Abstract
Tumor necrosis factor (TNF) receptor 6/decoy receptor 3 (TR6/DcR3) is an antiapoptosis soluble receptor of the TNF family produced by tumor cells. In this study, TR6 expression in human immune cells was investigated. TR6 mRNA and protein were detectable in selected antigen-presenting cells. Monocytes and myeloid-derived dendritic cells (MDC) released the protein exclusively following stimulation of Toll-like receptor 2 (TLR2) and TLR4 by gram-positive and gram-negative bacterial antigens. Plasmacytoid dendritic cells, activated by bacterial antigens via TLR9 or by viral infection, did not produce the protein. Similarly, activated T cells did not release TR6. The release of TR6 by MDC was dependent on the activation of p42/p44 mitogen-activated protein kinases, Src-like protein tyrosine kinases, and phosphatidylinositol 3-kinase, signaling pathways important for MDC maturation and survival. In agreement with the in vitro data, TR6 levels in serum were significantly elevated in patients with bacterial infections. Overall, these data suggest a novel role for TR6 in immune responses to bacteria.
Tumor necrosis factor (TNF) receptor 6 (TR6), also called decoy receptor 3 (DcR3) or M68, is a member of the TNF receptor family that is produced as a secreted protein (3, 25, 35). Similar to other members of the family, TR6 binds with high affinity to multiple ligands, including Fas ligand (FasL), LIGHT, and TL1A (18, 25, 35). Since TR6 lacks a transmembrane domain, it can function as an inhibitor by competing with the signal-transducing receptor for the ligand. The recombinant protein was indeed able to inhibit in vitro and in vivo FasL-induced apoptosis (5, 25). Because of TR6 overexpression in a substantial number of tumors (23, 28), it has been postulated that the decoy receptor promotes the survival of tumor cells by helping them to escape FasL-dependent cell death (3, 25). In addition, several studies have suggested a role for TR6 in immunity, through the modulation of T-cell responses. TR6 was found to inhibit the interaction of TL1A, a T-cell costimulatory cytokine, with its receptor DR3 (18). Soluble protein was also shown to downmodulate the cytotoxic activity of T lymphocytes and ameliorate heart allograft rejection in mice, possibly by interfering with LIGHT/TR2 binding (36). In another study, solid-phase TR6 was reported to stimulate proliferation and cytokine production in T lymphocytes by reverse signaling through LIGHT (32). Lastly, TR6 was reported to modulate dendritic cell maturation (10).
Little is known about the regulation of TR6 expression in nonmalignant cells. TR6 mRNA is expressed in endothelial cells and keratinocytes (16, 35), and the protein was detected in epithelial cells of the colon (3). The mRNA was also detected at low levels in other healthy human tissues, such as stomach, lymph node, lung, and spleen tissues (3). The involvement of TR6 in immune responses raised the possibility that the expression of the protein may be regulated in cells of the immune system. Therefore, the aim of our study was to investigate the expression and release of TR6 by immune cells.
MATERIALS AND METHODS
Reagents.
Cytokines were purchased from PeproTech (Rocky Hill, N.J.); Staphylococcus aureus Cowan I, PD98059, SB203580, herbimycin A, and wortmannin were purchased from Calbiochem (San Diego, Calif.); phytohemagglutinin (PHA), lipopolysaccharide (LPS), and lipoteichoic acids (LTA) were purchased from Sigma-Aldrich (St. Louis, Mo.); and CD40 ligand (CD40L) and a TNF-α enzyme-linked immunosorbent assay (ELISA) kit were purchased from R&D Systems (Minneapolis, Minn.).
Cell cultures.
Monocytes were obtained from human peripheral blood mononuclear cells (PBMC) by centrifugation of leukopheresis preparations (BRT Inc., Baltimore, Md.) through Histopaque gradients (Sigma-Aldrich) followed by counterflow centrifugal elutriation. Cell purity was greater than 92%. Cells were cultured in complete medium consisting of RPMI 1640 medium (GIBCO BRL, Rockville, Md.) supplemented with 10% heat-inactivated fetal bovine serum, 2 mM l-glutamine, and 50 μg of gentamicin (Biofluids Inc., Rockville, Md.)/ml. Myeloid dendritic cells (MDC) were obtained by culturing monocytes for 7 to 10 days in complete medium supplemented with 1% nonessential amino acids, 1% sodium pyruvate (Biofluids), 5 × 10−5 M 2-mercaptoethanol (Sigma), 50 ng of granulocyte macrophage-colony stimulating factor/ml, and 20 ng of interleukin-4 (IL-4)/ml. Over 90% of the cells were shown by flow cytometry to express high levels of CD1a and low levels of CD14 and showed characteristic dendrite formations on examination with phase-contrast light microscopy. Plasmacytoid dendritic cells (PDC) were purified from PBMC by magnetic-bead separation (Miltenyi Biotec, Auburn, Calif.) as previously reported (7). T, B, and natural killer (NK) cells were purified from PBMC by the magnetic-bead procedure. For the determination of TR6 and TNF-α release, cells were incubated with LPS or LTA for 24 h at a concentration of 106 cells/ml (for monocytes and T cells) or 0.5 × 106 cells/ml (for MDC and PDC) in 0.5 ml/well.
Quantitative RT-PCR.
Total RNA (25 ng) was used in a one-step quantitative reverse transcription (RT)-PCR with a 25-μl reaction mixture. To control for genomic contamination, parallel reactions were set up without reverse transcriptase. The abundance of TR6-specific mRNA relative to that of 18S rRNA was measured with the Applied Biosystems Prism 7700 sequence detection system. Reactions were carried out at 48°C for 30 min and 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. Reactions were performed in triplicate. Total RNA was purified from cells, reverse transcribed, and amplified with the TR6 probe 5′-TCTACATCCTTGGCACCCCACTTGCA-3′ and the primers 5′-CTGATCCTGGCCCCCTCTTA-3′ and 5′-TTCTTCTATTTAAAAAAAAGCCTCTTTCA-3′. Probes were labeled at the 5′ end with the reporter dye 6-FAM (6-carboxyfluorescein) and at the 3′ end with the quencher dye TAMRA (6-carboxytetramethylrhodamine; Biosource International, Camarillo, Calif.).
TR6 ELISA.
Anti-TR6 monoclonal antibodies (MAbs) were generated by the fusion of mouse myeloma P3X63Ag8.653 cells with splenocytes from BALB/c mice immunized with TR6.Fc protein. For TR6-specific ELISA, MAb 9E02 (3 μg/ml) was used to coat Maxisorp plates (Nunc, Rochester, N.Y.) overnight at 4°C. The plates were washed and blocked with 3% bovine serum albumin in phosphate-buffered saline. Serial dilutions of culture supernatants or serum samples were prepared in diluent buffer (phosphate-buffered saline containing 0.05% Tween 20 and 0.1% bovine serum albumin) and incubated for 2 h at room temperature on the coated microplates. The plates were washed, and biotinylated anti-TR6 MAb 12B03 (200 ng/ml) was added for 2 h at room temperature. After washing, streptavidin-peroxidase conjugate (1:2,000 [vol/vol]; Vector Laboratories, Burlingame, Calif.) was added for 1 h. The peroxidase reaction was developed with the 3,3′,5,5′-tetramethylbenzidine substrate (Kirkegaard & Perry, Gaithersburg, Md.). Light absorbance was measured at 450 nm with a SpectraMax 3000 plate reader (Molecular Devices, Sunnyvale, Calif.). Each value was calculated as the mean ± standard deviation for triplicate samples. The sensitivity limit of the ELISA is typically <30 pg/ml.
RESULTS AND DISCUSSION
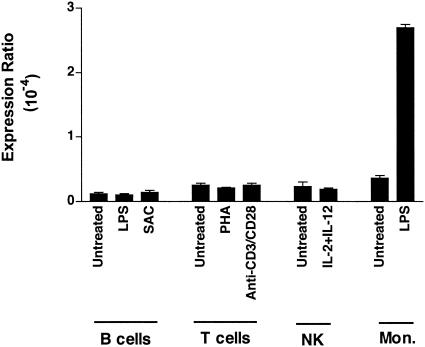
To identify immune cells with the potential to release the decoy receptor, TR6 mRNA levels in purified cell populations obtained from PBMC from healthy donors were analyzed by quantitative RT-PCR (Fig. 1). The addition of specific activating factors to B cells (LPS or S. aureus Cowan I), T cells (PHA or CD3/CD28 stimulation), and NK cells (IL-2 and IL-12) did not significantly increase the low basal level of TR6 transcript in the cells. In contrast, the treatment of monocytes with the bacterial antigen LPS produced a significant increase in the TR6 mRNA level. This observation suggests that the recognition of pathogen-associated molecular patterns by antigen-presenting cells (APC) might induce TR6 expression. Thus, our subsequent work focused on the analysis of the receptor's expression in monocytes and in the two subsets of dendritic cells, MDC and PDC.
FIG. 1.
TR6 mRNA expression in resting or stimulated immune cells. B cells were treated for 18 to 20 h with LPS (5 μg/ml) or S. aureus Cowan I (SAC) (10−5 dilution), T cells were treated with PHA (5 μg/ml) or immobilized anti-CD3 (1 μg/ml) and anti-CD28 (10 μg/ml) MAb, NK cells were treated with IL-2 (100 U/ml) and IL-12 (5 ng/ml), and monocytes (Mon.) were treated with LPS (100 ng/ml). TR6 mRNA expression was evaluated by quantitative RT-PCR. Results for one of three donors are shown.
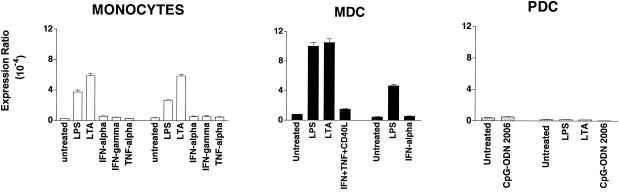
APC respond to bacterial and viral infections through stimulation of specific Toll-like receptors (TLR) (11, 12, 31). Monocytes and MDC express all the known TLR, except TLR7 and TLR9. They respond to pathogen-associated molecular patterns of gram-positive bacteria through TLR2 and to those of gram-negative bacteria through TLR4. PDC, which, in contrast, express only TLR7 and TLR9, are activated by bacterial DNA sequences via ligation of TLR9 (9). In our experiments with APC, the cells were incubated with LPS or LTA, components of the cell wall of gram-negative or gram-positive bacteria, respectively. These two bacterial products share many pathophysiological properties and the usage of CD14 and TLR (8). Their effect was compared to those of the stimulatory cytokines gamma interferon (IFN-γ), IFN-α, and TNF-α. As shown in Fig. 2 (left panel), only LPS and LTA treatment affected TR6 mRNA expression in monocytes, inducing average increases of 10- and 15-fold, respectively, in the donors tested (n = 4). Similarly, stimulation of MDC with LPS or LTA induced approximately a ninefold increase in TR6 expression, while stimulation with T-cell-derived signals (CD40L in combination with IFN-γ and TNF-α) or IFN-α induced a marginal increase in TR6 mRNA expression (Fig. 2, middle panel). In contrast and as expected, treatment of PDC with LPS or LTA was ineffective (Fig. 2, right panel), since the cells lack TLR2 and TLR4. In addition, incubation of PDC with a stimulatory bacterial DNA sequence, that of CpG-oligodeoxynucleotide (CpG-ODN) 2006 (4), did not upregulate TR6 transcript.
FIG. 2.
Bacterial antigens induce TR6 mRNA expression in monocytes and MDC. Quantitative RT-PCR was conducted with RNA from cells stimulated for 18 to 20 h with bacterial antigens recognized by TLR2 (LTA, prepared from B. subtilis and used at 1 μg/ml), TLR4 (LPS, 100 ng/ml), or TLR9 (CpG-ODN 2006, 1 μg/ml). Cells were also stimulated with IFN-γ (100 U/ml), TNF-α (50 ng/ml), CD40L (3 μg/ml), and IFN-α (100 ng/ml), singly or in combination. Results for two of four donors are shown for each cell type.
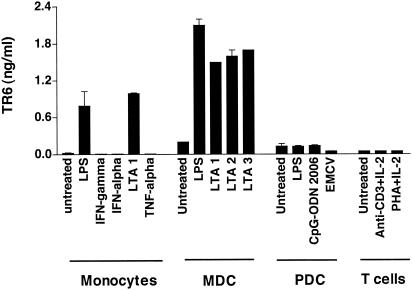
To provide evidence that enhanced TR6 mRNA levels resulted in secretion of the soluble receptor, conditioned media from APC cultures were tested in a TR6-specific ELISA (Fig. 3). TR6 was found in the supernatants of monocytes and MDC activated by LPS or LTA. In contrast, TR6 release was not enhanced by the stimulation of monocytes with the cytokines IFN-γ, TNF-α, or IFN-α or by the stimulation of MDC with CD40L plus IFN-γ plus TNF-α (data not shown). Costimulation of the MDC cultures with IFN-γ and the bacterial products resulted in slight inhibition of LPS- and LTA-induced TR6 release (data not shown). MDC stimulated with LTA purified from various strains of bacteria, including Bacillus subtilis, S. aureus, and Streptococcus faecalis, released similar concentrations of the soluble receptor. A comparable effect was observed following treatment with peptidoglycan, another microbial ligand for TLR2 (data not shown). In contrast to MDC, PDC did not release substantial levels of TR6 when stimulated with CpG-ODN 2006 or with viral infection, although the cells were activated by the treatments, since they released large amounts of IFN-α (>2 ng/ml). In agreement with the mRNA data shown in Fig. 1, anti-CD3- or PHA-treated T cells did not release significant amounts of TR6. Similar results were also obtained with conditioned media of stimulated Th1 and Th2 cells (data not shown). In summary, our results demonstrate that TR6 is specifically released in vitro by APC of the myeloid lineage following bacterial stimulation of TLR2 and TLR4.
FIG. 3.
Release of TR6 by immune cell types. Protein release was evaluated for monocytes, MDC, PDC, and T cells stimulated for 24 h with IFN-γ (100 U/ml); LPS (100 ng/ml); LTA derived from B. subtilis (LTA 1), S. aureus (LTA 2), or S. faecalis (LTA 3) (all used at 1 μg/ml); CpG-ODN 2006 (1 μg/ml); 5 × 104 PFU of encephalomyocarditis virus (EMCV); immobilized anti-CD3 MAb (1 μg/ml); and PHA (5 μg/ml) in the presence of IL-2 (100 U/ml). Cell-free supernatants were collected and assayed for the presence of soluble TR6 receptor by ELISA. Results obtained for one of four donors are shown.
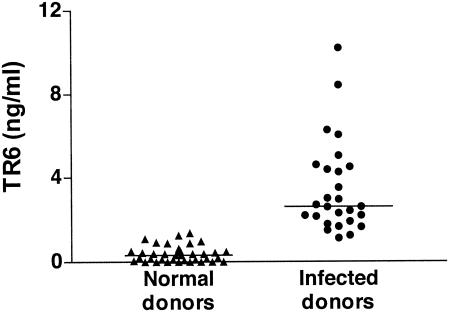
To substantiate the in vitro results, we analyzed serum samples from 27 patients with bacterial infections and 36 healthy donors (Fig. 4). The diagnoses of the patients included bacteremia, cellulitis, osteomyelitis, and urosepsis. Interestingly, serum TR6 levels in the patients were significantly higher (mean, 3.46 ng/ml) than those in healthy donors (mean, 0.38 ng/ml), indicating that upregulation of TR6 release occurs in vivo during infections. It is possible that TR6-expressing cells other than APC, e.g., endothelial cells, epithelial cells, and keratinocytes (3, 16, 35), contribute to the enhanced levels of the protein in the patients' blood. Nonetheless, these results indicate that TR6 production cannot be considered an exclusive marker for malignant tumors. An increase in the relative expression level of the TR6 gene was also reported for silicosis patients (24).
FIG. 4.
Elevated levels of TR6 in the sera of patients with bacterial infections. Peripheral blood was obtained from patients (n = 27) with various infectious diseases admitted to the Georgetown University Hospital. Control samples were drawn from healthy blood donors (n = 36). Levels of TR6 in serum (nanograms per milliliter) were measured by ELISA. The horizontal bars indicate the average for each group: 0.38 ± 0.06 ng/ml for healthy donors and 3.46 ± 0.42 ng/ml for infected donors (mean ± standard error of the mean). The P value (<0.0001) was determined by the Mann-Whitney U test.
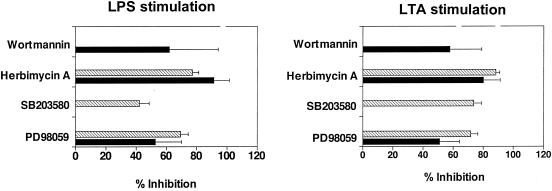
The aim of the next series of experiments was to identify signaling pathways regulating TR6 release. LPS has been shown to activate multiple signaling pathways in MDC, including the p42/p44 mitogen-activated protein kinase (MAPK), p38 stress-activated protein kinase, and phosphatidylinositol 3-kinase (PI3K) pathways (2). While p42/p44 and p38 MAPKs mediate various aspects of MDC maturation, such as cytokine release (15, 27, 29, 15), PI3K is important for the survival of LPS-stimulated cells (2). In our experiments, MDC were treated with LPS or LTA in the absence or presence of defined kinase inhibitors. Specifically, we used PD98059, an inhibitor of p42/p44 MAPK; SB203580, an inhibitor of p38 MAPK; herbimycin A, an inhibitor of Src-like protein tyrosine kinases; or wortmannin, an inhibitor of PI3K. The conditioned media from the cell cultures were then collected and assayed by ELISA to determine the TR6 and TNF-α levels (Fig. 5). Exposure to PD98059 or herbimycin A reduced both TR6 and TNF-α release in LPS- or LTA-stimulated MDC. Treatment with wortmannin did not inhibit the release of TNF-α as reported previously (29); in contrast, it suppressed the release of TR6. Conversely, treatment with SB203580 inhibited TNF-α release but did not affect TR6 release. Thus, TLR-induced TR6 release is dependent on the activation of p42/p44 MAPKs, protein tyrosine kinases, and PI3K. The results obtained in the present study, moreover, indicate that the release of TR6 and TNF-α follows the activation of similar but not identical signaling pathways in MDC. These data are in agreement with those of a previous report showing that in MDC, PI3K is part of the signaling pathway activated by TLR2 and TLR4 and regulating cytokine production (26). Recruitment of PI3K by TLR2 was also demonstrated in an additional study, although it was not observed for TLR4 (1). In our experiments, the same pattern of inhibition by the chemicals was observed for both LPS- and LTA-induced TR6 release, indicating that the triggering of different TLRs by gram-positive and gram-negative bacteria activates a common cascade of intracellular events leading to TR6 production. This conclusion may not apply to all of the cytokines released by MDC, since it was reported that activation of the cells by TLR2 or TLR4 agonists promoted the production of different sets of cytokines (26).
FIG. 5.
Effects of signal transduction inhibitors on TR6 and TNF-α release from LPS- or LTA-stimulated MDC. The cells were incubated with LPS or LTA (from B. subtilis) in the absence or presence of 50 μM PD98059 (p42/p44 MAPK inhibitor), 1.25 μM SB203580 (p38 MAPK inhibitor), 1.25 μM herbimycin A (tyrosine kinase inhibitor), or 0.05 μM wortmannin (PI3K inhibitor). The release of TR6 (black bars) and TNF-α (hatched bars) in the culture supernatants was analyzed by ELISA. Data shown represent mean values ± standard deviations for independent experiments conducted with cells of three donors.
The results of our study demonstrate that TR6 is produced by immune cells, specifically by myeloid APC, in response to bacterial products. The specific pattern of TR6 production (i.e., release by myeloid-derived APC, which control the differentiation of T helper 1 cells, and not by PDC, which control the differentiation of T helper 2 cells [14, 19]) suggests that the protein preferentially plays a role in cell-mediated immune responses. An appropriate expression of pro- and antiapoptotic molecules is implicated in the regulation of the inflammatory reaction to pathogens (6). In this context, TR6 release might decrease Fas-mediated deaths of effector cells or lessen Fas-related damage of the healthy tissues surrounding the sites of infection (13, 17, 20, 30, 34). Alternatively, production of the decoy receptor could be a mechanism used by the bacteria to escape the immune system by inhibiting Fas-dependent apoptosis of infected cells and dampening T-cell responses (21, 22, 33). Further investigation of the potential involvement of TR6 in antibacterial immunity may increase our understanding of the factors regulating the interaction between innate and acquired immunity following bacterial challenge.
Acknowledgments
We thank V. Kao and D. Shah for technical assistance, H. Olsen for providing CpG-ODN 2006, and D. Russell, D. Hilbert, and M. Bowen for helpful discussions.
This work was funded by Human Genome Sciences, Inc.
Editor: F. C. Fang
REFERENCES
- 1.Arbibe, L., J. P. Mira, N. Teusch, L. Kline, M. Guha, N. Mackman, P. J. Godowski, R. J. Ulevitch, and U. G. Knaus. 2000. Toll-like receptor 2-mediated NF-kappa B activation requires a Rac1-dependent pathway. Nat. Immunol. 1:533-540. [DOI] [PubMed] [Google Scholar]
- 2.Ardershna, K. M., A. R. Pizzey, S. Devereux, and A. Khwaja. 2000. The PI3 kinase, p38 SAP kinase, and NF-κB signal transduction pathways are involved in the survival and maturation of lipopolysaccharide-stimulated human monocyte-derived dendritic cells. Blood 96:1039-1046. [PubMed] [Google Scholar]
- 3.Bai, C., B. Connolly, M. L. Metzker, C. A. Hilliard, X. Liu, V. Sandig, A. Soderman, S. M. Galloway, Q. Liu, C. P. Austin, and C. T. Caskey. 2000. Overexpression of M68/TR6 in human gastrointestinal tract tumors independent of gene amplification and its location in a four-gene cluster. Proc. Natl. Acad. Sci. USA 97:1230-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bauer, M., V. Redecke, J. W. Ellwart, B. Ascherer, J.-P. Kremer, H. Wagner, and G. B. Lipford. 2001. Bacterial CpG-DNA triggers activation and maturation of human CD11c−, CD123+ dendritic cells. J. Immunol. 166:5000-5007. [DOI] [PubMed] [Google Scholar]
- 5.Connolly, K., Y. H. Cho, R. Duan, J. Fikes, T. Gregorio, D. W. LaFleur, Z. Okoye, T. W. Salcedo, G. Santiago, S. Ullrich, P. Wei, K. Windle, E. Wong, X. T. Yao, Y. Q. Zhang, G. Zheng, and P. A. Moore. 2001. In vivo inhibition of Fas ligand mediated killing by TR6, a Fas ligand decoy receptor. J. Pharmacol. Exp. Ther. 298:25-33. [PubMed] [Google Scholar]
- 6.Dockrell, D. H. 2001. Apoptotic cell death in the pathogenesis of infectious diseases. J. Infect. 42:227-234. [DOI] [PubMed] [Google Scholar]
- 7.Dzionek, A., Y. Sohma, J. Nagafune, M. Cella, M. Colonna, F. Facchinetti, G. Gunther, I. Johnston, A. Lanzavecchia, T. Nagasaka, T. Okada, W. Vermi, G. Winkels, T. Yamamoto, M. Zysk, Y. Yamaguchi, and J. Schmitz. 2001. BDCA-2, a novel plasmacytoid dendritic cell-specific type II C-type lectin, mediates antigen capture and is a potent inhibitor of interferon α/β production. J. Exp. Med. 194:1823-1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ginsburg, I. 2002. Role of lipoteichoic acid in infection and inflammation. Lancet 2:171-179. [DOI] [PubMed] [Google Scholar]
- 9.Hemmi, H., O. Takeuchi, T. Kawai, T. Kaisho, S. Sato, H. Sanjo, M. Matsumoto, K. Hoshino, H. Wagner, K. Takeda, and S. Akira. 2000. A Toll-like receptor recognizes bacterial DNA. Nature 408:740-745. [DOI] [PubMed] [Google Scholar]
- 10.Hsu, T.-L., Y.-C. Chang, S.-J. Chen, Y.-J. Liu, A. W. Chiu, C.-C. Chio, L. Chen, and S.-L. Hsieh. 2002. Modulation of dendritic cell differentiation and maturation by decoy receptor 3. J. Immunol. 168:4846-4853. [DOI] [PubMed] [Google Scholar]
- 11.Jarrossay, D., G. Napolitani, M. Colonna, F. Sallusto, and A. Lanzavecchia. 2001. Specialization and complementarity in microbial molecule recognition by human myeloid and plasmacytoid dendritic cells. Eur. J. Immunol. 31:3388-3393. [DOI] [PubMed] [Google Scholar]
- 12.Kadowaki, N., S. Ho, S. Antonenko, R. de Waal Malefyt, R. A. Kastelein, F. Bazan, and Y.-J. Liu. 2001. Subsets of human dendritic precursors express different Toll-like receptors and respond to different microbial antigens. J. Exp. Med. 194:863-869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kremer, L., J. Estaquier, I. Wolowczuk, F. Biet, J.-C. Ameisen, and C. Locht. 2000. Ineffective cellular immune response associated with T-cell apoptosis in susceptible Mycobacterium bovis BCG-infected mice. Infect. Immun. 68:4264-4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu, Y. J., N. Kadowaki, M. C. Rissoan, and V. Soumelis. 2000. T cell activation and polarization by DC1 and DC2. Curr. Top. Microbiol. Immunol. 251:149-159. [DOI] [PubMed] [Google Scholar]
- 15.Lu, H. T., D. D. Yang, M. Wysk, E. Gatti, I. Mellman, R. J. Davis, and R. A. Flavell. 1999. Defective IL-12 production in mitogen-activated protein (MAP) kinase kinase 3 (Mkk3)-deficient mice. EMBO J. 18:1845-1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maeda, T., C. Hao, and V. A. Tron. 2001. Ultraviolet light (UV) regulation of the TNF family decoy receptors DcR2 and TR6 in human keratinocytes. J. Cutan. Med. Surg. 5:294-298. [DOI] [PubMed] [Google Scholar]
- 17.Matute-Bello, G., C. W. Frevert, W. C. Liles, M. Nakamura, J. T. Ruzinski, K. Ballman, V. A. Wong, C. Vathanaprida, and T. R. Martin. 2001. Fas/Fas ligand system mediates epithelial injury, but not pulmonary host defenses, in response to inhaled bacteria. Infect. Immun. 69:5768-5776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Migone, T.-S., J. Zhang, X. Luo, L. Zhuang, C. Chen, B. Hu, J. S. Hong, J. W. Perry, S.-F. Chen, J. X. Zhou, Y. H. Cho, S. Ullrich, P. Kanakaraj, J. Carrell, E. Boyd, H. S. Olsen, G. Hu, L. Pukac, D. Liu, J. Ni, S. Kim, R. Gentz, P. Feng, P. A. Moore, S. M. Ruben, and P. Wei. 2002. TL1A is a TNF-like ligand for DR3 and TR6/TR6 and functions as a T cell costimulator. Immunity 16:479-492. [DOI] [PubMed] [Google Scholar]
- 19.Moser, M., and K. M. Murphy. 2000. Dendritic cell regulation of TH1-TH2 development. Nat. Immunol. 1:199-205. [DOI] [PubMed] [Google Scholar]
- 20.Mukasa, A., M. Lahn, S. Fleming, B. Freiberg, E. Pflum, M. Vollmer, A. Kupfer, R. O'Brien, and W. Born. 2002. Extensive and preferential Fas/Fas ligand-dependent death of gammadelta T cells following infection with Listeria monocytogenes. Scand. J. Immunol. 56:233-247. [DOI] [PubMed] [Google Scholar]
- 21.Nwakoby, I. E., K. Reddy, P. Patel, N. Shah, S. Sharma, M. Bhaskaran, N. Gibbons, A. A. Kapasi, and P. C. Singhal. 2001. Fas-mediated apoptosis of neutrophils in sera of patients with infection. Infect. Immun. 69:3343-3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oddo, M., T. Renno, A. Attinger, T. Bakker, H. R. MacDonald, and P. R. A. Meylan. 1998. Fas ligand-induced apoptosis of infected human macrophages reduces the viability of intracellular Mycobacterium tuberculosis. J. Immunol. 160:5448-5454. [PubMed] [Google Scholar]
- 23.Ohshima, K., S. Haraoka, M. Sugihara, J. Suzumiya, C. Kawasaki, M. Kanda, and M. Kikuchi. 2000. Amplification and expression of a decoy receptor for Fas ligand (TR6) in virus (EBV or HTLV-1) associated lymphomas. Cancer Lett. 160:89-97. [DOI] [PubMed] [Google Scholar]
- 24.Otsuki, T., A. Tomokuni, H. Sakaguchi, T. Aikoh, T. Matsuki, Y. Isozaki, F. Hyodoh, H. Ueki, M. Kusaka, S. Kita, and A. Ueki. 2000. Over-expression of the decoy receptor 3 (DcR3) gene in peripheral blood mononuclear cells (PBMC) derived from silicosis patients. Clin. Exp. Immunol. 119:323-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pitti, R. M., S. A. Marsters, D. A. Lawrence, M. Roy, F. C. Kischkel, P. Dowd, A. Huang, C. J. Donahue, S. W. Sherwood, D. T. Baldwin, P. J. Godowski, W. I. Wood, A. L. Gurney, K. J. Hillan, R. L. Cohen, A. D. Goddard, D. Botstein, and A. Ashkenazi. 1998. Genomic amplification of a decoy receptor for Fas ligand in lung and colon cancer. Nature 396:699-703. [DOI] [PubMed] [Google Scholar]
- 26.Re, F., and J. L. Strominger. 2001. Toll-like receptor 2 (TLR2) and TLR4 differentially activate human dendritic cells. J. Biol. Chem. 276:37692-37699. [DOI] [PubMed] [Google Scholar]
- 27.Rescigno, M., F. Granucci, S. Citterio, M. Foti, and P. Ricciardi-Castagnoli. 1999. Coordinated events during bacteria-induced DC maturation. Immunol. Today 20:200-203. [DOI] [PubMed] [Google Scholar]
- 28.Roth, W., S. Isenmann, M. Nakamura, M. Platten, W. Wick, P. Kleihues, M. Bahr, H. Ohgaki, A. Ashkenazi, and M. Weller. 2001. Soluble decoy receptor 3 is expressed by malignant gliomas and suppresses CD95 ligand-induced apoptosis and chemotaxis. Cancer Res. 61:2759-2765. [PubMed] [Google Scholar]
- 29.Termeer, C., F. Benedix, J. Sleeman, C. Fieber, U. Voith, T. Ahrens, K. Miyake, M. Freudenberg, C. Galanos, and J. C. Simon. 2002. Oligosaccharides of hyaluronan activate dendritic cells via Toll-like receptor 4. J. Exp. Med. 195:99-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Um, H.-D., J. M. Orenstein, and S. M. Wahl. 1996. Fas mediated apoptosis in human monocytes by a reactive oxygen intermediate dependent pathway. J. Immunol. 156:3469-3477. [PubMed] [Google Scholar]
- 31.Visintin, A., A. Mazzoni, J. H. Spitzer, D. H. Wyllie, S. K. Dower, and D. M. Segal. 2000. Regulation of Toll-like receptors in human monocytes and dendritic cells. J. Immunol. 166:249-255. [DOI] [PubMed] [Google Scholar]
- 32.Wan, X., J. Zhang, H. Luo, G. Shi, E. Kapnik, S. Kim, P. Kanakaraj, and J. Wu. 2002. A TNF family member LIGHT transduces costimulatory signals into human T cells. J. Immunol. 169:6813-6821. [DOI] [PubMed] [Google Scholar]
- 33.Wang, J., E. G. Brooks, K. B. Bamford, T. L. Denning, J. Pappo, and P. B. Ernst. 2001. Negative selection of T cells by Helicobacter pylori as a model for bacterial strain selection by immune evasion. J. Immunol. 167:926-934. [DOI] [PubMed] [Google Scholar]
- 34.Wang, J., X. Fan, C. Lindholm, M. Bennett, J. O'Connoll, F. Shanahan, E. G. Brooks, V. E. Reyes, and P. B. Ernst. 2000. Helicobacter pylori modulates lymphoepithelial cell interactions leading to epithelial cell damage through Fas/Fas ligand interactions. Infect. Immun. 68:4303-4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu, K.-Y., B. Kwon, J. Ni, Y. Zhai, R. Ebner, and B. S. Kwon. 1999. A newly identified member of tumor necrosis factor receptor superfamily (TR6) suppresses LIGHT-mediated apoptosis. J. Biol. Chem. 274:13733-13736. [DOI] [PubMed] [Google Scholar]
- 36.Zhang, J., T. W. Salcedo, X. Wan, S. Ullrich, B. Hu, T. Gregorio, P. Feng, S. Qi, H. Chen, Y. H. Cho, Y. Li, P. A. Moore, and J. Wu. 2001. Modulation of T-cell responses to alloantigens by TR6/DcR3. J. Clin. Investig. 107:1459-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]