Abstract
Antibody-mediated neutralization of pertussis toxin-induced proliferation of human peripheral blood mononuclear cells (PBMC) was assessed using alamarBlue and compared with results from the Chinese hamster ovary (CHO) cell assay using sera from vaccinated adults and convalescent children. Neutralization values for the CHO assay were similar for vaccinated and convalescent subjects; however. the convalescent group had higher titers in the PBMC assay. Results for pertussis toxin neutralization with the CHO assay appear to be distinct from those with the PBMC assay.
In spite of high vaccination rates, the number of cases of pertussis in the United States has increased steadily since the 1980s (39). Bordetella pertussis, the causative agent of whooping cough, is a gram-negative bacterium that remains localized to the respiratory tract throughout infection. However, systemic effects of the infection, such as lymphocytosis, demonstrate a toxin-mediated component of the disease (23). Pertussis toxin, one of the toxins of B. pertussis, is essential for virulence (8, 37) and has been included as an antigen in all acellular vaccines. The acellular vaccines are effective in preventing severe disease; however, they appear to be less effective at preventing mild disease. Identifying serological correlates of immunity to B. pertussis may facilitate development of improved pertussis vaccines.
Antibody levels can be measured in a quantitative or a functional manner. Associations between quantitative antibody titers to the component antigens and protective immunity have been studied. Storsaeter et al. (30) and Cherry et al. (4) found that protection from pertussis correlated with levels of circulating antibodies to pertactin and fimbriae and to a lesser extent to pertussis toxin but that protection did not correlate with levels of antibody to filamentous hemagglutinin (FHA). However, the pertussis toxoid vaccines used in the Sweden-Göteborg trial (35) and in a more recent study (34) were shown to be efficacious, indicating that protection against severe disease can be achieved without pertactin or fimbriae. A combined pertussis toxoid-FHA vaccine seemed to be superior to a monocomponent pertussis toxoid vaccine in a long-term follow-up study (31), suggesting a role for FHA in acellular vaccines.
None of the aforementioned studies evaluated the mode of action of protective antibodies or the ratio of protective to nonprotective antibodies. Without this information, it is unlikely that the true serological correlate of immunity can be gleaned. A functional assay such as the one being presented might provide a better correlate.
PBMC mitogenicity assay.
We wanted to develop an assay that would measure the ability of human antibodies to neutralize the activity of pertussis toxin on human cells that are known to be targeted in human disease. A simple nonradioactive proliferation assay to assess the ability of antibody to neutralize pertussis toxin-induced mitogenicity for peripheral blood mononuclear cells (PBMC) was developed by using alamarBlue as an indicator of metabolic activity. alamarBlue has been used to detect lymphocyte proliferation with the use of cell lines and both murine and human PBMC (1, 5) and is a useful alternative to [3H]thymidine incorporation assays (1, 18). It is both a colorimetric and fluorimetric dye. The oxidized form is blue and nonfluorescent. When added to cells, it is converted to a reduced form by mitochondrial enzyme activity (6). Reduced alamarBlue is pink and fluorescent.
PBMC were obtained from healthy human donors. Blood was collected by venipuncture, combined with 0.11 ml of 3.8% sodium citrate per ml of blood to prevent coagulation, and centrifuged for 20 min at 100 × g to remove plasma. The blood was incubated with 0.6% dextran (final concentration) in 0.9% saline for 1 h. PBMC were further separated by centrifugation at 450 × g for 25 min through a Ficoll-Paque (Amersham Pharmacia, Uppsala, Sweden) gradient. Cell numbers were determined with a hemocytometer, and viability was assessed by trypan blue exclusion. PBMC were suspended in RPMI 1640 (Gibco Invitrogen, Carlsbad, Calif.) containing 10% fetal bovine serum, 100 U of penicillin/ml, 100 μg of streptomycin/ml, and 2 mM l-glutamine and added to each well of a 96-well tissue culture-treated plate (Corning Costar, Acton, Mass.).
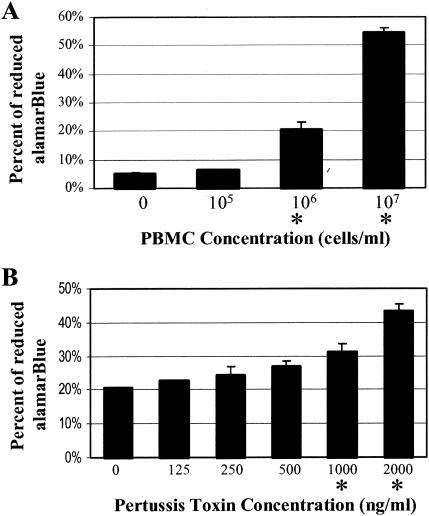
In preliminary studies, various concentrations of PBMC were plated. alamarBlue (10 μl) (Trek Diagnostic Systems, Westlake, Ohio) was added, and the PBMC were incubated for 6 h at 37°C in a 5% CO2 atmosphere. Absorbance was measured at 570 and 595 nm for each well, and the percent chemical reduction of alamarBlue was calculated according to the manufacturer's protocols (Fig. 1A). Increased cell density resulted in increased chemical reduction of alamarBlue. Cell densities of 106 cells/ml and higher displayed a significant increase (P < 0.05) in chemical reduction of alamarBlue relative to that for the control media. The cells were plated at 106 cells/ml for all subsequent assays.
FIG. 1.
Detection by alamarBlue of PBMC proliferation due to pertussis toxin. (A) PBMC were plated at the indicated density and incubated for 3 days. (B) Pertussis toxin was added as indicated to initial cell populations of 106 cells/ml, and the cells were incubated for 3 days. The percent chemical reduction of alamarBlue compared to that for no-cell controls was determined. Data are expressed as the mean ± standard deviation of at least three independent trials. An asterisk denotes a significant difference (Student t test, P < 0.05) from the results for no-toxin controls.
The ability of pertussis toxin to increase cell numbers was examined (Fig. 1B). Pertussis toxin or purified B-pentamer of pertussis toxin (List Biological Laboratories, Campbell, Calif.) was added to the PBMC seeded in 96-well tissue culture plates and incubated at 37°C for 3 days in a 5% CO2 atmosphere. Cell density was determined by using alamarBlue as described above. Increasing concentrations of pertussis toxin exhibited a dose-dependent increase in cell numbers, becoming measurably significant (P < 0.05) at pertussis toxin concentrations of 1,000 and 2,000 ng/ml (Fig. 1B). In studies using [3H]thymidine incorporation assays, lower concentrations of pertussis toxin have been shown to mediate proliferation (15), but we used a pertussis toxin concentration of 2,000 ng/ml for all of the alamarBlue assays.
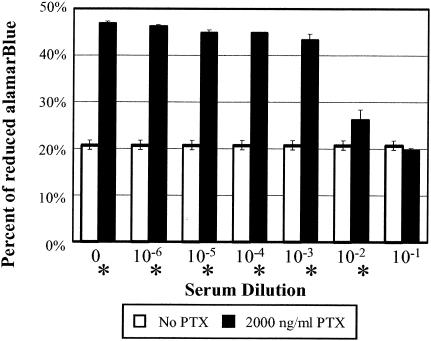
To assess antibody neutralization, pertussis toxin was incubated with serial dilutions of a human serum sample known to have high titers of antibody to pertussis toxin before being added to PBMC (Fig. 2). The pertussis toxin was diluted to 36 ng/μl in Hanks' balanced salt solution (BioWhittaker, Rockland, Md.) buffered with 10 mM HEPES (Sigma, St. Louis, Mo.), and 10 μl was added to 20 μl of serial dilutions of sera in Hanks' balanced salt solution buffered with 10 mM HEPES and incubated at 37°C for 1 h with agitation. The pertussis toxin-sera solutions (20 μl) were then added to the PBMC seeded in 96-well tissue culture plates and incubated at 37°C for 3 days in a 5% CO2 atmosphere.
FIG. 2.
Neutralization of pertussis toxin by human serum. Pertussis toxin was incubated with increasing concentrations of human serum as indicated and added to PBMC at a concentration of 2,000 ng/ml. The cells were incubated for 3 days, and toxin neutralization was observed as production of less-reduced alamarBlue. Data are expressed as the mean ± standard deviation of at least three independent trials. An asterisk denotes a significant difference (Student t test, P < 0.05) from results for the no-toxin control wells at the same serum concentration. PTX, pertussis toxin
The samples (assayed in triplicate) were compared to the negative (cells with or without human test serum in the absence of pertussis toxin) and positive (cells treated with pertussis toxin in the absence of human test serum) controls. A decrease in mitogenicity was observed at high serum concentrations. Similar experiments using the purified B-pentamer of pertussis toxin yielded the same results (data not shown), consistent with previous reports that B-pentamer alone induces PBMC proliferation (32, 33). Whole toxin was used for all subsequent assays in order to assess antibodies to the S1 subunit that may affect B-pentamer activity. Pertussis toxin neutralization was defined as the lowest serum concentration that resulted in a 50% reduction of the mitogenic response based on the difference in the percentages of alamar Blue reduction between the positive control (pertussis toxin without serum) and the negative control (serum without pertussis toxin).
Determination of pertussis toxin antibody and neutralization titers in human sera.
Two sources of human sera were evaluated for antibody responses to pertussis toxin. One group consisted of pre- and postvaccination sera from a subset of volunteers in the APERT study, a prospective, randomized, double-blind trial conducted at eight National Institutes of Health study sites in the United States. Over a 2-year period, 2,781 subjects aged 15 to 65 were recruited and randomized to receive either a three-component (FHA, pertussis toxoid, and pertactin) acellular pertussis vaccine lacking diphtheria or tetanus antigens manufactured by SmithKline Beecham or a hepatitis A vaccine. For the study reported here, volunteers recruited at the Cincinnati site agreed to donate a larger volume of blood (approximately 10 ml) at pre- and postimmunization (approximately 30 days later) to permit a more in-depth characterization of their immune responses. We obtained 34 paired pre- and postimmunization samples as part of the blinded study, 15 from volunteers who received the pertussis vaccine.
The second group of sera was collected from culture-confirmed pertussis-infected children (19). The subjects were identified through ongoing surveillance at the Cincinnati Children's Hospital Medical Center. Following informed consent and assent, 51 subjects with culture-confirmed pertussis were recruited to participate in the study, which was approved by the Institutional Review Boards of the Cincinnati Children's Hospital Medical Center and the University of Cincinnati. Serum samples were collected as soon as possible following diagnosis. The subjects ranged in age from 6 to 17, with a mean age of 11.5 years. All had received pertussis vaccine, and 46 of 51 had completed the recommended series of pertussis immunizations. The time between initiation of coughing and collection of the serum sample was recorded for 48 of 51 subjects. The mean value was 59 days, with a minimum of 16 days and a maximum of 145 days. Thus, these sera reflect convalescent responses more accurately than acute responses. All serum samples used in this study were heat-inactivated at 56°C for 30 min to remove complement activity.
Relationships between different measures of antibody response to pertussis toxin.
Neutralization of pertussis toxin toxicity to Chinese hamster ovary (CHO) cells was determined as previously described (7, 10). An enzyme-linked immunosorbent assay (ELISA) was used to measure immunoglobulin G (IgG) antibodies to pertussis toxin. A Food and Drug Administration reference serum (17), control serum, and subject serum specimens were added to each plate, and ELISA units were computed using UnitCalc Software (Stockholm, Sweden) based on the reference line method. IgG titers to pertussis toxin were determined by ELISA for the subjects enrolled in the vaccine study, and pertussis toxin neutralization in the CHO cell and PBMC assays was determined for all samples. The logarithmic means were compared (Table 1). A statistically significant (P < 0.05) increase in IgG titers to pertussis toxin was observed following immunization, with mean values increasing by more than 10-fold. Mean postvaccination titers were significantly greater than prevaccination titers (P < 0.05) for pertussis toxin neutralization as determined by either the CHO cell cytotoxicity assay or the PBMC mitogenicity assay. When the two immune groups (postvaccine and convalescent) were compared, mean neutralization values determined by the CHO assay did not differ (P = 0.25). In contrast, the mean neutralization of pertussis toxin determined by the PBMC assay was significantly greater for the convalescent group than for the postvaccination group (P < 0.05).
TABLE 1.
Mean logarithmic titers to pertussis toxin for different study groups, as determined by three different assays
| Assay | Study group
|
||
|---|---|---|---|
| Prevaccine | Postvaccine | Convalescent | |
| IgG-ELISA | 0.51 ± 0.03 | 1.75 ± 0.08a | |
| CHO neutralization | 1.94 ± 0.20 | 3.85 ± 0.11a | 4.04 ± 0.11 |
| PBMC neutralization | 1.34 ± 0.04 | 1.47 ± 0.03a | 1.68 ± 0.03b |
Postvaccination titers significantly greater than prevaccination titers (P < 0.05).
Convalescent titers significantly greater than postvaccination titers (P < 0.05).
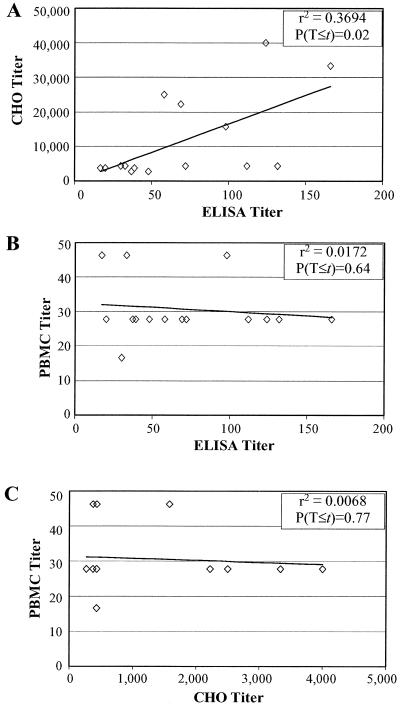
Correlations between the three measures of antibody responses to pertussis toxin (ELISA, neutralization titers for CHO cells, and PBMC) were compared for the immunized and convalescent individuals. For the postvaccination samples, pertussis toxin neutralization as determined by CHO cell cytotoxicity was plotted against the anti-pertussis toxin titer determined by ELISA (Fig. 3A). A weak correlation based on the Pearson product-moment correlation coefficient of determination (r2 = 0.369) was found to be significant (P = 0.02) when evaluated against a random t distribution (df = 13; df = n − 2 for all correlation data presented). Furthermore, the correlation (r2 = 0.562) remained significant (P < 0.05, df = 28) with a combined pre- and postvaccination data set.
FIG. 3.
Correlation of titers for postvaccination sera. Each data point represents results from one individual, with identical values represented by a single point. The statistical significance of the correlation was determined by the t value of the Pearson product-moment correlation coefficient with df = n − 2. (A) CHO assay titers plotted versus ELISA titers for postvaccination sera. (B) PBMC titers plotted versus ELISA titers for postvaccination sera. (C) PBMC assay titers plotted versus CHO cell assay titers for postvaccination sera. T, titer.
For the postvaccination samples, neutralization values in the PBMC assay were plotted against ELISA titers and neutralization values in the CHO assay. PBMC values and ELISA values for the postvaccine sera (Fig. 3B) did not display a significant (P = 0.64, df = 13) correlation (r2 = 0.017), and a lack of correlation was also observed for the combined data set (r2 = 0.064, P = 0.18, df = 28). The correlation (r2 = 0.007) for PBMC values and CHO values for postvaccination samples (Fig. 3C) was also not significant (P = 0.77, df = 13), as was the correlation (r2 = 0.039) for the combined data set (P = 0.30, df = 28).
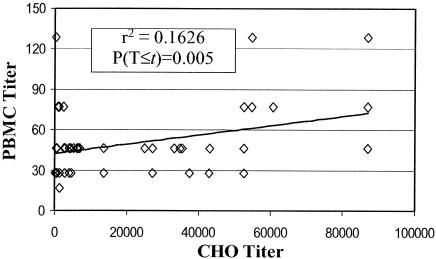
Pertussis toxin neutralization titers for the convalescent samples, as determined by PBMC mitogenicity plotted against neutralization as determined by CHO cell cytotoxicity (Fig. 4), revealed a significant (P < 0.005, df = 49) correlation (r2 = 0.163), in contrast to neutralization titers for the postvaccination samples.
FIG. 4.
PBMC assay titers plotted versus CHO cell assay titers for convalescent individuals. Data are expressed and statistical significance was calculated as described in the legend to Fig. 3. T, titer.
Defining a serologic correlate of immunity to pertussis has been difficult. While recent studies have shown that levels of antibody to pertactin and fimbriae have some predictive value, levels of antibody to pertussis toxin were less predictive of protection (4, 30). Since immunization with pertussis toxoid appears to mediate protection from whooping cough (34, 35), the fact that the level of circulating antibodies to pertussis toxin did not correlate with protection suggests that protective antibodies were not being measured. Most studies have used gross titers determined by ELISA to measure antibody levels. In this study, sera from children with recent pertussis disease and adults participating in a pertussis vaccine study were characterized for pertussis toxin-neutralizing responses by two different functional assays.
A tremendous variation in response was observed for the immunized and convalescent individuals. ELISA values (determined only for the vaccine group) ranged from 17 to 166, neutralization titers in the CHO cell assay ranged from about 100 to 87,000, and neutralization titers in the PBMC assay ranged from 17 to 128. While a weak correlation was observed between ELISA and neutralization in the CHO assay for the vaccinated individuals, neutralization in the PBMC assay did not correlate with either ELISA titers (Fig. 3B) or neutralization titers in the CHO cell assay (Fig. 3C), and in fact all but 4 of the 15 individuals had the same PBMC titer. The highest titers in the PBMC assay were seen in convalescent-phase sera from individuals with pertussis infection. The three individuals with the highest titers in the PBMC assay had a huge variation in neutralization titers in the CHO assay, with a low of 278 to a high of 86,957 (Fig. 4). One possible explanation for the limited ability of sera from the vaccinated individuals to neutralize pertussis toxin in the PBMC assay compared to that from the convalescent group could be that pertussis toxoid is a less effective immunogen than pertussis toxin. While the whole-cell pertussis vaccine has been shown to contain active pertussis toxin, no one advocates using active pertussis toxin in the acellular vaccines. However, it may be possible to develop improved methods to inactivate the toxin and still retain critical epitopes.
Pertussis toxin is a complex AB5 toxin which is comprised of the enzymatically active A subunit, S1, and the binding B-pentamer, which is composed of subunits S2, S3, S4, and S5, found in a 1:1:2:1 ratio. The S1 subunit catalyzes the transfer of an ADP-ribosyl group to GTP-binding proteins of mammalian cells, disrupting intracellular signaling (11), which results in unchecked cyclic AMP production (11), reduced phosphatidylinositol hydrolysis (3), inhibition of arachidonate release (2), or altered calcium mobilization (21, 22). Many of the physiological effects of the toxin are known to be due to the ADP-ribosyltransferase activity of the S1 subunit (16, 20).
Classically, the B portion of AB toxins is thought to transport the A subunit to the target cell and not participate in toxicity. However, the B-pentamer of pertussis toxin has been shown to have activity in addition to its role in facilitating entry of S1 into the cytoplasm of the target cell. The B-pentamer is mitogenic for human T cells and murine splenocytes (32, 33), induces oxidation of glucose in rat adipocytes (33), alters macrophage adhesion (38), and mimics the effects of selectins on the regulation of leukocyte trafficking (24). A recent report showed that an S3-S4 dimer was as strongly mitogenic as the B-pentamer, and in vitro stimulation of naive lymphocytes by the S3-S4 dimer resulted in reversal of the normal CD4+/CD8+ T-cell ratio (12). The complex structure of the B-pentamer allows it to bind to multiple targets (9, 13, 24, 26-29, 36). Most of the receptor binding sites map to residues in S2 and S3, yet despite a 70% amino acid sequence identity, S2 and S3 mediate different binding specificities of the toxin (14). The presence of multiple binding sites suggests that antibodies to different epitopes may be needed to neutralize the different toxic activities of pertussis toxin.
The CHO cell cytotoxicity assay was the first in vitro assay for assessing neutralizing antibodies to pertussis toxin (7, 10). However, neutralization of pertussis toxin with the CHO cell assay is a poor predictor of protection from disease. In one study, monoclonal antibodies that protected mice from pertussis toxin-mediated lymphocytosis conferred protection from aerosol challenge, while monoclonal antibodies that neutralized pertussis toxin with the CHO cell assay did not mediate protection from aerosol challenge (25). On the molecular level, mutations at positions 101 and 102 of S2 and S3 abolish activity with the CHO cell assay without altering the ability of the mutant toxin to promote lymphocytosis or lymphocyte mitogenicity (15).
Mutagenesis studies (15) and biochemical studies (12) suggest that amino acids on the S3 subunit of pertussis toxin are required for pertussis toxin to bind to T cells and promote lymphocytosis and PBMC mitogenicity. Mutation of the unique tyrosine at position 82 of S3 reduced pertussis toxin activity in all toxin assays (15). Furthermore, the demonstration that purified S3-S4 dimer is sufficient to promote mitogenicity and alters T-cell ratios (12) suggests that antibodies to S3, perhaps tyrosine 82, may be critical in preventing lymphocytosis and other pertussis toxin-mediated manifestations observed in human disease. We suggest that maintaining the integrity of these epitopes in toxoided preparations of pertussis toxin may be critical for inducing an antibody response that will prevent these toxin-mediated manifestations as well as human disease.
While it is not possible to examine neutralization of pertussis toxin-mediated lymphocytosis in humans, the toxin-binding sites that are required for lymphocytosis are also required for PBMC mitogenicity. From this study, it is clear that ELISA and neutralization in the CHO and PBMC assays measure distinct antibodies to pertussis toxin. The ability of neutralizing antibody to pertussis toxin to serve as a serologic correlate of protection from whooping cough should be reevaluated in light of these results.
Acknowledgments
This work was supported by NIH Institute of Allergy and Infectious Diseases grant RO1 AI38415 to A.A.W, NO1-A1-45252 and NO1-2549 to D.I.B., and NO1-A1-25463 and NO-1-A1-45249 to J.I.W.
Editor: J. T. Barbieri
REFERENCES
- 1.Ahmed, S. A., R. M. Gogal, Jr., and J. E. Walsh. 1994. A new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: an alternative to [3H]thymidine incorporation assay. J. Immunol. Methods 170:211-224. [DOI] [PubMed] [Google Scholar]
- 2.Bokoch, G. M., and A. G. Gilman. 1984. Inhibition of receptor-mediated release of arachidonic acid by pertussis toxin. Cell 39:301-308. [DOI] [PubMed] [Google Scholar]
- 3.Brandt, S. J., R. W. Dougherty, E. G. Lapetina, and J. E. Niedel. 1985. Pertussis toxin inhibits chemotactic peptide-stimulated generation of inositol phosphates and lysosomal enzyme secretion in human leukemic (HL-60) cells. Proc. Natl. Acad. Sci. USA 82:3277-3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cherry, J. D., J. Gornbein, U. Heininger, and K. Stehr. 1998. A search for serologic correlates of immunity to Bordetella pertussis cough illnesses. Vaccine 16:1901-1906. [DOI] [PubMed] [Google Scholar]
- 5.de Fries, R., and M. Mitsuhashi. 1995. Quantification of mitogen induced human lymphocyte proliferation: comparison of alamarBlue assay to 3H-thymidine incorporation assay. J. Clin. Lab. Anal. 9:89-95. [DOI] [PubMed] [Google Scholar]
- 6.Fields, R. D., and M. V. Lancaster. 1993. Dual-attribute continuous monitoring of cell proliferation/cytotoxicity. Am. Biotechnol. Lab. 11:48-50. [PubMed] [Google Scholar]
- 7.Gillenius, P., E. Jaatmaa, P. Askelof, M. Granstrom, and M. Tiru. 1985. The standardization of an assay for pertussis toxin and antitoxin in microplate culture of Chinese hamster ovary cells. J. Biol. Stand. 13:61-66. [DOI] [PubMed] [Google Scholar]
- 8.Goodwin, M. S., and A. A. Weiss. 1990. Adenylate cyclase toxin is critical for colonization and pertussis toxin is critical for lethal infection by Bordetella pertussis in infant mice. Infect. Immun. 58:3445-3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heerze, L. D., P. C. Chong, and G. D. Armstrong. 1992. Investigation of the lectin-like binding domains in pertussis toxin using synthetic peptide sequences. Identification of a sialic acid binding site in the S2 subunit of the toxin. J. Biol. Chem. 267:25810-25815. [PubMed] [Google Scholar]
- 10.Hewlett, E. L., K. T. Sauer, G. A. Myers, J. L. Cowell, and R. L. Guerrant. 1983. Induction of a novel morphological response in Chinese hamster ovary cells by pertussis toxin. Infect. Immun. 40:1198-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katada, T., and M. Ui. 1982. Direct modification of the membrane adenylate cyclase system by islet-activating protein due to ADP-ribosylation of a membrane protein. Proc. Natl. Acad. Sci. USA 79:3129-3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Latif, R., N. Kerlero de Rosbo, T. Amarant, R. Rappuoli, G. Sappler, and A. Ben-Nun. 2001. Reversal of the CD4+/CD8+ T-cell ratio in lymph node cells upon in vitro mitogenic stimulation by highly purified, water-soluble S3-S4 dimer of pertussis toxin. Infect. Immun. 69:3073-3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lobet, Y., C. Feron, G. Dequesne, E. Simoen, P. Hauser, and C. Locht. 1993. Site-specific alterations in the B oligomer that affect receptor-binding activities and mitogenicity of pertussis toxin. J. Exp. Med. 177:79-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Locht, C., and J. M. Keith. 1986. Pertussis toxin gene: nucleotide sequence and genetic organization. Science 232:1258-1264. [DOI] [PubMed] [Google Scholar]
- 15.Loosmore, S., G. Zealey, S. Cockle, H. Boux, P. Chong, R. Yacoob, and M. Klein. 1993. Characterization of pertussis toxin analogs containing mutations in B-oligomer subunits. Infect. Immun. 61:2316-2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luttrell, L. M., E. L. Hewlett, G. Romero, and A. D. Rogol. 1988. Pertussis toxin treatment attenuates some effects of insulin in BC3H-1 murine myocytes. J. Biol. Chem. 263:6134-6141. [PubMed] [Google Scholar]
- 17.Lynn, F., G. F. Reed, and B. D. Meade. 1996. Collaborative study for the evaluation of enzyme-linked immunosorbent assays used to measure human antibodies to Bordetella pertussis antigens. Clin. Diagn. Lab. Immunol. 3:689-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maurer, H. R. 1981. Potential pitfalls of [3H]thymidine techniques to measure cell proliferation. Cell Tissue Kinet. 14:111-120. [DOI] [PubMed] [Google Scholar]
- 19.Mobberley-Schuman, P. S., B. L. Connelly, and A. A. Weiss. 2003. Phagocytosis of Bordetella pertussis incubated with convalescent serum. J. Infect. Dis. 187:1646-1653. [DOI] [PubMed] [Google Scholar]
- 20.Munoz, J. J., H. Arai, and R. L. Cole. 1981. Mouse-protecting and histamine-sensitizing activities of pertussigen and fimbrial hemagglutinin from Bordetella pertussis. Infect. Immun. 32:243-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakamura, T., and M. Ui. 1985. Simultaneous inhibitions of inositol phospholipid breakdown, arachidonic acid release, and histamine secretion in mast cells by islet-activating protein, pertussis toxin. A possible involvement of the toxin-specific substrate in the Ca2+-mobilizing receptor-mediated biosignaling system. J. Biol. Chem. 260:3584-3593. [PubMed] [Google Scholar]
- 22.Okajima, F., and M. Ui. 1984. ADP-ribosylation of the specific membrane protein by islet-activating protein, pertussis toxin, associated with inhibition of a chemotactic peptide-induced arachidonate release in neutrophils. A possible role of the toxin substrate in Ca2+-mobilizing biosignaling. J. Biol. Chem. 259:13863-13871. [PubMed] [Google Scholar]
- 23.Pittman, M. 1979. Pertussis toxin: the cause of the harmful effects and prolonged immunity of whooping cough. A hypothesis. Rev. Infect. Dis. 1:401-412. [DOI] [PubMed] [Google Scholar]
- 24.Rozdzinski, E., W. N. Burnette, T. Jones, V. Mar, and E. Tuomanen. 1993. Prokaryotic peptides that block leukocyte adherence to selectins. J. Exp. Med. 178:917-924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sato, H., Y. Sato, and I. Ohishi. 1991. Comparison of pertussis toxin (PT)-neutralizing activities and mouse-protective activities of anti-PT mouse monoclonal antibodies. Infect. Immun. 59:3832-3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saukkonen, K., W. N. Burnette, V. L. Mar, H. R. Masure, and E. I. Tuomanen. 1992. Pertussis toxin has eukaryotic-like carbohydrate recognition domains. Proc. Natl. Acad. Sci. USA 89:118-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmidt, M. A., and W. Schmidt. 1989. Inhibition of pertussis toxin binding to model receptors by antipeptide antibodies directed at an antigenic domain of the S2 subunit. Infect. Immun. 57:3828-3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmidt, W., and M. A. Schmidt. 1989. Mapping of linear B-cell epitopes of the S2 subunit of pertussis toxin. Infect. Immun. 57:438-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stein, P. E., A. Boodhoo, G. D. Armstrong, L. D. Heerze, S. A. Cockle, M. H. Klein, and R. J. Read. 1994. Structure of a pertussis toxin-sugar complex as a model for receptor binding. Nat. Struct. Biol. 1:591-596. [DOI] [PubMed] [Google Scholar]
- 30.Storsaeter, J., H. O. Hallander, L. Gustafsson, and P. Olin. 1998. Levels of anti-pertussis antibodies related to protection after household exposure to Bordetella pertussis. Vaccine 16:1907-1916. [DOI] [PubMed] [Google Scholar]
- 31.Storsaeter, J., and P. Olin. 1992. Relative efficacy of two acellular pertussis vaccines during three years of passive surveillance. Vaccine 10:142-144. [DOI] [PubMed] [Google Scholar]
- 32.Strnad, C. F., and R. A. Carchman. 1987. Human T lymphocyte mitogenesis in response to the B oligomer of pertussis toxin is associated with an early elevation in cytosolic calcium concentrations. FEBS Lett. 225:16-20. [DOI] [PubMed] [Google Scholar]
- 33.Tamura, M., K. Nogimori, M. Yajima, K. Ase, and M. Ui. 1983. A role of the B-oligomer moiety of islet-activating protein, pertussis toxin, in development of the biological effects on intact cells. J. Biol. Chem. 258:6756-6761. [PubMed] [Google Scholar]
- 34.Taranger, J., B. Trollfors, T. Lagergard, V. Sundh, D. A. Bryla, R. Schneerson, and J. B. Robbins. 2000. Correlation between pertussis toxin IgG antibodies in postvaccination sera and subsequent protection against pertussis. J. Infect. Dis. 181:1010-1013. [DOI] [PubMed] [Google Scholar]
- 35.Trollfors, B., J. Taranger, T. Lagergard, L. Lind, V. Sundh, G. Zackrisson, C. U. Lowe, W. Blackwelder, and J. B. Robbins. 1995. A placebo-controlled trial of a pertussis-toxoid vaccine. N. Engl. J. Med. 333:1045-1050. [DOI] [PubMed] [Google Scholar]
- 36.van't Wout, J., W. N. Burnette, V. L. Mar, E. Rozdzinski, S. D. Wright, and E. I. Tuomanen. 1992. Role of carbohydrate recognition domains of pertussis toxin in adherence of Bordetella pertussis to human macrophages. Infect. Immun. 60:3303-3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weiss, A. A., E. L. Hewlett, G. A. Myers, and S. Falkow. 1984. Pertussis toxin and extracytoplasmic adenylate cyclase as virulence factors of Bordetella pertussis. J. Infect. Dis. 150:219-222. [DOI] [PubMed] [Google Scholar]
- 38.Wong, W. S., D. I. Simon, P. M. Rosoff, N. K. Rao, and H. A. Chapman. 1996. Mechanisms of pertussis toxin-induced myelomonocytic cell adhesion: role of Mac-1(CD11b/CD18) and urokinase receptor (CD87). Immunology 88:90-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zanardi, L., F. B. Pascual, K. Bisgard, T. Murphy, M. Wharton, and E. Maurice. 2002. Pertussis—United States, 1997-2000. Morb. Mortal. Wkly. Rep. 51:73-76. [PubMed] [Google Scholar]