Abstract
In this study, we examined the immune response during gonococcal infection to the individual transferrin binding proteins by using a quantitative enzyme-linked immunosorbent assay (ELISA). Recombinant transferrin binding protein A (rTbpA) and rTbpB were purified under nondenaturing conditions for use as ELISA antigens. Sera and secretions from culture-positive individuals were analyzed for antibodies to rTbpA and rTbpB and compared to samples from individuals with no history of gonococcal infection. Although antibodies to both rTbpA and rTbpB were detected in serum, in most cases the antibody levels were not significantly different from those measured in the control population. Also, previous history of gonococcal infection did not increase antibody levels in serum, suggesting the lack of an anamnestic response. Analysis of secretion samples revealed antibody levels that were generally below the limits of detection in our assay. Overall, this study demonstrated a paucity of systemic and local antibody responses to rTbps as a result of natural infection and represents a baseline over which a protective antibody response will have to be generated in order to develop an efficacious gonococcal vaccine.
Neisseria gonorrhoeae, the causative agent of the sexually transmitted disease (STD) gonorrhea, is one of the most common sexually transmitted pathogens worldwide. In the United States, an estimated 361,700 cases were reported in 2001 (9); however, this number is likely an underrepresentation due to incomplete reporting and the high prevalence of asymptomatic infections (9, 26). Gonorrhea manifests as urethritis in men and as endocervicitis and/or urethritis in women. Ascension to the upper genital tract can occur in both sexes; in males this can result in epididymitis or prostatitis. In females, ascension can result in pelvic inflammatory disease, from which complications such as infertility, fallopian tube scarring, and subsequent ectopic pregnancy can occur. Disseminated gonococcal infection can also result from infection, although this outcome is less common. One of the hallmarks of this disease is a mucopurulent discharge associated with symptomatic infection. Microscopic examination of the discharge reveals that it is characterized by large numbers of polymorphonuclear leukocytes associated with extracellular and intracellular diplococci. Although a robust inflammatory response ensues during symptomatic infection, no apparent protective immunity is developed following infection, as shown in a male human challenge study (44) and by the high incidence of recidivism among patients attending sexually transmitted disease clinics (4, 21, 35, 40).
The search for a vaccine against gonorrhea has been largely disappointing. In human vaccine trials, partially lysed gonococci, purified pilin, and purified porin were shown to be immunogenic, but all failed to elicit protection upon subsequent natural exposure (7, 19, 46). The lack of protective immunity is likely due, in part, to the capacity of many gonococcal surface antigens to undergo high-frequency phase and antigenic variation (38). With the increasing emergence of drug-resistant strains (9) and the disturbing finding that human immunodeficiency virus transmission is increased with concurrent gonococcal infection (11, 27), the search for effective vaccine antigens has become more urgent. The gonococcal transferrin binding proteins (Tbps) have garnered interest as potential vaccine antigens due to their surface accessibility, sequence conservation, and ubiquitous expression in all strains studied to date (12, 13, 34, 39, 42). Expression of the transferrin receptor has been shown to be necessary for establishment of infection in humans (16). In the closely related species Neisseria meningitidis, murine vaccine studies using the Tbps as vaccine antigens demonstrated that mice immunized with TbpA or TbpA plus TbpB were protected upon subsequent lethal challenge (49).
Immunity to meningococcal infection is thought to develop as a result of the generation of bactericidal antibodies following carriage and/or infection. Convalescent-phase sera from individuals recovering from meningococcal infections contain antibodies specific for the Tbps (1, 29, 33); however, it is not known whether these antibodies contribute to protection. By contrast, little is known about the immunogenicity of the Tbps during natural gonococcal infections. However, recent studies investigating the antibody response to the whole gonococcus indicate that local and systemic antibody responses during and following gonococcal infection were not robust compared to the level of antibodies present in a control population (21, 22). These observations are consistent with those of recent investigations described by Boulton and Gray-Owen (8) documenting down regulation of T-cell activation as a result of interaction between Opa-expressing gonococci and CEACAM1-expressing T cells. By down regulating T cells, the gonococcus could potentially limit the antibody response by inhibiting T-cell helper functions. These findings are consistent with the observation that individuals recovering from gonococcal disease do not develop immunity to further episodes (4, 21, 35, 40).
In the present study, we measured antibody levels to the individual gonococcal Tbps in serum and secretions during natural infections. Previous studies conducted by Hedges et al. (21, 22) illustrated a paucity of antibodies to the whole gonococcus; however, these studies failed to evaluate specific antibody responses to individual antigens. Furthermore, gonococcal cells used as antigens by Hedges et al. were not grown in iron-depleted media. Consequently, iron-repressible proteins were not maximally expressed, and hence a portion of the antibody repertoire may not have been evaluated in the previous studies. To fully characterize the specific immune response to the individual Tbps during natural infection, we expressed and purified recombinant Tbps (rTbps) and used them as antigens in quantitative enzyme-linked immunosorbent assays (ELISAs). We analyzed sera and secretions from infected patients and compared the antibody levels to those detected in naïve individuals.
MATERIALS AND METHODS
Specimen collection and processing.
Twenty serum samples from a previous longitudinal study of N. gonorrhoeae infection at an STD clinic in Wilson County, North Carolina (17), were screened in the present study. Additional serum samples and genital secretions were collected prospectively for this study from subjects attending the STD clinic of the Wake County Department of Health and Human Services in Wake County, North Carolina. The samples were collected at the time of patient presentation, and infection with N. gonorrhoeae was subsequently confirmed by culture by using routine laboratory methods. Sera from adult volunteers with no history of gonorrhea were also used as uninfected controls. Clinical histories were obtained, including previous gonococcal infection and the time since last infection; however, no specific information was obtained regarding the onset or duration of symptoms. Informed consent was obtained from all subjects and volunteers, and research was approved by the Institutional Review Boards of Virginia Commonwealth University and the University of North Carolina at Chapel Hill. Research protocols complied with all relevant federal guidelines and institutional policies.
Sera from peripheral venous blood was aliquoted and frozen at −70°C. Semen was allowed to liquefy at room temperature for 10 to 30 min, aliquoted, and frozen at −70°C until analysis. After being thawed, the semen was centrifuged at 1,000 × g for 5 min, and antibody concentrations were measured in the supernatant seminal plasma. Swabs containing cervical mucous were resuspended in 0.5 ml of phosphate-buffered saline (PBS) and frozen at −70°C. After being thawed, the cervical secretions were centrifuged at 1,000 × g for 5 min, and antibody concentrations were measured in the supernatant fluid.
Construction of expression plasmids.
The tbpA expression plasmid, pUNCH412, was described previously (14). The tbpB expression plasmid, pVCU705, was constructed by PCR amplification of the genomic copy of tbpB from gonococcal strain FA19 by using a proofreading Taq polymerase (Platinum Pfx; Invitrogen). The forward primer, oVCU170 (CATATGAACAATCCATTGGTGAATCAGG), contained an NdeI site (shown in bold) and amplified the tbpB native signal sequence. The reverse primer, oVCU172 (CTCGAGTTTCACAAGCTTTTGGC), contained an XhoI restriction site (shown in bold) and encoded the terminal lysine of TbpB from gonococcal strain FA19. The PCR product was ligated into the pET-22b(+) expression vector (Novagen). The resultant plasmid, pVCU705, contained the full-length tbpB gene under control of a T7 promoter, as well as a region encoding a six-histidine tag immediately 3′ of tbpB. The expression host for both plasmids was the Escherichia coli strain BL21(DE3) (Novagen).
Recombinant protein expression and purification.
One-liter cultures containing Luria-Bertani broth (LB) (pH 7.5), 1% glucose, and 500 μg of carbenicillin/ml were inoculated with recombinant E. coli strains and grown at 37°C with shaking. When the cultures reached an optical density at 600 nm of 0.4 to 0.6, they were centrifuged for 15 min at 6,000 × g to pellet the bacteria. The supernatants were decanted, and 1 liter of fresh LB containing glucose and carbenicillin was added. IPTG (isopropyl-β-d-thiogalactopyranoside) was added at 1 mM for 3 h at 37°C to induce protein expression. After induction, the cultures were removed and bacterial cells were pelleted and stored at −80°C.
For purification, the pellets were thawed on ice and resuspended in buffer containing 100 mM Tris (pH 8.0)-0.5 M NaCl (Tris buffer). Elugent (Calbiochem) was added to a final concentration of 2%. A protease inhibitor cocktail (Calbiochem) was added, along with lysozyme, DNase, and RNase to promote cell lysis and to reduce viscosity. Solubilized preparations were subjected to high speed centrifugation at 39,000 × g for 40 min. For rTbpA purification, the supernatant was added to a transferrin affinity matrix (30); for rTbpB purification, thesupernatant was added to a nickel-nitriloacetic affinity resin (QIAGEN). The rTbpA-transferrin column was washed with 20-bed volumes of Tris buffer and then eluted by using 50 mM glycine (pH 2.0)-0.5 M NaCl-1% octylglucoside (n-octyl-β-d-glucopyranoside; Calbiochem). The eluted proteins were immediately neutralized in collection tubes containing 1 M Tris (pH 8.0). rTbpB was processed similarly except that the column was washed with 20-bed volumes of 50 mM NaH2PO4-300 mM NaCl-20 mM imidazole (pH 8.0) and eluted by using a buffer containing 50 mM NaH2PO4-300 mM NaCl-250 mM imidazole (pH 8.0).
Amino-terminal sequencing of rTbpB.
For N-terminal sequencing of rTbpB, samples were prepared as previously described (30). Sequencing was performed by Midwest Analytical, Inc. (St. Louis, Mo.)
Western blotting and solid-phase transferrin-binding assays.
Western blotting was performed by using iron-stressed N. gonorrhoeae strain MCV601 (30), iron stressed N. meningitidis strain FAM2 (47), or purified recombinant proteins transferred onto nitrocellulose (Schleicher & Schuell). To detect TbpA, the blots were probed with either a polyclonal rabbit serum raised against a denatured TbpA (15) or a polyclonal serum raised against recombinant TbpA. The latter serum was elicited by immunization of New Zealand White Elite rabbits (Covance Research Products, Denver, Pa.) with recombinant TbpA, purified as described above. For TbpB detection, polyclonal rabbit serum raised against recombinant TbpB (kindly provided by Christopher Thomas and P. Frederick Sparling) or, alternatively, peroxidase-conjugated human transferrin (HRP-Tf; Jackson Immunoresearch) was used. The blots were developed with nitroblue tetrazolium-5-bromo-4-chloro-3-indolyl phosphate (BCIP) or with Opti-4CN (Bio-Rad). For solid-phase transferrin binding assays, purified rTbpA or rTbpB was applied to nitrocellulose supports, which were subsequently probed with HRP-Tf. Dot blots were developed by using the colorimetric substrate Opti-4CN.
ELISAs.
Serum and mucosal secretions were screened for antibodies specific to rTbpA and rTbpB. TbpA, TbpB, or tetanus toxoid (Calbiochem) was diluted in coating buffer (0.1 M NaCl, 0.05 M boric acid, 0.0012 M sodium tetraborate decahydrate, pH 8.2) to a concentration of 1 μg/ml, and then the proteins were applied to individual wells in 96-well microtiter plates (Nunc) overnight at 4°C. Following binding, the plates were washed with PBS containing 0.15% Tween 20 (Sigma) and then blocked for 2 h with PBS containing Tween 20 and 5% skim milk to reduce nonspecific binding. Sera or secretion samples were diluted in PBS with Tween 20 and 5% skim milk, and the dilutions were added to the plates and incubated overnight at 4°C. The plates were again washed and blocked as described above, and then goat anti-human alkaline phosphatase-conjugated antibodies (Jackson Immunoresearch and Southern Biotechnology Associates) were used to detect antigen-specific immunoglobulin G (IgG), IgA, or IgM. For each plate, a standard curve was generated by using anti-human immunoglobulins of known isotypes (Jackson Immunoresearch and Southern Biotechnology Associates). Known concentrations of human Igs (Sigma and Jackson Immunoresearch) were added and serially diluted to generate the standard curve. The plates were developed with p-nitrophenylphosphate substrate (Sigma) in carbonate buffer (0.05 M sodium carbonate, 1 mM MgCl2, pH 9.8), and the reaction was allowed to proceed until sufficient color developed. The reactions were stopped with the addition of 2 M NaOH, and the plates were analyzed using a Multiskan Ascent microplate reader (Thermo Labsytems, Helsinki, Finland). Ig concentrations in the samples were interpolated from the standard curve by using Ascent software (Thermo Labsystems). The samples were analyzed at multiple dilutions when applicable to confirm parallelism with the standard curve.
Statistics.
Statistical analysis was performed by using NCSS 2000/Pass 2000 statistics software. Data analysis between groups included the Mann-Whitney U test and the Kolmogorov-Smirnov test. Determination of the most appropriate test depended on data normality and the variance between the groups being measured. A P value of <0.05 was considered significant.
RESULTS AND DISCUSSION
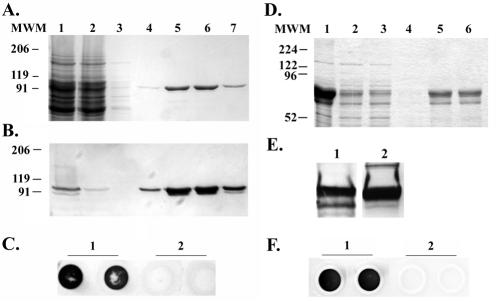
We expressed and purified recombinant TbpA and TbpB under nondenaturing conditions (Fig. 1) and utilized the overexpressed proteins as ELISA antigens in order to evaluate sera and secretions for the presence of Tbp-specific antibodies. The purification conditions used in this study enabled us to obtain recombinant protein that retained ligand-binding properties similar to those of the native proteins (Fig. 1C and F). This condition was an important aspect of the analysis, as antibodies recognizing conformational epitopes could still be measured. It is believed that conformational epitopes represent the vast majority of epitopes in proteins (2), and it has been shown that the use of denatured proteins in ELISAs can reduce the sensitivity of the assay (23, 31).
FIG. 1.
Expression and purification of rTbps. (A) Coomassie blue-stained SDS-polyacrylamide gel containing rTbpA protein. Lane 1 contains the soluble fraction after detergent solubilization of induced E. coli. Lanes 2 through 7 represent rTbpA purification fractions. Lane 2 contains the column flowthrough fraction after overnight incubation with a human transferrin-bound affinity column. Lane 3 contains the wash fraction. Lanes 4 through 7 contain purified rTbpA column elution fractions. Molecular weight standards (MWM) are indicated at the left. (B) Western blot of the above SDS-PAGE probed with an anti-TbpA antibody. The positions of molecular weight standards are indicated on the left. (C) Solid-phase transferrin binding assay of purified rTbpA probed with HRP-transferrin (1 μg/ml). Lane 1 contains duplicate spots of purified rTbpA. Lane 2 contains duplicate spots of buffer only. (D) Coomassie blue-stained SDS-PAGE containing rTbpB protein. Lane 1 contains a whole-cell lysate of IPTG-induced E. coli. Lane 2 contains the soluble fraction of IPTG-induced, detergent-solubilized E. coli. Lanes 3 through 6 represent rTbpB purification fractions. Lane 3 contains the flowthrough after overnight incubation with a nickel-affinity resin. Lane 4 contains the wash fraction. Lanes 5 through 6 contain purified rTbpB fractions. Molecular weight standards (MWM) are indicated at the left. (E) Western blots of purified rTbpB. Panel 1 was probed with an anti-TbpB antibody. Panel 2 was probed with HRP-transferrin (1 μg/ml). (F) Solid-phase transferrin binding assay of purified rTbpB probed with HRP-transferrin (1 μg/ml). Lane 1 contains duplicate spots of rTbpB. Lane 2 contains duplicate spots of buffer only.
Expression and purification of rTbpB resulted in two major bands when analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting (Fig. 1D and E). Both bands reacted with a rabbit anti-TbpB serum, and both bound horseradish peroxidase (HRP)-transferrin, although the smaller species did so weakly (Fig. 1E). Because both protein bands reacted with an anti-histidine tag antibody (data not shown), the target for which was located at the carboxy terminus of the protein, the difference in their apparent molecular weights was expected to be the result of alterations of the amino terminus. N-terminal sequencing of the smaller species confirmed that it was indeed a truncated form of TbpB, 110 amino acids shorter in length than the mature TbpB protein, initiated with the sequence MYTSP. Since both protein species were TbpB derived, we continued with these recombinant protein preparations as ELISA target antigens.
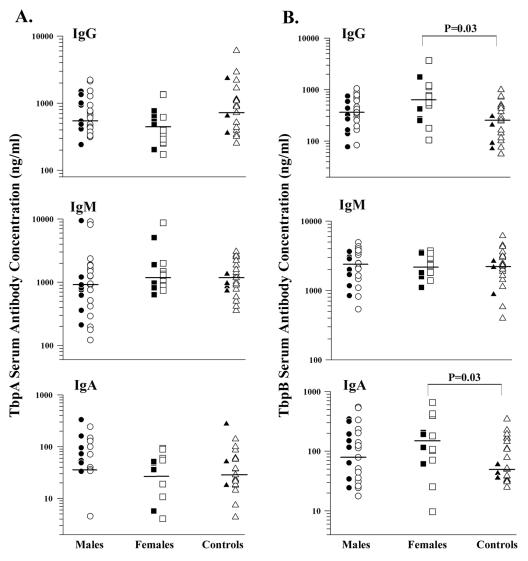
To quantify levels of antibodies specific for rTbpA and rTbpB,we examined sera from 27 males and 14 females who were culture positive for gonorrhea in a quantitative ELISA. The antibody concentrations of infected individuals were compared to those of a control group of adult volunteers with no history of gonococcal disease and of individuals attending an STD clinic who were culture negative for gonorrhea without a prior history of gonococcal disease. Both genders were included in the control group. Concentrations of IgG, IgM, and IgA in serum were analyzed for specificity to TbpA and TbpB (Fig. 2). Although we were able to measure antibodies to rTbpA and rTbpB, the concentrations were not dramatically different from those detected among individuals in the negative control group. Statistical analysis of the antibody concentrations in serum detected in infected women indicated that only TbpB-specific IgG and IgA Ig classes were significantly different from those detected in the control group (P = 0.03 for both isotypes, as determined by the Kolmogorov-Smirnov test) (Fig. 2B). This statistical correlation occurred only in women, and the median antibody concentration detected among this group of infected individuals was only slightly higher than that determined within the uninfected control group. This finding was corroborated in Western blots in which a subset of the female sera was screened (Fig. 3). Western blot reactivities against TbpB, whether in purified form or in the context of total-membrane preparations, were greater when screened with female sera than with male or uninfected control sera (Fig. 3A and C). One explanation for the detection of higher antibody concentrations in females could be gleaned from the nature of gonococcal infection. Typically, women have higher rates of asymptomatic infection than do men (6, 26); additionally, women often suffer longer latent periods before symptoms of gonorrhea appear (25). This longer latent period may give the immune system more time to mount a response before treatment is sought (10). However, clearly many of these samples from infected females fell well within the antibody levels of the controls, with only a few individuals having slightly higher antibody titers than the controls (Fig. 2B). The more significant response elicited toward TbpB than towards TbpA could be explained by the relative immunogenicity of these two molecules in the context of a natural infection. The greater sequence variability seen among TbpBs than among TbpAs (12, 13) is consistent with the hypothesis that TbpB is more immunogenic than TbpA.
FIG. 2.
IgG, IgM, and IgA antibody responses in serum. (A) Antibody responses against rTbpA. (B) Antibody responses against rTbpB. The infected groups are divided into those with a first gonococcal infection (filled symbols) and those with at least one prior episode of gonorrhea (open symbols). The control group is divided into volunteer lab personnel (filled symbols) and individuals attending an STD clinic who were culture negative with no previous history of gonococcal disease (open symbols). The horizontal bars indicate median values. Differences between the groups were compared with the Mann-Whitney U test or the Kolmogorov-Smirnov test where appropriate. Note the logarithmic scales.
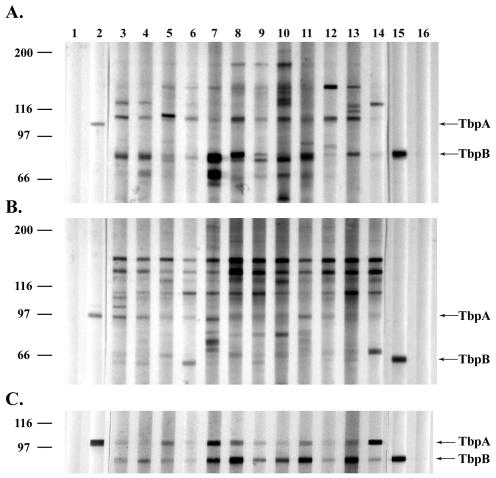
FIG. 3.
Western blot analysis of patient sera. (A) Western blot of a total-membrane preparation of iron-stressed N. gonorrhoeae probed with patient sera diluted 1:150. (B) Western blot of a total membrane preparation of iron-stressed N. meningitidis probed with the same dilution of patient sera as in panel A. (C) Western blot of purified gonococcal TbpA and TbpB probed with patient sera diluted at 1:100. Lanes 1 and 16 are negative controls with no antibody. Lane 2 was probed with an anti-TbpA antibody. Lanes 3 through 5 are female control sera. Lanes 6 and 7 are male control sera. Lanes 8 and 9 are sera from females infected with gonorrhea for the first time. Lanes 10 and 11 contain sera from females with at least one prior gonococcal infection. Lane 12 contains sera from a male infected with gonorrhea for the first time. Lanes 13 and 14 contain sera from males with at least one prior gonococcal infection. Lane 15 was probed for TbpB by using HRP-transferrin.
Because both the infected individuals and the negative controls had similar serum antibody levels specific for rTbpA and rTbpB, we explored the possibility that both were the result of cross-reactive antibodies elicited during meningococcal carriage. Hicks et al. demonstrated that antibodies elicited following meningococcal colonization were cross-reactive with the N. gonorrhoeae antigens, pili and H.8 (24). We performed similar analyses using iron-stressed membrane proteins from meningococcal strain FAM2 and an lbp mutant of gonococcal strain FA19, MCV601 (Fig. 3A and B). The meningococcal strain chosen for this analysis expressed a TbpB of the low-molecular-weight class. This family of proteins is quite diverse from the high-molecular-weight class, to which all gonococcal TbpBs belong (13), and thus represents a stringent test for cross-reactivity. The analysis presented in Fig. 3 demonstrates that although most of the individuals screened appeared to have antibodies reactive against meningococcal TbpA, TbpB-specific antibodies were more reactive against the gonococcal protein. Thus, based on these reactivities, we cannot definitively establish whether the antibodies detected were elicited by meningococcal carriage or by the current gonococcal infection. However, we can conclude that if the antibodies were elicited as a result of colonization with N. meningitidis, the colonizing strains likely expressed a TbpB of the high-molecular-weight isotype, which is more similar to TbpBs expressed by N. gonorrhoeae and more frequently encountered in nature (42). Moreover, we can also conclude that the presence of these antibodies in serum did not protect the subjects from acquisition of N. gonorrhoeae expressing a cross-reactive TbpB protein.
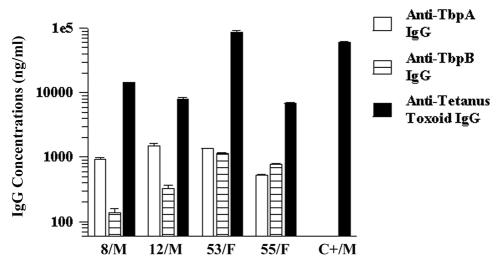
To correlate the Tbp-specific serum antibodies detected in this study with what would constitute a protective antibody level, we analyzed randomly selected serum samples for the presence and concentration of antibodies against a common vaccine antigen, tetanus toxoid (Fig. 4). The immune response elicited against tetanus toxoid is robust (3), and we reasoned that everyone in our study population had been immunized with this antigen at some time previously. Moreover, the presence of tetanus toxoid-specific antibodies would establish that the infected individuals in this study were capable of mounting a humoral immune response. Since we had no documentation of immunization histories on the study subjects and since tetanus toxoid antibodies are known to decrease over time (18, 48), we obtained serum from an individual who had recently been immunized as a positive control for this experiment. Even those individuals whose antibody responses to tetanus toxoid were modest compared to those of the control individual had antitetanus toxoid antibody concentrations 5- to 10-fold higher than those of either TbpA- or TbpB-specific antibodies. The greatest differential between anti-Tbp and anti-tetanus toxoid antibodies detected among this study population was 100-fold.
FIG. 4.
Comparison of serum IgG responses to rTbpA, rTbpB, and tetanus toxoid. Patients' identification numbers and genders are indicated on the x axis. Antibodies to rTbpA or rTbpB were not measured in the control serum (C+/M). Note the logarithmic scale.
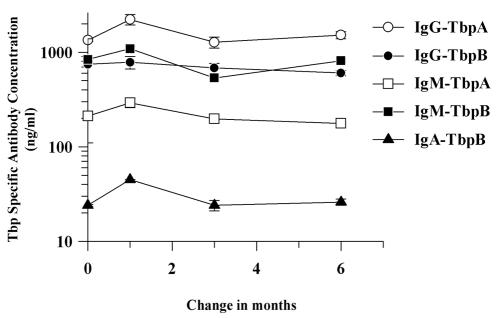
We analyzed one individual's serum antibody responses following four separate episodes of gonorrhea over a period of 6 months (Fig. 5) to determine if recurrent gonococcal infection increased the serum antibody response to TbpA or TbpB. Although there was a small, transient increase recorded during the second infection, overall Tbp-specific antibody levels remained relatively constant, highlighting the lack of an anamnestic antibody response.
FIG. 5.
Longitudinal serum antibody response. Serum antibody responses to rTbpA and rTbpB were detected from an infected male individual following four separate episodes of gonorrhea over a 6-month period. Following the initial infection, subsequent infections occurred at 1, 3, and 6 months. Note the logarithmic scale.
We analyzed cervical mucus from females and seminal plasma from males to determine if secretory Tbp-specific antibodies were correlated with antibody concentrations in serum. Total Ig levels were determined (Table 1), and we found no significant differences in total antibody levels between the female groups. In the men, there was a significant difference in total IgA concentrations between the infected individuals and the controls, with the controls having higher levels of IgA (P = 0.014, as determined by the Kolmogorov-Smirnov test). However, caution must be taken in interpreting this data since only a small number of male uninfected control samples were available for analysis.
TABLE 1.
Total genital tract immunoglobulin levels from uninfected and infected volunteers
| Sample type and antibody class | Median Ig levels (μg/ml) (range [n])
|
|
|---|---|---|
| No previous history | Current Infection | |
| Cervical mucus | ||
| IgG | 15.6 (0.1-178.6 [14]) | 6.1 (0.3-89.8 [11]) |
| IgM | 0.3 (0.0-64.0 [14]) | 4.6 (0.1-34.5 [11]) |
| IgA | 9.6 (0.2-121.5 [14]) | 8 (0.0-88.5 [11]) |
| Seminal plasma | ||
| IgG | 75.7 (49.5-187.0 [4]) | 75.5 (34.6-727.5 [9]) |
| IgM | 1.2 (0.4-7.5 [4]) | 3.5 (1.1-47.0 [9]) |
| IgA | 79.3 (54.4-80.1 [4]) | 42.5a (27.0-348.7 [9]) |
P < 0.05 compared with results for uninfected controls (as determined by the Kolmogorov-Smirnov test).
While we were able to detect Tbp-specific antibodies in the sera from all individuals tested, we were unable to detect any Tbp-specific antibodies in the secretions from any individual infected with N. gonorrhoeae. This observation suggests that circulating antibodies in the serum were not of sufficient concentration to allow transudation from serum to genital mucosa. Also, local production of antibodies was not induced to either Tbp protein during infection, as shown by the lack of detectable IgA against either antigen. It is striking that the total antibody concentrations in these infected females was low, even though there was an active infection occurring at the time of sample collection. In general, the males had much greater total antibody titers than the females, yet we were unable to detect antigen-specific responses in any of the males. To rule out the possibility of interfering substances in the secretions, we performed an ELISA by using secretion specimens spiked with TbpA-specific rabbit antiserum. We detected no decreased antibody-antigen recognition in the presence of secretions from either males or females (data not shown).
One secretion sample from a culture-negative, female STD clinic attendee contained measurable secretory antibodies specific for TbpA (Table 2). These antibodies, of both IgA and IgM classes, appeared to be locally produced, as evidenced by higher IgA concentrations in the secretions than in the serum samples (Table 2). Furthermore, if these antibodies were from serum transudation, we should have been able to detect rTbpA-specific IgG, but we were unable to do so. We were also unable to measure detectable anti-TbpB antibodies in this individual, which could be the result of poor antigenic cross-reactivity between gonococcal TbpBs (12, 13). More importantly, why did this subject have anti-TbpA antibodies if she was not infected? One possible explanation is that she did have a gonococcal infection but was culture negative. Another possibility is that this individual was infected with N. gonorrhoeae but the infection was in the latent or eclipse phase, during which culture of viable organisms is difficult. The detection of high concentrations of TbpA-specific IgM in the secretions is consistent with the suggestion that the infection was likely recently acquired or in its early stages (37).
TABLE 2.
Ig levels in serum and secretion from control subject
| Sample type and antigen | Antigen-specific antibody levels (ng/ml) of:
|
||
|---|---|---|---|
| IgG | IgM | IgA | |
| Serum | |||
| rTbpA | 2194 | 3037 | 23 |
| rTbpB | 143 | 3560 | 33 |
| Cervical mucus | |||
| rTbpA | NDa | 1076 | 71 |
| rTbpB | ND | ND | ND |
ND, not detected.
Previous studies have shown modest secretory antibody responses to the whole gonococcus in both males and females (21, 22, 35, 36, 45) and to specific antigens in vaginal fluids (28, 32). So why were we unable to measure genital antibody responses to the Tbps? One possible explanation for the lack of a genital-tract immune response to these antigens during a natural infection is that these proteins may be hidden from the immune system. The bacterium may coat itself with human transferrin in an effort to hide the Tbps from immune surveillance. Also, these antigens may not be immunogenic in the context of a whole organism, or other diversional antigens may elicit relatively greater antibody responses (28, 32). Another interesting possibility relates to the finding that N. gonorrhoeae has the ability to down regulate T cells through Opa-CEACAM1 interactions (8). Down regulating T cells could, in turn, suppress antibody production against T-cell-dependent protein antigens. This effect could also inhibit the development of memory responses to these antigens, which is consistent with our finding that multiple episodes of gonococcal disease did not correlate with an increase in Tbp-specific serum antibody levels.
Conclusions.
Although anti-Tbp antibodies were detected in the sera of patients suffering from gonococcal disease, the response was not robust compared to that elicited against tetanus toxoid, a known protective antigen. Furthermore, the response did not appear to be significantly increased with additional gonococcal exposure, indicating the lack of an anamnestic response. Because infected individuals and the uninfected controls had similarly low levels of Tbp-specific serum antibodies, we suggest that pre-existing antibodies, possibly elicited by meningococcal carriage, while cross-reactive, were not protective in the context of a gonococcal infection. We also demonstrated a complete lack of detectable antibodies specific for either TbpA or TbpB in the secretions of any infected individual. This finding has important implications for vaccine development. It is clear from these data that gonococcal infection did not elicit Tbp-specific antibodies in the genital tract, which would likely be the first line of defense. However, elicitation of a mucosal immune response in the genital tract by using a purified antigen, such as the Tbps, could be protective even though these same antigens presented in the context of a live gonococcus do not elicit a protective response. Intranasal vaccination has proven a good strategy in the generation of antibody responses in the genital tracts and sera of rodents, primates, and humans (5, 20, 43). A recent study showed protection from gonococcal colonization of mice following intranasal immunization using gonococcal outer membrane preparations (41). Studies in our laboratory are currently under way to test whether induction of an antibody response in the genital tract by vaccination with TbpA and/or TbpB could prevent colonization and/or multiplication in an exposed individual.
Acknowledgments
This work was supported by Public Health Service grants from the National Institute of Allergy and Infectious Diseases. Grant AI47141 is to C.N.C., and grant AI31496 supports the Microbiology Core Laboratory of the University of North Carolina STD Cooperative Research Center.
Polyclonal antiserum specific for gonococcal TbpB was kindly provided by Christopher Thomas and P. Frederick Sparling. We gratefully acknowledge Michael Russell for advice on ELISA implementation and for critically reading the manuscript. We also thank Peter Leone and Gail Lieblang for subject enrollment and specimen collection, Andrew Gorringe for his insight into Tbp purification, and John Tew for advice and kind donation of antitetanus sera.
Editor: J. T. Barbieri
REFERENCES
- 1.Ala'Aldeen, D. A., P. Stevenson, E. Griffiths, A. R. Gorringe, L. I. Irons, A. Robinson, S. Hyde, and S. P. Borriello. 1994. Immune responses in humans and animals to meningococcal transferrin-binding proteins: implications for vaccine design. Infect. Immun. 62:2984-2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnon, R., and M. H. Van Regenmortel. 1992. Structural basis of antigenic specificity and design of new vaccines. FASEB J. 6:3265-3274. [DOI] [PubMed] [Google Scholar]
- 3.Bayas, J. M., A. Vilella, M. J. Bertran, J. Vidal, J. Batalla, M. A. Asenjo, and L. L. Salleras. 2001. Immunogenicity and reactogenicity of the adult tetanus-diphtheria vaccine. How many doses are necessary? Epidemiol. Infect. 127:451-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beller, M., J. Middaugh, B. Gellin, and D. Ingle. 1992. The contribution of reinfection to gonorrhea incidence in Alaska, 1983 to 1987. Sex. Transm. Dis. 19:41-46. [DOI] [PubMed] [Google Scholar]
- 5.Bergquist, C., E. L. Johansson, T. Lagergard, J. Holmgren, and A. Rudin. 1997. Intranasal vaccination of humans with recombinant cholera toxin B subunit induces systemic and local antibody responses in the upper respiratory tract and the vagina. Infect. Immun. 65:2676-2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biro, F. M., S. L. Rosenthal, and M. Kiniyalocts. 1995. Gonococcal and chlamydial genitourinary infections in symptomatic and asymptomatic adolescent women. Clin. Pediatr. 34:419-423. [DOI] [PubMed] [Google Scholar]
- 7.Boslego, J. W., E. C. Tramont, R. C. Chung, D. G. McChesney, J. Ciak, J. C. Sadoff, M. V. Piziak, J. D. Brown, C. C. Brinton, Jr., S. W. Wood, et al. 1991. Efficacy trial of a parenteral gonococcal pilus vaccine in men. Vaccine 9:154-162. [DOI] [PubMed] [Google Scholar]
- 8.Boulton, I. C., and S. D. Gray-Owen. 2002. Neisserial binding to CEACAM1 arrests the activation and proliferation of CD4+ T lymphocytes. Nat. Immunol. 3:229-236. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. 2002. Sexually transmitted disease surveillance, 2001. U.S. Department of Health and Human Services. Centers for Disease Control and Prevention, Atlanta, Ga.
- 10.Cohen, I. R., D. S. Kellogg, Jr., and L. C. Norins. 1969. Serum antibody response in experimental human gonorrhoea. Immunoglobulins G, A, and M. Br. J. Vener. Dis. 45:325-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen, M. S., I. F. Hoffman, R. A. Royce, P. Kazembe, J. R. Dyer, C. C. Daly, D. Zimba, P. L. Vernazza, M. Maida, S. A. Fiscus, and J. J. Eron, Jr. 1997. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. AIDSCAP Malawi Research Group. Lancet 349:1868-1873. [DOI] [PubMed] [Google Scholar]
- 12.Cornelissen, C. N., J. E. Anderson, I. C. Boulton, and P. F. Sparling. 2000. Antigenic and sequence diversity in gonococcal transferrin-binding protein A. Infect. Immun. 68:4725-4735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cornelissen, C. N., J. E. Anderson, and P. F. Sparling. 1997. Characterization of the diversity and the transferrin-binding domain of gonococcal transferrin-binding protein 2. Infect. Immun. 65:822-828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cornelissen, C. N., G. D. Biswas, and P. F. Sparling. 1993. Expression of gonococcal transferrin-binding protein 1 causes Escherichia coli to bind human transferrin. J. Bacteriol. 175:2448-2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cornelissen, C. N., G. D. Biswas, J. Tsai, D. K. Paruchuri, S. A. Thompson, and P. F. Sparling. 1992. Gonococcal transferrin-binding protein 1 is required for transferrin utilization and is homologous to TonB-dependent outer membrane receptors. J. Bacteriol. 174:5788-5797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cornelissen, C. N., M. Kelley, M. M. Hobbs, J. E. Anderson, J. G. Cannon, M. S. Cohen, and P. F. Sparling. 1998. The transferrin receptor expressed by gonococcal strain FA1090 is required for the experimental infection of human male volunteers. Mol. Microbiol. 27:611-616. [DOI] [PubMed] [Google Scholar]
- 17.Fox, K. K., J. C. Thomas, D. H. Weiner, R. H. Davis, P. F. Sparling, and M. S. Cohen. 1999. Longitudinal evaluation of serovar-specific immunity to Neisseria gonorrhoeae. Am. J. Epidemiol. 149:353-358. [DOI] [PubMed] [Google Scholar]
- 18.Gergen, P. J., G. M. McQuillan, M. Kiely, T. M. Ezzati-Rice, R. W. Sutter, and G. Virella. 1995. A population-based serologic survey of immunity to tetanus in the United States. N. Engl. J. Med. 332:761-766. [DOI] [PubMed] [Google Scholar]
- 19.Greenberg, L., B. B. Diena, F. A. Ashton, R. Wallace, C. P. Kenny, R. Znamirowski, H. Ferrari, and J. Atkinson. 1974. Gonococcal vaccine studies in Inuvik. Can. J. Public Health 65:29-33. [PubMed] [Google Scholar]
- 20.Hajishengallis, G., S. K. Hollingshead, T. Koga, and M. W. Russell. 1995. Mucosal immunization with a bacterial protein antigen genetically coupled to cholera toxin A2/B subunits. J. Immunol. 154:4322-4332. [PubMed] [Google Scholar]
- 21.Hedges, S. R., M. S. Mayo, J. Mestecky, E. W. Hook, III, and M. W. Russell. 1999. Limited local and systemic antibody responses to Neisseria gonorrhoeae during uncomplicated genital infections. Infect. Immun. 67:3937-3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hedges, S. R., D. A. Sibley, M. S. Mayo, E. W. Hook, III, and M. W. Russell. 1998. Cytokine and antibody responses in women infected with Neisseria gonorrhoeae: effects of concomitant infections. J. Infect. Dis. 178:742-751. [DOI] [PubMed] [Google Scholar]
- 23.Heegaard, E. D., C. J. Rasksen, and J. Christensen. 2002. Detection of parvovirus B19 NS1-specific antibodies by ELISA and Western blotting employing recombinant NS1 protein as antigen. J. Med. Virol. 67:375-383. [DOI] [PubMed] [Google Scholar]
- 24.Hicks, C. B., J. W. Boslego, and B. Brandt. 1987. Evidence of serum antibodies to Neisseria gonorrhoeae before gonococcal infection. J. Infect. Dis. 155:1276-1281. [DOI] [PubMed] [Google Scholar]
- 25.Hook, E. W. I., and H. H. Handsfield. 1999. Gonococcal infections in the adult, p. 451-466. In K. K. Holmes, P. Mardh, P. F. Sparling, S. M. Lemon, W. E. Stamm, P. Piot, and J. N. Wasserheit (ed.), Sexually transmitted diseases, 3rd ed. McGraw-Hill, New York, N.Y.
- 26.Hook, E. W. I., M. S. Pate, S. R. Hedges, M. W. Russell, and J. Mestecky. 1999. Mucosal immunology of sexually transmitted diseases, p. 1463-1481. In P. L. Ogra, M. E. Lamm, J. Bienenstock, J. Mestecky, W. Strober, and J. R. McGhee (ed.), Mucosal immunology, 2nd ed. Academic Press, San Diego, Calif.
- 27.Ison, C. A., J. A. Dillon, and J. W. Tapsall. 1998. The epidemiology of global antibiotic resistance among Neisseria gonorrhoeae and Haemophilus ducreyi. Lancet 351:8-11. [DOI] [PubMed] [Google Scholar]
- 28.Ison, C. A., S. G. Hadfield, C. M. Bellinger, S. G. Dawson, and A. A. Glynn. 1986. The specificity of serum and local antibodies in female gonorrhoea. Clin. Exp. Immunol. 65:198-205. [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson, A. S., A. R. Gorringe, A. J. Fox, R. Borrow, and A. Robinson. 1997. Analysis of the human Ig isotype response to individual transferrin binding proteins A and B from Neisseria meningitidis. FEMS Immunol. Med. Microbiol. 19:159-167. [DOI] [PubMed] [Google Scholar]
- 30.Kenney, C. D., and C. N. Cornelissen. 2002. Demonstration and characterization of a specific interaction between gonococcal transferrin binding protein A and TonB. J. Bacteriol. 184:6138-6145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kerr, S., G. O'Keeffe, C. Kilty, and S. Doyle. 1999. Undenatured parvovirus B19 antigens are essential for the accurate detection of parvovirus B19 IgG. J. Med. Virol. 57:179-185. [DOI] [PubMed] [Google Scholar]
- 32.Lammel, C. J., R. L. Sweet, P. A. Rice, J. S. Knapp, G. K. Schoolnik, D. C. Heilbron, and G. F. Brooks. 1985. Antibody-antigen specificity in the immune response to infection with Neisseria gonorrhoeae. J. Infect. Dis. 152:990-1001. [DOI] [PubMed] [Google Scholar]
- 33.Lehmann, A. K., A. R. Gorringe, K. M. Reddin, K. West, I. Smith, and A. Halstensen. 1999. Human opsonins induced during meningococcal disease recognize transferrin binding protein complexes. Infect. Immun. 67:6526-6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Masri, H. P., and C. N. Cornelissen. 2002. Specific ligand binding attributable to individual epitopes of gonococcal transferrin binding protein A. Infect. Immun. 70:732-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McMillan, A., G. McNeillage, and H. Young. 1979. Antibodies to Neisseria gonorrhoeae: a study of the urethral exudates of 232 men. J. Infect. Dis. 140:89-95. [DOI] [PubMed] [Google Scholar]
- 36.McMillan, A., G. McNeillage, H. Young, and S. R. Bain. 1979. Serum immunoglobulin response in uncomplicated gonorrhoea. Br. J. Vener. Dis. 55:5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McMillan, A., G. McNeillage, H. Young, and S. S. Bain. 1979. Secretory antibody response of the cervix to infection with Neisseria gonorrhoeae. Br. J. Vener. Dis. 55:265-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meyer, T. F., J. Pohlner, and J. P. van Putten. 1994. Biology of the pathogenic Neisseriae. Curr. Top. Microbiol. Immunol. 192:283-317. [DOI] [PubMed] [Google Scholar]
- 39.Mickelsen, P. A., and P. F. Sparling. 1981. Ability of Neisseria gonorrhoeae, Neisseria meningitidis, and commensal Neisseria species to obtain iron from transferrin and iron compounds. Infect. Immun. 33:555-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Noble, R. C., N. M. Kirk, W. A. Slagel, B. J. Vance, and G. W. Somes. 1977. Recidivism among patients with gonococcal infection presenting to a venereal disease clinic. Sex. Transm. Dis. 4:39-43. [DOI] [PubMed] [Google Scholar]
- 41.Plante, M., A. Jerse, J. Hamel, F. Couture, C. R. Rioux, B. R. Brodeur, and D. Martin. 2000. Intranasal immunization with gonococcal outer membrane preparations reduces the duration of vaginal colonization of mice by Neisseria gonorrhoeae. J. Infect. Dis. 182:848-855. [DOI] [PubMed] [Google Scholar]
- 42.Rokbi, B., G. Renauld-Mongenie, M. Mignon, B. Danve, D. Poncet, C. Chabanel, D. A. Caugant, and M. J. Quentin-Millet. 2000. Allelic diversity of the two transferrin binding protein B gene isotypes among a collection of Neisseria meningitidis strains representative of serogroup B disease: implication for the composition of a recombinant TbpB-based vaccine. Infect. Immun. 68:4938-4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Russell, M. W., Z. Moldoveanu, P. L. White, G. J. Sibert, J. Mestecky, and S. M. Michalek. 1996. Salivary, nasal, genital, and systemic antibody responses in monkeys immunized intranasally with a bacterial protein antigen and the cholera toxin B subunit. Infect. Immun. 64:1272-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmidt, K. A., H. Schneider, J. A. Lindstrom, J. W. Boslego, R. A. Warren, L. Van de Verg, C. D. Deal, J. B. McClain, and J. M. Griffiss. 2001. Experimental gonococcal urethritis and reinfection with homologous gonococci in male volunteers. Sex. Transm. Dis. 28:555-564. [DOI] [PubMed] [Google Scholar]
- 45.Tapchaisri, P., and S. Sirisinha. 1976. Serum and secretory antibody responses to Neisseria gonorrhoeae in patients with gonococcal infections. Br. J. Vener. Dis. 52:374-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tramont, E. C. 1989. Gonococcal vaccines. Clin. Microbiol. Rev. 2(Suppl.):S74-S77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsai, J., D. W. Dyer, and P. F. Sparling. 1988. Loss of transferrin receptor activity in Neisseria meningitidis correlates with inability to use transferrin as an iron source. Infect Immun. 56:3132-3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Viljanen, M. K., and S. Nieminen. 1980. Immunity to tetanus in Finland. Scand. J. Infect. Dis. 12:211-213. [DOI] [PubMed] [Google Scholar]
- 49.West, D., K. Reddin, M. Matheson, R. Heath, S. Funnell, M. Hudson, A. Robinson, and A. Gorringe. 2001. Recombinant Neisseria meningitidis transferrin binding protein A protects against experimental meningococcal infection. Infect. Immun. 69:1561-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]