Abstract
Asplenic individuals are known to be at increased risk of infection with encapsulated bacteria. Recent United Kingdom recommendations stated that this at-risk group should receive one dose of the meningococcal serogroup C conjugate (MCC) vaccine. However, the immune response of asplenic individuals to MCC vaccine is unknown. The immune response of asplenics (n = 130) to immunization with the MCC vaccine was investigated. Asplenic individuals had a significantly lower geometric mean titer (GMT) (157.8; 95% confidence interval [CI], 94.5 to 263.3) of bactericidal antibody in serum (SBA) than an age-matched control group (n = 48) (1448.2; 95% CI, 751.1 to 2792.0). However, 80% of asplenic individuals achieved the proposed protective SBA titer of ≥8. No differences were observed between the two groups in the serogroup C-specific immunoglobulin G geometric mean concentration. A significant reduction in SBA GMT or the number of responders achieving an SBA titer of ≥8 was observed if the reason for splenectomy was a medical cause or if MCC vaccination occurred <10 years after splenectomy. Individuals (n = 29) who did not achieve an SBA titer of ≥16 were offered a second dose of MCC vaccine. Analysis of the SBA response revealed that 61% (14 of 23) of the individuals who received a second dose achieved a protective titer. In total, 93% of asplenic individuals achieved a titer of ≥8 following MCC vaccination (one or two doses combined). We recommend that, following vaccination of asplenics, either the level of functional antibody should be determined, with a second dose of MCC vaccine offered to nonresponders, or two doses of MCC vaccine should be routinely offered.
Asplenic individuals are at an increased risk of infection from encapsulated bacteria, including Neisseria meningitidis (9, 15). Overwhelming postsplenectomy infection has a high mortality rate, between 40% and 70% (7). Immunization with the 23-valent pneumococcal plain polysaccharide and Haemophilus influenzae type b (Hib) conjugate (29) vaccines is recommended for individuals with functional or anatomic asplenia. In 2001, the United Kingdom Department of Health extended this recommendation to include vaccination with meningococcal serogroup C conjugate (MCC) vaccine, although no data on immune responses are available to date for MCC vaccines in this group (6).
Various studies have provided conflicting evidence on the immune response to polysaccharide antigens in asplenic individuals (12, 21), and the limitations of plain polysaccharide vaccines are well documented (13, 17, 18, 29). Meningococcal serogroup C plain polysaccharide vaccines are not immunogenic in those under the age of 2 years (13, 17), and repeated doses can lead to hyporesponsiveness (2, 13, 18, 29). MCC vaccines that have been shown to be highly immunogenic and to generate immune memory in all age groups from infants to adults are now available (10, 25-29). MCC vaccines have been shown to be efficacious in all the age groups targeted during the vaccination campaign initiated in 1999 in the United Kingdom and elicit herd immunity (23).
Hib conjugate vaccines have been shown to be immunogenic in children with congenital asplenia (31), but the response to MCC vaccines in asplenic individuals is unknown. To address this issue, meningococcal serogroup C-specific immunoglobulin G (IgG) concentrations and bactericidal antibody titers in serum were assessed in splenectomized individuals before and after MCC vaccination. The effects of the reason for splenectomy, the presence of chronic illness, antibiotics, previous plain polysaccharide vaccination, and the time since splenectomy on the immune response to MCC vaccination were also analyzed.
(This work was presented in part at the Pathogenic Neisseria Conference, September 2002, Oslo, Norway.)
MATERIALS AND METHODS
Study population.
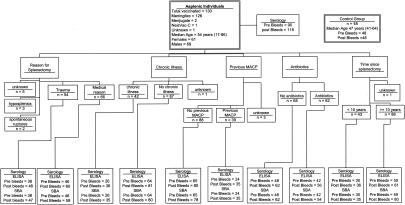
A total of 130 splenectomized individuals from South Cheshire, United Kingdom, were monitored. Details of the study population are given in Fig. 1. Historical data from 48 age-matched individuals with functional spleens were included as the control (2). All vaccinees were asked to complete a record form that provided information on age; the reason for asplenia; the date of splenectomy; the date of previous meningococcal serogroup A and C plain polysaccharide (MACP) vaccination; the history of meningococcal disease; present medication(s), including immunoglobulin, cytotoxic drugs, and azothiaprine; details of any chronic illness; the date of MCC vaccination; the brand of vaccine used; and the dates of blood samples taken.
FIG. 1.
Study population history and serology.
Vaccines.
Splenectomized individuals received one dose of one of the three licensed MCC vaccines (depending upon availability), namely, Meningitec (Wyeth Vaccines, Pearl River, N.Y.), Menjugate (Chiron Vaccines, Siena, Italy), or NeisVac-C (Baxter Vaccines, Beltsville, Md.). Individuals in the control group received only Meningitec. Blood samples were obtained prior to and after vaccination (when asplenic individuals returned for a follow up visit and 1 month postvaccination for the control group). A subset of individuals received a second dose of MCC vaccine if protective responses (for this study, a titer of ≥16 of bactericidal antibody in serum [SBA]) were not achieved. A blood sample was obtained 1 month after the second dose of MCC vaccine. All blood samples were stored at −20°C until assayed.
Antibody assays.
The level of functional antibody was determined by serum bactericidal assay, as described elsewhere (19), using baby rabbit complement (Pel-Freeze Incorporated, Rodgerson, Ariz.) as an exogenous source of complement. The target strain for the assay was C11 (C:16:P1.7-1,1). β-Lactamase was incorporated into the serum bactericidal assay to address the possible presence of antibiotics in the samples obtained. Results are expressed as geometric mean titers (GMT) with 95% confidence intervals. An SBA titer of <4 was considered negative and assigned a titer of 2 for statistical analysis. For the purpose of this study, an SBA titer of ≥16 was considered to be protective, as at the time of this investigation the exact protective level for an SBA using baby rabbit complement was not known but was thought to be between 8 to 64 titers (3). Asplenic individuals who had an SBA titer of <16 were offered a second dose of MCC vaccine. At this visit, their antibiotic status was reviewed and travel advice was given. The protective cutoff has recently been reevaluated and proposed to be an SBA titer of ≥8 (1).
Sera were analyzed for serogroup C-specific IgG by enzyme-linked immunosorbent assay (ELISA) as previously described (11). Results are expressed as geometric mean concentrations (GMC) with 95% confidence intervals. The lower limit of quantitation for the serogroup C-specific IgG by ELISA was 0.05 μg/ml.
Statistical analysis.
The asplenic group was compared to the control group by using Fisher's exact test for comparing the proportion with SBA titers of <8 and by using a Student's t test for comparing the log10 pre- and post-MCC IgG concentrations and post-MCC vaccination SBA titers. GMTs for post-MCC vaccination SBA results and GMCs for pre- and post-MCC vaccination IgG results were calculated along with 95% confidence intervals (CI). Within the asplenic group, single-variable logistic regression was used to examine the effect of the parameters listed in the study population on whether or not the SBA titer was <8, and linear regression was used to examine the effect of the listed parameters on the log10 pre- and post-MCC vaccination IgG concentrations and post-MCC vaccination SBA titers. The pre-MCC vaccination SBA titer could not be treated as a continuous variable in a linear regression analysis because of the large proportion of titers of <4. To examine the independent effects of variables of interest in the single-variable analysis, multivariable models were fitted with all those variables with P values of <0.1 in the single-variable analysis. Paired Student's t tests were used to compare the post-first-dose and post-second-dose responses.
RESULTS
Population data.
One hundred thirty asplenic individuals were monitored, with 126 receiving Meningitec, 2 receiving Menjugate, 1 receiving NeisVac-C, and 1 receiving an unknown brand of MCC vaccine. The median age of the study population was 54 years (range, 17 to 86 years), and there were 61 females and 69 males (Fig. 1). The median age of the control group was 47 years (range, 41 to 64 years). The median age at the time of splenectomy was 29.7 years (range, 2.0 to 84.4 years), and the median time from splenectomy to MCC vaccination was 18.0 years (range, −0.19 to 53 years). The study population included two individuals who received MCC vaccine prior to splenectomy. Thirty-nine asplenic individuals had previously been immunized with an MACP vaccine (20 individuals in the control group had previously received MACP vaccine), and the median time from MACP immunization to MCC immunization was 4.76 years (range, 0.75 to 9.2 years). A total of 103 (79%) asplenic individuals were on medication, including 62 (48%) who were taking prescribed antibiotics, two who were on azothiaprine (plus two individuals with unknown azothiaprine status), one who was undergoing cytotoxic treatment (plus two individuals with unknown cytotoxic treatment status), and two who were on immunoglobulin (plus two individuals with unknown immunoglobulin treatment status). Forty-two individuals reported ongoing chronic illness requiring treatment.
For analysis of SBA, a total of 90 prevaccination and 116 postvaccination results were available. For ELISA, a total of 90 prevaccination and 118 postvaccination results were available. The median time from MCC vaccination to the collection of the postvaccination sample was 28 days (range, 14 to 637 days). Antibody levels are known to persist for 6 months to 1 year following meningococcal vaccination (26, 27). All those individuals with a sample taken more than 6 months following vaccination (n = 5) had an SBA titer of ≥8 (range, 32 to 1,024).
Bactericidal antibody titers in serum.
GMTs in SBA were significantly lower (P < 0.001) in the asplenic group (GMT, 157.8; 95% CI, 94.5 to 263.3) than in the control group (GMT, 1,448.2; 95% CI, 751.1 to 2,792.0) following MCC vaccination (Table 1). Multivariable analysis incorporating the pre-MCC titers gives an adjusted estimate of the reduction in post-MCC vaccination titers in the asplenic group of 83%. However, 87 of 116 (75%) of the asplenic group achieved an SBA titer of ≥16, the protective cutoff titer for this follow-up. Using the newly established serologic correlate of protection, an SBA titer of ≥8 (1), 80% of the asplenic group achieved a protective response. Twenty percent of asplenic individuals did not generate an SBA titer of ≥8 in response to MCC vaccination. One individual in the control group did not achieve an SBA titer of ≥8 (Table 1).
TABLE 1.
Serogroup C serum bactericidal antibody GMT with 95% CI and number of nonresponders with an SBA titer of <8, before and after one dose of MCC vaccine
| Group | Pre-MCC vaccination
|
Post-MCC vaccination
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of patients | No. with SBA titer of <8 (%) | P value | No. of patients | GMT | 95% CI | P value | No. with SBA titer of <8 (%) | P value | |
| Asplenic | 90 | 67 (74) | 116 | 157.8 | 94.5-263.3 | 23 (20) | |||
| Sex | |||||||||
| Female | 43 | 35 (81) | 0.054 | 56 | 98.7 | 47.2-206.3 | 0.069 | 15 (27) | 0.08 |
| Male | 47 | 31 (66) | 60 | 244.4 | 119.6-499.5 | 8 (13) | |||
| Chronic illness | |||||||||
| Yes | 26 | 21 (81) | 0.38 | 35 | 84.4 | 30.9-230.8 | 0.13 | 10 (29) | 0.13 |
| No | 64 | 46 (72) | 80 | 199.1 | 109.4-362.4 | 13 (16) | |||
| Reason for removal of spleen | |||||||||
| Trauma | 38 | 25 (66) | 0.20 | 47 | 354.1 | 157.4-796.5 | 0.014 | 6 (13) | 0.15 |
| Medical | 46 | 36 (78) | 59 | 92.1 | 45.1-188.0 | 14 (24) | |||
| Time since splenectomy (yrs) | |||||||||
| <10 | 30 | 22 (73) | 0.90 | 35 | 75.0 | 27.7-203.2 | 0.054 | 12 (34) | 0.011 |
| ≥10 | 59 | 44 (75) | 80 | 222.9 | 122.3-406.0 | 11 (14) | |||
| Previous MACP | |||||||||
| Yes | 24 | 14 (58) | 0.038 | 35 | 98.9 | 37.7-259.6 | 0.30 | 7 (20) | 0.95 |
| No | 65 | 52 (80) | 78 | 179.4 | 95.7-336.2 | 16 (21) | |||
| Antibiotics | |||||||||
| Yes | 42 | 33 (79) | 0.40 | 54 | 185.7 | 90.5-381.2 | 0.56 | 8 (15) | 0.21 |
| No | 48 | 34 (71) | 62 | 136.9 | 65.1-287.6 | 15 (24) | |||
| Control | 48 | 25 (52) | 0.008b | 48 | 1448.2 | 751.1-2792.0 | <0.001a | 1 (2) | 0.003b |
Compared with all asplenic individuals.
Determined by Fisher's exact test of the results for the controls versus those for all asplenics.
The results of the single-variable analysis of factors associated with pre- and post-MCC vaccination SBA titers are shown in Table 1. Factors not shown in Table 1 that were also looked at were the age at vaccination, medications taken, and the time from vaccination to collection of postvaccination blood, but none of these factors were found to have a significant influence on post-MCC vaccination SBA titers.
In the multivariable analysis, those who had received a previous MACP vaccine were less likely to have a pre-MCC vaccination SBA titer of <8 (P = 0.035), as were males less likely than females (P = 0.034). Analysis of the post-MCC vaccination titers showed that the reason for removal of the spleen and the time since splenectomy were the only parameters that remained significant or close to significant. Those who were asplenic due to a medical condition showed evidence of lower titers than those who were asplenic due to trauma (P = 0.053), and those who had a splenectomy within the past 10 years were more likely to have titers of <8 than those whose splenectomy was done more than 10 years before vaccination (P = 0.053).
As expected, post-MCC vaccination titers were found to depend on the titers in pre-MCC sera. On average, those whose prevaccination titer was ≥8 had a 10-fold-greater postvaccination SBA titer than those with prevaccination titers of <8 (P < 0.001). Given this fact, it is interesting that previous MACP vaccination was associated with higher pre-MCC vaccination titers but the post-MCC vaccination GMT was actually (nonsignificantly) lower in those who had received previous MACP vaccination (Table 1).
Meningococcal serogroup C-specific IgG antibody.
Geometric mean concentrations of IgG antibody to serogroup C polysaccharide were similar for the asplenic (GMC, 9.4; 95% CI, 6.7 to 13.3) and control (GMC, 13.3; 95% CI, 8.7 to 20.3) groups (Table 2), with significant rises in GMC observed from pre- to postvaccination. In the single-variable analysis, significant differences in the pre-MCC vaccination IgG concentration were associated only with previous MACP vaccination and chronic illness (Table 2). Multivariable analysis revealed that only previous MACP vaccination remained significant (P < 0.001), with chronic illness no longer significant (P = 0.54). The post-MCC vaccination IgG concentration was not associated with any of the factors examined.
TABLE 2.
Meningococcal serogroup C-specific IgG GMC with 95% CI prior to and following one dose of MCC vaccine
| Group | Pre-MCC vaccination
|
Post-MCC vaccination
|
||||||
|---|---|---|---|---|---|---|---|---|
| No. of patients | ELISA GMC | 95% CI | P value | No. of patients | ELISA GMC | 95% CI | P value | |
| Asplenic | 90 | 1.1 | 0.7-1.6 | 118 | 9.4 | 6.7-13.3 | ||
| Sex | ||||||||
| Female | 43 | 1.1 | 0.6-1.9 | 0.95 | 57 | 7.3 | 4.6-11.7 | 0.16 |
| Male | 47 | 1.1 | 0.6-2.0 | 61 | 11.9 | 7.2-19.8 | ||
| Chronic illness | ||||||||
| Yes | 26 | 0.5 | 0.3-0.9 | 0.018 | 36 | 7.4 | 3.8-14.7 | 0.43 |
| No | 64 | 1.5 | 0.9-2.5 | 81 | 10.0 | 6.7-15.0 | ||
| Reason for removal of spleen | ||||||||
| Trauma | 38 | 1.2 | 0.6-2.4 | 0.97 | 48 | 12.9 | 7.4-22.7 | 0.17 |
| Medical reason | 46 | 1.2 | 0.7-2.0 | 60 | 7.8 | 4.9-12.5 | ||
| Time since splenectomy (yrs) | ||||||||
| <10 | 30 | 1.3 | 0.6-2.8 | 0.58 | 36 | 7.2 | 3.7-14.1 | 0.30 |
| ≥10 | 59 | 1.0 | 0.6-1.6 | 81 | 10.7 | 7.1-16.1 | ||
| Previous MACP | ||||||||
| Yes | 24 | 4.5 | 2.0-10.5 | <0.001 | 35 | 10.1 | 5.0-20.0 | 0.75 |
| No | 65 | 0.7 | 0.4-1.0 | 80 | 8.9 | 6.0-13.4 | ||
| Antibiotics | ||||||||
| Yes | 42 | 1.0 | 0.5-1.9 | 0.84 | 56 | 9.7 | 5.8-16.4 | 0.85 |
| No | 48 | 1.1 | 0.6-2.0 | 62 | 9.1 | 5.7-14.6 | ||
| Control | 48 | 1.5 | 0.8-2.6 | 0.40a | 48 | 13.3 | 8.7-20.3 | 0.26a |
Compared with all asplenic individuals.
Response of asplenic individuals to a second dose of MCC vaccine.
Asplenic individuals with an SBA titer of <16 in response to an initial dose of MCC vaccine were offered a second dose. Twenty-nine individuals had an SBA titer of <16, 25 received a second dose of MCC vaccine, and a post-second- dose blood sample was available for 23, of whom 15 received Meningitec, 7 received NeisVac-C, and 1 received Menjugate. The subgroup of asplenic individuals receiving a second dose of MCC vaccine included 16 females and 7 males with a median age of 61 years (range, 27 to 86 years). A total of 9 had a chronic illness, 9 had previously received MACP vaccination, 13 had had their spleens removed <10 years before MCC vaccination, 14 had had their spleens removed for a medical reason, and 6 had had their spleens removed due to trauma. There was a small but significant increase (P = 0.005) in the serogroup C-specific IgG GMC from post-primary dose (GMC, 1.4; 95% CI, 0.8 to 2.7) to post-second dose (GMC, 2.7; 95% CI, 1.4 to 5.4). The SBA GMT significantly increased (P < 0.001) post-primary dose (GMT, 3.3; 95% CI, 2.6 to 4.3) to post-second dose (GMT, 38.3; 95% CI, 13.1 to 111.8). However, the SBA GMT was significantly lower than the overall SBA GMT after the primary dose for the asplenic and control groups. Of the 23 individuals, 61% (14 of 23) had an SBA titer of ≥8, leaving 39% (9 of 23) of the group with a functional antibody level below the protective cutoff. This group comprised seven females and two males with a median age of 61 years (range, 50 to 85 years). A total of three had a chronic illness, five had previously received MACP vaccination, seven had had their spleens removed <10 years before MCC vaccination, five had had their spleens removed for a medical reason, and two had had their spleens removed due to trauma.
DISCUSSION
The response of asplenic individuals to MCC vaccination was analyzed to determine if the absence of a functional spleen impairs the response to MCC vaccination. The level of functional antibody generated by asplenic individuals was significantly lower than that of the control group. However, 80% of asplenic individuals did achieve an SBA titer of ≥8. The extended period between vaccination and sample collection for several individuals did not account for any of the patients who failed to achieve an SBA titer of ≥8. The importance of a functional spleen in the response to polysaccharide vaccines has previously been demonstrated (4, 20). However, polysaccharide-protein conjugate vaccines have been shown to overcome dependence upon the presence of a functional spleen (4, 14, 30). Hib conjugate vaccines have been shown to be immunogenic in splenectomized children and adolescents (14) and in children with congenital asplenia (30). Patients with Hodgkin's disease, a high proportion of whom undergo splenectomy, have been shown to respond to pneumococcal conjugate vaccination (5). A significant proportion of the asplenic individuals reported here achieved a titer of functional antibody greater than the protective threshold, demonstrating that MCC vaccination can induce protective responses in this patient group, albeit at a significantly lower level than in the control group.
The reason for the impaired generation of functional antibodies by asplenic individuals may be that the spleen is the primary site for response to bacterial polysaccharides, especially a primary antibody response, which would be observed following immunization with a nonconjugated polysaccharide. A particular subset of circulating memory B cells, IgM memory B cells, has been shown to have an important role in the response to bacterial polysaccharides (16). IgM memory B cells are absent in congenital asplenics and are severely depleted immediately following splenectomy (16). It is thought that the IgM memory B cells are dependent upon a functional spleen for their generation and/or survival and are responsible for the T-cell-independent response to bacterial polysaccharides. Conjugation of the meningococcal capsular polysaccharide to a protein carrier converts the nature of the antipolysaccharide responses from T cell independent to T cell dependent, which may aid secondary lymphoid tissues in compensating for the absence of a spleen (4) and may stimulate a distinct subset of memory B cells, switched memory B cells, that are involved in cognate interactions with T cells in germinal centers (16). Hence, the poor response to bacterial polysaccharides due to an absence of IgM memory B cells is bypassed, and an SBA response can be generated in the absence of a functional spleen.
It has previously been reported that asplenic individuals generated normal IgG levels in response to nonconjugated polysaccharide vaccines (1). This response was observed in the results reported here, with asplenic individuals and the control group having comparable levels of IgG. However, the previous study did not measure functional antibody levels, and it appears that although the level of meningococcal serogroup C-specific IgG is not influenced by the absence of a functional spleen, the generation of bactericidal antibody in serum is.
Several factors were identified as having potential to influence the response of asplenic individuals to MCC vaccination. Those individuals who had a splenectomy due to a medical condition rather than to trauma had a significantly lower SBA GMT and a greater proportion of postvaccination SBA titers of <8. The presence of a chronic illness also appeared to result in lower SBA GMT and more individuals with an SBA titer of <8. This result is perhaps to be expected, with the presence of a chronic illness exerting additional stress on the immune system. The time since splenectomy appears to influence the SBA response, with those splenectomized less than 10 years before MCC vaccination generating lower SBA titers. Interestingly, individuals who have been recently splenectomized have substantially depleted circulating memory B cells, but the switched memory B cells are replaced over time (16). This particular subset of memory B cells may be responsible for the response to MCC vaccination and may account for the influence of the time since splenectomy on SBA responses.
High pre-MCC vaccination SBA titers resulted in a significantly higher post-MCC vaccination SBA titer. However, a previous MACP vaccination, which did result in a higher pre-MCC vaccination SBA titer, reduced the post-MCC vaccination SBA titer by 71%, an observation that is similar to that of a previous study (2). Therefore, the positive effect of higher pre-MCC vaccination SBA titers in those with a previous MACP vaccination is cancelled out, and no overall effect of a previous MACP vaccination on post-MCC vaccination levels was observed.
It is interesting that only 62 out of 130 (48%) asplenic individuals were currently taking antibiotics despite this being a recommendation for this patient group (8, 32). This figure appears to be low compared with that from a retrospective cross-sectional study of asplenic patients in Lothian, Scotland, where 75% of patients were being prescribed antibiotic prophylaxis (22). However, antibiotics did not influence the response to MCC vaccination.
Those asplenic individuals who had a post-MCC vaccination SBA titer of <16 were offered a second dose. The overall SBA GMT of the group who received a second dose was still significantly lower than the SBA GMT for the control group after one dose of MCC vaccine. However, 61% of the asplenic individuals receiving a second dose achieved an SBA titer of ≥8. Overall, following two doses of MCC vaccine, 93% of the asplenic group achieved an SBA titer of ≥8.
Future studies are required to investigate immunologic memory following MCC vaccination in asplenic individuals. Such studies might then demonstrate whether revaccination with MCC was required once antibody levels declined. With tetravalent conjugate vaccines for serogroups A, C, Y, and W135 nearing licensure (24), the immunogenicity of these vaccines will also need to be investigated for this patient group.
The current United Kingdom recommendations state that asplenics should receive a single dose of MCC vaccine, but the data described in this report suggest that either determination of circulating functional antibody following MCC vaccination and offering a second dose if required or administering two doses of MCC vaccine may be more appropriate.
Acknowledgments
We thank Roger Simpson, Director of Public Health (South Cheshire Health Authority), for development work and General Practices in South Cheshire for collecting data. We are grateful to Elizabeth Miller (Health Protection Agency, Immunisation Division, CDSC, Colindale, United Kingdom) for critical reading of the manuscript.
We also thank South Cheshire Health Authority for funding this investigation.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Andrews, N., R. Borrow, and E. Miller. 2003. Validation of serological correlate of protection for meningococcal C conjugate vaccine using efficacy estimates from post-licensure surveillance in England. Clin. Diagn. Lab. Immunol. 10:780-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borrow, R., J. Southern, N. Andrews, N. Peake, R. Rahim, M. Acuna, S. Martin, E. Miller, and E. Kaczmarski. 2001. Comparison of antibody kinetics following meningococcal serogroup C conjugate vaccine between healthy adults previously vaccinated with meningococcal A/C polysaccharide vaccine and vaccine-naive controls. Vaccine 19:3043-3050. [DOI] [PubMed] [Google Scholar]
- 3.Borrow, R., N. Andrews, D. Goldblatt, and E. Miller. 2001. Serological basis for use of meningococcal serogroup C conjugate vaccines in the United Kingdom: reevaluation of correlates of protection. Infect. Immun. 69:1568-1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Breukels, M. A., A. Zandvoort, G. P. J. M. van den Dobblesteen, A. van den Muijsenberg, M. E. Lodewijk, M. Beurret, P. A. Klok, W. Timens, and G. T. Rijkers. 2001. Pneumococcal conjugate vaccines overcome splenic dependency of antibody response to pneumococcal polysaccharides. Infect. Immun. 69:7583-7587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan, C. Y., D. C. Molrine, S. George, N. J. Tarbell, P. Mauch, L. Diller, R. C. Shamberger, N. R. Phillips, A. Goorin, and D. M. Ambrosino. 1996. Pneumococcal conjugate vaccine primes for antibody responses to polysaccharide pneumococcal vaccine after treatment of Hodgkin's disease. J. Infect. Dis. 173:256-258. [DOI] [PubMed] [Google Scholar]
- 6.Chief Medical Officer, Chief Nursing Officer, and Chief Pharmaceutical Officer. 2001. Current vaccine and immunisation issues. In Chief medical officer letters PL/CMO/2001/1, PL/CNO/2001/1, and PL/CPHO/2001/1. Department of Health, London, United Kingdom.
- 7.Davidson, R. N., and R. A. Wall. 2001. Prevention and management of infections in patients without a spleen. Clin. Microbiol. Infect. 7:657-660. [DOI] [PubMed] [Google Scholar]
- 8.Davies, J. M., R. Barnes, and D. Milligan. 2002. Update of guidelines for the prevention and treatment of infection in patients with an absent or dysfunctional spleen. Clin. Med. 2:440-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eraklis, A. J., S. V. Kevy, L. K. Diamond, and R. E. Gross. 1967. Hazard of overwhelming infection after splenectomy in childhood. N. Engl. J. Med. 276:1225-1229. [DOI] [PubMed] [Google Scholar]
- 10.Fairley, C. K., N. Begg, R. Borrow, A. J. Fox, D. M. Jones, and K. Cartwright. 1996. Conjugate meningococcal serogroup A and C vaccine: reactogenicity and immunogenicity in United Kingdom infants. J. Infect. Dis. 174:1360-1363. [DOI] [PubMed] [Google Scholar]
- 11.Gheesling, L. L., G. M. Carlone, L. B. Pais, P. F. Holder, S. E. Maslanka, B. D. Plikaytis, M. Achtman, P. Densen, C. E. Frasch, and H. Kayhty. 1994. Multicenter comparison of Neisseria meningitidis serogroup C anti-capsular polysaccharide antibody levels measured by a standardized enzyme-linked immunosorbent assay. J. Clin. Microbiol. 32:1475-1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giebink, G. S., J. E. Foker, Y. Kim, and G. Schiffman. 1980. Serum antibody and opsonic responses to vaccination with pneumococcal capsular polysaccharide in normal and splenectomized children. J. Infect. Dis. 141:404-412. [DOI] [PubMed] [Google Scholar]
- 13.Gold, R., M. L. Lepow, I. Goldschneider, T. L. Draper, and E. C. Gotschlich. 1975. Clinical evaluation of group A and group C meningococcal polysaccharide vaccines in infants. J. Clin. Investig. 56:1536-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kristensen, K. 1992. Antibody response to a Haemophilus influenzae type b polysaccharide tetanus toxoid conjugate vaccine in splenectomized children and adolescents. Scand. J. Infect. Dis. 24:629-632. [DOI] [PubMed] [Google Scholar]
- 15.Krivit, W. 1977. Overwhelming postsplenectomy infection. Am. J. Hematol. 2:193-201. [DOI] [PubMed] [Google Scholar]
- 16.Kruetzmann, S., M. M. Rosado, H. Weber, U. Germing, O. Tournilhac, H. H. Peter, R. Berner, A. Peters, T. Boehm, A. Plebani, I. Quinti, and R. Carsetti. 2003. Human immunoglobulin M memory B cells controlling Streptococcus pneumoniae infections are generated in the spleen. J. Exp. Med. 197:939-945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leach, A., P. A. Twumasi, S. Kumah, W. S. Banya, S. Jaffar, B. D. Forrest, D. M. Granoff, D. E. Libutti, G. M. Carlone, L. B. Pais, C. V. Broome, and B. M. Greenwood. 1997. Induction of immunologic memory in Gambian children by vaccination in infancy with a group A plus group C meningococcal-protein conjugate vaccine. J. Infect. Dis. 175:200-204. [DOI] [PubMed] [Google Scholar]
- 18.Macdonald, N. E., S. A. Halperin, B. J. Law, B. Forrest, L. E. Danzig, and D. M. Granoff. 1998. Induction of immunologic memory by conjugated vs plain meningococcal C polysaccharide vaccine in toddlers. JAMA 280:1685-1689. [DOI] [PubMed] [Google Scholar]
- 19.Maslanka, S. E., L. L. Gheesling, D. E. Libutti, K. B. Donaldson, H. S. Harakeh, J. K. Dykes, F. F. Arhin, S. J. Devi, C. E. Frasch, J. C. Huang, P. Kriz-Kuzemenska, R. D. Lemmon, M. Lorange, C. C. Peeters, S. Quataert, J. Y. Tai, and G. M. Carlone. 1997. Standardization and a multilaboratory comparison of Neisseria meningitidis serogroup A and C serum bactericidal assays. Clin. Diagn. Lab. Immunol. 4:156-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Molrine, D. C., G. R. Siber, Y. Samra, D. S. Shevy, K. MacDonald, R. Cieri, and D. M. Ambrosino. 1999. Normal IgG and impaired IgM response to polysaccharide vaccines in asplenic patients. J. Infect. Dis. 179:513-517. [DOI] [PubMed] [Google Scholar]
- 21.Pedersen, F. K., J. Henrichsen, and G. Schiffman. 1982. Antibody response to vaccination with pneumococcal capsular polysaccharides in splenectomized children. Acta Paediatr. Scand. 71:451-455. [DOI] [PubMed] [Google Scholar]
- 22.Pickering, J., and H. Campbell. 2000. An audit of the vaccination and antibiotic prophylaxis practices amongst patients splenectomised in Lothian. Health Bull. (Edinburgh) 58:390-395. [PubMed] [Google Scholar]
- 23.Ramsay, M. E., N. J. Andrews, C. L. Trotter, E. B. Kaczmarski, and E. Miller. 2003. Herd immunity from meningococcal serogroup C conjugate vaccination in England: database analysis. Br. Med. J. 326:365-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rennels, M., J. King, R. Ryall, S. Manoff, T. Papa, A. Weddle, and J. Froeschle. 2002. Dose escalation, safety and immunogenicity study of a tetravalent meningococcal polysaccharide diphtheria conjugate vaccine in toddlers. Pediatr. Infect. Dis. J. 21:978-979. [DOI] [PubMed] [Google Scholar]
- 25.Richmond, P., R. Borrow, J. Findlow, S. Martin, C. Thornton, K. Cartwright, and E. Miller. 2001. Evaluation of de-O-acetylated meningococcal C polysaccharide-tetanus toxoid conjugate vaccine in infancy: reactogenicity, immunogenicity, immunologic priming, and bactericidal activity against O-acetylated and de-O-acetylated serogroup C strains. Infect. Immun. 69:2378-2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richmond, P., R. Borrow, D. Goldblatt, J. Findlow, S. Martin, R. Morris, K. Cartwright, and E. Miller. 2001. Ability of 3 different meningococcal C conjugate vaccines to induce immunologic memory after a single dose in UK toddlers. J. Infect. Dis. 183:160-163. [DOI] [PubMed] [Google Scholar]
- 27.Richmond, P., R. Borrow, E. Miller, S. Clark, F. Sadler, A. Fox, N. Begg, R. Morris, and K. Cartwright. 1999. Meningococcal serogroup C conjugate vaccine is immunogenic in infancy and primes for memory. J. Infect. Dis. 179:1569-1572. [DOI] [PubMed] [Google Scholar]
- 28.Richmond, P., D. Goldblatt, P. C. Fusco, J. D. S. Fusco, L. Heron, S. Clark, R. Borrow, and F. Michon. 2000. Safety and immunogenicity of a new Neisseria meningitidis serogroup C tetanus toxoid conjugate vaccine in healthy adults. Vaccine 18:641-646. [DOI] [PubMed] [Google Scholar]
- 29.Richmond, P., E. Kaczmarski, R. Borrow, J. Findlow, S. Clark, R. McCann, J. Hill, M. Barker, and E. Miller. 2000. Meningococcal C polysaccharide vaccine induces immunologic hyporesponsiveness in adults that is overcome by meningococcal C conjugate vaccine. J. Infect. Dis. 181:761-764. [DOI] [PubMed] [Google Scholar]
- 30.Salisbury, D. M., and N. T. Begg (ed.) 1996. Immunisation against infectious diseases. HMSO, London, United Kingdom.
- 31.Webber, S. A., C. G. Sandor, M. W. Patterson, L. A. Mitchell, D. Scheifele, J. J. Ochnio, and P. H. McVerry. 1993. Immunogenicity of Haemophilus influenzae type b conjugate vaccine in children with congenital asplenia. J. Infect. Dis. 167:1210-1213. [DOI] [PubMed] [Google Scholar]
- 32.Working Party of the British Committee for Standards in Haematology Clinical Haematology Task Force. 1996. Guidelines for the prevention and treatment of infection in patients with an absent or dysfunctional spleen. Br. Med. J. 312:430-434. [DOI] [PMC free article] [PubMed] [Google Scholar]