Abstract
Peptide mimotopes of capsular polysaccharides have been proposed as antigens for vaccines against encapsulated pathogens. In this study, we determined the antibody response to and efficacy of P13, a peptide mimetic of the Cryptococcus neoformans capsular polysaccharide glucuronoxylomannan (GXM), in mice that produce human antibodies. P13 was conjugated to tetanus toxoid (TT) or diphtheria toxoid (DT) and administered subcutaneously in Alhydrogel with or without CpG to mice transgenic for human immunoglobulin loci (XenoMouse mice) and expressing either immunoglobulin G2 (IgG2) (G2 mice) or IgG4 (G4 mice). Mice were vaccinated and revaccinated two or three times. The serum antibody responses of the mice to GXM and P13 and antibody idiotype expression were analyzed by an enzyme-linked immunosorbent assay. The results showed that both P13-TT and P13-DT were antigenic, inducing a mimetic response to P13 in both G2 and G4 mice, and immunogenic, inducing a mimotope response including VH3 (idiotype)-positive antibodies to GXM in G2 but not G4 mice. CpG led to higher titers of IgG to P13 and GXM in P13-TT-vaccinated G2 mice. C. neoformans challenge of P13-protein conjugate-vaccinated and control G2 mice induced anamnestic IgG- and VH3-positive responses to GXM and was associated with a significantly decreased risk of death and a prolongation of survival in P13-DT-vaccinated mice compared to phosphate-buffered saline-treated or protein carrier-vaccinated mice. These findings reveal that P13 elicited a human antibody response with VH3 expression in human immunoglobulin transgenic mice that has been observed for human antibodies to GXM and support the concept that peptide mimotope-based vaccines may hold promise for the treatment of C. neoformans infections.
Cryptococcus neoformans is an encapsulated fungal pathogen that causes significant morbidity and mortality in immunocompromised individuals, including patients with AIDS and other immune defects (54). Despite the availability of antifungal agents that are active against C. neoformans, cryptococcosis is largely incurable in individuals with immune impairment because the organism cannot be completely eradicated. Treatment failures and high recurrence rates have led to the need for lifelong prophylaxis to prevent recrudescent disease. The use of azole prophylaxis and the introduction of highly active antiretroviral therapy for human immunodeficiency virus (HIV) infection have reduced the incidence of HIV-associated cryptococcosis in the developed world (5). However, cryptococcosis is an emerging problem in other immunocompromised patient populations (33) and remains a major cause of meningoencephalitis in the developing world (7).
Cryptococcosis is rare in individuals with normal immunity. Hence, modalities that enhance or provide components of the protective immune response to C. neoformans represent a rational approach to the management of cryptococcosis (12, 13). As such, immune-based adjunctive antibody-based therapies are promising modalities because of their ability to augment host defense mechanisms against C. neoformans (12, 14). The ability of specific antibodies to the C. neoformans capsular polysaccharide glucuronoxylomannan (GXM) to prolong survival in lethal experimental cryptococcosis has been established by four independent groups (19, 23, 48, 50). In light of evidence that specific antibodies can stimulate host defense mechanisms and effector cell activity against C. neoformans (12, 14), a vaccine that could elicit antibodies to GXM might prevent the development of or ameliorate cryptococcosis. Unfortunately, GXM has limitations as a vaccine antigen. First, it is a T-cell-independent type 2 antigen that does not induce affinity maturation, class switching, or memory T cells (44, 71). Second, although GXM-protein conjugates increase the immunogenicity of GXM in mice (12, 18), GXM-tetanus toxoid (TT) elicited disease-enhancing and nonprotective antibodies in addition to protective antibodies to GXM in mice (49).
Protective and nonprotective antibodies to GXM can be distinguished by their specificity in certain experimental models (51). Hence, the most desirable vaccine antigens are those that elicit only protective antibodies, but the GXM epitopes that elicit protective antibodies are not known. Since defined oligosaccharide epitopes of GXM are not yet available, various groups have investigated the feasibility of using peptide mimotopes of GXM as surrogates for the GXM epitopes that elicit protective responses (10, 11, 21, 80). Zhang et al. previously described a peptide mimetic (P13) of GXM that was selected from a random peptide phage display library by use of a protective human monoclonal antibody (MAb) to GXM (80). Vaccination studies with P13-protein conjugates in mice established that P13 was a GXM mimotope, in that it elicited an antibody response to GXM and the conjugates prolonged survival after C. neoformans challenge (21). In this study, we investigated the immunogenicity of P13-protein conjugates in mice transgenic for human immunoglobulin loci (XenoMouse mice).
(Parts of this work were presented at the 101st General Meeting of the American Society for Microbiology, Orlando, Fla., May 2001 [abstr. E-40, p. 337] and at the 102nd General Meeting of the American Society for Microbiology, Salt Lake City, Utah, May 2002 [abstr. F-58, p. 211].)
MATERIALS AND METHODS
XenoMouse mice, peptide conjugates, and adjuvants.
The animal research presented in this study complied with all federal, local, and institutional regulations controlling animal use. XenoMouse mice were obtained from Abgenix (Fremont, Calif.) and maintained in the barrier facility of the Albert Einstein College of Medicine. The two mouse strains used are transgenic for the same human heavy- and light-chain-variable-region genes (43) but differ in the human immunoglobulin G (IgG) subclasses that they express. G2 mice express human IgG2, and G4 mice express human IgG4. Both strains express the same human IgM and human light-chain kappa but not light-chain lambda loci (43). TT was obtained from the University of Massachusetts Biologic Laboratories, Worcester, and diphtheria toxoid (DT) was obtained from Sigma, St. Louis, Mo. P13 conjugates consisting of P13 conjugated to TT, to DT, and to dextran (DEX) were synthesized as previously described (21). Alhydrogel was obtained from Accurate Chemical and Scientific Corp., Westbury, N.Y. A CpG preparation (ImmuneEasy) consisting of short oligonucleotides that contain unmethylated cytosine-guanine dinucleotide repeats was obtained from Qiagen, Valencia, Calif.
Vaccination and bleeding protocols.
G2 and G4 mice in groups of five each were vaccinated subcutaneously at the base of the tail with 100 μl of either a P13-protein conjugate or a control treatment (phosphate-buffered saline [PBS]). In one experiment, G2 mice were vaccinated with 10 μg of P13-TT in 50 μl of Alhydrogel with or without 10 μl of the CpG preparation on day 0 and were revaccinated with the same dose on days 14, 38, and 73. The doses of CpG were based on the manufacturer's recommendation of 1 μl of antigen/μg. In another experiment, groups of G2 and G4 mice were vaccinated with 10 μg of P13-DT in Alhydrogel with 10 μl of the CpG preparation on day 0 and were revaccinated with the same dose on days 14 and 39. Mice were bled on days 0, 7, 15, 21, 28, 35, 44, 59, 66, 80, and 106 (P13-TT vaccinations) and on days 0, 7, 14, 21, 28, 36, 42, 49, 56, and 70 (P13-DT vaccinations). In another experiment, groups of G2 mice were vaccinated with either P13-TT or P13-DT in Alhydrogel with CpG on days 0, 14, and 28 and were challenged with C. neoformans (see below).
Serological studies. (i) Determination of titers of antibodies to GXM and P13.
Sera were separated by centrifugation of blood at 3,000 rpm for 10 min and stored at −20°C until analyzed. An antigen capture enzyme-linked immunosorbent assay (ELISA) was used to detect antibodies to GXM and P13 as previously described (21). Briefly, 96-well polystyrene ELISA plates (Corning Glass Works, Corning, N.Y.) were coated with 10 μg of C. neoformans serotype D GXM (strain 24067; American Type Culture Collection, Manassas, Va.)/ml, with 10 μg of serotype A GXMs (strains H99 and SB4; provided by A. Casadevall, Albert Einstein College of Medicine)/ml, or with 10 μg of P13-DEX/ml for 3 h at room temperature (RT). The plates then were washed and blocked with PBS-0.1% Tween 20 (Sigma) overnight at 4°C. Beginning at a dilution of 1:50 or 1:500, sera were serially diluted 1:3 and incubated with the plates for 1 h at 37°C. The plates were washed with PBS- 0.01% Tween 20 by using a SkanWasher 400 (Molecular Devices, Sunnyvale, Calif.) and then were incubated with a 1:1,000 dilution of alkaline phosphatase (AP)-conjugated antibodies to human IgM, IgG, or human light chain kappa (Southern Biotechnology, Birmingham, Ala.) or to mouse light chain lambda (Fisher, Pittsburgh, Pa.) for 1 h at 37°C. Antibody binding was detected with p-nitrophenyl phosphate substrate (Sigma). The plates were washed and developed, and the absorbance was read at 405 nm. The antibody titer was defined as the average of duplicate wells of the highest serum dilution that gave an absorbance of greater than 0.1 after subtraction of 1.5 times the background.
(ii) Determination of serum antibody idiotype expression.
It was previously shown that human antibodies to GXM use VH3 genes, including gene elements that express the VH3 determinants recognized by mouse anti-human MAbs 16.84 and D12 (22, 23, 57). To investigate the VH responses of XenoMouse mice to vaccination, titers of serum antibodies expressing the VH3 determinants recognized by MAbs D12, 16.84, and B6 and the VH1 determinants recognized by MAbs G6 and G8 were determined by an ELISA as previously described (3, 22, 23, 70). To detect antibodies expressing the VH3 determinants recognized by MAbs D12 (D12-positive antibodies) and 16.84 (16.84-positive antibodies), ELISA plates were coated for 3 h at RT with 5 μg of each of the mouse anti-human MAbs/ml and blocked overnight at 4°C with 1% bovine serum albumin-PBS. The plates were incubated for 1 h at 37°C with pre- and postvaccination sera serially diluted 1:3 beginning at a dilution of 1:100. The plates were washed, incubated, with a 1:1,000 dilution of AP-conjugated goat anti-human IgM or IgG for 1 h at 37°C, and developed, and the absorbance was read as described above. Endpoint titers were defined as the first absorbance value after which the slope of the titration curve did not change. To determine the VH expression of specific antibodies, ELISA plates coated with GXM (strain 24067, H99, or SB4) or P13-DEX as described above were incubated with a 1:50 dilution of pre- or postvaccination sera for 1 h at 37°C. After being washed, the plates were incubated with 5 μg of mouse MAb D12 or 16:84/ml, and MAb binding was detected after incubation with a 1:1,000 dilution of AP-conjugated goat anti-mouse IgG (heavy and light chains) (Southern Biotechnology) as described above. Control mice received CpG and Alhydrogel and are referred to here after as the CpG group.
C. neoformans challenge of P13 conjugate-vaccinated and control mice.
C. neoformans challenge experiments were performed to assess the efficacy of the P13 conjugates. Mice in groups of five were each vaccinated subcutaneously on day 0 and revaccinated on days 14 and 28 after primary vaccination with (i) PBS, (ii) with 10 μg of P13-TT, P13-DT, TT, or DT in 50 μl of Alhydrogel and 10 μl of the CpG preparation, or (iii) with the CpG preparation and Alhydrogel. All injections were given in a 100-μl volume. On day 35 after primary vaccination, the mice received 5 × 106 CFU of C. neoformans strain 24067 intraperitoneally, and survival was monitored daily. On day 65 after C. neoformans challenge (day 100 after primary vaccination), all surviving mice were rechallenged with 107 CFU of the same C. neoformans strain intraperitoneally, and survival was monitored daily. Mice were bled on days 0, 7, and 30 after primary vaccination, and surviving mice were bled on day 65 after the initial C. neoformans challenge (day 100 after primary vaccination) and on days 5 and 35 after rechallenge (days 70 and 100 after the initial C. neoformans challenge, respectively, or days 105 and 135 after primary vaccination, respectively). Pooled sera from these bleedings were used to determine titers of specific and VH3-positive antibodies to GXM and P13 (as detailed above) and serum GXM levels. Inhibition ELISAs that used P13 and GXM as soluble inhibitors of serum antibody binding to GXM and to P13, respectively, were performed as previously described (21).
Determination of serum GXM concentrations.
The GXM concentrations in pooled serum samples from the C. neoformans challenge experiment were determined for samples taken on day 65 after the original challenge and on days 5 and 30 after rechallenge (days 70 and 100 after the first challenge) by using an antigen capture ELISA as described previously (17, 21). Briefly, 96-well microtiter plates were coated for 3 h at RT with 1 μg of a mouse IgM MAb to GXM (2D10; provided by A. Casadevall)/ml, blocked overnight with 1% bovine serum albumin in PBS, and incubated with twofold dilutions of purified GXM from C. neoformans strain 24067 to generate a standard curve. The plates were incubated for 1 h at 37°C with serum samples serially diluted after an initial dilution of 1:100, washed, and incubated for 1 h at 37°C with a mouse IgG1 MAb to GXM (2H1; provided by A. Casadevall). Bound 2H1 was detected by incubating the plates with AP-conjugated goat anti-mouse IgG (Southern Biotechnology) for 1 h at 37°C. The plates were developed and read as described for the serological studies. The standard curve was drawn by using the absorbance values of the standard dilutions, and the GXM concentrations in test samples were calculated by extrapolation from the absorbance values in the linear portion of the titration curve.
Statistical analysis.
Comparisons between groups at different times and within groups at different times were performed with a Mann-Whitney U test. We focused our comparisons on the differences between the responses of G2 and G4 mice to vaccination, between CpG-vaccinated and non-CpG-vaccinated mice, and between P13-TT-vaccinated and P13-DT-vaccinated mice. To increase the stringency of the number of comparisons made, the level of significance was chosen at 0.01 to include a 99% confidence interval (CI). Therefore, a P value of <0.01 was considered highly significant (6), except when otherwise noted. Changes in serum GXM concentrations in different groups after C. neoformans challenge were evaluated by using the unpaired t test, with P < 0.05 being considered significant. Comparisons of serum antibody levels in the C. neoformans challenge experiments (using pooled samples) were performed with a one-way analysis of variance (ANOVA), followed by the Bonferroni posttest for pairwise comparisons, computed only when the overall differences in mean titers were significant (Prism version 3.0.2; Graphpad Software, Inc., San Diego, Calif.). Animal survival was analyzed by using the Kaplan-Meier log rank test (Prism) and Cox proportional hazard analysis (SPSS version 8.0 for Windows; SPSS Inc., Chicago, Ill.). A P value of <0.05 was considered significant for the ANOVA and survival analyses.
RESULTS
Serological studies.
All of the antibodies produced in response to vaccination with P13-TT and P13-DT in G2 and G4 mice were human, based on their expression of both human light chain kappa and IgM or IgG and their undetectable reactivity with anti-mouse light-chain lambda reagents (data not shown).
(i) Antibodies to P13.
P13-TT elicited IgM and IgG to P13 in G2 and G4 mice (Fig. 1A to D). Compared to P13-TT-vaccinated G2 mice that did not receive CpG, G2 mice that received CpG had significantly higher titers of IgM and IgG to P13 on days 7 to 28 and on days 21, 28, and 44, respectively (P = 0.008, 99% CI); G4 mice that received CpG had higher titers of IgM and IgG to P13 on days 15 to 44 and 80 and on days 21 to 44, respectively (P = 0.008, 99% CI), than did those that did not receive CpG. P13-DT, which was administered only with CpG, also elicited IgM and IgG to P13 in G2 and G4 mice (Fig. 1E to H). P13-TT-vaccinated G2 mice that received CpG had significantly higher titers of IgM and IgG to P13 on days 7 and 59 than did P13-DT-vaccinated G2 mice on days 7 and 56 (P ≤ 0.004, 99% CI). P13-TT-vaccinated G4 mice that received CpG had higher titers of IgM to P13 on days 7, 44, and 59 than did P13-DT-vaccinated mice on days 7, 42, and 56 (P = 0.008, 99% CI); P13-TT-vaccinated mice that received CpG had higher titers of IgG to P13 on days 21, 28, 35, and 44 than did P13-DT-vaccinated mice on days 21, 28, 36, and 42 (P = 0.008, 99% CI).
FIG. 1.
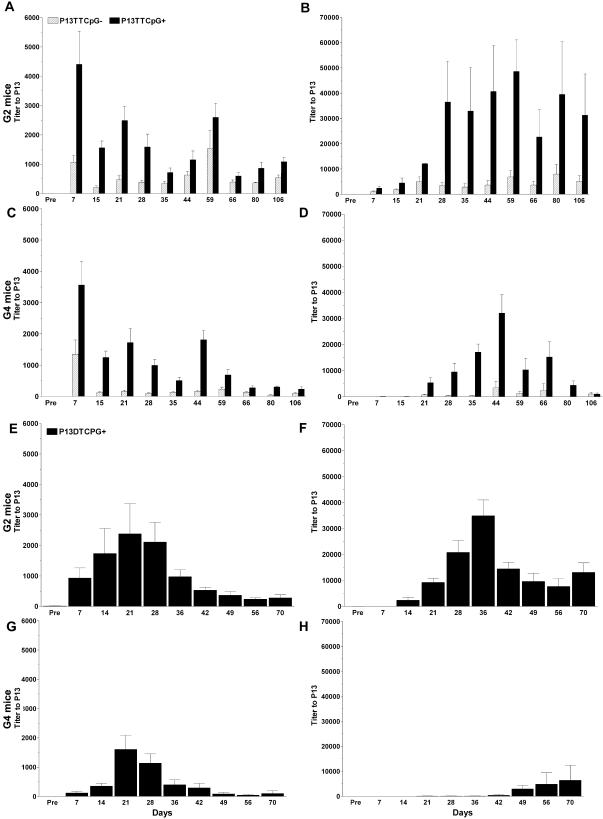
Titers of IgM (A, C, E, and G) and IgG (B, D, F, and H) to P13 in sera from P13-TT-vaccinated (A to D) and P13-DT-vaccinated (E to H) G2 and G4 mice. The y axis shows the inverse titer for the times after primary vaccination shown on the x axis. Hatched bars and black bars represent mice that did not and mice that did receive CpG, respectively. Overall, CpG increased the antigenicity of P13, the antigenicities of P13-TT and P13-DT were comparable, and G2 mice had a more robust response than G4 mice. Error bars represent the standard error of the mean of replicate samples. Statistically significant differences between CpG-treated and untreated mice, between P13-TT- and P13-DT-vaccinated mice, and between G2 and G4 mice are described in the text. There were five mice per group. Pre, preimmunization.
In a comparison of G2 and G4 mice that received P13-TT with CpG, G2 mice had significantly higher titers of IgM and IgG to P13 on days 80 and 106 (P = 0.008, 99% CI); at other times, G2 mice had higher titers (significant at a lower CI; P = 0.016, 95% CI) of IgM to P13 on days 59 and 66 and of IgG to P13 on days 7, 15, 28, and 59. For P13-TT-vaccinated mice that did not receive CpG, G2 mice had significantly higher titers of IgM to P13 on days 28, 44, 59, 80, and 106 and of IgG to P13 on days 7 to 28 (P = 0.008, 99% CI). For mice that received P13-DT, G2 mice had higher titers (significant at a lower CI; P < 0.031, 95% CI) of IgM to P13 than did G4 mice on days 7, 49, and 56 and of IgG to P13 on days 21 to 42 (P = 0.001, 99% CI).
In summary, CpG increased the titers of IgM and IgG to P13 in P13-TT-vaccinated mice, the titers induced by P13-TT and P13-DT (when both were given with CpG) were comparable, and the titers induced by both P13 conjugates were lower in G4 mice than in G2 mice. All statistical comparisons were performed with the Mann-Whitney U test.
(ii) Antibodies to GXM.
IgM to GXM was detectable after vaccination with P13-TT (Fig.2A and C). The titers of IgG to GXM were low (Fig. 2B and D), regardless of CpG use or XenoMouse strain. For mice that received CpG, the titers of IgM to GXM were significantly higher in G2 mice than in G4 mice on days 21, 44, and 66 to 106 (P = 0.008, 99% CI) and on days 7, 15, 28, 35, and 59 at a lower CI (P < 0.032, 95% CI). For mice that did not receive CpG, the titers of IgM to GXM were significantly higher in G2 mice than in G4 mice on days 15 to 106 (P = 0.008, 99% CI) and on day 7 at a lower CI (P = 0.032, 95% CI). IgM to GXM was detectable on days 7 to 49 in P13-DT-vaccinated G2 mice (all of which received CpG) (Fig. 2E). The titers of IgM to GXM in P13-TT-vaccinated G2 mice that received CpG and P13-DT-vaccinated G2 mice were comparable, except that the titers in P13-TT-vaccinated mice on day 44 were higher than those in P13-DT-vaccinated mice on day 42 (P = 0.004, 99% CI). The titers of IgG to GXM were low in both P13-TT- and P13-DT-vaccinated G2 mice; however, P13-TT-vaccinated mice that received CpG had higher titers of IgG to GXM than did those that did not (on day 28, the difference was highly significant; P = 0.008, 99% CI). The titers of P13-TT-elicited IgG were significantly higher than those of P13-DT-elicited IgG on day 28 (P = 0.007, 99% CI), whereas the titers of P13-DT-elicited IgG were higher on day 36 than were the IgG titers in P13-TT-vaccinated mice on day 35 (P = 0.007, 99% CI). Vaccination of G4 mice with either P13-TT or P13-DT did not elicit a response to GXM, as the titers of IgM and IgG to GXM did not rise above preimmunization levels (Fig. 2C, D, G, and H). Antibody detection with an IgG4-specific reagent similarly did not reveal an IgG4 response to GXM (data not shown).
FIG. 2.
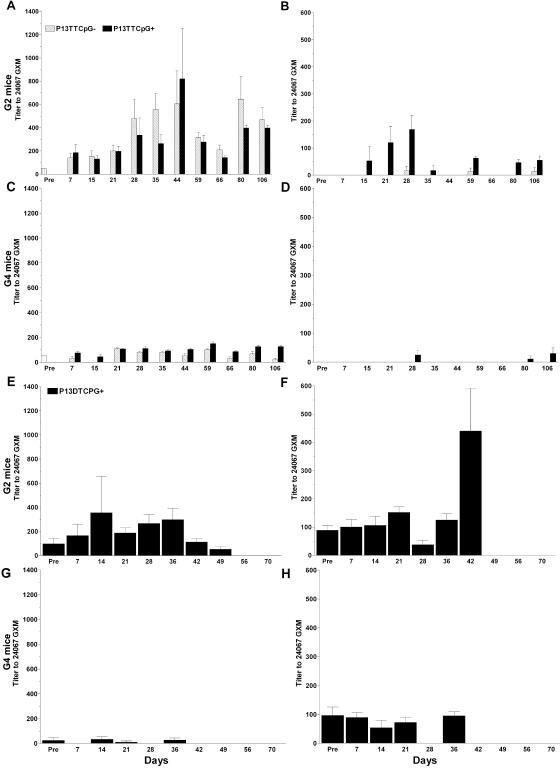
Titers of IgM (A, C, E, and G) and IgG (B, D, F, and H) to GXM (serotype D, 24067) in sera from P13-TT-vaccinated (A to D) and P13-DT-vaccinated (E to H) G2 and G4 mice. The y axis shows the inverse titer for the times after primary vaccination shown on the x axis. Hatched bars and black bars represent mice that did not and mice that did receive CpG, respectively. Overall, CpG increased the IgG response to GXM, the immunogenicities of P13-TT and P13-DT were comparable, and G4 mice did not manifest a GXM response. Error bars represent the standard error of the mean of replicate samples. Statistically significant differences between CpG-treated and untreated mice and between P13-TT- and P13-DT-vaccinated mice are described in the text. There were five mice per group. Pre, preimmunization.
In summary, CpG increased the P13-TT-elicited IgG response to GXM, only G2 mice generated responses to GXM, and the GXM responses of G2 mice to P13-TT and P13-DT were mostly comparable. All statistical comparisons were performed with the Mann-Whitney U test.
(iii) Antibody specificity.
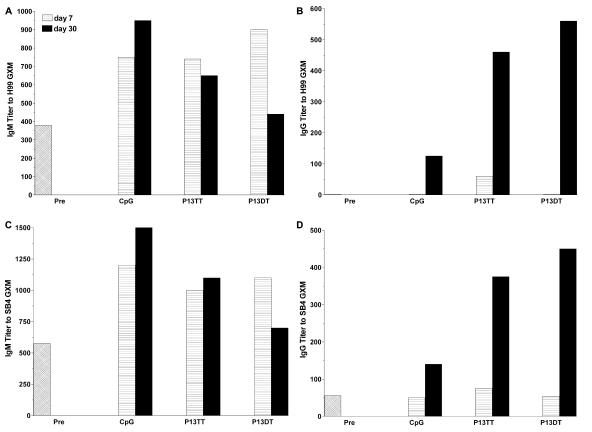
GXM is responsible for the serotype specificity of C. neoformans strains (34). Serotype A is the most prevalent C. neoformans serotype in the United States (54), whereas serotype D strains cause disease globally, including in Europe (20). To determine whether the P13 conjugates elicited antibodies to serotype A GXM, we examined the binding of pre- and postvaccination sera to the serotype A strains H99 and SB4. P13-TT- and P13-DT-induced antibodies in pooled sera from G2 mice in the C. neoformans challenge experiment (see below) bound to both serotype A strains (Fig. 3).
FIG. 3.
Reactivity of sera from CpG- or P13-protein conjugate-vaccinated G2 mice with GXM from serotype A C. neoformans strain H99 or SB4. The y axis shows the inverse titer of antibodies for the immunogens on the x axis for sera obtained either 7 days or 30 days after primary vaccination in the C. neoformans challenge experiment. Each bar represents pooled sera from five vaccinated mice. Pre, preimmunization.
(iii) Total serum idiotype expression.
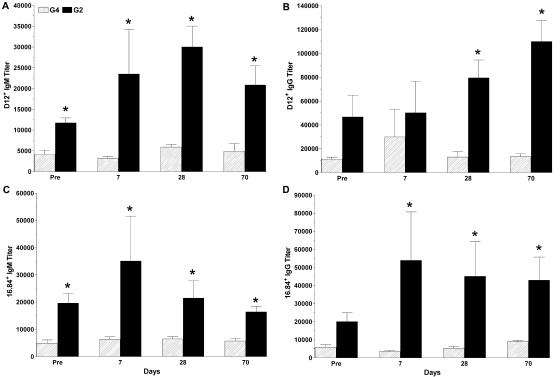
Higher levels of serum antibodies expressing the VH3 determinants recognized by MAbs D12 and 16.84 were observed in G2 mice than in G4 mice (Fig. 4). For mice that received P13-DT, the prevaccination titers of D12- and 16.84-positive IgM and the postvaccination titers on days 7, 28, and 70 were significantly higher in G2 mice than in G4 mice (P = 0.029, 95% CI, and P = 0.008, 99% CI, respectively). The prevaccination titers of D12-positive and 16.84-positive IgG were also higher in G2 mice than in G4 mice, but the difference was not significant. The titers of D12-positive IgG were significantly higher in G2 mice than in G4 mice on days 28 and 70 (P = 0.008, 99% CI), and the titers of 16.84-positive IgG were significantly higher on days 7, 28, and 70 (P = 0.008, 99% CI). There were no statistically significant differences between pre- and postvaccination titers of D12-positive or 16.84-positive IgM or IgG in P13-DT-vaccinated G2 mice. P13-TT-vaccinated G2 mice had higher pre- and postvaccination titers of D12-positive and 16.84-positive IgM and IgG than did G4 mice; these were higher in CpG-vaccinated mice (data not shown). Neither the conjugate-induced nor the CpG-induced antibodies expressed the determinants recognized by MAb G6 or G8 (VH1) or by MAb B6 (VH3) at the levels seen in prevaccination sera (data not shown). All comparisons were performed with the Mann-Whitney U test.
FIG. 4.
Titers of antibodies expressing the VH3 determinants recognized by mouse MAbs D12 and 16.84 in P13-DT-vaccinated G2 and G4 mice. The y axis shows the inverse titer of antibodies for the times after primary vaccination shown on the x axis for G4 and G2 mice. Error bars represent the standard error of the mean. An asterisk indicates a P value of <0.05 for a comparison of titers in G2 and G4 mice, as determined by the Mann-Whitney U test. There were five mice per group. Pre, preimmunization.
C. neoformans challenge of vaccinated mice. (i) Survival.
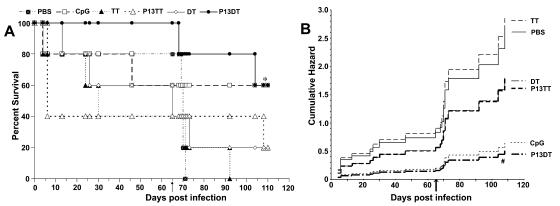
There were no significant differences in the survival of control (treated with CpG, DT, TT, or PBS) and P13-vaccinated (P13-TT or P13-DT) G2 mice after the first C. neoformans challenge (Fig. 5A). After rechallenge with C. neoformans 65 days after the first challenge, the survival of P13-DT-vaccinated G2 mice was significantly longer than that of PBS-, DT-, or TT-treated control G2 mice (P < 0.03; Kaplan-Meier log rank survival test) (Fig. 5A). The survival of CpG-treated and P13-TT-vaccinated mice was not statistically different from that of the control mice. Comparison of the survival of the vaccination and control groups by Cox hazard regression analysis revealed that P13-DT vaccination increased the likelihood of survival after C. neoformans rechallenge by 81%, CpG vaccination did so by 76%, and P13-TT vaccination did so by 32%, compared to vaccination with PBS (P = 0.03 for the reduction in the likelihood of death after P13-DT vaccination; P = 0.06 for the overall reduction in the risk of death after P13-DT vaccination) (Fig. 5B).
FIG. 5.
Survival of P13-TT-, P13-DT-, and CpG-vaccinated and control G2 mice after C. neoformans infection. (A) The y axis shows the Kaplan-Meier survival plot for mice on the days after primary vaccination shown on the x axis. Mice were challenged 35 days after primary vaccination with C. neoformans strain 24067 and rechallenged 65 days later (on day 100 postvaccination), as shown by the arrow. An asterisk indicates a P value of <0.03 for a comparison of the survival of P13-DT-vaccinated mice to that of PBS-, DT-, and TT-treated mice, as determined by a Kaplan-Meier log rank test. (B) Cox hazard regression analysis of the survival data shown in panel A, depicting the increasing likelihood of death over time for each of the groups. A number sign indicates a P value of 0.031 for the reduction in the likelihood of death for P13-DT vaccination compared to PBS vaccination and a P value of 0.0571 for the overall reduction in risk of death for P13-DT vaccination compared to PBS vaccination. There were five mice per group.
(ii) Serum GXM levels after C. neoformans challenge.
GXM was detectable in the sera of all mice 65 days after the first challenge and 5 days after rechallenge with C. neoformans (day 70 after the initial challenge) (Table 1). There were no significant differences in the GXM levels in the vaccination (P13-DT and P13-TT) and control (DT, TT, CpG, and PBS) groups after the first challenge (Table 1). Both P13-DT-vaccinated and CpG-treated G2 mice had lower GXM levels than did P13-TT-vaccinated mice 5 days after rechallenge (P < 0.03; unpaired t test). The differences in GXM levels at these times for the other groups either were not statistically significant or could not be determined because only one mouse remained in a group (Table 1).
TABLE 1.
Serum GXM concentrations in control and P13-vaccinated G2 micea
| Treatment | Mean ± SEM serum GXM concn (μg/ml) at the following day:
|
||
|---|---|---|---|
| 65 after initial challenge | 5 after rechallenge | 35 after rechallenge | |
| PBS | 544 ± 251 | ||
| CpG | 1,030 ± 459 | 491 ± 64 | 379 ± 137 |
| TT | 1,789 ± 1,671 | 1,973 ± 0 | |
| P13-TT | 400 ± 237 | 2,440 ± 600 | 422 ± 0 |
| DT | 1,124 ± 146 | 587 ± 0 | 1,085 ± 0 |
| P13-DT | 725 ± 261 | 1,044 ± 181 | 311 ± 134 |
Serum GXM concentrations in G2 mice vaccinated with the indicated treatments were determined on day 30 after the initial C. neoformans challenge (100 days after initial vaccination) and on days 5 and 35 after rechallenge. PBS-treated mice died after rechallenge, and no values could be obtained for them. The P value was <0.03 for a comparison of P13-DT-treated mice at 35 and 5 days after rechallenge and for a comparison of CpG- and P13-TT-treated mice at 5 days after rechallenge, as determined by the unpaired t test. The decrease seen in the CpG-treated mice at 5 days after rechallenge was not statistically significant compared with the value seen in the CpG-treated mice at 65 days after the initial challenge (P = 0.08). The TT-, DT-, and P13-TT-treated groups in which the standard error of the mean was zero had one surviving mouse, precluding statistical analyses.
(iii) Antibodies to GXM in mice in the C. neoformans challenge experiment.
Compared to prevaccination titers, the titers of IgM to GXM were higher on day 30 after primary vaccination in CpG- and P13-TT-vaccinated mice (preimmunization serum versus day 30: P < 0.001) and on day 7 in P13-DT-vaccinated mice (preimmunization serum versus day 7: P < 0.001). The titer of IgM to GXM after C. neoformans rechallenge was higher than the titer on day 30 after primary vaccination (P < 0.001). The titer of IgG to GXM after rechallenge was comparable to that on day 30 after primary vaccination, which were higher than those on day 7, but this increase was not statistically significant (Fig. 6 A and B). P13-DT-vaccinated mice manifested an increase in the titer of IgM to GXM after both C. neoformans challenges compared to the titer on day 30 after primary vaccination, but the titer on day 7 was higher (Fig. 6A). P13-DT-vaccinated mice also manifested a large increase in the titers of IgG to GXM after C. neoformans rechallenge compared to the titers on day 30 after primary vaccination (P < 0.01) and the first challenge (P < 0.001) (Fig. 6B). CpG-vaccinated mice manifested a significant increase in the titer or IgM to GXM after both C. neoformans challenges compared to the titers on days 7 and 30 after primary vaccination (P < 0.001); however, the titers of IgG to GXM did not increase from the titers on day 30 after primary vaccination (Fig. 6A and B).
FIG. 6.
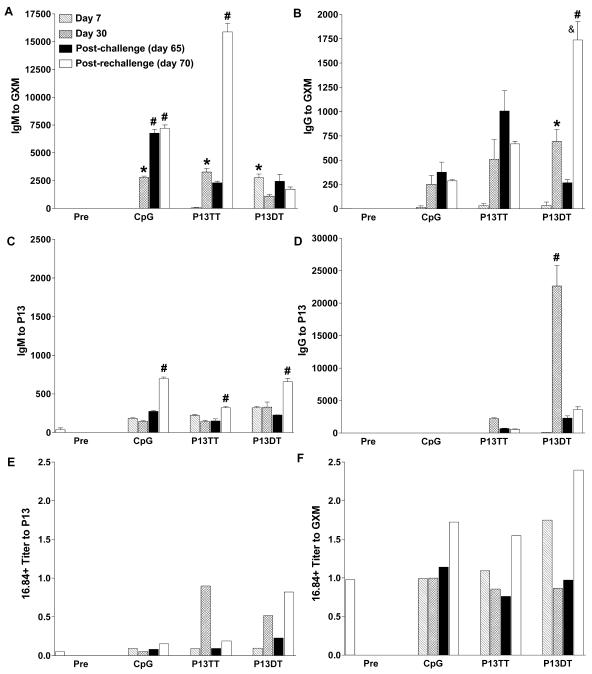
Serum antibody profiles of P13-DT-, P13-TT-, and CpG-vaccinated G2 mice in the C. neoformans challenge experiment depicted in Fig. 5. Days 7 and 30 represent days after primary vaccination, and days 65 and 70 represent days after C. neoformans challenge. The y axis shows the inverse titer for the immunogens shown on the x axis at the designated times. GXM was from strain 24067. Error bars represent the standard error of the mean. An asterisk indicates a P value of ≤0.01 for a comparison of preimmunization (Pre) sera and sera obtained at days 7 and/or 30 after primary vaccination. A number sign indicates a P value of ≤0.01 for a comparison of day 30 after primary vaccination and days 65 and/or 70 after C. neoformans challenge. An ampersand indicates a P value of ≤0.01 for a comparison of day 65 after C. neoformans challenge and day 70 after the first C. neoformans challenge (day 5 after rechallenge). Comparisons were made by a one-way ANOVA with the Bonferroni posttest.
(iv) Antibodies to P13 in mice in the C. neoformans challenge experiment.
Vaccination increased the titers of IgM to P13 in all groups (prevaccination titers compared to day 7 titers in CpG-, P13-TT-, and P13-DT-vaccinated mice: P < 0.05), although the overall titers were lower than those of IgM to GXM. Following C. neoformans rechallenge, there was a significant increase in the titers of IgM to P13 in CpG-vaccinated (day 30 versus day 70: P < 0.001), P13-TT-vaccinated (day 30 versus day 70: P < 0.01) and P13-DT-vaccinated (day 30 versus day 70: P < 0.001) mice (Fig. 6C). The titers of IgG to P13 decreased after C. neoformans challenge compared to the titers on day 30 after primary vaccination in P13-TT- and P13-DT-vaccinated mice (Fig. 6D). This decrease was significant for the P13-DT-vaccinated group (day 30 versus postchallenge and rechallenge: P < 0.001). All studies were performed with pooled sera, and statistical comparisons were made by an ANOVA with the Bonferroni posttest for pairwise comparisons of quadruplicate determinations for each time.
(v) Idiotype expression of antibodies to GXM and P13 in mice in a challenge experiment.
The prevaccination sera of G2 mice contained antibodies that reacted with GXM (24067) and expressed the VH3 determinants recognized by MAb 16.84 (Fig. 6E) but not by MAb D12 (data not shown). P13-DT-vaccinated mice manifested an increase in the level of GXM-specific, 16.84-positive antibodies on day 7 after primary vaccination that decreased on day 30 after primary vaccination and the first C. neoformans challenge and then increased to greater than the day 7 level after rechallenge (Fig. 6E). CpG- and P13-TT-vaccinated mice also manifested an increase in the level of GXM-specific, 16.84-positive antibodies after rechallenge compared to pre- and postvaccination levels (Fig. 6E). P13-DT and P13-TT also elicited P13-specific, 16.84-positive antibodies on day 30 after primary vaccination, but only P13-DT elicited P13-specific, 16.84-positive antibodies after C. neoformans rechallenge, and the reactivity of these antibodies was lower than that of GXM-specific antibodies (Fig. 6F).
In summary, CpG-, P13-TT-, and P13-DT-vaccinated mice in the challenge experiment had more robust IgG responses to GXM than did mice that were vaccinated in the experiments shown in Fig. 1 and 2. P13-DT-vaccinated mice had a greater IgG response to P13 and greater 16.84-positive responses to P13 and GXM than did P13-TT-vaccinated mice. The control treatments (TT and DT) did not elicit specific antibody responses to GXM or P13. Hence, the serological findings for the mice in the challenge experiment are most consistent with the interpretation that both P13 conjugates elicited mimetic (P13) and mimotope (GXM) responses and primed the mice to produce an IgM response to GXM after C. neoformans challenge; however, only P13-DT primed the mice to produce an anamnestic IgG response. Inhibition ELISAs revealed that P13-DEX inhibited 35% of the binding of P13-DT-elicited serum IgG to GXM from day 30 after primary vaccination but that GXM did not inhibit the binding of serum IgG to P13-DEX (data not shown). The amount of serum obtained from the P13-TT-vaccinated mice was insufficient to perform inhibition studies.
DISCUSSION
The results presented here show that both P13-protein conjugates that were used in this study induced a human antibody response to P13 in human immunoglobulin transgenic mice expressing either IgG2 or IgG4. P13-TT-vaccinated G2 and G4 mice produced IgG to P13 with similar kinetics, although the titers in G4 mice were lower and waned by day 44 after primary vaccination. P13-DT-vaccinated G4 mice showed delayed kinetics, which have been described for other human IgG4 responses (1). In contrast, an antibody response to GXM was observed only in G2 mice. Although antibody titers and isotype switching depend strongly on the nature of the immunogen within each mouse strain, IgG subclass expression has been shown to influence binding to GXM and other polysaccharide antigens (16, 42). For example, variable-region-identical mouse-human chimeric MAbs to GXM manifested IgG subclass-dependent differences in GXM specificity and opsonic activity (42). IgG2 is the predominant subclass of human antibodies to capsular polysaccharides (56), including GXM (17, 55, 82), and it has been proposed that the preferential use of IgG2 in antibodies to capsular polysaccharides (56) is a function of its avidity and ability to polymerize (78). Since human serum IgG4 is functionally monovalent (2, 65), it might not be able to bind the relevant GXM epitope for which P13 is a surrogate antigen when monovalency eliminates avidity, whereas the avidity of IgG2 might enhance binding. Along the same lines, lower levels of IgM to GXM in G4 mice might reflect epitope blocking by monomeric IgG4, but further investigation is required to identify the mechanisms responsible for differences in the GXM reactivities of sera from G4 and G2 mice.
We found that the levels of antibodies expressing D12- and 16.84-positive VH3 determinants were higher in G2 mice than in G4 mice, although total serum immunoglobulin levels were comparable in the two strains (data not shown). Since CpG, P13-TT, and P13-DT each elicited 16.84-positive antibodies to GXM in G2 mice, but G4 mice did not produce detectable antibodies to GXM, our data suggest that a threshold level of VH3 expression might be needed for XenoMouse mice to generate an antibody response to GXM. While the functional importance of VH3 use by antibodies to GXM is unknown, it has been proposed that antibodies with shared specificities are encoded by defined VH genes (36, 75). Along these lines, it was previously shown that human antibodies to GXM use VH3 gene elements, including those that express D12- and 16.84-reactive VH3 determinants (22, 23, 57), and that HIV-infected individuals who developed cryptococcal meningitis had lower levels of 16.84-positive antibodies than did those who did not (22). Since the eliciting antigen for the GXM response in this study was either a peptide or an adjuvant, our data support the concept that the use of VH3 by polysaccharide-specific antibodies (3, 22, 55) is driven by conformational constraints on antigen binding (75, 79) rather than by the nature of the antigen. Although this hypothesis requires further investigation, it has the potentially important ramification that peptide surrogates may not overcome impaired polysaccharide responses that result from antibody repertoire defects (55).
The ability of peptides to elicit antibodies to polysaccharides has been attributed to molecular mimicry, whereby polysaccharide binding is acquired through affinity maturation of antibodies induced by the peptides (11, 45). However, the data provided in this study and an earlier study (21) show that P13-induced antibody responses to GXM and P13 arose essentially simultaneously. We also found that even when a robust IgG response to GXM was induced (in the challenge experiment), only 30 to 40% of P13-DT-elicited antibodies to GXM cross-reacted with P13 in inhibition ELISAs. Limitations of solid-phase assays notwithstanding, these observations suggest that antibodies to GXM may not arise exclusively from affinity maturation of the P13 response. In light of evidence that primary and secondary antibody responses can arise from different B-cell subsets (39, 40), distinct molecular mechanisms might be responsible for P13 mimotope-induced responses to GXM and P13. The idea that one peptide could elicit independent antibody responses is supported by evidence that peptide mimetics of capsular polysaccharides can assume different conformations and bind antibodies with different specificities (66). Conformational analysis of P13 and molecular analysis of P13-conjugate-elicited GXM- and P13-specific antibodies are required to reveal whether or not they are derived from common or distinct B-cell precursors.
Protein carriers can influence the specificity, function, avidity, and idiotype of the antibody response to polysaccharide-protein conjugate vaccines (8, 27, 41, 58, 67). Serological results from the challenge experiment showed that P13-DT primed for a C. neoformans-induced IgG anamnestic response to GXM and prolonged survival after C. neoformans rechallenge, whereas P13-TT vaccination primed for an IgM response. Other peptide mimotopes also have been shown to prime anamnestic responses to encapsulated pathogens (28, 46), but there are very few reports of mimotope efficacy against microbial pathogens (21, 26, 53). The magnitude of the IgG response to C. neoformans rechallenge in P13-DT-vaccinated mice compared to that in mice that received the other immunogens suggests that a threshold level of defined, specific antibodies might be required for protection against C. neoformans, as described for a mimotope of another pathogen (53). However, high levels of mimotope-induced antibodies to polysaccharides have not been reliable predictors of vaccine efficacy in vivo, particularly for capsular polysaccharide mimotopes (10, 29, 30, 74). In addition, for C. neoformans, antibody-mediated immunity might involve regulation of the cellular immune response (14), and the amount of antibody needed for this function is unknown. It has been shown that protective and nonprotective mouse IgM antibodies to GXM have specificity differences (51), that nonprotective antibodies can reduce the efficacy of protective antibodies (52), and that large amounts of antibodies can cause a prozone-like phenomenon that results in a lack of antibody efficacy against C. neoformans (72, 73). Specificity is difficult to decipher in polyclonal sera, and defined antibody reagents will be needed to determine the specificity of P13-conjugate-induced antibodies. However, the height of the IgM response to GXM in P13-TT-vaccinated mice after C. neoformans rechallenge, almost 10 times greater than that in P13-DT-vaccinated mice, suggests the possibility that a prozone-like phenomenon mitigated antibody efficacy. Although the mechanisms of mimotope efficacy remain uncertain, our data suggest that efficacy is not a straightforward function of the induced mimotope titer at the time of microbial challenge; therefore, further studies are needed to identify the most suitable protein carrier for GXM mimotopes and useful surrogates for mimotope efficacy.
Both P13 conjugates elicited antibodies that bound to GXM of serotype A (H99 and SB4) C. neoformans strains in addition to serotype D (24067). Although protective mouse and human antibodies to GXM elicited by the investigational conjugate vaccine GXM-TT bound serotype A GXM and serotype D GXM (23, 47, 50, 57), GXM-TT also elicited nonprotective and deleterious antibodies as well as protective antibodies (12). Hence, a GXM mimotope vaccine may hold promise for broadening the response to different C. neoformans serotypes and strains, while focusing the response on epitopes that elicit protective antibodies. Although the degree of protection that we observed for P13-DT was modest, the scope of this study did not permit us to examine additional variables that could influence mimotope efficacy, such as the conjugate dose, the route of administration, and the timing of and number of vaccinations. In addition, the challenge-rechallenge model that we used might have affected our findings. However, we are encouraged by the results obtained with this model, because it has similarities to the pathogenesis of human C. neoformans infection, which is thought to be acquired in childhood (4, 25), to remain latent, and to undergo reactivation in HIV- and immunosuppression-associated cryptococcosis (24, 69). The species difference in our model, i.e., induced antibodies were human and cellular receptors were murine, also could have influenced our findings. Limited information is available on mouse Fc receptor signaling or complement activation by immune complexes containing human immunoglobulin. However, it has been demonstrated that human IgM antibodies to C. neoformans and S. pneumoniae are protective in mice and that antibody efficacy requires mouse complement (15, 81). Furthermore, human IgG interacts with mouse Fc receptors (61) and complement (64); complement is required for the efficacy of type-specific human IgG1 and IgG2 against S. pneumoniae in mice (64); and several human antibody preparations, including intravenous immunoglobulin, type-specific immune sera, and monoclonal IgG1 and IgG2 antibodies, have been shown to be protective against the relevant pathogen in mice (59, 60, 63, 64). Hence, there is experimental evidence supporting the ability of human antibodies to mediate protection in mice. The efficacy of P13-DT in G2 mice underscores the promise of some peptide mimotopes as potential C. neoformans vaccine antigens and highlights the suitability of XenoMouse mice for study of the human antibody response to microbial pathogens (15, 31, 62) and in the area of vaccine development.
CpG was used as an adjuvant with Alhydrogel the P13 conjugates in this study. CpG enhances the immunogenicity of Alhydrogel (77), T-cell-dependent antibody responses to polysaccharide and protein antigens (76), and T-cell-independent responses to some polysaccharide antigens (38). CpG also enhances Th1 immunity (32), which is beneficial in the host defense against C. neoformans (37) and which has been shown to be elicited by specific antibodies to GXM (reviewed in reference 14). CpG had at least two effects in this study. First, it enhanced the antigenicity of P13-TT in G2 and G4 mice. Second, it elicited GXM-specific antibodies and primed for C. neoformans-induced IgM and 16.84-positive anamnestic responses to GXM, which were associated with reduced serum GXM levels and a trend toward increased survival after C. neoformans rechallenge. Notably, CpG has been shown to augment resistance to another fungal pathogen (68). Since CpG enhances innate immunity and stimulates expansion and differentiation of IgM and other B-cell subsets (9, 35, 46), our data suggest that it might independently augment the production of naturally occurring antibodies, including GXM- and P13-specific IgM, which might in turn enhance resistance to C. neoformans. Naturally occurring antibodies to GXM are found in practically all human sera, and various investigations have demonstrated qualitative, quantitative, and specificity differences in the serum antibody profiles of non-HIV-infected and HIV-infected individuals, who are relatively resistant and susceptible to cryptococcosis, respectively (17, 22, 25, 80). Further investigation of the specificities and characteristics of CpG- and P13-DT-elicited antibodies may reveal an interplay between innate and acquired antibody- and cell-mediated immune responses in vaccine-mediated protection against C. neoformans.
Acknowledgments
This work was supported by National Institutes of Health grants R01AI035370, R01AI045459, and R01AI044374 (to L.-a.P.) and by Individual National Research service award F31AI010129 (to R.W.M.).
We thank Arturo Casadevall, Marta Feldmesser, Geoff Davis, Mike Gallo, Sirid Kellermann, Mary Haak-Frendscho, and Chris Hare for critical review of the manuscript.
Editor: T. R. Kozel
REFERENCES
- 1.Aalberse, R. C., and J. Schuurman. 2002. IgG4 breaking the rules. Immunology 105:9-19. [DOI] [PMC free article] [PubMed]
- 2.Aalberse, R. C., J. Schuurman, and R. Van Ree. 1999. The apparent monovalency of human IgG4 is due to bispecificity. Int. Arch. Allergy Immunol. 118:187-189. [DOI] [PubMed] [Google Scholar]
- 3.Abadi, J., J. Friedman, R. Jefferis, R. A. Mageed, and L. Pirofski. 1998. Human antibodies elicited by a pneumococcal vaccine express idiotypic determinants indicative of VH3 gene segment usage. J. Infect. Dis. 178:707-716. [DOI] [PubMed] [Google Scholar]
- 4.Abadi, J., and L. Pirofski. 1999. Antibodies reactive with the cryptococcal capsular polysaccharide glucuronoxylomannan are present in sera from children with and without HIV infection. J. Infect. Dis. 180:915-919. [DOI] [PubMed] [Google Scholar]
- 5.Aberg, J. A., R. W. Price, D. M. Heeren, and B. Bredt. 2002. A pilot study of the discontinuation of antifungal therapy for disseminated cryptococcal disease in patients with acquired immunodeficiency syndrome, following immunologic response to antiretroviral therapy. J. Infect. Dis. 185:1179-1182. [DOI] [PubMed] [Google Scholar]
- 6.Altman, D. G. 1999. Practical statistics for medical research, p. 165-175. Chapman & Hall/CRC Press, New York, N.Y.
- 7.Banerjee, U., K. Datta, T. Majumdar, and K. Gupta. 2001. Cryptococcosis in India: the awakening of a giant? Med. Mycol. 39:51-67. [DOI] [PubMed] [Google Scholar]
- 8.Batzloff, M. R., W. A. Hayman, M. R. Davies, M. Zeng, S. Pruksakorn, E. R. Brandt, and M. F. Good. 2003. Protection against group A streptococcus by immunization with J8-diphtheria toxoid: contribution of J8- and diphtheria toxoid-specific antibodies to protection. J. Infect. Dis. 187:1598-1608. [DOI] [PubMed] [Google Scholar]
- 9.Bauer, S., C. J. Kirschning, H. Hacker, V. Redecke, S. Hausmann, S. Akira, H. Wagner, and G. B. Lipford. 2001. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc. Natl. Acad. Sci. USA 98:9237-9242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beenhouwer, D. O., R. J. May, P. Valadon, and M. D. Scharff. 2002. High affinity mimotope of the polysaccharide capsule of Cryptococcus neoformans identified from an evolutionary phage peptide library. J. Immunol. 169:6992-6999. [DOI] [PubMed] [Google Scholar]
- 11.Beenhouwer, D. O., P. Valadon, R. May, and M. D. Scharff. 2000. Peptide mimicry of the polysaccharide capsule of Cryptococcus neoformans, p. 143-160. In M. W. Cunningham and R. S. Fujinami (ed.), Molecular mimicry, microbes, and autoimmunity. ASM Press, Washington, D.C.
- 12.Casadevall, A., M. Feldmesser, and L. A. Pirofski. 2002. Induced humoral immunity and vaccination against major human fungal pathogens. Curr. Opin. Microbiol. 5:386-391. [DOI] [PubMed] [Google Scholar]
- 13.Casadevall, A., and L. Pirofski. 2001. Adjunctive immune therapy for fungal infections. Clin. Infect. Dis. 33:1048-1057. [DOI] [PubMed] [Google Scholar]
- 14.Casadevall, A., and L. Pirofski. 2003. Antibody mediated regulation of cellular immunity and the inflammatory response. Trends Immunol. 24:474-478. [DOI] [PubMed] [Google Scholar]
- 15.Chang, Q., Z. Zhong, A. Lees, M. Pekna, and L. Pirofski. 2002. Structure-function relationships for human antibodies to pneumococcal capsular polysaccharide from transgenic mice with human immunoglobulin loci. Infect. Immun. 70:4977-4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cooper, L. J., J. C. Schimenti, D. D. Glass, and N. S. Greenspan. 1991. H chain C domains influence the strength of binding of IgG for streptococcal group A carbohydrate. J. Immunol. 146:2659-2663. [PubMed] [Google Scholar]
- 17.DeShaw, M., and L. Pirofski. 1995. Antibodies to Cryptococcus neoformans capsular polysaccharide glucuronoxylomannan are ubiquitous in the serum of HIV+ and HIV− individuals. Clin. Exp. Immunol. 99:425-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Devi, S. J. 1996. Preclinical efficacy of a glucuronoxylomannan-tetanus toxoid conjugate vaccine of Cryptococcus neoformans in a murine model. Vaccine 14:841-844. [DOI] [PubMed] [Google Scholar]
- 19.Dromer, F., J. Charreire, A. Contrepois, C. Carbon, and P. Yeni. 1987. Protection of mice against experimental cryptococcosis by an anti-Cryptococcus neoformans monoclonal antibody. Infect. Immun. 55:749-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dromer, F., S. Mathoulin, B. Dupont, L. Letenneur, O. Ronin, et al. 1996. Individual and environmental factors associated with infection due to Cryptococcus neoformans serotype D. Clin. Infect. Dis. 23:91-96. [DOI] [PubMed] [Google Scholar]
- 21.Fleuridor, R., A. Lees, and L. Pirofski. 2001. A cryptococcal capsular polysaccharide mimotope prolongs the survival of mice with Cryptococcus neoformans infection. J. Immunol. 166:1087-1096. [DOI] [PubMed] [Google Scholar]
- 22.Fleuridor, R., R. H. Lyles, and L. Pirofski. 1999. Quantitative and qualitative differences in the serum antibody profiles of HIV-infected persons with and without Cryptococcus neoformans meningitis. J. Infect. Dis. 180:1526-1536. [DOI] [PubMed] [Google Scholar]
- 23.Fleuridor, R., Z. Zhong, and L. Pirofski. 1998. A human IgM monoclonal antibody prolongs survival of mice with lethal cryptococcosis. J. Infect. Dis. 178:1213-1216. [DOI] [PubMed] [Google Scholar]
- 24.Garcia-Hermoso, D., G. Janbon, and F. Dromer. 1999. Epidemiologic evidence for dormant Cryptococcus neoformans infection. J. Clin. Microbiol. 37:3204-3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldman, D. L., H. Khine, J. Abadi, D. L. Lindenberg, L. Pirofski, R. Niang, and A. Casadevall. 2001. Serologic evidence for Cryptococcus neoformans infection in early childhood. Pediatrics 107:e66. [DOI] [PubMed] [Google Scholar]
- 26.Grabowska, A. M., R. Jennings, P. Laing, M. Darsley, C. L. Jameson, L. Swift, and W. L. Irving. 2000. Immunisation with phage displaying peptides representing single epitopes of the glycoprotein G can give rise to partial protective immunity to HSV-2. Virology 269:47-53. [DOI] [PubMed] [Google Scholar]
- 27.Granoff, D. M., P. G. Shackelford, S. J. Holmes, A. H. Lucas, et al. 1993. Variable region expression in the antibody responses of infants vaccinated with Haemophilus influenzae type b polysaccharide-protein conjugates. Description of a new lambda light chain-associated idiotype and the relation between idiotype expression, avidity, and vaccine formulation. J. Clin. Investig. 91:788-796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grothaus, M. C., N. Srivastava, S. L. Smithson, T. Kieber-Emmons, D. B. Williams, G. M. Carlone, and M. A. J. Westerink. 2000. Selection of an immunogenic peptide mimic of the capsular polysaccharide of Neisseria meningitidis serogroup A using a peptide display library. Vaccine 18:1253-1263. [DOI] [PubMed] [Google Scholar]
- 29.Harris, S. L., A. S. Dagtas, and B. Diamond. 2002. Regulating the isotypic and idiotypic profile of an anti-PC antibody response: lessons from peptide mimics. Mol. Immunol. 39:263-272. [DOI] [PubMed] [Google Scholar]
- 30.Harris, S. L., M. K. Park, M. H. Nahm, and B. Diamond. 2000. Peptide mimic of phosphorylcholine, a dominant epitope found on Streptococcus pneumoniae. Infect. Immun. 68:5778-5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hemachandra, S., K. Kamboj, J. Copfer, G. Pier, L. L. Green, and J. R. Schreiber. 2001. Human monoclonal antibodies against Pseudomonas aeruginosa lipopolysaccharide derived from transgenic mice containing megabase human immunoglobulin loci are opsonic and protective against fatal pseudomonas sepsis. Infect. Immun. 69:2223-2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hemmi, H., O. Takeuchi, T. Kawai, T. Kaisho, S. Sato, H. Sanjo, M. Matsumoto, K. Hoshino, H. Wagner, K. Takeda, and S. Akira. 2000. A Toll-like receptor recognizes bacterial DNA. Nature 408:740-745. [DOI] [PubMed] [Google Scholar]
- 33.Husain, S., M. M. Wagener, and N. Singh. 2001. Cryptococcus neoformans infection in organ transplant recipients: variables influencing clinical characteristics and outcome. Emerg. Infect. Dis. 7:375-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ikeda, R., T. Shinoda, Y. Fukazawa, and L. Kaufman. 1982. Antigenic characterization of Cryptococcus neoformans serotypes and its application to serotyping of clinical isolates. J. Clin. Microbiol. 16:22-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jung, J., A. K. Yi, X. Zhang, J. Choe, L. Li, and Y. S. Choi. 2002. Distinct response of human B cell subpopulations in recognition of an innate immune signal, CpG DNA. J. Immunol. 169:2368-2373. [DOI] [PubMed] [Google Scholar]
- 36.Kirkham, P. M., R. F. Mortari, J. A. Newton, and H. W. Schroeder. 1992. Immunoglobulin VH clan and family identity predicts variable domain structure and may influence antigen binding. EMBO J. 11:603-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koguchi, Y., and K. Kawakami. 2002. Cryptococcal infection and Th1-Th2 cytokine balance. Int. Rev. Immunol. 21:423-438. [DOI] [PubMed] [Google Scholar]
- 38.Li, W. M., M. B. Bally, and M. P. Schutze-Redelmeier. 2001. Enhanced immune response to T-independent antigen by using CpG oligodeoxynucleotides encapsulated in liposomes. Vaccine 20:148-157. [DOI] [PubMed] [Google Scholar]
- 39.Linton, P. J. L., D. J. Decker, and N. R. Klinman. 1989. Primary antibody-forming cells and secondary B cells are generated from separate precursor cell subpopulations. Cell 59:1049-1059. [DOI] [PubMed] [Google Scholar]
- 40.Lu, Y. F., M. Singh, and J. Cerny. 2001. Canonical germinal center B cells may not dominate the memory response to antigenic challenge. Int. Immunol. 13:643-655. [DOI] [PubMed] [Google Scholar]
- 41.Lucas, A. H., and D. M. Granoff. 1995. Functional differences in idiotypically defined IgG1 anti-polysaccharide antibodies elicited by vaccination with Haemophilus influenzae type b polysaccharide-protein conjugates. J. Immunol. 154:4195-4202. [PubMed] [Google Scholar]
- 42.McLean, G. R., M. Torres, N. Elguezabal, A. Nakouzi, and A. Casadevall. 2002. Isotype can affect the fine specificity of an antibody for a polysaccharide antigen. J. Immunol. 169:1379-1386. [DOI] [PubMed] [Google Scholar]
- 43.Mendez, M. J., L. L. Green, J. R. Corvalan, X. C. Jia, C. E. Maynard-Currie, X. D. Yang, M. L. Gallo, D. M. Louie, D. V. Lee, K. L. Erickson, J. Luna, C. M. Roy, Abderrahim, H., F. Kirschenbaum, M. Noguchi, D. H. Smith, A. Fukushima, J. F. Hales, Finer, M. H., C. G. Davis, K. M. Zsebo, and A. Jakobovits. 1997. Functional transplant of megabase human immunoglobulin loci recapitulates human antibody response in mice. Nat. Gen. 15:146-156. [DOI] [PubMed] [Google Scholar]
- 44.Mond, J. J., A. Lees, and C. M. Snapper. 1995. T cell-independent antigens type 2. Annu. Rev. Immunol. 13:655-692. [DOI] [PubMed] [Google Scholar]
- 45.Monzavi-Karbassi, B., G. Cunto-Amesty, P. Luo, and T. Kieber-Emmons. 2002. Peptide mimotopes as surrogate antigens of carbohydrates in vaccine discovery. Trends Biotechnol. 20:207-214. [DOI] [PubMed] [Google Scholar]
- 46.Monzavi-Karbassi, B., S. Shamloo, M. Kieber-Emmons, F. Jousheghany, P. Luo, K. Y. Lin, G. Cunto-Amesty, D. B. Weiner, and T. Kieber-Emmons. 2003. Priming characteristics of peptide mimotopes of carbohydrate antigens. Vaccine 21:753-760. [DOI] [PubMed] [Google Scholar]
- 47.Mukherjee, J., A. Casadevall, and M. D. Scharff. 1993. Molecular characterization of the antibody responses to Cryptococcus neoformans infection and glucuronoxylomannan-tetanus toxoid conjugate immunization. J. Exp. Med. 177:1105-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mukherjee, J., T. R. Kozel, and A. Casadevall. 1998. Monoclonal antibodies reveal additional epitopes of serotype D Cryptococcus neoformans capsular glucuronoxylomannan that elicit protective antibodies. J. Immunol. 161:3557-3567. [PubMed] [Google Scholar]
- 49.Mukherjee, J., G. Nussbaum, M. D. Scharff, and A. Casadevall. 1995. Protective and nonprotective monoclonal antibodies to Cryptococcus neoformans originating from one B cell. J. Exp. Med. 181:405-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mukherjee, J., M. D. Scharff, and A. Casadevall. 1992. Protective murine monoclonal antibodies to Cryptococcus neoformans. Infect. Immun. 60:4534-4541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakouzi, A., P. Valadon, J. D. Nosanchuk, N. Green, and A. Casadevall. 2001. Molecular basis for immunoglobulin M specificity to epitopes in Cryptococcus neoformans polysaccharide that elicit protective and nonprotective antibodies. Infect. Immun. 69:3398-3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nussbaum, G., W. Cleare, A. Casadevall, M. D. Scharff, and P. Valadon. 1997. Epitope location in the Cryptococcus neoformans capsule is a determinant of antibody efficacy. J. Exp. Med. 185:685-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Olszewska, W., O. E. Obeid, and M. W. Steward. 2000. Protection against measles-virus induced encephalitis by anti-mimotope antibodies: the role of antibody affinity. Virology 272:98-105. [DOI] [PubMed] [Google Scholar]
- 54.Perfect, J. R., and A. Casadevall. 2002. Cryptococcosis. Infect. Dis. Clin. North Am. 16:837-874. [DOI] [PubMed] [Google Scholar]
- 55.Pirofski, L. 2001. Polysaccharides, mimotopes and vaccines for encapsulated pathogens. Trends Microbiol. 9:445-452. [DOI] [PubMed] [Google Scholar]
- 56.Pirofski, L., and A. Casadevall. 1998. The use of licensed vaccines for active immunization of the immunocompromised host. Clin. Microbiol. Rev. 11:1-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pirofski, L., R. Lui, M. DeShaw, A. B. Kressel, and Z. Zhong. 1995. Analysis of human monoclonal antibodies elicited by vaccination with a Cryptococcus neoformans glucuronoxylomannan capsular polysaccharide vaccine. Infect. Immun. 63:3005-3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Puumalainen, T., R. Dagan, T. Wuorimaa, R. Zeta-Capeding, M. Lucero, J. Ollgren, H. Kayhty, and H. Nohynek. 2003. Greater antibody responses to an eleven valent mixed carrier diphtheria- or tetanus-conjugated pneumococcal vaccine in Filipino than in Finnish or Israeli infants. Pediatr. Infect. Dis. J. 22:141-149. [DOI] [PubMed] [Google Scholar]
- 59.Ramisse, F., P. Binder, M. Szatanik, and J.-M. Alonso. 1996. Passive and active immunotherapy for experimental pneumococcal pneumonia by polyvalent human immunoglobulin or F(ab′)2 fragments administered intranasally. J. Infect. Dis. 173:1123-1128. [DOI] [PubMed] [Google Scholar]
- 60.Ramisse, F., M. Szatanik, P. Binder, and J.-M. Alonso. 1993. Passive local immunotherapy of experimental staphylococcal pneumonia with human intravenous immunoglobulin. J. Infect. Dis. 168:1030-1033. [DOI] [PubMed] [Google Scholar]
- 61.Ravetch, J. V., and J. P. Kinet. 1991. Fc receptors. Annu. Rev. Immunol. 9:457-492. [DOI] [PubMed] [Google Scholar]
- 62.Russell, N., J. R. Corvalan, M. L. Gallo, C. G. Davis, and L. Pirofski. 2000. Production of protective human antipneumococcal antibodies by transgenic mice with human immunoglobulin loci. Infect. Immun. 68:1820-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Saeland, E., G. Vidarsson, and I. Jonsdottir. 2000. Pneumococcal pneumonia and bacteremia model in mice for the analysis of protective antibodies. Microb. Pathog. 29:81-91. [DOI] [PubMed] [Google Scholar]
- 64.Saeland, E., G. Vidarsson, J. H. Leusen, E. Van Garderen, M. H. Nahm, H. Vile-Weekhout, V. Walraven, A. M. Stemerding, J. S. Verbeek, G. T. Rijkers, W. Kuis, E. A. Sanders, and J. G. Van de Winkel. 2003. Central role of complement in passive protection by human IgG1 and IgG2 anti-pneumococcal antibodies in mice. J. Immunol. 170:6158-6164. [DOI] [PubMed] [Google Scholar]
- 65.Schuurman, J., R. Van Ree, G. J. Perdok, H. R. Van Doorn, K. Y. Tan, and R. C. Aalberse. 1999. Normal human immunoglobulin G4 is bispecific: it has two different antigen-combining sites. Immunology 97:693-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shin, J. S., J. Yu, J. Lin, L. Zhong, K. L. Bren, and M. H. Nahm. 2002. Peptide mimotopes of pneumococcal capsular polysaccharide of 6B serotype: a peptide mimotope can bind to two unrelated antibodies. J. Immunol. 168:6273-6278. [DOI] [PubMed] [Google Scholar]
- 67.Sigurdardottir, S. T., G. Ingolfsdottir, K. Davidsdottir, T. Gudnason, S. Kjartansson, K. G. Kristinsson, F. Bailleux, O. Leroy, and I. Jonsdottir. 2002. Immune response to octavalent diphtheria- and tetanus-conjugated pneumococcal vaccines is serotype- and carrier-specific: the choice for a mixed carrier vaccine. Pediatr. Infect. Dis. J. 21:548-554. [DOI] [PubMed] [Google Scholar]
- 68.Souza, M. C., M. Correa, S. R. Almeida, J. D. Lopes, and Z. P. Camargo. 2001. Immunostimulatory DNA from Paracoccidioides brasiliensis acts as T-helper 1 promoter in susceptible mice. Scand. J. Immunol. 54:348-356. [DOI] [PubMed] [Google Scholar]
- 69.Spitzer, E. D., S. G. Spitzer, L. F. Freundlich, and A. Casadevall. 1993. Persistence of initial infection in recurrent Cryptococcus neoformans meningitis. Lancet 341:595-596. [DOI] [PubMed] [Google Scholar]
- 70.Subramaniam, K. S., R. Segal, R. H. Lyles, M. C. Rodriguez-Barradas, and L. A. Pirofski. 2003. Qualitative change in antibody responses of human immunodeficiency virus-infected individuals to pneumococcal capsular polysaccharide vaccination associated with highly active antiretroviral therapy. J. Infect. Dis. 187:758-768. [DOI] [PubMed] [Google Scholar]
- 71.Sundstrom, J. B., and R. Cherniak. 1992. The glucuronoxylomannan of Cryptococcus neoformans serotype A is a type 2 T-cell-independent antigen. Infect. Immun. 60:4080-4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Taborda, C., and A. Casadevall. 2001. Immunoglobulin M efficacy against Cryptococcus neoformans: mechanism, dose dependence, and prozone-like effects in passive protection experiments. J. Immunol. 166:2100-2107. [DOI] [PubMed] [Google Scholar]
- 73.Taborda, C. P., J. Rivera, O. Zaragoza, and A. Casadevall. 2003. More is not necessarily better: prozone-like effects in passive immunization with IgG. J. Immunol. 170:3621-3630. [DOI] [PubMed] [Google Scholar]
- 74.Valadon, P., G. Nussbaum, J. Oh, and M. D. Scharff. 1998. Aspects of antigen mimicry revealed by immunization with a peptide mimetic of Cryptococcus neoformans polysaccharide. J. Immunol. 161:1829-1836. [PubMed] [Google Scholar]
- 75.Vargas-Madrazo, E., F. Lara-Ochoa, and J. C. Almagro. 1995. Canonical structure repertoire of the antigen-binding site of immunoglobulins suggests strong geometrical restrictions associated to the mechanism of immune recognition. J. Mol. Biol. 254:497-504. [DOI] [PubMed] [Google Scholar]
- 76.von Hunolstein, C., S. Mariotti, R. Teloni, G. Alfarone, G. Romagnoli, G. Orefici, and R. Nisini. 2001. The adjuvant effect of synthetic oligodeoxynucleotide containing CpG motif converts the anti-Haemophilus influenzae type b glycoconjugates into efficient anti-polysaccharide and anti-carrier polyvalent vaccines. Vaccine 19:3058-3066. [DOI] [PubMed] [Google Scholar]
- 77.Weeratna, R. D., M. J. McCluskie, Y. Xu, and H. L. Davis. 2000. CpG DNA induces stronger immune responses with less toxicity than other adjuvants. Vaccine 18:1755-1762. [DOI] [PubMed] [Google Scholar]
- 78.Yoo, E. M., L. A. Wims, L. A. Chan, and S. L. Morrison. 2003. Human IgG2 can form covalent dimers. J. Immunol. 170:3134-3138. [DOI] [PubMed] [Google Scholar]
- 79.Young, N. M., M. A. J. Gidney, B.-M. E. Gudmundsson, C. R. MacKenzie, R. To, D. C. Watson, and D. R. Bundle. 1999. Molecular basis for the lack of mimicry of Brucella polysaccharide antigens by Ab2gamma antibodies. Mol. Immunol. 36:339-347. [DOI] [PubMed] [Google Scholar]
- 80.Zhang, H., Z. Zhong, and L. Pirofski. 1997. Peptide epitopes recognized by a human anticryptococcal glucuronoxylomannan antibody. Infect. Immun. 65:1158-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhong, Z., T. Burns, Q. Chang, M. Carroll, and L. Pirofski. 1999. Molecular and functional characteristics of a protective human monoclonal antibody to serotype 8 Streptococcus pneumoniae capsular polysaccharide. Infect. Immun. 67:4119-4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhong, Z., and L. Pirofski. 1996. Opsonization of Cryptococcus neoformans by human antiglucuronoxylomannan antibodies. Infect. Immun. 64:3446-3450. [DOI] [PMC free article] [PubMed] [Google Scholar]