Abstract
Background and Aims:
Sepsis management remains a great challenge for intensive care medicine. The aim of this study was to evaluate the effect of adding dobutamine versus epinephrine to norepinephrine in treating septic shock patients refractory to fluid therapy.
Materials and Methods:
Sixty adult patients with the diagnosis of septic shock were included in this study. Norepinephrine infusion was started at a dose of 0.05 μg/kg/min, and increased gradually up to 0.1 μg/kg/min. Upon reaching this dose, patients with mean arterial pressure <70 mmHg were further divided randomly into two equal groups. In group I: the patients continued on norepinephrine and dobutamine was added at a starting dose of 3 μg/kg/min and increased in increments of 2 μg/kg/min up to 20 μg/kg/min. In group II: the patients continued on norepinephrine and epinephrine was added in a starting dose of 0.05 μg/kg/ min and increased in increments of 0.03 μg/kg/min up to 0.3 μg/kg/min.
Results:
Group II patients developed significantly better cardiovascular parameters, lower arterial pH and higher serum lactate and urine output; however, the 28-day mortality and major adverse effects were comparable in both groups.
Conclusions:
The addition of epinephrine to norepinephrine has positive effects on the cardiovascular parameters but negative results on the serum lactate concentration and systemic pH compared with the addition of dobutamine to norepinephrine.
Keywords: Cardiovascular support, dobutamine, epinephrine, norepinephrine, septic shock
Introduction
Sepsis remains a major challenge for intensive care medicine despite great advances in medication. High mortality from sepsis and septic shock continues despite slight improvement in outcomes.[1,2] For a long time, there has been a debate about the underlying pathophysiology of sepsis. Sepsis triggers systemic vasodilatation, which leads to maldistribution in regional blood flow and development of shock.[3,4] Sepsis imposes significant global tissue hypoxia, although vital signs may be normal. High-risk patients with low tissue perfusion may be identified through metabolic markers, which include serum lactate, sodium bicarbonate and arterial acid–base deficits.[5,6]
Resuscitation of septic shock patients involves volume replacement in addition to some vasoactive agents that help in raising the arterial blood pressure rapidly to a satisfactory level. Vasoactive drug therapy in the treatment of septic shock aims to increase organ perfusion pressure or oxygen delivery or both by using vasopressors, inotropic agents or a combination of both.[7–11] Norepinephrine is the first drug of choice in septic shock patients, whereas epinephrine is considered a good option in patients who are still hypotensive with cardiac depression, but it may cause side-effects such as gut ischemia, arrhythmia and tachycardia.[12] Dobutamine is considered as another option in septic shock patients with myocardial depression, but it may produce hypotension through its vasodilator effect.[13] We cannot predict the patient outcome through the use of a specific catecholamine. Adequate-sized, prospective, randomized studies are lacking. The question of the ideal vasopressor agent in septic shock patient thus remains controversial and unanswered.
The present study was aimed to evaluate the effect of adding dobutamine versus epinephrine to norepinephrine in treating septic shock patients refractory to fluid therapy.
Materials and Methods
This prospective, randomized, double-blind study was carried out in the intensive care unit (ICU) after a written informed consent was obtained from the patients or their next of kin and institutional review board approval was obtained. Sixty adult patients with the diagnosis of septic shock (sepsis plus hypotension refractory to an initial fluid challenge) were included in this study between January/2008 and April/2010. The patients known with the following diseases (cardiac diseases, chronic renal or hepatic impairment, peripheral vascular diseases, coagulopathy and burns) were excluded from this study. All patients were scored by the Sequential Organ Failure Assessment (SOFA) score and monitored by ECG, pulse oximetry, central venous pressure (CVP), invasive blood pressure (IBP), arterial blood gases (ABG) sampling and echocardiography.
All patients received traditional treatment of sepsis (fluids, antibiotics, glucose control and respiratory support), and the fluid therapy was in the form of crystalloid infusion till achieving a normal CVP. In patients who were still hypotensive, norepinephrine infusion was started in a dose of 0.05 μg/kg/min, and the dose was increased gradually up to 0.1 μg/kg/min. After reaching this dose, patients with mean arterial pressure (MAP) <70 mmHg were further divided randomly into two groups, with 30 patients each.
Group I (norepinephrine plus dobutamine)
The patients continued on norepinephrine in a dose of 0.1 μg/kg/min and dobutamine was added in a starting dose of 3 μg/kg/min using syringe pump. Dobutamine was increased in increments of 2 μg/kg/min up to 20 μg/kg/min and, upon reaching a cardiac index (CI) >2.5 L/min/m2 and/ or MAP >70 mmHg, the incremental doses were stopped.
Group II (norepinephrine plus epinephrine)
The patients continued on norepinephrine in a dose of 0.1 μg/kg/min and epinephrine was added in a starting dose of 0.05 μg/kg/min using syringe pump. Epinephrine was increased in increments of 0.03 μg/kg/min up to 0.3 μg/kg/min and, upon reaching a CI >2.5 L/min/m2 and/or MAP >70 mmHg, the incremental doses were stopped.
The patients were randomly assigned, in a 1:1 ratio, according to a computer-generated random list. Randomization was done centrally by an independent statistician to ensure appropriate concealment. An investigator who was responsible for the collection of the data was blinded with respect to the study protocol during the whole process of data evaluation. Any additional management was left to the discretion of the patients’ primary physicians.
Parameters of assessment
The primary outcome measures were SOFA score and cardiovascular effects: heart rate (HR), MAP, CVP, CI, systemic vascular resistance index (SVRI), ejection fraction (EF), left ventricular end diastolic volume (LVEDV) and oxygen delivery, whereas secondary outcome measures included metabolic measurements (arterial pH, serum lactate and urine output), 28-day all-cause mortality and major adverse effects.
Statistical analysis
With a two-sided type I error of 5% and study power at 80%, a mean sample size of 30 patients in each group was found sufficient to demonstrate a 25% difference in the measured parameters with an expected standard deviation of 25%, which was based on a previous pilot study in our hospital. Two-way ANOVA was used to identify the difference between groups at different time points. The data were analyzed with a software program (Sigma Stat version 1.01; Jandel Scientific, San Rafael, CA, USA). Mann-Whitney U test was used for comparison of nonparametric data. Categorical variables were compared by Chi-square test or Fisher's exact test, as appropriate. The data were expressed as mean ± SD or median (interquartile range) for continuous variables and number (%) for categorical variables with 95% confidence interval. P <0.05 was considered significant.
Results
The patient groups were comparable at baseline regarding demographic data and characteristics of infections (P > 0.05), as shown in Table 1. Also, the two treatment groups were well balanced as regards the general characteristics at randomization (P > 0.05), as shown in Table 2.
Table 1.
Demographic data and characteristics of infections of the studied groups
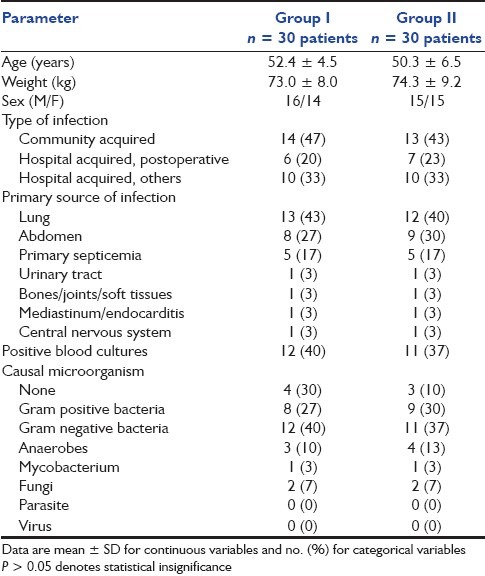
Table 2.
General characteristics at randomization

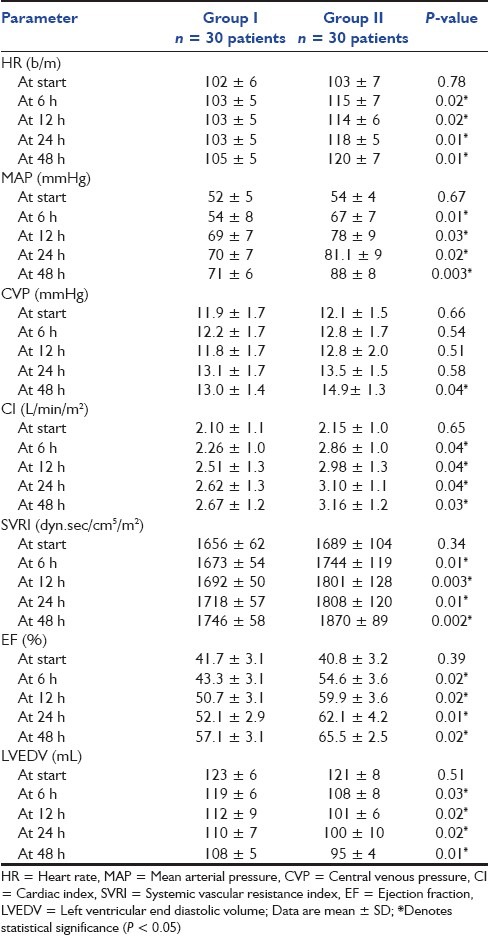
Both HR and MAP were significantly higher in group II (P < 0.05) at 6, 12, 24 and 48 h, and MAP reached the target level of >70 mmHg in group II at 12 h versus 24 h in group I [Table 3]. In a view to the CVP, there was an insignificant difference at start, 6, 12 and 24 h (P > 0.05), whereas it was significantly higher in group II at 48 h (P < 0.05) [Table 3]. CI, SVRI and EF were significantly higher in group II (P < 0.05) at 6, 12, 24 and 48 h, and CI reached the target level of >2.5 L/min/m2 in group II at 6 h, whereas it reached the target level in group I at 12 h [Table 3]. LVEDV was significantly higher in group I (P < 0.05) at 6, 12, 24 and 48 h [Table 3].
Table 3.
Hemodynamic parameter changes in the studied groups
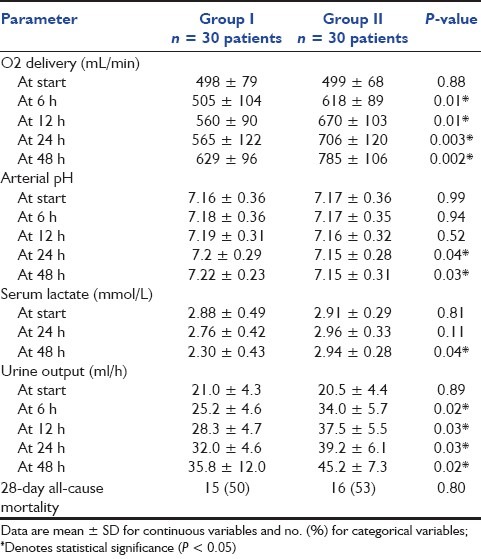
Oxygen delivery was comparable in both groups at start (P > 0.05); however, it was significantly higher in group II (P < 0.05) at 6, 12, 24 and 48 h [Table 4]. Arterial pH was significantly lower in group II (P < 0.05) at 24 and 48 h [Table 4]. Serum lactate was significantly higher in group II (P < 0.05) at 48 h [Table 4]. In view of the urine output, this was noted to be significantly higher in group II (P < 0.05) at 6, 12, 24 and 48 h [Table 4]. The 28-day all-cause mortality was comparable in both groups [Table 4].
Table 4.
Oxygen, metabolic parameters, urine output and 28-day all-cause mortality in both groups
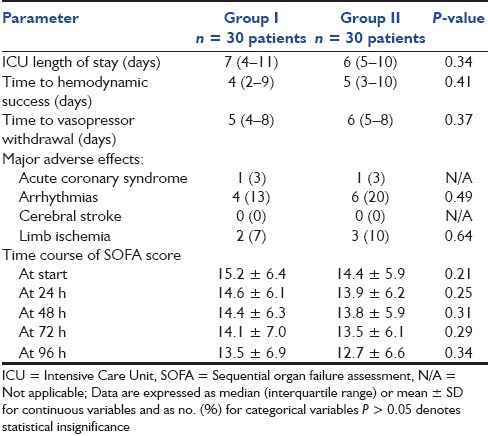
Both groups were comparable with respect to ICU length of stay as well as time to hemodynamic success and vasopressor withdrawal. Furthermore, there were no significant differences between both groups regarding the incidence of acute coronary syndrome, arrhythmias, cerebral stroke, limb ischemia or any other adverse events related to catecholamine administration. SOFA score improved over time, and was comparable in both groups [Table 5].
Table 5.
Length of ICU stay, time to hemodynamic success and vasopressor withdrawal, major adverse effects and time course of SOFA score in both groups
Discussion
The results of the present study showed that the addition of epinephrine in a dose of 0.05–0.3 μg/kg/min to norepinephrine in a dose of 0.1 μg/kg/min in patients with septic shock unresponsive to fluid resuscitation had positive effects on the hemodynamics but negative results on the serum lactate concentration and systemic pH compared with the addition of dobutamine in a dose of 3–20 μg/kg/min. Both groups were comparable regarding the progress of SOFA score and 28-day mortality.
This was in agreement with many studies, one of them being a study done by Minneci et al.,[14] which compared the different effects of epinephrine, norepinephrine and vasopressin in septic shock canines. Cardiovascular parameters were significantly increased with epinephrine compared with the other two groups.
Kreijci et al.[15] studied the effects of epinephrine, norepinephrine and phenylephrine on the microcirculatory blood flow of the gastrointestinal tract in a septic shock model in pigs. They increased the perfusion pressure to the gastrointestinal tract but without concomitant increase in the microcirculatory blood flow. Systemic blood flow was increased by both epinephrine and norepinephrine. These findings were explained by the fact that both epinephrine and norepinephrine divert blood flow away from the mesenteric circulation with a consequent decrease in the microcirculatory blood flow. On the other hand, phenylephrine supports the systemic blood pressure without changing distribution of blood flow.
In another study,[16] dobutamine prevented vasopressin-induced splanchnic lactate release and hyperlactatemia and maintained cardiac output in a porcine endotoxic shock model.
The previous results can be explained by the fact that dobutamine is an adrenergic agent stimulating both β1 and β2 receptors only and so it is a good inotrope but has no constrictive effects in contrast to epinephrine, which has potent α and β effects that makes it a good pressor and inotrope and, therefore, increases the SVR, MAP, HR and cardiac output.[17]
Wilson and coworkers[18] reported that epinephrine can restore global hemodynamics in septic shock primarily through increasing stroke volume and CI, together with more modest elevation in HR and SVR. In another study,[19] epinephrine was used as a first-line agent where a linear relationship was shown between epinephrine dose and MAP, HR, left ventricular stroke work index and CI, as well as oxygen consumption and delivery. No adverse cardiac side-effects were reported despite elevation of oxygen consumption.
In contrast with our results, the study done by Le Tulzo et al.[20] reported that epinephrine in a dose of 0.1 μg/kg/min improved right ventricular function in patients unresponsive to dopamine and dobutamine, but the SVR remained unchanged. These differences may also be explained by the lower dose of epinephrine used compared with the dose used in our study, which was up to 0.3 μg/kg/min. We used a higher dose according to the concept that improvement in MAP and oxygen delivery induced by epinephrine in septic shock patients may offset its ischemic adverse effects.
Serum lactate was statistically significantly higher and arterial pH was significantly lower in the norepinephrine plus epinephrine group, which is in agreement with the study done by Seguin et al.,[21] which studied the effect of dopexamine plus norepinephrine versus epinephrine on gastric perfusion in patients with septic shock. This study found that serum lactate was increased with epinephrine but not significantly, and explained this by the fact that epinephrine diverts blood flow away from the gastrointestinal mucosa with subsequent increase in regional lactate.
Another explanation for the decrease of lactic acid by the use of dobutamine and its increase with the use of epinephrine was cleared by a study done by Durantea and coworkers,[22] who attributed this finding to the splanchnic vasoconstrictor action of epinephrine through its α effects, which leads to gut ischemia in contrast to dobutamine, which has a splanchnic vasodilator action due to its β2-relaxing effect, which leads to elevation of the splanchnic blood flow and gastric perfusion with consequent decrease of serum lactate.
A recent study done by Levy[23] has considered increase of lactate by administration of epinephrine to be a physiological metabolic response to epinephrine stimulation. Epinephrine activates glycogenolysis and glycolysis, which leads to elevation of ATP levels and activation of Na+/K+-ATPase, resulting in ATP consumption and ADP generation, activation of phosphofructokinase, further activation of glycolysis and with consequent formation of pyruvate and its conversion into lactate. Another study[24] has reported elevation of skeletal muscle lactate production in patients with sepsis during administration of epinephrine. The authors attributed this effect to be a metabolic response to epinephrine administration that is mediated by Na+/K+-ATPase and, accordingly, raised the debate about clinical relevance of increased lactate levels in septic shock patients subjected to epinephrine administration. However, up till now, elevation of lactate levels in septic patients is still considered a bad omen, reflecting anaerobic glycolysis in hypoperfused tissues that is associated with a worse prognosis, although it may be in part a physiological response to severe sepsis resulting from increased aerobic glycolysis in skeletal muscle induced by epinephrine-activated Na+/K+-ATPase activity.
The urine outputwas significantly increased in the norepinephrine plus epinephrine group compared with the norepinephrine plus dobutamine group.
This result is supported and explained by many open-label clinical series done by Bourgoin and colleagues.[25] The authors attributed elevation of the renal perfusion pressure and urine output to the increase of MAP to a level of 75 mmHg; however, other studies referred that to increase of MAP from 60 to 65 mmHg.
It should be noticed that up till now, there is no adequate-sized, prospective randomized clinical study indicating that one catecholamine is superior to the other during septic shock. The patient outcome cannot be predicted through the use of a specific catecholamine. Consequently, the question of the ideal vasoactive agent in a septic shock patient remains controversial and unanswered.
In conclusion, the addition of epinephrine in a dose of 50–300 ng/kg/min to norepinephrine in a dose of 100 ng/kg/min in patients with septic shock unresponsive to fluid resuscitation had positive effects on the hemodynamics but negative effects on serum lactate and systemic pH compared with the addition of dobutamine in a dose of 3–20 μg/kg/min. However, the metabolic effects had no influence on the time to hemodynamic stabilization or recovery of organ dysfunction.
Acknowledgments
The authors pay great gratitude to the ICU staff of Minoufiya University Hospital for their effort in patient care. Thanks are also due to Dr. Amal El-Batanouni (Prof. of Biostatistics) for her help in completing the statistical analysis. In addition, the authors would also like to thank staff of the Cardiology Department, Minoufiya University Hospital, for their help in performing the echocardiography. The Minoufiya University funded this research.
Footnotes
Source of Support: The Minoufiya University funded this research
Conflict of Interest: None declared.
References
- 1.Shapiro NI, Howell M, Talmor D. A blue print for a sepsis protocol. Acad Emerg Med. 2005;12:352–9. doi: 10.1197/j.aem.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 2.Blanco J, Muriel-Bombín A, Sagredo V, Taboada F, Gandía F, Tamayo L, et al. Incidence, organ dysfunction and mortality in severe sepsis: A Spanish multicentre study. Crit Care. 2008;12:R158. doi: 10.1186/cc7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Makwana N, Baines PB. Myocardial dysfunction in meningococcal septic shock. Curr Opin Crit Care. 2005;11:418–23. doi: 10.1097/01.ccx.0000176699.51456.13. [DOI] [PubMed] [Google Scholar]
- 4.Ragaller M. Microcirculation in sepsis and septic shock-therapeutic options? Anaesthesiol Intensivmed Notfallmed Schmerzther. 2008;43:48–53. doi: 10.1055/s-2008-1038091. [DOI] [PubMed] [Google Scholar]
- 5.Lin SM, Huang CD, Lin HC, Liu CY, Wang CH, Kuo HP. A modified goal-directed protocol improves clinical outcomes in intensive care unit patients with septic shock: A randomized controlled trial. Shock. 2006;26:551–7. doi: 10.1097/01.shk.0000232271.09440.8f. [DOI] [PubMed] [Google Scholar]
- 6.Nfor TK, Walsh TS, Prescott RJ. The impact of organ failures and their relationship with outcome in intensive care: Analysis of a prospective multicentre database of adult admissions. Anaesthesia. 2006;61:731–8. doi: 10.1111/j.1365-2044.2006.04707.x. [DOI] [PubMed] [Google Scholar]
- 7.Vieillard Baron A, Schmitt JM, Beauchet A, Augarde R, Prin S, Page B, et al. Early preload adaptation in septic shock? A transesophageal echocardiographic study. Anesthesiology. 2001;94:400–6. doi: 10.1097/00000542-200103000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Beale RJ, Hollenberg SM, Vincent JL, Parrillo JE. Vasopressor and inotropic support in septic shock: An evidence-based review. Crit Care Med. 2004;32:S455–65. doi: 10.1097/01.ccm.0000142909.86238.b1. [DOI] [PubMed] [Google Scholar]
- 9.Myburgh JA. An appraisal of selection and use of catecholamines in septic shock – old becomes new again. Crit Care Resusc. 2006;8:353–60. [PubMed] [Google Scholar]
- 10.Jones AE, Brown MD, Trzeciak S, Shapiro NI, Garrett JS, Heffner AC, et al. The effect of a quantitative resuscitation strategy on mortality in patients with sepsis: A meta-analysis. Crit Care Med. 2008;36:2734–9. doi: 10.1097/CCM.0b013e318186f839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rudis MI, Rowland KL. Current concepts in severe sepsis and septic shock. J Pharmacol Pract. 2005;18:351–62. [Google Scholar]
- 12.Hernandez G, Bruhn A, Romero C, Larrondo FJ, De La Fuente R, Cornejo R, et al. Implementation of a norepinephrine-based protocol for management of septic shock: A pilot feasibility study. J Trauma. 2006;60:77–81. doi: 10.1097/01.ta.0000202062.49814.f4. [DOI] [PubMed] [Google Scholar]
- 13.Annane D, Vignon P, Renault A, Bollaert PE, Charpentier C, Martin C, et al. Norepinephrine plus dobutamine versus epinephrine alone for management of septic shock: A randomized trial. Lancet. 2007;370:676–84. doi: 10.1016/S0140-6736(07)61344-0. [DOI] [PubMed] [Google Scholar]
- 14.Minneci PC, Deans KJ, Banks SM, Costello R, Csako G, Eichacker PQ, et al. Differing effects of epinephrine, norepinephrine and vasopressin on survivals in a canine model of septic shock. Am J Physiol Heart Cric Physiol. 2004;287:H 2545–54. doi: 10.1152/ajpheart.00450.2004. [DOI] [PubMed] [Google Scholar]
- 15.Kreijci V, Hiltebrand LB, Sigurdsson GH. Effects of epinephrine, norepinephrine and phenylephrine on microcirculation blood flow in the gastrointestinal tract in sepsis. Crit Care Med. 2006;34:1456–63. doi: 10.1097/01.CCM.0000215834.48023.57. [DOI] [PubMed] [Google Scholar]
- 16.Martikainen TJ, Uusaro A, Tenhunen JJ, Ruokonen E. Dobutamine compensates deleterious hemodynamic and metabolic effects of vasopressin in the splanchnic region in endotoxin shock. Acta Anaesthesiol Scand. 2004;48:935–43. doi: 10.1111/j.0001-5172.2004.00435.x. [DOI] [PubMed] [Google Scholar]
- 17.Dries DJ. Cardiovascular support in septic shock. Air Med J. 2007;26:240–7. doi: 10.1016/j.amj.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 18.Wilson W, Lipman J, Scribante J, Kobilski S, Lee C, Krause P, et al. Septic shock: Does adrenaline have a role as a first-line inotropic agent? Anaesth Intensive Care. 1992;20:470–4. doi: 10.1177/0310057X9202000413. [DOI] [PubMed] [Google Scholar]
- 19.Moran JL, O’Fathartaigh MS, Peisach AR, Chapman MJ, Leppard P. Epinephrine as an inotropic agent in septic shock: A dose-profile analysis. Crit Care Med. 1993;21:70–7. doi: 10.1097/00003246-199301000-00015. [DOI] [PubMed] [Google Scholar]
- 20.Le Tulzo Y, Seguin P, Gacouin A, Camus C, Suprin E, Jouannic I, et al. Effects of epinephrine on right ventricular function in patients with severe septic shock and right ventricular failure: A preliminary descriptive study. Intensive Care Med. 1997;23:664–70. doi: 10.1007/s001340050391. [DOI] [PubMed] [Google Scholar]
- 21.Seguin P, Laviolle B, Guinet P, Morel I, Mallédant Y, Bellissant E. Dopexamine and norepinephrine versus epinephrine on gastric perfusion in patients with septic shock: A randomized study. Crit Care. 2006;10:R32. doi: 10.1186/cc4827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Durantea J, Sitbon P, Teboul JL, Vicaut E, Anguel N, Richard C, et al. The effect of epinephrine, norepinephrine or the combination of norepinephrine and dobutamine on gastric mucosa in septic shock. Crit Care Med. 1999;27:893–900. doi: 10.1097/00003246-199905000-00021. [DOI] [PubMed] [Google Scholar]
- 23.Levy B. Lactate and shock: The metabolic view. Curr Opin Crit Care. 2006;12:315–21. doi: 10.1097/01.ccx.0000235208.77450.15. [DOI] [PubMed] [Google Scholar]
- 24.Levy B, Gibot S, Franke P, Cravoisy A, Bollaert PE. Relation between muscle Na+ K+ ATPase and raised lactate concentration in septic shock: A prospective study. Lancet. 2005;365:871–5. doi: 10.1016/S0140-6736(05)71045-X. [DOI] [PubMed] [Google Scholar]
- 25.Bourgoin A, Leone M, Delmas A, Garnier F, Albanèse J, Martin C. Increasing mean arterial pressure in patients with septic shock: Effects on oxygen variables and renal function. Crit Care Med. 2005;33:780–6. doi: 10.1097/01.ccm.0000157788.20591.23. [DOI] [PubMed] [Google Scholar]


