Abstract
There have been rapid technological advances in blood banking in South Asian region over the past decade with an increasing emphasis on quality and safety of blood products. The conventional test tube technique has given way to newer techniques such as column agglutination technique, solid phase red cell adherence assay, and erythrocyte-magnetized technique. These new technologies are adaptable to automation and major manufacturers in this field have come up with semi and fully automated equipments for immunohematology tests in the blood bank. Automation improves the objectivity and reproducibility of tests. It reduces human errors in patient identification and transcription errors. Documentation and traceability of tests, reagents and processes and archiving of results is another major advantage of automation. Shifting from manual methods to automation is a major undertaking for any transfusion service to provide quality patient care with lesser turnaround time for their ever increasing workload. This article discusses the various issues involved in the process.
Keywords: Automation, immunohematology, serology
Introduction
There have been rapid technological advances in blood banking in our region over the past decade with an increasing emphasis on quality and safety of blood products. The conventional pre-transfusion testing techniques in immunohematolgy are quite cumbersome. The most commonly employed, tube technique, though still considered as a gold standard has some inherent limitations in the form of; elution of low affinity antibodies during washing, variability in the red cell concentrations, improper cell serum ratio, and lack of consistency in reporting the results due to inter observer variability.[1,2] The introduction of newer techniques such as column agglutination technique (CAT), Solid Phase Red Cell Adherence Assay (SPRCA), and erythrocyte-magnetized technique (EMT) have tried to overcome these short comings and bring about an improvement in the quality of testing and the reproducibility of results.[1–3] These new technologies are amenable to automation and major manufacturers in this field have come up with user-friendly semi-automated and fully automated equipments for immunohematology tests with varying throughputs depending upon the work load of a blood bank. We, here, present a brief introduction of major equipments available and the requirements for shifting from the conventional technology to the automated systems.
Automation in Blood bank serology was introduced in the developed countries in the 1960s.[4] Automation in blood banks is being adopted by more and more centers and is rapidly becoming a standard testing technology in developed nations.[5] In India automation has come up in a big way with the larger centers shifting to totally automated platforms for serologic testing. Automation provides the advantage of improving quality of testing by:[6,7]
Decreasing human errors in sample identification which has often been quoted as a significant cause of near miss events and transfusion reactions due to mismatched blood transfusion.[6]
Reducing human errors while performing tests and subjective variations during interpretation of results.
Preventing transcription errors during documentation of results.
Improving objectivity, reproducibility, and storage and retrieval of results of immunohematology tests.
Improving traceability of all variables during testing including, samples, reagents and operating staff.
It reduces manual input and therefore results in manpower economy.
High throughput devices with lesser turnaround time improve the quality of services in large tertiary care settings.
The selection of the optimum equipment for a blood center depends on the workload, resources, and space available. In India at present the following manufacturers are providing fully automated and semi-automated Immunohematology platforms:
BIO-RAD (Switzerland) - IH-1000/Techno Twin Station/Saxo ID Reader.
DIAGAST (France) - Qwalys 3/FREELYS Mini Lab.
Ortho-Clinical Diagnostics (Johnson & Johnson, USA) - Autovue Innova/Biovue.
IMMUCOR (USA) - Galileo/NEO.
Grifols (Singapore) - WADIANA/Semi-automated.
The automated platforms from BIO-RAD, Ortho-Clinical Diagnostics, and Grifols are based on the column agglutination technology. The IMMUCOR platform is based on SPRCA principle, while DIAGAST is based on the innovative erythro-magnetic technology. The detailed methods are described below:
Column Agglutination Technology[1,8] : The column agglutination test system consists of a plastic card with six to eight inbuilt microtubes. The microtubes have a broad reaction chamber in the upper part, while the lower part contains either a clear gel (BIO-RAD and Grifols) or a glass microbead matrix (Ortho-Clinical). Antihuman globulin (AHG) or other antisera are incorporated in the gel or microbead matrix as per requirements for a particular test. A weak suspension (0.8–1%) of red cells is prepared in a low ionic strength solution (LISS) and a measured amount of red blood cell (RBC) suspension is mixed with plasma. The cards are incubated if required and then centrifuged. The sensitized red cells agglutinate in the presence of AHG in the gel/bead matrix and get trapped while unsensitized cells form a button at the bottom of the microtube. The reactions may be graded from 1 + to 4 + and a card reader if used provides objectivity to the grading. Omission of the washing steps during testing reduces the turnaround time and elution of antibodies. The tests results may be preserved for up to 24 h in the testing card, however, they can be stored for longer periods in electronic formats. The column agglutination system is an open system and can fulfill a variety of red cell serology testing requirements.
Solid Phase Red Cell Adherence Assay[1,8,9]: This is a technique in which one of the components of an antigen–antibody reaction is immobilized onto a solid medium and after reaction with a free antigen/antibody the end point of the reaction is indicated by use of red cells, which may be a part of the antigen–antibody reaction or may be added as indicator cells. In forward grouping U shaped micro plate wells are coated with Anti-A antiserum, Anti-B antiserum, and Anti-D antiserum, A drop of 0.5% bromelin-treated red cells are added to the well. On centrifugation antigen positive cells spread out while antigen negative cells form a button at the bottom of the well. In case of reverse grouping a monolayer of RBC membrane is attached to the bottom of the well and plasma to be tested is added after incubation for 5 min, the excess plasma is blotted and anti-IgG bound indicator red cells are added to give a visible reaction. SPRCA may be adapted to other red cell serology tests such as antibody screening, identification, and cross matching. It may also be adapted to platelet serology.
Erythro-Magnetic Technology[2,10] : This technology is based on the magnetization of RBCs. Paramagnetic particles are adsorbed on to the surface of RBCs. Once antibodies in plasma/antisera react with antigens on RBCs in a micro plate well a magnetic force is applied at the bottom of the microplate using a magnetic plate, this causes the RBCs to be pulled toward the bottom of the microplate. In this manner the magnetic force replaces the centrifugation step. On shaking/resuspension the reactions may be deciphered. In forward grouping the test RBCs are suspended in a solution of iron chloride and bromelin, then the RBC suspension is dispensed into the microplate well precoated with antisera. This is followed by gentle shaking and incubation for 10 min, and then the microplate is put on a magnetic plate. The magnetized RBCs gather at the bottom of the plate. On shaking after this step the free RBCs are resuspended while agglutinated RBCs form a button at the bottom of the well. In case of reverse grouping premagnetized RBCs are mixed with test plasma in the microplate wells followed by the same steps as above. This technology may also be adapted to antibody screening and identification.
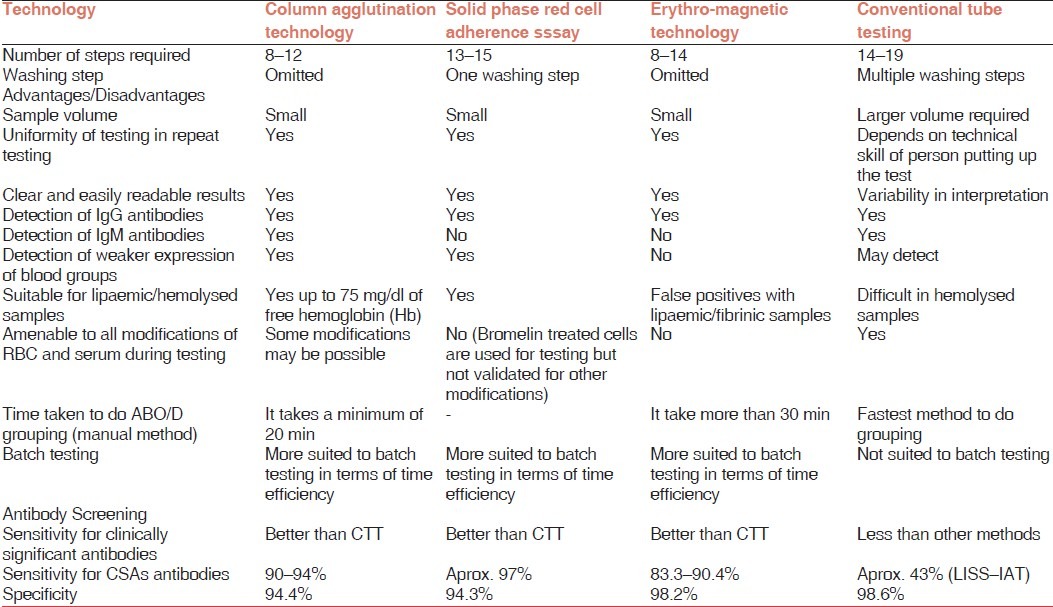
All the above described methods have reduced the manual input in putting up various serology tests and hence improved the laboratory efficiency. The gel (CAT) has shown a sensitivity as compared with conventional test tube (CTT) methods (93.5-100% for CAT vs. 50% for CTT).[11–13] The sensitivity of SPRCA has been found to be superior to CTT and comparable with that of CAT.[14] EMT is a relatively recent technology and not much has been published about it. In the available studies, it has been shown a performance comparable with that of gel CAT.[3] A comparison of the newer technologies with the conventional tube testing has been compiled in Table 1.[1,7,8,10,14,15] The newer methods have added to the quality of testing but none of the methods has been found to be unequivocally superior to others. Detection of all antibodies during antibody screening is not the goal of antibody screening; rather detecting all clinically significant antibodies should be the aim for any technology, as detection of insignificant antibodies adds to the burden of further work up and delay in providing blood to the patients.[8] While selecting an appropriate technology for automation there are a number of other issues to be considered and are discussed later.
Table 1.
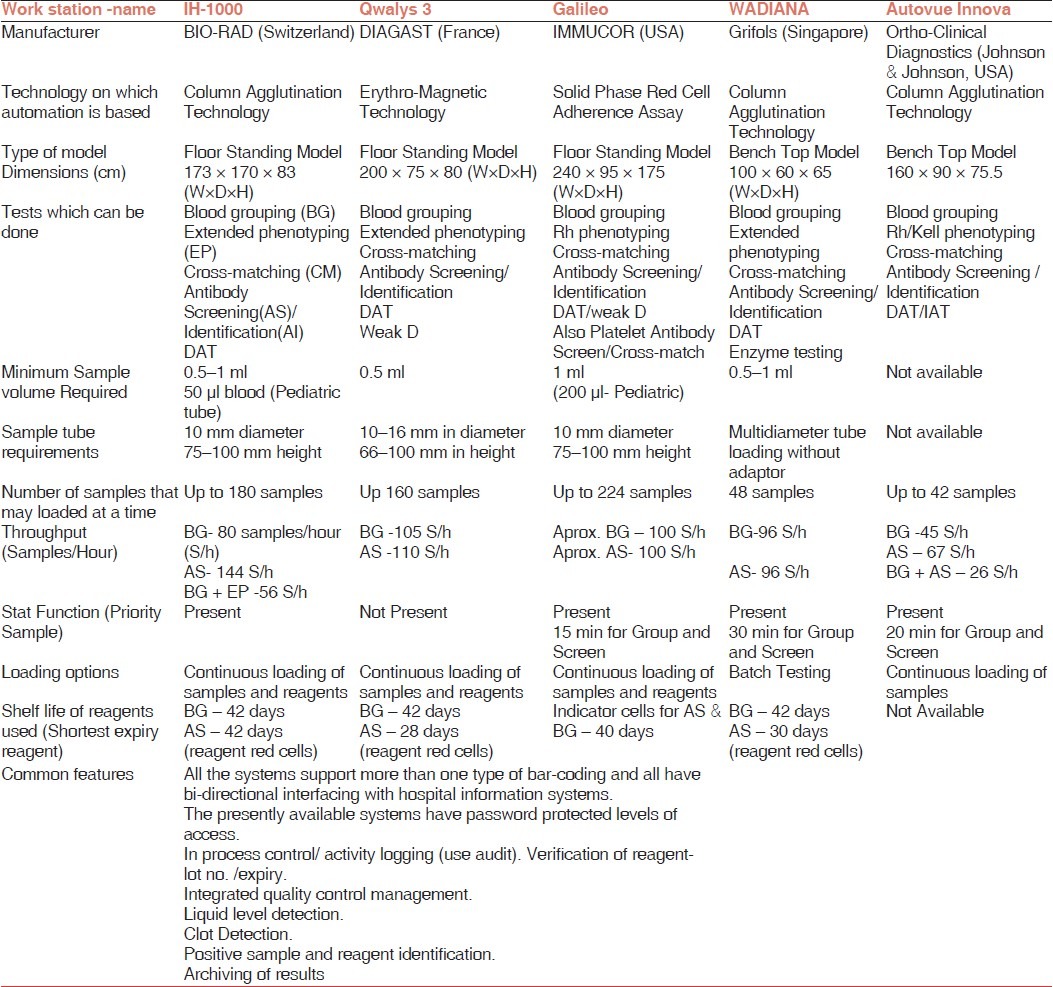
There are several fully automated immunohematology work stations available at present which differ in the technology used, configuration of the immunohematology tests, throughput, turnaround time, sample loading options, priority sample facility. A comparison of the major fully automated immunohematology work stations available in our region has been compiled in Table 2. The information given in the table has been taken from the product literatures and the vendors. The decision to buy an automated system depends on the location of the blood bank, type of services provided; cost issues, space availability, staff competency, and feedback regarding the equipment and services of the vendor. We will be discussing the major issues one by one.
Table 2.
Comparison of Fully Automated Immunohematology Workstations
Semi-Automated or Fully Automated
Transfusion services with a small workload may often prefer to go for semi-automated system rather than fully automated. It must be realized that although the number of steps may be reduced, there is still a substantial amount of manual input involved in semi-automated systems.[2] There is improvement in the objectivity and reproducibility of results as well as the time taken to perform certain tests.[8] The major issue that remains with semi-automated systems is the lower level of safety features than those available in fully automated systems and scope of human errors due to manual steps involved in sample labeling, dilution, reagent addition, and interpretation of results. Lack of interfacing with hospital information systems (HIS) in some of the equipments may lead to manual transcription errors while handling the data. In view of the above, semi-automated systems may be appropriate for small blood bank settings, however, for transfusion centers with high workload, a fully automated system is always a better option.
Cost Issues
Automation in immunohematology is expensive and usually requires a large initial investment. In places where purchase policies permit and the workload is substantial, a ‘reagent rental agreement’ can be worked out. Under this agreement the vendor provides the equipment and maintenance for free in return for a promised workload and the client buys reagents from the vendor at a negotiated rate contract. The initial costing should also include the change to be made in the space (restructuring the room), reagent storage facility (refrigerators, shelves), furniture, etc., required. The cost should also include the cost of hardware and software required for interfacing the equipment with the HIS. The cost of testing is difficult to compare due to the type of testing done and individual centers plan to follow. The cost per test usually decreases as the number of samples processed increases.
Feedback From Users
Taking a feedback from current users is one of the most important steps while deciding for equipment. However good an equipment may look on paper, it is the actual user who may provide a reasonable useful information on the following aspects.
Installation-related Issues (time taken from completion of installation to actual regular use of equipment, additional requirement, and problems faced during installation such as air conditioning, electrical refitting requirement of a water purification plant, etc.).
After sales Service support (turnaround time of a service call, competency of vendors service staff, staff training, etc.).
Reagents supply chain– (whether there is down time due to delay in receiving reagents from vendor, gap between date of receiving reagent and expiry of reagent).
Miscellaneous end user problems
Hospital Information System/laboratory Information System Interfacing
The equipment purchased should be compatible with the HIS/LIS of the hospital/institute. An agreement with the vendor regarding the interfacing should be done during negotiations as sometimes this is delayed due to the HIS vendor not being competent or willing to complete the interfacing. The client is not able to take full advantage of automation in terms of decreased documentation until this process is complete.
Back-Up for the Automated Equipment
Automated equipment will invariably have a down time at some point of time; therefore it is always advisable to have a backup system. Most vendors provide semi-automated equipment along with fully automated equipment, which may be helpful for a moderate work-load setting, however, a fully automated standby equipment is a better option. The important issue here is to keep the backup equipment in working order and the concerned staff should be trained to use both the systems. This may be done by placing the semi-automated equipment at another place and assigning a certain category of tests to be done on that equipment or using the systems alternatively.
Staff Training
Training of staff is an important component while introducing a new laboratory technique or technology and this holds true for the automation as well. Protocols for staff training must be discussed and planned with the vendor beforehand in a manner so that the routine patient care does not suffer. Staff may have to be given time off during initial training. A comprehensive training program, which makes the staff comfortable with the new technology, is imperative to make full use of the new technology; otherwise the staff is often overwhelmed by the new technology and tends to fall back on traditional methods of testing.
Sample Collection and Sample Flow
Automated systems may have stringent sample requirements to be loaded on to the system. Uniformity in donor samples, collected in the blood bank is easier to achieve but bringing uniformity in patient bedside sample collection may require an extra effort. It may not be possible to bring about uniformity if samples are also received from other hospitals in large regional blood centers that caters the blood and component needs of that region. Therefore, new standard operating procedures (SOPs) and workflow charts should be designed with the requisite information to be circulated in that area.
Validation of Equipment
The purpose of validation of an automated system is to test the competence of an automated system and demonstrate control over the processes executed by the automated system. It is also to ensure compliance to the accuracy and safety standards and enhance knowledge regarding maintenance and calibration of the equipment. A detailed discussion regarding validation is beyond the preview of this article and is explained in detail in the ÍSBT Guidelines for Validation Of Automated Systems in Blood Establishments.[16]
Continuous Quality Assurance
To continuously maintain the quality of work and improve performance a continuous appraisal of the following should be done.
Audit use of the equipment: To ensure optimum use of the equipment and shift additional tasks to the automated system as the staff becomes more comfortable with its use.
Optimization of reagent inventory: To ensure all reagents are used and there is minimal wastage due to expiry of reagents especially reagent red cells, which are short expiry products
Staff competence in performing the procedures: This should be tested from time to time and refresher courses undertaken specially when the system hardware/software is upgraded at any point of time.
Audit of results and downtime: A record of all samples which are flagged or reported as a discrepancy/unsuitable, should be recorded and corrective action taken wherever necessary. A record of downtime is essential to audit performance of the system and a record of the same should be maintained by the staff in-charge of the equipment.
Adopting automation in immunohematology is expected to bring about major changes in the pretransfusion testing laboratories and an overall facelift in the working of a transfusion service setup. Nevertheless, the newer challenges would be to keep pace with ever changing technologies with concurrent staff training, to make optimum use of automation, and to ensure a safe blood supply to patients.
Footnotes
Source of Support: Nil
Conflict of Interest: No.
References
- 1.Rumsey DH, Ciesielski DJ. New protocols in serologic testing: A review of techniques to meet today's challenges. Immunohematology. 2000;16:131–7. [PubMed] [Google Scholar]
- 2.Duguid JK, Bromilow IM. New technology in hospital blood banking. J Clin Pathol. 1993;46:585–8. doi: 10.1136/jcp.46.7.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouix O, Ferrera V, Delamaire M, Redersdorff JC, Roubinet F. Erythrocyte-magnetized technology: An original and innovative method for blood group serology. Transfusion. 2008;48:1878–85. doi: 10.1111/j.1537-2995.2008.01790.x. [DOI] [PubMed] [Google Scholar]
- 4.Sturgeon P, Cedergren B, McQuiston D. Automation of routine blood typing procedures. Vox Sang. 1963;8:438–51. doi: 10.1111/j.1423-0410.1963.tb04163.x. [DOI] [PubMed] [Google Scholar]
- 5.Garratty G. Advances in red blood cell immunology 1960 to 2009. Transfusion. 2010;50:526–35. doi: 10.1111/j.1537-2995.2009.02493.x. [DOI] [PubMed] [Google Scholar]
- 6.Morelati F, Barcellini W, Manera MC, Paccapelo C, Revelli N, Villa MA, et al. New technologies in immunohaematology. Blood Transfus. 2007;5:58–65. doi: 10.2450/2007.0006-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butch SH. Automation in the transfusion service. Immunohematology. 2008;24:86–92. [PubMed] [Google Scholar]
- 8.Casina TS. In search of the Holy Grail: Comparison of antibody screening methods Immunohematology. 2006;22:196–202. [PubMed] [Google Scholar]
- 9.Ching E. Solid Phase Red Cell Adherence Assay: A tubeless method for pretransfusion testing and other applications in transfusion science. Transfus Apher Sci. 2012;46:287–91. doi: 10.1016/j.transci.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 10.Schoenfeld H, Bulling K, von Heymann C, Neuner B, Kalus U, Kiesewetter H, et al. Evaluation of immunohematologic routine methods using the new erythrocyte-magnetized technology on the QWALYS 2 system. Transfusion. 2009;49:1347–52. doi: 10.1111/j.1537-2995.2009.02136.x. [DOI] [PubMed] [Google Scholar]
- 11.Nathalang O, Chuansumrit A, Prayoonwiwat W, Siripoonya P, Sriphaisal T. Comparison between the conventional tube technique and the gel technique in direct antiglobulin tests. Vox Sang. 1997;72:169–71. doi: 10.1046/j.1423-0410.1997.7230169.x. [DOI] [PubMed] [Google Scholar]
- 12.Das SS, Chaudhary R, Khetan D. A comparison of conventional tube test and gel technique in evaluation of direct antiglobulin test. Hematology. 2007;12:175–8. doi: 10.1080/10245330601111862. [DOI] [PubMed] [Google Scholar]
- 13.Novaretti MC, Jens E, Pagliarini T, Bonifacio SL, Dorlhiac-Llacer PE, Chamone DA. Comparison of conventional tube test technique and gel microcolumn assay for direct antiglobulin test: A large study. J Clin Lab Anal. 2004;18:255–8. doi: 10.1002/jcla.20033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weisbach V, Kohnhäuser T, Zimmermann R, Ringwald J, Strasser E, Zingsem J, et al. Comparison of the performance of microtube column systems and solid-phase systems and the tube low-ionic-strength solution additive indirect antiglobulin test in the detection of red cell alloantibodies. Transfus Med. 2006;16:276–84. doi: 10.1111/j.1365-3148.2006.00674.x. [DOI] [PubMed] [Google Scholar]
- 15.Schoenfeld H, Pretzel KJ, von Heymann C, Neuner B, Kalus U, Kiesewetter H, et al. Validation of a hospital-laboratory workstation for immunohematologic methods. Transfusion. 2010;50:26–31. doi: 10.1111/j.1537-2995.2009.02359.x. [DOI] [PubMed] [Google Scholar]
- 16.ISBT Guidelines for validation of automated systems in blood establishments. Validation. Task Force of the International Society of Blood Transfusion Working Party on Information Technology. Vox Sang. 2010;98(Suppl 1):S1–15. doi: 10.1111/j.1423-0410.2009.01287.x. [DOI] [PubMed] [Google Scholar]


