Abstract
Background and aims
Alcohol intake is a strong and well-established risk factor for esophageal squamous cell carcinoma (ESCC), but the association with esophageal adenocarcinoma (EA) or adjacent tumors of the esophagogastric junction (EGJA), remains unclear. Therefore, we determined the association of alcohol intake with ESCC, EA, and EGJA in nine case-control studies and two cohort studies of the Barrett’s Esophagus and Esophageal Adenocarcinoma Consortium (BEACON).
Materials and methods
We collected information on alcohol intake, age, sex, education, body mass index, gastroesophageal reflux, and tobacco smoking from each study. Along with 10,854 controls, 1,821 EA, and 1,837 EGJA, seven studies also collected ESCC cases (n=1,016). Study-specific odds ratios (OR) and 95% confidence intervals (CI) were calculated from multivariate-adjusted logistic regression models for alcohol intake in categories compared to non-drinkers. Summary risk estimates were obtained by random effects models.
Results
We observed no increase in risk of EA or EGJA for increasing levels of any of the alcohol intake measures examined. ORs for the highest frequency category (≥7 drinks per day) were 0.97 (95% CI = 0.68-1.36) for EA and 0.77 (95% CI = 0.54-1.10) for EGJA. Suggestive findings linked moderate intake (e.g. 0.5 to <1 drinks per day) to decreased risk of EA (OR = 0.63 95% CI = 0.41-0.99) and EGJA (OR = 0.78; 95% CI = 0.62-0.99). In contrast, alcohol intake was strongly associated with increased risk of ESCC (OR for ≥7 drinks per day= 9.62, 95%CI=4.26-21.71).
Conclusions
In contrast to ESCC, higher alcohol consumption was not associated with increased risk of either EA or EGJA. The apparent inverse association observed with moderate alcohol intake should be evaluated in future prospective studies.
Keywords: Alcohol Drinking, Esophageal Neoplasms, Stomach Neoplasms, Epidemiology
INTRODUCTION
Esophageal cancer is the sixth leading cause of cancer-related mortality worldwide[1] and occurs as two predominant histologic subtypes, esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EA).[2] Whereas incidence rates of EA have increased rapidly in many Western countries over the past three decades, rates of ESCC have concurrently declined.[3]
Alcohol intake is a strong and well-established risk factor for ESCC.[2, 4-12] The association of alcohol with EA or adjacent adenocarcinomas overlapping the esophagogastric junction (EGJA), however, remains unclear. Results have been inconsistent in previous studies, [6, 7, 9, 10, 12-16] regardless of whether analyzed as total alcoholic beverage intake or the individual beverage types of beer, liquor, and wine. Previous studies were limited in size, precluding precise quantification of modest effects and offering limited power to compare associations for EA with EGJA. [2, 3, 17]
To overcome these limitations, and to examine possible effect modification by known risk factors, such as sex, body mass index, gastroesophageal reflux disease, or tobacco smoking.[2, 3, 17, 18], we performed pooled analyses of the association between alcohol intake with EA and EGJA using data from nine case-control and two cohort studies of the international Barrett’s Esophagus and Esophageal Adenocarcinoma Consortium (BEACON).
METHODS
Study population
Analyses included participants from eleven BEACON studies. Population-based case-control studies included the Population Health Study,[14] the Larynx/Esophagus/Oral cavity (LEO) Study,[12] the United States (US) Multi-Center Study,[7] the nationwide Swedish Esophageal and Cardia Cancer (SECC) study,[9] the Los Angeles County Multi-ethnic Case-control Study,[16] the Nebraska Health Study II,[19] the Nova Scotia Barrett Esophagus Study (NSBES),[15] the Factors Influencing the Barrett’s Adenocarcinoma Relationship (FINBAR) Study,[13] and the nationwide Australian Cancer Study (esophageal cancer component).[10] Analyses also included eligible cases from two prospective cohort studies, the National Institutes of Health-AARP Diet and Health (NIH-AARP) study with follow-up through 2003[6] and the Kaiser-Permanente Multiphasic Health Checkup Study with follow-up until 2006.[20] We drew nested control sets randomly from the cohorts in a four to one control-case ratio from the NIH-AARP study and in an eight to one ratio from the Kaiser Multiphasic Health Checkup Study.
Combining all eleven studies, 4,140 cases [2,064 EA, 2,076 EGJA], and 13,676 controls were available for analysis. Among participants with available data on alcohol intake, we restricted our analyses to white non-Hispanic study participants (3,658 cases: 1,821 EA, 1,837 EGJA, and 10,854 controls) due to low numbers of cases in participants from other ethnic groups (50 Black, 112 Hispanic, and 71 other). All studies collected EA cases and ten studies collected EGJA cases.[6, 7, 9, 10, 12-14, 16, 19, 20] Seven participating studies also collected ESCC cases, [6, 7, 9, 10, 12, 14, 20] for which 1,016 cases and 9,253 controls were available for analysis.
Study specific case numbers and characteristics are presented in Table 1.
Table 1.
Characteristics of the eleven studies participating in this analysis.
| Controls | Cases | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Study | Year | Design | Available data† | No. Alcohol use* | Site | No. | Alcohol use* |
| EA | 354 | 1.7, 0.6-3.4 | |||||
| Australian Cancer Study, esophageal cancer component [10] |
2001-2005 | case-control | drinks/day, duration, types |
1470 1.0, 0.3-2.5 | EGJA | 414 | 1.4, 0.6-3.2 |
|
| |||||||
| ESCC | 284 | 1.9, 0.4-4.3 | |||||
| Factors INfluencing the Barrett’s Adenocarcinoma Relationship (FINBAR), Ireland[13] |
2002-2004 | case-control | drinks/day, types |
260 1.2, 0.0-4.4 | EA | 127 | 1.0, 0.0-4.0 |
| EGJA | 89 | 0.5, 0.0-2.2 | |||||
|
| |||||||
| Larynx/Esophagus/Oral cavity (LEO), USA[12] | 1983-1990 | case-control | drinks/day, duration, types |
685 0.4, 0.1-1.0 | EA | 108 | 0.6, 0.1-1.9 |
| EGJA | 132 | 0.6, 0.1-2.1 | |||||
| ESCC | 75 | 1.7, 0.4-4.5 | |||||
|
| |||||||
| Los Angeles County Multi-ethnic, USA[16] | 1993-1999 | case-control | drinks/day, duration, types |
838 0.9, 0.1-2.3 | EA | 171 | 0.9, 0.1-2.4 |
| EGJA | 209 | 1.0, 0.1-3.1 | |||||
|
| |||||||
| Nebraska Health Study II, USA[19] | 1992-1994 | case-control | drinks/day, duration, types |
405 0.1, 0.0-0.7 | EA | 87 | 0.6, 0.0-2.0 |
| EGJA | 38 | 0.4, 0.0-2.0 | |||||
|
| |||||||
| Nova Scotia Barrett Esophagus Study, Canada[15] | 2001-2003 | case-control | drinks/day, duration, types |
98 0.7, 0.1-1.8 | EA | 56 | 1.3, 0.2-5.1 |
|
| |||||||
| Population Health Study, USA[14] | 1986-1989 | case-control | drinks/day, duration, types |
725 1.1, 0.1-2.9 | EA | 58 | 2.0, 0.7-4.6 |
| EGJA | 113 | 1.3, 0.1-4.0 | |||||
| ESCC | 122 | 5.1, 2.2-8.4 | |||||
|
| |||||||
| Swedish Esophageal Cancer Study[9] | 1994-1997 | case-control | drinks/day, types |
820 0.8, 0.2-2.1 | EA | 189 | 0.7, 0.1-2.4 |
| EGJA | 262 | 0.7, 0.2-2.4 | |||||
| ESCC | 167 | 2.0, 0.6-4.0 | |||||
|
| |||||||
| US Multi-Center Study[7] | 1993-1995 | case-control | drinks/day, duration, types |
516 1.0, 0.3-2.7 | EA | 220 | 1.4, 0.3-4.0 |
| EGJA | 197 | 1.3, 0.5-3.2 | |||||
| ESCC | 148 | 5.0, 2.0-9.8 | |||||
|
| |||||||
| Kaiser-Permanente Multiphasic Health, USA[20] | 1964-2006 | cohort | drinks/day | 1884 0.5, 0.5-0.5‡ | EA | 78 | 0.5, 0.5-0.5‡ |
| EGJA | 80 | 0.5, 0.5-0.5‡ | |||||
| ESCC | 77 | 0.5, 0.5-3.9‡ | |||||
|
| |||||||
| National Institutes of Health AARP Diet and Health (NIH-AARP) study, USA[6] |
1995-2003 | cohort | drinks/day, types |
3153 0.2, 0.0-0.9 | EA | 373 | 0.3, 0.1-1.3 |
| EGJA | 303 | 0.2, 0.0-1.2 | |||||
| ESCC | 143 | 1.1, 0.0-4.8 | |||||
Number of drinks/day: Median, interquartile range
Available data: drinks/day, typical alcohol intake in drinks per day; duration, years of drinking alcohol; types, information on beer, liquor, and wine
The Kaiser study assessed alcohol intake in categories: (non-drinker, <0 to <, 3 to <6, 6 to <9, ≥9 drinks per day). For each category in this cohort, we assigned the median alcohol intake within the stated ranges of other BEACON studies. 62.3% of controls, 60.5% of EA cases, 55.9% of EGJA cases, and 41.6% of ESCC cases were in the <0 to 3 drinks per day category, for which we imputed a value of 0.45 drinks per day.
Numbers of cases and controls listed are those with alcohol data after restricting to white non-Hispanic participants.
Abbreviations: No., number; EA, esophageal adenocarcinoma; EGJA, esophagogastric junction adenocarcinoma; ESCC, esophageal squamous cell carcinoma; US, United States; USA, United States of America.
Data acquisition was approved by the Institutional Review Board or Research Ethics Committee of each participating institution providing data for the study; permission to participate in the BEACON consortium was also provided by these boards if required by a study’s home institution.
Study variables
Each study provided a questionnaire, study methods, and a de-identified dataset including information on alcohol consumption, age, sex, body mass index (BMI; weight divided by height squared, in kg/m2), education, gastroesophageal reflux, tobacco smoking, and study center (for multi-center studies).[7, 12, 14] We compared the data provided by each study with published data; any apparent inconsistencies were resolved with study investigators.
Though all studies included questions on typical alcohol intake, the method of assessment and the wording of questions differed across studies. Seven studies assessed alcohol intake by interviewer administered questionnaire,[7, 9, 12, 14-16, 19, 20] two studies used computer based questionnaires,[9, 13] and two studies administered questionnaires by mail.[6, 10] Typical adult alcohol intake was assessed by six studies,[7, 12, 14-16, 19] whereas, one study assessed alcohol drinking at the age intervals of 20 to <30 years, 30 to <50 years, and ≥ 50 years;[10] for this study the average of these data were used for the pooled analysis. In these seven studies,[7, 10, 12, 14-16, 19] the non-drinking category was restricted to life-long never drinkers. The remaining four studies assessed typical alcohol intake at a specific time-point: twenty years[9] or five years before interview,[13] or in the past 12 months for the two cohort studies.[6, 20] In these four studies,[6, 9, 13, 20] therefore, non-drinkers were those who were not drinking at the reference timepoint. One of these four studies assessed alcohol intake in categories (non-drinker, 0< to <3, 3 to <6, 6 to <9, ≥9 drinks per day).[20] For each category in this study, we assigned the median alcohol intake within the stated ranges of other BEACON studies.
Alcohol intake across studies was standardized to a single drink of 14 grams of ethanol (one 12 ounce beer, 5 ounce glass of wine, or 1.5 ounces of liquor). Intakes of beer, wine, and liquor were available separately for ten studies.[6, 7, 9, 10, 12-16, 19] Duration of alcohol drinking was available for seven studies (Table 1).[7, 10, 12, 14-16, 19]
Statistical analysis
Risk estimates were adjusted for known and suspected risk factors for EA and EGJA, namely age (years; <50, 50 to <60, 60 to <70, ≥70), total cigarette smoking exposure (pack-years; 0, 0< to <15, 15 to <30, 30 to <45, ≥45), BMI (<25, 25 to <30, ≥30 ), gastroesophageal reflux status (yes versus no), and study center (for multi-center studies). For education, we used study-specific variables. Data on gastroesophageal reflux was available for five studies.[7, 9, 10, 13, 16] Because one study did not collect information on the age at smoking initiation at study baseline,[6] we estimated duration by subtracting the median age at smoking initiation in a subset of the cohort (17 years) from current age or for former smokers the age at smoking cessation. This duration variable was then used to calculate pack-years. All other variables were available for all studies.
We analyzed alcohol intake in categories. Study specific relative risk estimates were estimated by logistic regression models in Stata 10.0 (College Station, Texas) and then pooled using random effects models.[21] Results for fixed effect models were similar (data not shown). Heterogeneity between studies was assessed by the I2 statistic.[22] Heterogeneity that can be explained by chance is indicated by an I2 of 0%. P-values less than 0.05 were considered significant and all tests were two-sided.
Total alcohol exposure was assessed by drink-years, created by multiplying years of alcohol drinking in lifetime by typical drinks per day. Linear trend tests were also assessed in random effects models. In each study, we fitted a trend variable with the median intake per alcohol category; pooled results were then obtained by random effects meta-analysis of the study-specific risk estimates. Risk estimates for beer, wine, and liquor used non-drinkers of any alcohol type as the referent and were adjusted for categories of total alcohol intake in order to investigate beverage specific effects independent of ethanol content. As a sensitivity analysis, we examined risk estimates that were unadjusted for total alcohol intake and results were similar. We also stratified analyses by categories of sex, BMI, gastroesophageal reflux, and cigarette smoking to identify potential modifiers of an association with alcohol intake.
RESULTS
Details on study design and assessment of alcohol use are shown in Table 1. Alcohol use among controls ranged from a median of 0.1 drinks per day in the Nebraska study to 1.2 drinks per day in the FINBAR study.
Table 2 provides ORs for frequency (drinks per day), duration (years) and drink-years of alcohol intake, relative to non-drinking. Overall, relative risk estimates had low to moderate heterogeneity with a majority of I2 below 40%. Not even the highest amount of alcohol intake (≥7 drinks per day) was associated with increased risk of EA (OR = 0.97; 95% CI = 0.68-1.36) or EGJA (OR = 0.77; 95%CI= 0.54-1.10). No evidence for a dose-response for either endpoint was observed and tests for linear trend were not statistically significant (EA: p=0.21; EGJA: p=0.88). Relative to non-drinkers, ORs for most categories of intake were below one.
Table 2.
Adjusted odds ratios and 95% confidence intervals for the association of alcohol drinking and risk of esophageal and esophagogastric junction adenocarcinoma in the Barrett’s Esophagus and Esophageal Adenocarcinoma Consortium
| Alcohol category | Esophageal adenocarcinoma | Esophagogastric junction adenocarcinoma* | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Controls | Cases | OR† | 95% CI | I 2 | Controls | Cases | OR† | 95% CI | I 2 | |
| Drinks/day | ||||||||||
| None | 1831 | 249 | Referent | 1812 | 262 | Referent | ||||
| >0 - <0.5 | 3567 | 440 | 0.86 | 0.65-1.13 | 41 | 3526 | 425 | 0.83 | 0.68-1.00 | 0 |
| 0.5 - <1.0 | 1151 | 179 | 0.63 | 0.41-0.99 | 63 | 1104 | 215 | 0.78 | 0.62-0.99 | 0 |
| 1 - <3 | 2030 | 441 | 0.81 | 0.60-1.09 | 44 | 2010 | 432 | 0.77 | 0.62-0.94 | 0 |
| 3 - <5 | 846 | 182 | 0.86 | 0.59-1.24 | 47 | 837 | 215 | 0.93 | 0.73-1.19 | 3 |
| 5 - <7 | 268 | 95 | 0.93 | 0.66-1.31 | 0 | 261 | 87 | 0.95 | 0.69-1.32 | 0 |
| ≥7 | 381 | 117 | 0.97 | 0.68-1.36 | 16 | 379 | 102 | 0.77 | 0.54-1.10 | 21 |
| p-trend | 0.21 | 0.88 | ||||||||
|
| ||||||||||
| Duration (years) ‡ | ||||||||||
| None | 724 | 113 | Referent | 716 | 127 | Referent | ||||
| >0 - <10 | 140 | 32 | 0.94 | 0.56-1.57 | 0 | 153 | 36 | 0.98 | 0.62-1.55 | 0 |
| 10 - <20 | 326 | 61 | 1.15 | 0.77-1.74 | 1 | 318 | 75 | 1.07 | 0.73-1.57 | 2 |
| 20 -<30 | 696 | 127 | 0.83 | 0.55-1.24 | 24 | 671 | 140 | 0.80 | 0.58-1.10 | 0 |
| 30 - <40 | 1045 | 252 | 0.76 | 0.53-1.07 | 17 | 1024 | 241 | 0.76 | 0.57-1.01 | 0 |
| 40- <50 | 1053 | 267 | 0.74 | 0.49-1.10 | 37 | 1035 | 287 | 0.81 | 0.61-1.07 | 0 |
| ≥50 | 561 | 164 | 0.71 | 0.48-1.05 | 22 | 551 | 147 | 0.64 | 0.46-0.89 | 0 |
| p-trend | 0.02 | 0.003 | ||||||||
|
| ||||||||||
| Drink-years ‡ | ||||||||||
| None | 727 | 114 | Referent | 715 | 126 | Referent | ||||
| >0 - <25 | 1503 | 259 | 0.75 | 0.56-0.99 | 0 | 1464 | 274 | 0.80 | 0.61-1.04 | 0 |
| 25- <50 | 651 | 127 | 0.66 | 0.44-0.99 | 26 | 637 | 146 | 0.79 | 0.58-1.07 | 0 |
| 50- <100 | 723 | 184 | 0.75 | 0.54-1.02 | 0 | 711 | 191 | 0.74 | 0.54-1.00 | 0 |
| 100- <200 | 567 | 152 | 0.67 | 0.48-0.94 | 0 | 557 | 177 | 0.84 | 0.58-1.21 | 25 |
| 200- <300 | 174 | 84 | 1.04 | 0.69-1.57 | 0 | 170 | 69 | 0.98 | 0.66-1.45 | 0 |
| ≥300 | 151 | 57 | 0.97 | 0.50-1.88 | 45 | 149 | 53 | 0.83 | 0.54-1.27 | 0 |
| p-trend | 0.25 | 0.72 | ||||||||
Unavailable for the Nova Scotia Barrett Esophagus Study.[15]
Summary OR and 95% CI from random effect models. OR adjusted for sex, age (categorical: <50, 50-<60, 60-<70, ≥70), body mass index (categorical: <25, 25-<30, ≥30), education (study-specific), pack-years of smoking (categorical: 0, <0-<15, 15-<30, 30-<45, ≥45), and where available, for gastroesophageal reflux.
Analysis for duration and drink-years include seven studies: Australian Cancer Study, esophageal cancer component,[10] Larynx/Esophagus/Oral cavity,[12] Los Angeles County Multi-ethnic,[16] Nebraska Health Study II,[19] Nova Scotia Barrett Esophagus Study,[15] Population Health Study,[14] and US Multi-Center Study.[7]
Abbreviations: OR, odds ratio; CI, confidence interval.
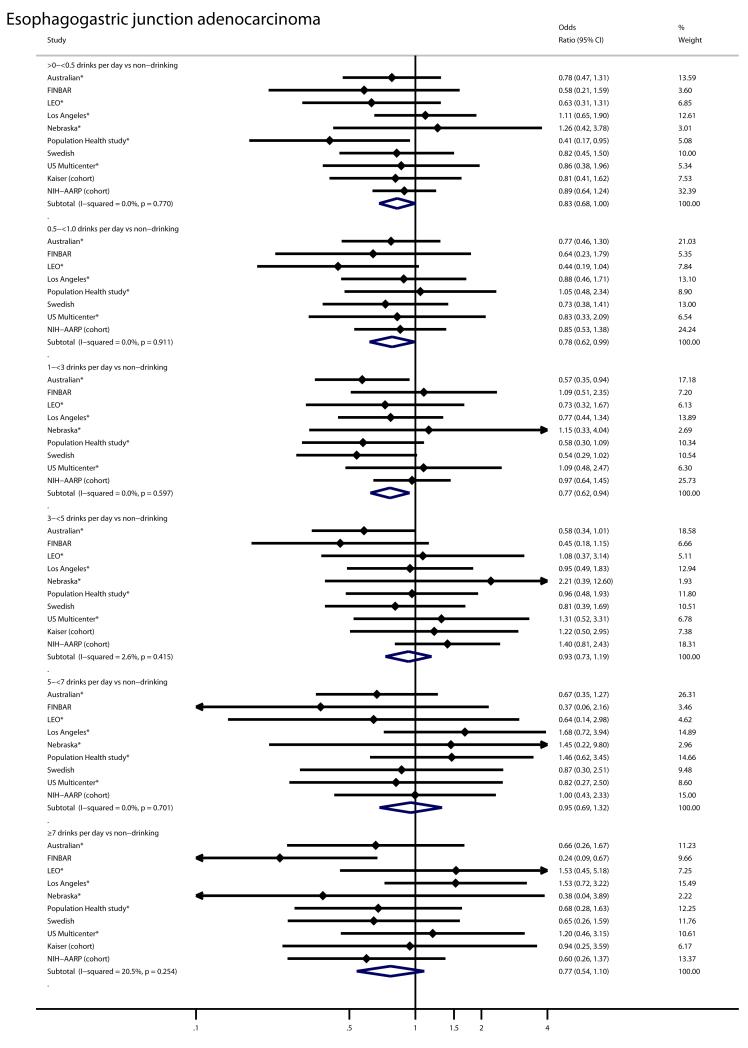
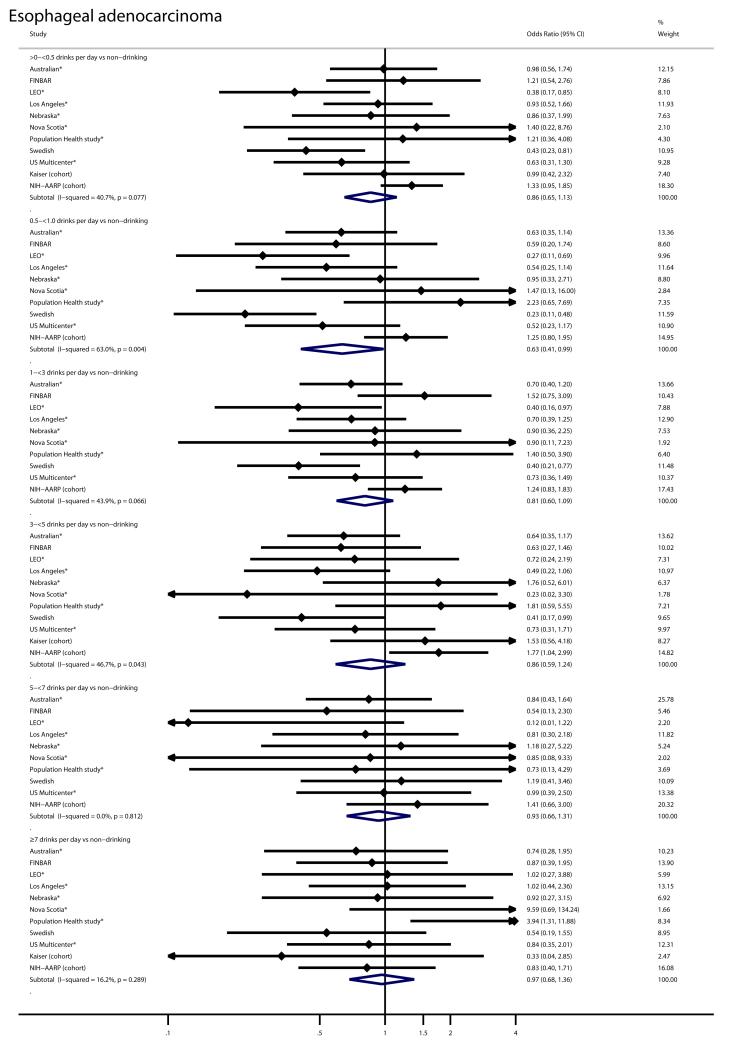
For example, participants who reported typically drinking 0.5 to <1 drinks per day had lower risk of both EA (OR = 0.63; 95% CI = 0.41-0.99) and EGJA (OR = 0.78; 95% CI = 0.62-0.99). Risk estimates from the seven participating studies[7, 10, 12, 14-16, 19] which restricted the non-drinking referent category to lifetime never drinkers were similar to the four participating studies that could not make this restriction (Figure 1 and Figure 2). To increase power, we also looked at a combined endpoint which included both EA and EGJA cases. Alcohol intake was not associated with increased risk of this combined endpoint either (OR for ≥7 drinks per day=0.86; 95%CI: 0.66-1.11; I2=12%).
Figure 1.
Forest plot for the association of alcohol intake (drinks per day) with risk of esophageal adenocarcinoma in the Barrett’s Esophagus and Esophageal Adenocarcinoma Consortium. Odds ratios are shown for each category of alcohol intake relative to non-drinking and are adjusted for age (categorical: <50, 50-<60, 60-<70, ≥70), body mass index (categorical: <25, 25-<30, ≥30), education (study-specific), pack-years of smoking (categorical: 0, <0-<15, 15-<30, 30-<45, ≥45), and where available, for gastroesophageal reflux. Large black unfilled diamonds indicate the overall point estimate. Small black filled diamonds represent the point estimate for each study. Horizontal lines represent 95% confidence intervals (CI). The solid vertical line indicates a relative risk of 1. Studies in which the non-drinking referent group is further restricted to lifetime never drinkers are marked with a star. The two included cohort studies are also marked.
Figure 2.
Forest plot for the association of alcohol intake (drinks per day) with risk of esophagogastric junction adenocarcinoma in the Barrett’s Esophagus and Esophageal Adenocarcinoma Consortium. Odds ratios are shown for each category of alcohol intake relative to non-drinking and are adjusted for age (categorical: <50, 50-<60, 60-<70, ≥70), body mass index (categorical: <25, 25-<30, ≥30), education (study-specific), pack-years of smoking (categorical: 0, <0-<15, 15-<30, 30-<45, ≥45), and where available, for gastroesophageal reflux. Large black unfilled diamonds indicate the overall point estimate. Small black filled diamonds represent the point estimate for each study. Horizontal lines represent 95% confidence intervals (CI). The solid vertical line indicates a relative risk of 1. Studies in which the non-drinking referent group is further restricted to lifetime never drinkers are marked with a star. The two included cohort studies are also marked.
We observed evidence for a modest inverse association between years of alcohol drinking and EA and EJGA, with p-value for linear trends of 0.02 and 0.003, respectively. Relative to never drinking, ORs for drinking for ≥50 years were 0.71 (95%CI=0.48-1.05) for EA and 0.64 (95%CI=0.46-0.89) for EGJA. Overall, results for drink-years, reflecting duration of alcohol drinking and typical drinks per day, were null with little evidence for a dose-response association. The p-value for linear trend was not-significant for each endpoint. Yet relative to never-drinking, there was evidence for a non-linear association. For example, the OR for 25 to <50 drink years was 0.66 for EA and 0.79 for EGJA, whereas the corresponding ORs for >200 to <300 drink-years were 1.04 and 0.98, respectively.
In order to distinguish possible effects of individual types of alcoholic beverages from a possible generic effect of ethanol, we examined ORs for categories of beer, liquor, and wine consumption after adjustment for total alcohol intake (Table 3). Beer intake had an apparent inverse association with EA and EGJA risk, though the p-value for linear trend was not significant for either EA (p=0.12) or EGJA (p=0.06). Associations for liquor intake centered around unity, whereas there was suggestive evidence for a non-linear association with wine.
Table 3.
Adjusted odds ratios and 95% confidence intervals for the association of beer, liquor, and wine (drinks per day) with the risk of esophageal and esophagogastric junction adenocarcinoma in the Barrett’s Esophagus and Esophageal Adenocarcinoma Consortium
| Alcohol category |
Esophageal adenocarcinoma | Esophagogastric junction adenocarcinoma* | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Controls | Cases | OR† | 95% CI | I 2 | Controls | Cases | OR† | 95% CI | I 2 | |
| Beer‡ | ||||||||||
| None§ | 1556 | 242 | Referent | 1544 | 250 | Referent | ||||
| >0 - <0.5 | 2828 | 480 | 0.75 | 0.53-1.07 | 51 | 2794 | 427 | 0.72 | 0.58-0.89 | 0 |
| 0.5 - <1.0 | 752 | 153 | 0.71 | 0.44-1.15 | 43 | 716 | 196 | 0.81 | 0.58-1.13 | 4 |
| 1 - <3 | 1100 | 292 | 0.72 | 0.51-1.04 | 8 | 1085 | 271 | 0.64 | 0.46-0.90 | 0 |
| 3 - <5 | 375 | 107 | 0.60 | 0.36-1.01 | 16 | 367 | 134 | 0.70 | 0.40-1.24 | 34 |
| ≥5 | 288 | 105 | 0.63 | 0.40-0.99 | 0 | 287 | 86 | 0.58 | 0.34-1.00 | 0 |
| p-trend | 0.12 | 0.06 | ||||||||
| Liquor ‡ | ||||||||||
| None§ | 1556 | 242 | Referent | 1544 | 250 | Referent | ||||
| >0 - <0.5 | 3529 | 542 | 0.71 | 0.49-1.02 | 56 | 3470 | 599 | 0.77 | 0.62-0.95 | 0 |
| 0.5 - <1.0 | 527 | 121 | 0.95 | 0.66-1.35 | 4 | 517 | 113 | 0.73 | 0.51-1.04 | 0 |
| 1 - <3 | 664 | 184 | 1.09 | 0.60-1.97 | 59 | 671 | 196 | 1.06 | 0.76-1.47 | 0 |
| 3 - <5 | 172 | 53 | 1.27 | 0.75-2.13 | 0 | 173 | 45 | 0.84 | 0.50-1.41 | 0 |
| ≥5 | 132 | 46 | 1.52 | 0.82-2.80 | 0 | 121 | 40 | 0.99 | 0.53-1.84 | 0 |
| p-trend | 0.097 | 0.70 | ||||||||
| Wine ‡ | ||||||||||
| None§ | 1556 | 242 | Referent | 1544 | 250 | Referent | ||||
| >0 - <0.5 | 3182 | 493 | 0.67 | 0.45-0.99 | 57 | 3155 | 492 | 0.71 | 0.56-0.92 | 13 |
| 0.5 - <1.0 | 701 | 94 | 0.59 | 0.39-0.88 | 11 | 694 | 111 | 0.64 | 0.45-0.90 | 0 |
| 1 - <3 | 676 | 112 | 0.71 | 0.49-1.03 | 5 | 672 | 137 | 0.72 | 0.52-1.02 | 0 |
| ≥3 | 70 | 28 | 1.49 | 0.80-2.78 | 0 | 64 | 22 | 1.18 | 0.51-2.72 | 36 |
| p-trend | 0.40 | 0.15 | ||||||||
Unavailable for the Nova Scotia Barrett Esophagus Study.[15]
Summary OR and 95% CI from random effect models. OR adjusted for sex, age (categorical: <50, 50-<60, 60-<70, ≥70), body mass index (categorical: <25, 25-<30, ≥30), education (study-specific), pack-years of smoking (categorical: 0, <0-<15, 15-<30, 30-<45, ≥45), alcohol intake (0<-<0.5, 0.5-<1.0,1-<3, 3-<5, 5-<7, ≥7), and where available, for gastroesophageal reflux.
Beer, liquor, and wine were unavailable for the Kaiser-Permanente Multiphasic Health study.[20]
Referent group is non-drinkers of alcohol
Abbreviations: OR, odds ratio; CI, confidence interval.
Relative to non-drinking, the OR for drinking 0.5 to <1 drinks of wine per day was 0.59 (95%CI=0.39-0.88) for EA and 0.64 (95%CI=0.45-0.90) for EGJA. In the highest category of wine intake (≥3 drinks per day), the OR was 1.49 (95%CI=0.80-2.78) for EA and 1.18 (95%CI=0.51-2.72) for EGJA.
Next, we examined the association of alcohol (drinks per day) with cancer risk separately in men and women and by stratum of gastroesophageal reflux, body mass index, and tobacco smoking (Online Table 1 and Online Table 2). Risk estimates generally appeared similar and 95% confidence intervals overlapped across most strata. One exception was an increased risk for EA (OR = 4.25; 95% CI = 1.60-11.30, 14 cases; p for linear trend = 0.13) among women drinking ≥3 alcoholic beverages per day, but no evidence for EGJA (OR=0.99, 95%CI: 0.41-2.35, 11 cases; p for linear trend=0.90). No such patterns were observed in men.
In contrast to results for EA and EGJA, we observed a strong dose-response association between alcohol intake and ESCC among the seven BEACON studies which also included ESCC cases (Table 4). Relative to non-drinking, the OR for drinking ≥7 drinks per day was 9.62 (95%CI=4.26-21.71; p-for linear trend <0.0001).
Table 4.
Adjusted odds ratios and 95% confidence intervals for the association of alcohol drinking and risk of esophageal squamous cell carcinoma in the Barrett’s Esophagus and Esophageal Adenocarcinoma Consortium
| Alcohol category | Esophageal squamous cell carcinoma* | ||||
|---|---|---|---|---|---|
| Controls | Cases | OR† | 95% CI | I 2 | |
| Drinks/day ‡ | |||||
| None | 1412 | 89 | Referent | ||
| >0 - <0.5 | 3160 | 149 | 0.80 | 0.56-1.14 | 18 |
| 0.5 - <1.0 | 939 | 68 | 1.23 | 0.55-2.74 | 67 |
| 1 - <3 | 1623 | 224 | 2.56 | 1.10-5.96 | 79 |
| 3 - <5 | 694 | 161 | 4.56 | 2.32-8.96 | 67 |
| 5 - <7 | 215 | 100 | 7.17 | 2.98-17.25 | 69 |
| ≥7 | 284 | 161 | 9.62 | 4.26-21.71 | 71 |
| p-trend | <0.0001 | ||||
Available for seven studies: Australian Cancer Study, esophageal cancer component,[10] Kaiser-Permanente Multiphasic Health study,[20] Larynx/Esophagus/Oral cavity,[12] NIH-AARP Diet and Health study,[6] Population Health Study,[14] Swedish Esophageal Cancer Study,[9]and US Multi-Center Study.[7]
Summary OR and 95% CI from random effect models. OR adjusted for sex, age (categorical: <50, 50-<60, 60-<70, ≥70), body mass index (categorical: <25, 25-<30, ≥30), education (study-specific), pack-years of smoking (categorical: 0, <0-<15, 15-<30, 30-<45, ≥45), and where available, for gastroesophageal reflux.
Abbreviations: OR, odds ratio; CI, confidence interval.
DISCUSSION
In this large pooled analysis of over 15,000 participants in eleven studies of the BEACON consortium, we found no evidence for an association between higher alcohol intake and increased risk of EA or EGJA. Indeed, risks among alcohol drinkers tended to be below those among non-drinkers, albeit mostly statistically non-significant. We found no evidence that any particular type of beverage (beer, liquor, or wine) was especially associated with increased or decreased cancer risk. Further, we exploited the large numbers of cases and controls in the BEACON dataset to examine associations by stratum of sex, gastroesophageal reflux disease, cigarette smoking, and body mass index. Of the examined strata, alcohol intake was associated with increased EA risk in just one, women who drank ≥ 3 drinks per day. Given that the category comprised only 14 cases, the p-for linear trend was not significant, and no association was observed with less intake, with EGJA, or in men, we conclude that this single association is most likely a chance finding.
Our results for EA and EJGA stand in remarkable contrast to results for ESCC in this and previously published studies.[2, 4-12] The ESCC results, generated from the same studies as the EA and EGJA results, should allay concerns about non-differential misclassification of alcohol intake, recall bias, and reverse causation as explanations for our null findings. Associations between other environmental risk factors and esophageal cancer have also been shown to vary by histologic type. For example, higher body mass index is a consistent risk factor for EA but not ESCC risk.[23] Cigarette smoking is a risk factor for both tumor sites, though the magnitude of the association appears greater for ESCC than EA.[6, 7, 9, 11, 12, 24] Distinct associations between Helicobacter pylori infection and ESCC and EA risk have also been observed.[25] Together with opposing incidence trends over time,[3] these results indicate distinct etiologies for ESCC and EA.
In our study, the association for alcohol intake was generally similar for EA and EGJA. It is difficult to distinguish these sites clinically[26] and whether the etiology of these sites are similar or different is unclear. Whereas EA were defined in our study as those above the esophagogastric junction, EGJA were more heterogeneous and included tumors overlapping the junction. Even so, results were generally similar for both endpoints and only modest heterogeneity was observed between studies which used distinct diagnostic criteria. Together, these data provide little evidence for an association between alcohol intake and increased risk of tumors either proximal or overlapping the esophagogastric junction.
We observed suggestive evidence that modest alcohol drinking, particularly less than one drink per day, might be associated with reduced EA and EGJA risk. Such findings may be due to chance, particularly as multiple comparisons were made. As in all observational studies, the observed inverse association with modest alcohol drinking may also reflect additional unknown or poorly measured confounders. For example, moderate alcohol drinking could be associated with aspects of a healthy lifestyle, such that the observed association reflects confounding. In our study, similar results were observed in smokers and in participants with a BMI between 18.5 to <25 and those with a BMI ≥ 25. Yet, other aspects of a healthy lifestyle could still be responsible for these findings.
Inverse associations may also reflect recall bias or reverse causality as nine of the eleven studies participating in Beacon had a case-control design. Patients diagnosed with cancer may alter their alcohol intake or their report of it. In addition, individuals with undetected tumors or their precursor conditions, such as gastroesophageal reflux, might avoid alcohol because it provokes symptoms. Supporting this hypothesis, reflux symptoms have been shown to be associated with less alcohol intake in past studies [13].
Of the two participating prospective cohorts, results from the NIH-AARP Diet and Health study[6] and the Kaiser-Permanente Multiphasic Health Checkup Study[20] showed little evidence for an inverse association (Figure 1 and Figure 2). Published results from two other cohorts are mixed. Risk estimates for EA were 1.17 (0.69-1.98) for drinking up to 5 grams (~ 1/3 a drink) per day and 0.91 (0.51-1.60) for 5-<15 grams (~ 1 drink) per day relative to non-drinking in the Netherlands Cohort Study.[11] In the Million Women Study, risk estimates were 0.78 (0.61-0.99) for drinking ≤ 2 drinks per week relative to not drinking.[27] Future prospective studies are needed.
Alternatively, inverse associations with moderate alcohol drinking may reflect contamination of the non-drinking referent group with formerly heavy drinkers. But, we found no evidence for increased cancer risk with higher alcohol intake in our study, even among those drinking ≥ 7 drinks per day, so contamination with heavy drinkers would not alter the associations in this manner. Furthermore, seven studies restricted the non-drinking category to those who reported never drinking alcohol.[7, 10, 12, 14-16, 19] Inverse associations with modest alcohol drinking persisted in these seven studies (Figure 1 and Figure 2).
It is also possible that these results reflect a true association. For example, ethanol intake may have beneficial effects on insulin resistance or levels of serum lipids and lipoproteins,[28] which might be important for EA and EGJA risk. Also, wine,[29] and to some extent beer,[30] is thought to contain antioxidants which could affect cancer risk. Intriguingly, similar results have been observed for alcohol intake in two recent studies of Barrett’s esophagus,[13, 31] a precursor of EA.
One striking aspect of EA and EGJA epidemiology is the profoundly higher incidence in men relative to women.[3] As such, previous studies have had little power to examine the association of alcohol with EA and EGJA in men and women separately. In sex-stratified analyses, we generally found comparable results between men and women, with the exception of EA risk among women drinking ≥3 drinks per day. As there were just 14 EA cases in this group, this result may be due to chance variation. Either way, since men typically drink more alcoholic beverages than women, our results suggest differences in alcohol use are very unlikely to explain differences in the incidence of these cancers between men and women.
Strengths of our study include its large size, inclusion of both population based case-control and cohort studies, inclusion of both histologic types of esophageal cancer and adjacent adenocarcinomas of the esophagogastric junction, and adjustment for major EA risk factors. We defined variables for alcohol intake in the same way for each study. Furthermore, risk estimates from each study were adjusted for a standard set of covariates. We also investigated risk estimate heterogeneity between studies. Alcohol intake was comprehensively assessed, with analyses of drinks per day, duration, drink-years, and beverage types. Furthermore, we exploited the large dataset to examine possible differences by major risk factors, including body mass index, cigarette smoking, and gastroesophageal reflux disease. Finally, we found similar risk estimates in studies performed in different regions of the world and with different study designs, adding credence to our findings.
Limitations include possible recall and selection bias, as most participating studies had a case-control design. However, results were generally similar for the two cohort studies. In addition, though our study is the largest to date, case numbers were small in some strata of the stratified analyses, such as women, and we lacked ability to assess associations in non-Caucasian ethnic groups.
In conclusion, in contrast to results for ESCC, we observed little evidence for an association between higher alcohol consumption with either EA or EGJA risk. Moderate alcohol consumption was associated with reduced EA and EGJA cancer risk, though these findings need to be examined further in future prospective cohort studies.
Supplementary Material
SUMMARY BOX.
What is already known about this topic
Esophageal cancer is the sixth leading cause of cancer-related mortality worldwide and occurs as two predominant histologic subtypes, esophageal squamous cell carcinoma and esophageal adenocarcinoma.
Whereas incidence rates of esophageal adenocarcinoma have increased rapidly in many Western countries over the past three decades, rates of esophageal squamous cell carcinoma have concurrently declined.
Heavy alcohol consumption is an established cause of esophageal squamous cell carcinoma, but associations with esophageal adenocarcinoma have been inconsistent in past studies.
Previous studies of esophageal adenocarcinoma have been too small to detect modest associations.
What are the new findings
Heavy alcohol consumption, even consuming seven or more drinks per day, was not associated with increased risk of esophageal adenocarcinoma in the eleven studies and more than 1800 cases of the Barrett’s Esophagus and Esophageal Adenocarcinoma Consortium
Heavy alcohol intake was also not associated with adjacent adenocarcinomas of the esophagogastric junction.
Modest alcohol consumption appeared to be associated with reduced risk for adenocarcinomas of both the esophagus and esophagogastric junction.
How might it impact on clinical practice in the foreseeable future?
These results indicate that heavy alcohol consumption is not a risk factor for adenocarcinomas of the esophagus and esophagogastric junction, highlighting substantial differences in the etiology of esophageal squamous cell carcinoma and esophageal adenocarcinoma, the two most common histological types of esophageal cancer.
Our findings may provide clinicians with further data to address patient queries about the causes of their cancer.
ACKNOWLEDGEMENTS
We thank the participants and staff of each participating study.
FUNDING: This work was supported in part by the Intramural Program of the National Institutes of Health. The Population Health Study was funded by the Intramural Program of the National Institutes of Health. The Larynx, Esophagus, and Oral Cavity (LEO) Study was funded by grants R01-CA30022 and R37-CA41530 (both awarded to TLV, David Thomas, Scott Davis, Bonnie Worthington Roberts, Ruth Little, and Mary Rogers). The US Multi-Center Study was funded by grants U01-CA57949 (awarded to TLV), U01-CA57983 (awarded to MDG), and U01-CA57923 (awarded to HAR). The Swedish Esophageal Cancer Study was funded by grant number R01 CA57947–03 (awarded to ON and Hans-Olov Adami). The Los Angeles County Multi-ethnic Case-control Study was funded by grants 3RT-0122 (‘Smoking and Risk of Proximal Vs. Distal Gastric Cancer’, awarded to AHW) and 10RT-0251 (‘Smoking, microsatellite instability & gastric cancers’, awarded to AHW) from the California Tobacco Related Research Program and grant CA59636 (awarded to LB) from the National Cancer Institute. The Nebraska Health Study was funded by the Intramural Program of the National Institutes of Health. The Nova Scotia Barrett Esophagus Study was supported by the Nova Scotia Health Research Foundation (‘Molecular mechanisms and lifestyle risk factor interactions in the pathogenesis of human esophageal adenocarcinoma’, N419, awarded to AGC). The Factors Influencing the Barrett’s Adenocarcinoma Relationship (FINBAR) study was funded by an Ireland-Northern Ireland Co-operation Research Project Grant sponsored by the Northern Ireland Research & Development Office, and the Health Research Board, Ireland (All-Ireland case-control study of Oesophageal Adenocarcinoma and Barrett’s Oesophagus, awarded to LJM and Harry Comber). The Australian Cancer Study was supported by the Queensland Cancer Fund and the National Health and Medical Research Council (NHMRC) of Australia (Program no. 199600, awarded to DCW, Adele C. Green, Nicholas K. Hayward, Peter G. Parsons, David M. Purdie, and Penelope M. Webb). NIH-AARP was funded by the Intramural Program of the National Institutes of Health. Reported analyses with the Kaiser-Permanente Multiphasic Health Checkup Study were funded by NIH grant number R01 DK063616 (Epidemiology and Incidence of Barrett’s Esophagus, Kaiser Permanente, awarded to DAC) and NIH grant R21DKO77742 (Barrett’s Esophagus: Risk Factors in Women, awarded to DAC and Nicholas J. Shaheen).
ABBREVIATIONS
- (ESCC)
Esophageal squamous cell carcinoma
- (EA)
Esophageal adenocarcinoma
- (EGJA)
Esophagogastric junction
- (BEACON)
Barrett’s Esophagus and Esophageal Adenocarcinoma Consortium
- (OR)
Odds ratios
- (CI)
Confidence intervals
- (LEO)
Larynx/Esophagus/Oral cavity Study
- (US)
United States
- (SECC)
Nationwide Swedish Esophageal and Cardia Cancer
- (NSBES)
Nova Scotia Barrett Esophagus Study
- (FINBAR)
Factors Influencing the Barrett’s Adenocarcinoma Relationship Study
- (NIH-AARP)
National Institutes of Health-AARP Diet and Health
- (USA)
United States of America
- (BMI)
body mass index
Footnotes
COMPETING INTERESTS: None.
COPYRIGHT LICENCE STATEMENT: The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, a non-exclusive licence on a worldwide basis to the BMJ Group and co-owners or contracting owning societies (where published by the BMJ Group on their behalf), and its Licensees to permit this article (if accepted) to be published in GUT and any other BMJ Group products and to exploit all subsidiary rights, as set out in our licence.
REFERENCES
- 1.Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349:2241–52. doi: 10.1056/NEJMra035010. [DOI] [PubMed] [Google Scholar]
- 3.Brown LM, Devesa SS, Chow WH. Incidence of adenocarcinoma of the esophagus among white Americans by sex, stage, and age. J Natl Cancer Inst. 2008;100:1184–7. doi: 10.1093/jnci/djn211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown LM, Hoover RN, Greenberg RS, et al. Are racial differences in squamous cell esophageal cancer explained by alcohol and tobacco use? J Natl Cancer Inst. 1994;86:1340–5. doi: 10.1093/jnci/86.17.1340. [DOI] [PubMed] [Google Scholar]
- 5.Castellsague X, Munoz N, De SE, et al. Independent and joint effects of tobacco smoking and alcohol drinking on the risk of esophageal cancer in men and women. Int J Cancer. 1999;82:657–64. doi: 10.1002/(sici)1097-0215(19990827)82:5<657::aid-ijc7>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 6.Freedman ND, Abnet CC, Leitzmann MF, et al. A prospective study of tobacco, alcohol, and the risk of esophageal and gastric cancer subtypes. Am J Epidemiol. 2007;165:1424–33. doi: 10.1093/aje/kwm051. [DOI] [PubMed] [Google Scholar]
- 7.Gammon MD, Schoenberg JB, Ahsan H, et al. Tobacco, alcohol, and socioeconomic status and adenocarcinomas of the esophagus and gastric cardia. J Natl Cancer Inst. 1997;89:1277–84. doi: 10.1093/jnci/89.17.1277. [DOI] [PubMed] [Google Scholar]
- 8.Hashibe M, Boffetta P, Janout V, et al. Esophageal cancer in Central and Eastern Europe: tobacco and alcohol. Int J Cancer. 2007;120:1518–22. doi: 10.1002/ijc.22507. [DOI] [PubMed] [Google Scholar]
- 9.Lagergren J, Bergstrom R, Lindgren A, et al. The role of tobacco, snuff and alcohol use in the aetiology of cancer of the oesophagus and gastric cardia. Int J Cancer. 2000;85:340–6. [PubMed] [Google Scholar]
- 10.Pandeya N, Williams G, Green AC, et al. Alcohol consumption and the risks of adenocarcinoma and squamous cell carcinoma of the esophagus. Gastroenterology. 2009;136:1215–2. doi: 10.1053/j.gastro.2008.12.052. [DOI] [PubMed] [Google Scholar]
- 11.Steevens J, Schouten LJ, Goldbohm RA, et al. Alcohol consumption, cigarette smoking and risk of subtypes of oesophageal and gastric cancer: a prospective cohort study. Gut. 2010;59:39–48. doi: 10.1136/gut.2009.191080. [DOI] [PubMed] [Google Scholar]
- 12.Vaughan TL, Davis S, Kristal A, et al. Obesity, alcohol, and tobacco as risk factors for cancers of the esophagus and gastric cardia: adenocarcinoma versus squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 1995;4:85–92. [PubMed] [Google Scholar]
- 13.Anderson LA, Cantwell MM, Watson RG, et al. The association between alcohol and reflux esophagitis, Barrett’s esophagus, and esophageal adenocarcinoma. Gastroenterology. 2009;136:799–805. doi: 10.1053/j.gastro.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 14.Brown LM, Silverman DT, Pottern LM, et al. Adenocarcinoma of the esophagus and esophagogastric junction in white men in the United States: alcohol, tobacco, and socioeconomic factors. Cancer Causes Control. 1994;5:333–40. doi: 10.1007/BF01804984. [DOI] [PubMed] [Google Scholar]
- 15.Veugelers PJ, Porter GA, Guernsey DL, et al. Obesity and lifestyle risk factors for gastroesophageal reflux disease, Barrett esophagus and esophageal adenocarcinoma. Dis Esophagus. 2006;19:321–8. doi: 10.1111/j.1442-2050.2006.00602.x. [DOI] [PubMed] [Google Scholar]
- 16.Wu AH, Wan P, Bernstein L. A multiethnic population-based study of smoking, alcohol and body size and risk of adenocarcinomas of the stomach and esophagus (United States) Cancer Causes Control. 2001;12:721–32. doi: 10.1023/a:1011290704728. [DOI] [PubMed] [Google Scholar]
- 17.Kamangar F, Chow WH, Abnet CC, et al. Environmental causes of esophageal cancer. Gastroenterol Clin North Am. 2009;38:27–57. vii. doi: 10.1016/j.gtc.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cook MB, Kamangar F, Whiteman DC, et al. Cigarette smoking and adenocarcinomas of the esophagus and esophagogastric junction: a pooled analysis from the international BEACON consortium. J Natl Cancer Inst. 2010;102:1344–53. doi: 10.1093/jnci/djq289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ward MH, Sinha R, Heineman EF, et al. Risk of adenocarcinoma of the stomach and esophagus with meat cooking method and doneness preference. Int J Cancer. 1997;71:14–9. doi: 10.1002/(sici)1097-0215(19970328)71:1<14::aid-ijc4>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 20.Corley DA, Kubo A, Zhao W. Abdominal obesity and the risk of esophageal and gastric cardia carcinomas. Cancer Epidemiol Biomarkers Prev. 2008;17:352–8. doi: 10.1158/1055-9965.EPI-07-0748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith-Warner SA, Spiegelman D, Ritz J, et al. Methods for pooling results of epidemiologic studies: the Pooling Project of Prospective Studies of Diet and Cancer. Am J Epidemiol. 2006;163:1053–64. doi: 10.1093/aje/kwj127. [DOI] [PubMed] [Google Scholar]
- 22.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Renehan AG, Tyson M, Egger M, et al. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–78. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 24.Pandeya N, Williams GM, Sadhegi S, et al. Associations of duration, intensity, and quantity of smoking with adenocarcinoma and squamous cell carcinoma of the esophagus. Am J Epidemiol. 2008;168:105–14. doi: 10.1093/aje/kwn091. [DOI] [PubMed] [Google Scholar]
- 25.Islami F, Kamangar F. Helicobacter pylori and esophageal cancer risk: a meta-analysis. Cancer Prev Res (Phila Pa) 2008;1:329–38. doi: 10.1158/1940-6207.CAPR-08-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Corley DA, Kubo A. Influence of site classification on cancer incidence rates: an analysis of gastric cardia carcinomas. J Natl Cancer Inst. 2004;96:1383–7. doi: 10.1093/jnci/djh265. [DOI] [PubMed] [Google Scholar]
- 27.Allen NE, Beral V, Casabonne D, et al. Moderate alcohol intake and cancer incidence in women. J Natl Cancer Inst. 2009;101:296–305. doi: 10.1093/jnci/djn514. [DOI] [PubMed] [Google Scholar]
- 28.Agarwal DP. Cardioprotective effects of light-moderate consumption of alcohol: a review of putative mechanisms. Alcohol Alcohol. 2002;37:409–15. doi: 10.1093/alcalc/37.5.409. [DOI] [PubMed] [Google Scholar]
- 29.Brown L, Kroon PA, Das DK, et al. The biological responses to resveratrol and other polyphenols from alcoholic beverages. Alcohol Clin Exp Res. 2009;33:1513–23. doi: 10.1111/j.1530-0277.2009.00989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gerhauser C. Beer constituents as potential cancer chemopreventive agents. Eur J Cancer. 2005;41:1941–54. doi: 10.1016/j.ejca.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 31.Kubo A, Levin TR, Block G, et al. Alcohol types and sociodemographic characteristics as risk factors for Barrett’s esophagus. Gastroenterology. 2009;136:806–15. doi: 10.1053/j.gastro.2008.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.