Abstract
Asthma is a chronic inflammatory airway disease associated with increased generation of reactive oxidant species and disturbed antioxidant defenses. NRF2 is the master transcription factor that regulates the expression of Phase II antioxidant and detoxifying enzymes. Disruption of NRF2 augments oxidative stress and inflammation in a mouse model of asthma suggesting a protective role of NRF2 in the lungs in vivo. Yet, little is known about the regulation and function of NRF2 in human asthmatics. Using segmental allergen challenge, a well established experimental model of IgE-mediated asthma exacerbation in human atopic asthmatics, we investigated the effect of a specific allergen and the modulatory role of vitamin E on NRF2 and a NRF2-target gene, superoxide dismutase, in alveolar macrophages recovered from the airways at 24h after allergen instillation in vivo. Allergen-provoked airway inflammation in sensitive asthmatics caused a profound inhibition of macrophage NRF2 activity and superoxide dismutase, rendering them incapable of responding to the NRF2 inducers. Prolonged treatment with high doses of the antioxidant vitamin E lessened this allergen-induced drop in alveolar macrophage NRF2. These results are the first to demonstrate that NRF2 expression in human asthmatics is compromised upon allergen challenge but can be rescued by vitamin E in vivo.
Keywords: Atopic asthma, human, alveolar macrophage, oxidative stress, NRF2, superoxide dismutase, antioxidant, vitamin E, segmental allergen challenge, BAL fluid, in vivo
INTRODUCTION
Asthma is a chronic obstructive airway disorder characterized by inflammation and hyperresponsiveness that affects more than 300 million children and adults worldwide [1]. The economic impact of asthma in the USA is estimated in billions of dollars [2]. Human asthma is linked to increased generation of reactive oxidant species (ROS) and impaired antioxidant defenses [3,4]. Yet, current understanding of the pathophysiologic role of oxidative stress in asthma is incomplete.
Nuclear factor (erythroid-derived 2)-like 2, (NRF2) is a transcription factor that heterodimerizes at cis-acting antioxidant response elements (ARE) located in proximal promoters of target genes that encode proteins involved in managing adaptive antioxidant defense, xenobiotic detoxification, proteasome maintenance, cell proliferation, and cell cycle regulation [5]. In addition, studies suggest indirect modulatory effects of NRF2 on various immune and inflammatory pathways [6]. Under basal conditions, constitutively expressed NRF2 undergoes rapid ubiquitylation and proteasome-dependent degradation, a consequence of NRF2’s binding to the CUL3 adaptor protein Keap-1. In cells exposed to oxidative stress, Keap1-dependent ubiquitylation of NRF2 is inhibited and NRF2 is allowed to translocate into the nucleus, heterodimerize with bZip transcription factors, and bind to ARE elements [7]. Disruption of NRF2 expression augments airway inflammation and hyperresponsiveness in a mouse model of allergic asthma, suggesting an important protective role of the NRF2 pathway in allergic and asthmatic responses in vivo [8]. Indeed, recent studies employing a model of mouse dendritic cells exposed to environmental particulate matter [9] and ragweed allergen [10] suggest a restraining function of NRF2 on the pro-allergic phenotype of dendritic cells in vitro [9,10]. Currently, little is known about the regulation and role of NRF2 in the pathophysiology of allergic asthma in humans in vivo.
Vitamin E (α-tocopherol) is a potent lipid soluble ROS scavenging and chain-breaking antioxidant [11] that may prevent the development of atopy and asthma [12,13]. Although vitamin E has previously been investigated in asthma, the lack of evidence for alteration of oxidant stress in prior studies limits conclusions concerning its role [14,15].
In the current study, we investigated the effect of allergic inflammation on the activity and expression of NRF2 in alveolar macrophages and modulation of this effect by prolonged treatment with high doses of vitamin E in human atopic asthmatics in vivo. Segmental allergen challenge is an established experimental model of asthma exacerbation that provides important information regarding the pathophysiology of the IgE-mediated asthmatic inflammation in humans in vivo [16]. The new method of vitamin E supplementation employed in this study was guided by a recent investigation showing that prolonged treatment and high dose of α-tocopherol is necessary for its antioxidant effect in vivo [17].
MATERIALS AND METHODS
Subjects
Nine mild non-smoking allergic asthmatics by NAEPP guidelines [18] and five healthy non-smoking volunteers were recruited from the Nashville community (Table 1). All asthmatic volunteers had positive skin tests with common aeroallergens, methacholine challenge, and inhaled allergen challenge during screening prior to enrollment into the study. Oral antihistamines were discontinued at least 48 h prior to bronchoscopy. No volunteer used inhaled or systemic corticosteroids. Pregnant women were excluded by urine HCG testing. No volunteer had history of a viral respiratory infection in the 6 weeks preceding the study. Patients consented to the protocol verbally and in writing. The protocol was approved by the Vanderbilt University Committee for the Protection of Human Subjects.
Table I.
Patient demographics, baseline characteristics and history of atopy, asthma, and asthma medication use
| Gender, F/M | 8/1 |
| Average age (years), F/M | 27.8 (20–38)/28 |
| Race | |
| Caucasian | 8 |
| African American | 1 |
| Family history of atopy | 9 |
| History of allergic rhinitis | 9 |
| Frequency of daily asthma symptoms | |
| ≤ 2 days/week | 6 |
| > 2 days/week but not daily | 3 |
| Nighttime awakenings | |
| ≤ 2 days/week | 9 |
| > 2 days/week but not daily | 0 |
| Baseline spirometry (%predicted) | |
| FEV1 (%predicted) | 90 (75–109) |
| FEV1/FVC | 0.77 (0.68–0.84) |
| Methacholine PC20 (mg/ml) | 4.1 (0.15–10) |
| Medications | |
| H1-antihistamine (daily or as needed) | 9 |
| Albuterol ≤ 2 days/week | 9 |
| Inhaled corticosteroids | 0 |
| Inhaled long-acting β-agonist | 0 |
| Leukotriene modifying agents | 0 |
| Cromolyns | 0 |
Study protocol
Volunteers underwent bronchoscopy with baseline (before allergen challenge) bronchoalveolar lavage (BAL) in the lingua of the left upper lobe followed by segmental allergen challenge (SAC) in a subsegment of the right middle lobe. The allergen challenged subsegment was lavaged 24 h later. The patients took 1,500 IU of natural vitamin E (d-α-tocopheryl acetate from soy, E-Gems, Carlson Lab. Inc, Arlington Heights, IL, USA) daily for at least 16 weeks with meals to ensure its optimal absorption [19]. At the end of the treatment two bronchoscopies with SAC and collection of BAL were accomplished using the identical protocol. In addition, 5 healthy (non-atopic, non-asthmatic) volunteers underwent baseline bronchoscopy with BAL in the lingua of the left upper lobe (allergen challenge was not performed in normal subjects).
Allergy skin test
Screening prick skin testing was performed with diluent and histamine controls in parallel with a battery of standardized aeroallergens, Dermatophagoides pteronyssinus, Dermatophagoides farinae, cat hair, Bermuda grass, Kentucky bluegrass, fescue meadow grass, orchard grass, redtop grass, ryegrass, sweet vernal grass, timothy grass, short ragweed Greer Laboratories, Lenoir, NC) [20]. The allergen which showed the strongest wheal reaction was selected and subsequently tested intradermally. Intradermal testing was done by injecting of 0.02 ml of serially diluted antigen extracts from 10−5 to 10−3 w/v dilutions. The diluent and histamine 0.01 mg/ml were used as a negative and positive control, respectively.
Bronchoscopy
Volunteers were fasted overnight. Midazolam 1–2 mg and fentanyl 50–100 mcg were administered intravenously if patients wished conscious sedation. Topical lidocaine (≤ 7 mg/kg) was used for airway anesthesia. BAL was performed using three 50 ml aliquots of sterile normal saline and immediately suctioned back. SAC was done using two incremental doses of the allergen to which the volunteers had positive skin test and inhaled allergen challenge, each dose in a 5 ml aliquot of normal saline. The first dose of the allergen was 10-fold higher than the concentration that gave 2+ skin reaction on intradermal test (6–8 mm wheal, 20–30 mm erythema). None of the volunteers developed visible local inflammation after the first dose of allergen. Thus, in each subject the second dose of the same allergen was instilled at concentration 100-fold higher than the 2+ skin reaction, and the bronchoscope was then removed [21]. Allergen used for challenge included (number of subjects in brackets): Dermatophagoides farinae (1), cat hair (6), Bermuda grass (1), ryegrass (1).
Methacholine challenge
Methacholine (Provocholine, Methapharm Inc., Brantford, Ontario, Canada) challenge was undertaken according to the American Thoracic Society guidelines [22] using a Salter dosimeter (Salter Labs, Arvin, CA) and Flow Screen computerized spirometer (VIASYS Healthcare GmbH, Hoechberg, Germany).
Inhaled allergen challenge
Allergen inhalation challenge was carried out as previously described [23]. Allergen aerosols were generated using a Wright nebulizer connected to a 2-way Hans Rudolph valve mouthpiece (Roxon, Montreal, Canada) attached to a wall oxygen source at 50 psi. Spirometry was performed using Flow Screen computerized spirometer.
Measurement of F2-isoprostanes in BAL
F2-isoprostanes, markers of oxidative stress in vivo, were analyzed by stable isotope dilution method in conjunction with gas chromatography-negative ion chemical ionization-mass spectrometry (GC/NICI/MS) [24].
Analysis of vitamin E in plasma
Vitamin E in plasma was analyzed by HPLC as previously described [25].
Isolation of alveolar macrophages from BAL
BAL aliquots were pooled, filtered through loose gauze, and centrifuged at 400 × g for 10 min at 4°C. The BAL fluid cells were resuspended in DMEM culture medium, counted, plated 1×106 cell/ml, and allowed to adhere for 2 h at 37 °C in 5% CO2 incubator. After elimination of non-adherent cells with triple PBS wash, alveolar macrophages were used for experiments [26].
Stimulation of alveolar macrophages with sulforaphane and CDDO ex vivo
Alveolar macrophages isolated from BAL obtained at baseline and 24 h after SAC in vivo were stimulated with synthetic sulforaphane (Sigma-Aldrich Co., St. Louis, USA) and 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oic acid (CDDO) (a kind gift of Dr. Brahm Segal, Buffalo University) at concentration 5 µmol/ml and 1 µmol/ml, respectively, for 2 h at 37°C ex vivo. Sulforaphane was dissolved in 10% DMSO and CDDO in a 10% DMSO 10% cremaphor-EL PBS solution.
Isolation of nuclear and whole cell protein extracts
Cytoplasmic and nuclear extracts were obtained using the NE-PER kit (Pierce, Rockford, IL) in the presence of Complete mini, EDTA-free protease inhibitor cocktail (Roche Diagnostics, Switzerland) at 4°C. Samples were stored at −80°C.
Measurement of DNA binding activity of NRF2
NRF activity in nuclear extracts was measured using a commercial ELISA-based TransAM NRF2 kit (Active Motif North America, Carlsbad, CA). In this assay NRF2 present in nuclear extract binds specifically to ARE-containing oligonucleotide and is detected through use of an antibody against NRF2. Spectrophotometric quantification is accomplished after addition of secondary antibody conjugated to horseradish peroxidase.
Real-time quantitative PCR analysis of NRF2 and SOD-1
RNA was isolated using RNeasy Mini Kit (QIAGEN). 1 ug of total RNA was used to make cDNA by using iScript cDNA Synthesis Kit (BIO-RAD) after treatment with DNA-free (Ambion). Real-time PCR was performed on AB Applied Biosystems Real-time PCR detection system using iQ SYBR Green Supermix (BIO-RAD). All data were normalized to GAPDH expression. Amplification confiscations were 95°C for 5 min, followed by 35 cycles of 95°C for 30s, 55°C for 30s, and 72°C for 30s. Nrf2, SOD1 and GAPDH cDNAs were amplified using the following primers: NRF2f (5’- GATCTTGGAGTAAGTCGAGAAG-3’), NRF2r (5’- GCAAAGTGATAGATCAGAAACATC-3’), SOD-1f (5’- GATCCCAATACACCACAAGC-3’), SOD-1r (5’- CATGTTCATGAGTTTGGAGA-3’), GAPDHf (5’-(TGCACCACCAACTGCTTAGC-3’), GAPDHr (5’- GGCATGCACTGTGGTCATGAG-3’). The relative mRNA amount in each sample was calculated based on its threshold cycle (Ct) in comparison to the threshold cycle of housekeeping gene GAPDH. The relative mRNA expression was presented as 2^ (Ct(housekeeping gene)-Ct(target genes)).
Analysis of NRF2 protein by Western blot
Protein concentration was measured with BCA protein assay kit (Pierce, Rockford, IL). Cell lysate proteins were separated by SDS-polyacrylamide gel electrophoresis in 4–20% Tris-HCl ready gels (Bio-Rad). Resolved proteins then were transferred to polyvinylidene difluoride membranes, which were blocked with 5% milk in TBST buffer (20 mM Tris HCl, pH 7.5, 200 mM NaCl, 0.1% Tween 20) and then probed with anti-actin or anti-GAPDH (Zymed Laboratories Inc.), and anti-NRF2 (Santa Cruz, Santa Cruz, CA) in 5% milk in TBST buffer. After treatment with the appropriate secondary antibody conjugated to horseradish peroxidase (Santa Cruz), immunostained proteins were detected by enhanced chemiluminescence with Western blotting luminol reagent (Santa Cruz) [27].
Statistical analysis
Wilcoxon signed rank test was used for comparisons within the asthmatic group and unpaired t test for comparison of asthmatics with healthy controls. The data regarding the effect of CDDO on NRF2 ex vivo were calculated employing paired t test. Analysis was performed using software package SPSS (SPSS Inc, Chicago, IL). Data were expressed as means ± standard error of mean (SEM). Significance was accepted when two-tailed p < 0.05.
RESULTS
Segmental allergen challenge provoked augmentation of airway eosinophilia (1.07±0.5 vs. 28.4±10.0, baseline vs. allergen, p= 0.015) and neutrophilia (4.3±1.2 vs. 6.6±17.1, p=0.06), and induction of F2-isoprostanes in BAL fluid (1.5±0.1 vs. 4.8±2.6 pg/ml, baseline vs. allergen, p= 0.01). These results demonstrate that allergen challenge provoked airway inflammation and oxidative stress in the airways of asthmatics participating in the study. The DNA binding activity of NRF2 in asthmatic alveolar macrophages recovered at 24 h after allergen instillation was markedly reduced compared to baseline macrophages in vivo. Noteworthy, the post-allergen DNA binding activity of NRF2 in the asthmatic macrophages was significantly lower compared to baseline macrophages obtained from 5 non-smoking healthy volunteers (Fig1). Next we found, that inhibition of the NRF2 DNA binding activity was not related to suppression of the NRF2 gene analyzed by RT-PCR (Fig 2). Because it has been suggested, that use of agents inducing NRF2 could be beneficial in human asthmatics [3], we determined the effect of two potent NRF2 inducers on the NRF2 expression in asthmatic alveolar macrophages. The NRF2 protein in asthmatic macrophages recovered at baseline was modestly stimulated by sulforaphane ex vivo. However, sulforaphane failed to induce the NRF2 protein in cells exposed to allergen in vivo (Fig3A). In another set of experiments, exposure to CDDO ex vivo failed to rescue and upregulate NRF2 binding activity in allergen-challenged alveolar macrophages in vivo (Fig3B). These experiments demonstrate that allergen inhibits activity and expression of the NRF2 protein in asthmatic alveolar macrophages and renders them insensitive to NRF2 inducers. To study the effect of the IgE-mediated inflammation on NRF2-dependent genes, we analyzed expression of SOD-1 by RT-PCR. These experiments showed inhibition of the SOD-1 gene expression in asthmatic alveolar macrophages challenged with allergen in vivo (Fig4). Recent studies have demonstrated protective effects of antioxidants on NRF2 and NRF2-dependent genes in cells exposed to prolonged oxidative stress in vitro [28,29]. Therefore, we determined the effect of vitamin E on NRF2 activity and expression in asthmatic alveolar macrophages in vivo. Prolonged supplementation of vitamin E resulted in augmentation of vitamin E concentrations in plasma (24.0±3.8 vs. 31±3.8 µM, p=0.035, before vs. after supplementation), and significantly reduced levels of F2-isoprostanes in BAL at baseline (1.5±0.1 vs. 0.8±0.2 pg/ml, p=0.04, before vs. after vitamin E), and after allergen challenge (4.8±2.6 vs. 2.2±0.9 pg/ml, p=0.03), thus providing evidence of inhibition of oxidative stress in asthmatic airways in vivo. Studies concerning the effect of vitamin E on NRF2 showed alleviation of the loss of NRF2 activity (Fig5), and the NRF2 protein in the allergen-challenged asthmatic alveolar macrophages in vivo (Fig6).
Fig1.
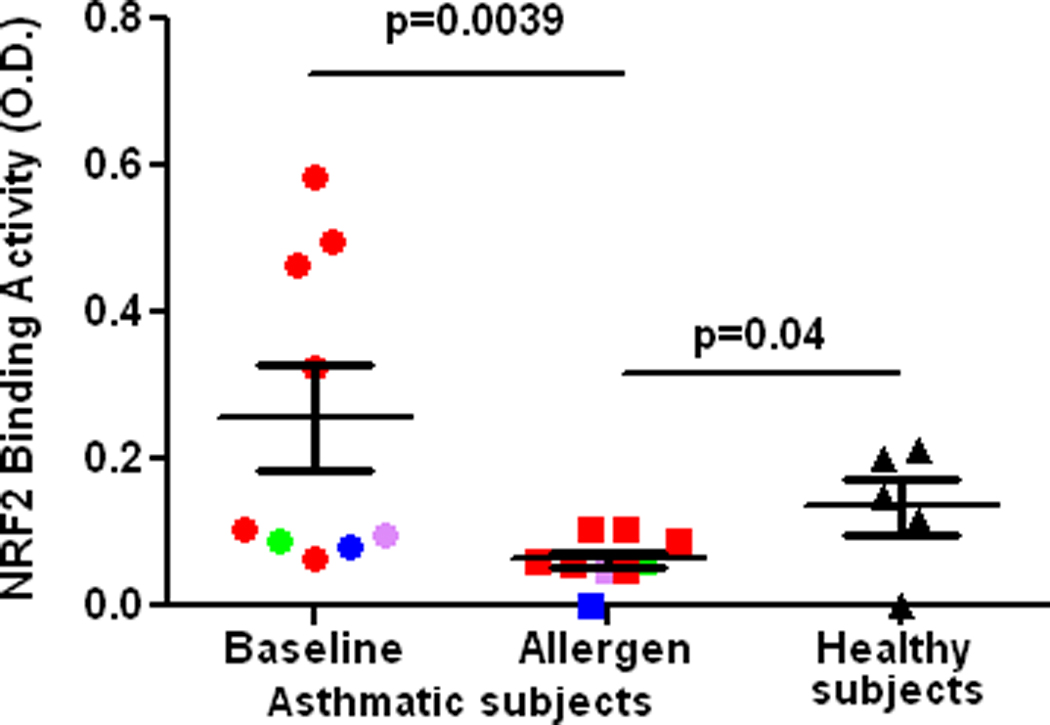
DNA binding activity of NRF2 in alveolar macrophages from the control lung segment (Baseline) and at 24 h after allergen challenge in atopic asthmatics (Allergen), and 5 healthy subjects (non-atopic and non-asthmatic) (Healthy subjects) in vivo. Segmental allergen challenge was not performed in normal volunteers. Red, green, purple, and blue colors indicate various allergens used for challenge, cat, dust mite, and ryegrass, and Bermuda grass, respectively.
Fig2.
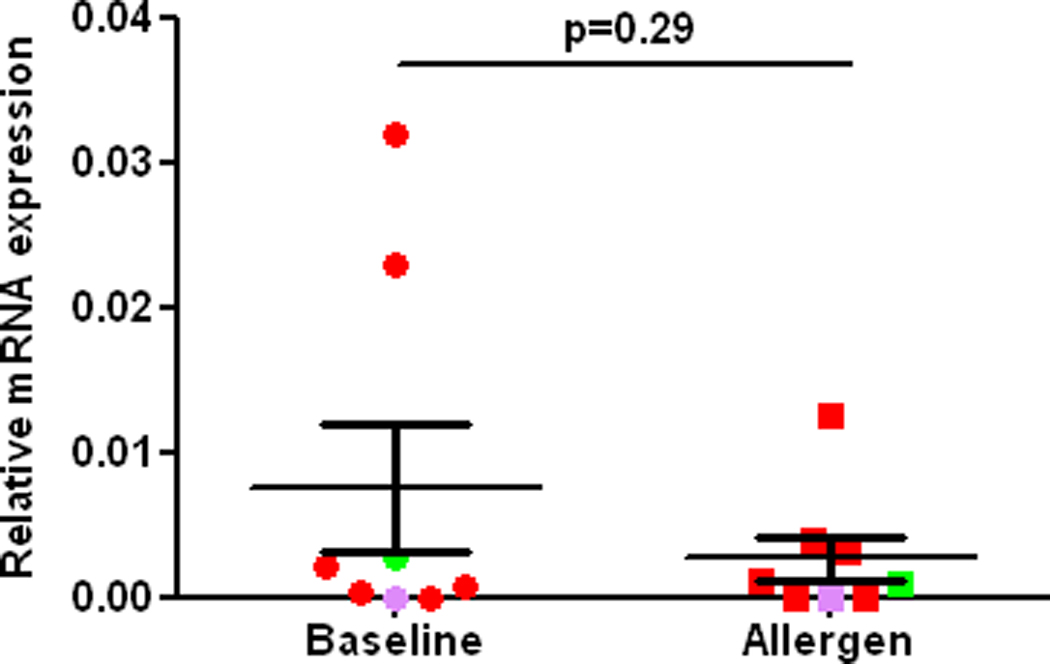
RT-PCR of the NRF2 gene in alveolar macrophages from the control lung segment (Baseline) and at 24 h after allergen challenge (Allergen) in atopic asthmatics in vivo. NRF2 expression is normalized to expression of GADPH. Red, green, and purple, colors indicate various allergens used for challenge, cat, dust mite, and ryegrass, respectively.
Fig3.
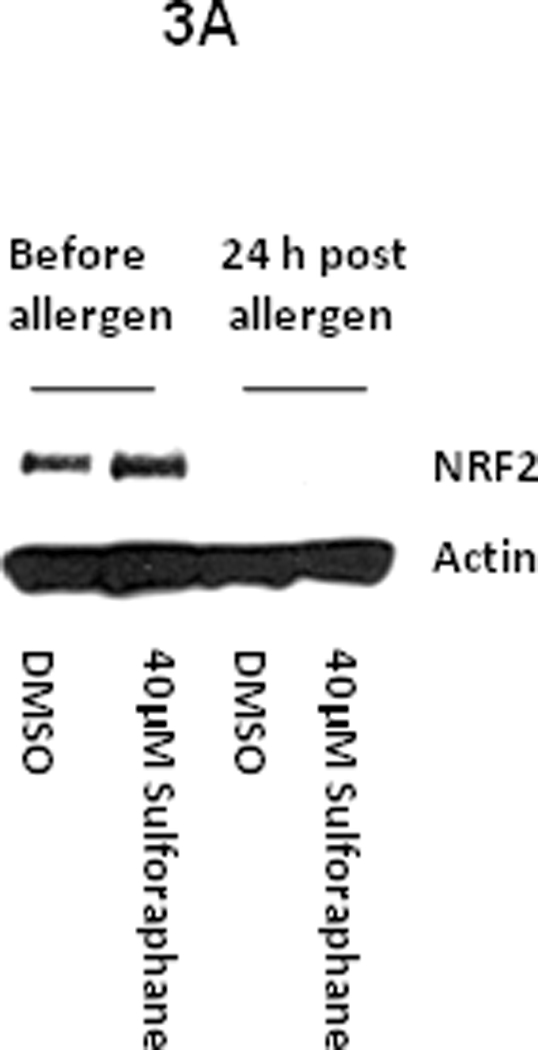
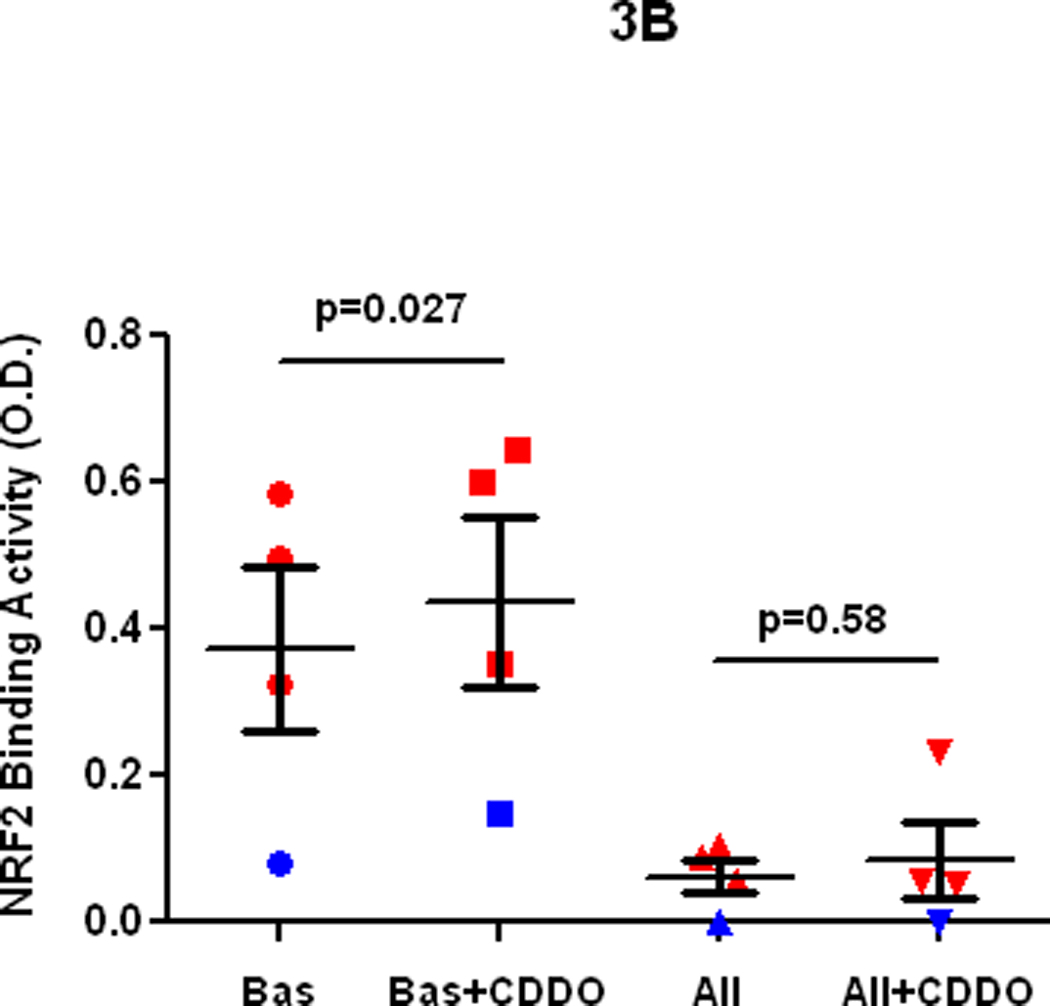
A. Ex vivo effect of sulforaphane on NRF2 protein expression in alveolar macrophages from an atopic asthmatic at baseline and at 24 h after allergen challenge in vivo. B. Ex vivo effect of CDDO on DNA binding activity of NRF2 in alveolar macrophages from the control lung segment and at 24 h after allergen challenge in atopic asthmatics in vivo.
Fig4.
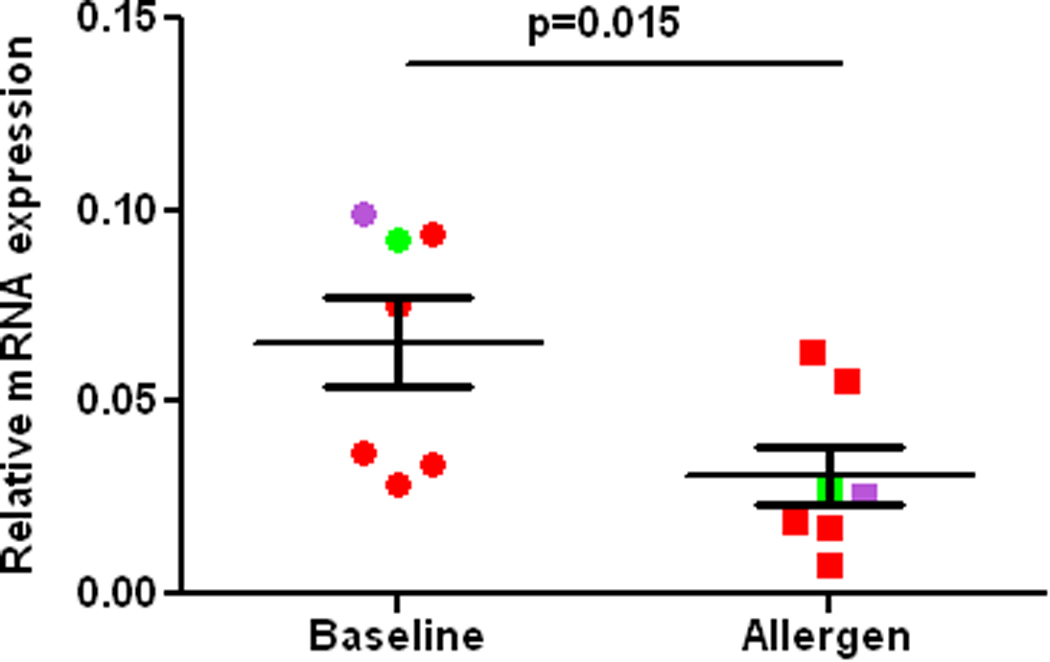
RT-PCR of the SOD-1 gene in alveolar macrophages from the control lung segment (Baseline) and at 24 h after allergen challenge (Allergen) in atopic asthmatics in vivo. SOD1 expression is normalized to expression of GADPH. Red, green and purple colors indicate various allergens used for challenge, cat, dust mite, and ryegrass, respectively.
Fig5.
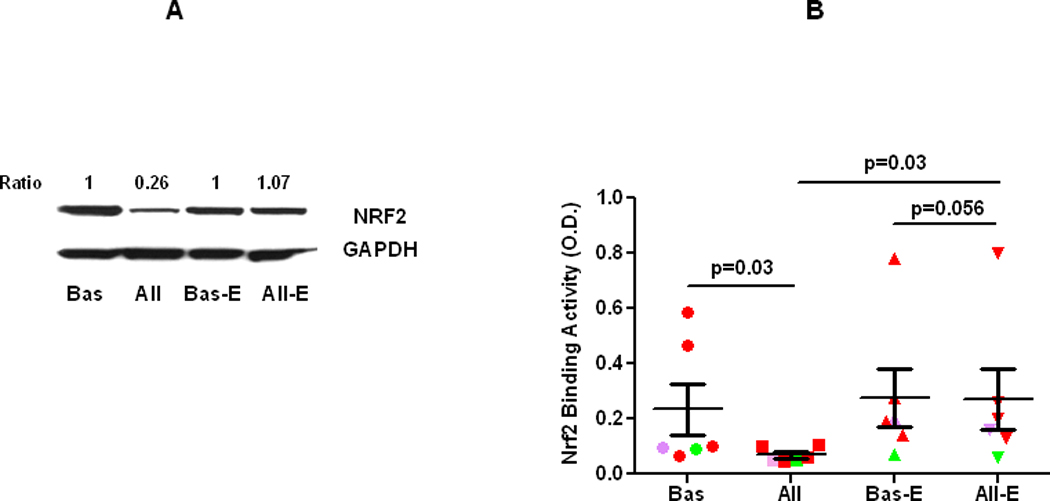
A. Comparison of NRF2 protein expression in alveolar macrophages from the control lung segment and at 24 h after allergen challenge, before (Bas and All, respectively) and after supplementation of vitamin E (Bas-E and All-E, respectively) in atopic asthmatics in vivo. B. Comparison of DNA binding activity of NRF2 in alveolar macrophages from the control lung segment and at 24 h after allergen challenge, before (Bas and All, respectively) and after supplementation of vitamin E (Bas-E and All-E, respectively) in atopic asthmatics in vivo. Red, green, purple, colors indicate various allergens used for challenge, cat, dust mite, and ryegrass, respectively”.
DISCUSSION
The purpose of the study was to determine the effect of allergic inflammation on the activity and expression of NRF2 in alveolar macrophages and modulation of this effect by prolonged treatment with high doses of vitamin E in human atopic asthmatics in vivo. Alveolar macrophages have multifaceted effector and modulatory roles in allergic and asthmatic responses [30], and display high expression of low affinity IgE receptor (FcεRII or CD23) which induces cellular activation after stimulation with specific allergen in vitro and in vivo [26,31,32]. Atopic alveolar macrophages primed with specific allergen in vivo generate greater amounts of superoxide compared with control cells ex vivo [33]. Growing evidence suggests important role of ROS as secondary signaling messengers in macrophages [34], and a protective and modulatory role of the NRF2 and NRF2-regulated genes on macrophage function [35,36].
We demonstrate here that allergic inflammation in vivo causes a profound inhibition of macrophage NRF2 activity resulting in inhibition of the downstream target gene, SOD-1. Given the importance of the alveolar macrophage in airway inflammation, this alteration in oxidant response may have significant effects. The inhibition of NRF2 activity appears to be mediated by ROS-dependent injury, since vitamin E mitigates the reduction in NRF2 activity caused by allergen. However, presently it is unknown if the damaging effect on NRF2 is caused directly by ROS or ROS-generated compounds, because vitamin E as a peroxyl radical scavenger, terminates chain reactions leading to generation of various signaling molecules such as lipid peroxidation products [11,37]. Recently, we have showed that an identical method of prolonged use of high dose of vitamin E inhibited allergen-induced airway oxidative stress and modulated allergic inflammation in human asthmatics in vivo [38]. The dose and timing of vitamin E supplementation in asthmatics was dictated by a recent study showing that a long time and high dose of vitamin E is necessary for it to exert its antioxidant effect in patients with hypercholesterolemia and oxidative stress in vivo [17]. Furthermore, we demonstrate that allergic inflammation renders the macrophage incapable of responding to potent NRF2 stimulation by sulforaphane or CDDO ex vivo [39,40]. Thus, our data suggest that the IgE-mediated inflammation disrupts the fundamental mechanism regulating activation of NRF2 and expression of NRF2-directed genes in the airway cells in vivo. We further surmise that inhibition of NRF2 activity and impairment of critical antioxidant forces in alveolar macrophages and perhaps other airway cells may enhance and prolong oxidant stress, promoting a vicious cycle that contributes to pathogenesis of allergic inflammation and consequently allergen-provoked asthma exacerbation in atopic asthmatics. Our hypothesis offers a unifying explanation for decreased activity of several Phase II enzymes that has been documented in human asthmatics [41].
Currently, neither the molecular mechanism of allergen-induced inhibition of NRF2 activity nor the mechanism of the rescue effect of vitamin E on NRF2 activity in alveolar macrophages during allergic inflammation is known, although our data suggest that allergen does not significantly affect transcriptional regulation of the NRF2 gene. In as much as vitamin E is considered a potent lipid soluble free radical scavenging and chain-breaking antioxidant [11] one could infer that abrogation of the NRF2 activity by allergen is mediated by an excessive generation of ROS. This supposition is supported by recent papers showing that while acute exposure to oxidants induces NRF2 activation, prolonged oxidative stress can result in inhibition of NRF2 activity and reduced expression of NRF2 target genes in cells in vitro [28,29,42]. Given that allergen triggers early [43] and prolonged oxidant stress in the asthmatic airways [44], a similar process could lead to inhibition of NRF2 in airway cells causing protracted oxidant stress associated with asthma exacerbation in atopic asthmatics in vivo. Important in the context of our findings are new studies in human smokers demonstrating downregulation of the NRF2 pathway, likely due to inhibition of DJ-1, a NRF2 stabilizing protein [45], in alveolar macrophages recovered from aged smokers and patients with chronic obstructive pulmonary disease (COPD) [44,46], an airway disorder that is also associated with chronic oxidative stress [47].
At present it is unknown if the late phase of IgE-mediated inflammation affects NRF2 and NRF2-target genes in other structural, immune and inflammatory cells involved in the asthmatic inflammation. This is a vital question in the light of recent data showing that the NRF2 pathway modulates the function of several cell types that are critically important for the pathomechanism of asthma and allergies, such as airway epithelial cells [28,32], dendritic cells [9,10], and B cell lymphocytes [48]. Likewise, our study does not provide information whether downregulation of NRF2 is specific for the IgE-mediated inflammation because subjects with non-allergic chronic inflammatory airway disorders have not been investigated. These questions will be addressed in future studies.
In summary, this is the first demonstration of attenuation of NRF2 ac tivity and its rescue by vitamin E in alveolar macrophages during the late phase of IgE-mediated inflammation in human atopic asthmatics in vivo. Our study suggests that asthmatics may differ in their capacity to deal with an oxidant burden as compared with normal subjects. Inhibition of NRF2 activity and impairment of the critical antioxidant forces in the alveolar macrophages and perhaps other airway cells involved in innate and adaptive immunity by allergen and other pro-inflammatory and pro-oxidant environmental factors may play a significant role in the pathogenesis of allergen-provoked asthma exacerbation in atopic asthmatics. On the other hand, stabilization of the NRF2 action by vitamin E may lessen allergic and asthmatic responses explaining the protective role of vitamin E against developing allergies and asthma as suggested by epidemiologic studies in humans [12,13], and animal research [49]. Further research is warranted to enlighten complex interactions between oxidative stress and the critical components of the antioxidant defenses in human asthmatic airways in vivo.
ACKNOWLEDGMENTS
Supported by NIH grants K23 HL080030, M01 RR-00095, P30 ES000267, 5RO1 CA104590, 5RO1 CA 115556, and 5RO1 CA102353.
We thank Dr. Pierre Massion (Vanderbilt University) for providing normal human BAL fluid samples, and Dr. James May (Vanderbilt University) for analysis of vitamin E in plasma.
LIST OF ABBREVIATIONS
- BAL
Bronchoalveolar lavage
- DMSO
Dimethyl sulfoxide
- FVC
Forced Volume Capacity
- FEV1
Forced Expiratory Volume in 1 second
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
- GC/NICI/MS
Gas Chromatography/Negative Ion Chemical Ionization/Mass Spectrometry
- HCG
Human chorionic gonadotropin
- HPLC
High Pressure Liquid Chromatography
- IgE
Immunoglobulin E
- PBS
Phosphate buffered saline
- PC20
Provocative concentration causing 20% fall in forced expiratory volume in 1 second
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Eder W, Ege MJ, von Mutius E. The asthma epidemic. N Eng J Med. 2006;355:2226–2235. doi: 10.1056/NEJMra054308. [DOI] [PubMed] [Google Scholar]
- 2.Weiss KB, Sullivan SD. The health economics of asthma and rhinitis, I: assessing the economic impact. J Allergy Clin Immunol. 2001;107:3–8. doi: 10.1067/mai.2001.112262. [DOI] [PubMed] [Google Scholar]
- 3.Riedl MA, Nel A. Importance of oxidative stress in the pathogenesis and treatment of asthma. Curr Opin Allergy Clin Immunol. 2008;8:49–56. doi: 10.1097/ACI.0b013e3282f3d913. [DOI] [PubMed] [Google Scholar]
- 4.Nadeem A, Masood A, Siddiqui N. Oxidant-antioxidant imbalance in asthma: scientific, epidemiological data and possible options. Ther Adv Respir Dis. 2008;2:215–235. doi: 10.1177/1753465808094971. [DOI] [PubMed] [Google Scholar]
- 5.Malhotra D, Portales-Casamar E, Singh A, Srivastava S, Arenillas D, Happel C, Shyr C, Wakabayashi N, Kensler TW, Wasserman WW, Biswal S. Global mapping of binding sites of Nrf2 identifies novel targets in cell survival response through ChIP-Seq profiling and network analysis. Nucleic Acid Res. 2010;38:5718–5734. doi: 10.1093/nar/gkq212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wakabayashi N, Slocum SL, Skoko JJ, Shin S, Kensler TW. When Nrft2 talks, who’s listening? Antioxidant & Redox Signaling. 2010;113:1649–1663. doi: 10.1089/ars.2010.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 8.Rangasamy T, Guo J, Mitzner WA, Roman J, Singh A, Fryer AD, Yamamoto M, Kensler TW, Tuder RM, Georas SN, Biswell S. Disruption of Nrf2 enhances susceptibility to severe airway inflammation and asthma in mice. J Exp Med. 2005;202:47–59. doi: 10.1084/jem.20050538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams MA, Rangasamy T, Bauer SM, Killendar S, Karp M, Kensler TW, Yamamoto M, Breysse P, Biswall S, Georas SN. Disruption of the transcription factor Nrf2 promotes pro-oxidative dendritic cells that stimulate Th2-like immunoresponsiveness upon activation by ambient particulate matter. J Immunol. 2008;181:4545–4559. doi: 10.4049/jimmunol.181.7.4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rangasamy T, Williams MA, Bauer S, Trush MA, Emo J, Georas SN, Biswal S. Nuclear erythroid 2 p45-related factor 2 inhibits the maturation of murine dendritic cells by ragweed extracts. Am J Respir Cell Mol Biol. 2010;43:276–285. doi: 10.1165/rcmb.2008-0438OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schneider C. Chemistry and biology of vitamin E. Mol Nutr Food Res. 2005;49:7–30. doi: 10.1002/mnfr.200400049. [DOI] [PubMed] [Google Scholar]
- 12.Devereux G, Turner SW, Craig LCA, McNeill G, Martindale S, Harbour PJ, Helms PJ, Seaton A. Low maternal vitamin E intake during pregnancy is associated with asthma in 5-year old children. Am J Respire Crit Care Med. 2006;174:499–507. doi: 10.1164/rccm.200512-1946OC. [DOI] [PubMed] [Google Scholar]
- 13.Wood LG, Gibson PG. Reduced circulating antioxidant defenses are associated with airway hyper-responsiveness, poor control and severe disease pattern in asthma. Brit J Nutr. 2010;103:735–741. doi: 10.1017/S0007114509992376. [DOI] [PubMed] [Google Scholar]
- 14.Romieu I, Sienra-Monge JJ, Ramírez-Aguilar M, Tellez-Rojo MM, Moreno-Macias H, Reyes-Ruíz NI, del Río-Navarro BE, Ruíz-Navarro MX, Hatch G, Slade R, Hernández-Avila M. Antioxidant supplementation and lung functions among children with asthma exposed to high levels of air pollutants. Am J Respir Crit Care Med. 2002;166:703–709. doi: 10.1164/rccm.2112074. [DOI] [PubMed] [Google Scholar]
- 15.Pearson P, Lewis S, Britton J, Fogarty A. Vitamin E supplements in asthma: a parallel group randomized placebo controlled trial. Thorax. 2004;59:652–656. doi: 10.1136/thx.2004.022616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Busse WW, Wanner A, Adams K, Reynolds HY, Castro M, Chowdhury B, Kraft M, Levine RJ, Peters SP, Sullivan EJ. Investigative bronchoprovocation and bronchoscopy in airway disease. Am J Respir Crit Care Med. 2005;172:807–816. doi: 10.1164/rccm.200407-966WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roberts LJ, II, Oates JA, Linton MF, Fazio S, Meador BP, Gross MD, Shyr Y, Morrow JD. The relationship between dose of vitamin E and suppression of oxidative stress in humans. Free Radical Biol & Med. 2007;43:1388–1393. doi: 10.1016/j.freeradbiomed.2007.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Asthma Education and Prevention Program. Experts Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Bethesda, MD: National Institute of Health, National Heart, Lung, and Blood Institute; 2007. Pub no. 08-5846. [Google Scholar]
- 19.Iuliano L, Micheletta F, Maranghi M, Frati G, Diczfalusy U, Violi F. Bioavailability of vitamin E as function of food intake in healthy subjects: effects on plasma peroxide-scavenging activity and cholesterol-oxidation products. Arteriosclerosis, Thromb & Vasc Biol. 2001;21:E34–E37. doi: 10.1161/hq1001.098465. [DOI] [PubMed] [Google Scholar]
- 20.American College of Allergy, Asthma and Immunology, American Academy of Allergy, Asthma and Immunology, and the Joint Council of Allergy, Asthma and Immunology. Practice Parameters for Allergy Diagnostic Testing. Ann Allergy Asthma Immunol. 1995;75:543–625. [PubMed] [Google Scholar]
- 21.Liu MC, Hubbard WC, Proud D, Stealey BA, Galli SJ, Kagey-Sobotka A, Bleecker ER, Lichtenstein LM. Immediate and late inflammatory responses to ragweed antigen challenge of the peripheral airways in allergic asthmatics: cellular, mediator, and permeability changes. Am Rev Respir Dis. 1991;144:51–58. doi: 10.1164/ajrccm/144.1.51. [DOI] [PubMed] [Google Scholar]
- 22.American Thoracic Society. Guidelines for methacholine and exercise challenge testing. Am J Respir Crit Care Med. 2000;161:309–329. doi: 10.1164/ajrccm.161.1.ats11-99. [DOI] [PubMed] [Google Scholar]
- 23.Cockcroft DW, Davis BE, Boulet LP, Deschesnes F, Gauvreau GM, O’Byrne PM, Watson RM. The links between allergen skin test sensitivity, airway responsiveness and airway response to allergen. Allergy. 2005;60:56–59. doi: 10.1111/j.1398-9995.2004.00612.x. [DOI] [PubMed] [Google Scholar]
- 24.Roberts LJ, Morrow JD. Measurement of F2-isoprostanes as an index of oxidative stress in vivo. Free Radic Biol Med. 2000;28:505–513. doi: 10.1016/s0891-5849(99)00264-6. [DOI] [PubMed] [Google Scholar]
- 25.Lang JK, Gohil K, Packer L. Simultaneous determination of tocopherols, ubiquinols, and ubiquinones in blood, plasma, tissue homogenates, and subcellular fractions. Anal Biochem. 1986;157:106–116. doi: 10.1016/0003-2697(86)90203-4. [DOI] [PubMed] [Google Scholar]
- 26.Joseph M, Tonnel A-B, Torpier G, Capron A. Involvement of immunoglobulin E in the secretory process of alveolar macrophages from asthmatic patients. J Clin Invest. 1983;71:221–230. doi: 10.1172/JCI110762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hong F, Sekhar KR, Freeman ML, Liebler DC. Specific patterns of electrophile adduction trigger Keap 1 ubiquitination and Nrf2 activation. J Biol Chem. 2005;280:31768–31775. doi: 10.1074/jbc.M503346200. [DOI] [PubMed] [Google Scholar]
- 28.Kode A, Rajendrasozhan S, Caito S, Yang S-R, Megson IL, Rahman I. Resveratrol induces glutathione epithelial cells. Am J Physiol.: Lung Cell Mol Physiol. 2008;294:L478–L488. doi: 10.1152/ajplung.00361.2007. [DOI] [PubMed] [Google Scholar]
- 29.Feng Z, Liu Z, Li X, Jia H, Sun L, Tian C, Jia L, Liu J. α-Tocopherol is an effective Phase II enzyme inducer: protective effects on acrolein-induced oxidative stress and mitochondrial dysfunction in human retinal pigment epithelial cells. J Nutr Biochem. 2010;21:1222–1231. doi: 10.1016/j.jnutbio.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 30.Holt PG, Strickland DH, Wikström ME, Jahnsen FL. Regulation of immunological homeostasis in the respiratory tract. Nat Rev Immunol. 2008;8:142–152. doi: 10.1038/nri2236. [DOI] [PubMed] [Google Scholar]
- 31.Rankin JA, Hitchcock M, Merrill W, Bach MK, Brashler JR, Askenase PW. IgE-dependent release of leukotriene C4 from alveolar macrophages. Nature. 1982;297:329–331. doi: 10.1038/297329a0. [DOI] [PubMed] [Google Scholar]
- 32.Williams J, Johnson S, Mascali JJ, Smith H, Rosenwasser LJ, Borish L. Regulation of low affinity IgE receptor (CD23) expression on mononuclear phagocytes in normal and asthmatic subjects. J Immunol. 1992;149:2823–2829. [PubMed] [Google Scholar]
- 33.Calhoun WJ, Reed HE, Moest DR, Stevens CA. Enhanced superoxide production by alveolar macrophages and air-space cells, airway inflammation, and alveolar macrophage density changes after segmental antigen bronchoprovocation in allergic subjects. Am Rev Respir Dis. 1992;145:317–325. doi: 10.1164/ajrccm/145.2_Pt_1.317. [DOI] [PubMed] [Google Scholar]
- 34.Gwinn MR, Vallyathan V. Respiratory burst; role in signal transduction in alveolar macrophages. J Toxicol Environ Health B Crit Rev. 2006;9:27–39. doi: 10.1080/15287390500196081. [DOI] [PubMed] [Google Scholar]
- 35.Li N, Alam J, Venkatesan MI, Eiguren-Fernandez A, Schmitz D, Di Stefano E, Slaughter N, Killeen E, Wang X, Huang A, Wang M, Miguel AH, Cho A, Sioutas C, Nel AE. Nrf2 is a key transcription factor that regulates antioxidant defense in macrophages and epithelial cells: protecting against the pro-inflammatory and oxidizing effects of diesel exhaust particles. J Immunol. 2004;173:3467–3481. doi: 10.4049/jimmunol.173.5.3467. [DOI] [PubMed] [Google Scholar]
- 36.Zhu H, Jia Z, Zhang L, Yamamoto M, Misra HP, Trush MA, Li Y. Antioxidants and Phase 2 enzymes in macrophages: regulation by Nrf2 signaling and protection against oxidative and electrophilic stress. Exp Biol Med. 2008;33:463–474. doi: 10.3181/0711-RM-304. [DOI] [PubMed] [Google Scholar]
- 37.Traber MG, Atkinson J. Vitamin E, antioxidant and nothing more. Free Radical Biol & Med. 2007;43:4–15. doi: 10.1016/j.freeradbiomed.2007.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dworski R, Hoskins A, Borntrager HL, Sanchez SC, Milne GL, Sheller JR, Summar M, Roberts LJ., II Suppression of allergen-induced oxidant stress by vitamin E in atopic asthmatic airways in vivo. Am J Respir Crit Care Med. 2010;81:A5622. [Google Scholar]
- 39.Juge N, Mithen RF, Traka M. Molecular basis for chemoprevention by sulforaphane: a comprehensive review. Cell Mol Life SCi. 2007;64:1105–1127. doi: 10.1007/s00018-007-6484-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liby K, Hock T, Yore MM, Suh N, Place AE, Risingsong R, Williams CR, Royce DB, Honda T, Honda Y, Gribble GW, Hill-Kapturczak N, Agarwal A, Sporn MB. The synthetic triterpenoids, CDDO and CDDO-imidazolide, are potent inducers of heme oxygenase-1 and Nrf2/ARE signaling. Cancer Res. 2005;65:4789–4798. doi: 10.1158/0008-5472.CAN-04-4539. [DOI] [PubMed] [Google Scholar]
- 41.Li N, Nel AE. Role of the Nrf2-mediated signaling pathway as a negative regulator of inflammation: implications for the impact of particulate pollutants on asthma. Antioxidants & Redox Signaling. 2006;8:88–98. doi: 10.1089/ars.2006.8.88. [DOI] [PubMed] [Google Scholar]
- 42.Goven D, Boutten A, Leçon-Malas V, Boczkowski J, Bonay M. Prolonged cigarette smoke exposure decreases heme oxygenase-1 and alters Nrf2 and Bach1 expression in human macrophages: roles of the synthesis by activation of Nrf2 and protects against cigarette-mediated oxidative stress in human lung e MAP kinases ERK½ and JNK. FEBS Lett. 2009;583:3508–3518. doi: 10.1016/j.febslet.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 43.Dworski R, Hoskins A, Sheller JR, Summar M, Roberts LJ., II Isofurans, novel lipid peroxidation products, are increased in bronchoalveolar lavage (BAL), and exhaled breath condensate (EBC) after allergen challenge in human allergic asthmatics in vivo. Am J Respir Crit Care Med. 2009;179:A2513. [Google Scholar]
- 44.Dworski R, Murray JJ, Roberts LJ, II, Oates JA, Morrow JD, Fisher l, Sheller JR. Allergen-induced synthesis of F(2)-isoprostanes in atopic asthmatics. Evidence for oxidant stress. Am J Respir Crit Care Med. 1999;160:1947–1951. doi: 10.1164/ajrccm.160.6.9903064. [DOI] [PubMed] [Google Scholar]
- 45.Malhotra D, Thimmulappa R, Navas-Acien A, Sandford A, Elliott M, Singh A, Chen L, Zhuang X, Hogg J, Pare P, Tuder RM, Biswal S. Decline in NRF2-regulated antioxidants in chronic obstructive pulmonary disease lungs due to loss of its positive regulator, DJ-1. Am J Respir Crit Care Med. 2008;178:592–604. doi: 10.1164/rccm.200803-380OC. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46.Suzuki M, Betsuyaku T, Ito Y, Nagai K, Nasuhara Y, Kaga K, Kondo S, Nashimura M. Down-regulated NF-E2-Related factor 2 in pulmonary macrophages of aged smokers and patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;39:673–682. doi: 10.1165/rcmb.2007-0424OC. [DOI] [PubMed] [Google Scholar]
- 47.MacNee W. Pulmonary and systemic oxidant/antioxidant imbalance in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2005;2:50–60. doi: 10.1513/pats.200411-056SF. [DOI] [PubMed] [Google Scholar]
- 48.Bertolotti M, Yim SH, Garcia-Manteiga JM, Masciarelli S, Kim YJ, Kang MH, Iuchi Y, Fujii J, Vené R, Rubartelli A, Rhee SG, Sitia R. B- to plasma-cell terminal differentiation entails oxidative stress and profound reshaping of the antioxidant responses. Antioxid Redox Signal. 2010;13:1133–1144. doi: 10.1089/ars.2009.3079. [DOI] [PubMed] [Google Scholar]
- 49.Mabalirajan U, Aich J, Leishangthem GD, Sharma SK, Dinda AK, Ghosh B. Effect of vitamin E on mitochondrial dysfunction and asthma features in an experimental allergic murine model. J Appl Physiol. 2009;107:1285–1292. doi: 10.1152/japplphysiol.00459.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]


