Abstract
Dendritic cells can initiate antimicrobial responses by CD1-mediated presentation of pathogen-derived glycolipids. We show that the protozoan Leishmania donovani inhibits CD1 expression and prevents activation of CD1-restricted T cells by dendritic cells. Evasion of presentation by CD1 may represent a Leishmania survival strategy to avoid recognition of abundant parasite glycolipids.
Parasites of the genus Leishmania generate severe clinical syndromes, ranging from self-healing cutaneous to often fatal visceral leishmaniasis, which afflict millions of people worldwide (2). All Leishmania species express a surface glycocalyx, composed of related glycolipids, including lipophosphoglycan and glycoinositol phospholipids, and this glycocalyx is involved in many aspects of parasite pathogenicity (15, 26, 30). Components of this surface coat are likely to be involved in the activation of innate immunity and may thus also have an important influence on the induction of the adaptive immune response against Leishmania parasites (6, 7, 9, 11, 16, 18).
The CD1 family consists of nonpolymorphic antigen-presenting molecules that are expressed on antigen-presenting cells and induced on peripheral blood monocytes (PBMs) by exposure to granulocyte-macrophage colony-stimulating factor (GM-CSF) and other cytokines (21, 23). Group I CD1 molecules, which include human CD1a, CD1b, and CD1c, have been shown to present mycobacterial lipids and glycolipids to specific T cells. These can potentially contribute to antimicrobial resistance by production of gamma interferon (IFN-γ) (24) and the antimicrobial protein granulysin (27). The group I CD1 proteins are thus likely to be involved in the protective immune response against complex intracellular pathogens such as Leishmania species, which are know to harbor abundant glycolipid antigens.
Intracellular pathogens, however, have evolved various survival strategies to avoid immune recognition of infected host cells. These include down-regulation of antigen-presenting molecules by Mycobacterium and Leishmania (19, 28), capture and degradation of antigen-presenting molecules by Leishmania mexicana and Leishmania amazonensis amastigotes (1), and interference with intracellular antigen-loading by Leishmania major promastigotes (10). Here we demonstrate that Leishmania donovani promastigotes block lipid antigen presentation in infected human DCs and down-regulate group I CD1 expression. Both processes may potentially be associated with the establishment of a disease-promoting immune response and chronic infection.
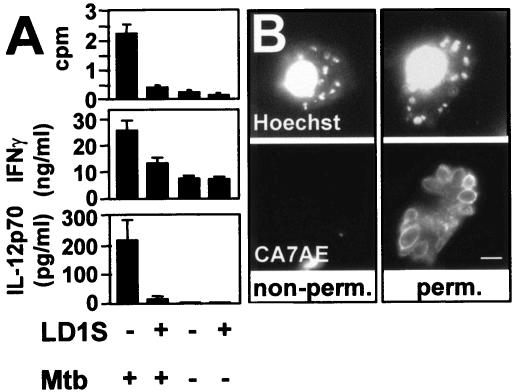
We tested in vitro the ability of Leishmania-infected dendritic cells (DCs) to present antigen to a well-characterized CD1b-restricted T-cell line (DN1) (17), which is specific for the mycobacterial lipid mycolic acid (4). Human blood monocytes were isolated from random donor leukocyte concentrates and differentiated to immature DCs by a 3-day incubation with GM-CSF (300 U/ml; Immunex Corporation, Seattle, Wash.) and interleukin 4 (IL-4; 200 U/ml; gift from Schering Corporation, Kenilworth, N.J.). Irradiated (5,000 rads) immature DCs were infected with stationary-phase L. donovani promastigotes (MHOM/SD/1S-2D, referred to as LD1S) at a multiplicity of 10 parasites per host cell, and infected cells as well as uninfected controls were incubated at a 1:1 ratio with DN1 T cells in the presence of 5 μg of Mycobacterium tuberculosis sonicate per ml. Uninfected DCs efficiently presented mycobacterial mycolic acids, as judged by incorporation of radiolabeled thymidine into proliferating activated T cells (Fig. 1A, top) and their robust secretion of IFN-γ (middle). In contrast, infected DCs were unable to stimulate T-cell proliferation, and a substantial decrease in T-cell IFN-γ production was observed. T cells were not activated by incubation with infected DCs alone or Leishmania sonicate (not shown), a result expected from the known specificity of the DN1 T-cell line to mycobacterial mycolic acid (4).
FIG. 1.
Leishmania-infected DCs inhibit CD1b-restricted T-cell activation. (A) T-cell proliferation and activation assay. L. donovani LD1S-infected DCs were incubated 2 days postinfection with CD1b-restricted T cells (DN1 cells) in the presence or absence of 5 μg of M. tuberculosis (Mtb) sonicate per ml, and proliferation (top) and cytokine secretion (middle) were assessed. At least three independent experiments with similar outcomes were performed, and triplicate values from one representative experiment are shown. (B) Immunofluorescence analysis. Infected human DCs were immobilized on poly-l-lysine-coated glass coverslips, fixed in 4% paraformaldehyde (left), or fixed and permeabilized with ice cold ethanol (right) and sequentially incubated with the monoclonal antiphosphoglycan antibody CA7AE and Texas red-conjugated anti-mouse immunoglobulin G secondary antibody. Host and parasite DNAs were counterstained with 0.5 μg of Hoechst 33342 per ml. Bar, 5 μm.
Leishmania infection had also a profound effect on DC cytokine production induced by exposure to the M. tuberculosis sonicate. Antigen-treated, uninfected DCs produced increased levels of IL-12 as a result of their maturation following CD1b-T-cell-receptor ligation. In contrast, IL-12 production was completely abolished in the presence of intracellular Leishmania (Fig. 1A, bottom). Together, these data indicated that Leishmania disrupts the primary immune recognition of lipid antigens in infected human DCs and subverts the protective immune response by inhibition of T-cell activation and host cell IL-12 production.
Since the ability of DCs to phagocytose Leishmania promastigotes in culture has been questioned (31), we confirmed that infection of DCs under the conditions used in our experiments led to intracellular parasitism. As determined by nuclear staining, 85 to 90% of DCs were generally infected in our experiments (Fig. 1B, top). Only a minor fraction of the parasites were associated with the host cell surface, as shown by immunofluorescence staining of nonpermeabilized DCs with antiphosphoglycan antibody CA7AE (29). More than 90% of the parasites were labeled only following host cell permeabilization, indicating predominant intracellular parasite localization.
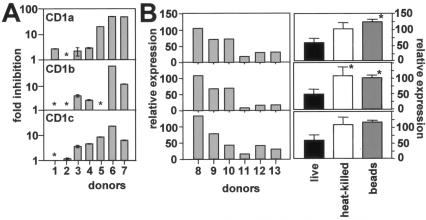
We next tested if inhibition of the CD1-mediated responses was associated with transcriptional down-regulation of CD1 expression. Human DCs obtained from seven healthy random donors were infected with stationary-phase promastigotes as described above, and total RNA was isolated with Trizol reagent (Gibco BRL, Carlsbad, Calif.) 2 days after the infection. Abundance of CD1a, -b, and -c mRNA was determined by quantitative reverse transcription (RT)-PCR. Three distinct patterns of regulation were observed (Fig. 2A): two donors (donors 1 and 2) showed no or only moderate down-regulation of the three CD1 isoforms, two donors (donors 3 and 4) showed substantial inhibition of CD1 expression ranging from two- to fivefold, and three donors (donors 5, 6, and 7) showed strong down-regulation above 20-fold compared to the uninfected control. With the exception of donor 5, the level of inhibition was comparable between the three isoforms, suggesting a process of coordinate down-regulation of the genes. However, the overall heterogeneity of this phenomenon between the various donors suggests the presence of unknown host factors that determine susceptibility to CD1 down-regulation.
FIG. 2.
Leishmania-infected DCs down-regulate CD1 expression. (A) RT-PCR analysis. Infected DCs and uninfected controls from seven donors were cultured for 48 h, and total RNA was extracted. TaqMan one-step RT-PCR (Applied Biosystems) was carried out with the ABI Prism 7900HT apparatus (Perkin-Elmer). CD1a, -b, and -c specific primer oligonucleotides and an internal probe oligonucleotide labeled with both a reporter (VIC; Applied Biosystems) and a quencher dye (TAMRA; 6-carboxy-tetramethyl-rhodamine) were added to the RT-PCR mix at the beginning of the reaction. GAPDH (glyceraldehyde-3-phosphate dehydrogenase) mRNA levels were measured as an internal control with the human GAPDH TaqMan one-step kit (Applied Biosystems). Sequences for CD1 forward primers, reverse primers, and probes (respectively) were as follows: CD1A, 5′-ATCTCCAGCGGTCAAGTGAAG-3′, 5′-ACACGGGCTTTGGGTAGAATC-3′, and VIC-5′-CCTAGGCCTGGCTGTCCCATG-3′-TAMRA; CD1B, 5′-GGGTGAAGCACACAGCAGTTTAGA-3′, 5′-CCAAACAATTGAGCCAATGG-3′, and VIC-5′-AGGACATCATCTCTACTGGAGAAACCCCAC-3′-TAMRA; CD1C, 5′-GGGAGATTCAATACCATGCAA-3′, 5′-CAGCCCGCTTTCACCTGTAC-3′, and VIC-5′-TCAAGATTACTCGAAATATCCCTTTGA-3′-TAMRA. Inhibition of CD1 mRNA expression of the infected DCs relative to that of the uninfected DCs from the same donor is shown. *, no inhibition. Analysis was done in quadruplicate, and bars indicate standard deviations. (B) Flow cytometry. DCs obtained from six donors (left) were infected with stationary-phase LD1S promastigotes, and surface marker expression was assessed by flow cytometry. The inhibition of the mean fluorescence intensity relative to that of autologous uninfected DCs (percent) is shown. Stationary-phase, live promastigotes, heat-killed promastigotes, or 1-nm latex beads (Sigma) were incubated at 37°C at a 10:1 ratio with immature DCs (left). Surface marker expression was quantified 48 h later and compared to that in autologous uninfected controls. *, statistically significant difference relative to live-Leishmania-infected cells (P < 0.05). Statistical analysis was performed with the Mann-Whitney U test.
Consistent with reduced abundance of CD1 mRNA, we observed a reduction in CD1 surface expression in Leishmania-infected DCs. Surface expression of CD1a (using monoclonal antibody OKT6 [20]), CD1b (using monoclonal antibody BCD1b3 [5]), and CD1c (using monoclonal antibody F10/21A3 [22]) was determined by flow cytometry in DCs 2 days after infection with stationary-phase promastigotes and compared to that in uninfected controls. As seen at the transcriptional level, the response of the various donors was heterogenous (Fig. 2B, left), including no down-regulation (donor 8), moderate (2-fold) down-regulation (donors 9 and 10), or strong (5- to 10-fold) down-regulation (donors 11 to 13). Inhibition of CD1 expression was not observed following incubation of DCs with 1 nm latex beads (Sigma, St. Louis, Mo.) or heat-inactivated Leishmania promastigotes (56°C, 30 min) (Fig. 2B, right). Thus, CD1 down-regulation appeared to be an active process driven by live Leishmania and not a consequence of phagocytosis of inert particles or nonviable parasite-derived material.
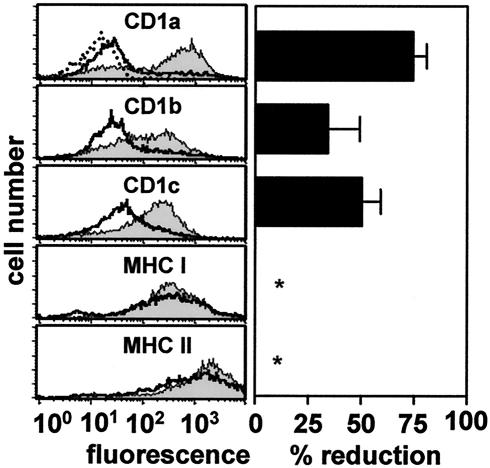
Visceral Leishmania heavily colonizes the bone marrow of the infected host, where it may interfere with the differentiation of immune effector cells. We therefore tested the effect of intracellular Leishmania on induction of CD1 expression during differentiation of PBMs into immature DCs. PBMs of three donors were infected with stationary-phase promastigotes and incubated for 3 days with GM-CSF-IL-4. Surface marker expression of CD1a, -b, and -c, as well as major histocompatibility complex (MHC) class I (using monoclonal antibody PA2.6 [(8]) and class II (using monoclonal antibody L243 [13]), was quantified thereafter by flow cytometry and compared to results for cytokine-treated uninfected controls. As expected, uninfected PBMs differentiated into immature DCs and induced expression of all three group I CD1 isoforms (Fig. 3, left). In contrast, PBMs showed markedly reduced induction of CD1a, -b, and -c expression if previously infected with Leishmania (Fig. 3, right). No effect of either cytokine-treatment or Leishmania infection on MHC class I and class II expression was observed.
FIG. 3.
Leishmania inhibits cytokine-induced CD1 expression in PBMs. (Left) Infected (open histograms) and uninfected (closed histograms) PBMs were incubated with GM-CSF and IL-4 and analyzed for group I CD1, MHC class I, and MHC class II expression by flow cytometry. The panel shows one representative histogram from three independent experiments performed. (Right) Percent reduction of cell surface expression in infected versus uninfected monocytes obtained from three donors. Data are averages, bars indicate standard deviations, and asterisks indicate that no inhibition was observed.
Expression of CD1 is a hallmark of DCs (7), and our results on the inhibition of CD1 expression by Leishmania-infected DCs suggest a pathway for immune evasion by these parasites. Indeed, visceral Leishmania infection has been shown to impair DC functions and has been associated with depletion of follicular DCs from germinal centers in infected spleens (3, 25). DCs have a high turnover rate with an average life span of approximately 2 days (12, 14), and thus, a constant supply of de novo differentiated cells is required to maintain immune functions. Leishmania bone marrow infection may potentially disrupt this supply or modify the functional properties of DCs, including their antigen presentation and T-cell-activation capacities. Our study supports this hypothesis and suggests that L. donovani actively modulates the expression pattern of infected immature DCs and leukocyte precursors toward an immune-suppressive phenotype, processes that may have important implications for innate and acquired antileishmanial immunity and may be relevant for the pathophysiology of chronic leishmaniasis.
Acknowledgments
GFP-tagged L. donovani was kindly provided by Albert Descoteaux, University of Quebec (Quebec, Canada). The CA7AE antibody was kindly provided by Sam Turco, University of Kentucky (Lexington).
This work was supported by grants AI48933 and AI45889 from NIH/NIAID. J. Amprey was supported by NIH MSTP grant GM07739. G. Späth was supported by the Starr Foundation.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Antoine, J. C., T. Lang, E. Prina, N. Courret, and R. Hellio. 1999. H-2M molecules, like MHC class II molecules, are targeted to parasitophorous vacuoles of Leishmania-infected macrophages and internalized by amastigotes of L. amazonensis and L. mexicana. J. Cell Sci. 112:2559-2570. [DOI] [PubMed] [Google Scholar]
- 2.Ashford, R., P. Desjeux, and P. Raadt. 1996. Estimation of population at risk of infection and number of cases of leishmaniasis. Parasitol. Today 8:104-105. [DOI] [PubMed] [Google Scholar]
- 3.Basu, A., G. Chakrabarti, A. Saha, and S. Bandyopadhyay. 2000. Modulation of CD11C+ splenic dendritic cell functions in murine visceral leishmaniasis: correlation with parasite replication in the spleen. Immunology 99:305-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beckman, E. M., S. A. Porcelli, C. T. Morita, S. M. Behar, S. T. Furlong, and M. B. Brenner. 1994. Recognition of a lipid antigen by CD1-restricted αβ+ T cells. Nature 372:691-694. [DOI] [PubMed] [Google Scholar]
- 5.Behar, S. M., S. A. Porcelli, E. M. Beckman, and M. B. Brenner. 1995. A pathway of costimulation that prevents anergy in CD28- T cells: B7-independent costimulation of CD1-restricted T cells. J. Exp. Med. 182:2007-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bendelac, A., M. N. Rivera, S. H. Park, and J. H. Roark. 1997. Mouse CD1-specific NK1 T cells: development, specificity, and function. Annu. Rev. Immunol. 15:535-562. [DOI] [PubMed] [Google Scholar]
- 7.Blumberg, R. S., D. Gerdes, A. Chott, S. A. Porcelli, and S. P. Balk. 1995. Structure and function of the CD1 family of MHC-like cell surface proteins. Immunol. Rev. 147:5-29. [DOI] [PubMed] [Google Scholar]
- 8.Brodsky, F. M., P. Parham, C. J. Barnstable, M. J. Crumpton, and W. F. Bodmer. 1979. Monoclonal antibodies for analysis of the HLA system. Immunol. Rev. 47:3-61. [DOI] [PubMed] [Google Scholar]
- 9.Brossay, L., N. Burdin, S. Tangri, and M. Kronenberg. 1998. Antigen-presenting function of mouse CD1: one molecule with two different kinds of antigenic ligands. Immunol. Rev. 163:139-150. [DOI] [PubMed] [Google Scholar]
- 10.Fruth, U., N. Solioz, and J. A. Louis. 1993. Leishmania major interferes with antigen presentation by infected macrophages. J. Immunol. 150:1857-1864. [PubMed] [Google Scholar]
- 11.Gumperz, J. E., and M. B. Brenner. 2001. CD1-specific T cells in microbial immunity. Curr. Opin. Immunol. 13:471-478. [DOI] [PubMed] [Google Scholar]
- 12.Kamath, A. T., J. Pooley, M. A. O'Keeffe, D. Vremec, Y. Zhan, A. M. Lew, A. D'Amico, L. Wu, D. F. Tough, and K. Shortman. 2000. The development, maturation, and turnover rate of mouse spleen dendritic cell populations. J. Immunol. 165:6762-6770. [DOI] [PubMed] [Google Scholar]
- 13.Lampson, L. A., and R. Levy. 1980. Two populations of Ia-like molecules on a human B cell line. J. Immunol. 125:293-299. [PubMed] [Google Scholar]
- 14.Leenen, P. J., K. Radosevic, J. S. Voerman, B. Salomon, N. van Rooijen, D. Klatzmann, and W. van Ewijk. 1998. Heterogeneity of mouse spleen dendritic cells: in vivo phagocytic activity, expression of macrophage markers, and subpopulation turnover. J. Immunol. 160:2166-2173. [PubMed] [Google Scholar]
- 15.McConville, M. J., T. A. Collidge, M. A. Ferguson, and P. Schneider. 1993. The glycoinositol phospholipids of Leishmania mexicana promastigotes. Evidence for the presence of three distinct pathways of glycolipid biosynthesis. J. Biol. Chem. 268:15595-15604. [PubMed] [Google Scholar]
- 16.Moody, D. B., T. Ulrichs, W. Muhlecker, D. C. Young, S. S. Gurcha, E. Grant, J. P. Rosat, M. B. Brenner, C. E. Costello, G. S. Besra, and S. A. Porcelli. 2000. CD1c-mediated T-cell recognition of isoprenoid glycolipids in Mycobacterium tuberculosis infection. Nature 404:884-888. [DOI] [PubMed] [Google Scholar]
- 17.Porcelli, S., C. T. Morita, and M. B. Brenner. 1992. CD1b restricts the response of human CD4-8- T lymphocytes to a microbial antigen. Nature 360:593-597. [DOI] [PubMed] [Google Scholar]
- 18.Porcelli, S. A. 1995. The CD1 family: a third lineage of antigen-presenting molecules. Adv. Immunol. 59:1-98. [DOI] [PubMed] [Google Scholar]
- 19.Reiner, N. E., W. Ng, T. Ma, and W. R. McMaster. 1988. Kinetics of gamma interferon binding and induction of major histocompatibility complex class II mRNA in Leishmania-infected macrophages. Proc. Natl. Acad. Sci. USA 85:4330-4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reinherz, E. L., R. E. Hussey, and S. F. Schlossman. 1980. A monoclonal antibody blocking human T cell function. Eur. J. Immunol. 10:758-762. [DOI] [PubMed] [Google Scholar]
- 21.Romani, N., S. Gruner, D. Brang, E. Kampgen, A. Lenz, B. Trockenbacher, G. Konwalinka, P. O. Fritsch, R. M. Steinman, and G. Schuler. 1994. Proliferating dendritic cell progenitors in human blood. J. Exp. Med. 180:83-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosat, J. P., E. P. Grant, E. M. Beckman, C. C. Dascher, P. A. Sieling, D. Frederique, R. L. Modlin, S. A. Porcelli, S. T. Furlong, and M. B. Brenner. 1999. CD1-restricted microbial lipid antigen-specific recognition found in the CD8+ αβ T cell pool. J. Immunol. 162:366-371. [PubMed] [Google Scholar]
- 23.Sallusto, F., and A. Lanzavecchia. 1994. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J. Exp. Med. 179:1109-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sieling, P. A., D. Chatterjee, S. A. Porcelli, T. I. Prigozy, R. J. Mazzaccaro, T. Soriano, B. R. Bloom, M. B. Brenner, M. Kronenberg, P. J. Brennan, et al. 1995. CD1-restricted T cell recognition of microbial lipoglycan antigens. Science 269:227-230. [DOI] [PubMed] [Google Scholar]
- 25.Smelt, S. C., C. R. Engwerda, M. McCrossen, and P. M. Kaye. 1997. Destruction of follicular dendritic cells during chronic visceral leishmaniasis. J. Immunol. 158:3813-3821. [PubMed] [Google Scholar]
- 26.Spath, G. F., L. Epstein, B. Leader, S. M. Singer, H. A. Avila, S. J. Turco, and S. M. Beverley. 2000. Lipophosphoglycan is a virulence factor distinct from related glycoconjugates in the protozoan parasite Leishmania major. Proc. Natl. Acad. Sci. USA 97:9258-9263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stenger, S., D. A. Hanson, R. Teitelbaum, P. Dewan, K. R. Niazi, C. J. Froelich, T. Ganz, S. Thoma-Uszynski, A. Melian, C. Bogdan, S. A. Porcelli, B. R. Bloom, A. M. Krensky, and R. L. Modlin. 1998. An antimicrobial activity of cytolytic T cells mediated by granulysin. Science 282:121-125. [DOI] [PubMed] [Google Scholar]
- 28.Stenger, S., K. R. Niazi, and R. L. Modlin. 1998. Down-regulation of CD1 on antigen-presenting cells by infection with Mycobacterium tuberculosis. J. Immunol. 161:3582-3588. [PubMed] [Google Scholar]
- 29.Tolson, D. L., S. J. Turco, and T. W. Pearson. 1990. Expression of a repeating phosphorylated disaccharide lipophosphoglycan epitope on the surface of macrophages infected with Leishmania donovani. Infect. Immun. 58:3500-3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turco, S. J., and A. Descoteaux. 1992. The lipophosphoglycan of Leishmania parasites. Annu. Rev. Microbiol. 46:65-94. [DOI] [PubMed] [Google Scholar]
- 31.von Stebut, E., Y. Belkaid, T. Jakob, D. L. Sacks, and M. C. Udey. 1998. Uptake of Leishmania major amastigotes results in activation and interleukin 12 release from murine skin-derived dendritic cells: implications for the initiation of anti-Leishmania immunity. J. Exp. Med. 188:1547-1552. [DOI] [PMC free article] [PubMed] [Google Scholar]