXZ7, resulting in pXZ8. The suicide plasmid pXZ8 was mobilized into V. cholerae JBK70 and CVD114 by conjugation with E. coli SM10λpir. Deletion mutants were obtained by first selecting for resistance to sucrose (10% wt/vol) and tetracycline and then by screening for sensitivity to ampicillin as described by Mey and Payne (32). The hap mutants were verified by PCR and Southern blotting (data not shown).
Construction of the rtx mutant.
To construct an rtxA mutant, 1-kb fragments upstream and downstream of rtxA were generated by using primer K2995 with K2994 and primer K2996 with K2993, respectively. The two fragments were digested by BgIII/XbaI and XbaI/SacI, respectively, and the upstream fragment was cloned into the BglII/XbaI site of plasmid pHM5. The resulting plasmid was then digested and cloned into the XbaI/SacI sites of pHM5, yielding pDQW1. pDQW1 was transformed into E. coli SM10λpir, and the resulting strain was conjugated with CVD110. Transconjugants were selected on Luria agar plates containing ampicillin (100 μg/ml) and polymyxin B (50 μg/ml). Colonies were grown overnight in LB at 37°C and plated onto Luria agar containing 10% sucrose. Isolates that were ampicillin sensitive were screened for rtx mutation by PCR using primers K2992 and K2994.
Infection of T84 cells with V. cholerae.
The infection protocol was followed as previously described (9) with modification. Briefly, prior to infection, bacteria were washed twice, resuspended, and adjusted to approximately 2 × 109 CFU/ml in sterile phosphate-buffered saline (PBS; pH 7.4). The suspensions were added to T84 cells at various multiplicities of infection (MOI). To synchronize infection, the 24-well plate was centrifuged for 10 min at 2,000 rpm with an Allegra 6R centrifuge (Beckman). After incubation for 1 h at 37°C with 5% CO2, the cells were washed twice with DMEM/F-12 medium. To avoid bacterial overgrowth, 1 ml of DMEM/F-12 serum-free medium containing 100 μg of gentamicin/ml was added to each well and incubated for 18 h at 37°C with 5% CO2. For stimulation by supernatant, bacterial culture supernatants were collected by centrifugation and filter sterilized with 0.2-μm-pore-size syringe filters. A total of 100 μl of the supernatant was added to each well and incubated for 18 h at 37°C with 5% CO2.
Azocasein assay.
The azocasein assay was carried out as described by Mel et al. with modifications (31). Briefly, V. cholerae strains were grown overnight in TSB at 30°C with shaking at 200 rpm; the overnight culture was subcultured to 5 ml of TSB in 50-ml conical tubes at a dilution of 1:1,000 and grown for 18 h at 30°C with shaking. A total of 100 μl of azocasein (5 mg/ml) in 100 mM Tris (pH 8.0) was incubated with 100 μl of filter-sterilized cell culture supernatants for 1 h at 37°C. The reaction was stopped by the addition of 400 μl of 10% trichloroacetic acid. After centrifugation, the trichloroacetic acid supernatant was transferred to 700 μl of 525 mM NaOH, and the optical density (OD) was determined at 442 nm. One azocasein unit was defined as the amount of enzyme producing an increase of 0.01 OD unit per hour.
ELISA.
The IL-8 concentration was measured by enzyme-linked immunosorbent assay (ELISA) as described previously (57). Briefly, the coated plates containing anti-human IL-8 antibody were washed with PBS containing 0.1% Tween 20 (PBST) and then blocked in PBST containing 5% skim milk for 1 h at room temperature (RT). After the plate was washed four times with PBST, 100 μl of samples and a series of twofold dilutions of standard were added to 96-well plates and incubated for 2 h at RT. One hundred microliters of diluted (1:2,000) biotinylated mouse anti-human IL-8 monoclonal antibody was added to each well after washing and then incubated for 1 h at RT. After the cells were washed, 100 μl of diluted (1:2,000) avidin-horseradish peroxidase conjugate was added to each well. The reaction was developed by tetramethylbenzidine (TMB) substrate reagent and stopped with 2 N H2SO4. ODs (at 405 nm) were measured with a Labsystems Multiskan Plus reader (Fisher Scientific). Recombinant human IL-8 was used as a standard. The biotinylated mouse anti-human IL-8 monoclonal antibody, avidin-horseradish peroxidase conjugate, and TMB substrate reagent set were all obtained from BD PharMingen (San Diego, Calif.).
Cytotoxicity assay.
Lactate dehydrogenase (LDH) is a cytoplasmic enzyme whose presence in the culture medium reflects the loss of plasma membrane integrity. LDH activity in the culture supernatants was measured at specific intervals with the colorimetric CytoTox96 kit (Promega Corp., Madison, Wis.) according to the manufacturer's instructions. The percentage of cytotoxicity was calculated by using the following formula: 100 × [(experimental release − spontaneous release)/(total release − spontaneous release)]. Spontaneous release represents the amount of LDH activity in the supernatant of uninfected cells, while total release represents the LDH activity in cell lysates.
SDS-PAGE and Western blotting.
To determine the production of Hap, the supernatants from V. cholerae were harvested, filter sterilized, and concentrated by being passed through Centricon-10 centrifugal filters (Amicon Bioseparations) and separated by sodium dodecyl sulfate-12% polyacrylamide gel electrophoresis (SDS-PAGE) (4). The proteins were transferred to a polyvinylidene difluoride membrane in standard transfer buffer (25 mM Tris [pH 8.3], 192 mM glycine, and 20% methanol) at 30 V for 20 min with a Bio-Rad semidry transfer cell. After the transfer was completed, the membranes were blocked for 1 h with 2% skim milk and PBST at RT, probed with a rabbit Hap antiserum, and developed with peroxidase-conjugated goat anti-rabbit immunoglobulin G.
Motility assay.
Overnight cultures of V. cholerae were stabbed into LB containing 0.3% agar plates. The plates were incubated for 8 h at 37°C and the diameters of the motility zones were measured.
Protein assay and statistical analysis.
Protein concentrations were determined with a bicinchoninic acid assay (Pierce, Rockford, Ill). Data are presented as the means ± standard deviations of at least two separate experiments of duplicates, except where results of blots are shown, in which case a representative experiment is depicted in the figure. Comparisons between two values were analyzed by a Student's t test. Differences were considered significant at P values of <0.05.
RESULTS
Construction and characterization of hap mutants.
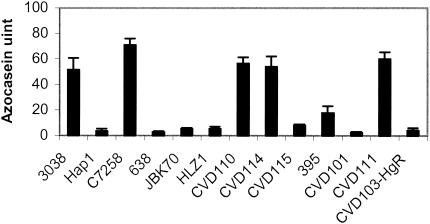
Hap has been implicated as a factor involved in reactogenicity in human volunteer trials (3) and in the induction of IL-8 in intestinal epithelial cells (38). We created isogenic mutants with deletions of hap to test any possible role hap may have on IL-8 induction. Mutations in hap were constructed in JBK70 and CVD114, resulting in HLZ1 and CVD115, respectively. To confirm the hap deletion in HZL1 and CVD115, the protease activity of their supernatants was measured with an azocasein assay as described in Materials and Methods. As shown in Fig. 1, azocasein activity was diminished in V. cholerae hap mutants Hap1, 638, and CVD115 compared to their wild-type parent strains 3038, C7258, and CVD110, which elicited high activity (3, 15). Similarly, the protease activity of CVD115, the hap mutant of CVD114, was nearly abolished. Interestingly, both JBK70 and its isogenic hap mutant, HZ1A, had no detectable azocasein activity. The parent strain of JBK70, N16961, did not elicit activity (data not shown). The result is consistent with the report that a frameshift mutation occurred in hapR, the gene encoding the positive regulator of hap (58).
FIG. 1.
Azocasein activity of V. cholerae strains. The total protease activity was measured in culture supernatants prepared from the indicated strains grown for 18 h at 30°C with shaking.
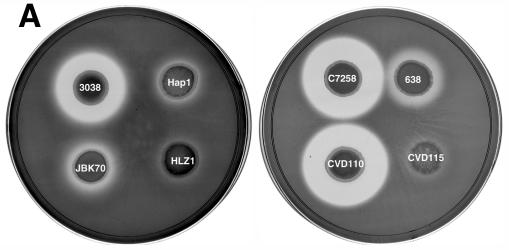
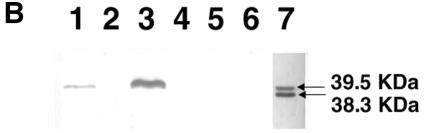
Since the azocasein assay only detects protease activity in a 1-h incubation period, we also tested the protease activity of V. cholerae strains on nutrient agar plates containing 1% skim milk by adding 10 μl of their overnight culture supernatants onto the plate and incubating them for 48 h at 37°C. The diameter (in centimeters) of the clear zone of N16961 was similar to that of JBK70 (1.5 ± 0.01). The results shown in Fig. 2A suggest that Hap is the major protease in the wild-type strains 3038, C7258, and CVD110, since protease activity was greatly diminished in all isogenic hap mutants. Consistent with the data from the azocasein assays, strains N16961 and JBK70 were significantly defective in protease activity compared to strains 3038, C7258, and CVD110. HLZ1 was even more defective in protease activity compared to JBK70. A Westen blot analysis was performed to further confirm the presence of Hap in these strains. As shown in Fig. 2B, the Hap protein was detected in the supernatants of strains 3038 and C7258 but not in their isogenic hap mutants, Hap1 and 638, nor in JBK70 and HLZ1.
FIG. 2.
(A) Protease activity of V. cholerae hap mutants and their isogenic parent strains on milk-containing medium. Bacteria were grown for 16 h at 37°C, pelleted by centrifugation, and 10 μl of the supernatant was spotted onto the plates and incubated for 48 h at 37°C. (B) Western blot analysis of Hap production. Overnight bacterial cultures were subcultured (at a dilution of 1:1,000) into 5 ml of TSB medium, and incubated at 30°C with shaking (200 rpm) for 20 h. The cultures were centrifuged, filtered, and concentrated through Centricon-30 centrifugal filters. The retentate was adjusted to 0.05 ml, 50-μl aliquots were subjected to SDS-12% PAGE, and the proteins were transferred to a polyvinylidene difluoride membrane. Hap was detected by using a rabbit anti-Hap serum and peroxidase-conjugated goat anti-rabbit immunoglobulin G. Lane 1, C7258; lane 2, 638; lane 3, 3038; lane 4, Hap1; lane 5, JBK70; lane 6, HLZ1; lane 7, pure Hap (2.5 μg). Molecular masses were calculated with reference to SDS molecular mass standards from Bio-Rad laboratories.
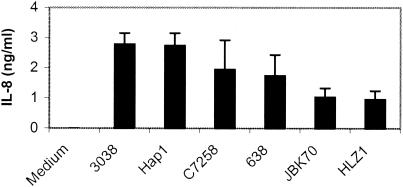
V. cholerae stimulates IL-8 production in T84 cells.
Recently, it was suggested that there might be a correlation between the ability of V. cholerae strains to induce IL-8 in vitro and reactogenicity (38). Additionally, V. cholerae strain 638, a hap and CTXφ deletion mutant, is deficient in inducing IL-8 production elicited in HT29 cells (38). To test whether a hap mutation affects IL-8 induction in T84 cells, we compared the IL-8 production elicited by wild-type strains to that elicited by their isogenic hap mutants. As shown in Fig. 3, hap mutants induced amounts of IL-8 similar to those elicited their parent wild-type strains at an MOI of 10. Similar results were obtained at an MOI of 25 (data not shown). The results suggested that Hap may not be involved in the induction of IL-8 in this system. It is interesting that the levels of IL-8 stimulated by 3038 and C7258 were higher than that induced by the highly reactogenic JBK70. This result suggests that the IL-8 inducer is not evenly distributed or secreted among different V. cholerae strains, at least under the in vitro culture conditions employed here. It should be also noted that IL-8 is only one of many chemokines that may participate in gut inflammation.
FIG. 3.
Induction of IL-8 by V. cholerae strains and their isogenic hap mutants in T84 cells. V. cholerae were grown in 5 ml of LB at 37°C overnight and washed twice in sterile PBS. T84 cells were infected with V. cholerae strains at an MOI of 10 in serum-free DMEM/F-12 medium for 1 h. The medium was then replaced by serum-free DMEM/F-12 medium containing gentamicin (100 μg/ml) and incubated for 18 h. The levels of IL-8 in the supernatants were measured by ELISA.
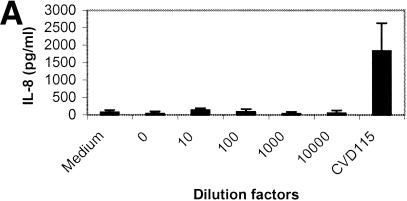
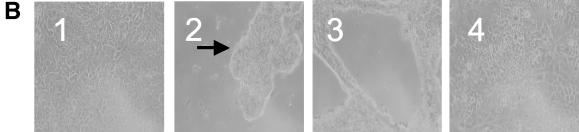
V. cholerae El Tor strains secrete many factors that are cytotoxic to epithelial cells, including Rtx (27) and hemolysin (6). The cytotoxicity interferes with the induction of IL-8 production by some V. cholerae strains. To determine whether Hap is able to stimulate IL-8 in T84 cells, we constructed CVD114, an rtx mutant of CVD110. The cytotoxicity induced by CVD114 in T84 cells is greatly reduced (data not shown), which is consistent with the report that rtx leads to rapid cell death in epithelial cells (27). To determine whether Hap is able to stimulate IL-8 in T84 cells, CVD114 was grown under conditions optimized for hap expression as described in Material and Methods, and the supernatant was tested in the azocasein assay, indicating a level of activity of approximately 55 units (Fig. 1). The supernatant was serially diluted and applied to T84 cells, and the induction of IL-8 and cytotoxicity in T84 cells after treatment are shown in Fig. 4A and B, respectively. As expected, the supernatant caused T84 cell detachment, forming cell clumps at higher concentrations (Fig. 4B, panel 2), and the effect was diminished when the dilution was more than 1:100 (Fig. 4B, panel 4). However, there was no significant difference in cytotoxicity as measured by an LDH assay, and most detached cells were still alive at the end of the experiment (data not shown). The result is consistent with the report that Hap induces morphological changes in epithelial cells (54). However, at doses that did not cause cell detachment, the supernatant did not induce IL-8 production (Fig. 4A), suggesting that Hap either negatively regulated IL-8 expression or may not directly induce IL-8 production in T84 cells.
FIG. 4.
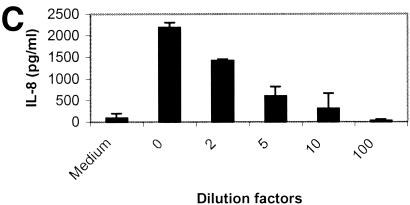
(A) IL-8 induction by culture supernatant of CVD114 in T84 cells. An overnight culture of V. cholerae CVD114 was subcultured (at a dilution of 1:1,000) and grown in 5 ml of LB broth for 18 h at 30°C. The supernatant was diluted, and 100 μl of the supernatant dilutions was added to T84 cells in 24-well tissue culture plate. Supernatant from CVD115 was used as a control. The levels of IL-8 were determined by ELISA, and the cytotoxicity was determined by trypan blue staining. (B) T84 cells were either not treated (panel 1) or treated with undiluted (panel 2), 10-fold-diluted (panel 3), or 100-fold-diluted (panel 4) supernatant containing Hap. The arrow shows the detached cell clumps. (C) Supernatant from CVD115 stimulates IL-8 in T84 cells in a dose-dependent manner. An overnight culture of V. cholerae CVD115 was subcultured (at a dilution of 1:1,000) and grown in 5 ml of LB broth for 18 h at 30°C. The supernatant was diluted, and 100 μl of each diluted supernatant was added to T84 cells in a 24-well tissue culture plate. The levels of IL-8 were determined by ELISA.
Supernatant from CVD115 induces IL-8 in T84 cells.
When CVD114 was grown under the optimized conditions for hap expression (4), its supernatant was not able to induce IL-8 production in T84 cells (Fig. 4A). The result, however, does not exclude the possibility that there is an IL-8 stimulator in the supernatant of CVD115 since Hap causes detachment in T84 cells at a high concentration. Furthermore, our results suggested the existence of a Hap-independent IL-8 stimulator in strains Hap1, C7258, and JBK70 (Fig. 3). CVD115 was deficient in azocasein activity (Fig. 1) and was not able to form a clear zone on the casein agar plate (Fig. 2A). Furthermore, the supernatant of CVD115 was not cytotoxic to T84 cells as measured by the LDH assay (data not shown) and did not cause cell detachment (data not shown). As shown in Fig. 4C, culture supernatants from CVD115 induced IL-8 production in a dose-dependent manner, indicating the presence of at least one Hap-independent IL-8 stimulator.
Effect of temperature and aeration on the induction of IL-8 by culture supernatants of CVD115.
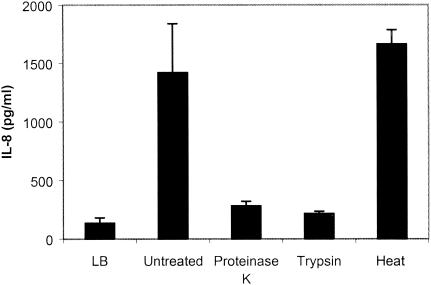
To determine whether the IL-8 inducer is regulated by temperature or aeration, CVD115 was grown with shaking or under static conditions at either 37°C or 30°C, and the levels of IL-8 induced by their supernatants was measured by ELISA. The levels of IL-8 stimulated by the supernatant of CVD115 were appreciably higher when CVD115 was grown under conditions of shaking than under static conditions (data not shown); however, it appears that temperature may not affect the expression of the IL-8 inducer significantly. It seems that the ability of the supernatant of CVD115 to stimulate IL-8 correlates with the culture density of CVD115 instead of with temperature and aeration (data not shown). To determine whether the induction of IL-8 by the supernatant of CVD115 is dependent on the growth phase of the bacteria, we measured the level of IL-8 induced by supernatants of CVD115 grown to different growth phases and found that the supernatant from cells grown to stationary phase induced the highest amount of IL-8 (data not shown). To further investigate whether the IL-8 stimulator is a protein, we pretreated the supernatant with proteinase K, trypsin, or heat at 95°C for 30 min before stimulating T84 cells. As shown in Fig. 5, the IL-8 inducer was resistant to heat treatment and sensitive to proteinase K and trypsin, suggesting that the IL-8 inducer from the supernatant of CVD115 is a protein.
FIG. 5.
Stability of IL-8 inducer in CVD115 culture supernatant. T84 cells were incubated for 18 h with control LB medium, V. cholerae CVD115 supernatant, or heat-treated supernatant (30 min at 95°C) or were incubated for 2 h with 40 μg of trypsin/ml or 200 μg of proteinase K/ml (34), followed by heat treatment.
Correlation of reactogenicity and protease activity.
Since Hap appears to be the major protease in V. cholerae, as shown in Fig. 1 and 2, and protease activity has been associated with an increase in membrane permeability in epithelial cells (31), we investigated the role of hap in the reactogenicity of V. cholerae vaccine strains by measuring the azocasein activity of both classical and El Tor vaccine candidate strains and correlating the results with the reactogenicity in volunteer trials. As shown in Fig. 1, azocasein activity was barely detected in the El Tor strain JBK70 and in the classical strains CVD101 and CVD103-HgR. However, JBK70 and CVD101 are highly reactogenic in human volunteer trials (21, 46). Both CVD110 and CVD111 have high azocasein activity; however, CVD110 was highly reactogenic (46), whereas CVD111 is only slightly reactogenic (44). These results showed that no correlation exists between human reactogenicity and protease activity as measured under these in vitro conditions, although protease activity measured in vitro may differ from that in vivo.
Correlation of motility and reactogenicity.
To determine whether there is a correlation between motility and reactogenicity, we tested the motility of strain 638. CVD103-HgR and 638 are well tolerated in human volunteers; however, the molecular basis of their nonreactogenicity is not known. Since spontaneous nonmotile mutants of some reactogenic V. cholera vaccine candidates became nonreactogenic (8, 23), it was proposed that motility may play an important role in causing reactogenicity in volunteers (52). The reactogenic vaccine candidates, CVD110, CVD101, JBK70, CVD111, and CVD112, were highly motile (N. Stokes and J. B. Kaper, unpublished data). However, CVD103-HgR was deficient in motility, suggesting that the nonreactogenicity of this strain may be at least in part due to reduced motility (N. R. Stokes, X. Zhou, S. J. Meltzer, and J. B. Kaper, submitted for publication). Interestingly, strain 638 was not deficient in motility compared to its parent strain C7258 (data not shown), suggesting that a factor other than motility is important for its nonreactogenicity.
DISCUSSION
Despite years of research in the development of live oral attenuated cholera vaccines, the reactogenic factor(s) causing mild diarrhea and other symptoms in North American volunteers has remained elusive. Recently, strain 638, a CTXφ and hap deletion mutant, was reported to be well tolerated in volunteer trials in Cuba (3), suggesting that hap might play a role in reactogenicity. Furthermore, it was reported that reactogenic V. cholerae strains induced higher levels of IL-8 in vitro than nonreactogenic strains such as 638 and CVD103-HgR. These data suggest that the nonreactogenicity of these strains may be due to their diminished ability to induce IL-8 production in intestinal epithelial cells (38). However, our results did not support a direct role of Hap in causing inflammation in vitro. First, V. cholerae C7258, JBK70, and 3038 induced amounts of IL-8 similar to those induced by their isogenic hap mutants in T84 cells. Second, the supernatant of CVD115, a rtx and hap mutant of CVD110, induced IL-8 production in a dose-dependent manner. Third, the supernatant of CVD114, which contains Hap, did not induce IL-8 production at a variety of tested doses. Fourth, no correlation was found between protease activity in vitro and reactogenicity among the vaccine candidates tested. Our finding that strain 638 induced similar amounts of IL-8 in T84 cells appears to be inconsistent with the report that 638 is diminished in IL-8 induction in HT29 cells (38). T84 cells are a human colonic carcinoma which maintains vectorial electrolyte transport and are more similar to enterocytes; however, HT29-18N2 was subcloned from the HT-29 human colonic adenocarcinoma parental cell line, which has numerous mucous granules which degranulate in the presence of parasympathomimetic drugs, suggesting that the subclone is similar to goblet cells (19). Enterocytes are much more numerous than goblet cells in the intestine, and so we performed our experiments with T84 cells. We suspect that the difference in IL-8 induction by 638 and its parent strain observed by Rodriguez et al. (38) was due to the different protocol and cell line used. Since there is a mucus layer on epithelial cells in vivo, it would be interesting to examine the effect of Hap on inflammation in human intestinal tissue samples. So far, it is unclear what cell type may be contributing to chemokine production in vivo during V. cholerae infection. Recently, Fullner et al. (14) reported that a hap mutant is more lethal in a pulmonary murine model and caused more severe histopathologic damage than its wild-type parent in the lungs of survivors, although no difference was seen in the induction of inflammation. It is also important to note that classical strains do not have Hap activity (22) and naturally lack the rtxA gene (27). However, classical vaccine candidate strains that were deleted of ctxA still caused reactogenicity. Furthermore, an El Tor vaccine candidate strain, JBK70, which does not have detectable Hap in vitro, was reactogenic (21). Taken together, our data suggest that Hap may not be responsible for the reactogenicity caused by V. cholerae.
We demonstrated that there is at least one IL-8 stimulating factor secreted by V. cholerae CVD115. The difficulty in identifying reactogenic factors in V. cholerae is mainly due to the poor understanding of the mechanisms of reactogenicity and, as a result, the lack of an in vitro indicator of reactogenicity. Furthermore, V. cholerae El Tor strains produce many cytotoxic factors, such as Rtx (27) and hemolysin (6), that are cytotoxic to epithelial cells, and this fact has complicated the identification of the IL-8 stimulator. In this study, we generated CVD115 by deleting genes encoding all known cytotoxic factors, hly, rtx, and hap, in an El Tor strain. As expected, the resulting strain, CVD115, is not cytotoxic to T84 cells as measured by the LDH assay (data not shown) (Fig. 4C), and the supernatant from CVD115 is able to induce IL-8. These results indicated that a secreted factor(s) from V. cholerae, other than Rtx, Hap, and Hly, can induce IL-8 in T84 cells. Recently, flagellin (16) and lipoprotein (2) have been shown to recognize Toll-like receptor 5 and Toll-like receptor 2, respectively. Both flagellin (16) and lipoprotein (1) are secreted into the supernatant; therefore, it is possible that flagellin and lipoprotein may be the stimulators. Lipoprotein is present in both gram-positive and gram-negative bacteria, and it is resistant to protease and heat treatment (51, 53). Flagellin is resistant to heat treatment but sensitive to proteinase K treatment (34). Our results suggested that the IL-8 stimulator in V. cholerae is resistant to heat treatment but sensitive to proteinase treatment; therefore, the IL-8 inducer in V. cholerae may be a protein. Despite the fact that flagella are present in all V. cholerae strains and are secreted into the supernatant, supernatants from different V. cholerae strains induced significantly different levels of IL-8 in T84 cells (data not shown). Therefore, it is possible that factors other than flagellin and lipoprotein in V. cholerae can trigger inflammatory responses. Recently, many factors secreted by bacteria have been shown to induce IL-8 induction in epithelial cells, for example, α-hemolysin of uropathogenic Escherichia coli (50), the cytolethal distending toxin of Campylobacter jejuni (18), SipA, a secreted protein of Salmonella enterica serovar Typhimurium (24), and N-3-oxododecanoyl homoserine lactone, an autoinducer of Pseudomonas aeruginosa (42). Identification of the IL-8 stimulator in V. cholerae may be helpful in understanding the mechanisms of reactogenicity of cholera vaccine candidates in volunteers.
Hap is thought to mediate the detachment of V. cholerae from mucus (5). We were not able to detect Hap activity in JBK70. Zhu et al. recently reported that N16961 has a frameshift mutation in hapR (58), a positive regulator for hap (20). Alignment of hapR sequences from V. cholerae 3038 (GenBank accession number AF000716) and N16961 shows that at position 119 of hapR, a nucleotide, T, is missing in N16961, resulting in a frameshift mutation in hapR in N16961. Since HapR is required for hap expression (20), it is possible that a hapR mutation may lead to the lack of azocasein (Fig. 1A) and protease activity (Fig. 1C) in both N16961 and JBK70, accounting for the inability to detect Hap in these two strains (Fig. 1B). In the present study, we showed that Hap leads to the detachment of T84 cells; however, it does not cause significant cell death as measured by the LDH assay. This finding is consistent with the reports that Hap activity causes morphological change (54) and correlated with increased cell membrane permeability in T84 cells (31). Although the hap gene is upregulated in rabbit ileum loops (56), the actual role of hap in vivo remains to be determined.
Motility is important for V. cholerae to penetrate the mucus layer and to adhere to intestinal epithelial cells (13). Since spontaneous nonmotile mutants of reactogenic strains Peru-3 and Bengal-3 are minimally reactogenic (8, 23), Waldor and Mekalanos proposed the mucus penetration model (52). This model hypothesizes that penetration of V. cholerae through the mucus layer and the contact of V. cholerae with intestinal epithelial cells lead to reactogenicity (52). We found that the nonreactogenic strain CVD103-HgR (N. R. Stokes et al., submitted) is deficient in motility. However, strain 638 appears to be as motile as its wild-type parent. Therefore, the nonreactogenicity of 638 is probably attributed to a factor(s) other than motility. So far, the spontaneous mutation in Peru-15 and Bengal-15 leading to the nonmotile and nonreactogenic phenotype is still not characterized. Furthermore, the molecular and cellular mechanisms of V. cholerae interaction with epithelial cells are poorly understood. Recently, a high-density host gene array showed that reactogenic V. cholerae strains trigger higher levels of proinflammatory cytokine gene transcriptions than nonreactogenic strains in T84 cells (N. Stokes and J. B. Kaper, submitted for publication); the genes include those encoding macrophage inflammatory protein 3 alpha, tumor necrosis factor alpha, etc. It is possible that the proinflammatory cytokines induced by live, motile V. cholerae strains in intestinal epithelial cells may be the cause of the reactogenicity observed in human trials, which is in agreement with reports of others (38, 52).
Among the proinflammatory cytokines, IL-8 is especially important since it is a potent chemoattractant for PMN and can recruit PMN into the infected site and promote their infiltration of the epithelial layer, resulting in the opening of epithelial cell tight junctions (29). It was shown that IL-8 induction plays an important role in gut inflammation during infection by S. enterica serovar Typhimurium (reviewed in reference 28) and enteropathogenic E. coli, among other organisms (40). We hypothesize that a heat-resistant and proteinase- sensitive factor(s) of V. cholerae may account for the gut inflammation seen in volunteers in cholera vaccine trials by inducing the IL-8 production in epithelial cells. Currently, we are further investigating the nature of the IL-8 inducer.
In the present study, our data suggested that hap may not be involved in the induction of IL-8 production in T84 cells. The vaccine candidate 638 is nonreactogenic probably due to a factor(s) other than motility. At least one unidentified IL-8 inducer of CVD115 was secreted into the supernatant. The inducer is heat resistant and secreted abundantly in stationary phase. The identification of the IL-8 inducer may shed light on the mechanisms of reactogenicity caused by V. cholerae vaccine candidates and may provide important information for the development of live attenuated vaccines.
Acknowledgments
We thank the entire Kaper laboratory for encouragement and support, particularly Alfredo Torres for assistance with the mutagenesis of V. cholerae, and Shelly Payne at the University of Texas at Austin for sharing the mutagenesis protocol. We thank Alfredo Torres, Laura Q. Leverton, Jennifer Smart, and Kristen Kanack for their critical reading of the text and Diana Gomez for the excellent assistance with cell culture.
This work was supported by grant AI19716 from the National Institutes of Health.
Editor: A. D. O'Brien
REFERENCES
- 1.Aliprantis, A. O., D. S. Weiss, J. D. Radolf, and A. Zychlinsky. 2001. Release of Toll-like receptor-2-activating bacterial lipoproteins in Shigella flexneri culture supernatants. Infect. Immun. 69:6248-6255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aliprantis, A. O., R. B. Yang, M. R. Mark, S. Suggett, B. Devaux, J. D. Radolf, G. R. Klimpel, P. Godowski, and A. Zychlinsky. 1999. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science 285:736-739. [DOI] [PubMed] [Google Scholar]
- 3.Benitez, J. A., L. Garcia, A. Silva, H. Garcia, R. Fando, B. Cedre, A. Perez, J. Campos, B. L. Rodriguez, J. L. Perez, T. Valmaseda, O. Perez, M. Ramirez, T. Ledon, M. D. Jidy, M. Lastre, L. Bravo, and G. Sierra. 1999. Preliminary assessment of the safety and immunogenicity of a new CTXΦ- negative, hemagglutinin/protease-defective El Tor strain as a cholera vaccine candidate. Infect. Immun. 67:539-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benitez, J. A., A. J. Silva, and R. A. Finkelstein. 2001. Environmental signals controlling production of hemagglutinin/protease in Vibrio cholerae. Infect. Immun. 69:6549-6553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benitez, J. A., R. G. Spelbrink, A. Silva, T. E. Phillips, C. M. Stanley, M. Boesman-Finkelstein, and R. A. Finkelstein. 1997. Adherence of Vibrio cholerae to cultured differentiated human intestinal cells: an in vitro colonization model. Infect. Immun. 65:3474-3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coelho, A., J. R. Andrade, A. C. Vicente, and V. J. DiRita. 2000. Cytotoxic cell vacuolating activity from Vibrio cholerae hemolysin. Infect. Immun. 68:1700-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen, M. B., R. A. Giannella, J. Bean, D. N. Taylor, S. Parker, A. Hoeper, S. Wowk, J. Hawkins, S. K. Kochi, G. Schiff, and K. P. Killeen. 2002. Randomized, controlled human challenge study of the safety, immunogenicity, and protective efficacy of a single dose of Peru-15, a live attenuated oral cholera vaccine. Infect. Immun. 70:1965-1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coster, T. S., K. P. Killeen, M. K. Waldor, D. T. Beattie, D. R. Spriggs, J. R. Kenner, A. Trofa, J. C. Sadoff, J. J. Mekalanos, and D. N. Taylor. 1995. Safety, immunogenicity, and efficacy of live attenuated Vibrio cholerae O139 vaccine prototype. Lancet 345:949-952. [DOI] [PubMed] [Google Scholar]
- 9.DiMango, E., H. J. Zar, R. Bryan, and A. Prince. 1995. Diverse Pseudomonas aeruginosa gene products stimulate respiratory epithelial cells to produce interleukin-8. J. Clin. Investig. 96:2204-2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fasano, A., B. Baudry, D. W. Pumplin, S. S. Wasserman, B. D. Tall, J. M. Ketley, and J. B. Kaper. 1991. Vibrio cholerae produces a second enterotoxin, which affects intestinal tight junctions. Proc. Natl. Acad. Sci. USA 88:5242-5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finkelstein, R. A., M. Boesman-Finkelstein, and P. Holt. 1983. Vibrio cholerae hemagglutinin/lectin/protease hydrolyzes fibronectin and ovomucin: F. M. Burnet revisited. Proc. Natl. Acad. Sci. USA 80:1092-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finkelstein, R. A., and L. F. Hanne. 1982. Purification and characterization of the soluble hemagglutinin (cholera lectin) produced by Vibrio cholerae. Infect. Immun. 36:1199-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freter, R., P. C. O'Brien, and M. S. Macsai. 1981. Role of chemotaxis in the association of motile bacteria with intestinal mucosa: in vivo studies. Infect. Immun. 34:234-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fullner, K. J., J. C. Boucher, M. A. Hanes, G. K. Haines III, B. M. Meehan, C. Walchle, P. J. Sansonetti, and J. J. Mekalanos. 2002. The contribution of accessory toxins of Vibrio cholerae O1 El Tor to the proinflammatory response in a murine pulmonary cholera model. J. Exp. Med. 195:1455-1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Häse, C. C., and R. A. Finkelstein. 1991. Cloning and nucleotide sequence of the Vibrio cholerae hemagglutinin/protease (HA/protease) gene and construction of an HA/protease-negative strain. J. Bacteriol. 173:3311-3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayashi, F., K. D. Smith, A. Ozinsky, T. R. Hawn, E. C. Yi, D. R. Goodlett, J. K. Eng, S. Akira, D. M. Underhill, and A. Aderem. 2001. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410:1099-1103. [DOI] [PubMed] [Google Scholar]
- 17.Heidelberg, J. F., J. A. Eisen, W. C. Nelson, R. A. Clayton, M. L. Gwinn, R. J. Dodson, D. H. Haft, E. K. Hickey, J. D. Peterson, L. Umayam, S. R. Gill, K. E. Nelson, T. D. Read, H. Tettelin, D. Richardson, M. D. Ermolaeva, J. Vamathevan, S. Bass, H. Qin, I. Dragoi, P. Sellers, L. McDonald, T. Utterback, R. D. Fleishmann, W. C. Nierman, and O. White. 2000. DNA sequence of both chromosomes of the cholera pathogen Vibrio cholerae. Nature 406:477-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hickey, T. E., A. L. McVeigh, D. A. Scott, R. E. Michielutti, A. Bixby, S. A. Carroll, A. L. Bourgeois, and P. Guerry. 2000. Campylobacter jejuni cytolethal distending toxin mediates release of interleukin-8 from intestinal epithelial cells. Infect. Immun. 68:6535-6541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huet, C., C. Sahuquillo-Merino, E. Coudrier, and D. Louvard. 1987. Absorptive and mucus-secreting subclones isolated from a multipotent intestinal cell line (HT-29) provide new models for cell polarity and terminal differentiation. J. Cell Biol. 105:345-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jobling, M. G., and R. K. Holmes. 1997. Characterization of hapR, a positive regulator of the Vibrio cholerae HA/protease gene hap, and its identification as a functional homologue of the Vibrio harveyi luxR gene. Mol. Microbiol. 26:1023-1034. [DOI] [PubMed] [Google Scholar]
- 21.Kaper, J. B., H. Lockman, M. M. Baldini, and M. M. Levine. 1984. Recombinant nontoxinogenic Vibrio cholerae strains as attenuated cholera vaccine candidates. Nature 308:655-658. [DOI] [PubMed] [Google Scholar]
- 22.Kaper, J. B., J. G. Morris, Jr., and M. M. Levine. 1995. Cholera. Clin. Microbiol. Rev. 8:48-86. (Erratum, 8: 316.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kenner, J. R., T. S. Coster, D. N. Taylor, A. F. Trofa, M. Barrera-Oro, T. Hyman, J. M. Adams, D. T. Beattie, K. P. Killeen, D. R. Spriggs, et al. 1995. Peru-15, an improved live attenuated oral vaccine candidate for Vibrio cholerae O1. J. Infect. Dis. 172:1126-1129. [DOI] [PubMed] [Google Scholar]
- 24.Lee, C. A., M. Silva, A. M. Siber, A. J. Kelly, E. Galyov, and B. A. McCormick. 2000. A secreted Salmonella protein induces a proinflammatory response in epithelial cells, which promotes neutrophil migration. Proc. Natl. Acad. Sci. USA 97:12283-12288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levine, M. M., R. E. Black, M. L. Clements, L. Cisneros, D. R. Nalin, and C. R. Young. 1981. Duration of infection-derived immunity to cholera. J. Infect. Dis. 143:818-820. [DOI] [PubMed] [Google Scholar]
- 26.Levine, M. M., J. B. Kaper, D. Herrington, J. Ketley, G. Losonsky, C. O. Tacket, B. Tall, and S. Cryz. 1988. Safety, immunogenicity, and efficacy of recombinant live oral cholera vaccines, CVD 103 and CVD 103-HgR. Lancet ii:467-470. [DOI] [PubMed] [Google Scholar]
- 26a.Levine, M. M., J. B. Kaper, D. Herrington, G. Lonsonsky, J. G. Morris, Jr., M. L. Clements, R. E. Black, B. Tall, and R. Hall. 1988. Volunteer studies of deletion mutants of Vibrio cholerae O1 prepared by recombinant techniques. Infect. Immun. 56:161-167. [DOI] [PMC free article] [PubMed]
- 27.Lin, W., K. J. Fullner, R. Clayton, J. A. Sexton, M. B. Rogers, K. E. Calia, S. B. Calderwood, C. Fraser, and J. J. Mekalanos. 1999. Identification of a Vibrio cholerae RTX toxin gene cluster that is tightly linked to the cholera toxin prophage. Proc. Natl. Acad. Sci. USA 96:1071-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCormick, B. A. 2003. Salmonella spp.: masters of inflammation, p. 439-454. In G. Hecht (ed.), Microbial pathogenesis and the intestinal epithelial cell. ASM Press, Washington D.C.
- 29.McCormick, B. A., C. A. Parkos, S. P. Colgan, D. K. Carnes, and J. L. Madara. 1998. Apical secretion of a pathogen-elicited epithelial chemoattractant activity in response to surface colonization of intestinal epithelia by Salmonella typhimurium. J. Immunol. 160:455-466. [PubMed] [Google Scholar]
- 30.Mekalanos, J. J., D. J. Swartz, G. D. Pearson, N. Harford, F. Groyne, and M. de Wilde. 1983. Cholera toxin genes: nucleotide sequence, deletion analysis and vaccine development. Nature 306:551-557. [DOI] [PubMed] [Google Scholar]
- 31.Mel, S. F., K. J. Fullner, S. Wimer-Mackin, W. I. Lencer, and J. J. Mekalanos. 2000. Association of protease activity in Vibrio cholerae vaccine strains with decreases in transcellular epithelial resistance of polarized T84 intestinal epithelial cells. Infect. Immun. 68:6487-6492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mey, A. R., and S. M. Payne. 2001. Haem utilization in Vibrio cholerae involves multiple TonB-dependent haem receptors. Mol. Microbiol. 42:835-849. [DOI] [PubMed] [Google Scholar]
- 33.Miller, J. R., L. J. Barrett, K. Kotloff, and R. L. Guerrant. 1994. A rapid test for infectious and inflammatory enteritis. Arch. Intern. Med. 154:2660-2664. [DOI] [PubMed] [Google Scholar]
- 34.Ogushi, K., A. Wada, T. Niidome, N. Mori, K. Oishi, T. Nagatake, A. Takahashi, H. Asakura, S. Makino, H. Hojo, Y. Nakahara, M. Ohsaki, T. Hatakeyama, H. Aoyagi, H. Kurazono, J. Moss, and T. Hirayama. 2001. Salmonella enteritidis FliC (flagella filament protein) induces human beta-defensin-2 mRNA production by Caco-2 cells. J. Biol. Chem. 276:30521-30526. [DOI] [PubMed] [Google Scholar]
- 35.O'Malley, S. M., S. L. Mouton, D. A. Occhino, M. T. Deanda, J. R. Rashidi, K. L. Fuson, C. E. Rashidi, M. Y. Mora, S. M. Payne, and D. P. Henderson. 1999. Comparison of the heme iron utilization systems of pathogenic vibrios. J. Bacteriol. 181:3594-3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qadri, F., R. Raqib, F. Ahmed, T. Rahman, C. Wenneras, S. K. Das, N. H. Alam, M. M. Mathan, and A. M. Svennerholm. 2002. Increased levels of inflammatory mediators in children and adults infected with Vibrio cholerae O1 and O139. Clin. Diagn. Lab. Immunol. 9:221-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Richie, E. E., N. H. Punjabi, Y. Y. Sidharta, K. K. Peetosutan, M. M. Sukandar, S. S. Wasserman, M. M. Lesmana, F. F. Wangsasaputra, S. S. Pandam, M. M. Levine, P. P. O'Hanley, S. J. Cryz, and C. H. Simanjuntak. 2000. Efficacy trial of single-dose live oral cholera vaccine CVD 103-HgR in North Jakarta, Indonesia, a cholera-endemic area. Vaccine 18:2399-2410. [DOI] [PubMed] [Google Scholar]
- 38.Rodriguez, B. L., A. Rojas, J. Campos, T. Ledon, E. Valle, W. Toledo, and R. Fando. 2001. Differential interleukin-8 response of intestinal epithelial cell line to reactogenic and nonreactogenic candidate vaccine strains of Vibrio cholerae. Infect. Immun. 69:613-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 40.Savkovic, S. D., A. Koutsouris, and G. Hecht. 1997. Activation of NF-kappaB in intestinal epithelial cells by enteropathogenic Escherichia coli. Am. J. Physiol. 273:C1160-1167. [DOI] [PubMed] [Google Scholar]
- 41.Silva, T. M., M. A. Schleupner, C. O. Tacket, T. S. Steiner, J. B. Kaper, R. Edelman, and R. Guerrant. 1996. New evidence for an inflammatory component in diarrhea caused by selected new, live attenuated cholera vaccines and by El Tor and O139 Vibrio cholerae. Infect. Immun. 64:2362-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith, R. S., E. R. Fedyk, T. A. Springer, N. Mukaida, B. H. Iglewski, and R. P. Phipps. 2001. IL-8 production in human lung fibroblasts and epithelial cells activated by the Pseudomonas autoinducer N-3-oxododecanoyl homoserine lactone is transcriptionally regulated by NF-kappa B and activator protein-2. J. Immunol. 167:366-374. [DOI] [PubMed] [Google Scholar]
- 43.Tacket, C. O., M. B. Cohen, S. S. Wasserman, G. Losonsky, S. Livio, K. Kotloff, R. Edelman, J. B. Kaper, S. J. Cryz, R. A. Giannella, G. Schiff, and M. M. Levine. 1999. Randomized, double-blind, placebo-controlled, multicentered trial of the efficacy of a single dose of live oral cholera vaccine CVD 103-HgR in preventing cholera following challenge with Vibrio cholerae O1 El Tor Inaba three months after vaccination. Infect. Immun. 67:6341-6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tacket, C. O., K. L. Kotloff, G. Losonsky, J. P. Nataro, J. Michalski, J. B. Kaper, R. Edelman, and M. M. Levine. 1997. Volunteer studies investigating the safety and efficacy of live oral El Tor Vibrio cholerae O1 vaccine strain CVD 111. Am. J. Trop. Med. Hyg. 56:533-537. [DOI] [PubMed] [Google Scholar]
- 45.Tacket, C. O., G. Losonsky, J. P. Nataro, L. Comstock, J. Michalski, R. Edelman, J. B. Kaper, and M. M. Levine. 1995. Initial clinical studies of CVD 112 Vibrio cholerae O139 live oral vaccine: safety and efficacy against experimental challenge. J. Infect. Dis. 172:883-886. [DOI] [PubMed] [Google Scholar]
- 46.Tacket, C. O., G. Losonsky, J. P. Nataro, S. J. Cryz, R. Edelman, A. Fasano, J. Michalski, J. B. Kaper, and M. M. Levine. 1993. Safety and immunogenicity of live oral cholera vaccine candidate CVD 110, a ΔctxAΔzotΔace derivative of El Tor Ogawa Vibrio cholerae. J. Infect. Dis. 168:1536-1540. [DOI] [PubMed] [Google Scholar]
- 47.Tauxe, R., L. Seminario, R. Taipia, and M. O. Libel. 1994. The Latin American epidemic, p. 321-344. In I. K. Wachsmuth, P. A. Blake, and Ø. Olsvik (ed.), Vibrio cholerae and cholera: molecular to global perspectives. ASM Press, Washington, D.C.
- 48.Taylor, D. N., K. P. Killeen, D. C. Hack, J. R. Kenner, T. S. Coster, D. T. Beattie, J. Ezzell, T. Hyman, A. Trofa, M. H. Sjogren, A. Friedlander, J. J. Mekalanos, and J. C. Sadoff. 1994. Development of a live, oral, attenuated vaccine against El Tor cholera. J. Infect. Dis. 170:1518-1523. [DOI] [PubMed] [Google Scholar]
- 49.Trucksis, M., J. E. Galen, J. Michalski, A. Fasano, and J. B. Kaper. 1993. Accessory cholera enterotoxin (Ace), the third toxin of a Vibrio cholerae virulence cassette. Proc. Natl. Acad. Sci. USA 90:5267-5271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Uhlen, P., A. Laestadius, T. Jahnukainen, T. Soderblom, F. Backhed, G. Celsi, H. Brismar, S. Normark, A. Aperia, and A. Richter-Dahlfors. 2000. Alpha-haemolysin of uropathogenic E. coli induces Ca2+ oscillations in renal epithelial cells. Nature 405:694-697. [DOI] [PubMed] [Google Scholar]
- 51.Vidal, V., I. G. Scragg, S. J. Cutler, K. A. Rockett, D. Fekade, D. A. Warrell, D. J. Wright, and D. Kwiatkowski. 1998. Variable major lipoprotein is a principal TNF-inducing factor of louse-borne relapsing fever. Nat. Med. 4:1416-1420. [DOI] [PubMed] [Google Scholar]
- 52.Waldor, M. K., and J. Mekalanos. 1996. Progress toward live-attenuated cholera vaccines, p. 229-240. In H. Kiyono, P. L. Ogra, and J. R. McGhee (ed.), Mucosal vaccines. Academic Press, San Diego, Calif.
- 53.Wooten, R. M., T. B. Morrison, J. H. Weis, S. D. Wright, R. Thieringer, and J. J. Weis. 1998. The role of CD14 in signaling mediated by outer membrane lipoproteins of Borrelia burgdorferi. J. Immunol. 160:5485-5492. [PubMed] [Google Scholar]
- 54.Wu, Z., D. Milton, P. Nybom, A. Sjo, and K. E. Magnusson. 1996. Vibrio cholerae hemagglutinin/protease (HA/protease) causes morphological changes in cultured epithelial cells and perturbs their paracellular barrier function. Microb. Pathog. 21:111-123. [DOI] [PubMed] [Google Scholar]
- 55.Wu, Z., P. Nybom, and K. E. Magnusson. 2000. Distinct effects of Vibrio cholerae haemagglutinin/protease on the structure and localization of the tight junction-associated proteins occludin and ZO-1. Cell Microbiol. 2:11-17. [DOI] [PubMed] [Google Scholar]
- 56.Xu, Q., M. Dziejman, and J. J. Mekalanos. 2003. Determination of the transcriptome of Vibrio cholerae during intraintestinal growth and midexponential phase in vitro. Proc. Natl. Acad. Sci. USA 100:1286-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou, X., J. A. G., A. G. Torres, J. A. Crawford, E. Negree, S. N. Vogel, and J. B. Kaper. 2003. Flagellin of enteropathogenic Escherichia coli stimulates interleukin-8 production in 84 cells. Infect. Immun. 71:2120-2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhu, J., M. B. Miller, R. E. Vance, M. Dziejman, B. L. Bassler, and J. J. Mekalanos. 2002. Quorum-sensing regulators control virulence gene expression in Vibrio cholerae. Proc. Natl. Acad. Sci. USA 99:3129-3134. [DOI] [PMC free article] [PubMed] [Google Scholar]