Abstract
Corneal transplantation is the oldest, most common, and arguably the most successful form of organ transplantation. In uncomplicated first-time cases, corneal allografts enjoy a success rate of up to 90% even though the transplants are performed without HLA matching or the use of systemic immunosuppressive drugs. In rodents, corneal allografts transplanted across entire MHC and multiple minor histocompatibility barriers enjoy long-term survival in >50% of the hosts, while skin grafts invariably undergo immune rejection. These observations are the basis for “immune privilege” of corneal transplants. In spite of this immune privilege, immune rejection can occur and remains the leading cause of corneal graft failure. Rodent models of penetrating keratoplasty have facilitated studies that have challenged, and in some cases, refuted prevailing dogmas. The long-held belief that CD4+ T helper 1 (Th1) cells were the sole mediators of corneal allograft rejection has fallen to the wayside based on studies in interferon-γ (IFN-γ)−/− mice. The dogma that biasing the alloimmune response down a Th2 pathway would enhance graft survival has also been disproven, and in fact, compelling evidence indicates that Th2-based immune rejection of corneal allografts is swifter and more intense than Th1-based rejection. Animal studies have also preempted emerging dogmas including the hypothesis that Th17 cells play a crucial role in allograft rejection. Instead, IL-17A appears to be necessary for corneal allograft survival. Finally, IFN-γ, and IL-17A, which were normally viewed as proinflamma-tory, exert the opposite effect in the context of corneal transplantation and are necessary for corneal allograft survival.
Introduction
Over 100,000 keratoplasties are performed annually making corneal transplantation the most common form of solid organ transplantation worldwide (Ehlers, 1997). Compared to other forms of solid organ transplantation, which require systemic immunosuppressive treatment and HLA typing, corneal transplantation commonly enjoys a success rate of up to 90% while relying only on the use of topical corticosteroids as the sole immunosuppressive modality to prevent immune rejection (Group, 1992). This success is even more remarkable when one considers that keratoplasty occurs in the absence of HLA histocompatibility matching. Prospective studies in animal models have shown that in the absence of immunosuppressive agents, only 50% of fully allogeneic corneal allografts (i.e., mismatches at the entire MHC, plus the full array of minor histocompatibility alleles) undergo immune rejection compared to a 100% incidence of rejection for skin grafts involving the same donor/host rodent strains (Niederkorn, 2003; Niederkorn, 2006a; Niederkorn, 2007a). These apparent violations of the laws of transplantation are the basis for the notion that corneal allografts are endowed with “immune privilege.” Studies in rodent models of keratoplasty have shown that immune privilege of corneal allografts is attributed to three conditions: a) immunosuppressive molecules within the aqueous humor that prevent immune cell activation and inhibit production of inflammatory mediators; b) corneal cell membrane-bound molecules that block complement activation and induce apoptosis of immune cells; and c) regulatory T cells (Tregs) that suppress immune effector cells involved in corneal transplant rejection (Niederkorn, 2003; Niederkorn, 2006a). In spite of this immune privilege, up to 20% of the keratoplasties performed each year in the United States undergo immune-mediated rejection (www.nei.nih.gov/health/cornealdisease).
Investigations on corneal allografts in rodents have revealed that in vivo depletion of CD4+ T cells dramatically reduces the incidence of immune rejection (Ayliffe et al., 1992; He et al., 1991; Hegde et al., 2005; Yamada et al., 1999a). Additional evidence through adoptive transfer studies confirmed that CD4+ T cells can independently mediate corneal allograft rejection (Cunnusamy et al., 2010a; Hegde et al., 2005). Similarly, downregulation of CD4+ T cell immune responses have been correlated with enhanced graft survival (Hattori et al., 2007). Since its first association with allograft rejection, the CD4+ T cell subset has expanded significantly into several discrete populations. Among the most extensively characterized subsets are the Th1, Th2, Th17, and CD4+CD25+ T regulatory cell lineages. Th1 cells are characterized by their activation of the T-bet transcription factor and their production of interferon-γ (IFN-γ), while Th2 cells express the GATA-3 transcription factor and produce IL-4, IL-5, and IL-13. It has become well recognized that Th1 and Th2 cells cross-regulate each other and that the overexpression of Th2 cytokines prevents the emergence of Th1 immune responses (Mosmann et al., 1986; Mosmann and Sad, 1996). A third category of CD4+ T helper cells, termed Th17 cells, has recently been described based on the secretion of the cytokine IL-17A and the expression of the transcription factor RORγt (Miossec, 2009; Miossec et al., 2009). The current dogma posits that CD4+ Th1 immune cells are the primary, if not the sole, T cell population required for corneal allograft rejection. Moreover, it was previously proposed that biasing the systemic immune response toward a Th2 pathway would cross-regulate the Th1 arm of the alloimmune response and enhance corneal allograft survival. However, studies completed during the past five years have challenged these paradigms and have provided compelling evidence that multiple pathways exist for the immune rejection of corneal allografts and that some maneuvers that were predicted to enhance corneal allograft survival, in fact, exacerbate immune-mediated rejection.
The Evolving Role for Th1 Cells in Corneal Allograft Rejection
Th1 cells are defined by their production of IFN-γ, which can have multiple effects that stimulate macrophages and endothelial cells to produce proinflammatory factors (Cua et al., 1996; Pober et al., 1986). The notion that Th1 cells were the primary mediators for corneal allograft rejection stemmed from observations in human keratoplasty patients and in rodent models of corneal transplantation, which noted the involvement of CD4+ T cells and IFN-γ in rejecting corneal allografts (Pepose et al., 1985; Torres et al., 1996a). Th1 cells mediate delayed-type hypersensitivity (DTH), which is closely associated with corneal allograft rejection, while maneuvers that downregulate donor-specific DTH correlate with long-term corneal allograft survival (Niederkorn and Mellon, 1996; She et al., 1990; Sonoda and Streilein, 1992; Yamada et al., 1999a). Moreover, corneal allograft rejection is dramatically reduced or absent in CD4−/− mice and in rodents treated with depleting anti-CD4 antibodies (Ayliffe et al., 1992; He et al., 1991; Hegde et al., 2005; Hegde and Niederkorn, 2000; Yamada et al., 1999a). Adoptive transfer of allospecific CD4+ T cells to immunoincompetent nude mice results in swift rejection of corneal allografts (Hegde et al., 2005).
The precise effector mechanism by which the Th1 subset mediates graft rejection remains elusive. Possible candidates include soluble inflammatory mediators normally released by Th1 cells during DTH reactions. Indeed, increased protein and mRNA levels of TNF-α and IFN-γ have been detected in rejected corneas (Torres et al., 1996b; Zhu et al., 1999). Also, corneal endothelial cells exposed to these cytokines undergo nitric oxide-induced apoptosis (Sagoo et al., 2004). By contrast, corneal allograft rejection proceeds unimpeded in mice with deletion of genes encoding either TNF-α receptor 1 (TNFRI) or TNF-α receptor 2 (TNFRII) thus highlighting redundancies in the rejection mechanisms (Yamada et al., 1999b). It has also been suggested that Th1-dependent allograft rejection might be mediated by the recruitment of accessory cells such as mononuclear cells. For instance, treatment of corneal cells with TNF-α and IFN-γ has been shown to upregu-late expression of the cell adhesion molecules VCAM-1, ICAM-1, and E-selectin, which are required for recruiting mononuclear cells to the graft site (Goldberg et al., 1994; Iwata et al., 1997; Whitcup et al., 1993).
The weight of evidence described above would suggest that the Th1 cell subset is the sole mediator of allograft rejection. However, several recent reports would suggest otherwise. Indeed, inhibition of the Th1 subset either by gene deletion in IFN-γ−/− mice or systemic depletion with the anti-IFN-γ antibody significantly exacerbates allograft rejection, suggesting that the Th1 subset is not necessary for graft rejection (Cunnusamy et al., 2010a; Hargrave, 2004). Instead, it appears that additional CD4+ T cell subsets might be involved and that IFN-γ might even have a beneficial role in establishing corneal immune privilege (Cunnusamy et al., 2010a).
Th2-based Alloimmune Responses Exacerbate Corneal Allograft Rejection
In the classical T helper cell paradigm, CD4+ Th2 cells produce IL-4, IL-5, and IL-13, which cross-regulate Th1 cells and presumably suppress Th1-mediated immune responses (Mosmann et al., 1986). Accordingly, it has been suggested that skewing the Th1 alloimmune response to the Th2 pathway would enhance graft survival. The first hint that this was not the case stemmed from experiments in IFN-γ−/− mice that cannot generate classical Th1 cells. Instead of abolishing or delaying corneal allograft rejection, elimination of the Th1 pathway by deletion of the IFN-γ gene resulted in an exacerbation of corneal allograft rejection (Hargrave et al., 2002). Moreover, wild-type mice treated with anti-IFN-γ display a similar exacerbation of corneal allograft rejection (Cunnusamy et al., 2010a). CD4+ T cells isolated from anti-IL-17A-treated mice following corneal allograft rejection display a monolithic Th2 cytokine pattern when confronted with donor alloantigens in vitro, yet these hosts reject >90% of their corneal allografts (Cunnusamy et al., 2010a). Similarly, in atopic diseases such as allergic conjunctivitis and allergic airway hyperreactivity, which are characterized by Th2-based immune responses, the incidence and tempo of allograft rejection increase dramatically (Beauregard et al., 2005; Niederkorn et al., 2010; Niederkorn et al., 2009). Interestingly, while corneal allograft rejection in the IFN-γ-deficient host appears to be mediated solely by the Th2 subset, in atopic hosts, allograft rejection appears to be mediated by a combination of allospecific Th1 and Th2 cells which act synergistically to exacerbate allograft rejection (Niederkorn et al., 2010). It is interesting to note that only co-adoptive transfer of Tim3+CD4+ T cells (Th1 cells) and T1ST2+CD4+ T cells (Th2 cells) to SCID mice was able to mirror the tempo and incidence of rejection observed in the atopic hosts (Niederkorn et al., 2010). Thus, there are multiple pathways and mechanisms by which CD4+ T cells can mediate corneal allo-graft rejection (Table 1).
Table 1.
The Role of CD4+ T Cells in Keratoplasty
| CD4+ T Cell | T Helper 1 Cell (Th1) | T Helper 2 Cell (Th2) | T Helper 17 Cell (Th17) | Regulatory T Cell (Treg) |
|---|---|---|---|---|
| Effector cytokine | IFNγ, TNFα, IL-2 | IL-4, IL-5, IL-13 | IL-17A, IL-17F, IL-21, IL-22 | IL-10, TGFβ1 |
| Obligatory transcription factor | T-bet | GATA3 | RORγT | Foxp3 |
| Effector function | Classical mediator of allograft rejection | Exacerbate allograft rejection in atopic hosts | Promote allograft survival | Promote allograft survival |
| Possible effector mechanisms |
|
|
|
|
The mechanisms used by Th2 cells to mediate corneal allograft rejection remain to be elucidated. However, recent observations suggest that Th2-mediated corneal allograft rejection might occur through accessory cells that are activated by allospecific Th2 cells (Goldman et al., 2001). Indeed, in allergic conjunctivitis-associated corneal allograft rejection in mice, significant infiltration of Th2 cells and eosinophils was noted at the rejection site (Beauregard et al., 2005). Moreover, eosinophils, the inflammatory cell population associated with Th2-based inflammation, have been detected in corneal allografts that have undergone rejection in humans with allergic conjunctivitis (Hargrave et al., 2003). It was thought that allospecific Th2 cells migrate to the graft site where they produce IL-5 and recruit eosinophils. Once at the tissue, the eosinophils might mediate damage to the corneal allograft through multiple effector mechanisms (Trocme et al., 1997). Eosinophils secrete an array of cytotoxic granule cationic proteins such as major basic protein (MBP), eosinophil cationic protein (ECP), eosinophil peroxidase (EPO), and eosinophil-derived neurotoxin (EDN), which are capable of inducing tissue damage and dysfunction (Gleich et al., 1993). Eosinophils can also release a variety of cytokines including IFN-γ and TNF-α, which can directly damage the corneal cells (Throsby et al., 2000). Finally, eosinophils can serve as APCs that present antigen to T cells and cause mast cell degranulation, thereby amplifying the inflammatory response locally (MacKenzie et al., 2001; Piliponsky et al., 2002; Shi et al., 2000). Thus, eosinophils possess a plethora of mechanisms that could account for the exaggerated rejection of corneal allografts in hosts with allergic conjunctivitis.
However, two observations indicate that the eosinophil is not necessary, and probably not even involved in corneal allograft rejection in hosts with a Th2-biased alloimmune response. The first observation is based on a simple experiment in which allergic conjunctivitis was induced in one eye and a corneal allograft was placed in the contralateral eye that was not challenged with allergen and did not manifest allergic conjunctivitis (Beauregard et al., 2005). Although the eye that was challenged with allergen expressed allergic conjunctivitis and contained abundant numbers of eosinophils, the contralateral eye was free of eosinophils, yet the tempo and incidence of corneal allograft rejection in the non-allergic eye replicated the exacerbated rejection that occurred when corneal allografts were placed onto eyes with active allergic conjunctivitis (Beauregard et al., 2005). Thus, the most plausible explanation for the exacerbation of corneal allograft rejection in this setting suggests that allergic conjunctivitis produces a systemic effect that denies the corneal allograft its immune privilege. A second observation supporting the hypothesis that allergic diseases exacerbate corneal allograft rejection by exerting a systemic effect stems from studies using a mouse model of airway hyperreactivity (AHR), which is a model of allergic asthma. Like the case with allergic conjunctivitis, mice with short ragweed (SRW) pollen-induced AHR displayed a dramatic increase in the tempo and incidence of corneal allograft rejection compared to non-allergic hosts (Niederkorn et al., 2009). Thus, allergic disease, even in an organ distant from the eye exacerbates corneal allograft rejection leading to the inescapable conclusion that allergic diseases exert a systemic effect that abolishes immune privilege of corneal allografts.
Th17 and IL-17A Promote Immune Privilege of Corneal Allografts
Recently, a newly identified CD4+ Th17 helper cell subset has blurred the distinction between Th1 and Th2 cell-mediated inflammation (Harrington et al., 2005; Park et al., 2005). Characterized by the expression of its signature transcription factor RORγT, and secretion of interleukin 17A, the Th17 cell subset has been implicated in the resistance to certain bacterial pathogens (Harrington et al., 2005; Miossec, 2009; Park et al., 2005). Several reports have also linked Th17 cells to the pathogenesis of several autoimmune diseases and transplant rejection, which were previously thought to be Th1-mediated processes (Cua et al., 2003; Hirota et al., 2007). However, recent reports suggest that the Th17 subset might not be required for allograft rejection (Chen et al., 2009; Cunnusamy et al., 2010a; Yamada et al., 2009). Instead, there is compelling evidence that IL-17A derived from CD4+ T cells is necessary for corneal allograft survival (Cunnusamy et al., 2010a). Indeed, systemic depletion of IL-17A by in vivo treatment with monoclonal antibodies increases the incidence of allograft rejection from 50% to 90% (Cunnusamy et al., 2010a). Subsequent characterization of the CD4+ T cell subsets from the IL-17A-deficient corneal allograft rejector mice demonstrated that the alloreactive CD4+ T cells exclusively produced Th2 cytokines when confronted with corneal allograft alloantigens in vitro and could independently mediate allograft rejection when adoptively transferred in vivo (Cunnusamy et al., 2010a). Although significant infiltration of Th2 cells was found within the rejected corneal allografts in anti-IL-17A-treated mice, very sparse eosinophilic infiltrates were detected, further supporting the notion that Th2-based alloimmune inflammation is neither eosinophil-mediated nor eosinophil-dependent. It is well-established that Th1 and Th17 cell populations cross-regulate each other (Harrington et al., 2005; Park et al., 2005). However, it appears that in the context of keratoplasty, allospecific Th17 cells serve a unique role whereby they cross-regulate Th2 cells. Although this remains to be confirmed, it is clear that blocking IL-17A abolishes the immune privilege of corneal allografts and hastens immune rejection. This is clearly a shift in the paradigm which proposes that Th17 cells are solely a proinflammatory T helper cell subset.
Tregs and Immune Privilege of Corneal Allografts
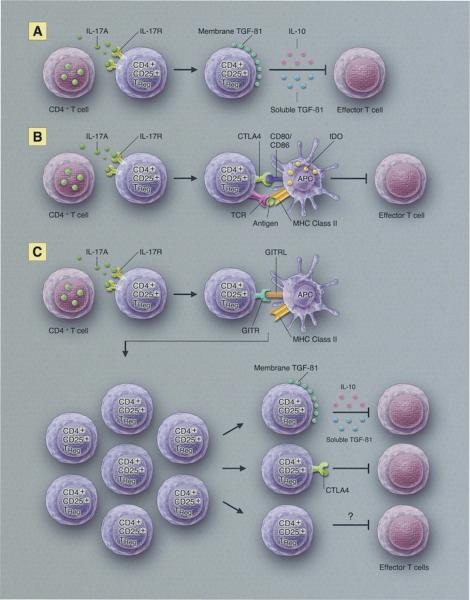
The proposition that T cells might regulate or suppress the immune response was suggested in the 1970s by the Gershon and colleagues, but became mired in controversy with some investigators challenging the very existence of “suppressor T cells,” as they were known at the time (Gershon et al., 1972; Gershon and Kondo, 1971; Moller, 1988). Suppressor T cells languished in the periphery of mainstream immunology until 1995 when Sakaguchi and co-workers published their seminal studies demonstrating the existence of T cells that maintained self-tolerance (Sakaguchi et al., 1995; Sakaguchi et al., 2008). These tolerance-conveying cells were described as “regulatory T cells,” a euphemism that helped distance them from the moniker “suppressor cells” that had provoked >20 years of controversy. Regulatory T cells (Tregs) are now firmly established in mainstream immunology and are associated with various forms of immunologic tolerance including the immune privilege of corneal allografts. CD4+CD25+ Tregs promote allograft survival in mice and have been implicated in the success of organ allografts in clinical settings (Akl et al., 2008; Graca et al., 2002; Joffre et al., 2008; Yoshizawa et al., 2005). CD4+CD25+ Tregs make up 5–10% of the CD4+ T cell population and are identified by the expression of their signature transcription factor Foxp3 (Hori et al., 2003; Vignali et al., 2008). CD4+CD25+ Tregs are involved in a wide range of activities that maintain immunological homeostasis and prevent autoimmune diseases. CD4+CD25+ Tregs can come in two basic varieties: natural Tregs and induced Tregs. Natural Tregs display a T cell receptor (TCR) repertoire that is specific for self antigens and block autoimmune responses. By contrast, induced Tregs are generated in response to specific antigens and utilize a limited TCR repertoire and, as such, have been implicated in alloantigen-specific tolerance (Vignali et al., 2008). In the context of corneal transplantation, CD4+CD25+ Tregs are induced during keratoplasty and have a profound influence on the fate of corneal allografts (Chauhan et al., 2009; Cunnusamy et al., 2010b). Interestingly, the survival of corneal allografts is closely correlated with the level of Foxp3 expression in CD4+CD25+ Tregs and not with the number of CD4+CD25+ Tregs as has been reported with other categories of organ allografts (Gregori et al., 2001; Li et al., 2004; Wang et al., 2008). Further examination of the corneal allograft-induced Tregs revealed that these cells promote graft survival via their production of two well-known soluble immunosuppressive molecules, transforming growth factor-β1 (TGF-β1) and interleukin (IL-10), which directly inhibit T cell proliferation (Figure 1A).
Figure 1.
Potential pathways for IL-17-dependent T regulatory cell enhancement of corneal allograft survival.
As mentioned earlier, IL-17A is required for the induction of alloantigen-specific CD4+CD25+ Tregs and the maintenance of corneal immune privilege. In vivo depletion of IL-17A inhibits Treg activity by downregulating the expression of three well-characterized suppressive molecules that are tethered to the cell membrane: cytotoxic T-lymphocyte antigen-4 (CTLA-4), glucocorticoid-induced tumor-necrosis factor receptor family-related gene (GITR), and membrane-bound TGF-β1. In vitro blocking assays have shown that the inflammatory cytokine, IL-17A, is required for activating Tregs. While it has not been mechanistically described in the corneal allograft models, interaction between CTLA-4 derived from CD4+CD25+ Tregs and CD80/86 present on dendritic cells is thought to upregulate the expression of indoleamine 2,3-dioxygenase (IDO) (Fallarino et al., 2003). IDO catabolizes the amino acid tryptophan, which is essential for CD4+ T cell survival. In addition to starving the T cells, L-kynurenine, a tryptophan metabolite, can render effector T cells apoptotic (Figure 1B). The role of IDO in promoting corneal allograft survival is unresolved. It has been reported that human corneal cells express IDO mRNA and protein and inhibit the proliferation of T cells (Ryu and Kim, 2007). In mice, IDO gene transfer to corneal allografts results in a significant prolongation of corneal allograft survival in a donor/host combination with an exceptionally high incidence of rejection (Beutelspacher et al., 2006). However, it is not known if IDO is generated directly or indirectly by Tregs or the degree to which it influences corneal allograft survival. It is possible that Tregs acting in situ might generate IDO or induce corneal cells to produce IDO, which would disable effector T cells that infiltrate the corneal allograft and thereby promote graft survival.
GITR is the third membrane-bound molecule that is employed by corneal allograft-induced Tregs to mediate suppression. The dynamics of CD4+CD25+ Tregs and GITR ligand (GITRL) interaction that results in alloimmune suppression is currently still under investigation. CD4+CD25+ Tregs are known to constitutively express high levels of GITR (Kanamaru et al., 2004; McHugh et al., 2002). The current paradigm as described in Figure 1C suggests that during an inflammatory response, CD4+CD25+ Tregs expressing GITR interact with GITRL expressed on APCs. This interaction, in conjunction with IL-2 produced by effector cells, leads to an expansion of the CD4+CD25+ Tregs, which subsequently suppress the effector T cell population (Shevach and Stephens, 2006). Although the current pathway has not been defined in the context of corneal allograft transplantation, a recent study by Hori et al. (2010) suggests that GITRL expressed on corneal endothelial cells can stimulate a local expansion in the Treg subset leading to allograft acceptance.
Orthotopic corneal allografts are in direct contact with the anterior chamber (AC) of the eye and are beneficiaries of the immune privilege that occurs at this site. Immune privilege in the AC of the eye has been recognized for over 130 years and within the last 25 years it has been clear that three conditions contribute to the immune privilege in the AC: a) the immunosuppressive and anti-inflammatory molecules that are present in the aqueous humor; b) cell membrane molecules that decorate the cells lining the AC and that induce apoptosis of infiltrating inflammatory cells; and c) a unique form of immune tolerance that is induced when antigens are introduced into the AC -- a phenomenon termed anterior chamber-associated immune deviation (ACAID) (Niederkorn, 2006a; Niederkorn, 2006b; Niederkorn, 2007b). One of the hallmarks of ACAID is the profound downregulation of alloantigen-specific DTH that is induced when alloantigens are injected into the AC. AC injection of donor alloantigenic cells prior to corneal transplantations produces a remarkable downregulation of donor-specific DTH responses and a steep reduction in corneal allograft rejection (Niederkorn, 2006a). It is noteworthy that orthotopic corneal allografts are placed over the AC and are in direct contact with the aqueous humor of the eye. The close proximity of the corneal allograft to the AC, and the likelihood that corneal alloantigens are shed into the AC during surgery has led many to suspect that corneal allografts induce ACAID. Indeed, maneuvers that are known to ablate ACAID invariably lead to corneal allograft rejection (Niederkorn, 2006a). Likewise, hosts with long-term surviving corneal allografts have donor alloantigen-specific suppression of DTH that resembles the suppression that occurs in ACAID (Sonoda and Streilein, 1993). Thus, the weight of evidence would suggest that corneal allografts induce ACAID, which contributes to their survival. However, a recent comparison of the Tregs induced by corneal allografts and those induced by AC injection of alloantigens (i.e., ACAID) revealed important differences between the two Treg populations (Cunnusamy et al., 2010b). For example, hosts with allergic diseases such as allergic conjunctivitis or airway hyperreactivity (AHR) have a profound loss of corneal allograft-induced Treg activity and experience a two-fold increase in corneal allograft rejection, yet have no impairment in their capacity to develop ACAID (Cunnusamy et al., 2010b). Likewise, in vivo treatment with anti-IL-17A abolishes corneal allograft-induced Tregs and results in the immune rejection of 90–100% of the corneal allografts, even though anti-IL-17A-treated mice can develop normal ACAID Tregs (Cunnusamy et al., 2010a; Cunnusamy et al., 2010b). By contrast, in vivo treatment with anti-CD8 antibody abolishes ACAID, but has no effect on corneal allograft survival (Cunnusamy et al., 2010b). Thus, the previously held paradigm that corneal allografts induced ACAID and that their survival was inextricably linked to ACAID must be re-evaluated. It appears that two distinct populations of Tregs can influence the fate of corneal allografts: a) ACAID Tregs that are induced when donor alloantigenic cells are injected into the AC prior to orthotopic corneal transplantation and b) corneal allograft-induced Tregs induced during keratoplasty. Harnessing both Treg populations could have enormous impact on corneal allograft survival.
Conclusions
Research on the pathobiology of allograft rejection has benefited immensely from the advent of the mouse model of penetrating keratoplasty and the wealth of reagents and genetically manipulated hosts that this model brings to bear on these research topics. These tools have provided important insights into the immune privilege of corneal allografts and the conditions that circumvent it. Results from studies with these models compel us to reconsider long-held paradigms. The hypothesis that corneal allograft rejection occurs solely by Th1 cells is no longer tenable. While it is clear that allospecific Th1 cells can mediate corneal allograft rejection, they are not required for rejection. In fact, corneal allograft rejection not only occurs in the absence of conventional Th1 immune responses, but it proceeds at an accelerated tempo and at a higher incidence in Th2-biased hosts (Cunnusamy et al., 2010a; Hargrave, 2004). These results suggest that not only is the Th1 cytokine IFN-γ not needed for immune rejection, but instead, it may be required for maintaining immune privilege of corneal allografts. It was previously believed that Th2 cells cross-regulate Th1 immune responses and that skewing the alloimmune response towards a Th2 pathway would enhance corneal allograft survival. However, IFN-γ−/− mice, or mice with atopic diseases develop robust Th2 immune responses to donor alloantigens and produce the signature Th2 cytokines IL-4, IL-5, and IL-13 when confronted with alloantigens expressed on their corneal allografts, yet the Th2-biased hosts express accelerated corneal allograft rejection (Beauregard et al., 2005; Cunnusamy et al., 2010a), Thus, tilting the alloimmune response in a Th2 direction does not enhance corneal allograft survival, but in fact, has the opposite effect.
The recently discovered Th17 cell population, with its widely recognized proinflammatory properties, was assumed by some to be a potential mediator of corneal allograft rejection. However, closer scrutiny has revealed that not only are Th17 cells unnecessary for corneal allograft rejection, they may instead be needed for maintaining immune privilege. We have recently discovered that IL-17A is required for the function of corneal allograft-induced Tregs and ultimately, for corneal allograft survival (Cunnusamy et al., manuscript in preparation). Thus, IL-17A, like IFN-γ, produces pleiotropic effects that, in some circumstances, provoke inflammation, yet in other settings, exert anti-inflammatory activity and may be necessary for the generation and function of corneal allograft-induced Tregs.
Results from animal models have taught us that perhaps other paradigms need to be challenged. For example, in both the rat and mouse models of penetrating keratoplasty 50% of CD4−/− mice and >50% of rats and mice treated with anti-CD4 antibodies reject their corneal allografts (Ayliffe et al., 1992; He et al., 1991; Niederkorn et al., 2006; Yamada et al., 1999a). A widely held perception is that CD4+ T cells are the primary default pathway for corneal allograft rejection, yet the data are suggesting that in the absence of CD4+ T cells, the immune system has other options for mediating corneal allograft rejection. Thus, disabling CD4+ T cells is not a foolproof strategy for preventing corneal allograft rejection and only uncovers a new set of challenges. Moreover, recent characterization of two novel T helper subsets, Th9 and Th22, makes the task ever more daunting.
Acknowledgments
The work was supported NIH Grants EY 007641 and EY 020799, and an unrestricted grant from Research to Prevent Blindness.
Footnotes
Disclosures The authors have no financial conflicts of interest to disclose.
References
- Akl A, Jones ND, Rogers N, Bakr MA, Mostafa A, El Shehawy El M, Ghoneim MA, Wood KJ. An investigation to assess the potential of CD25highCD4+ T cells to regulate responses to donor alloantigens in clinically stable renal transplant recipients. Transpl Int. 2008;21(1):65–73. doi: 10.1111/j.1432-2277.2007.00560.x. [DOI] [PubMed] [Google Scholar]
- Ayliffe W, Alam Y, Bell EB, Mcleod D, Hutchinson IV. Prolongation of rat corneal graft survival by treatment with anti-CD4 monoclonal antibody. Br J Ophthalmol. 1992;76(10):602–606. doi: 10.1136/bjo.76.10.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauregard C, Stevens C, Mayhew E, Niederkorn JY. Cutting edge: atopy promotes Th2 responses to alloantigens and increases the incidence and tempo of corneal allograft rejection. J Immunol. 2005;174(11):6577–6581. doi: 10.4049/jimmunol.174.11.6577. [DOI] [PubMed] [Google Scholar]
- Beutelspacher SC, Pillai R, Watson MP, Tan PH, Tsang J, Mcclure MO, George AJ, Larkin DF. Function of indoleamine 2,3-dioxygenase in corneal allograft rejection and prolongation of allograft survival by over-expression. Eur J Immunol. 2006;36(3):690–700. doi: 10.1002/eji.200535238. [DOI] [PubMed] [Google Scholar]
- Chauhan SK, Saban DR, Lee HK, Dana R. Levels of Foxp3 in regulatory T cells reflect their functional status in transplantation. J Immunol. 2009;182(1):148–153. doi: 10.4049/jimmunol.182.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Wang W, Xie H, Xu X, Wu J, Jiang Z, Zhang M, Zhou L, Zheng S. A pathogenic role of IL- 17 at the early stage of corneal allograft rejection. Transpl Immunol. 2009;21(3):155–161. doi: 10.1016/j.trim.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Cua DJ, Coffman RL, Stohlman SA. Exposure to T helper 2 cytokines in vivo before encounter with antigen selects for T helper subsets via alterations in antigen-presenting cell function. J Immunol. 1996;157(7):2830–2836. [PubMed] [Google Scholar]
- Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, Zurawski S, Wiekowski M, Lira SA, Gorman D, Kastelein RA, Sedgwick JD. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421(6924):744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- Cunnusamy K, Chen PW, Niederkorn JY. IL-17 promotes immune privilege of corneal allografts. J Immunol. 2010a;185(8):4651–4658. doi: 10.4049/jimmunol.1001576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunnusamy K, Paunicka K, Reyes NJ, Yang W, Chen PW, Niederkorn JY. Orthotopic corneal allografts and alloantigens introduced into the anterior chamber induce two different regulatory T cell populations that promote corneal allograft survival. Invest Ophthalmol Vis Sci. 2010b Aug. doi: 10.1167/iovs.10-6161. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers N. World eye banking. Acta Ophthalmol Scand. 1997;75(5):481. doi: 10.1111/j.1600-0420.1997.tb00146.x. [DOI] [PubMed] [Google Scholar]
- Fallarino F, Grohmann U, Hwang KW, Orabona C, Vacca C, Bianchi R, Belladonna ML, Fioretti MC, Alegre ML, Puccetti P. Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol. 2003;4(12):1206–1212. doi: 10.1038/ni1003. [DOI] [PubMed] [Google Scholar]
- Gershon RK, Cohen P, Hencin R, Liebhaber SA. Suppressor T cells. J Immunol. 1972;108(3):586–590. [PubMed] [Google Scholar]
- Gershon RK, Kondo K. Infectious immunological tolerance. Immunology. 1971;21(6):903–914. [PMC free article] [PubMed] [Google Scholar]
- Gleich GJ, Adolphson CR, Leiferman KM. The biology of the eosinophilic leukocyte. Annu Rev Med. 1993;44:85–101. doi: 10.1146/annurev.me.44.020193.000505. [DOI] [PubMed] [Google Scholar]
- Goldberg MF, Ferguson TA, Pepose JS. Detection of cellular adhesion molecules in inflamed human corneas. Ophthalmology. 1994;101(1):161–168. doi: 10.1016/s0161-6420(94)31370-4. [DOI] [PubMed] [Google Scholar]
- Goldman M, Le Moine A, Braun M, Flamand V, Abramowicz D. A role for eosinophils in transplant rejection. Trends Immunol. 2001;22(5):247–251. doi: 10.1016/s1471-4906(01)01893-2. [DOI] [PubMed] [Google Scholar]
- Graca L, Cobbold SP, Waldmann H. Identification of regulatory T cells in tolerated allografts. J Exp Med. 2002;195(12):1641–1646. doi: 10.1084/jem.20012097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregori S, Casorati M, Amuchastegui S, Smiroldo S, Davalli AM, Adorini L. Regulatory T cells induced by 1 alpha,25-dihydroxyvitamin D3 and mycophenolate mofetil treatment mediate transplantation tolerance. J Immunol. 2001;167(4):1945–1953. doi: 10.4049/jimmunol.167.4.1945. [DOI] [PubMed] [Google Scholar]
- Group CCTSR The collaborative corneal transplantation studies (CCTS). Effectiveness of histocompatibility matching in high-risk corneal transplantation. Arch Ophthalmol. 1992;110(10):1392–1403. [PubMed] [Google Scholar]
- Hargrave S, Chu Y, Mendelblatt D, Mayhew E, Niederkorn J. Preliminary findings in corneal allograft rejection in patients with keratoconus. Am J Ophthalmol. 2003;135(4):452–460. doi: 10.1016/s0002-9394(02)02055-x. [DOI] [PubMed] [Google Scholar]
- Hargrave SL, Hay C, Mellon J, Mayhew E, Niederkorn JY. Fate of MHC-matched corneal allografts in Th1-deficient hosts. Invest Ophthalmol Vis Sci. 2004;45:1188–1193. doi: 10.1167/iovs.03-0515. [DOI] [PubMed] [Google Scholar]
- Hargrave SL, Mellon J, Niederkorn J. MHC matching improves corneal allograft survival in mice with Th2-immune bias. Transplant Proc. 2002;34(8):3413–3415. doi: 10.1016/s0041-1345(02)03612-6. [DOI] [PubMed] [Google Scholar]
- Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6(11):1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- Hattori T, Usui Y, Okunuki Y, Sonoda Y, Usui M, Takada E, Mizuguchi J, Yagita H, Okumura K, Akiba H, Takeuchi M. Blockade of the OX40 ligand prolongs corneal allograft survival. Eur J Immunol. 2007;37(12):3597–3604. doi: 10.1002/eji.200636975. [DOI] [PubMed] [Google Scholar]
- He YG, Ross J, Niederkorn JY. Promotion of murine orthotopic corneal allograft survival by systemic administration of anti-CD4 monoclonal antibody. Invest Ophthalmol Vis Sci. 1991;32(10):2723–2728. [PubMed] [Google Scholar]
- Hegde S, Beauregard C, Mayhew E, Niederkorn JY. CD4(+) T-cell-mediated mechanisms of corneal allograft rejection: role of Fas-induced apoptosis. Transplantation. 2005;79(1):23–31. doi: 10.1097/01.tp.0000147196.79546.69. [DOI] [PubMed] [Google Scholar]
- Hegde S, Niederkorn JY. The role of cytotoxic T lymphocytes in corneal allograft rejection. Invest Ophthalmol Vis Sci. 2000;41(11):3341–3347. [PubMed] [Google Scholar]
- Hirota K, Hashimoto M, Yoshitomi H, Tanaka S, Nomura T, Yamaguchi T, Iwakura Y, Sakaguchi N, Sakaguchi S. T cell self-reactivity forms a cytokine milieu for spontaneous development of IL-17+ Th cells that cause autoimmune arthritis. J Exp Med. 2007;204(1):41–47. doi: 10.1084/jem.20062259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori J, Taniguchi H, Wang M, Oshima M, Azuma M. GITR ligand-mediated local expansion of regulatory T cells contributes to immune privilege of corneal allografts. Invest Ophthalmol Vis Sci. 2010 Aug. doi: 10.1167/iovs.09-4959. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299(5609):1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- Iwata M, Sawada S, Sawa M, Thoft RA. Mechanisms of lymphocyte adhesion to cultured human corneal epithelial cells. Curr Eye Res. 1997;16(8):751–760. doi: 10.1076/ceyr.16.8.751.8983. [DOI] [PubMed] [Google Scholar]
- Joffre O, Santolaria T, Calise D, Al Saati T, Hudrisier D, Romagnoli P, Van Meerwijk JP. Prevention of acute and chronic allograft rejection with CD4+CD25+Foxp3+ regulatory T lymphocytes. Nat Med. 2008;14(1):88–92. doi: 10.1038/nm1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanamaru F, Youngnak P, Hashiguchi M, Nishioka T, Takahashi T, Sakaguchi S, Ishikawa I, Azuma M. Costimulation via glucocorticoid-induced TNF receptor in both conventional and CD25+ regulatory CD4+ T cells. J Immunol. 2004;172(12):7306–7314. doi: 10.4049/jimmunol.172.12.7306. [DOI] [PubMed] [Google Scholar]
- Li Y, Koshiba T, Yoshizawa A, Yonekawa Y, Masuda K, Ito A, Ueda M, Mori T, Kawamoto H, Tanaka Y, Sakaguchi S, Minato N, Wood KJ, Tanaka K. Analyses of peripheral blood mononuclear cells in operational tolerance after pediatric living donor liver transplantation. Am J Transplant. 2004;4(12):2118–2125. doi: 10.1111/j.1600-6143.2004.00611.x. [DOI] [PubMed] [Google Scholar]
- Mackenzie JR, Mattes J, Dent LA, Foster PS. Eosinophils promote allergic disease of the lung by regulating CD4(+) Th2 lymphocyte function. J Immunol. 2001;167(6):3146–3155. doi: 10.4049/jimmunol.167.6.3146. [DOI] [PubMed] [Google Scholar]
- Mchugh RS, Whitters MJ, Piccirillo CA, Young DA, Shevach EM, Collins M, Byrne MC. CD4(+)CD25(+) immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity. 2002;16(2):311–323. doi: 10.1016/s1074-7613(02)00280-7. [DOI] [PubMed] [Google Scholar]
- Miossec P. IL-17 and Th17 cells in human inflammatory diseases. Microbes Infect. 2009;11(5):625–630. doi: 10.1016/j.micinf.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. N Engl J Med. 2009;361(9):888–898. doi: 10.1056/NEJMra0707449. [DOI] [PubMed] [Google Scholar]
- Moller G. Do suppressor T cells exist? Scand J Immunol. 1988;27(3):247–250. doi: 10.1111/j.1365-3083.1988.tb02344.x. [DOI] [PubMed] [Google Scholar]
- Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136(7):2348–2357. [PubMed] [Google Scholar]
- Mosmann TR, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17(3):138–146. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- Niederkorn JY. The immune privilege of corneal grafts. J Leukoc Biol. 2003;74(2):167–171. doi: 10.1189/jlb.1102543. [DOI] [PubMed] [Google Scholar]
- Niederkorn JY. Anterior chamber-associated immune deviation and its impact on corneal allograft survival. Curr Opin Organ Transplant. 2006a;11:360–365. [Google Scholar]
- Niederkorn JY. See no evil, hear no evil, do no evil: the lessons of immune privilege. Nat Immunol. 2006b;7(4):354–359. doi: 10.1038/ni1328. [DOI] [PubMed] [Google Scholar]
- Niederkorn JY. Immune mechanisms of corneal allograft rejection. Curr Eye Res. 2007a;32(12):1005–1016. doi: 10.1080/02713680701767884. [DOI] [PubMed] [Google Scholar]
- Niederkorn JY. The induction of anterior chamber-associated immune deviation. Chem Immunol Allergy. 2007b;92:27–35. doi: 10.1159/000099251. [DOI] [PubMed] [Google Scholar]
- Niederkorn JY, Chen PW, Mellon J, Stevens C, Mayhew E. Allergic conjunctivitis exacerbates corneal allograft rejection by activating Th1 and th2 alloimmune responses. J Immunol. 2010;184(11):6076–6083. doi: 10.4049/jimmunol.0902300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederkorn JY, Chen PW, Mellon J, Stevens C, Mayhew E. Allergic airway hyperreactivity increases the risk for corneal allograft rejection. Am J Transplant. 2009;9(5):1017–1026. doi: 10.1111/j.1600-6143.2009.02603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederkorn JY, Mellon J. Anterior chamber-associated immune deviation promotes corneal allograft survival. Invest Ophthalmol Vis Sci. 1996;37(13):2700–2707. [PubMed] [Google Scholar]
- Niederkorn JY, Stevens C, Mellon J, Mayhew E. CD4+ T-cell-independent rejection of corneal allografts. Transplantation. 2006;81(8):1171–1178. doi: 10.1097/01.tp.0000203140.70742.cb. [DOI] [PubMed] [Google Scholar]
- Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6(11):1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepose JS, Nestor MS, Gardner KM, Foos RY, Pettit TH. Composition of cellular infiltrates in rejected human corneal allografts. Graefes Arch Clin Exp Ophthalmol. 1985;222(3):128–133. doi: 10.1007/BF02173536. [DOI] [PubMed] [Google Scholar]
- Piliponsky AM, Gleich GJ, Bar I, Levi-Schaffer F. Effects of eosinophils on mast cells: a new pathway for the perpetuation of allergic inflammation. Mol Immunol. 2002;38(16–18):1369. doi: 10.1016/s0161-5890(02)00090-1. [DOI] [PubMed] [Google Scholar]
- Pober JS, Gimbrone MA, Jr., Lapierre LA, Mendrick DL, Fiers W, Rothlein R, Springer TA. Overlapping patterns of activation of human endothelial cells by interleukin 1, tumor necrosis factor, and immune interferon. J Immunol. 1986;137(6):1893–1896. [PubMed] [Google Scholar]
- Ryu YH, Kim JC. Expression of indoleamine 2,3-dioxygenase in human corneal cells as a local immunosuppressive factor. Invest Ophthalmol Vis Sci. 2007;48(9):4148–4152. doi: 10.1167/iovs.05-1336. [DOI] [PubMed] [Google Scholar]
- Sagoo P, Chan G, Larkin DF, George AJ. Inflammatory cytokines induce apoptosis of corneal endothelium through nitric oxide. Invest Ophthalmol Vis Sci. 2004;45(11):3964–3973. doi: 10.1167/iovs.04-0439. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155(3):1151–1164. [PubMed] [Google Scholar]
- Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133(5):775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- She SC, Steahly LP, Moticka EJ. Intracameral injection of allogeneic lymphocytes enhances corneal graft survival. Invest Ophthalmol Vis Sci. 1990;31(10):1950–1956. [PubMed] [Google Scholar]
- Shevach EM, Stephens GL. The GITR-GITRL interaction: co-stimulation or contrasuppression of regulatory activity? Nat Rev Immunol. 2006;6(8):613–618. doi: 10.1038/nri1867. [DOI] [PubMed] [Google Scholar]
- Shi HZ, Humbles A, Gerard C, Jin Z, Weller PF. Lymph node trafficking and antigen presentation by endobronchial eosinophils. J Clin Invest. 2000;105(7):945–953. doi: 10.1172/JCI8945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda Y, Streilein JW. Orthotopic corneal transplantation in mice–evidence that the immunogenetic rules of rejection do not apply. Transplantation. 1992;54(4):694–704. doi: 10.1097/00007890-199210000-00026. [DOI] [PubMed] [Google Scholar]
- Sonoda Y, Streilein JW. Impaired cell-mediated immunity in mice bearing healthy orthotopic corneal allografts. J Immunol. 1993;150(5):1727–1734. [PubMed] [Google Scholar]
- Throsby M, Herbelin A, Pleau JM, Dardenne M. CD11c+ eosinophils in the murine thymus: developmental regulation and recruitment upon MHC class I-restricted thymocyte deletion. J Immunol. 2000;165(4):1965–1975. doi: 10.4049/jimmunol.165.4.1965. [DOI] [PubMed] [Google Scholar]
- Torres PF, De Vos AF, Van Der Gaag R, Martins B, Kijlstra A. Cytokine mRNA expression during experimental corneal allograft rejection. Exp Eye Res. 1996a;63(4):453–461. doi: 10.1006/exer.1996.0135. [DOI] [PubMed] [Google Scholar]
- Torres PF, De Vos AF, Van Der Gaag R, Martins B, Kijlstra A. Cytokine mRNA expression during experimental corneal allograft rejection. Exp Eye Res. 1996b;63(4):453–461. doi: 10.1006/exer.1996.0135. [DOI] [PubMed] [Google Scholar]
- Trocme SD, Hallberg CK, Gill KS, Gleich GJ, Tyring SK, Brysk MM. Effects of eosinophil granule proteins on human corneal epithelial cell viability and morphology. Invest Ophthalmol Vis Sci. 1997;38(3):593–599. [PubMed] [Google Scholar]
- Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8(7):523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Jiang J, Guan Q, Lan Z, Wang H, Nguan CY, Jevnikar AM, Du C. Reduction of Foxp3-expressing regulatory T cell infiltrates during the progression of renal allograft rejection in a mouse model. Transpl Immunol. 2008;19(2):93–102. doi: 10.1016/j.trim.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Whitcup SM, Nussenblatt RB, Price FW, Jr, Chan CC. Expression of cell adhesion molecules in corneal graft failure. Cornea. 1993;12(6):475–480. doi: 10.1097/00003226-199311000-00003. [DOI] [PubMed] [Google Scholar]
- Yamada J, Hamuro J, Fukushima A, Ohteki T, Terai K, Iwakura Y, Yagita H, Kinoshita S. MHC-matched corneal allograft rejection in an IFN-gamma/IL-17-independent manner in C57BL/6 mice. Invest Ophthalmol Vis Sci. 2009;50(5):2139–2146. doi: 10.1167/iovs.08-2993. [DOI] [PubMed] [Google Scholar]
- Yamada J, Kurimoto I, Streilein JW. Role of CD4+ T cells in immuno-biology of orthotopic corneal transplants in mice. Invest Ophthalmol Vis Sci. 1999a;40(11):2614–2621. [PubMed] [Google Scholar]
- Yamada J, Streilein JW, Dana MR. Role of tumor necrosis factor receptors TNFR-I (P55) and TNFR-II (P75) in corneal transplantation. Transplantation. 1999b;68(7):944–949. doi: 10.1097/00007890-199910150-00008. [DOI] [PubMed] [Google Scholar]
- Yoshizawa A, Ito A, Li Y, Koshiba T, Sakaguchi S, Wood KJ, Tanaka K. The roles of CD25+CD4+ regulatory T cells in operational tolerance after living donor liver transplantation. Transplant Proc. 2005;37(1):37–39. doi: 10.1016/j.transproceed.2004.12.259. [DOI] [PubMed] [Google Scholar]
- Zhu S, Dekaris I, Duncker G, Dana MR. Early expression of proinflammatory cytokines interleukin-1 and tumor necrosis factor-alpha after corneal transplantation. J Interferon Cytokine Res. 1999;19(6):661–669. doi: 10.1089/107999099313811. [DOI] [PubMed] [Google Scholar]