Abstract
Background & Aims
Progressive Familial Intrahepatic Cholestasis 1 (PFIC1) results from mutations in ATP8B1 (also known as FIC1), a putative aminophospholipid flippase. However conflicting hypotheses have been proposed for the pathogenesis of PFIC1. The aim of this study was to determine whether ATP8B1-deficiency produces cholestasis by altering the activity of the nuclear receptor FXR or by impairing the structure of the canalicular membrane
Methods
ATP8B1/Atp8b1 was knocked down in human and rat hepatocytes, and Caco2 cells using adenoviral and oligonucleotide siRNAs.
Results
ATP8B1 mRNA and protein expression was greatly reduced in human and rat hepatocytes and Caco2 cells. In contrast, FXR expression and several FXR dependent membrane transporters (BSEP, MRP2) were unchanged at mRNA or protein levels in ATP8B1-deficient cells, whereas Mrp3 and Mrp4 were up-regulated in rat hepatocytes. FXR activity remained intact in these cells as evidenced by 6-ECDCA mediated induction of SHP, BSEP and MDR3/Mdr2. Fluorescent substrate excretion assays indicate that Bsep function was significantly reduced in Atp8b1-deficient rat hepatocytes although Bsep remained localized to the canalicular membrane. Exposure to the hydrophobic bile acid, CDCA resulted in focal areas of canalicular membrane disruption by electron microscopy and luminal accumulation of NBD-phosphatidylserine consistent with Atp8b1’s function as an aminophospholipid flippase.
Conclusion
ATP8B1- deficiency predisposes to cholestasis by favoring bile acid-induced injury in the canalicular membrane, but does not directly affect FXR expression, which may occur in PFIC1 as a secondary phenomenon associated to bile acid accumulation.
Introduction
Progressive familial intrahepatic cholestasis type 1 (PFIC1), also called Byler’s disease, is caused by mutations in the ATP8B1 (also known as FIC1) gene(1). Patients with PFIC1 are characterized by progressive cholestatic liver disease, low biliary bile salt concentrations, elevated serum bile salt and bilirubin levels, and low serum cholesterol and γ-glutamyl transfersase levels(2). Immunohistochemical studies indicate that the bile salt export pump (BSEP, ABCB11), multidrug resistance-associate protein 2 (MRP2, ABCC2) and multidrug resistant protein 1 (MDR1, ABCB1) all remain localized on the bile canalicular membrane of hepatocytes, despite its compromised morphological appearance and characteristic ultrastructural abnormalities including “Byler’s bile”(3–5). In contrast to PFIC2 (due to mutations in BSEP), ATP8B1-deficient patients have milder liver damage, and also often suffer from extrahepatic complications, including secretory diarrhea and pancreatitis(2).
Sequence homologies place ATP8B1 as a member of the P4 subfamily of P-type ATPases which function in yeast as aminophospholipid (APL) flippases(1). These flippases translocate APLs from the outer to the inner leaflet of the plasma membrane and certain subcellular vesicles to maintain membrane lipid asymmetry, which is important in maintaining cell signaling and other cell functions. There are 14 members of the P4 P-type ATPase in man with substantial overlap in tissue expression. ATP8B1 is expressed abundantly in the bladder, stomach, and intestine, and to a lesser extent in the liver and pancreas(1). It is localized on the apical membrane of epithelial cells including hepatocytes, cholangiocytes and enterocytes(3). Functional studies using heterologously expressed ATP8B1 in UPS-1 cells (a CHO-K1 mutant cell line with a defect in the nonendocytic uptake of the NBD-PS analog) indicate that ATP8B1 can function as a phosphatidylserine flippase(6; 7), where CDC50 is required for its membrane targeting (7). However, it is not clear how ATP8B1-deficiency leads to cholestasis.
Recently, two groups have reported that PFIC1 is associated with decreased expression and activity of the bile acid nuclear receptor Farnesoid X receptor (FXR, NR1H4), suggesting that ATP8B1 regulates FXR activity and that impaired FXR signaling results in PFIC1(8–10). However, reduced expression of FXR and its target genes are also seen in other cholestatic liver diseases, including PFIC2 and Biliary Atresia, suggesting that alteration of FXR gene expression may be secondary to the effects of cholestasis rather than its primary cause(11). Furthermore, Atp8b1-deficient mice (Atp8b1G308V/G308V) retain expression of Fxr and its target genes (Bsep, Ntcp, Asbt, Cyp7A1). These mice have disturbances in bile salt homeostasis, but no significant impairment in bile secretion unless infused with cholic acid(12). The transport of hydrophobic bile salts into bile is impaired in Atp8b1-deficient perfused mice livers, in association with increased biliary excretion of cholesterol and phosphatidylserine(4). These studies suggest that Atp8b1-deficiency renders the canalicular membrane less resistant to hydrophobic bile salts which subsequently results in impairment in bile salt excretion and cholestasis.
To further elucidate the pathogenesis of PFIC1, we have examined if ATP8B1 plays a role in FXR expression and function by knocking down ATP8B1/Atp8b1 in human cells and rat hepatocytes. We have investigated if ATP8B1-deficiency affects both the activity and membrane localization of transport proteins or results in functional impairment of bile acid transport and/or ultrastructural damage to the bile canalicular membrane. Our findings indicate that ATP8B1 does not play a direct role in FXR function, but that ATP8B1-deficiency predisposes the canalicular membrane to injury by hydrophobic bile acids, findings that can explain the development of cholestasis in patients with PFIC1.
Materials and methods
Materials
Chemicals were purchased from Sigma, except where otherwise specified. Cell culture media (DMEM and William E), fetal bovine serum (FBS), penicillin/streptomycin, trypsin and phosphate buffered saline (PBS) were from Invitrogen (Carlsbad, CA). HMM medium is from Lonza (Walkersville, MD). Collagen coated plates, collagen and Matrigel were purchased from BD Sciences (Bedford, MA). ECL reagents were from Amersham. DNA oligos and sequencing were provided by the Keck Biotechnology Center at Yale University. Cholylglycylamidofluorescein (CGamF) was a gift from Dr. Alan Hofmann (San Diego, CA). 5-chloromethylfluorescein diacetate (CMFDA) was purchase from Molecular Probes (Eugene, OR). 1-palmitoyl-2-[6-[(7–nitrobenz-2-oxa-1,3-diazol-4-yl)amino]caproyl]-sn-glycero-3-phosphatidylserine (NBD-PS) was obtained from Avanti Polar Lipids, Inc. (Alabaster, Alabama).
Small interference RNA (siRNA) oligonucleotides and Adenoviral siRNA (Ad-siRNA) constructs
Four sets of double-strand siRNA oligos that target human ATP8B1 were chemically synthesized and annealed to duplex by Dharmacon, Inc. (Chicago, IL) (Supplementary materials, Table S1). Control RNA oligo, siCONTROL RISC-Free siRNA (sequence proprietary) was also purchased from Dharmacon. Four double-strand DNA oligos that target both human and rat ATP8B1/Atp8b1 were synthesized (Supplementary materials, Table S1) and used for Ad-siRNA constructs. These constructs were made using Adeno-X system from BD Sciences, and the virus was generated and titrated according to the manufacturer’s instruction. All four viral constructs were tested in both human and rat hepatocytes, and ATP8B1 Ad-siRNA2 and 4 demonstrated high knockdown efficiency.
Cell culture, transfection and infection
Human hepatocytes were obtained from the Liver Tissue Procurement and Distribution System of the NIH (Dr. Stephen Strom, University of Pittsburgh), and maintained on collagen coated plates using HHM medium and overlaid with Matrigel. Rat hepatocytes were isolated by collagenase perfusion and maintained in a collagen sandwich culture system as previously described(13). Hepatocytes were infected with Ad-siRNA at 15 MOI. Before harvesting the cells, the culture medium was collected for assays for lactate dehyrogenase (LDH) activity, as a measure of cytotoxicity, using a kit from Thermo Electron (Louisville, CO). Caco2 cells were grown in DMEM medium with 20% FBS in a 37°C 5% CO2 incubator. Cells were transfected when 70% confluent using Lipofectamine 2000 (Invitrogen) in serum-free medium for 16 hrs. Specifically, for each well in a 6-well plate, 25 pmoles of RNA oligo were mixed with 12 µl of Lipofectamine 2000 in 500 µl Opti-MEM for 20 min. The mixture was applied to cells. After transfection, cells were maintained in 5% FBS DMEM for an additional 24, 48, or 72 hrs. To test for FXR activation, 2µM 6-ECDCA, or 50µM CDCA or DMSO (vehicle control) was used to treat the cells for an additional 24 hr.
Quantitative Real-time PCR Analysis
Total RNA was extracted, purified and converted to cDNA as template. TaqMan real-time PCR was used to detect each gene mRNA expression. The detail information is described in supplementary materials (Table S2).
Western blot Analyses
Protein was extracted from samples and analyzed as we previously described(13). The sources and dilution of antibodies were listed in supplementary materials (Table S3).
Confocal microscopy
Caco2 cells and rat hepatocytes grown on coverslips were fixed using cold methanol. After 5% BSA blocking, the coverslips were incubated with primary antibody for 2 hr at room temperature or overnight at 4°C, and followed by fluorescence conjugated secondary antibody. The images were visualized using Zeiss LSM 510 confocal microscope. FXR, Bsep and Mrp2 antibodies were used at 1:25, 1:50, and 1:100 dilution, respectively.
CGamF and CMFDA excretion assay
5 days rat hepatocytes on coverslips were briefly washed twice using warm (37°C) HEPES buffer, followed by 10 min incubation in warm HEPES buffer containing 1 µM CGamF at 37°C. Then the coverslips were washed twice using ice-cold HEPES buffer. Six fluorescent images were blindly obtained from each coverslip. Similar procedure was used for detection of CMFDA excretion except CMFDA (5 µM) was loaded for 10 min first, after a brief wash the cells were incubated at 37°C in HEPES buffer for additional 10 min before visualization using the confocal microscope. Image J software was used to analyze the canalicular fluorescence intensity. Briefly, fluorescent pseudocanaliculi were delineated because of the striking contrast between them and surrounding cells. The mean value of the fluorescent intensity from each pseudocanaliculus was measured, and the average values of fluorescent intensity of all pseudocanaliculi from each experiment were compared.
NBD-PS assay
Canalicular NBD-PS fluorescent intensity was measured as previously described (14) with the following modifications. Hepatocyte collagen sandwich cultures on coverslips were loaded with 4 µM NBD-PS in HEPES buffer for 20 minutes on ice followed by a 20 minute incubation at 37°C for internalization of the fluorescent lipid. Then the HEPES buffer was replaced with 5% FBS William E medium containing either 5 µM CDCA or UDCA or medium without bile acid for 16 hr. The coverslips were coded by SYC and six images from each coverslip, each showing phase contrast and fluorescence, were obtained by CJS in a blinded fashion. The phase contrast image was used to identify and delineate the canalicular spaces and this outline was then superimposed over the identical fluorescent image. The mean fluorescence intensity of the luminal space was determined using Image J software. The mean fluorescent values were binned into 2 groups, either above or below 20 fluorescent units, based upon the control levels. The percent canalicular lumens in each group were then determined for each of the treatment conditions.
Electron Microscopy
Rat hepatocytes sandwich cultures on coverslips were fixed with 2% paraformaldehyde/2.5% glutaraldehyde and postfixed with 1% osmium tetroxide. The cultures were blocked stained with 2% uranyl acetate, dehydrated in acetone series and epon embedded. Thin sections were stained with lead citrate and uranyl acetate. Electron micrographs were acquired on a Philips 410 electron microscope (FEI, Hillsboro, OR) and evaluated blindly by JLB.
Statistical Analysis
Data are expressed as means ± standard deviation (SD). Differences between experimental groups were assessed for significance using the two-tailed paired Student t-test. A P value of < 0.05 was considered to be statistically significant.
Results
ATP8B1-deficient human cells maintained FXR expression and function
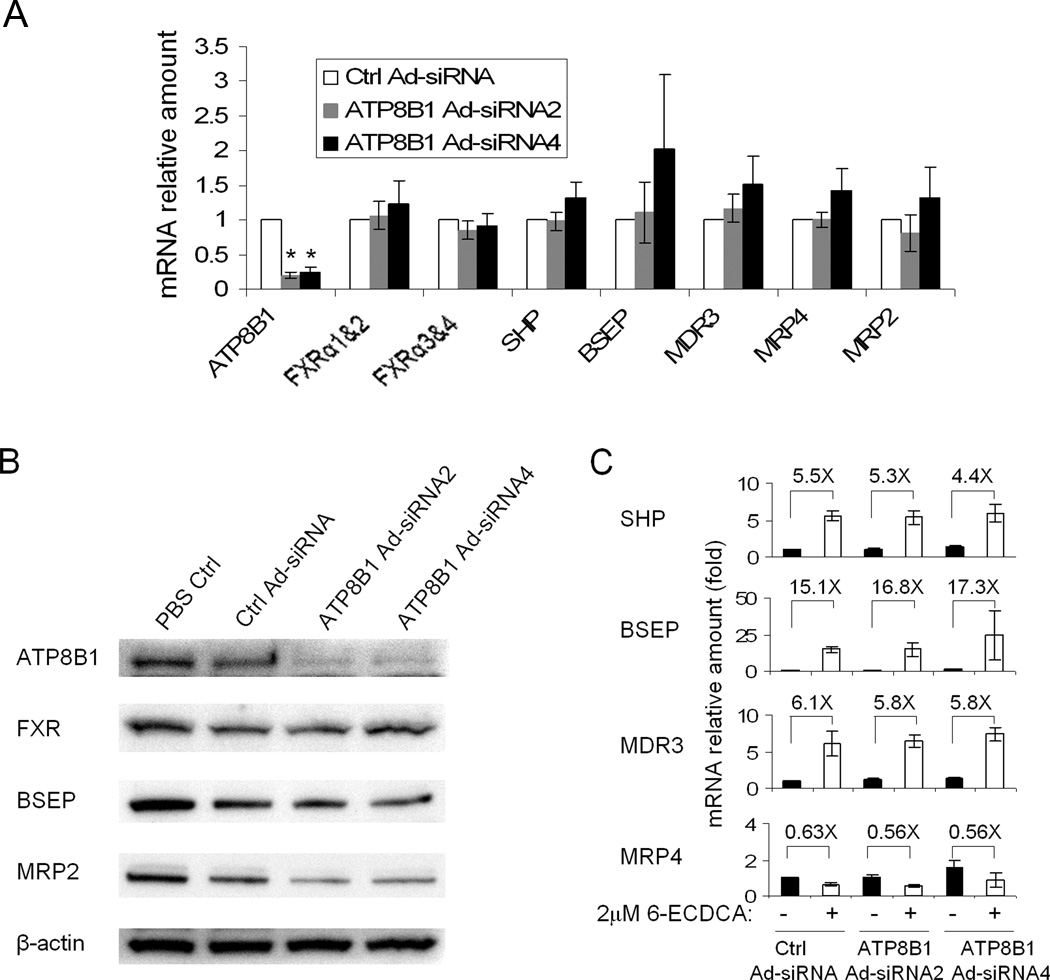
To determine if ATP8B1 plays a direct role in FXR expression and activity, we first knocked down ATP8B1 expression in human hepatocytes using an adenoviral vector. Both mRNA and protein expression of ATP8B1 were reduced to less than 25% by 4 days compared to control virus infection (Fig. 1A &B). In contrast, the basal mRNA and/or protein expression of FXR, SHP, BSEP, MRP2, MDR3 and MRP4 were not significantly changed (Fig. 1A &B and data not shown). To further examine if FXR functional activity is compromised in ATP8B1-deficient human hepatocytes, we treated these cells with 2µM 6-ECDCA (a potent FXR agonist) for 24hr after 3 days of virus infection. Q-PCR analysis demonstrated that FXR targets SHP, BSEP, and MDR3 mRNA expression were all markedly induced to very similar extents by 6-ECDCA compared to their correspondent medium controls, whereas MRP4 mRNA expression was slightly decreased by 6-ECDCA treatment (Fig. 1C). Together, these results indicate that FXR expression and functional activity are intact in ATP8B1-deficient human hepatocytes, in contrast to prior reports in Caco2 cells(8).
Figure 1.
mRNA and protein expression in ATP8B1 knockdown human hepatocytes. Hepatocytes were treated with scrambled Ad-siRNA (Ctrl Ad-siRNA), ATP8B1 Ad-siRNA2, or ATP8B1 Ad-siRNA4 and cultured for 4 days with Matrigel overlay. A, The basal mRNA expression of ATP8B1, FXR and its targets. Data are normalized to GAPDH, and the mRNA amount of each gene from Ctrl Ad-siRNA is set as 1. Data represent the means ±SD of 4 independent experiments, *p<0.001. B, Western blot of FXR, BSEP and MRP2, and β-actin is shown as loading control. C, The FXR agonist, 6-ECDCA (2 µM for 24 hr) stimulated BSEP, SHP, and MDR3 mRNA expression, but not MRP4 expression in ATP8B1 knockdown cells. The fold changes (X) are relative to their own medium control. Data represent the means ±SD of 3 independent experiments.
To verify if these differences are due to tissue specificity in gene expression, we knocked down ATP8B1 expression in Caco2 cells by transfection of synthetic siRNA oligonucleotides, as the adenovirus had a very low infection efficiency in these cells. Again, Q-PCR analysis revealed that ATP8B1 mRNA expression was decreased to about 27% compared to its control 40 hr after transfection, and remained at that level 64hr and 88hr after transfection (Supplementary data, Figure S1). Western blot analysis demonstrated that ATP8B1 protein expression was also markedly reduced 64hr and 88hr after transfection (Figure S1). In contrast, the basal mRNA expression of FXR and its target gene IBABP were essentially unchanged at all time points. When treated with CDCA or 6-ECDCA 40hr after transfection for an additional 24hr, Q-PCR analysis revealed that 50 µM CDCA and 2 µM 6-ECDCA both induced IBABP and SHP mRNA expression about 20-fold and 2-fold, respectively, in ATP8B1 knockdown Caco2 cells when compared to their medium treatment controls (data not shown). These results are consistent with our observation from ATP8B1-deficent human hepatocytes. In addition, immunofluorescent staining demonstrated that nuclear localization of FXR protein remained the same as with control oligos (siCONTROL) transfected in Caco2 cells (data not shown).
Intact Fxr expression and function in Atp8b1-deficient rat hepatocytes
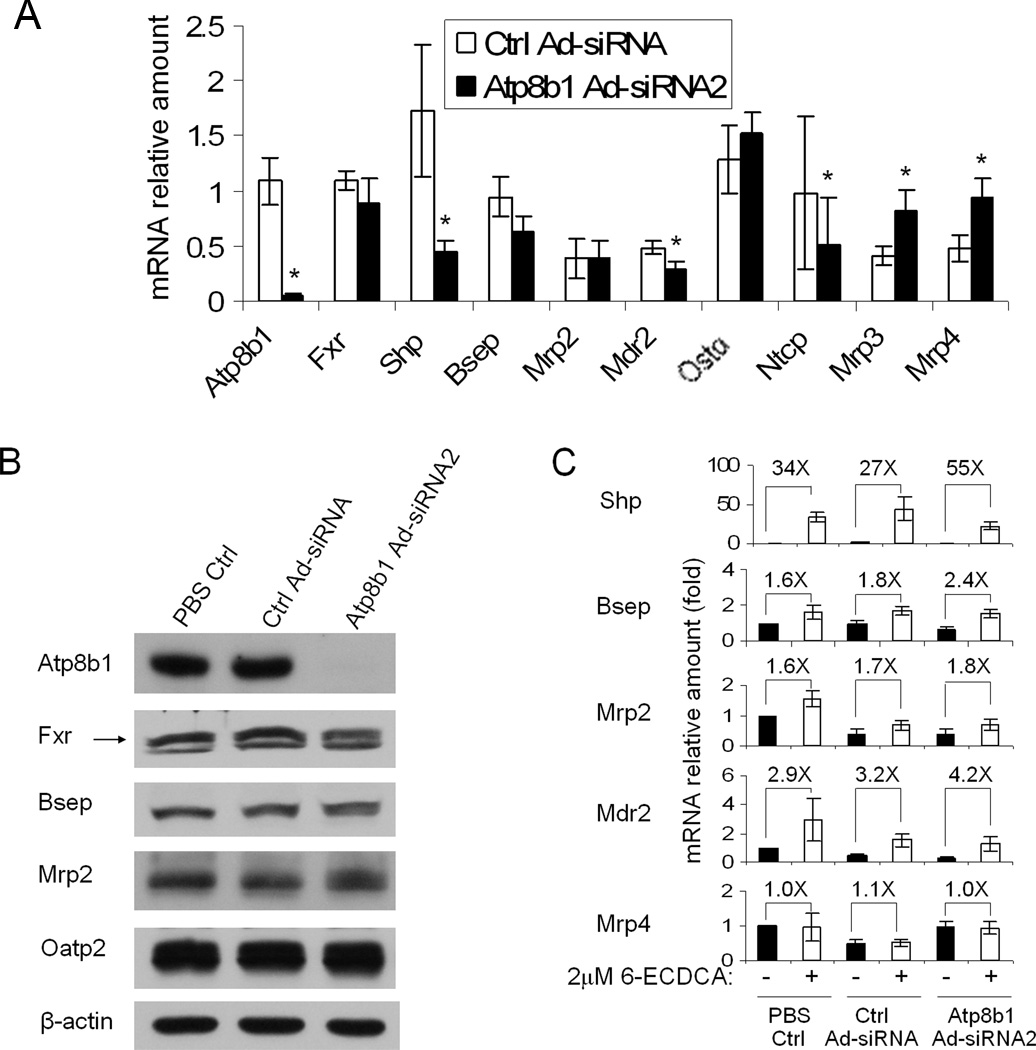
Because there was considerable variability in the quality and the tendency to form bile canaliculi in the human hepatocyte cultures, we sought to repeat these experiments using rat hepatocytes in a collagen gel sandwich configuration. Rat hepatocytes in sandwich culture maintain a highly polarized and differentiated phenotype, both morphologically and functionally, and therefore are an excellent in vitro model of the intact liver(15). To confirm these findings in rat hepatocytes, we again knocked down Atp8b1 expression using adenovirus. In this culture system, we were able to knockdown Atp8b1 mRNA and protein expression levels by more than 90% by 5 days after infection (Fig. 2A&B). As in human cells, we did not observe any significant changes in mRNA and/or protein expression levels in Atp8b1 knockdown cells either of Fxr or Fxr regulated genes, Bsep, Mrp2, and Ostα compared to the control virus infected cells. However, there was significant down-regulation (paired analysis, p<0.05) in the expression of Shp (76%), Mdr2 (38%) and Ntcp (47%) and up-regulation of Mrp3 (99%), and Mrp4 (98%) expression in these experiments. In addition, Cyp7A1 mRNA expression was substantially repressed in these Atp8b1-deficient cells (data not shown). All of these changes in gene expression with the exception of Mdr2 are similar to the characteristic adaptive responses in transporter expression that have been observed in cholestatic animal models in an attempt to decrease hepatic toxicity by reducing the hepatic uptake, synthesis and retention of bile salts (16).
Figure 2.
mRNA and protein expression in Atp8b1 knockdown rat hepatocytes in collagen sandwich gel. Rat hepatocytes were treated with PBS, scrambled Ad-siRNA (Ctrl Ad-siRNA), or ATP8B1 Ad-siRNA2 and cultured for 5 days. Data represent the means ±SD of 4 independent experiments. A, Basal mRNA expression of Atp8b1, Fxr, Shp, Bsep, Mrp2, Mdr2, Ostα, Ntcp, Mrp3 and Mrp4. Data is normalized to GAPDH, and the amount of mRNA of each gene from the PBS control is set as 1. *p<0.05, n=4. B, Western blot of Fxr, Bsep, Mrp2 and Oatp2, β-actin is shown as loading control. C, The FXR agonist (2µM 6-ECDCA for 24 hr) stimulated Bsep, Shp, Mrp2, and Mdr2 mRNA expression, but not Mrp4 expression in Atp8b1 knockdown cells. The fold changes (X) are relative to their own medium control.
Furthermore, the intact Fxr activation by 6-ECDCA is also confirmed in these Atp8b1-deficent rat hepatocytes. As seen in Figure 2C, 2 µM 6E-CDCA stimulated Shp mRNA expression by 34-, 27-, and 55-fold in uninfected, scrambled Ad-siRNA infected, and Atp8b1 Ad-siRNA2 infected cells respectively compared to their medium controls. A similar induction pattern was also observed for other Fxr targets, including Bsep, Mdr2, and Mrp2 (Fig. 2C). In contrast, the expression of two non-Fxr targets Mrp3 (data not shown) and Mrp4 (Fig. 2C) were unchanged by this treatment.
Reduced Bsep activity in Atp8b1-deficient rat hepatocytes
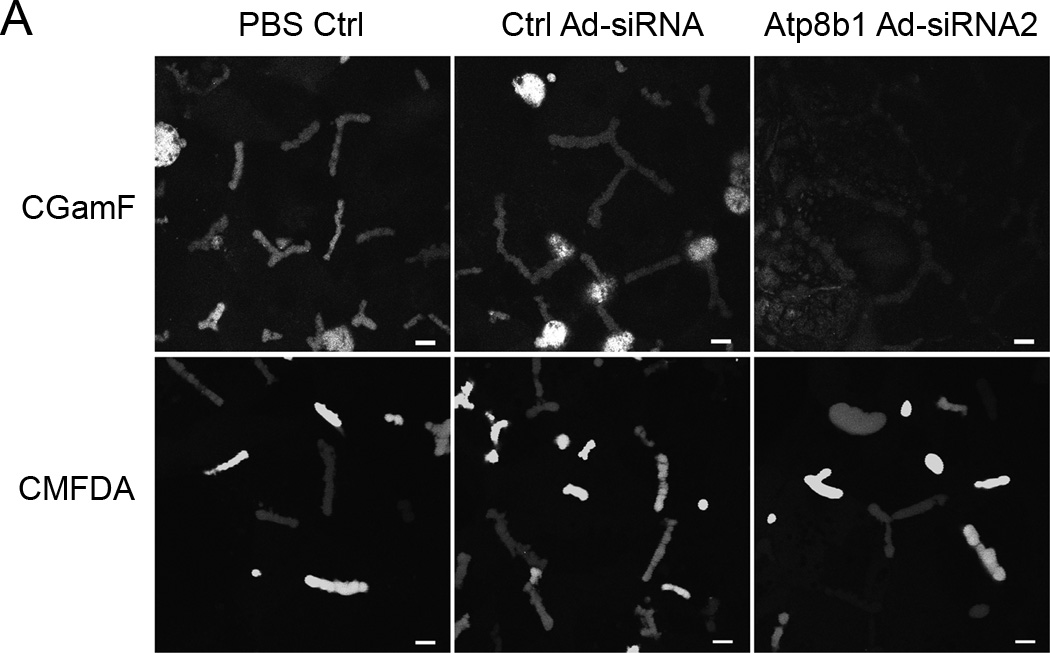
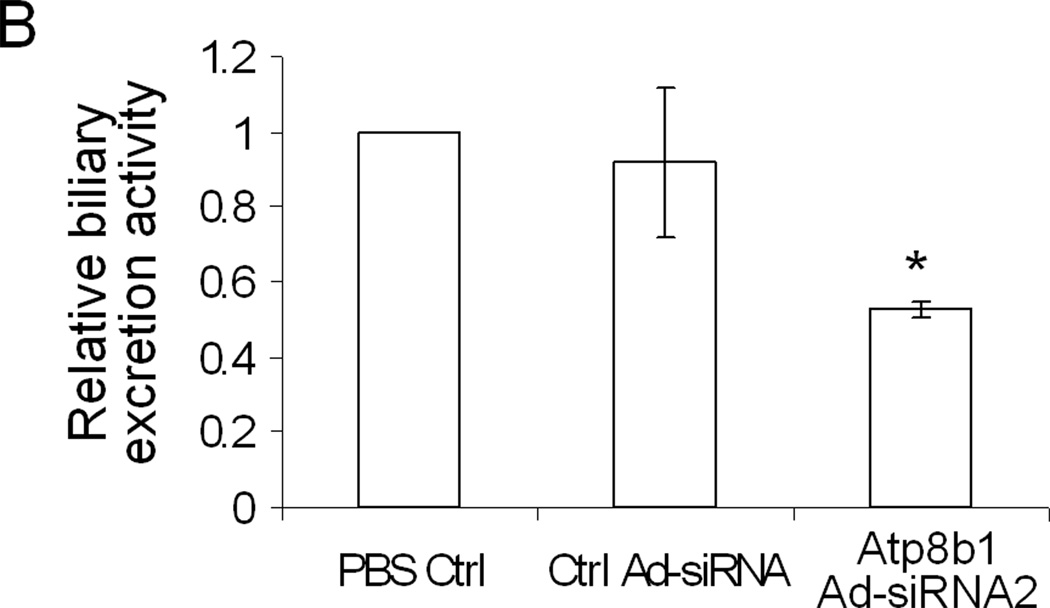
Bsep and Mrp2 are two ABC transporters on the hepatic canalicular membrane that are major determinants of bile salt dependent and independent bile flow, respectively. To assess if Atp8b1-deficiency altered transport activity for Bsep and/or Mrp2, fluorescent substrate excretion assays were performed using confocal microscopy. Rat hepatocyte collagen sandwich cultures form closed bile canalicular spaces allowing assessment of the ability of these cell cultures to excrete these fluorescent substrates into pseudocanaliculi. An analysis of confocal images show that CGamF (a Bsep substrate) secretion was reduced to about 40% compared to viral control cells, suggesting that Bsep transport activity was reduced in the Atp8b1-deficient cells (Figure 3A&B). In contrast, we did not see significant changes in CMFDA secretion (an Mrp2 substrate), although canalicular excretion of CMFDA was quite variable (Fig. 3A), which might account for these negative findings.
Figure 3.
Canalicular excretion of CGamF, but not CMFDA is reduced in Atp8b1 knockdown rat hepatocytes. The cells were treated with PBS, scrambled Ad-siRNA (Ctrl Ad-siRNA), or ATP8B1 Ad-siRNA2 and cultured for 5 days in collagen sandwich gel configuration. A, Cells were incubated with CGamF or CMFDA, and visualized by confocal microscopy. B, A quantitative measurement of the mean fluorescent CGamF intensity in canaliculi was obtained using Image J software by delineating the fluorescent canaliculi from the surrounding cells. Data represent the means ±SD of 3 independent experiments, *p<0.05. Bar, 10µm.
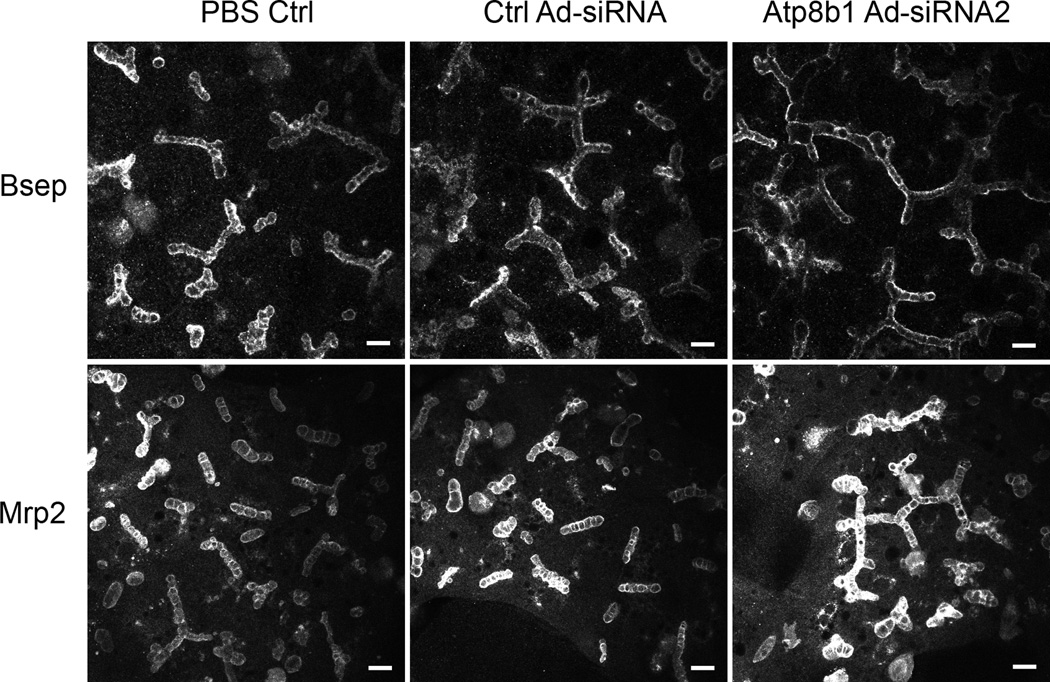
Bsep and Mrp2 remain localized to the canalicular membrane in Atp8b1-deficient rat hepatocytes
To examine if Bsep and Mrp2 cellular localization is changed in Atp8b1-deficient cells, rat hepatocytes were stained with specific antibodies to Bsep and Mrp2, and visualized by confocal microscopy. Both Bsep and Mrp2 appear to remain localized on the canalicular membrane in 5-day Atp8b1 knockdown cells as compared to control cells (Figure 4). Furthermore when these cell cultures were treated with 5 µM CDCA for an additional 24 hr after Atp8b1 Ad-siRNA2 infection for 4 days, confocal images again demonstrated that this hydrophobic bile acid did not alter the cellular localization of either Bsep or Mrp2 (data not shown). Thus, these findings in Atp8b1-deficient rat hepatocytes agree with observations in PFIC1 patients and in Atp8b1-deficient mice indicating that these transporter proteins also remain at the canalicular apical membrane in these conditions.
Figure 4.
Bsep and Mrp2 proteins remain localized to the canalicular membrane in Atp8b1 knockdown rat hepatocytes. The cells were treated with PBS, scrambled Ad-siRNA (Ctrl Ad-siRNA), or ATP8B1 Ad-siRNA2 and cultured for 5 days in collagen sandwich gel configuration. Indirect immunofluorescence was carried out as in Methods and visualized using confocal microscopy. Bar, 10µm.
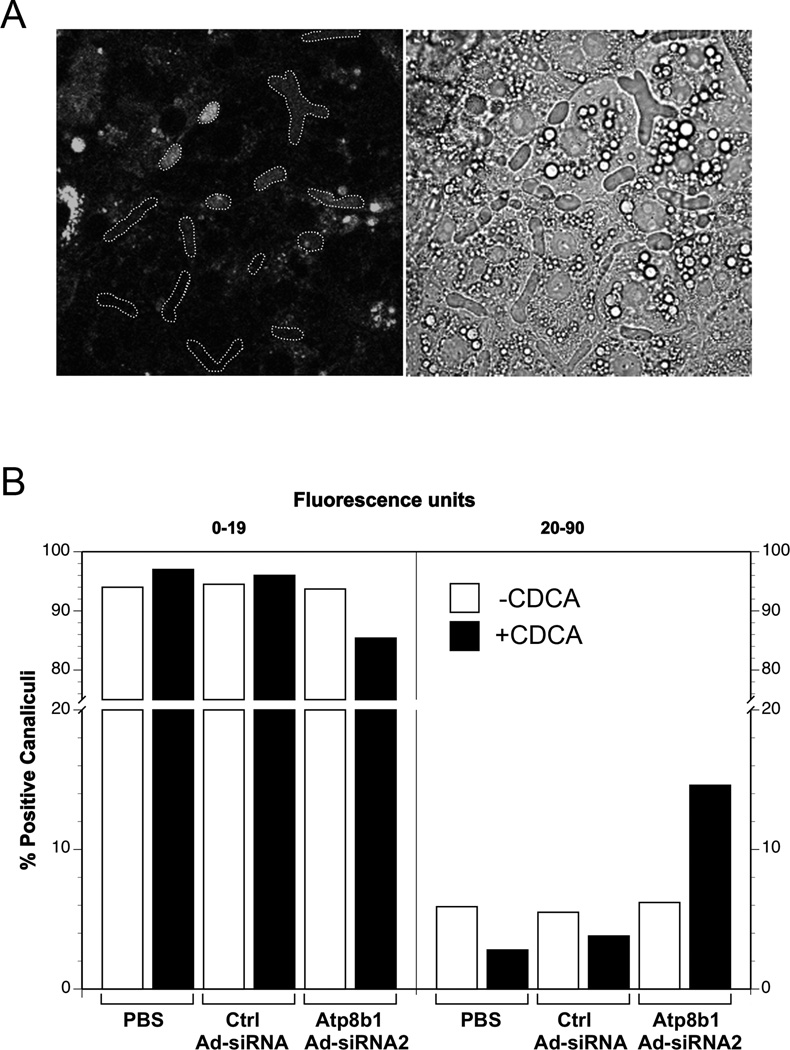
NBD-Phosphatidylserine is detected within the lumen of bile canaliculi in Atp8b1-deficient rat hepatocytes
Normally, Phosphatidylserine (PS) is localized to the inner leaflet of the membrane bilayer and is not excreted into bile. To determine if Atp8b1 knock down altered the membrane localization of PS, we examined Atp8b1 knock down rat hepatocytes by confocal microscopy after exposure of the cultures to fluorescent NBD-PS, with or without 5 µM CDCA for 16 hr. All cells demonstrated fluorescence in intracellular punctuate, vesicular structures, but only a subgroup showed positive fluorescence within the lumens of the pseudocanaliculi (Fig. 5A). Quantitative analysis of the luminal fluorescent intensity revealed that a greater percentage of positive pseudocanaliculi were present in the Atp8b1-deficient cells compared to the control cells, especially after the CDCA treatment (Fig. 5B). To ensure that the presence of NBD-PS in the bile canaliculi was not due to the cytotoxic effects of Atp8b1 Ad-siRNA2, we assayed LDH activity in the culture media. No significant differences were observed among uninfected, scrambled Ctrl Ad-siRNA infected, or Atp8b1 Ad-siRNA2 infected cells, demonstrating that the effects of CDCA on the extraction of this aminophospholipid were clearly secondary to the loss of Atp8b1. Together, these results not only provide evidence for Atp8b1’s function as an aminophospholipid flippase, but also demonstrate that hydrophobic bile acids such as CDCA are capable of detergent-like effects on the luminal leaflet of the canalicular membrane when the localization of this lipid in the inner membrane bilayer is disturbed.
Figure 5.
Increased amounts of fluorescent labeled phosphatidylserine (NBD-PS) were observed within bile canaliculi in Atp8b1 knockdown hepatocytes. The cells were treated with PBS, scrambled Ad-siRNA (Ctrl Ad-siRNA), or ATP8B1 Ad-siRNA2 and cultured for 5 days in collagen sandwich gel configuration. Prior to confocal microscopy, the cells were loaded with NBD-PS, and then incubated with or without 5µM CDCA for 16 hr. A. Pseudocanaliculi were first delineated in the phase image and then the outlines were superimposed over the fluorescent image. Shown is one example with positive lumens. B. NBD-PS fluorescence within the outlines was quantitated using Image J. Based on the PBS control levels, canaliculi were divided into 2 groups, below or above the basal fluorescent level of 19. The percentage of canaliculi whose fluorescence intensity fell above or within the PBS control level were then determined for each treatment group. Data represent all canaliculi from 3–4 independent experiments. Bar, 20µm.
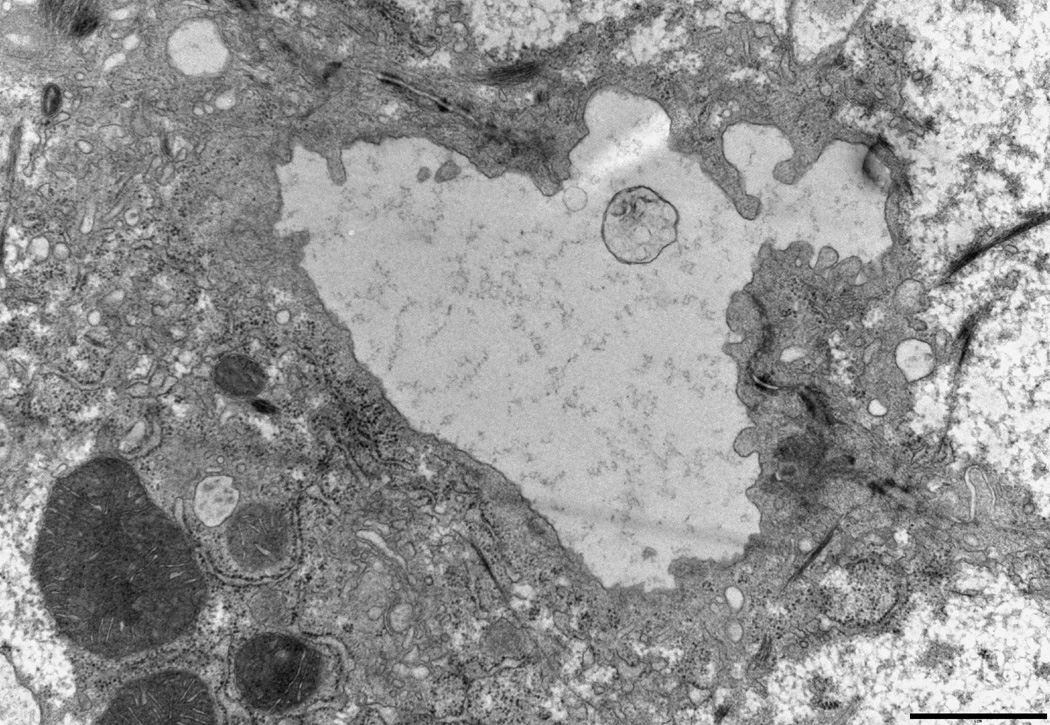
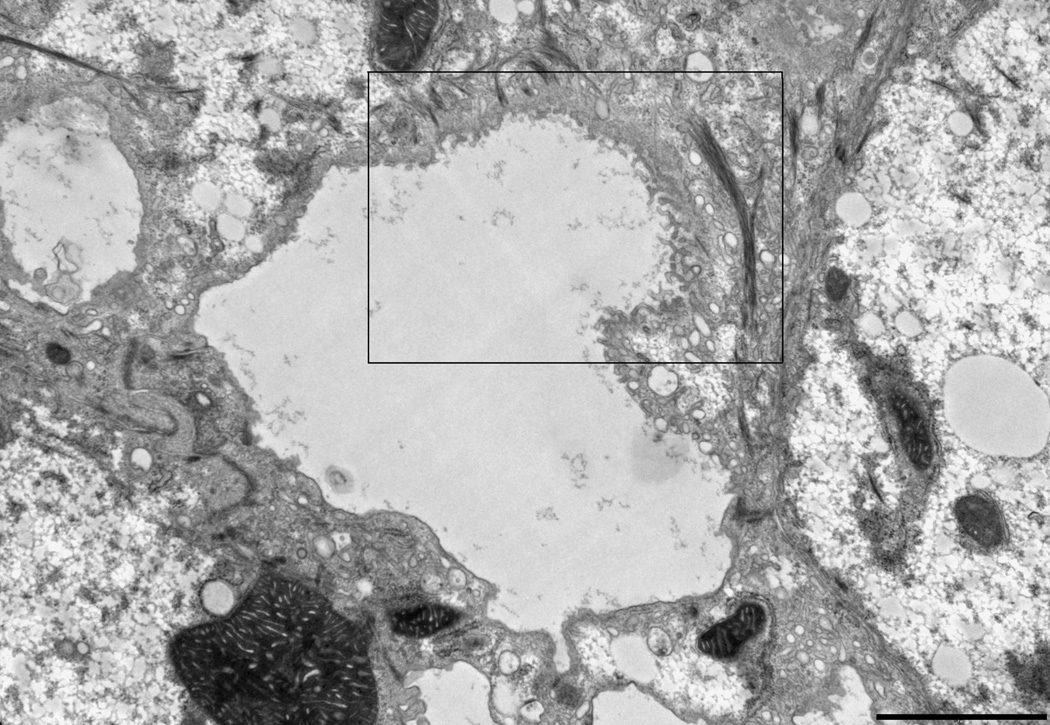
CDCA disrupts the canalicular bilayer membrane structure in Atp8b1-deficient rat hepatocytes
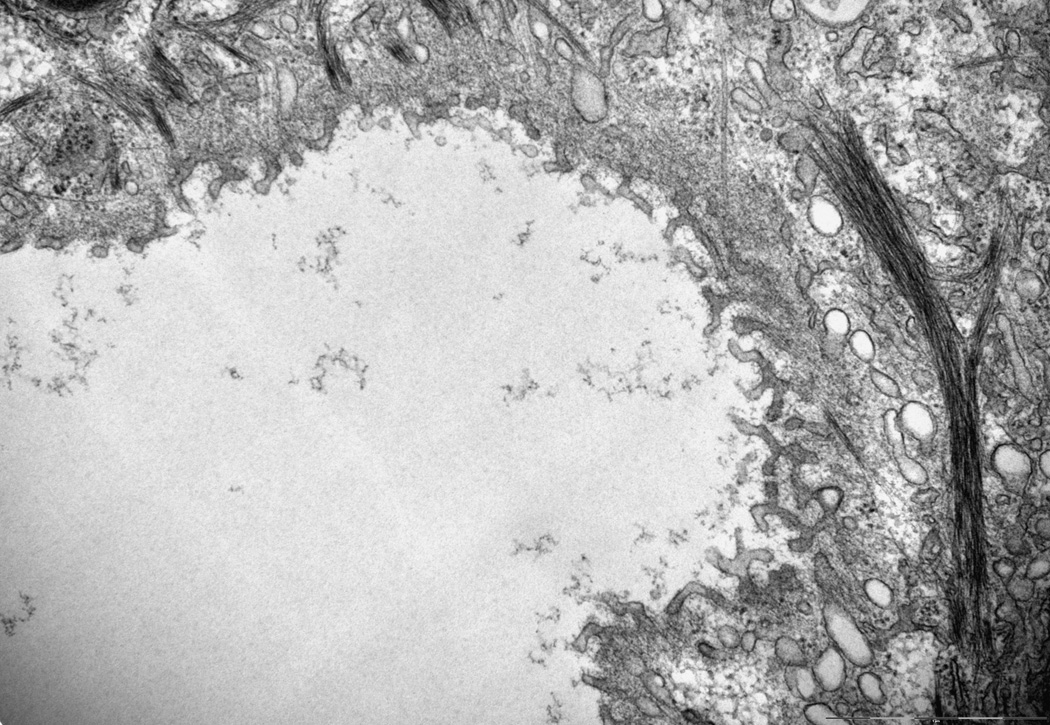
Byler’s Bile and focal disruptions in the canalicular membrane are hallmarks of the ultrastructure of the PFIC1 liver as has been demonstrated by electron microscopy(5). To examine if Atp8b1-deficiency leads to any changes in the ultrastructure of the bile canalicular membrane in these rat hepatocyte cultures, we blindly examined electron micrographs obtained from Atp8b1 knocked down rat hepatocyte cultures. Pseudocanaliculi of Ctrl Ad-siRNA infected cells were dilated and few microvilli were noted (Fig.6A), as has been previously described in this sandwich culture system where the pseudocanaliculi are a closed system(13). When these cells were treated with 5 µM CDCA for 20 hours, no marked changes were observed in Ctrl Ad-siRNA infected cells. However, we found a number of areas in the canalicular membranes where there were foci with striking morphological abnormalities in Atp8b1 knockdown cells. These included clubbed and bifurcating finger-like microvilli, and interruptions in the continuity of the plasma membrane, reminiscent of ultrastructural abnormalities seen in PFIC1 patients (Fig.6B)(5). In contrast, these findings were not observed in Atp8b1-deficient hepatocytes following treatment with 5 µM UDCA for 20 hours, a hydrophilic bile acid commonly used to treat certain cholestatic disorders. This result indicates that the bile canalicular membrane is susceptible to injury from hydrophobic bile acid in ATP8B1-deficient hepatocytes. Intra-canalicular debris, characteristic of “Byler’s bile” in patients with PFIC1 disease, was not observed in these micrographs.
Figure 6.
The canalicular membrane is disrupted in Atp8b1 knockdown rat hepatocytes after CDCA treatment in 5 day rat hepatocytes in collagen sandwich culture. Cells were treated with 5µM CDCA for 16 hr before fixation. A, An electron microscopic image of a bile canaliculus from a scrambled Ad-siRNA infection. This image is similar to previously described canaliculi in rat hepatocyte sandwhich cultures(13). B, Disrupted canalicular membrane in Atp8b1 knockdown cells exposed to CDCA. The microvilli from one of the hepatocytes that form the canalicular space are blunted, bifurcated and club shaped. Other areas (not shown) demonstrated loss of the electron dense lining of the luminal membrane that is normally seen. C, This is a higher magnification of the disrupted area seen in B. Bar, 2µm.
Discussion
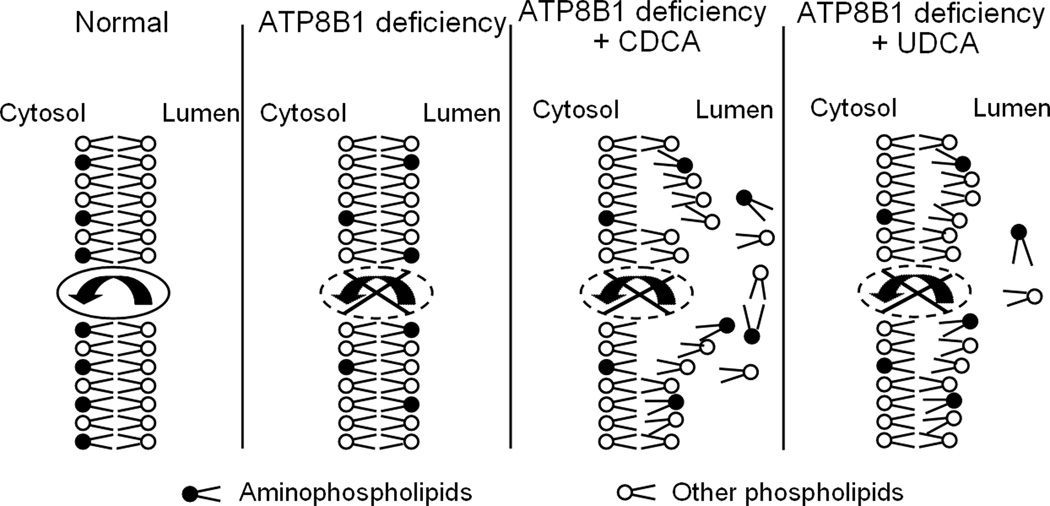
Conflicting hypotheses have been proposed to explain the pathogenesis of PFIC1. The first hypothesis, supported by two groups(8–10), suggests that ATP8B1 is involved in bile acid nuclear receptor FXR signaling, and that reduced FXR expression and function lead to impairment of bile acid excretion and mal-absorption of bile acids in the intestine. The second hypothesis, supported by several other studies(4; 11; 12), claims that ATP8B1 functions as an aminophospholipid flippase and that loss of membrane lipid asymmetry predisposes the canalicular membrane to injury from the detergent effects of hydrophobic bile acids. In contrast to the first hypothesis, the findings in the present study clearly demonstrate that FXR expression and functional activity are maintained in ATP8B1/Atp8b1-deficient human cells and rat hepatocytes (Fig. 1 and 2), indicating that dysregulation of FXR is unlikely to be the primary pathogenic factor in PFIC1. Rather we find that when Atp8b1 is knocked down in rat hepatocyte sandwich cultures, adaptive responses in bile transporters occurs (Fig. 2A), bile salt excretion is reduced (Fig.3), and when exposed to hydrophobic bile acids, the bile canalicular membrane bilayer structure is disrupted and phosphatidylserine accumulates within the canalicular lumen (Fig. 6). These results support the second hypothesis and provide a clear explanation for the development of progressive cholestasis in patients with PFIC1 (Fig.7).
Figure 7.
A proposed model for ATP8B1 function at the bile canalicular membrane. Deficiency of ATP8B1 leads to loss of maintenance of aminophospholipids on the cytoplasmic side of the canalicular membrane bilayer making the canalicular membrane susceptible to disruption by hydrophobic bile acids, such as CDCA, a primary human bile acid. In contrast, the hydrophilic bile acid, UDCA does not have the same disruptive effect.
In contrast to several previous reports, our data demonstrate that FXR expression and activity remain intact in ATP8B1-deficient cells, suggesting that reductions in FXR expression and activity are likely to be secondary phenomenon associated with the cholestatic syndrome. Alternatively it is possible that the small amounts of ATP8B1/Atp8b1 protein expression that remain in our knockdown experiment could still have an effect on FXR and its target genes expression. However, our findings are in full agreement with Atp8b1-deficient (Atp8b1G308V/G308V) mice where Fxr and its target gene expression also remain intact, thus making this possibility unlikely.
Proinflammatory cytokines such as TNFα and IL-1β are often elevated in cholestasis(17; 18) and can result in loss of expression of nuclear receptors(19). For example, FXR mRNA expression is significantly reduced in Hep3B human hepatoma cells when treated with TNFα and IL-1β and in vivo in mice after lipopolysacaride (LPS) administration(20). In unpublished studies we can also confirm that FXR mRNA expression is reduced in TNFα treated HepG2 cells. In addition, previous published studies in mouse hepatocytes demonstrate that TNFα and IL-1β result in translocation of RXR from the nucleus to the cytoplasm where this obligate heterodimeric partner for Class I nuclear receptors is degraded(21). Together, these observations suggest that reduced FXR expression in cholestatic patients is likely secondary to similar cytokine mediated events, although the details of this mechanism remain to be elucidated. However since FXR/RXR heterodimers are major transcriptional regulators of the expression of genes involved in bile acid synthesis, metabolism and transport, down regulation of FXR would be expected to contribute to further worsening of cholestatic syndromes.
Several previous studies suggest that ATP8B1 functions as a phospholipid flippase at the canalicular membrane of hepatocytes. First, immunofluorescent studies indicate that ATP8B1/Atp8b1 is localized to the canalicular apical membrane of the hepatocytes(3) where it presumably functions to help maintain the asymmetry of the membrane’s lipid composition. Second, cell-based assays from polarized HepG2 cells indicate that the canalicular membrane does contain aminophospholipid flippase activity(14). Finally Atp8b1-deficient (Atp8b1G308V/G308V) mice excrete PS and cholesterol into bile only when isolated perfused livers from these mice are infused with taurocholic acid(4) suggesting that absence of Atp8b1 destabilizes the canalicular membrane lipid symmetry resulting in PS excretion into bile as a direct result of the detergent effects of bile acids. The findings in the present study provide direct evidence for this phenomenon by demonstrating the accumulation of fluorescent PS within the canalicular lumen in Atp8b1 knockdown rat hepatocytes sandwich cultures, especially after exposure to CDCA (Fig.5).
PS is an important component of the plasma membrane that may be involved in many cellular functions including membrane fluidity, protein docking and membrane signaling(22; 23). PS normally resides in the inner leaflet of cell membrane and is maintained there by the aminophospholipid flippase. ATP8B1 is expressed in many human tissues, but is more abundant in the bladder, stomach and intestine, than in the liver or pancreas. Despite its abundance in the bladder or stomach, no injury has been reported in these organs in patients with PFIC1. Rather, ATP8B1 mutations lead to cholestasis, pancreatitis and diarrhea. This suggests that the function of ATP8B1 is most critical for hepatocytes and intestinal epithelia.
When ATP8B1 is dysfunctional, PS would be expected to be scrambled to the outer leaflet of the plasma membrane. Unlike the stomach, the outer leaflet of the hepatocyte and cholangiocyte luminal membrane is exposed to the highest concentrations of bile salts in the body. These strong biological detergents can extract lipids into the bile if they are located in the external membrane leaflet. This process accounts for the normal physiological excretion of phosphatidylcholine (PC) from the external membrane leaflet into bile and the formation of mixed micelles. In MDR3/Mdr2-deficiency, cholestasis results because PC asymmetry can not be maintained. In these disorders, PC is not excreted into bile, resulting in bile acid induced injury to hepatocytes and cholangiocyte membranes. Similarly, depletion of PS in hepatocytes should also result in an imbalance of PS in the canalicular membrane, which as shown in this study, destabilizes the membrane resulting in bile acid induced tissue injury.
Loss of PS membrane asymmetry with ATP8B1/Atp8b1-deficiency would be expected to alter the fluidity and perhaps the composition of lipid rafts and in turn affect the functional activity of certain canalicular membrane transporters. Indeed, Atp8b1 knockdown reduced CGamF excretion, suggesting Bsep function is compromised (Fig. 3). Reduced Bsep activity has also been reported in Atp8b1G308V/G308V mice(4), where increased biliary cholesterol output also occurs(24). Reductions in Bsep activity would result in bile acid retention in hepatocytes and contribute further to the development of cholestasis.
The absence of significant canalicular membrane injury in Atp8b1-deficient (Atp8b1G308V/G308V) mice can be explained by the high concentrations of muricholic acid in the bile acid pool in mice which is much more hydrophilic than human bile acids. Finally, the beneficial effects of UDCA therapy in PFIC1 patients as well as the effectiveness of biliary drainage of bile can also be explained by this mechanism since both treatments will decrease the hydrophobicity of the bile acid pool, thereby reducing injury to the canalicular membrane.
In conclusion, our study indicates that ATP8B1 does not play a direct role in FXR expression or function but is a canalicular membrane PS flippase. Loss of ATP8B1 diminishes the resistance of the bile canalicular membrane to hydrophobic bile acids, leading to progressive cholestasis and the development of PFIC1.
Supplementary Material
Acknowledgement
This study was supported by the Yale Liver Center Pilot grant (to S.Y.C.), and National Institutes of Health Grants DK34989 (Yale Liver Center) and DK25636 (to J.L.B.).
Abbreviations used in this paper
- ATP8B1/Atp8b1 Ad-siRNA
adenovirus-mediated short interfering RNA for ATP8B1/Atp8b1
- BSEP/Bsep
bile salt export pump
- CDCA
chenodeoxycholic acid
- CGamF
Cholylglycylamidofluorescein
- CMFDA
5-chloromethylfluorescein diacetate
- Ctrl Ad-siRNA
Control adenovirus-mediated short interfering RNA using scrambled sequence
- 6-ECDCA
6α-ethyl CDCA
- FXR/Fxr
Farnesoid X receptor
- MRP2/Mrp2
multidrug resistance-associate protein 2
- NTCP/Ntcp
the Na+ taurocholate cotransporting polypeptide
- NBD-PS
1-palmitoyl-2-[6-[(7–nitrobenz-2-oxa-1,3-diazol-4-yl)amino]caproyl]-sn-glycero-3-phosphatidylserine
- PBS
phosphate-buffered saline
- PFIC1
Progressive Familial Intrahepatic Cholestasis 1
- PC
phosphatidylcholine
- PS
phosphatidylserine
- SHP/Shp
Small Heterodimer Partner
- UDCA
ursodeoxycholic acid
Footnotes
Disclosures: The following people have nothing to disclose: James L. Boyer, Shi-Ying Cai, Samir Gautam, Trong Nguyen, Christoph Rahner, Carol J. Soroka.
References
- 1.Bull LN, van Eijk MJ, Pawlikowska L, et al. A gene encoding a P-type ATPase mutated in two forms of hereditary cholestasis. Nat Genet. 1998;18:219–224. doi: 10.1038/ng0398-219. [DOI] [PubMed] [Google Scholar]
- 2.Bull LN, Carlton VE, Stricker NL, et al. Genetic and morphological findings in progressive familial intrahepatic cholestasis (Byler disease [PFIC-1] and Byler syndrome): evidence for heterogeneity. Hepatology. 1997;26:155–164. doi: 10.1002/hep.510260121. [DOI] [PubMed] [Google Scholar]
- 3.Eppens EF, van Mil SW, de Vree JM, et al. FIC1, the protein affected in two forms of hereditary cholestasis, is loc alized in the cholangiocyte and the canalicular membrane of the hepatocyte. J Hepatol. 2001;35:436–443. doi: 10.1016/s0168-8278(01)00158-1. [DOI] [PubMed] [Google Scholar]
- 4.Paulusma CC, Groen A, Kunne C, et al. Atp8b1 deficiency in mice reduces resistance of the canalicular membrane to hydrophobic bile salts and impairs bile salt transport. Hepatology. 2006;44:195–204. doi: 10.1002/hep.21212. [DOI] [PubMed] [Google Scholar]
- 5.De Vos R, Wolf-Peeters C, Desmet V, et al. Progressive intrahepatic cholestasis (Byler's disease): case report. Gut. 1975;16:943–950. doi: 10.1136/gut.16.12.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ujhazy P, Ortiz D, Misra S, et al. Familial intrahepatic cholestasis 1: studies of localization and function. Hepatology. 2001;34:768–775. doi: 10.1053/jhep.2001.27663. [DOI] [PubMed] [Google Scholar]
- 7.Paulusma CC, Folmer DE, Ho-Mok KS, et al. ATP8B1 requires an accessory protein for endoplasmic reticulum exit and plasma membrane lipid flippase activity. Hepatology. 2008;47:268–278. doi: 10.1002/hep.21950. [DOI] [PubMed] [Google Scholar]
- 8.Chen F, Ananthanarayanan M, Emre S, et al. Progressive familial intrahepatic cholestasis, type 1, is associated with decreased farnesoid X receptor activity. Gastroenterology. 2004;126:756–764. doi: 10.1053/j.gastro.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 9.Frankenberg T, Miloh T, Chen FY, et al. The membrane protein ATPase class I type 8B member 1 signals through protein kinase C zeta to activate the farnesoid X receptor. Hepatology. 2008 doi: 10.1002/hep.22431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alvarez L, Jara P, Sanchez-Sabate E, et al. Reduced hepatic expression of farnesoid X receptor in hereditary cholestasis associated to mutation in ATP8B1. Hum Mol Genet. 2004;13:2451–2460. doi: 10.1093/hmg/ddh261. [DOI] [PubMed] [Google Scholar]
- 11.Demeilliers C, Jacquemin E, Barbu V, et al. Altered hepatobiliary gene expressions in PFIC1: ATP8B1 gene defect is associated with CFTR downregulation. Hepatology. 2006;43:1125–1134. doi: 10.1002/hep.21160. [DOI] [PubMed] [Google Scholar]
- 12.Pawlikowska L, Groen A, Eppens EF, et al. A mouse genetic model for familial cholestasis caused by ATP8B1 mutations reveals perturbed bile salt homeostasis but no impairment in bile secretion. Hum Mol Genet. 2004;13:881–892. doi: 10.1093/hmg/ddh100. [DOI] [PubMed] [Google Scholar]
- 13.Wang W, Soroka CJ, Mennone A, et al. Radixin is required to maintain apical canalicular membrane structure and function in rat hepatocytes. Gastroenterology. 2006;131:878–884. doi: 10.1053/j.gastro.2006.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tannert A, Wustner D, Bechstein J, et al. Aminophospholipids have no access to the luminal side of the biliary canaliculus: implications for the specific lipid composition of the bile fluid. J Biol Chem. 2003;278:40631–40639. doi: 10.1074/jbc.M302131200. [DOI] [PubMed] [Google Scholar]
- 15.Wu Y, Zhang X, Bardag-Gorce F, et al. Retinoid X receptor alpha regulates glutathione homeostasis and xenobiotic detoxification processes in mouse liver. Mol Pharmacol. 2004;65:550–557. doi: 10.1124/mol.65.3.550. [DOI] [PubMed] [Google Scholar]
- 16.Boyer JL. New perspectives for the treatment of cholestasis: lessons from basic science applied clinically. J Hepatol. 2007;46:365–371. doi: 10.1016/j.jhep.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zachou K, Rigopoulou EI, Tsikrikoni A, et al. Autoimmune hepatitis type 1 and primary biliary cirrhosis have distinct bone marrow cytokine production. J Autoimmun. 2005;25:283–288. doi: 10.1016/j.jaut.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 18.Plebani M, Panozzo MP, Basso D, et al. Cytokines and the progression of liver damage in experimental bile duct ligation. Clin Exp Pharmacol Physiol. 1999;26:358–363. doi: 10.1046/j.1440-1681.1999.03042.x. [DOI] [PubMed] [Google Scholar]
- 19.Assenat E, Gerbal-Chaloin S, Larrey D, et al. Interleukin 1beta inhibits CAR-induced expression of hepatic genes involved in drug and bilirubin clearance. Hepatology. 2004;40:951–960. doi: 10.1002/hep.20387. [DOI] [PubMed] [Google Scholar]
- 20.Kim MS, Shigenaga J, Moser A, et al. Repression of farnesoid X receptor during the acute phase response. J Biol Chem. 2003;278:8988–8995. doi: 10.1074/jbc.M212633200. [DOI] [PubMed] [Google Scholar]
- 21.Ghose R, Zimmerman TL, Thevananther S, et al. Endotoxin leads to rapid subcellular re-localization of hepatic RXRalpha: A novel mechanism for reduced hepatic gene expression in inflammation. Nucl Recept. 2004;2:4. doi: 10.1186/1478-1336-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yeung T, Gilbert GE, Shi J, et al. Membrane phosphatidylserine regulates surface charge and protein localization. Science. 2008;319:210–213. doi: 10.1126/science.1152066. [DOI] [PubMed] [Google Scholar]
- 23.Martinez MC, Kunzelmann C, Freyssinet JM. Phosphatidylserine and signal transduction: who needs whom? Sci STKE. 2006;2006:e3. doi: 10.1126/stke.3182006pe3. [DOI] [PubMed] [Google Scholar]
- 24.Groen A, Kunne C, Jongsma G, et al. Abcg5/8 Independent Biliary Cholesterol Excretion in Atp8b1-Deficient Mice. Gastroenterology. 2008 doi: 10.1053/j.gastro.2008.02.097. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.