Abstract
We demonstrated previously that Actinobacillus actinomycetemcomitans leukotoxin (Ltx) is greatly able to induce apoptotic signaling in cells that are positive for lymphocyte function-associated antigen 1 (LFA-1), a cell receptor of Ltx. We investigated in this study whether inflammatory cytokines can regulate apoptosis of human leukemic HL-60 cells induced by Ltx. Of the cytokines tested, tumor necrosis factor alpha (TNF-α) significantly enhanced the Ltx-induced cell apoptosis. Northern and Western blotting analyses showed that TNF-α enhanced the expression of CD11a in the cells at both the mRNA and protein levels but did not do so for CD18 expression. TNF-α also enhanced the binding of Ltx to the cells. We also observed by measuring the mitochondrial transmembrane potential and the generation of superoxide anion that the cytokine enhanced Ltx-induced apoptosis in HL-60 cells. In addition, interleukin-1β significantly enhanced Ltx-induced cell apoptosis, although the enhancing activity was lower than that of TNF-α. These stimulatory effects of both cytokines were also observed for human polymorphonuclear leukocytes. The ability of TNF-α to increase cell susceptibility to Ltx could be inhibited by preincubation of the cells with a monoclonal antibody against TNF receptor 1 but not by preincubation of the cells with a monoclonal antibody against anti-TNF receptor 2. Furthermore, the results of an assay of caspase 3 intracellular activity (PhiPhiLuxG1D2) showed that Ltx-induced caspase 3 activation was completely neutralized by CD18 antibody treatment, although significant neutralization was also observed with anti-CD11a antibody. Taken together, the results of the present study indicate that TNF-α acts as a potent stimulator of Ltx-induced HL-60 cell apoptosis via TNF receptor 1-mediated upregulation of LFA-1 expression.
Apoptosis plays an important role in the inflammatory response, tumorigenesis, and embryonic development (10, 21). It has been shown that several pathogenic bacteria act as promoters or inhibitors of apoptosis of monocytes/macrophages (6, 9, 22). These observations suggest that several cell components and metabolic products of these bacteria are involved in an important pathogenic mechanism promoting inflammatory responses via apoptosis of monocytes/macrophages.
Actinobacillus actinomycetemcomitans is a gram-negative bacterium that has been identified in several human infectious diseases, such as endocarditis, meningitis, osteomyelitis (23), and aggressive periodontitis (33). It has been demonstrated by many studies that this organism produces a 116-kDa leukotoxin (Ltx) that destroys specific target cells via an apoptotic effect (16, 29). Interestingly, we clearly identified lymphocyte function-associated antigen 1 (LFA-1) as a β2 integrin, a cell receptor for Ltx (17). Also, we showed that the Ltx-induced apoptotic signal was initiated through LFA-1 binding of the toxin. These observations suggest that elimination of LFA-1-positive inflammatory cells such as monocytes/macrophages and neutrophils by the apoptotic effect of the toxin may allow A. actinomycetemcomitans to evade detection by the host immune system. Importantly, several studies have shown that the lipopolysaccharide of gram-negative bacteria is able to regulate the apoptosis of neutrophils and monocytes/macrophages through the effect of endogenous cytokines (4, 5, 35). Therefore, it was of interest to us to investigate the regulatory effect of inflammatory cytokines on Ltx target cell apoptosis occurring via the LFA-1-mediated signal because no such cytokine effect had been previously demonstrated in detail.
For this purpose, we investigated the regulatory effect of inflammatory cytokines in Ltx-induced HL-60 cell apoptosis occurring via LFA-1. We show herein that tumor necrosis factor alpha (TNF-α) and interleukin-1 (IL-1) enhanced the signal of Ltx-induced cell apoptosis through the same mechanism, by increasing LFA-1 expression, although no such stimulatory effect of IL-4 or IL-6 was observed.
MATERIALS AND METHODS
Ltx purification.
A. actinomycetemcomitans strain JP2 was grown in accordance with standard methods, and Ltx was extracted and purified by a modification (18) of a procedure described by Tsai et al. (27). The Limulus amebocyte lysate clotting activity (Toxicolor Test LS-6; Seikagaku Kogyo Co., Tokyo, Japan) of purified Ltx was undetectable at the concentrations used in this study.
Cell culture.
The Ltx-sensitive human promyelocytic leukemia cell line HL-60 (34) (RCB0041; Riken Gene Bank, Tsukuba, Japan) was used for experiments. Cells were grown in RPMI 1640 medium (Invitrogen Corp., Carlsbad, Calif.) supplemented with 10% fetal calf serum, 2 mM l-glutamine, and 50 μg of gentamicin per ml. Cultures were maintained at 37°C under 5% CO2.
Preparation of human polymorphonuclear leukocytes (HPMNs).
Heparinized human peripheral blood cells were obtained from a healthy adult volunteer. HPMNs were isolated by Mono-Poly Resolving Medium (ICN Biomedicals Japan Co., Tokyo, Japan), washed with phosphate-buffered saline (PBS; pH 7.3), and suspended in RPMI 1640 medium.
Reagents.
Human recombinant IL-1α (rIL-1α), human recombinant TNF-α, human rIL-4, anti-human TNF receptor 1 (TNF-R1) monoclonal antibody (MAb), anti-human TNF-R2 MAb, anti-human CD11a MAb, and anti-human CD18 MAb were purchased from R&D Systems, Inc. (Minneapolis, Minn.). Human rIL-1β was from Chemicon International Inc. (Temecula, Calif.), and human rIL-6 was from BD Biosciences (San Jose, Calif.). Normal mouse immunoglobulin G (IgG) was obtained from Upstate Biotechnology Inc. (Lake Placid, N.Y.). Sense and antisense primers for human CD11a, CD18, and glyceraldehydes-3-phosphate dehydrogenase (GAPDH) were synthesized on the basis of published DNA sequences (17, 31).
cDNA probes.
Human CD11a and CD18 cDNA plasmids were provided by M. K. Robinson (Celltech R&D Ltd.). The human GAPDH cDNA plasmid used was provided by S. Sakiyama (Chiba Cancer Center Research Institute and Hospital, Chiba, Japan). Probes for Northern blot assay were labeled with digoxigenin (DIG) by PCR as described previously (31).
Northern blot assay.
HL-60 cells were treated at a final concentration of 500 U of each cytokine per ml at 37°C for 30 min. Total RNA was then extracted from these cells by using TRIzol reagent (Invitrogen). Extracted RNA samples (20 μg) were electrophoresed on a 1.2% agarose gel containing 0.66 M formaldehyde and subsequently capillary blotted onto nylon membranes (Schleicher & Schuell, Inc., Keene, N.H.). The RNA bound to the membrane was cross-linked by exposure to UV light and then preincubated for 3 h at 50°C in a high-sodium dodecyl sulfate (SDS) hybridization buffer (31). Hybridization was performed with 100 ng of DIG-labeled PCR probes per ml in the high-SDS hybridization buffer at 50°C for 16 h. The hybridized probes were detected with a DIG luminescence detection kit (Roche Diagnostic Corp., Indianapolis, Ind.).
Western blotting.
Expression of human CD11a and CD18 was detected by immunoblotting (17, 30) with anti-human CD11a MAb (25.3.1.; Beckman Coulter Inc., Miami, Fla.) or anti-human CD18 MAb (KIM185; a kind gift from M. K. Robinson). Following incubation overnight at 4°C and washing, the blots were incubated with horseradish peroxidase-conjugated goat anti-mouse IgG (Zymed Laboratories Inc., San Francisco, Calif.) for 1 h at room temperature. Proteins were detected by using ECL Western blotting detection reagents (Amersham Biosciences Corp., Piscataway, N.J.).
Flow cytometric detection of CD11a.
HPMNs (106) were incubated with various concentrations of TNF-α or IL-1β at 37°C for 30 min. The cells were then washed with PBS and incubated at 4°C for 30 min with a fluorescein isothiocyanate-labeled anti-human CD11a MAb (25.3.1.; Beckman Coulter). The cells were washed with PBS and resuspended in 0.5 ml of PBS. Cells were analyzed by using an EPICS XL flow cytometer (Beckman Coulter) (5,000 cells were scored for green fluorescence on a log scale).
Biotinylation and binding assay of Ltx.
Biotinylated Ltx was prepared by a modification of the procedure described by Brown et al. (3) and Gretch et al. (8). Briefly, a mixture of NHS-LC-biotin (Pierce Chemical, Rockford, Ill.) and purified Ltx was incubated at room temperature for 1 h. Unbound biotin was then removed by passage of the mixture through a NAP-10 column (Amersham Biosciences) equilibrated with PBS (pH 7.2). The concentration of biotinylated Ltx protein was measured by the method of Bradford (2). Leukotoxic activity was assessed by incubating HL-60 cells for 1 h at 37°C with the biotinylated Ltx and then performing a trypan blue exclusion test (13). We found that biotinylation did not decrease the leukotoxic activity (3 × 10−10 M purified Ltx killed about 25% of 2 × 105 HL-60 cells in 1 h). Flow cytometric analysis of Ltx binding to HL-60 cells was performed as described by Brown et al. (3). Briefly, HL-60 cells (106) were preincubated for the desired periods at 37°C with 500 U of TNF-α per ml or heat-inactivated (100°C for 10 min) TNF-α (ΔI TNF-α) and then incubated for 10 min at 4°C with 3 × 10−10 M biotinylated purified Ltx. In addition, the cells were treated for 30 min at 4°C with streptavidin-phycoerythrin (Beckman Coulter) and then washed once with Ca2+- and Mg2+-free Hanks' balanced salt solution (HBSS) and resuspended in 0.5 ml of HBSS. The stained cells were analyzed with an EPICS XL flow cytometer (Beckman Coulter).
Flow cytometry for apoptosis assays.
Ltx-induced changes in mitochondrial transmembrane potential (ΔΨm) and generation of superoxide anion in HL-60 cells were analyzed by flow cytometry as described previously (14, 30). Caspase 3 activity was measured by using PhiPhiLuxG1D2 (Oncoimmunin Inc., Gaithersburg, Md.) with an EPICS XL flow cytometer as described earlier (14).
RESULTS
TNF-α enhances CD11a expression of HL-60 cells at both the gene and protein levels.
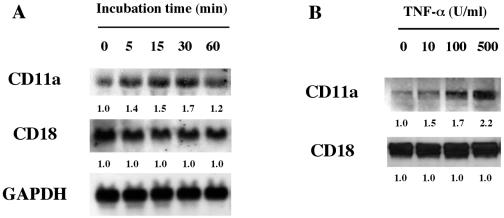
We demonstrated earlier that the β2 integrin LFA-1 is a cell surface receptor that mediates the toxicity of members of the RTX toxin family (17). Since CD11a and CD18 are the two β2 integrin subunits, to investigate the effect of TNF-α on Ltx-induced HL-60 apoptosis, we first examined the effect of TNF-α on the expression of the genes for CD11a and CD18 by Northern blot assay. The cells were left untreated or treated with TNF-α at 500 U/ml, and then their gene expressions were analyzed at several times after treatment initiation. As shown in Fig. 1A, TNF-α markedly enhanced the expression of the gene for CD11a in the cells at 15 and 30 min after the initiation of cytokine treatment. However, no such stimulatory effect on the expression of the gene for CD18 was observed.
FIG. 1.
TNF-α enhances human LFA-1 (CD11a/CD18) mRNA expression in and protein production by HL-60 cells in a time- and dose-dependent fashion. (A) TNF-α enhanced the human CD11a mRNA level in a time-dependent manner, but human CD18 mRNA was expressed constitutively over the same time course. (B) HL-60 cells (106) were treated with TNF-α at concentrations ranging from 10 to 500 U/ml for 30 min and lysed with detergent (1% Triton X-100 in 0.15 M NaCl), and the proteins from each cell lysate were electrophoresed on 7.5% SDS gels. Gels were blotted onto nitrocellulose membranes, and fluorographs were developed after incubation (overnight at 4°C) with either anti-human CD11a MAb or anti-human CD18 MAb. Relative band intensities were determined by using NIH Image version 1.62 software. The values below the bands are the mean fold increases in the expression levels in HL-60 cells incubated with TNF-α compared with the expression by unstimulated HL-60 cells.
Since Fig. 1A suggested the possibility that TNF-α may be able to stimulate the production of LFA-1 by HL-60 cells, we investigated the production of LFA-1 by TNF-α-treated cells by Western blotting assay. The cells were incubated with or without TNF-α, and then CD11a and CD18 production by the cells was monitored by using each MAb at 30 min after the initiation of cytokine treatment. We observed that the cytokine stimulated CD11a production in a dose-dependent fashion but did not affect CD18 production (Fig. 1B).
Taken together, these results clearly showed that TNF-α is able to enhance the expression of LFA-1, an Ltx receptor, in HL-60 cells.
TNF-α enhances Ltx binding to HL-60 cells.
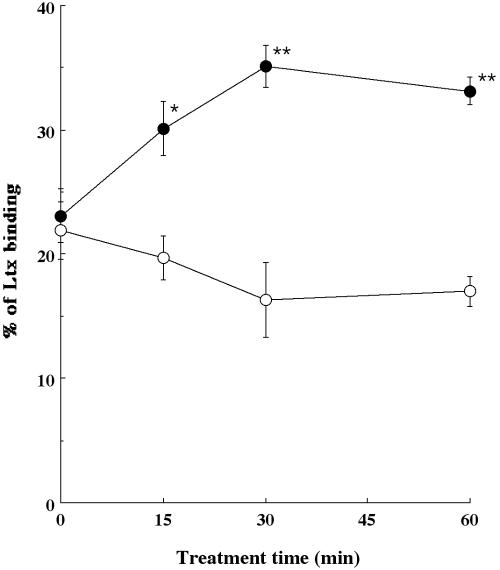
As described above, since TNF-α was able to enhance the expression of LFA-1 in HL-60 cells, we next examined whether the cytokine could also enhance the binding of Ltx to HL-60 cells. We treated HL-60 cells with TNF-α for various times at 37°C and then incubated them for 10 min at 4°C with 3 × 10−10 M biotinylated Ltx. Biotinylated, heat-inactivated (80°C for 30 min) Ltx (ΔI Ltx) did not bind the cells (data not shown). For non-cytokine-treated cells, the Ltx binding activity was 23.1% ± 2.2%. TNF-α increased Ltx binding to the cells in a treatment time-related manner when the cells were pretreated with the cytokine at 500 U/ml (Fig. 2). On the other hand, pretreatment with ΔI TNF-α did not enhance the binding of biotinylated Ltx to HL-60 cells over the same time course (Fig. 2).
FIG. 2.
Time course of binding of purified Ltx to HL-60 cells treated with TNF-α (•) or ΔI TNF-α (○). HL-60 cells (106) were incubated with TNF-α or ΔI TNF-α for 0, 15, 30, or 60 min at 37°C. The cells were then incubated with 3 × 10−10 M biotinylated purified Ltx for 10 min at 4°C. The cells were then washed with HBSS, stained with streptavidin-phycoerythrin for 30 min at 4°C, and analyzed by flow cytometry. Data are shown as the means ± standard errors of the means for five different experiments. Values significantly different from the value for Ltx binding at each time point, as determined by using Student's t test (*, P < 0.01; **, P < 0.001), are indicated.
TNF-α enhances Ltx-induced changes in ΔΨm and generation of superoxide anion by HL-60 cells.
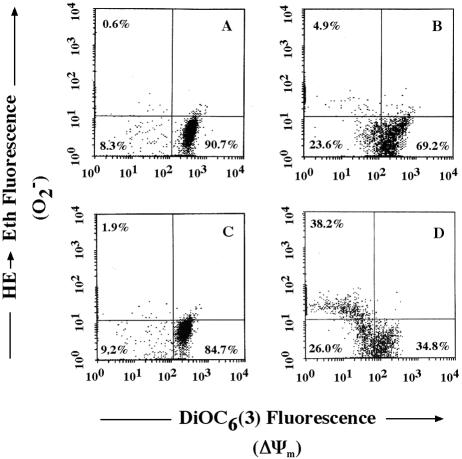
Ltx-induced changes in ΔΨm and generation of superoxide anion by HL-60 cells were measured by multiparameter fluorescence-activated cell sorter analysis by using a lipophilic, cationic fluorescent dye, 3,3′-dihexyloxacarbocyanine iodide [DiOC6(3)], and blue-fluorescing dihydroethidium (HE), respectively, as described previously (15, 30). For control cells treated with ΔI Ltx, multiparameter fluorescence-activated cell sorter analysis showed that 90.7% of the cells in the population exhibited relatively high DiOC6(3) and low ethidium (Eth) fluorescence [DiOC6(3)bright/Ethdim] (Fig. 3A). The percentage of this quadrant in untreated HL-60 cells was 94.8%. These phenotypes are characteristic of viable cells. In contrast, DiOC6(3) fluorescence, indicative of a reduction in ΔΨm, was decreased in the Ltx-treated cells (Fig. 3B). Pretreatment with TNF-α at 500 U/ml for 30 min enhanced mitochondrial changes (ΔΨm [34.4% decrease relative to treatment with Ltx alone] and superoxide anion production [33.3% increase relative to treatment with Ltx alone]) in HL-60 cells (Fig. 3D). When the cells were pretreated with TNF-α and then incubated with ΔI Ltx, there was no ΔΨm or change in superoxide anion production (Fig. 3C). These results show a stimulatory effect of TNF-α on Ltx-induced HL-60 apoptosis.
FIG. 3.
TNF-α enhances Ltx-induced changes in ΔΨm and superoxide anion generation. Ltx (3 × 10−10 M)-induced alterations in DiOC6(3) and HE staining of HL-60 cells were evaluated for 1 h at 37°C (B). As a control, cells were incubated with ΔI Ltx (A) for 1 h. Cells were pretreated with 500 U of TNF-α per ml for 30 min and then incubated with ΔI Ltx (C) or 3 × 10−10 M Ltx (D) for 1 h. Subsequently, cells were stained with DiOC6(3) and HE and analyzed by flow cytometry.
TNF-α enhances Ltx-induced caspase 3 activation in HL-60 cells.
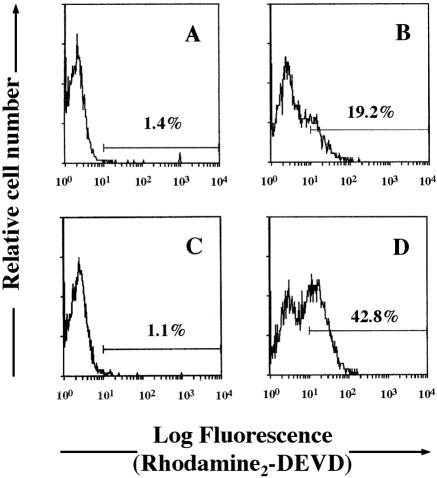
To confirm the stimulatory effect of TNF-α on Ltx-induced HL-60 apoptosis, we used a fluorogenic assay to measure the levels of active caspase 3 in cells exposed to Ltx (Fig. 4). HL-60 cells were incubated with a birhodamine-derivatized peptide containing the caspase 3 recognition sequence DEVD. Upon intracellular cleavage by activated caspase 3 or caspase 3-like enzymes, quenching of the rhodamine molecules is released and the resultant fluorescence can be detected by flow cytometry. Whereas substrate cleavage of ΔI Ltx-treated cells was only 1.4%, that of Ltx-treated cells was 19.2% (Fig. 4A and B). When the cells were pretreated with TNF-α and incubated with ΔI Ltx, there was no caspase 3 activation in them (Fig. 4C). Treatment of TNF-α alone did not affect caspase 3 activation of HL-60 cells (data not shown). On the other hand, the substrate cleavage of Ltx-treated cells increased to 42.8% when the cells were treated for 30 min with TNF-α at 500 U/ml (Fig. 4D). These results suggest that TNF-α is a potent stimulator of Ltx-induced HL-60 cell apoptosis, acting through multiple members of the caspase family, including caspase 3.
FIG. 4.
TNF-α enhances Ltx-induced activation of caspase 3 in HL-60 cells. A rhodamine-derivatized, cell-permeable, caspase 3-specific substrate (PhiPhiLuxG1D2) was used to evaluate whether activated caspase 3 was present in Ltx-treated cells. HL-60 cells were incubated with ΔI Ltx (A) or 3 × 10−10 M Ltx (B) for 1 h and then examined for cleavage of the substrate as determined by flow cytometry. Other cells were pretreated with 500 U of TNF-α per ml for 30 min and incubated with ΔI Ltx (C) or 3 × 10−10 M Ltx (D) for 1 h. These results are representative of three independent experiments; at least 5,000 cells were analyzed per experiment.
The CD18 molecule plays a central role in signal transduction for Ltx-induced caspase 3 activation in HL-60 cells.
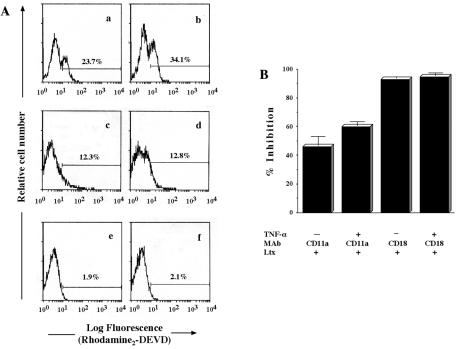
Since we showed previously (17) that RTX toxins bind to β2 integrin, it was of interest to us to examine which subunit of β2 integrin plays the central role in signal transduction for Ltx-induced apoptosis in target cells. As shown in Fig. 5A and B, although the Ltx-induced caspase 3 activation in HL-60 cells was significantly neutralized by CD11a antibody treatment, importantly, CD18 antibody completely neutralized the Ltx-induced caspase 3 activation. Even after TNF-α treatment, the inhibitory activity of the CD18 antibody was stronger than that of the CD11a antibody. A mouse IgG control did not inhibit Ltx-induced caspase 3 activation (data not shown). Interestingly, we also observed that Ltx-induced cell apoptosis was strongly inhibited by treatment of cells with the CD18 antibody (data not shown). These results strongly suggest an important role for CD18 (β chain) in the signaling of Ltx-induced apoptosis, although CD11a (α chain) may also be involved in part.
FIG. 5.
Effect of β2 integrin antibodies on Ltx-induced caspase 3 activation in HL-60 cells. (A) HL-60 cells were incubated with (b, d, f) or without (a, c, e) TNF-α (500 U/ml) for 30 min at 37°C and then pretreated with normal mouse IgG (10 μg/ml; a, b), anti-human CD11a antibody (10 μg/ml; c, d), or anti-human CD18 antibody (10 μg/ml; e, f) for 30 min at 4°C. After having been washed with HBSS, the cells were stimulated with 3 × 10−10 M Ltx for 1 h at 37°C. Caspase 3 activity was measured by using a PhiPhiLuxG1D2 kit and a flow cytometer and is expressed as the percentage of rhodamine-stained cells. (B) The results are expressed as the mean ± standard deviation percent inhibition of the value obtained for cells in the presence of normal mouse IgG. An identical experiment performed independently gave similar results.
Stimulatory signal of TNF-α for Ltx-induced caspase 3 activation in HL-60 cells is mediated via TNF-R1.
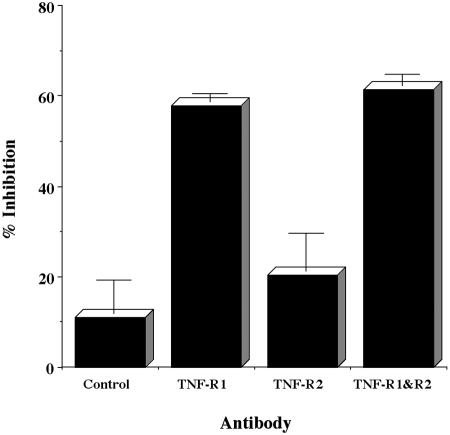
It is well known that TNF-α signal transduction operates via two distinct receptor molecules for TNF, TNF-R1, and TNF-R2 (28). Therefore, we explored which TNF-α receptor is involved in the cytokine stimulation of Ltx-induced caspase 3 activation. As shown in Fig. 6, anti-human TNF-R1 MAb significantly inhibited the stimulatory effect of TNF-α. However, such strong inhibitory activity was not observed with anti-human TNF-R2 MAb, although the activity was significant. These results suggest that TNF-R1-mediated signal transduction plays a predominant role in TNF-α stimulation of Ltx-induced HL-60 apoptosis.
FIG. 6.
Inhibitory effect of anti-human TNF-R1 MAb on Ltx-induced activation of caspase 3 after treatment of HL-60 cells with TNF-α. HL-60 cells (106) were pretreated with 10 μg of normal mouse IgG per ml, 10 μg of anti-human TNF-R1 MAb per ml, 10 μg of anti-human TNF-R2 MAb per ml, or 10 μg of anti-human TNF-R1 MAb per ml, and 10 μg of anti-human TNF-R2 MAb per ml for 1 h at 37°C and then incubated with 500 U of TNF-α per ml for 30 min. After having been washed with HBSS, the cells were stimulated with 3 × 10−10 M Ltx for 1 h at 37°C. Caspase 3 activity was measured by using a PhiPhiLuxG1D2 kit and a flow cytometer and was evaluated as the percentage of rhodamine-stained cells. The percentage of inhibition of caspase 3 activity was calculated by the following formula: percent inhibition = 100 × (percentage of Ltx-treated cells rhodamine positive after treatment with TNF-α − percentage of Ltx-treated cells rhodamine positive after treatment with MAb and TNF-α)/(percentage of Ltx-treated cells rhodamine positive after treatment with TNF-α − percentage of cells rhodamine positive after treatment with Ltx only). Data are shown as the means ± standard errors of the means for three experiments performed in duplicate.
IL-1 significantly enhances Ltx-induced HL-60 apoptosis.
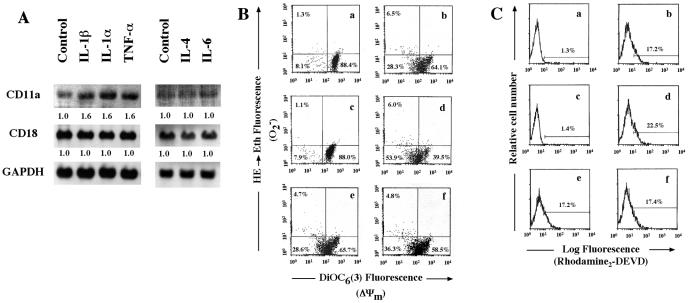
Although we demonstrated as described above that TNF-α is a powerful stimulator of Ltx-induced HL-60 cell apoptosis via LFA-1, in addition, we examined the effects of several cytokines on expression of the gene for CD11a, which is an important molecule as a trigger of Ltx-induced HL-60 cell apoptosis. As shown in Fig. 7A, IL-1α and IL-1β clearly increased expression of the gene for CD11a in the cells but not that of the gene for CD18. For IL-4 and IL-6, no stimulatory effects on the gene for either CD11a or CD18 were observed. These results suggested to us that IL-1 also acts as a stimulator of Ltx-induced HL-60 cell apoptosis via LFA-1. Therefore, by using IL-1β, we investigated the effect of IL-1β on mitochondrial changes and caspase 3 activation by using flow cytometry. IL-1β enhanced the drop in DiOC6 (3) fluorescence to 39.5% when the cells were pretreated for 30 min with the cytokine at 500 U/ml (Fig. 7B). Furthermore, the percentage of caspase 3-activated cells was clearly increased by IL-1β pretreatment (Fig. 7C). In contrast, as expected, IL-4 and IL-6 were ineffective in causing the Ltx-induced mitochondrial changes and caspase 3 activation (Fig. 7B and C).
FIG. 7.
Effects of three other inflammatory cytokines on CD11a/CD18 mRNA expression, Ltx-induced alterations in ΔΨm and superoxide anion generation, and Ltx-induced caspase 3 activation of HL-60 cells. (A) The cells were incubated with medium alone, IL-1β, IL-1α, TNF-α, IL-4, or IL-6 for 30 min, and total RNA was then analyzed by Northern blotting with DIG-labeled CD11a, CD18, and GAPDH PCR probes. Relative band intensities were determined by using NIH Image version 1.62 software. The values below the bands are the mean fold increases in the expression levels in HL-60 cells incubated with the cytokines compared with the expression by unstimulated HL-60 cells. (B) HL-60 cells were pretreated with medium alone (a, b), IL-1β (c, d), IL-4 (e), or IL-6 (f) for 30 min and then incubated with ΔI Ltx (a, c) or 3 × 10−10 M Ltx (b, d, e, f) for 1 h. Cells were stained with DiOC6(3) and HE to measure ΔΨm and superoxide anion production, respectively, and analyzed by flow cytometry. Data are plotted as DiOC6(3) fluorescence versus ethidium fluorescence. Bars indicate the setting for quadrant analysis; values represent the percentage of cells in each quadrant. Results are representative of three experiments. (C) HL-60 cells were pretreated with medium alone (a, b), IL-1β (c, d), IL-4 (e), or IL-6 (f) for 30 min and then incubated with ΔI Ltx (a, c) or 3 × 10−10 M Ltx (b, d, e, f) for 1 h. The cells were then assessed for caspase 3 activation by using a PhiPhiLuxG1D2 kit. Stained cells were analyzed by flow cytometry. Bars indicate region of positive fluorescence; the percentage of positive cells is presented in each panel. Results are representative of three experiments.
TNF-α and IL-1β induce the upregulation of CD11a on and stimulate Ltx-induced apoptosis of HPMNs.
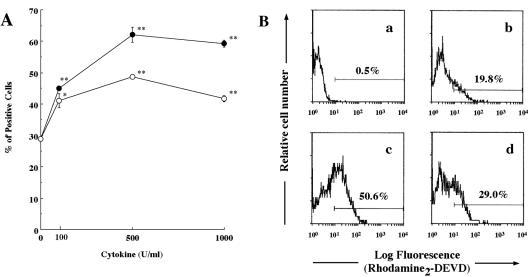
Finally, we investigated whether these stimulatory effects of TNF-α and IL-1β can be observed in normal HPMNs. As expected, both cytokines induced significant upregulation of CD11a in normal HPMNs (Fig. 8A) and also markedly enhanced Ltx-induced caspase 3 activation (Fig. 8B). Treatment with each cytokine alone did not affect Ltx-induced caspase 3 activation (data not shown). These results suggested that both TNF-α and IL-1β act as potent stimulators of Ltx-induced PMN apoptosis in vivo.
FIG. 8.
Effects of TNF-α or IL-1β on upregulation of CD11a and Ltx-induced caspase 3 activation of HPMNs. (A) Freshly isolated HPMNs (106/ml) were incubated with TNF-α (•) or IL-1β (○) at concentrations ranging from 100 to 1,000 U/ml or with medium (control) for 30 min at 37°C. The cells were then washed and incubated with fluorescein isothiocyanate-labeled anti-human CD11a MAb (30 min at 4°C) and analyzed by flow cytometry (5,000 cells were scored for green fluorescence). Data are shown as the mean percentages of positive cells ± standard deviations for duplicate tubes. The data for stimulated cells significantly different from the data for control cells, as determined by using Student's t test (*, P < 0.05; **, P < 0.01), are indicated. The experiments were performed three times, and similar results were obtained in each experiment. (B) Freshly isolated HPMNs were incubated with ΔI Ltx (a) or Ltx (b) for 1 h and then examined for cleavage of the substrate as determined by flow cytometry. Other cells were pretreated with TNF-α (c) or IL-1β (d) at 500 U/ml for 30 min and incubated with Ltx for 1 h. These results are representative of three independent experiments; at least 5,000 cells were analyzed per experiment.
DISCUSSION
Many studies have shown that A. actinomycetemcomitans cell components are able to induce production of inflammatory cytokines (IL-6, TNF-α, IL-1, MIP-1, MCP-1, and others) by several kinds of cells (1, 11, 31). On the basis of these observations, it was of interest to us to investigate whether these cytokines regulate Ltx-induced apoptosis of LFA-1-positive cells, which are sensitive to the cytotoxin. Therefore, in this study we investigated the regulatory effect of inflammatory cytokines on Ltx-induced apoptosis of cultured human HL-60 cells and demonstrated that TNF-α and IL-1 enhanced the Ltx-induced HL-60 cell apoptotic signal by stimulating LFA-1 expression.
Since we indicated earlier that β2 integrin is an Ltx cell receptor and that the toxin-induced cell apoptotic signal operates on cytotoxin-sensitive cells via this integrin, we first explored at both the gene and protein levels whether TNF-α would be able to stimulate the expression of CD11a on HL-60 cells. Our Northern and Western blot assays clearly showed that the cytokine stimulated CD11a expression by the cells at both levels. These data suggested to us that binding of Ltx to β2 integrin on the cells may be increased by TNF-α treatment. Therefore, we investigated this point by using biotinylated Ltx. Our flow cytometric analysis showed that the cytokine clearly increased Ltx binding to HL-60 cells at 30 min after the initiation of cytokine treatment.
The stimulatory effect of TNF-α on Ltx binding to HL-60 cells suggested to us the possibility that the cytokine enhances the toxin-induced apoptotic signal of the cells via the β2 integrin LFA-1. Perturbation of mitochondrial function is now accepted as playing a key regulatory role in the progression of apoptosis (14). Therefore, we tested this possibility by measuring ΔΨm and the generation of superoxide anion in HL-60 cells, because we had demonstrated earlier that Ltx induces initiation of the apoptotic signal by the mitochondrion-dependent pathway (30). Since we also observed previously that the Ltx-induced decrease in ΔΨm and hyperproduction of superoxide anion occurred concomitantly (30), reactive oxygen species, including superoxide anion, may participate as effector molecules in Ltx-induced HL60 cell apoptosis. As expected, we observed that TNF-α elicited a marked decrease in ΔΨm and an increased the generation of superoxide anion in Ltx-treated cells. These observations strongly suggest that the cytokine enhances the initiation stage of the apoptotic signal induced by the cytotoxin.
Caspase family members play an important role in the initiation and development of cell apoptosis. Of these, caspase 3 in particular is a key enzyme involved in the appearance of apoptosis (7). Therefore, to confirm the enhancing effect of TNF-α on Ltx-induced HL-60 cell apoptosis, it is very important to demonstrate whether the cytokine is able to stimulate Ltx-induced caspase 3 activation in these cells. We assessed this possibility by using a PhiPhiLuxG1D2 kit. Importantly, TNF-α clearly stimulated Ltx-induced activation of caspase 3 in these cells. These results strongly suggest that TNF-α enhances Ltx-induced apoptosis by stimulating caspase 3 activation and does so through the mitochondrion-dependent pathway.
We previously showed that RTX toxins bind to β2 integrin (CD11a/CD18) on target cells (17). Takeshita et al. (24) have demonstrated that the β chain (CD18) of these molecules plays an important role in signaling for the Porphyromonas gingivalis fimbria-induced expression of IL-1β and TNF-α genes in mouse peritoneal macrophages. In our present study, since CD18 antibody completely inhibited Ltx-induced caspase 3 activation (Fig. 5), these observations suggest a functional role of the β chain (CD18) in the initial stage of signaling in Ltx-induced apoptosis of HL-60 cells.
It is well known that TNF-α exhibits multipotential biological activities. Also, many studies (20, 26, 28) have shown that two distinct receptor molecules, TNF-R1 and TNF-R2, mediate the biological effects of TNF-α. Therefore, by using anti-human TNF receptor antibodies, we explored which receptor plays a central role in the potent effect of TNF-α on Ltx-induced HL-60 cell apoptosis. We observed that anti-human TNF-R1 antibody strongly inhibited Ltx-induced caspase 3 activation. However, TNF-R2 antibody exhibited no such inhibitory effect. Although several earlier studies (25, 28, 32) showed that the TNF-α signal for activation of JNK, p38, and ERK is mediated by TNF-R1, our result shows that TNF-R1 can also act as the predominant receptor of TNF-α for the signaling that enhances Ltx-induced HL-60 cell apoptosis.
Moreover, we were also interested in investigating whether other inflammatory-response-regulating cytokines also act as regulators of Ltx-induced apoptosis via stimulation of LFA-1 expression. We observed that IL-1α or -β exhibited the same enhancing effect on CD11a expression in HL-60 cells as TNF-α. However, no such enhancing effect of IL-4 or IL-6 was observed. Since Leite et al. (19) showed that IL-1β enhanced Mannheimia haemolytica Ltx-induced apoptosis of neutrophils and also did so for LFA-1 expression, their observations may support our conclusion that TNF-α and IL-1 enhanced Ltx-induced HL-60 cell and HPMN apoptosis by stimulating LFA-1 expression. We observed significant upregulation of CD11a expression in the responses of HPMNs that were incubated with each cytokine at concentrations of 100 to 1,000 U/ml. However, we cannot rule out the possibility that exposure to different cytokine concentrations further alters the PMN response to Ltx. Interestingly, Kesavalu et al. (12) investigated the effects of oral pathogens on the expression of TNF-α, IL-1β, and IL-6 in the murine calvaria in situ and consequently showed that A. actinomycetemcomitans and P. gingivalis induced the expression of the gene for TNF-α at the highest level (TNF-α ≫ IL-1β > IL-6) in the calvaria. These observations suggest that these cytokines play an important role in pathogenesis in the periodontal bacterium-infected diseases, because it is well documented that these cytokines are potent factors in host inflammatory responses.
In conclusion, our present results demonstrate that TNF-α enhanced Ltx-induced HL-60 cell apoptosis by causing upregulation of LFA-1 through TNF-R1. This effect suggests that TNF-α, in combination with Ltx, plays a functional role in a novel pathogenic mechanism operating in A. actinomycetemcomitans-associated infectious diseases.
Acknowledgments
We thank Martyn K. Robinson of Celltech R&D Ltd. for generously providing anti-human CD18 MAb and human CD11a/CD18 cDNAs.
Support for this research was provided by Grant-in-Aid for Scientific Research (C)14571953 from the Ministry of Education, Science, Sports, and Culture of Japan.
Editor: J. T. Barbieri
Footnotes
Dedicated to the memory of Toshihiko Koga.
REFERENCES
- 1.Agarwal, S., N. P. Piesco, L. P. Johns, and A. E. Riccelli. 1995. Differential expression of IL-1 beta, TNF-alpha, IL-6, and IL-8 in human monocytes in response to lipopolysaccharides from different microbes. J. Dent. Res. 74:1057-1065. [DOI] [PubMed] [Google Scholar]
- 2.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 3.Brown, J. F., F. Leite, and C. J. Czuprynski. 1997. Binding of Pasteurella haemolytica leukotoxin to bovine leukocytes. Infect. Immun. 65:3719-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Byrne, A., and D. J. Reen. 2002. Lipopolysaccharide induces rapid production of IL-10 by monocytes in the presence of apoptotic neutrophils. J. Immunol. 168:1968-1977. [DOI] [PubMed] [Google Scholar]
- 5.Bzowska, M., K. Guzik, K. Barczyk, M. Ernst, H. D. Flad, and J. Pryjma. 2002. Increased IL-10 production during spontaneous apoptosis of monocytes. Eur. J. Immunol. 32:2011-2020. [DOI] [PubMed] [Google Scholar]
- 6.Chen, Y., and A. Zychlinsky. 1994. Apoptosis induced by bacterial pathogens. Microb. Pathog. 17:203-212. [DOI] [PubMed] [Google Scholar]
- 7.Cohen, G. M. 1997. Caspases: the executioners of apoptosis. Biochem. J. 326:1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gretch, D. R., M. Suter, and M. F. Stinski. 1987. The use of biotinylated monoclonal antibodies and streptavidin affinity chromatography to isolate herpes virus hydrophobic proteins or glycoproteins. Anal. Biochem. 163:270-277. [DOI] [PubMed] [Google Scholar]
- 9.Hilbi, H., J. E. Moss, D. Hersh, Y. Chen, J. Arondel, S. Banerjee, R. A. Flavell, J. Yuan, P. J. Sansonetti, and A. Zychlinsky. 1998. Shigella-induced apoptosis is dependent on caspase-1 which binds to IpaB. J. Biol. Chem. 273:32895-32900. [DOI] [PubMed] [Google Scholar]
- 10.Jacobson, M. D., M. Weil, and M. C. Raff. 1997. Programmed cell death in animal development. Cell 88:347-354. [DOI] [PubMed] [Google Scholar]
- 11.Jiang, Y., and D. T. Graves. 1999. Periodontal pathogens stimulate CC-chemokine production by mononuclear and bone-derived cells. J. Periodontol. 70:1472-1478. [DOI] [PubMed] [Google Scholar]
- 12.Kesavalu, L., B. Chandrasekar, and J. L. Ebersole. 2002. In vivo induction of proinflammatory cytokines in mouse tissue by Porphyromonas gingivalis and Actinobacillus actinomycetemcomitans. Oral Microbiol. Immunol. 17:177-180. [DOI] [PubMed] [Google Scholar]
- 13.Korostoff, J., J. F. Wang, I. Kieba, M. Miller, B. J. Shenker, and E. T. Lally. 1998. Actinobacillus actinomycetemcomitans leukotoxin induces apoptosis in HL-60 cells. Infect. Immun. 66:4474-4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Korostoff, J., N. Yamaguchi, M. Miller, I. Kieba, and E. T. Lally. 2000. Perturbation of mitochondrial structure and function plays a central role in Actinobacillus actinomycetemcomitans leukotoxin-induced apoptosis. Microb. Pathog. 29:267-278. [DOI] [PubMed] [Google Scholar]
- 15.Kroemer, G., N. Zamzami, and S. A. Susin. 1997. Mitochondrial control of apoptosis. Immunol. Today 18:44-51. [DOI] [PubMed] [Google Scholar]
- 16.Lally, E. T., E. E. Golub, I. R. Kieba, N. S. Taichman, J. Rosenbloom, J. C. Rosenbloom, C. W. Gibson, and D. R. Demuth. 1989. Analysis of the Actinobacillus actinomycetemcomitans leukotoxin gene. Delineation of unique features and comparison to homologous toxins. J. Biol. Chem. 264:15451-15456. [PubMed] [Google Scholar]
- 17.Lally, E. T., I. R. Kieba, A. Sato, C. L. Green, J. Rosenbloom, J. Korostoff, J. F. Wang, B. J. Shenker, S. Ortlepp, M. K. Robinson, and P. C. Billings. 1997. RTX toxins recognize a β2 integrin on the surface of human target cells. J. Biol. Chem. 272:30463-30469. [DOI] [PubMed] [Google Scholar]
- 18.Lear, J. D., U. G. Furblur, E. T. Lally, and J. C. Tanaka. 1995. Actinobacillus actinomycetemcomitans leukotoxin forms large conductance, voltage-gated ion channels when incorporated into planar lipid bilayers. Biochim. Biophys. Acta 1238:34-41. [DOI] [PubMed] [Google Scholar]
- 19.Leite, F., S. O'Brien, M. J. Sylte, T. Page, D. Atapattu, and C. J. Czuprynski. 2002. Inflammatory cytokines enhance the interaction of Mannheimia haemolytica leukotoxin with bovine peripheral blood neutrophils in vitro. Infect. Immun. 70:4336-4343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Locksley, R. M., N. Killeen, and M. J. Lenardo. 2001. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell 104:487-501. [DOI] [PubMed] [Google Scholar]
- 21.Nagata, S. 1997. Apoptosis by death factor. Cell 88:355-365. [DOI] [PubMed] [Google Scholar]
- 22.Ozaki, K., and S. Hanazawa. 2001. Porphyromonas gingivalis fimbriae inhibit caspase-3-mediated apoptosis of monocytic THP-1 cells under growth factor deprivation via extracellular signal-regulated kinase-dependent expression of p21 Cip/WAF1. Infect. Immun. 69:4944-4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Page, M. I., and E. O. King. 1966. Infection due to Actinobacillus actinomycetemcomitans and Haemophilus aphrophilus. N. Engl. J. Med. 275:181-188. [DOI] [PubMed] [Google Scholar]
- 24.Takeshita, A., Y. Murakami, Y. Yamashita, M. Ishida, S. Fujisawa, S. Kitano, and S. Hanazawa. 1998. Porphyromonas gingivalis fimbriae use β2 integrin (CD11/CD18) on mouse peritoneal macrophages as a cellular receptor, and the CD18 β chain plays a functional role in fimbrial signaling. Infect. Immun. 66:4056-4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thoma, B., M. Grell, K. Pfizenmaier, and P. Scheurich. 1990. Identification of a 60-kD tumor necrosis factor (TNF) receptor as the major signal transducing component in TNF responses. J. Exp. Med. 172:1019-1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tracey, K. J., and A. Cerami. 1994. Tumor necrosis factor: a pleiotropic cytokine and therapeutic target. Annu. Rev. Med. 45:491-503. [DOI] [PubMed] [Google Scholar]
- 27.Tsai, C. C., B. J. Shenker, J. M. DiRienzo, D. Malamud, and N. S. Taichman. 1984. Extraction and isolation of a leukotoxin from Actinobacillus actinomycetemcomitans with polymyxin B. Infect. Immun. 43:700-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wajant, H., and P. Scheurich. 2001. Tumor necrosis factor receptor-associated factor (TRAF) 2 and its role in TNF signaling. Int. J. Biochem. Cell Biol. 33:19-32. [DOI] [PubMed] [Google Scholar]
- 29.Welch, R. A. 1991. Pore-forming cytolysins of gram-negative bacteria. Mol. Microbiol. 5:521-528. [DOI] [PubMed] [Google Scholar]
- 30.Yamaguchi, N., I. R. Kieba, J. Korostoff, P. S. Howard, B. J. Shenker, and E. T. Lally. 2001. Maintenance of oxidative phosphorylation protects cells from Actinobacillus actinomycetemcomitans leukotoxin-induced apoptosis. Cell. Microbiol. 3:811-823. [DOI] [PubMed] [Google Scholar]
- 31.Yamaguchi, N., Y. Yamashita, D. Ikeda, and T. Koga. 1996. Actinobacillus actinomycetemcomitans serotype b-specific polysaccharide antigen stimulates production of chemotactic factors and inflammatory cytokines by human monocytes. Infect. Immun. 64:2563-2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yuasa, T., S. Ohno, J. H. Kehrl, and J. M. Kyriakis. 1998. Tumor necrosis factor signaling to stress-activated protein kinase (SAPK)/Jun NH2-terminal kinase (JNK) and p38. Germinal center kinase couples TRAF2 to mitogen-activated protein kinase/ERK kinase kinase 1 and SAPK while receptor interacting protein associates with a mitogen-activated protein kinase kinase kinase upstream of MKK6 and p38. J. Biol. Chem. 273:22681-22692. [DOI] [PubMed] [Google Scholar]
- 33.Zambon, J. J. 1985. Actinobacillus actinomycetemcomitans in human periodontal disease. J. Clin. Periodontol. 12:1-20. [DOI] [PubMed] [Google Scholar]
- 34.Zambon, J. J., C. DeLuca, J. Slots, and R. J. Genco. 1983. Studies of leukotoxin from Actinobacillus actinomycetemcomitans using the promyelocytic HL-60 cell line. Infect. Immun. 40:205-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zychlinsky, A., and P. J. Sansonetti. 1997. Apoptosis as a proinflammatory event: what can we learn from bacteria-induced cell death? Trends Microbiol. 5:201-204. [DOI] [PubMed] [Google Scholar]