Abstract
Sera from normal adult humans may contain high levels of antibody reactive with Candida albicans mannan. This study examined selected biological activities of such antibodies, focusing on sera that were collected from 34 donors and analyzed individually. The results showed that antimannan titers were normally distributed. Reactivity as determined by enzyme-linked immunosorbent assay with serotype A mannan generally paralleled reactivity with serotype B. Analysis of the kinetics for activation of the complement system and deposition of complement component 3 (C3) onto serotype A and serotype B cells showed a decrease in the lag time that occurred before the onset of rapid accumulation of C3 that correlated with increasing antimannan titers. In contrast, there was a decrease in the overall rate of accumulation of C3 on serotype A cells that was strongly correlated with increasing antibody titers; serotype B cells showed no such decrease. An evaluation of the contribution of mannan antibody to opsonophagocytic killing showed that mannan antibody in individual sera and antimannan immunoglobulin G (IgG) affinity purified from human plasma contributed to killing by neutrophils in a dose-dependent fashion in the absence of a functional complement system. However, affinity-purified antibody in very high concentrations was inhibitory to both complement-dependent and complement-independent opsonophagocytosis, and this finding suggests a prozone-like effect. In contrast, if the complement system was functional, antimannan IgG was not needed for opsonophagocytic killing. These results suggest that naturally occurring mannan antibodies and the complement system are functionally redundant for opsonophagocytic killing by neutrophils.
Sera from normal, healthy adults may contain antibodies that are reactive with the mannan of Candida albicans. There is much variability among individual levels of antibody. Mannan antibody titers are normally distributed and may vary as much as 6,000-fold between different donors (6, 17, 21). The frequencies and levels of mannan antibodies in normal subjects vary with age; readily detectable levels are found in 18% of subjects 3 to 10 years of age, 48% of those 11 to 19 years of age, and 76% of those 20 to 80 years of age (2). Mannan antibodies in sera from normal donors are primarily immunoglobulin G (IgG) with much lower levels of IgM (6, 32).
Little is known about the epitope specificity of mannan antibodies in sera from normal subjects or the antigenic stimulus that leads to production of such antibody. Normal human serum contains antibodies reactive with the O-linked oligosaccharide (14). Antibodies reactive with C. albicans mannan are not removed by absorption with either purified Saccharomyces cerevisiae mannan (2) or whole cells of S. cerevisiae (19), and these findings raise the possibility that production of antibodies reactive with C. albicans mannan is due to specific stimulation with mannan from one or more Candida species. Notably, C. albicans mannan contains several epitopes that are absent in S. cerevisiae (27, 30). A likely stimulus for production of mannan antibodies is normal colonization by C. albicans. This explanation is consistent with the observation that laboratory rabbits and mice, for whom colonization is unlikely, lack mannan antibodies (17, 21).
Despite the widespread prevalence of naturally occurring mannan antibodies, little is known regarding the function of such antibodies. It has been previously reported that antimannan IgG in sera from normal adults mediates early deposition of component 3 (C3) onto the yeast via the classical complement pathway and facilitates the action of the alternative pathway (31, 32). Additional biological functions may include direct opsonization for phagocytosis and killing by neutrophils. The goals of the present study were to (i) further characterize the occurrence of mannan antibodies in normal human serum, (ii) determine the extent to which variability between individuals in levels of mannan antibodies influences the kinetics of C3 deposition via the classical and alternative pathways, and (iii) determine the role of naturally occurring antibodies in opsonophagocytic killing of C. albicans by polymorphonuclear leukocytes (PMN). The results showed that (i) the level of antimannan IgG in normal serum influences the kinetics for activation and binding of C3 but the effects of antibody titer differ for C. albicans serotypes A and B, (ii) mannan antibodies in sera from normal donors can function directly as an opsonin without the need for complement, and (iii) naturally occurring mannan antibody and the complement system play functionally redundant roles in opsonization of the yeast for killing by PMN.
MATERIALS AND METHODS
Yeast cells and isolation of mannan.
C. albicans serotype A (ATCC 36801) and C. albicans serotype B (ATCC 36803) were obtained from the American Type Culture Collection. Additional serotype A (3153A) and serotype B (CA-1) strains were provided by Jim E. Cutler (Research Institute for Children, Children's Hospital, New Orleans, La.). Stocks of yeast cells that had been stored at −80°C were used to start a fresh culture in GYEP (2% glucose, 0.3% yeast extract, and 1% peptone) broth. The yeast culture was passaged three times at 24-h intervals at 37°C. Complement activation studies used formalin-killed yeast cells. Yeast cells were killed by treatment overnight at 4°C with 1% formaldehyde, collected by centrifugation, washed, resuspended in phosphate-buffered saline (PBS), and stored at 4°C.
Yeast mannan was extracted by heating the yeast cells with water for 4 h at 121°C (24). Water-soluble mannan was precipitated with Fehling solution (13, 16, 24). Mannan was extracted from the precipitate by incubation with Amberlite IR-120 resin (Aldrich, Milwaukee, Wis.), dialyzed against water, and lyophilized. Mannan was coupled to CNBr-Sepharose 4B as described elsewhere (32) with the exception that mannan was used at a ratio of 0.1 mg per 3.5 ml of hydrated CNBr-Sepharose 4B. Examination of mannan from serotype A strains 36801 and 3153A and serotype B strains 36803 and CA-1 by enzyme-linked immunosorbent assay (ELISA) using monospecific antisera specific for each of the Candida antigenic factors (Iatron Laboratories, Inc., Tokyo, Japan) showed the expected result for each mannan; mannans from the serotype A strains were reactive with sera specific for factors 1, 4, 5, and 6, whereas mannans from the serotype B strains were reactive with sera specific for factors 1, 4, and 5 but not factor 6.
Serum samples and serum proteins.
Pooled sera were prepared from peripheral blood collected from at least 10 normal donors after informed consent and were stored at −80°C. Several experiments examined serum samples obtained from individual subjects. These individuals were normal adult donors drawn from students and laboratory personnel at the University of Nevada School of Medicine. Sera were heated for 30 min at 56°C for studies that required heat-inactivated sera. Several studies required removal of serum mannan antibodies and mannan binding lectin (MBL) by absorption with mannan-Sepharose 4B. Sera were absorbed at 0°C as described previously (32), separated from the beads by centrifugation, filtered through a 0.45-μm-pore-size filter, and frozen at −80°C. The concentrations of absorbed sera used in various experiments were corrected for the dilution that occurred during the absorption procedure. Depletion of mannan antibodies was confirmed by ELISA. Control experiments showed that the ability of absorbed sera to support activation and binding of C3 to an unrelated target, encapsulated cells of Cryptococcus neoformans, was not affected by the absorption procedure.
Naturally occurring human antimannan IgG was obtained from pooled human plasma. The IgG fraction of human plasma was isolated by differential precipitation with ammonium sulfate and caprylic acid as described previously (11). Antimannan antibody was purified from the IgG fraction by immunoaffinity chromatography using mannan-Sepharose 4B as described previously (22).
ELISA for assessment of mannan antibody titers.
Mannan antibody titers were determined by an ELISA in which purified mannan was used in the solid phase as described previously (32). Binding of human IgG, IgA, or IgM mannan antibodies was assessed by use of optimally diluted, horseradish peroxidase-labeled, heavy-chain-specific antibodies (Southern Biotechnology Associates, Birmingham, Ala.). Antibody titers were calculated by application of a linear regression to a plot of log optical density at 450 nm versus log serum dilution with background correction as described by Peterman (25). An end point optical density of 0.5 after 30 min of incubation with a substrate was used for calculation of antibody titers.
Kinetic analysis of C3 binding.
Binding of C3 to candidal yeast cells was determined by use of 125I-labeled C3 as described in previous studies (19, 31). Studies of C3 binding via the classical pathway used untreated sera and a binding medium that contained GBS2+ (sodium Veronal [5 mM]-buffered saline [142 mM], pH 7.3, containing 0.1% gelatin, 1.5 mM CaCl2, and 1 mM MgCl2). Studies of C3 binding via the alternative pathway used unabsorbed sera and a binding medium that contained GVB-Mg-EGTA (sodium Veronal [5 mM]-buffered saline [142 mM], pH 7.3, containing 0.1% gelatin, 5 mM EGTA, and 5 mM MgCl2). EGTA chelates Ca2+ that is required for classical pathway activity; consequently, any C3 deposition observed in Mg-EGTA-treated sera is due to the action of the alternative pathway (4, 26). Studies of antibody-independent binding via the alternative pathway used mannan-absorbed sera and a binding medium that contained GVB-Mg-EGTA. Samples were taken at various time intervals, the reaction was stopped by addition of a stop solution (PBS containing 0.1% sodium dodecyl sulfate and 20 mM EDTA), the cells were washed, and the bound radioactivity was assessed as described previously (31). Specific binding was determined by subtracting the radioactivities of samples which used heat-inactivated sera from the total binding observed with each sample.
Data for C3 binding are reported as the calculated number of C3 molecules bound per yeast cell at each time interval. The large number of individual serum samples that were subject to study required use of numerical measures of the kinetics for C3 binding as descriptors of the overall C3 binding curves. In this analysis, a pseudo first-order rate plot was constructed. The rationale and derivation of this approach are described in detail in previous studies of C3 binding to C. neoformans (20). Briefly, the process of activation and binding of C3 to each cell is viewed as an irreversible reaction with acceptor sites on the yeast cell. As the number of acceptor sites for C3 fragments is depleted over time, the observed velocity decreases. If t is defined as the elapsed time of the reaction, A0 is the total number of binding sites (estimated as the maximum number of C3 molecules bound to each cell after a 20-min incubation in normal serum), A is defined as the number of potential C3 binding sites at a given time t (A0 minus the number of bound C3 fragments at any given time), and k′ is the pseudo first-order rate constant, then we have the following equation: ln(A/A0) = k′t.
A plot of ln(A/A0) versus time yields a graph in which the slope of the linear portion of the curve is −k′. The nonzero intercept of the linear portion of the curve with the x axis is interpreted as an induction period prior to the onset of the pseudo first-order portion of the reaction (t0, or the lag time). In this way, the overall kinetics for binding of C3 fragments can be represented by the terms k′ and t0 (lag or induction period in minutes).
Opsonophagocytosis assay.
Neutrophils were isolated from blood drawn from normal adult donors. Neutrophils were isolated by sequential dextran sedimentation, sedimentation through Ficoll-Hypaque, and hypotonic lysis of erythrocytes (1). Following restoration of isotonicity, the neutrophils were collected by centrifugation at 250 × g and resuspended in PBS containing 10 mM glucose. Yeast cells to be used as target cells for opsonophagocytosis assays were passaged three times at 24-h intervals at 37°C on GYEP. The yeast cells were collected by centrifugation, washed three times with cold PBS, counted by use of a hemocytometer, and kept on ice. Opsonophagocytic killing assays were done in a 1.5-ml siliconized microfuge tube (Fisherbrand; Fisher Scientific, Pittsburgh, Pa.) with a 1-ml reaction mixture that contained (i) 50% concentrations of normal sera, heat-inactivated sera, or sera that had been absorbed with mannan-Sepharose 4B, (ii) 2 × 106 PMN, (iii) 2 × 106 yeast cells, and (iv) PBS containing 1.3 mM Ca2+, 0.8 mM Mg2+, and 5 mM glucose. The tubes were rotated end over end for 2 h at 37°C. At the end of the incubation period, 200 μl of Triton X-100 (0.1%) was added to lyse the PMN, triplicate samples were diluted in sterile distilled water, and aliquots were spread onto plates of YM agar (Becton Dickinson, Sparks, Md.) and incubated at 37°C. Numbers of CFU remaining in the opsonophagocytic mixture after the 2-h incubation were calculated for each treatment group.
Statistics.
Correlation coefficients were calculated as r, the Pearson product-moment coefficient. Simple pairwise comparisons that did not involve multiple comparisons were done by using Student's t test. Experiments that required multiple comparisons to a control group were analyzed by analysis of variance followed by post hoc analysis using the Bonferroni t test. All statistical analyses were done with the assistance of SigmaStat (SPSS Inc., Chicago, Ill.).
RESULTS
Mannan antibody levels in sera from normal adults.
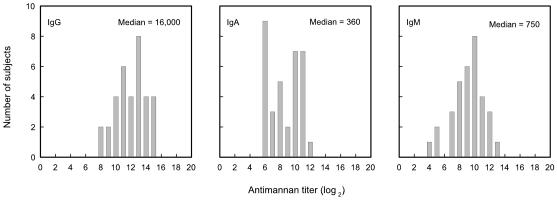
Sera from 34 normal, healthy adults were examined to determine the distribution of antimannan IgG, IgM, and IgA of serotype A. The results (Fig. 1) showed median titers of 1/16,000 for IgG (range, 1/340 to 1/120,000), 1/360 for IgA (range, 1/42 to 1/2,700), and 1/750 for IgM (range, 1/42 to 1/2,700). Overall, the titers were normally distributed around the medians, with the exception of IgA titers where a number of individuals showed very low antibody titers.
FIG. 1.
Histogram showing distribution of mannan antibodies in sera of 34 normal adult donors. Levels of IgG, IgA, or IgM antibodies were determined by ELISA using serotype A strain 36801 mannan in the solid phase.
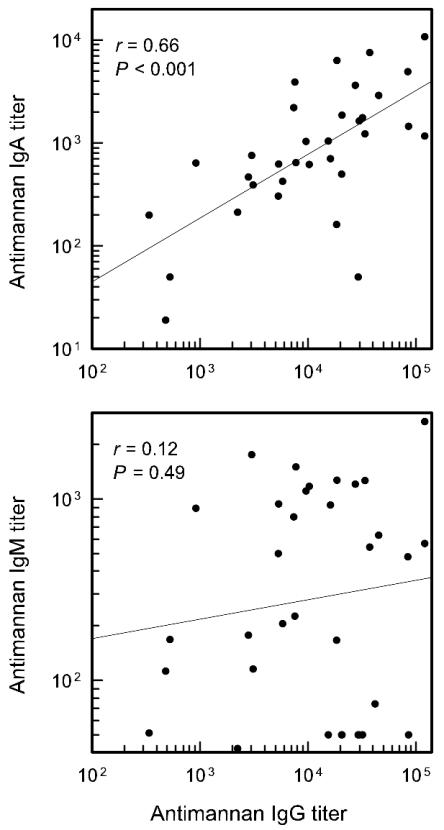
A correlation coefficient was used to assess the extent to which IgG antibody levels predicted levels of antimannan IgM or IgA. There was a significant correlation (P < 0.001) between IgG and IgA titers, but there was no correlation (P = 0.48) between IgG and IgM titers (Fig. 2). There were several donors who showed high levels of antimannan IgG but little antimannan IgM. If the sera with very low IgM titers were removed from the analysis, the results showed a significant correlation (P = 0.02) between antimannan IgG and IgM (data not shown), raising the possibility that there were two distinct sets of donors with regard to IgM antibody levels.
FIG. 2.
Correlation between IgG, IgA, and IgM antimannan titers in sera from 34 normal adults. Antimannan titers were determined by ELISA (serotype A strain 36801 mannan) for each subject and a correlation was determined for the group of subjects.
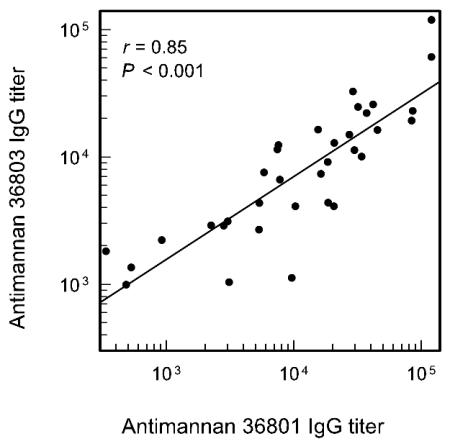
C. albicans occurs in two serotypes, serotype A and serotype B. The sera for which antibody distributions are shown in Fig. 1 were reassayed using serotype B mannan in the solid phase to determine the extent to which serotype A antibody titers are predictive of reactivity with serotype B mannan. The results (Fig. 3) showed a high degree of correlation (r = 0.85) between ELISA-determined titers found using serotype A mannan and those found using serotype B mannan in the solid phase. However, the geometric mean titers of serotype A antibody (1/11,000) were generally higher (P = 0.011 by paired t test) than serotype B antibody titers (1/7,500). In addition, there were some individual sera in which serotype A and serotype B antibody titers were largely independent of one another. For several sera, the serotype A antibody titer was approximately four times higher than the serotype B antibody titer; conversely, in several sera, the serotype B antibody titer was as much as three times higher than the serotype A antibody titer.
FIG. 3.
Correlation between IgG titers in sera of 34 normal adults as determined by ELISA using serotype A (strain 36801) or serotype B (strain 36803) mannan in the solid phase.
Influence of mannan antibody levels on the kinetics for activation and binding of C3 fragments via the classical and alternative pathways.
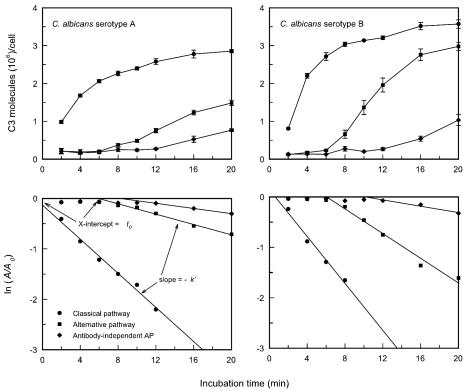
An initial experiment evaluated the kinetics for accumulation of C3 on C. albicans serotype A (strain 36801) and serotype B (strain 36803) cells via (i) the classical pathway (untreated sera), (ii) an alternative pathway (sera treated with Mg-EGTA), or (iii) an antibody-independent alternative pathway (sera treated with Mg-EGTA and absorbed to remove mannan antibody) by using a pool of sera from normal adult donors. This experiment had two goals, (i) to assess potential differences in the kinetics for C3 deposition onto cells of serotypes A and B and (ii) to evaluate the effectiveness of using t0 and k′ (see Materials and Methods) as measures that summarize the kinetics for accumulation of C3 by each pathway. The results (Fig. 4) showed that accumulation of C3 via the classical pathway began immediately after addition of yeast cells of either serotype to sera in which the classical pathway was operative. Blockade of the classical pathway by chelation of Ca2+ with Mg-EGTA extended the induction period to approximately 6 min (t0) before first-order accumulation of C3 became apparent. Absorption of sera to allow for only antibody-independent activation of the alternative pathway extended the induction period (t0) to 8 to 10 min. The rate constant for C3 accumulation (k′) was greatest when the classical pathway was active, lower when sera were treated with Mg-EGTA, and lowest when sera had been both absorbed and treated with Mg-EGTA. Therefore, the induction phase is extended and the first-order propagation phase is slowed simultaneously.
FIG. 4.
Kinetics for binding of C3 to cells of C. albicans serotype A or serotype B following incubation in PHS in which (i) the classical pathway was intact, (ii) the classical pathway had been blocked by treatment with Mg-EGTA, or (iii) both the classical pathway had been disabled by treatment with Mg-EGTA and antibody-dependent activation of the alternative pathway had been blocked by absorption of sera to remove mannan antibody (antibody-independent alternative pathway [AP]). Data are reported as the mean number ± standard error of the mean (SEM) of C3 molecules bound per cell at each incubation time in four independent experiments (upper panels) or as the results of a pseudo first-order kinetic analysis of the consumption of sites available for C3 binding where A0 is the maximum number of sites available for C3 binding and A is the number of unoccupied sites at each time interval (see Materials and Methods) (lower panels). The slope of the pseudo first-order plot yields −k′, the pseudo first-order rate constant, whereas the intercept of the plot with the x axis yields t0, in minutes, or the lag time before rapid accumulation of C3 occurs.
The results shown in Fig. 4 are the means of results from four independent experiments. Visual inspection of the results suggests that the rates of C3 accumulation via both the classical and alternative pathways were more rapid with cells of serotype B than with those of serotype A. A statistical analysis of results from the four experiments showed that this was, in fact, the case (Table 1). A comparison of the kinetics for deposition of C3 onto cells of C. albicans serotype A (36801) and serotype B (36803) showed that the rate of accumulation of C3 via the classical pathway, as expressed by k′, was slightly, but significantly, higher for cells of serotype B (Table 1). The most striking difference between the two strains was in the rates of accumulation of C3 in sera treated with Mg-EGTA, where k′ for the serotype B strain was three times higher than k′ for the serotype A strain.
TABLE 1.
Comparison of the kinetics for deposition of C3 onto cells of prototypic serotype A and serotype B strains via the classical and alternative pathwaysa
| Kinetics parameter | Value for classical pathwayb for:
|
P (A vs B) | Value for alternative pathwayc for:
|
P (A vs B) | ||
|---|---|---|---|---|---|---|
| Serotype A 36801 | Serotype B 36803 | Serotype A 36801 | Serotype B 36803 | |||
| k′d | 0.18 ± 0.04 | 0.22 ± 0.05 | 0.011 | 0.05 ± 0.007 | 0.15 ± 0.03 | 0.011 |
| t0e | −0.61 ± 0.69 | 0.65 ± 0.31 | 0.015 | 6.1 ± 1.0 | 6.2 ± 0.57 | 0.88 |
Results shown are the means ± standard deviations of results from four independent experiments. Analysis of each pair was done by Student's t test.
C3 deposition via the classical pathway was determined by incubation of C. albicans yeast cells in 40% untreated PHS.
C3 deposition via the alternative pathway was determined by incubation of C. albicans yeast cells in 40% PHS that had been treated with Mg-EGTA.
k′, pseudo first-order rate constant determined as described in the legend to Fig. 4.
t0, x intercept of a pseudo first-order rate plot. t0 is an estimate of the lag or induction period, in minutes, before the linear portion of the pseudo first-order rate plot begins (Fig. 4).
In order to confirm that the differences observed between strains 36801 and 36803 were due to serotype-specific differences rather than strain-specific differences, activation and binding of C3 fragments were assessed for additional strains of serotype A (3153A) and serotype B (CA-1). The results with the additional strains showed similar patterns; the rate of accumulation of C3 (k′) via both the classical (untreated sera) and alternative (sera treated with Mg-EGTA) pathways was greater for strains of serotype B than for strains of serotype A (data not shown).
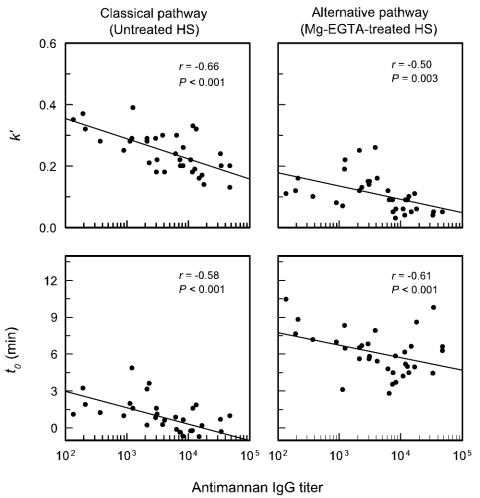
The kinetics for accumulation of C3 via the classical pathway (untreated sera) and the alternative pathway (Mg-EGTA-treated sera) by cells of serotype A strain 36801 and serotype B strain 36803 were determined for each of the 34 sera used for Fig. 1. Figure 5 shows the kinetics (k′ and t0) for accumulation of C3 onto cells of serotype A strain 36801, with the kinetics plotted as a function of the IgG mannan antibody titer of each serum sample. Analysis of the results showed a significant correlation (P < 0.001) between the antimannan IgG titer and t0; sera with high antibody titers exhibited shorter lags as expressed by t0. The results also showed a significant correlation (P < 0.001) between k′ and the antimannan IgG antibody titer. However, in the case of k′, high antibody levels were associated with slower overall rates of accumulation of C3 fragments. An analysis of the correlation of IgG mannan antibody titer with activation and binding of C3 via the alternative pathway showed similar results; there was a significant (P < 0.001) reduction in t0 with increasing antibody levels and a significant (P = 0.003) reduction in the overall rate of C3 accumulation (k′) with increasing antibody levels.
FIG. 5.
Correlation between the antimannan IgG titer (determined by ELISA with serotype A mannan in the solid phase) and the kinetics for C3 accumulation on serotype A strain 36801. The reported antimannan titers reflect the use of 40% serum concentrations in the assay. Each point is the result for 1 of 34 sera from normal adult donors. C3 accumulation via the classical pathway was assessed with untreated sera; sera were treated with Mg-EGTA to assess alternative pathway activity. The kinetics for deposition of C3 from each serum sample are expressed as k′, the pseudo first-order rate constant, and as t0, the intercept of the first-order rate plot with the x axis (as described in the legend to Fig. 4). HS, human sera.
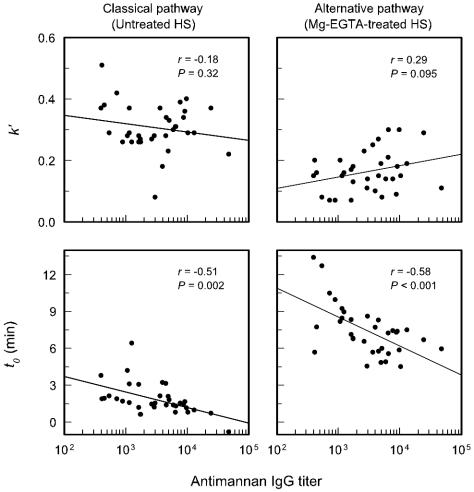
An evaluation of the impact of antimannan IgG antibody titers of individual sera on C3 binding to serotype B strain 36803 showed similarities to and important differences from results found with serotype A cells (Fig. 6). As with serotype A cells, there was a significant correlation between increasing antibody titers and a reduction of t0 for C3 binding to serotype B cells via the classical (P = 0.002) and alternative (P > 0.001) pathways. However, unlike results with serotype A cells, within the range of antibody titers represented by the individual sera there was no correlation between antibody titer and the overall rate of C3 accumulation (k′) on serotype B cells via either the classical (P = 0.32) or the alternative (P = 0.095) pathway.
FIG. 6.
Correlation between the antimannan IgG titer (determined by ELISA with serotype B mannan in the solid phase) and the kinetics for C3 accumulation on serotype B strain 36803. The reported antimannan titers reflect the use of 40% serum concentrations in the assay. Each point is the result for 1 of 34 sera from normal adult donors. C3 accumulation via the classical pathway was assessed with untreated sera; sera were treated with Mg-EGTA to assess alternative pathway activity. The kinetics for deposition of C3 from each serum sample are expressed as k′, the pseudo first-order rate constant, and as t0, the intercept of the first-order rate plot with the x axis (as described in the legend to Fig. 4). HS, human sera.
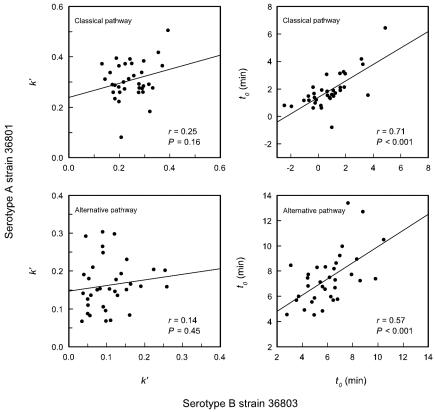
Results from examination of the dependence of k′ and t0 on antimannan IgG titers showed a dependence of t0 on antibody titers for activation and binding of C3 by both serotype A strain 36801 and serotype B strain 36803. In contrast, k′ showed a dependence on antimannan IgG antibody levels for cells of serotype A but not for cells of serotype B (Fig. 5 and 6). These results suggested that levels of antibody dependence of t0 may be similar for the two strains but that those of k′ are fundamentally different. To assess this possibility further, k′ and t0 for strain 36801 were plotted as functions of the same parameters as those for strain 36803 and correlation coefficients were determined for the individual sera. The results (Fig. 7) showed that there was no correlation between the k′ values for either the classical or alternative pathways for serotype A strain 36801 and serotype B strain 36803 when individual sera were examined. In contrast, a high degree of correlation (P < 0.001) was found between t0 values for the two yeast strains (both the classical and alternative pathways), suggesting that mannan antibody in normal sera plays similar roles in determining t0 for the two strains.
FIG. 7.
Correlation between the kinetics for deposition of C3 from individual sera onto C. albicans cells of serotype A (strain 36801) and serotype B (strain 36803). Each data point represents results from a different serum donor. Parameters measured were k′ (left panels) and t0 (right panels) for deposition of C3 from sera in which the classical pathway was intact (upper panels) or sera that had been treated with Mg-EGTA to block the classical pathway (lower panels).
Contributions of complement and naturally occurring mannan antibodies to opsonophagocytic killing.
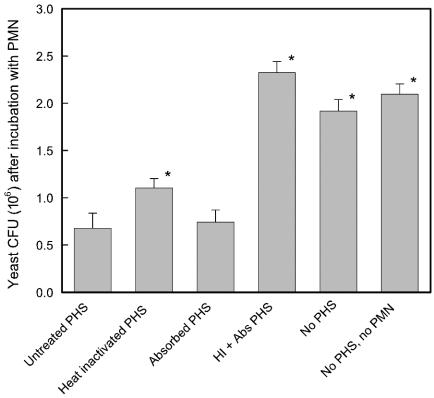
An initial experiment examined the ability of complement and naturally occurring antibodies found in pooled normal sera to support opsonophagocytic killing of C. albicans. Live cells of C. albicans serotype A strain 3153A were incubated for 2 h with neutrophils in the presence of (i) pooled human sera (PHS), (ii) heat-inactivated human sera (complement system disabled and antimannan IgG intact), (iii) pooled sera that had been absorbed with mannan-Sepharose (antibody-independent complement system intact and antimannan IgG removed), (iv) sera that had been both heat inactivated and absorbed with mannan-Sepharose, and (v) no sera. A control group consisted of C. albicans cells that were incubated in a manner identical to that for the various treatment groups but without either sera or PMN. The results are reported as the numbers of CFU at the end of the incubation period. In this system, a reduction in numbers of CFU is an indication of opsonophagocytic killing. The results (Fig. 8) showed no reduction in numbers of CFU relative to the those in the untreated control when C. albicans cells were incubated with PMN in the absence of sera or with sera that had been both heat inactivated and absorbed. In contrast, there was a near fourfold reduction in numbers of CFU if yeast cells were incubated with PMN in the presence of untreated sera, heat-inactivated sera, or sera that had been absorbed. The level of killing observed in the presence of absorbed sera did not differ from the level of killing found with untreated sera (P > 0.05). In contrast, opsonophagocytic killing in the presence of heat-inactivated sera was slightly, but significantly (P < 0.05), less effective than killing in the presence of untreated sera.
FIG. 8.
Contribution of PHS to opsonophagocytic killing of C. albicans serotype A cells by human neutrophils. Opsonophagocytic killing was determined in the presence of untreated PHS, heat-inactivated PHS, PHS that had been absorbed with mannan-Sepharose 4B to remove mannan antibody, or PHS that had been both heat inactivated and absorbed (HI + Abs PHS), in the absence of PHS, or in the absence of both PHS and PMN (control group). Results are presented as the numbers of CFU remaining after a 2-h assay. Data are reported as the means ± SEM of results from five independent experiments using neutrophils from different donors. *, P < 0.05 (versus PMN plus untreated PHS).
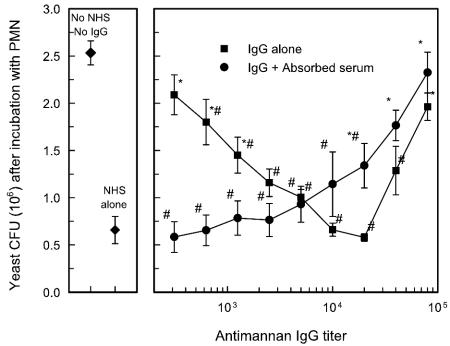
Results shown in Fig. 8 suggested that either an absorbable component of serum or the complement system can function individually to mediate opsonophagocytic killing. The opsonic component of PHS that mediated opsonophagocytic killing by heat-inactivated sera was presumed to be antimannan IgG. The ability of antimannan IgG in sera from normal adults to facilitate opsonophagocytic killing was directly assessed in an experiment in which immunoaffinity-purified antimannan IgG was used either alone or in combination with pooled sera that had been absorbed to remove antimannan IgG or MBL. The experiment was done in a dose-response manner in which affinity-purified antibody was added to the system to mimic the range of antimannan IgG levels found in our survey of individual donors (Fig. 1). The results (Fig. 9) showed that opsonization with affinity-purified IgG alone produced a dose-dependent increase in opsonophagocytic killing over a range of antibody concentrations that produced ELISA-determined titers of 1/200 to 1/10,000. Optimal killing occurred when the IgG titer was 1/10,000 to 1/20,000, antibody concentrations that bracket the median antimannan IgG titer of 1/16,000 found in the survey of sera from normal adults (Fig. 1). Further, maximum killing when yeast cells were opsonized with affinity-purified antibody alone was indistinguishable from the level of killing observed when yeast cells were opsonized with normal sera alone. Progressively less killing of C. albicans occurred when affinity-purified antibodies were present at concentrations that produced titers of 1/40,000 or 1/80,000.
FIG. 9.
Effect of affinity-purified IgG on opsonophagocytic killing of C. albicans serotype A. Yeast cells were incubated with PMN in the presence of affinity-purified antibody at concentrations that produced ELISA-determined titers over the range of 1/312 to 1/80,000 in the presence or absence of 50% PHS that had been absorbed with mannan-Sepharose to remove mannan antibody and MBL. Control groups comprised PMN incubated with C. albicans in the absence of pooled normal human sera (No NHS, No IgG) or in the presence of 50% concentrations of pooled normal human sera (NHS). Results are presented as the numbers of CFU remaining after a 2-h assay. Data are reported as the means ± SEM of results from three independent experiments using neutrophils from different donors. *, P < 0.05 (versus normal human sera alone); #, P < 0.05 (versus no normal human sera and no IgG).
Addition of affinity-purified antimannan IgG to absorbed sera had little impact on the ability of the absorbed sera to support opsonophagocytic killing at antibody levels of up to 1/10,000 (Fig. 9). The level of killing was similar to the reduction in numbers of CFU found by use of normal sera alone (P > 0.05), a result that was similar to results shown in Fig. 8. However, addition of affinity-purified antimannan IgG at concentrations equal to or greater than a concentration that produced an ELISA-determined titer of 1/20,000 produced a progressive and dose-dependent loss in opsonophagocytic killing (P < 0.05).
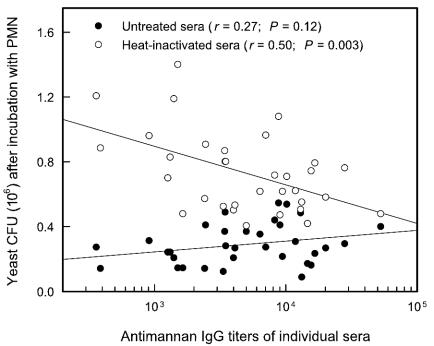
The contribution of antimannan IgG from normal sera to opsonophagocytic killing was examined further by an evaluation of the ability of sera from different donors to facilitate killing. Untreated or heat-inactivated sera for which antibody distributions are shown in Fig. 1 were examined individually to assess the ability of the sera to support opsonophagocytic killing. Untreated sera (50%) were used to evaluate the combined efficacy of opsonization by antibody and complement; 50% concentrations of heat-inactivated sera were used to evaluate opsonic activity in the absence of a functional complement system. Any opsonic activities of heat-inactivated sera were presumed to be due to the action of naturally occurring antibody. The results are shown in Fig. 10 as the numbers of CFU remaining after incubation with PMN in the presence of 50% serum concentrations as a function of the antimannan IgG titer of each serum sample. The results showed a significant correlation (P = 0.003) between an increasing antibody titer and increased opsonophagocytic killing by heat-inactivated sera. This dose-dependent effect occurred over the entire range of antibody titers. In contrast, there was no correlation (P = 0.12) between antibody titer and opsonophagocytic killing when yeast cells were incubated with sera that had not been heat inactivated; appreciable killing was observed with all sera.
FIG. 10.
Correlation between antimannan IgG titers and the abilities of sera from individual donors to support opsonophagocytic killing of C. albicans by neutrophils. Yeast cells were incubated with neutrophils in the presence of 50% concentrations of untreated sera (both complement system and antimannan IgG intact) or heat-inactivated sera (complement system disabled and antimannan IgG intact). Results shown are from one experiment representative of two experiments with similar results obtained by using neutrophils from different donors. Reported antimannan titers reflect the use of 50% serum concentrations in the assay. Results are plotted as the number of CFU remaining after incubation versus the antimannan IgG antibody titer of each serum sample. Each data point represents the results for an individual serum sample that was untreated or heat inactivated.
DISCUSSION
The objective of our study was to evaluate selected biological functions of mannan antibodies found in sera from normal adult donors. We examined the contribution of naturally occurring antibodies to activation and binding of C3 to C. albicans yeast cells and opsonophagocytic killing by neutrophils. Our studies confirmed previous reports that antimannan IgG is the primary antibody found in sera from normal adults and that antibody levels in a population of donors are normally distributed (21). Our studies also confirmed earlier reports that mannan antibodies found in PHS contribute to activation and binding of C3 via both the classical and alternative pathways (19, 31, 32). Our experimental design for examination of complement activation by C. albicans differed from those of previous studies in the use of (i) detailed kinetic analysis in the present report, (ii) an evaluation of possible serotype-specific regulation of complement activation, and (iii) an examination of the activities of a large number of individual sera. The primary new findings in the present study are that (i) antimannan IgA titers track IgG titers when sera from different individuals are examined but IgM levels are independent of either IgG or IgA, (ii) reactivities of individual sera as determined by ELISA with mannan from a single strain of serotype A generally parallel reactivities with mannan from a strain of serotype B but there are notable exceptions for such parallelism in sera from some individuals, (iii) cells of serotype A and serotype B differ in the effects of mannan antibodies on interactions with the complement system, (iv) the levels of antimannan IgG in individual sera influence the kinetics for activation and binding of C3 via both the classical and alternative pathways, and (v) naturally occurring antimannan IgG and the complement cascade are functionally redundant systems for opsonophagocytic killing of C. albicans by PMN.
It was previously reported that antimannan IgG in sera from normal donors mediates deposition of C3 onto the yeast by the classical pathway and is necessary for optimal activity of the alternative pathway (19, 31, 32). Results from the present study showed that individual variability in levels of antimannan IgG has consequences for the rate at which C3 accumulates on the yeast. Sera from individuals with low levels of antibody exhibit a lag or induction phase (t0) of approximately 2 to 3 min before rapid accumulation of C3 via the classical pathway begins on cells of serotype A. With higher antibody titers, there was a trend toward a shorter induction phase (P < 0.001). There was also an inverse correlation between antibody titer and the actual rate of first-order C3 accumulation (k′) on cells of serotype A.
Cells of serotype B were similar to those of serotype A in exhibiting a decrease in t0 that was dependent on the antimannan titers of the donor sera. In contrast, the rate of accumulation of C3 on serotype B cells, as reflected by k′, showed no dependence on the antimannan titers of the donor sera. The independence of k′ values obtained from the two serotypes (Fig. 7) suggests that the factors that influence k′ for cells of serotype A are not relevant to the propagation phase for accumulation of C3 on cells of serotype B. One explanation is the possibility that the antibodies that mediate the antibody titer-dependent decrease in k′ observed for serotype A are specific for a determinant that is not found with serotype B. Unlike that of k′ values, evaluation of t0 values produced by different sera showed very strong correlation (P < 0.001) between t0 values observed with cells of both serotypes A and B (Fig. 7). This strong correlation suggests that the roles of antibody titers in reducing t0 were similar for both cell types.
Our data do not provide an explanation for the apparent dichotomy in which high levels of antibody reduce the length of the induction phase but suppress the overall rate of C3 accumulation over the course of an extended incubation. One explanation for the antibody-dependent reduction in the induction phase is the possibility that antibody blocks binding of the regulatory protein factor H to the cell wall. Meri et al. recently found that factor H from normal serum binds to the surface of C. albicans (22). It is likely that factor H plays a critical role in suppressing the earliest phases of complement activation (15). As a consequence, suppression of the action of factor H would be critical for complement activation regardless of the Candida serotype. Once past the induction phase, it is possible that amplification proceeds in a manner that is less influenced by factor H. An explanation for the suppressive effect of high levels of antibody on the propagation phase of C3 accumulation is the possibility that high levels of antibody occupy sites that would normally be taken by C3 on serotype A cells. A high density of yeast-bound antibody would likely have little effect on early deposition but could exert an appreciable inhibitory effect at later stages of C3 deposition. This inhibitory effect would be particularly apparent when data are analyzed as a pseudo first-order rate constant, as was done in the present study.
A finding that there are differences in the abilities of naturally occurring mannan antibodies to support complement activation by cells of serotypes A and B is consistent with what is known about the two serotypes. Hasenclever and Mitchell found that C. albicans has two serotypes (12); serotype A contains all of the antigens found in serotype B as well as an additional antigen(s) not found in serotype B. Subsequent studies found that serotypes A and B share three antigenic determinants designated antigenic factors 1, 4, and 5 and serotype A has an additional determinant designated antigenic factor 6 (5, 27, 30). Structural studies found that antigenic factor 6 consists of mannoheptaose, -hexaose, and -pentaose which contain one, two, and three β-1,2 linkages at the nonreducing ends, respectively, in addition to three internal α-1,2 linkages (18). Finally, a caveat should be noted with regard to potential differences in the interactions of cells of serotypes A and B with the complement system. We examined the activities of a single strain of each serotype for most experiments. Both strains were examined for the presence of the antigenic factors expected of each serotype. Nevertheless, we cannot exclude the possibility that there are additional differences between the two strains that are unrelated to serotype.
An evaluation of the role of naturally occurring antibody in opsonophagocytic killing of C. albicans by PMN revealed that such antibody and the complement system are redundant systems; either system can mediate opsonophagocytic killing. PMN that were incubated with C. albicans in the absence of sera or in the presence of sera that had been both heat inactivated to block the complement cascade and absorbed to remove mannan antibody produced no reduction in the numbers of C. albicans CFU (Fig. 8). In contrast, a significant reduction in numbers of CFU was observed when PMN were incubated with C. albicans in the presence of untreated sera, heat-inactivated sera, or absorbed sera. Opsonophagocytic killing with absorbed sera was identical to that observed with untreated sera, whereas heat-inactivated sera was unable to reduce numbers of CFU completely to the level found with untreated sera, suggesting that naturally occurring mannan antibody is a less effective mediator of opsonophagocytic killing than the complement system. The importance of the complement system in opsonophagocytic killing of C. albicans has been widely reported (3, 23). To our knowledge, this is the first report that naturally occurring antimannan IgG found in sera from normal adults can also mediate opsonophagocytic killing.
The ability of naturally occurring antibody to function as an opsonin for killing of C. albicans by PMN independent of the complement system was confirmed by use of affinity-purified antibody from normal human plasma. Phagocytosis experiments done in the presence of increasing amounts of antimannan IgG showed a dose-dependent increase in killing at antibody concentrations up to those equivalent to ELISA-determined titers of 1/10,000 to 1/20,000. Importantly, this optimal level of killing brackets the median antimannan IgG titer found in the population of individual donors (Fig. 1). Surprisingly, a suppression of opsonophagocytic killing was observed at very high antibody titers. Indeed, no reduction in numbers of CFU was observed if the experiment was done with an antibody titer of 1/80,000, which is within the range found in normal sera (Fig. 1). An evaluation of the effect of affinity-purified antibody on the opsonophagocytic efficacy of mannan-absorbed sera showed that there was no effect of added antibody on the already efficient killing found with absorbed sera alone at antibody levels equivalent to titers as high as 1/10,000. However, as with opsonization with antimannan IgG alone, addition of very high levels of affinity-purified antibody to absorbed sera produced a dramatic reduction in opsonophagocytic killing.
An examination of the opsonic activities of sera from individuals with different levels of naturally occurring antibodies supported the finding that antimannan IgG and the complement system are functionally redundant systems in normal sera. There was a highly significant correlation (P = 0.003) between antimannan titer and opsonophagocytic killing (Fig. 10) when heat-inactivated sera were used as an opsonin, once again demonstrating the opsonic potential of antibody alone. In contrast, when untreated sera were used in phagocytosis assays, there was a high degree of killing mediated by all sera regardless of the antimannan titers. Notably, the suppression in opsonophagocytic activity observed with high levels of affinity-purified antimannan IgG (Fig. 9) was not observed with sera from individuals having antimannan titers of >1/20,000 (Fig. 10).
Prozone-like effects have been noted in studies of both C. albicans and C. neoformans. Taborda et al. found that anticapsular monoclonal antibodies (MAbs) are protective in a murine model of cryptococcosis when given in moderate doses (28, 29). However, administration of large antibody doses results in either no protection or enhanced infection. Similarly, Han et al. found that the antimannan IgM MAb 6.1 was protective in a murine model of disseminated candidiasis when given in moderate doses but that the same antibody exhibited reduced protection at high antibody doses (8). It has been previously reported that activation and binding of C3 to C. albicans is facilitated by moderate levels of affinity-purified naturally occurring mannan antibodies or by mannan MAbs; however, C3 deposition declines at very high antibody concentrations (8, 31).
Given the suppressive effects of high concentrations of affinity-purified polyclonal mannan antibodies or mannan MAbs on C3 deposition and the dramatic inhibitory effect of high levels of affinity-purified mannan antibodies on opsonophagocytosis (Fig. 9), the lack of consequences for opsonophagocytic killing using sera from normal donors with high levels of antimannan IgG is notable. One explanation for such discrepant results is the possibility that the suppressive effect for phagocytic killing is epitope specific. Use of variable amounts of affinity-purified mannan antibody may not duplicate the opsonic activities of sera from individuals with high mannan antibody titers. The high levels of antibody found in sera from some donors may be of a different epitope specificity or IgG subclass than the predominant antibodies found in pooled plasma. Unfortunately, serological assays that will identify the levels of antibodies in polyclonal sera that are reactive with the broad spectrum of distinct epitopes presented by Candida mannan are not available. As a consequence, this is a hypothesis that is not testable with existing technology. An alternate explanation for suppression of opsonophagocytosis at high concentrations of purified antimannan IgG is the possibility that the antibodies were altered during the purification process, leading to spurious results at high antibody inputs.
In summary, our results demonstrate that mannan antibodies found in sera from normal donors have biological activities that include activation of the classical pathway and facilitation of the alternative pathway as well as enhancement of opsonophagocytic killing. However, in the presence of a functioning complement system, such naturally occurring antibody is dispensable for optimal killing of C. albicans by PMN. These results suggest that low levels of naturally occurring mannan antibodies may not be a risk factor for disseminated candidiasis. Conversely, high levels of such antibodies may not appreciably enhance host resistance. However, our results do not preclude the possibility that antibodies of different epitope specificities will appreciably enhance host resistance either through active or passive immunization. Indeed, it is clear from studies of mannan MAbs that antibodies specific for some epitopes are protective whereas antibodies specific for other epitopes fail to protect (7, 9, 10). Technology is needed that will identify the epitopes that are recognized by mannan antibodies found in normal sera. Only then will we be able to place the biological activities of naturally occurring antibodies into the context of the biological activities and protective efficacy of mannan MAbs of various epitope specificities.
Acknowledgments
This work was supported by Public Health Service grants AI-14209, AI-37194, and AI-44786 from the National Institute for Allergy and Infectious Diseases.
Editor: J. T. Barbieri
REFERENCES
- 1.Clark, R. A., and W. M. Nauseef. 1996. Isolation and functional analysis of neutrophils, p. 7.23.1-7.23.3. In J. E. Coligan, A. M. Kruisbeek, D. H. Margulies, E. M. Shevach, and W. Strober (ed.), Current protocols in immunology. John Wiley & Sons, Inc., New York, N.Y. [DOI] [PubMed]
- 2.Faux, J. A., A. E. Agbarakwe, S. A. Misbah, and H. M. Chapel. 1992. A comparison of specific IgG antibody levels to the cell wall mannan of Candida albicans in normal individuals and in patients with primary antibody deficiency. J. Immunol. Methods 153:167-172. [DOI] [PubMed] [Google Scholar]
- 3.Ferrante, A., and Y. H. Thong. 1979. Requirement of heat-labile opsonins for maximal phgagocytosis of Candida albicans. Sabouraudia 17:293-297. [DOI] [PubMed] [Google Scholar]
- 4.Fine, D. P., S. R. Marney, Jr., D. G. Colley, J. S. Sergent, and R. M. Des Prez. 1972. C3 shunt activation in human serum chelated with EGTA. J. Immunol. 109:807-809. [PubMed] [Google Scholar]
- 5.Fukazawa, Y., K. Kagaya, M. Suzuki, and T. Shinoda. 1997. Serological specificity and biological activity of cell wall polysaccharides of medically important yeasts, p. 17-24. In S. Suzuki and M. Suzuki (ed.), Fungal cells in biodefense mechanism. Saikon Publishing, Tokyo, Japan.
- 6.Greenfield, R. A., J. L. Stephens, M. J. Bussey, and J. M. Jones. 1983. Quantitation of antibody to Candida mannan by enzyme-linked immunosorbent assay. J. Lab. Clin. Med. 101:758-771. [PubMed] [Google Scholar]
- 7.Han, Y., and J. E. Cutler. 1995. Antibody response that protects against disseminated candidiasis. Infect. Immun. 63:2714-2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han, Y., T. R. Kozel, M. X. Zhang, R. S. MacGill, R. J. Carroll, and J. E. Cutler. 2001. Complement is essential for protection by an IgM and an IgG3 monoclonal antibody against experimental, hematogenously disseminated candidiasis. J. Immunol. 167:1550-1557. [DOI] [PubMed] [Google Scholar]
- 9.Han, Y., M. H. Riesselman, and J. E. Cutler. 2000. Protection against candidiasis by an immunoglobulin G3 (IgG3) monoclonal antibody specific for the same mannotriose as an IgM protective antibody. Infect. Immun. 68:1649-1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han, Y., M. A. Ulrich, and J. E. Cutler. 1999. Candida albicans mannan extract-protein conjugates induce a protective immune response against experimental candidiasis. J. Infect. Dis. 179:1477-1484. [DOI] [PubMed] [Google Scholar]
- 11.Harlow, E., and D. Lane. 1996. Antibodies: a laboratory manual, p. 283-318. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 12.Hasenclever, H. F., and W. O. Mitchell. 1961. Antigenic studies of Candida. I. Observation of two antigenic groups in Candida albicans. J. Bacteriol. 82:570-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haworth, W. N., R. L. Heath, and S. Peat. 1941. The constitution of yeast mannan. J. Chem. Soc. 833-842.
- 14.Hayette, M. P., G. Strecker, C. Jaille, D. Dive, D. Camus, D. W. R. Mackenzie, and D. Poulain. 1992. Presence of human antibodies reacting with Candida albicans O-linked oligomannosides revealed by using an enzyme-linked immunosorbent assay and neoglycolipids. J. Clin. Microbiol. 30:411-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horstmann, R. D., M. K. Pangburn, and H. J. Muller-Eberhard. 1985. Species specificity of recognition by the alternative pathway of complement. J. Immunol. 134:1101-1104. [PubMed] [Google Scholar]
- 16.Jones, J. K. N., and R. J. Stoodley. 1965. Fractionation using copper complexes. Methods Carbohydr. Chem. 5:36-38. [Google Scholar]
- 17.Jones, J. M. 1980. Quantitation of antibody against cell wall mannan and a major cytoplasmic antigen of Candida in rabbits, mice, and humans. Infect. Immun. 30:78-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kobayashi, H., M. Komido, M. Watanabe, K. Matsuda, M. Suzuki, T. Ikeda, H. Oyamada, N. Shibata, and S. Suzuki. 1994. Structure of cell wall mannan of Candida kefyr IFO 0586. Infect. Immun. 62:4425-4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kozel, T. R., L. C. Weinhold, and D. M. Lupan. 1996. Distinct characteristics of initiation of the classical and alternative complement pathways by Candida albicans. Infect. Immun. 64:3360-3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kozel, T. R., M. A. Wilson, and W. H. Welch. 1992. Kinetic analysis of the amplification phase for activation and binding of C3 to encapsulated and nonencapsulated Cryptococcus neoformans. Infect. Immun. 60:3122-3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lehmann, P. F., and E. Reiss. 1980. Comparison by ELISA of serum anti-Candida albicans mannan IgG levels of a normal population and in diseased patients. Mycopathologia 70:89-93. [DOI] [PubMed] [Google Scholar]
- 22.Meri, T., A. Hartmann, D. Lenk, R. Eck, R. Wurzner, J. Hellwage, S. Meri, and P. F. Zipfel. 2002. The yeast Candida albicans binds complement regulators factor H and FHL-1. Infect. Immun. 70:5185-5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morrison, R. P., and J. E. Cutler. 1981. In vitro studies of the interaction of murine phagocytic cells with Candida albicans. J. Reticuloendothel. Soc. 29:23-34. [PubMed] [Google Scholar]
- 24.Peat, S., W. J. Whelan, and T. E. Edwards. 1961. Polysaccharides of baker's yeast. IV. Mannan. J. Chem. Soc. 1:29-34. [Google Scholar]
- 25.Peterman, J. H. 1991. Immunochemical considerations in the analysis of data from non-competitive solid-phase immunoassays, p. 47-65. In J. E. Butler (ed.), Immunochemistry of solid-phase immunoassay. CRC Press, Inc., Boca Raton, Fla.
- 26.Platts-Mills, T. A. E., and K. Ishizaka. 1974. Activation of the alternative pathway of human complement by rabbit cells. J. Immunol. 113:348-357. [PubMed] [Google Scholar]
- 27.Suzuki, S. 1997. Immunochemical study on mannans of genus Candida. I. Structural investigation of antigenic factors 1, 4, 5, 6, 8, 9, 11, 13, 13b and 34. Curr. Top. Med. Mycol. 8:57-70. [PubMed] [Google Scholar]
- 28.Taborda, C. P., and A. Casadevall. 2001. Immunoglobulin M efficacy against Cryptococcus neoformans: mechanism, dose dependence, and prozone-like effects in passive protection experiments. J. Immunol. 166:2100-2107. [DOI] [PubMed] [Google Scholar]
- 29.Taborda, C. P., J. Rivera, O. Zaragoza, and A. Casadevall. 2003. More is not necessarily better: prozone-like effects in passive immunization with IgG. J. Immunol. 170:3621-3630. [DOI] [PubMed] [Google Scholar]
- 30.Tsuchiya, T., Y. Fukazawa, M. Taguchi, T. Nakase, and T. Shinoda. 1974. Serologic aspects on yeast classification. Mycopathol. Mycol. Appl. 53:77-91. [DOI] [PubMed] [Google Scholar]
- 31.Zhang, M. X., and T. R. Kozel. 1998. Mannan-specific immunoglobulin G antibodies in normal human serum accelerate binding of C3 to Candida albicans via the alternative complement pathway. Infect. Immun. 66:4845-4850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang, M. X., D. M. Lupan, and T. R. Kozel. 1997. Mannan-specific IgG antibodies in normal human serum mediate classical pathway initiation of C3 binding to Candida albicans. Infect. Immun. 65:3822-3827. [DOI] [PMC free article] [PubMed] [Google Scholar]