Abstract
We report on an optimized method for the in vitro culture of tissue cyst-forming Neospora caninum bradyzoites in Vero cells and the separation of viable parasites from host cells. Treatment of tachyzoite-infected Vero cell cultures with 17 μM sodium nitroprusside for 8 days severely scaled down parasite proliferation, led to reduced expression of tachyzoite surface antigens, and induced the expression of the bradyzoite marker NcBAG1 and the cyst wall antigen recognized by the monoclonal antibody MAbCC2. Transmission electron microscopy demonstrated that intracellular parasites were located within parasitophorous vacuoles that were surrounded by a cyst wall-like structure, and the dense granule antigens NcGRA1, NcGRA2, and NcGRA7 were incorporated into the cyst wall. Adhesion-invasion assays employing purified tachyzoites and bradyzoites showed that tachyzoites adhered to, and invaded, Vero cells with higher efficiency than bradyzoites. However, removal of terminal sialic acid residues from either the host cell or the parasite surface increased the invasion of Vero cells by bradyzoites, but not tachyzoites.
Neospora caninum, an apicomplexan parasite that causes stillbirth and abortion in cattle and neuromuscular disorders in dogs (14, 15, 21), is phylogenetically closely related to Toxoplasma gondii but is different from Toxoplasma with regard to its natural host range (15), antigenicity (4, 22, 27), few ultrastructural features (25, 51), and differences in its host cell recognition (39, 40). Three stages are known in the life cycle of N. caninum (15). These are oocysts, the sexually produced stage that is shed with the feces of infected dogs (35); the rapidly proliferating tachyzoites that are present during the acute phase of the infection (reviewed in references 14, 15, and 21); and slowly proliferating and tissue cyst-forming bradyzoites (7, 28). Tissue cysts containing N. caninum bradyzoites can persist in the infected cow for several years without causing any clinical signs. Reactivation of quiescent tissue cysts in an immunocompromised situation, such as during pregnancy, may lead to bradyzoite-to-tachyzoite reconversion and subsequent infection of the placenta and/or the unborn fetus (28, 45).
In N. caninum, as in T. gondii, tachzyoites and bradyzoites can be differentiated at the ultrastructural level by transmission electron microscopy (TEM) (29, 51, 57) and through detection of stage-specific antigen expression. The major T. gondii surface antigens TgSAG1 and TgSAG2 are stage-specifically expressed in tachyzoites (30). Similarly, the two major immunodominant N. caninum tachyzoite surface antigens, NcSAG1 and NcSRS2 (9, 10, 48), were observed to be down-regulated during tachyzoite-to-bradyzoite stage conversion (17, 47, 55). Several bradyzoite-specific T. gondii antigens, such as TgBAG1 (5, 42), have been identified (6, 49, 52), and polyclonal antibodies directed against recombinant TgBAG1 were shown to cross-react with bradyzoites of N. caninum (36, 53, 55, 58). Furthermore, the monoclonal antibody MAbCC2, reacting with a 115-kDa T. gondii cyst wall protein (18), was recently demonstrated to cross-react with N. caninum tissue cysts (31, 55). In addition to stage-specifically expressed antigens, T. gondii dense granule proteins, which are secreted shortly after invasion and which are involved in the modification of the parasitophorous vacuole (11), have been shown to be differentially located in T. gondii tachyzoite and bradyzoite cysts (52). Several dense granule proteins in N. caninum have been described (1, 16, 32, 34), and one of them, NcGRA7 (32), formerly designated Nc-p33 (23), was found to be localized at the tissue cyst periphery (17).
Although procedures have been developed to obtain N. caninum bradyzoites from tissue cysts of experimentally infected animals (37), large numbers of bradyzoites are needed for scientific investigation. Thus, the development of in vitro culture techniques for inducing tachyzoite-to-bradyzoite stage conversion was anticipated. The methods used for induction of stage conversion in Toxoplasma are relatively inefficient for Neospora (58). Improved results were reported recently by Tunev et al. (53) upon the use of bovine monocytes as host cells. An efficient in vitro method for N. caninum bradyzoite-to-tachyzoite stage conversion in murine epidermal keratinocytes using sodium nitroprusside (SNP) has been established (8, 55). However, this culture system is not practical for obtaining larger numbers of N. caninum bradyzoites for biochemical, molecular, or functional studies due to high costs involved in culturing. In addition, in these keratinocyte host cells, tissue cysts are surrounded by thick keratin filament bundles which consistently obstruct attempts to purify the parasites (25). Close association of intermediate filaments with parasitophorous vacuoles and tissue cysts of T. gondii and N. caninum had also been observed in other host cells (19, 44, 56).
Here, we report the successful induction of N. caninum bradyzoites in Vero cells using a modified procedure. N. caninum tachyzoites of the Liverpool isolate (Nc-Liverpool) (2) were maintained in, and purified from, Vero cell monolayers as described by Hemphill et al. (24) and were immediately used for infection. Confluent monolayers, grown either on poly-l-lysine (100 μg/ml)-coated glass coverslips in 24-well tissue culture plates or in T75 or T175 tissue culture flasks, were overlaid with 1 ml/well (24-well plates) or 20 or 60 ml (T75 and T175 tissue culture flasks, respectively) of RPMI medium containing 10% fetal calf serum and 105 freshly purified tachyzoites/cm2. Different concentrations of SNP (17 to 70 μM) were added at the time of infection, and the cultures were maintained for 8 days at 37°C and 5% CO2. Each day, the medium was replaced with fresh medium containing the respective concentrations of SNP. We found that application of 20 to 70 μM SNP consistently resulted in detachment of Vero cells by day 4 of culture at the latest (data not shown), while treatment with 17 μM SNP did not visibly impair the structural integrity of the monolayer. Monitoring of N. caninum proliferation in Vero cells by quantitative real-time PCR (38, 55) revealed that parasite proliferation in SNP-treated cultures was heavily depressed (data not shown).
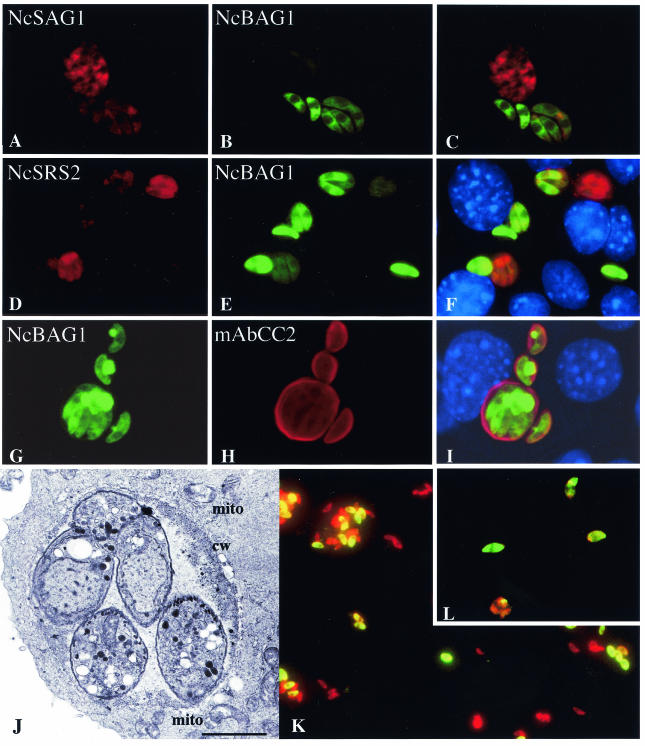
Immunofluorescence labeling of infected and SNP-treated Vero cell cultures was performed as previously described for keratinocytes (55). This revealed that the bradyzoite-specific antigen NcBAG1 (Fig. 1B, E, H, K, L) and the the cyst wall-associated antigen recognized by MAbCC2 (Fig. 1G to I) were expressed as efficiently as in murine epidermal keratinocytes. In addition, intracellular parasites expressing NcBAG1 consistently exhibited reduced staining with antibodies directed against NcSAG1 and NcSRS2 (Fig. 1A to F). Earlier, NcSAG1 and NcSRS2 had been shown to be involved in tachyzoite host cell invasion (20, 41), and the down-regulation of these surface antigens in bradyzoites suggests that they probably play a minor role in the biology of bradyzoites. Furthermore, TEM of SNP-treated cultures was performed as described previously (55) and showed that N. caninum bradyzoites generated in 17 μM SNP-treated Vero cells were enclosed within a parasitophorous vacuole and were surrounded by a peripheral accumulation of electron-dense material which resembled a cyst wall (Fig. 1J).
FIG. 1.
In vitro stage conversion in Vero cells. Vero cells were infected with Nc-Liverpool tachyzoites and treated with 17 μM SNP for 8 days, followed by immunofluorescence labeling. Note the down-regulation of NcSAG1- (A to C) and NcSRS2 expression (D to F) in those parasites expressing NcBAG1. Parasites within vacuoles expressing NcBAG1 also exhibit peripheral labeling with MAbCC2 (G to I). (J) TEM of a tissue cyst with bradyzoites, peripheral accumulation of electron-dense material reminiscent of a cyst wall (cw), and the presence of host cell mitochondria (mito) surrounding the cyst. Bar = 0.85 μm. (K and L) Purified parasites labeled with anti-BAG1 (green) and anti-SAG1 (red) antibodies.
Besides the reduced expression of the immunodominant surface antigens NcSAG1 and NcSRS2 in N. caninum parasites expressing NcBAG1 (Fig. 1), we also investigated the expression and localization of three dense granule proteins. The staining patterns in tachyzoites for NcGRA1 (1), NcGRA2 (16), and NcGRA7 (32) were identical for all three antigens, as exemplified in Fig. 2A to C for NcGRA1. Granular-type immunolabeling was found predominantly within the parasite cytoplasm at the anterior and posterior ends of the tachyzoites. In contrast, bradyzoites exhibited staining for NcGRA1 (see Fig. 4D to F) and NcGRA7 (see Fig. 4J to L) that had largely shifted toward the periphery of the vacuole. For NcGRA2 (see Fig. 4G to I), peripheral labeling was not as pronounced but was clearly evident as well. Immunogold labeling of sections of LR-White-embedded tachyzoites (Fig. 3A) and bradyzoites (Fig. 3B) confirmed these findings. In bradyzoites, distinct NcGRA1 staining of the cyst periphery could be observed, and similar results were obtained with anti-NcGRA2 and NcGRA7 antibodies (data not shown). Thus, upon SNP treatment, dense granule components were secreted and largely accumulated in the cyst wall, and NcGRA1, -2, and -7 could be involved in formation of the tissue cyst wall during stage conversion. In this respect, it is interesting that NcGRA1 and NcGRA2 were shown to elicit an immune response during chronic infection and could be used as markers to identify chronically infected animals by serological means (1, 16). Additionally, as in T. gondii, dense granule antigens are likely vaccine candidates, as they are expressed and secreted in both tachyzoites and bradyzoites (54).
FIG. 2.
Differential localization of dense granule antigens in tachyzoites and bradyzoites during in vitro culture. Localizations of NcGRA1, -2, and -7 in tachyzoite cultures were identical, as exemplified by NcGRA1 (A to C). During in vitro stage conversion, a strong shift of labeling toward the vacuole periphery was noted for NcGRA1 (D to F) and NcGRA7 (J to L), and also to a lesser extent for NcGRA2 (G to I).
FIG. 4.
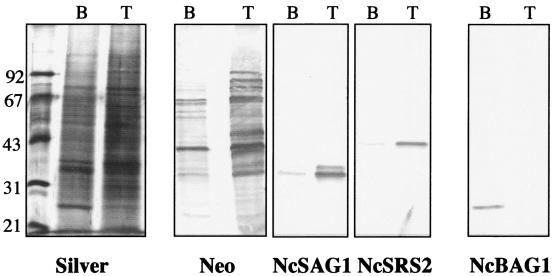
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (silver stain) and corresponding immunoblots of tachyzoite (T) and bradyzoite (B) extracts. Identical numbers of parasites were loaded. Note different banding patterns obtained in bradyzoite and tachyzoite extracts by silver staining and immunoblotting using an anti-N. caninum antiserum (Neo) (20), the down-regulation of NcSAG1 and NcSRS2 expression, and the appearance of NcBAG1 expression in bradyzoites.
FIG. 3.
Immunogold localization of NcGRA1 in N. caninum tachyzoites (A and B) and in vitro-cultured bradyzoites (C and D). The staining patterns for NcGRA2 and -3 were virtually identical (not shown). (A) Note the binding of gold particles with the tachyzoite dense granules (small arrows) and, to a lesser extent, with the vacuolar matrix. Bar = 0.23 μm. (B) Lower-magnification view of panel A. The arrow indicates the area magnified in panel A. Bar = 0.9 μm. (C) In bradyzoites, NcGRA1 antibodies are localized mainly at the tissue cyst periphery. Bar = 0.25 μm. (D) Lower-magnification view of panel C. Bar = 1.25 μm. The arrow indicates the area magnified in panel C.
In contrast to the situation in murine epidermal keratinocytes, Nc-Liverpool bradyzoites could be readily isolated from SNP-treated Vero cells after 8 days of culture by applying the previously described protocol for tachyzoites (24) with some modifications. Briefly, 8-day SNP-treated cultures were trypsinized and repeatedly passed through a 25-gauge needle, and following centrifugation at 600 × g at 4°C for 10 min, the pellet was resuspended in cold RPMI 1640 and washed twice by centrifugation at 600 × g. Under these conditions, cellular debris remained largely in the supernatant, while intact parasites were recovered in the pellet fraction. The final pellet was resuspended in 2 ml of cold RPMI 1640, and parasites were passed through PD-10 columns to separate parasites from still-intact host cells. The yield per T175 flask was typically ∼3 × 106 to 5 × 106 parasites. Immunofluorescence labeling of purified parasites demonstrated that a major portion (typically 50 to 80%) exhibited staining with anti-NcBAG1 antibodies. Thus, this purified population was heavily enriched in parasites undergoing stage conversion (Fig. 1K and L).
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotting (50) were used to assess the differences in protein expression by N. caninum tachyzoites and bradyzoites. Equal numbers of both parasite stages were loaded, and silver staining revealed partially distinct banding patterns for bradyzoite and tachyzoite extracts (Fig. 4). Immunoblotting confirmed the down-regulation of NcSAG1 and NcSRS2 expression, and the induction of NcBAG1 synthesis, in bradyzoites (Fig. 4).
The adhesive and invasive properties of N. caninum tachyzoites and bradyzoites were comparatively assessed using a recently described adhesion-invasion assay (40) that allows the determination of invasion rates (i.e., percentages of invaded parasites in relation to the overall number of parasites interacting with host cells in a given experiment). Only one representative result of one experiment is shown in Fig. 5; however, these assays were repeated twice and resulted in essentially identical results in all three experiments (data not shown). Equal numbers of tachyzoites and bradyzoites were incubated with Vero cell monolayers for 30 min, and the number of tachyzoites actually capable of either adhering to or invading Vero cells was consistently severalfold higher than that of bradyzoites (Fig. 5A). In addition, tachyzoites also exhibited a markedly higher invasion rate than bradyzoites (36 versus 25%, respectively). Differences in host cell invasion between tachyzoites and bradyzoites had also been described earlier for T. gondii, albeit at the ultrastructural level (46).
FIG. 5.
Adhesion and invasion of Vero cells by in vitro-cultured N. caninum tachyzoites and bradyzoites. The solid bars indicate the overall numbers of parasites interacting with Vero cells (adherent and invaded), while the open bars indicate the numbers of intracellular parasites only. The percentages above the bars indicate the invasion rates (percentages of invaded parasites in relation to the overall numbers). (A) Identical numbers of parasites were allowed to interact either with untreated Vero cells (no treatment) or with Vero cells which had been treated with neuraminidase. (B) Parasites were treated with neuraminidase prior to Vero cell interaction. The data are displayed as means plus standard deviations, and a representative experiment among three independent experiments is shown.
During natural infection, bradyzoites would invade epithelial cells of the dog gut to induce the sexual cycle (14). Intestinal epithelial cells are covered with mucin glycoproteins, whose carbohydrate chains are usually terminally modified by sialic acid (26). In order to investigate the role of sialic acid residues in the interaction between the two parasite stages and their host cells, Vero cell monolayers were treated with 0.05 IU of neuraminidase (Vibrio cholerae)/ml at 37°C for 2 h prior to the parasite incubation (13). Following this treatment, the invasion rate of bradyzoites almost doubled (from 25 to 46%), while tachyzoite invasion rates were only slightly elevated, by 6% (Fig. 5A). Similar experiments using T. gondii tachyzoites and macrophages had demonstrated that removal of sialic acid residues from the macrophage surface had increased invasion by T. gondii tachyzoites. Our results indicate that the presence or absence of host cell surface sialic acid residues could influence host cell invasion, most notably by N. caninum bradyzoites.
Pretreatment of tachyzoites and bradyzoites with 0.05 IU of neuraminidase/ml prior to host cell interaction resulted in largely similar findings. In this case, bradyzoite invasion rates were more than doubled (from 15 to 36%), while tachyzoite invasion rates were not affected at all (Fig. 5B). This result also implies that the two stages might employ different receptor-ligand interactions for invading their host cells. For another invasive protozoan parasite, Trypanosoma cruzi, removal of sialic acid residues from the surfaces of metacyclic trypomastigotes also enhanced host cell invasion (43, 59). Cleary et al. (12) used microarray analysis to study changes in transcript levels during T. gondii tachyzoite-to-bradyzoite conversion, and in addition to other developmentally regulated genes, they identified a gene coding for a putative mucin domain-containing bradyzoite surface molecule. Given the role of a close homologue in host cell invasion by another apicomplexan parasite, Cryptosporidium parvum (3), it is likely that mucin-like bradyzoite proteins are also functionally implicated in host cell invasion by N. caninum upon oral ingestion of tissue cysts. However, further studies are required to investigate this in more detail.
The optimized procedure for the culture of N. caninum bradyzoites presented here will facilitate the accessibility of this stage to studies of altered gene expression during stage conversion, e.g., through the construction of cDNA libraries and expressed sequence tag sequencing, as is currently done for a number of other apicomplexan parasites, including the N. caninum tachyzoite stage (33). This information would provide a better understanding of the events which occur during both the formation and reactivation of tissue cysts, both of which represent crucial events in the pathogenesis of Neospora infection.
Acknowledgments
Many thanks to Mark Jenkins (USDA, Beltsville, Md.), Milton McAllister (University of Iowa at Urbana), and Wolfgang Bohne and Uwe Gross (University of Göttingen) for their generous gifts of antibodies. Eliane Müller and Reto Caldelari (Institute of Animal Pathology) are acknowledged for help during the earlier stages of this project. We also thank Toni Wyler (Institute of Cell Biology) and Maja Suter (Institute of Animal Pathology) for letting us use their TEM facilities.
This work was financed through grants obtained through the Swiss National Science Foundation (no. 32-067782.02), the Foundation Research 3R (project no. 72/00), and the European Union (BBW grant no. 00.0498). C.B. was supported by the Swedish Council for Forestry and Agricultural Research. A.N. was supported by the Swiss Commission of Foreign Students and the Roche Research Foundation.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Atkinson, R. A., C. Ryce, C. M. Miller, S. Balu, P. A. Harper, and J. T. Ellis. 2001. Isolation of Neospora caninum genes detected during a chronic murine infection. Int. J. Parasitol. 31:67-71. [DOI] [PubMed] [Google Scholar]
- 2.Barber, J. S., O. J. Holmdahl, M. R. Owen, F. Guy, A. Uggla, and A. J. Trees. 1995. Characterization of the first European isolate of Neospora caninum (Dubey, Carpenter, Speer, Topper and Uggla). Parasitology 111:563-568. [DOI] [PubMed] [Google Scholar]
- 3.Barnes, D. A., A. Bonnin, J. X. Huang, L. Gousset, J. Wu, J. Gut, P. Doyle, J. F. Dubremetz, H. Ward, and C. Petersen. 1998. A novel multi-domain mucin-like glycoprotein of Cryptosporidium parvum mediates invasion. Mol. Biochem. Parasitol. 96:93-110. [DOI] [PubMed] [Google Scholar]
- 4.Björkman, C., and A. Hemphill. 1998. Characterization of Neospora caninum iscom antigens using monoclonal antibodies. Parasite Immunol. 20:73-80. [DOI] [PubMed] [Google Scholar]
- 5.Bohne, W., U. Gross, D. J. Ferguson, and J. Heesemann. 1995. Cloning and characterization of a bradyzoite-specifically expressed gene (hsp30/bag1) of Toxoplasma gondii, related to genes encoding small heat-shock proteins of plants. Mol. Microbiol. 16:1221-1230. [DOI] [PubMed] [Google Scholar]
- 6.Bohne, W., M. Holpert, and U. Gross. 1999. Stage differentiation of the protozoan parasite Toxoplasma gondii. Immunobiology 201:248-254. [DOI] [PubMed] [Google Scholar]
- 7.Buxton, D., M. M. McAllister, and J. P. Dubey. 2002. The comparative pathogenesis of neosporosis. Trends Parasitol. 18:546-552. [DOI] [PubMed] [Google Scholar]
- 8.Caldelari, R., M. M. Suter, D. Baumann, A. De Bruin, and E. Mueller. 2000. Long-term culture of murine epidermal keratinocytes. J. Investig. Dermatol. 114:1064-1065. [DOI] [PubMed] [Google Scholar]
- 9.Cannas, A., A. Naguleswaran, N. Muller, S. Eperon, B. Gottstein, and A. Hemphill. 2003. Vaccination of mice against experimental Neospora caninum infection using NcSAG1- and NcSRS2-based recombinant antigens and DNA vaccines. Parasitology 126:303-312. [DOI] [PubMed] [Google Scholar]
- 10.Cannas, A., A. Naguleswaran, N. Muller, B. Gottstein, and A. Hemphill. 2003. Reduced cerebral infection of Neospora caninum-infected mice after vaccination with recombinant microneme protein NcMIC3 and ribi adjuvant. J. Parasitol. 89:44-50. [DOI] [PubMed] [Google Scholar]
- 11.Carruthers, V. B., and L. D. Sibley. 1997. Sequential protein secretion from three distinct organelles of Toxoplasma gondii accompanies invasion of human fibroblasts. Eur. J. Cell Biol. 73:114-123. [PubMed] [Google Scholar]
- 12.Cleary, M. D., U. Singh, I. J. Blader, J. L. Brewer, and J. C. Boothroyd. 2002. Toxoplasma gondii asexual development: identification of developmentally regulated genes and distinct patterns of gene expression. Eukaryot. Cell 1:329-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Carvalho, L., C. Y. Yan, and W. de Souza. 1993. Effect of various digestive enzymes on the interaction of Toxoplasma gondii with macrophages. Parasitol. Res. 79:114-118. [DOI] [PubMed] [Google Scholar]
- 14.Dubey, J. P., and D. S. Lindsay. 1996. A review of Neospora caninum and neosporosis. Vet. Parasitol. 67:1-59. [DOI] [PubMed] [Google Scholar]
- 15.Dubey, J. P., B. C. Barr, J. R. Barta, I. Bjerkas, C. Bjorkman, B. L. Blagburn, D. D. Bowman, D. Buxton, J. T. Ellis, B. Gottstein, A. Hemphill, D. E. Hill, D. K. Howe, M. C. Jenkins, Y. Kobayashi, B. Koudela, A. E. Marsh, J. G. Mattsson, M. M. McAllister, D. Modry, Y. Omata, L. D. Sibley, C. A. Speer, A. J. Trees, A. Uggla, S. J. Upton, D. J. Williams, and D. S. Lindsay. 2002. Redescription of Neospora caninum and its differentiation from related coccidia. Int. J. Parasitol. 32:929-946. [DOI] [PubMed] [Google Scholar]
- 16.Ellis, J. T., C. Ryce, R. Atkinson, S. Balu, P. Jones, and P. A. Harper. 2000. Isolation, characterization and expression of a GRA2 homologue from Neospora caninum. Parasitology 120:383-390. [DOI] [PubMed] [Google Scholar]
- 17.Fuchs, N., S. Sonda, B. Gottstein, and A. Hemphill. 1998. Differential expression of cell surface- and dense granule-associated Neospora caninum proteins in tachyzoites and bradyzoites. J. Parasitol. 84:753-758. [PubMed] [Google Scholar]
- 18.Gross, U., H. Bormuth, C. Gaissmaier, C. Dittrich, V. Krenn, W. Bohneand, and D. J. Ferguson. 1995. Monoclonal rat antibodies directed against Toxoplasma gondii suitable for studying tachyzoite-bradyzoite interconversion in vivo. Clin. Diagn. Lab. Immunol. 2:542-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Halonen, S. K., L. M. Weiss, and F. C. Chiu. 1998. Association of host cell intermediate filaments with Toxoplasma gondii cysts in murine astrocytes in vitro. Int. J. Parasitol. 28:815-823. [DOI] [PubMed] [Google Scholar]
- 20.Hemphill, A. 1996. Subcellular localization and functional characterization of Nc-p43, a major Neospora caninum tachyzoite surface protein. Infect. Immun. 64:4279-4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hemphill, A. 1999. The host-parasite relationship in neosporosis. Adv. Parasitol. 43:47-104. [DOI] [PubMed] [Google Scholar]
- 22.Hemphill, A., R. Felleisen, B. Connolly, B. Gottstein, B. Hentrich, and N. Müller. 1997. Characterization of a cDNA-clone encoding Nc-p43, a major Neospora caninum tachyzoite surface protein. Parasitology 115:581-590. [DOI] [PubMed] [Google Scholar]
- 23.Hemphill, A., N. Gajendran, S. Sonda, N. Fuchs, B. Gottstein, B. Hentrich, and M. Jenkins. 1998. Identification and characterisation of a dense granule-associated protein in Neospora caninum tachyzoites. Int. J. Parasitol. 28:429-438. [DOI] [PubMed] [Google Scholar]
- 24.Hemphill, A., B. Gottstein, and H. Kaufmann. 1996. Adhesion and invasion of bovine endothelial cells by Neospora caninum. Parasitology 112:183-197. [DOI] [PubMed] [Google Scholar]
- 25.Hemphill, A., N. Vonlaufen, A. Naguleswaran, N. Guetg, S. Srinivsan, and F. Alaeddine. Tissue culture and explant approaches to study and visualize Neospora caninum and its interactions with the host cell. Microsc. Microanal., in press. [DOI] [PubMed]
- 26.Hicks, S. J., G. Theodoropoulos, S. D. Carrington, and A. P. Corfield. 2000. The role of mucins in host-parasite interactions. Part I—Protozoan parasites. Parasitol. Today 16:476-481. [DOI] [PubMed] [Google Scholar]
- 27.Howe, D. K., and L. D. Sibley. 1999. Comparison of the major antigens of Neospora caninum and Toxoplasma gondii. Int. J. Parasitol. 29:1489-1496. [DOI] [PubMed] [Google Scholar]
- 28.Innes, E. A., A. G. Andrianarivo, C. Bjorkman, D. J. Williams, and P. A. Conrad. 2002. Immune responses to Neospora caninum and prospects for vaccination. Trends Parasitol. 18:497-504. [DOI] [PubMed] [Google Scholar]
- 29.Jardine, J. E. 1996. The ultrastructure of bradyzoites and tissue cysts of Neospora caninum in dogs: absence of distinguishing morphological features between parasites of canine and bovine origin. Vet. Parasitol. 62:231-240. [DOI] [PubMed] [Google Scholar]
- 30.Kasper, L. H. 1989. Identification of stage-specific antigens of Toxoplasma gondii. Infect. Immun. 57:668-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keller, N., A. Naguleswaran, A. Cannas, N. Vonlaufen, M. Bienz, C. Bjorkman, W. Bohne, and A. Hemphill. 2002. Identification of a Neospora caninum microneme protein (NcMIC1) which interacts with sulfated host cell surface glycosaminoglycans. Infect. Immun. 70:3187-3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lally, N., M. Jenkins, S. Liddell, and J. P. Dubey. 1997. A dense granule protein (NCDG1) gene from Neospora caninum. Mol. Biochem. Parasitol. 87:239-243. [DOI] [PubMed] [Google Scholar]
- 33.Li, L., B. P. Brunk, J. C. Kissinger, D. Pape, K. Tang, R. H. Cole, J. Martin, T. Wylie, M. Dante, S. J. Fogarty, D. K. Howe, P. Liberator, C. Diaz, J. Anderson, M. White, M. E. Jerome, E. A. Johnson, J. A. Radke, C. J. Stoeckert, Jr., R. H. Waterston, S. W. Clifton, D. S. Roos, and L. D. Sibley. 2003. Gene discovery in the apicomplexa as revealed by EST sequencing and assembly of a comparative gene database. Genome Res. 13:443-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liddell, S., N. C. Lally, M. C. Jenkins, and J. P. Dubey. 1998. Isolation of the cDNA encoding a dense granule associated antigen (NCDG2) of Neospora caninum. Mol. Biochem. Parasitol. 93:153-158. [DOI] [PubMed] [Google Scholar]
- 35.McAllister, M. M., J. P. Dubey, D. S. Lindsay, W. R. Jolley, R. A. Wills, and A. M. McGuire. 1998. Dogs are definitive hosts of Neospora caninum. Int. J. Parasitol. 28:1473-1478. [PubMed] [Google Scholar]
- 36.McAllister, M. M., S. F. Parmley, L. M. Weiss, V. J. Welch, and A. M. McGuire. 1996. An immunohistochemical method for detecting bradyzoite antigen (BAG5) in Toxoplasma gondii-infected tissues cross-reacts with a Neospora caninum bradyzoite antigen. J. Parasitol. 82:354-355. [PubMed] [Google Scholar]
- 37.McGuire, A. M., M. M. McAllister, W. R. Jolley, and R. C. Anderson-Sprecher. 1997. A protocol for the production of Neospora caninum tissue cysts in mice. J. Parasitol. 83:647-651. [PubMed] [Google Scholar]
- 38.Müller, N., N. Vonlaufen, C. Gianinazzi, S. L. Leib, and A. Hemphill. 2002. Application of real-time fluorescent PCR for quantitative assessment of Neospora caninum infections in organotypic slice cultures of rat central nervous system tissue. J. Clin. Microbiol. 40:252-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Naguleswaran, A., A. Cannas, N. Keller, N. Vonlaufen, C. Bjorkman, and A. Hemphill. 2002. Vero cell surface proteoglycan interaction with the microneme protein NcMIC(3) mediates adhesion of Neospora caninum tachyzoites to host cells unlike that in Toxoplasma gondii. Int. J. Parasitol. 32:695-704. [DOI] [PubMed] [Google Scholar]
- 40.Naguleswaran, A., N. Müller, and A. Hemphill. 2003. Neospora caniunum and Toxoplasma gondii: a novel adhesion/invasion assay reveals distinct differences in tachyzoite-host cell interactions. Exp. Parasitol. 104:149-158. [DOI] [PubMed] [Google Scholar]
- 41.Nishikawa, Y., X. Xuan, H. Nagasawa, I. Igarashi, K. Fujisaki, H. Otsuka, and T. Mikami. 2000. Monoclonal antibody inhibition of Neospora caninum tachyzoite invasion into host cells. Int. J. Parasitol. 30:51-58. [DOI] [PubMed] [Google Scholar]
- 42.Parmley, S. F., L. M. Weiss, and L. Yang. 1995. Cloning of a bradyzoite-specific gene of Toxoplasma gondii encoding a cytoplasmic antigen. Mol. Biochem. Parasitol. 73:253-257. [DOI] [PubMed] [Google Scholar]
- 43.Pellegrin, J. L., E. Ortega-Barria, R. P. Prioli, M. Buerger, R. G. Strout, J. Alroy, and M. E. Pereira. 1993. Identification of a developmentally regulated sialidase in Eimeria tenella that is immunologically related to the Trypanosoma cruzi enzyme. Glycoconj. J. 10:57-63. [DOI] [PubMed] [Google Scholar]
- 44.Powell, H. C., C. J. Gibbs, Jr., A. M. Lorenzo, P. W. Lampert, and D. C. Gajdusek. 1978. Toxoplasmosis of the central nervous system in the adult. Electron microscopic observations. Acta Neuropathol. 41:211-216. [DOI] [PubMed] [Google Scholar]
- 45.Quinn, H. E., J. T. Ellis, and N. C. Smith. 2002. Neospora caninum: a cause of immune-mediated failure of pregnancy? Trends Parasitol. 18:391-394. [DOI] [PubMed] [Google Scholar]
- 46.Sasono, P. M., and J. E. Smith. 1998. Toxoplasma gondii: an ultrastructural study of host-cell invasion by the bradyzoite stage. Parasitol. Res. 84:640-645. [DOI] [PubMed] [Google Scholar]
- 47.Schares, G., J. F. Dubremetz, J. P. Dubey, A. Barwald, A. Loyens, and F. J. Conraths. 1999. Neospora caninum: identification of 19-, 38-, and 40-kDa surface antigens and a 33-kDa dense granule antigen using monoclonal antibodies. Exp. Parasitol. 92:109-119. [DOI] [PubMed] [Google Scholar]
- 48.Schares, G., M. Rauser, P. Sondgen, P. Rehberg, A. Barwald, J. P. Dubey, R. Edelhofer, and F. J. Conraths. 2000. Use of purified tachyzoite surface antigen p38 in an ELISA to diagnose bovine neosporosis. Int. J. Parasitol. 30:1123-1130. [DOI] [PubMed] [Google Scholar]
- 49.Smith, J. E. 1995. A ubiquitous intracellular parasite: the cellular biology of Toxoplasma gondii. Int. J. Parasitol. 25:1301-1309. [DOI] [PubMed] [Google Scholar]
- 50.Sonda, S., N. Fuchs, B. Gottstein, and A. Hemphill. 2000. Molecular characterization of a novel microneme antigen in Neospora caninum. Mol. Biochem. Parasitol. 108:39-51. [DOI] [PubMed] [Google Scholar]
- 51.Speer, C. A., J. P. Dubey, M. M. McAllister, and J. A. Blixt. 1999. Comparative ultrastructure of tachyzoites, bradyzoites, and tissue cysts of Neospora caninum and Toxoplasma gondii. Int. J. Parasitol. 29:1509-1519. [DOI] [PubMed] [Google Scholar]
- 52.Torpier, G., H. Charif, F. Darcy, J. Liu, M. L. Darde, and A. Capron. 1993. Toxoplasma gondii: differential location of antigens secreted from encysted bradyzoites. Exp. Parasitol. 77:13-22. [DOI] [PubMed] [Google Scholar]
- 53.Tunev, S. S., M. M. McAllister, R. C. Anderson-Sprecher, and L. M. Weiss. 2002. Neospora caninum in vitro: evidence that the destiny of a parasitophorous vacuole depends on the phenotype of the progenitor zoite. J. Parasitol. 88:1095-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vercammen, M., T. Scorza, K. Huygen, J. De Braekeleer, R. Diet, D. Jacobs, E. Saman, and H. Verschueren. 2000. DNA vaccination with genes encoding Toxoplasma gondii antigens GRA1, GRA7, and ROP2 induces partially protective immunity against lethal challenge in mice. Infect. Immun. 68:38-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vonlaufen, N., N. Müller, N. Keller, A. Naguleswaran, W. Bohne, M. M. McAllister, C. Björkman, E. Müller, R. Caldelari, and A. Hemphill. 2002. Exogenous nitric oxide triggers Neospora caninum tachyzoite-to-bradyzoite stage conversion in murine epidermal keratinocyte cell cultures. Int. J. Parasitol. 32:1253-1265. [DOI] [PubMed] [Google Scholar]
- 56.Vonlaufen, N., C. Gianinazzi, N. Mueller, F. Simon, C. Bjorkman, T. W. Jungi, S. L. Leib, and A. Hemphill. 2002. Infection of organotypic slice cultures from rat central nervous tissue with Neospora caninum: an alternative approach to study host-parasite interactions. Int. J. Parasitol. 32:533-542. [DOI] [PubMed] [Google Scholar]
- 57.Weiss, L. M., D. LaPlace, H. B. Tanowitz, and H. B. Wittner. 1992. Identification of Toxoplasma gondii bradyzoite-specific monoclonal antibodies. J. Infect. Dis. 166:213-215. [DOI] [PubMed] [Google Scholar]
- 58.Weiss, L. M., Y. F. Ma, S. Halonen, M. M. McAllister, and Y. W. Zhang. 1999. The in vitro development of Neospora caninum bradyzoites. Int. J. Parasitol. 29:1713-1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yoshida, N., M. L. Dorta, A. T. Rerreira, M. E. M. Oshiro, R. A. Mortara, A. Acosta-Serrano, and S. Favoreto, Jr. 1997. Removal of sialic acid from mucin-like surface molecules of Trypanosoma cruzi metacyclic trypomastigotes enhances parasite-host cell interaction. Mol. Biochem. Parasitol. 84:57-67. [DOI] [PubMed] [Google Scholar]