Abstract
In enteric bacteria, many small regulatory RNAs (sRNAs) associate with the RNA chaperone host factor Q (Hfq) and often require the protein for regulation of target mRNAs. Previous studies suggested that the hexameric Escherichia coli Hfq (HfqEc) binds sRNAs on the proximal site, whereas the distal site has been implicated in Hfq–mRNA interactions. Employing a combination of small angle X-ray scattering, nuclear magnetic resonance and biochemical approaches, we report the structural analysis of a 1:1 complex of HfqEc with a 34-nt-long subsequence of a natural substrate sRNA, DsrA (DsrA34). This sRNA is involved in post-transcriptional regulation of the E. coli rpoS mRNA encoding the stationary phase sigma factor RpoS. The molecular envelopes of HfqEc in complex with DsrA34 revealed an overall asymmetric shape of the complex in solution with the protein maintaining its doughnut-like structure, whereas the extended DsrA34 is flexible and displays an ensemble of different spatial arrangements. These results are discussed in terms of a model, wherein the structural flexibility of RNA ligands bound to Hfq stochastically facilitates base pairing and provides the foundation for the RNA chaperone function inherent to Hfq.
INTRODUCTION
The Escherichia coli host factor Q (Hfq) was originally identified as an accessory factor of the phage Qß replicase >40 years ago, whereas its role in bacterial post-transcriptional regulation became evident only more recently (1). Hexameric Hfq protein (protomer: 102 aa) belongs to the class of Sm-like proteins with multiple functions in eukaryotic RNA metabolism (2,3). In bacteria, small regulatory trans-acting RNAs (sRNAs) can modulate different stress responses through post-transcriptional regulation (4). In general, sRNAs either prevent ribosome loading onto the mRNA by base pairing with or in the vicinity of the ribosome binding site or act by an ‘anti-antisense’ mechanism and abrogate intramolecular inhibitory stem–loop structures that block ribosome binding (5). As many sRNAs display imperfect and non-contiguous target complementary, the requirement for Hfq in riboregulation has mainly been attributed to its RNA chaperone function, which appears to facilitate the interaction between the sRNA and the cognate mRNA (2,6).
Escherichia coli Hfq (HfqEc) homologues have been found in a number of Gram-negative and Gram-positive bacteria. The hexameric Hfq proteins possess an evolutionarily conserved common core consisting of amino acid (aa) residues 7–66, whereas there is considerable variation at the C-terminal end (7). Several high-resolution structures of Hfq proteins of different origin bound to RNA oligonucleotides have been published. The first 3D structure of the 77 aa Staphylococcus aureus Hfq protein in complex with the short 5′-AU5G-3′ oligoribonucleotide revealed that the poly(U) oligonucleotide was bound in a circular fashion along the inner basic rim of the central pore (8). Afterwards, a mutational analysis performed with HfqEc further indicated that sRNAs bind to the same site, which was termed the proximal face of the hexamer (9). In addition, the crystal structure of Salmonella typhimurium Hfq (protomer: 102 aa) in complex with an (U)6 RNA oligonucleotide not only corroborated poly(U) binding to the proximal side but also revealed that a free 3′ hydroxyl group of RNA is important for high-affinity binding to the protein, which in turn may impact on the stability of RNA substrates (10).
In contrast to uridine-containing sequences, a recent structural study revealed binding of a poly(A) tract to the distal face of Hfq through six tripartite binding motifs (11). Similarly, a crystallographic analysis demonstrated binding of an A(G)3A aptamer to the distal side of the Bacillus subtilis Hfq protein, but the overall RNA structure and protein–RNA interaction patterns differed from those of the HfqEc–poly(A) complex (12). Moreover, the crystal structure of a ternary complex between a C-terminally truncated HfqEc variant, ADP and an A(U)6A RNA oligonucleotide derived from the HfqEc binding region of the sRNA DsrA (see below) has been recently reported (13). In this structure, the ADP was bound on the distal R site(s) of HfqEc, similar to the adenines of poly(A) (11), while the (U)6A part of the RNA oligonucleotide was bound on the proximal side in a circular fashion, in a manner comparable to A(U)6G binding to S. aureus Hfq (8). Moreover, the 5′ proximal A nucleotide of A(U)6A was found inserted into a distal R site of another closely packed Hfq hexamer (13).
A paradigm for positive regulation by an sRNA entails the translational activation of E. coli rpoS mRNA (Supplementary Figure S1), encoding the stationary phase sigma-factor RpoS, by the sRNA DsrA. At low growth temperatures, the DsrA–rpoS mRNA interaction counteracts an inhibitory stem–loop structure that impedes ribosomal access to the ribosome binding site of rpoS (14). In vivo DsrA–rpoS duplex formation at low temperature requires Hfq (15) and the CsdA helicase (16) and creates an RNase III cleavage site within the duplex that would prevent reuse of DsrA (17). DsrA binds to Hfq with a 1:1 stoichiometry (18) on the proximal face (9), whereas rpoS mRNA is believed to be recruited on the distal side of Hfq via A-rich element(s) in the mRNA leader (11,19,20). Mechanistic insights into the sequence of events toward Hfq-mediated DsrA–rpoS duplex formation have been recently obtained by the Woodson laboratory. Soper et al. (6) provided evidence that Hfq increases the stability of the DsrA–rpoS complex by binding to the upstream A-rich regions, which is in line with a spectroscopic study (21), indicating that rapid co-binding of two RNA ligands and their release from Hfq precedes duplex formation. The need for Hfq to cycle off its binding site on DsrA before or during annealing with rpoS (22) could result from the fact that at least part of the Hfq binding site on DsrA (23) base pairs with rpoS mRNA (24). While the dedicated sRNA and mRNA binding surfaces on either site of the Hfq hexamer could readily serve to transiently increase the local concentration of two RNA substrates, the inherent capacity of Hfq to induce conformational changes in RNAs (25–28) could, together with the possibility of several RNA-binding modes within the hexamer, lead to different spatial arrangements of RNA substrates in individual Hfq–RNA complexes, which may in turn increase the likelihood for productive duplex formation.
To address this question, we used small angle X-ray scattering (SAXS), nuclear magnetic resonance (NMR) and biochemical studies together with available information on high-resolution X-ray structures to assess the biophysical parameters and shape of HfqEc and a truncated version thereof, HfqEc65 (aa 1–65), in complex with a 34-nt segment of DsrA domain II (DsrA34). Taken together, these data revealed that binding of DsrA34 to both the full-length and the truncated protein results in an ensemble of complexes where DsrA34 is bound in a structurally variable manner on the proximal face of a given hexamer. These results are discussed in light of the RNA chaperone function of HfqEc in riboregulation.
MATERIALS AND METHODS
Synthesis and purification of HfqEc and HfqEc65
The Hfq proteins used in this study were purified as previously described (29). For NMR experiments, the proteins were uniformly isotope labelled with 15N and 13C.
Enzymatic probing of DsrA34 with RNase V1
Aliquots containing 0.15 pmol of [32P]-5′end-labelled DsrA34 were incubated in buffer (50 mM bicine pH 8.8, 100 mM NaCl, 250 mM KCl and 0.5 mM MgCl2) in the presence or absence of HfqEc (1.5 pmol as hexamer) at 37°C for 10 min. Then, 7.5 × 10−3 (Figure 2B, lanes 2 and 4) and 1.5 × 10−2 (Figure 2B, lanes 3 and 5) units of RNase V1 were added and the incubation at 37°C was continued for 10 min. Then, 2× RNA loading dye was added to stop the reaction. For complete RNA hydrolysis, 0.15 pmol of radioactively labelled DsrA34 was incubated at 85°C for 10 min in 50 mM sodium carbonate (NaHCO3/Na2CO3), pH 9.0, in the presence of 1 µg yeast tRNA. The RNA hydrolysis was terminated by addition of 1 volume 2× RNA loading dye. The samples were analysed on a 10% polyacrylamide gel containing 8 M urea and the labelled RNA fragments were visualized using a PhosphoImager.
Figure 2.
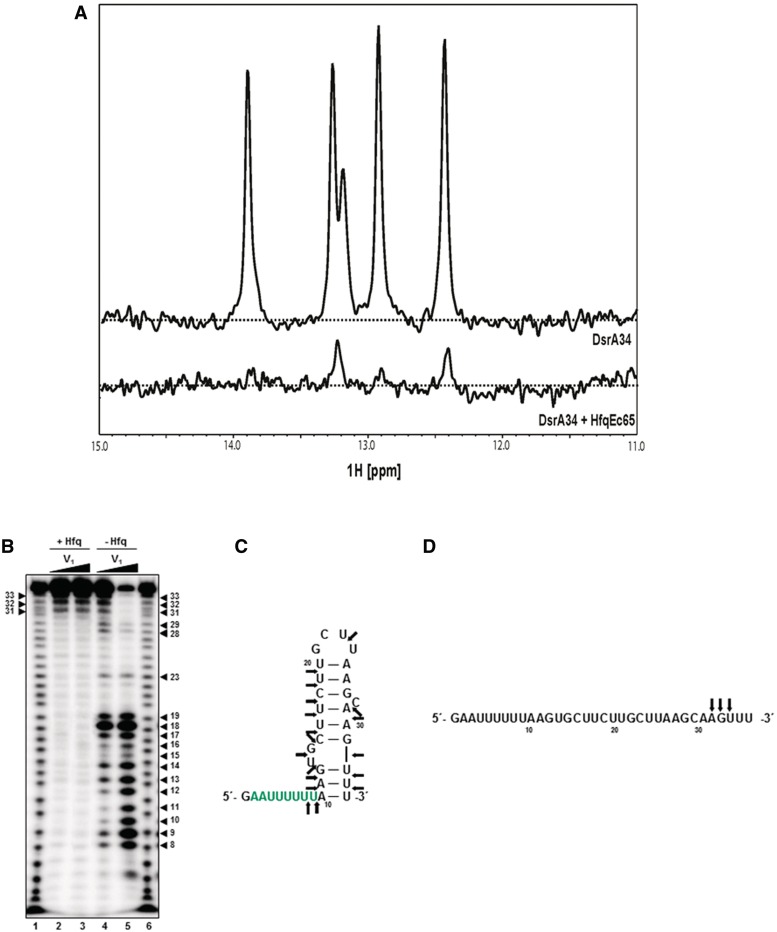
NMR spectra of DrsA34 and enzymatic probing of DsrA34 with RNase V1 in the presence and absence of HfqEc, respectively. (A) NMR spectra of free DrsA34 and in complex with HfqEc65. (B) In vitro RNase V1 cleavage of DsrA34 was performed in the presence (lanes 2 and 3) and in the absence (lanes 4 and 5) of HfqEc. 7.5 × 10−3 (lanes 2 and 4) and 1.5 × 10−2 (lanes 3 and 5) units of RNase V1 were added. Lanes 1 and 6, sequence ladder obtained by alkaline hydrolysis of [32P]-5′ end-labelled DsrA34. The numbers denote nucleotide positions in DsrA34. The arrows denote RNase V1 cleavage sites on DsrA34 in the absence (C) and presence (D) of HfqEc, respectively, derived from enzymatic probing.
HfqEc–DsrA34 complex preparation and screening of solution conditions
The DsrA34 RNA was chemically synthesized by Thermo Fisher Scientific, Inc., and was further purified on 8% polyacrylamide–8 M urea gels using standard procedures. DsrA34 samples were heated to 75°C for 15 min and immediately cooled down on ice, whereas HfqEc65 was kept at room temperature. Samples were centrifuged for 30 min at 13 000g at room temperature. Before protein–RNA complex assembling, protein and RNA concentrations were determined with a NanoDrop ND-1000 UV/Vis spectrophotometer at 280 and 260 nm, respectively.
To identify suitable conditions for long-term stabilization of HfqEc65–DsrA34 complexes, a series of sample buffer conditions were screened in 96-well plates (Intelli-Plate 96-3, Art Robbins Instruments, Inc.) containing 1 µl of HfqEc65 at a final concentration of 3.5 mg/ml in duplicate. These drops were set up using a Phoenix robot (Art Robbins Instruments, Inc.). Upon addition of stoichiometric amounts of DsrA34 to HfqEc65, the samples were left at room temperature (22°C) and visually examined at time 0 and over the course of 12 h using a Minstrel™ drop imager (Rigaku Americas Corporation). The drops without visible precipitation (14 conditions out of 96) were analysed by dynamic laser light scattering (DLS) experiment. The best four candidate conditions were selected on the basis of the lowest polydispersity index (PdI) derived from the DLS experiment. Then, a time course at different temperatures was performed using DLS to monitor the condition and the time point where the HfqEc65–DsrA34 complex samples acquired a PdI ≤ 15%. A long-term stability and monodispersity of HfqEc–DsrA34 and HfqEc65–DsrA34 complexes were achieved in 50 mM phosphate buffer, pH 7.2, containing 200 mM NaCl and in 50 mM bicine buffer, pH 8.8, containing 100 mM NaCl and 250 mM KCl.
Dynamic laser light scattering
Typically, samples with protein concentration ranging from 1 to 10 mg/ml were centrifuged 30 min at 13 000g to eliminate large aggregates. DLS was measured at a scattering angle of 90° on a DynaProNanoStar™ (Wyatt Technology) using a 10 μl microcuvette and a laser emitting at λ = 662 nm. The data collection was conducted with laser intensity in autoregulation mode, using 10 acquisition frames of 10 s/acquisition at 25 or 35°C. The data were analysed using online Wyatt software to determine the sample PdI, where sample with an average PdI ≤ 15% was considered as monodisperse and suitable for structural characterization. The apparent translational diffusion coefficient, D, calculated from the intensity autocorrelation functions fits, was converted into Stokes radii (Rs) according to the Stokes–Einstein equation (Eq. (1)):
| (1) |
with R being the gas constant, T the temperature, N Avogadro’s number and n the solvent viscosity. Control experiments carried out with HfqEc and HfqEc65 alone yielded experimental Stokes radii similar to the ones computed from atomic coordinates of rigid structures by the program HYDROPRO (30) that employs shell-model methodology. In this calculation, the crystal structure of E. coli Hfq (PDB accession code: 1HK9) (7) was used as the structural (PBD) file for HfqEc65, while the SAXS model described by Beich-Frandsen et al. (29) was employed for the HfqEc. The primary solution property derived here was translational diffusion coefficient (D) that was converted into Stokes radii (Rs) according to Eq. (1). For running HYDROPRO, the radius of the atomic elements (AER) was defined to be 2.9 Å. The number of values of the radius of the mini bead (NSIG) was set to be 6, and the radius of the mini beads in the shell was set to 2.0 for SIGMIN and 3.0 for SIGMAX. The solvent viscosity was defined to be 0.0200 poise. Partial specific volume (psv) for protein alone was set to be 0.732 cm3/g, while for HfqEc–DsrA34 and HfqEc65–DsrA34 complexes, the psv values were calculated to be 0.706 and 0.696 cm3/g according to the relative contribution of the pvs of RNA DsrA34 (0.550 cm3/g) in these respective protein–RNA complexes at stoichiometric ratio (1:1). The structural PDB files for the complexes were obtained from the SAXS models. The temperature was defined to be 298 or 308 K.
Nuclear magnetic resonance
For the NMR experiments, the complexes were prepared between unlabelled RNA and doubly 15N/13C isotope-labelled protein. All NMR experiments were performed at 37°C with a Varian Inova 600 MHz spectrometer and with a Varian Inova 800 MHz spectrometer. Before data collection, the HfqEc65–DsrA34 complex samples were incubated overnight to optimize the sample monodispersity level as suggested by the DLS experiment (Supplementary Figure S3). NMR spectra were processed with NMRPipe (31) and analysed with Sparky (32) software. The protein samples for the NMR experiments were prepared by size-exclusion chromatography in 50 mM Na3PO4, pH 7.2, 200 mM NaCl and concentrated to ∼1 mM HfqEc65 (with respect to the monomer). Samples were supplemented with 10% (vol/vol) D2O to provide the deuterium signal for the field-frequency lock, as well as 0.1–0.2% (wt/vol) NaN3 to inhibit bacterial growth. 1D 1H-NMR spectra were obtained using the WATERGATE (33) method for solvent suppression. Backbone signal assignment for the C-terminally truncated mutant HfqEc65 was obtained by a suite of standard (sensitivity enhanced) 3D triple resonance experiments: HNCA (34), HN (CO)CA (35), HNCACB (36) and HNCO (34) were recorded for sequential backbone chemical shift assignment of HfqEc65 as described by Beich-Frandsen et al. (29). 15N relaxation times (T1, T2) were measured using gradient sensitivity-enhanced 2D methods with 1H detection (37,38). Sequential backbone signal assignments in the apo form were available for HfqEc65 for aa residues 6–65 (29) and were used as starting points for signal assignment for the HfqEc65–DsrA34 complex. The fingerprint region of the 15N-HSQC spectra of HfqEc65 was superimposed onto the 15N-HSQC spectra of the HfqEc65–DsrA34 complex (Figure 1A). Both spectra were well resolved and largely similar, which allowed for sequential assignment of most of the resonances of the HfqEc65–DsrA34 complex spectra by simple peak comparison. The remainder of backbone signal assignment of the complex between HfqEc65 and DsrA34 was obtained employing HNCA (34)/HN(CO)CA (35) and HNCO (34) experiments. When necessary, ambiguous 1H–15N assignments of the HfqEc65–DsrA34 complex were resolved by inspection of their attached 13Cα or 13C′ shifts.
Figure 1.
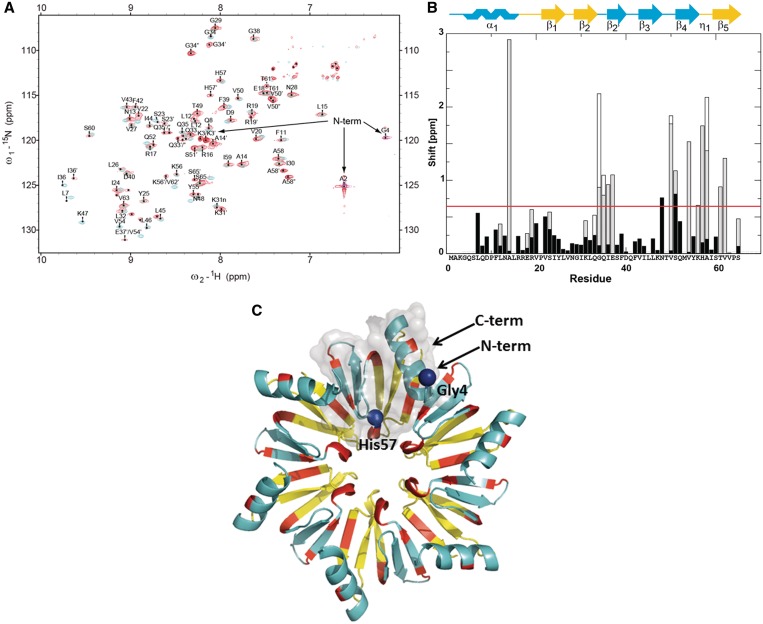
NMR analysis of the HfqEc65–DsrA34 complex. (A)13C-, 15N-labelled HfqEc65 was used for solution NMR studies. Assignments are indicated for the complex HfqEc65–DsrA34. Superposition of the 1H–15N HSQC spectra of HfqEc65 RNA free form (blue) and in complex with DsrA34 (red). (B) Chemical 1H–15N shift differences between HfqEc65 and HfqEc65–DsrA34 complex (calculated as sqrt(Δ15N2 + 25Δ1H2) for assigned peaks plotted against residue positions. Multiple sets of signals observed for certain residues are indicated in grey (two signals) and white (three signals), respectively. The HfqEc65 secondary structure is indicated and colour coded according to the position on the proximal (blue) and distal face (yellow). The distal and proximal portion of the β2-strand are termed β2 and β2′, respectively; η1 denotes the small 310-helix. The red line indicates the chemical shift differences threshold (double the average shift change) above which the chemical shift differences were considered significant. (C) Ribbon diagram of HfqEc65 hexamer. Residues with prominent chemical shift differences are colour coded in red and mapped onto the 3D structure of HfqEc65 derived from the crystal structure pdb1HK9 (7); residues belonging to proximal and distal sites are colour coded as in (B). Residues Gly4 and His57, used in SAXS modelling as contact points with DrsA34, are presented as blue spheres. Semitransparent solvent accessible surface of one HfqEc65 protomer is displayed in grey.
SAXS, ab initio shape determination and molecular modelling
SAXS experiments were performed at the EMBL BioSAXS beamline X33 (39,40) at the DORIS III synchrotron storage ring (DESY, Hamburg, Germany). A1M PILATUS detector (DECTRIS, Switzerland) was installed at a sample-to-detector distance of 2.7 m. At the X-ray wavelength λ = 1.5 Å, this beamline setup records the scattering profiles in the range of momentum transfers between 0.01 and 0.6 Å−1 (s = 4π sinθ/λ, where 2θ is the scattering angle). The data reduction and analysis followed the standard procedures using the ATSAS program package (41). All samples were in 50 mM bicine buffer, pH 8.8, containing 100 mM NaCl and 250 mM KCl and the measurements were performed at 35°C. HfqEc alone was measured at concentrations 4.1 and 15 mg/ml; the complex HfqEc–DsrA34 was measured at 2.3, 4.3 and 9.2 mg/ml, while HfqEc65 alone was measured at 2.5, 3.8 and 9.4 mg/ml and the complex HfqEc65–DsrA34 at 2.6, 4.0 and 5.1 mg/ml. The protein concentrations were determined with a Nanodrop ND-1000 UV/Vis spectrophotometer at 280 nm immediately before X-ray exposure.
Overall parameters, i.e. the forward scattered intensity I(0), radius of gyration Rg and the excluded volume of the particles, were calculated using PRIMUS (42). The program GNOM (43) was employed for calculating the pair distance distribution function and to estimate the maximum dimension (Dmax) of the particle. No concentration dependence was found for the HfqEc65–DsrA34 complex, whereas a continuous increase of overall parameters for HfqEc–DsrA34 complex was observed. For further analysis, the data from the lowest concentration of complexes, compatible with 1:1 stoichiometry of the complex, were used. The low-resolution shapes were reconstructed ab initio using the program DAMMIN (44).
To assess the flexibility of DsrA34 in the complexes, the ensemble optimization method (EOM) was used (45). A random pool of 10 000 models for the HfqEc–DsrA34 complex was generated from the atomic coordinates of HfqEc (29) and a chain of dummy residues representing the RNA molecule using RanCh (45). As the scattering density of a nucleotide is about 2.8 times larger than that of an aa residue, and as the volume of a nucleotide is two times larger than the volume of an aa residue, 136 dummy residues with the form-factor multiplier equal to one were used to represent DsrA34. This approach enables EOM to emulate flexible RNA moieties for the HfqEc–DsrA34 complex. A subset of the ensemble was selected using a genetic algorithm such that the calculated scattering of the mixture agreed with the experimental data. The Rg distributions of the selected ensembles were obtained by repeating the selection process multiple times. To better account for the relative difference in scattering density between RNA and protein, we generated a full-atom model of DsrA34 in unfolded conformation. This model on one hand was used to simulate a scattering profile of DsrA34 and on the other allowed us to validate the stoichiometry of the complexes and to estimate the effective difference in scattering density between the HfqEc–DsrA34 complex and HfqEc.
Finally, the rigid body modelling of the protein–RNA complexes was done using the program SASREF (46). To model DsrA34 in the complexes, a tentative 3D model was generated using the programme package RNABuilder (47,48) and subsequently divided into six fragments, in agreement with the persistency length of one unfolded single RNA molecule (49,50), generating in this way a chain of interconnected rigid subunits. Contacts were defined to guarantee the interactions of DsrA34 on the proximal face of Hfq hexamer, with the poly(U) stretch (Supplementary Figure S2) interacting with one of the ‘YKH’ motifs, while the next three nucleotides contact aa residues 2–4 of Hfq, as suggested by the NMR studies (Figure 1A–C). In the case of HfqEc65–DsrA34, the crystal structure of E. coli Hfq (PDB accession code: 1HK9) (7) was used as the rigid subunit, while the model described by Beich-Frandsen et al. (29) was used for the HfqEc–DsrA34 complex.
RESULTS
Hydrodynamic properties of Hfq–DsrA complexes assessed by DLS
The protein–RNA complexes were formed between HfqEc or HfqEc65 and a 34-nt fragment of DsrA (Supplementary Figure S2), spanning DsrA domain II (23). Binding of DsrA34 to both HfqEc and HfqEc65 was confirmed by electrophoretic mobility shift assays (not shown). DLS was used to determine the PdI and the Stokes’ radii (Rs) of both complexes (Supplementary Figure S3). Samples with average PdI values ≤15% were considered monodisperse and suitable for structural characterization. A long-term stability and monodispersity of HfqEc–DsrA34 and HfqEc65–DsrA34 complexes were achieved in 50 mM phosphate buffer, pH 7.2, containing 200 mM NaCl and in 50 mM bicine buffer, pH 8.8, containing 100 mM NaCl and 250 mM KCl. The DLS experiments revealed that the HfqEc–DsrA34 complex is monodisperse up to a final concentration of 3 mg/ml and displayed an Rs of 46 ± 1 Å, whereas the Rs for HfqEc alone was 35 ± 2 Å. The HfqEc65–DsrA34 complex showed monodispersity up to 9 mg/ml with an Rs of 37 ± 2 Å, whereas that of free HfqEc65 was 30 ± 2 Å. Furthermore, global analysis of the DLS autocorrelation functions (Supplementary Figure S3) corroborated the monodispersity of the complex assembled at 1:1 stoichiometry for the samples used for NMR and SAXS experiments.
NMR indicates binding of DsrA34 to the proximal face of the Hfq hexamer with 1:1 stoichiometry
NMR was used to gain more information on the structural and dynamic properties of the HfqEc65–DsrA34 complex in solution. The complex is in a ‘slow exchange situation’ reflecting the high affinity of DsrA34 for HfqEc65. Binding of the RNA to the symmetrical hexameric HfqEc65 changed the appearance of the NMR spectra. Upon the interaction with DsrA34, the original 6-fold symmetry of the HfqEc65 hexamer is broken and additional sets of cross-peaks, corresponding to the different subunits of the HfqEc65 hexamer, started to emerge in the 15N-HSQC spectra (Figure 1A). In theory, all the individual subunits of HfqEc65 are non-equivalent. However, in practice, only some of the subunit residues in direct contact with RNA experience significant shifts relative to apo-HfqEc65. Typically, only two or in very few cases three sets of signals were detected for a number of residues. This is compatible with a binding mode in which the DsrA34 predominantly interacts with one protomer of the hexameric HfqEc65. Due to the high affinity (nM) of the HfqEc65–DsrA34 interaction, no exchange between the different non-equivalent species could be detected by NMR. When compared with HfqEc65 alone, significant 15N–1HN chemical shift differences between the signal sets of the interacting and non-interacting subunits were observed in the HfqEc65–DsrA34 complex in the N-terminal α-helix, in β-strand β2′, in the proximity of the central pore, within the central pore that comprises the YKH motif (aa 55–57) and in the C-terminal β-strand β5 (Figure 1B and C). The YKH motif of S. aureus Hfq was shown to interact with poly-U (8) and is anticipated to serve as the primary binding site for sRNAs in HfqEc (9). In addition, upon complex formation with DsrA34, the amino acids A2–G4 of the partially disordered N-terminus (29) became apparently conformationally stabilized and thus detectable. As anticipated for close contacts with aromatic nucleobases, high upfield 15N shifts [∼95 (folded to 125) ppm for residue 2 and ∼90 (folded to 120) ppm for residue 4] were observed (Figure 1A). Hence, the proximal side residues A2 and G4 are apparently in contact with RNA.
Besides the N-terminus, a number of clusters of significant 15N–1HN chemical shift changes were identified. One large 15N–1HN chemical shift change maps to residue Ala14, residing in the central region of the N-terminal α-helix on its solvent-exposed side (Figure 1B and C). Ala14 could sense the conformational changes at the N-cap of the α-helix, caused by RNA interacting with the N-terminal aa residues 2–4. As expected, the YKH motif with a shell of surrounding residues experiences a difference in chemical environment upon binding of DsrA34 (Figure 1B and C). The third group of notable chemical shifts locates to the outer rim of the Hfq hexamer, i.e. to aa residues 34–37, in a conformationally labile region at the end of the β2-strand and turn connecting to the following β2′-strand (Figure 1B and C). In summary, the chemical shift analyses suggested that the RNA is bound to the central pore and that it emerges from the proximal side, tethered by interactions with the N-terminal aa residues 2–4, which precede the α1-helix on the proximal side and point toward the central pore (Figure 1B and C). In support, the HfqEcK31A mutant protein, which was shown to be strongly impaired in poly(A) binding to the distal site (51), was not impaired in binding to DsrA34, corroborating the chemical shift analyses (not shown).
The effect of adenine binding in the distal R binding site was independently investigated by titration with ATP (not shown). This ruled out simultaneous binding of DsrA34 to both the proximal and the distal side of HfqEc and a 2:1 stoichiometry as observed in complex with the RNA molecules used in the study by Wang et al. (13). This different behaviour can be best rationalized by the lack of a 5′ terminal A in our DsrA34 construct, which is obviously required for binding to the distal side (11).
Next, the 15N relaxation rates were measured to obtain information on the hydrodynamic radius, thus the effective molecular mass and consequently on the stoichiometry of the HfqEc65–DsrA34 complex. The NMR 15N relaxation measurements revealed for HfqEc65 (43.2 kDa) a T2 = 40.0 ± 2.1 ms (R2 = 25.0 ± 1.3 s−1) at 14.1 T (i.e. 600 MHz 1H frequency). For the HfqEc65–DsrA34 complex (50.5 kDa), T2 was determined with 29.0 ± 6.5 ms (R2 = 34.5 ± 7.7 s−1) at the same field strength for the signals of the core region. This agreed with the molecular weight increase expected for a 1:1 stoichiometry between HfqEc65 and DsrA34. Thus, the NMR studies strongly suggested that under these conditions, complex formation predominantly occurs in a 1:1 ratio. Moreover, no imino 1H resonances indicative of stable base pairs in double-stranded nucleic acids, which are observable in the 1D 1H NMR spectra of free DsrA34, were detected in the 1D 1H NMR spectra of the HfqEc65–DsrA34 complex (Figure 2A). The lack of stable base pairing in the HfqEc65–DsrA34 complex suggested an extended and single-stranded conformation of DsrA34, where the imino 1Hs are prone to solvent exchange in the absence of base pairing. Similarly, the H3 imino Hs of the poly U tract at the 5′-end of DsrA34, which are expected to be bound to the central pore of HfqEc65 (similar to A(U)6G binding to S. aureus Hfq (8)), are outward-facing towards the solvent and thus not expected to yield observable signals. To obtain additional experimental support for an extended conformation of DsrA bound to Hfq, enzymatic probing with the double-stand specific endoribonuclease V1 was performed. This analysis revealed that the stem–loop structure in DsrA34 is at least partially formed in the absence of Hfq, whereas no indications for base pairing were obtained when a 1:1 complex of HfqEc65 hexamer and DsrA34 was treated with the enzyme (Figure 2B–D). These chemical probing experiments are in agreement with a FRET study by Večerek et al. (28), showing that both HfqEc and HfqEc65 induce structural changes in full-length DsrA.
Low-resolution shape of HfqEc65–DsrA34 and HfqEc–DsrA34complexes
SAXS measurements were performed in parallel with the complexes HfqEc–DsrA34 and HfqEc65–DsrA34 at three different sample concentrations (HfqEc–DsrA34: 2.3, 4.3 and 9.2 mg/ml and HfqEc65–DsrA34: 2.6, 4.0 and 5.1 mg/ml). To avoid aggregation or concentration-induced artefacts, we used only the lowest concentrations of both complexes, corresponding to a 1:1 stoichiometry, which is in agreement with a study by Updegrove et al. (18) who reported that DsrA domain II and HfqEc form 1:1 complexes.
The processed experimental data are presented in Figure 3A and the overall molecular parameters are summarized in Table 1. The data recorded for HfqEc and HfqEc65 are fully compatible with the results obtained by Beich-Frandsen et al. (29). The calculated scattering profile of the crystal structure of HfqEc65 (PDB accession code: 1HK9) (7) agreed with the experimental SAXS data with a discrepancy χ = 1.06 (Figure 3A), indicating that HfqEc65 forms a stable hexamer in solution and that the doughnut shape of the crystal structure is retained. For the full-length protein, the model described by Beich-Frandsen et al. (29), where the C-terminal regions stretch outward from the central core, also agreed with the present experimental data (χ = 1.65; Figure 3A). The models generated by Beich-Frandsen et al. (29) were therefore used in the subsequent analysis of the HfqEc–DsrA34 and HfqEc65–DsrA34 complexes.
Figure 3.
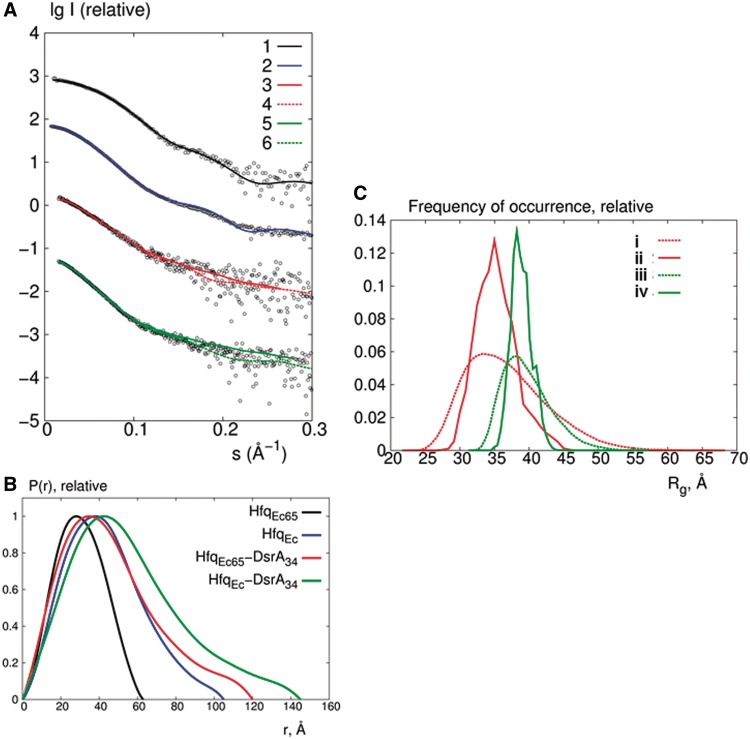
Analysis of the SAXS data. (A) Comparison of the experimental SAXS curves (empty circles) with the CRYSOL calculated scattering curves for HfqEc65 (curve 1) and HfqEc (curve 2) using the crystal structure pdb1HK9 (7) and the model reported by Beich-Frandsen et al. (29). Typical fits of the ab initio models (curves 3 and 5) and rigid body models (curves 4 and 6) against the experimental data for HfqEc65–DsrA34 (fits with red lines) and HfqEc–DsrA34 (fits with green lines) complexes, respectively. (B) Comparison of the pair distance distribution functions for HfqEc and HfqEc65 alone and the corresponding complexes with DsrA34. (C) Frequency distributions of Rg obtained in EOM to assess the flexibility of DsrA34 in complex with HfqEc65 (red) and HfqEc (green). Dashed curves (i) and (iii) correspond to the distributions for the random pool of 10 000 conformers and curves (ii) and (iv) to the distributions for the optimized ensembles.
Table 1.
SAXS parameters calculated from experimental data compared with Stokes radii from DLS experiment and with Stokes radii calculated from the ab initio models using the program HYDROPRO (30)
| Sample | Rg [SAXS] (Å) | Dmax [SAXS] (Å) | Rs [DLS] (Å) | Rs [HYDROPRO] ab initio model (Å) |
|---|---|---|---|---|
| HfqEc | 34 ± 1 | 105 ± 5 | 35 ± 2 | 36 |
| HfqEc–DsrA34 | 44 ± 2 | 145 ± 10 | 46 ± 1 | 48 |
| HfqEc65 | 23 ± 1 | 63 ± 3 | 30 ± 2 | 30 |
| HfqEc65–DsrA34 | 33 ± 2 | 120 ± 8 | 37 ± 2 | 38 |
As shown in Figure 3B, the distance distribution function P(r) of the HfqEc–DsrA34 complex revealed a skewed appearance typical for an elongated particle. The significant increase in Rg and Dmax after binding of DsrA34 again suggested that the 34-nt RNA molecule is fully extended after binding to the protein. The observed Rg and Dmax are incompatible with the presence of a stable stem–loop and can thus be explained by flexible and disordered single-stranded RNA (possibly in a fast exchange equilibrium with other loosely base-paired species). For HfqEc65 and the HfqEc65–DsrA34 complex, the changes in Rg and Dmax follow the same pattern as observed for HfqEc and the HfqEc–DsrA34 complex. The fact that truncation of the C-terminus did not change the behaviour of DsrA34 binding and that HfqEc65 was previously shown to be proficient in DsrA binding (28) suggested that C-terminal residues are not involved in Hfq–DsrA34 interactions.
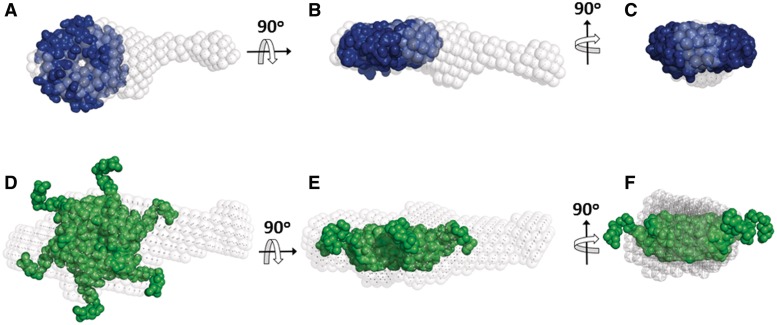
To obtain direct information on the structural changes upon RNA binding, low-resolution ab initio models (∼25 Å) were reconstructed from the SAXS data. The model of HfqEc65–DsrA34 shows an elongated particle consisting of a bulk region with an extended arm. The crystal structure of HfqEc (aa 1–71; PDB accession code: 1HK9) (7) can be docked into the bulk part of the model and the arm, apparently consisting of DsrA34, is stretching outward (Figure 4A–C). The elongated ab initio model for the complex is further supported by the agreement of a Stokes radius of 38 Å determined from this model by the program HYDROPRO (30) with the measured value (37 ± 2 Å) using DLS (Table 1). Similarly, the model for the HfqEc–DsrA34 complex displays an arm protruding outwards the protein part represented by the crystal structure of HfqEc with the C-terminal residues added as in (29) (Figure 4D–F).
Figure 4.
Ab initio models of HfqEc65–DsrA34 and HfqEc–DsrA34 derived from the SAXS data. (A–C) Models of HfqEc65 (blue) and (D–F) HfqEc (green) represented with their respective solvent accessible surfaces were docked manually into the bulk region of the ab initio shapes. The HfqEc65 atomic coordinates were derived from the crystal structure pdb1HK9 (7). The HfqEc structure was derived from the same atomic coordinates with the C-terminal segment modelled using SAXS data (29).
Flexible arrangements of DsrA34 in both complexes
As the NMR, SAXS and enzymatic probing studies suggested that DsrA34 is unfolded, extended and lacks defined secondary structure when bound to HfqEc and HfqEc65, the EOM (45) approach was used to assess the flexibility and the accessible conformational space of the complexes. EOM takes into account flexibility by allowing for the coexistence of different conformations of the complex in the population of molecules in solution, contributing to the experimental scattering pattern. In EOM, a large pool of random configurations was generated and ensembles were selected from this pool using a genetic algorithm, such that the average computed scattering over the ensemble fitted the experimental scattering data. As shown in Figure 3C, the Rg distributions of the random pools for both samples are rather broad, whereas the selected ensembles both for HfqEc–DsrA34 and HfqEc65–DsrA34 display relatively narrow distributions, where the most compact and most extended configurations are not present. The EOM ensembles fitted the scattering data rather well, with a discrepancy χ of 1.3 and 1.0 for HfqEc–DsrA34 and HfqEc65–DsrA34, respectively (fits not shown). This indicated that a confined range of conformationally variable HfqEc65–DsrA34 and HfqEc–DsrA34 complexes exists in solution. The major insight from EOM was that DsrA34 in the complex is unfolded, as the experimental data could not be fitted by compact RNA structures.
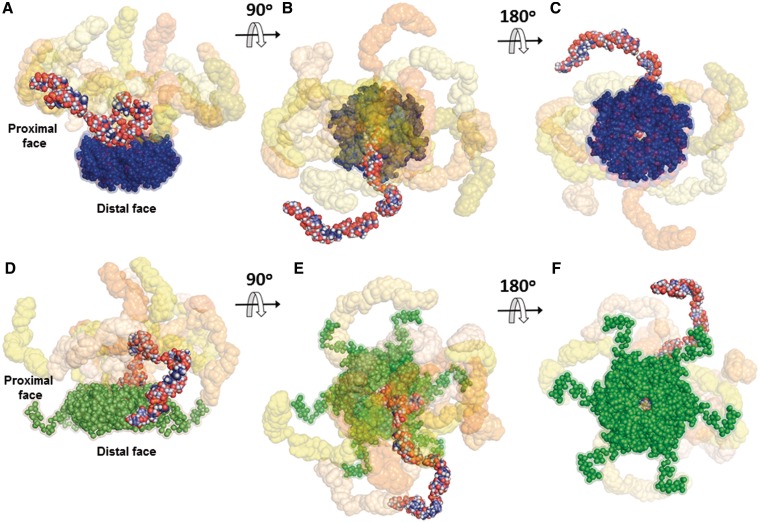
The confined flexibility of the complexes permitted rigid body modelling against the SAXS data to visualize the spatial arrangement of DsrA34 relative to the hexameric core of the protein. As shown in Figure 3A, the rigid body models generated by SASREF (46) agreed with the experimental data with a typical discrepancy χ = 1.2 for HfqEc–DsrA34 and χ = 1.1 for HfqEc65–DsrA34. Ten superimposed independently constructed rigid body models for HfqEc–DsrA34 and HfqEc65–DsrA34 revealed that in both complexes the RNA extends like an arm away from the hexamer core, thus leading to an elongated shape. The orientation and the contour length of the RNA arms may vary such that DsrA34 explores a manifold of configurations around the core (Figure 5). The obtained conformational space of the RNA molecule is confined to the proximal side of the hexamer, in agreement with the EOM results.
Figure 5.
Rigid body models of HfqEc65–DsrA34 and HfqEc–DsrA34 complexes. (A–C) Ten typical models for the HfqEc65–DsrA34 complex are superimposed and shown at three different plane rotations. HfqEc65 atomic coordinates were derived from the crystal structure pdb1HK9 (7) (solvent accessible surface representation in blue). The models were generated using SASREF (46) and SAXS data combined with constraints obtained from NMR. (D–F) Ten typical models of the HfqEc–DsrA34 complex are superimposed and shown at three different plane rotations. The HfqEc structure (solvent accessible surface representation in green) was derived from the high-resolution crystal structure pdb1HK9 (7) with the C-terminal segment modelled using SAXS data (29). In both complexes, one DsrA34 molecule is represented as full-atom model with atoms colour-coded to highlight the overall structure of the RNA in the complex, while the other nine models are presented with their solvent accessible surface (in different tonalities ranging from light yellow to deep orange). In both cases, the best DsrA34 models that fit the SAXS data display an extended conformation. The average root mean square deviation between the DsrA34 portions in the models, (60 ± 20 Å), is similar to that between the selected models in the EOM ensembles.
The theoretical Stokes radii (Rs) calculated for the HfqEc–DsrA34 and HfqEc65–DsrA34 complexes are equivalent to the Rs derived experimentally from the DLS data (Table 1) and agree with DsrA34 binding to HfqEc and to HfqEc65 in extended conformation with a 1:1 stoichiometry. Moreover, the RNA does not interact with the C-terminus of HfqEc (Figure 5D–F), suggesting that the C-terminal region of HfqEc may not affect the overall conformation of DsrA34 and vice versa. These results corroborated previous synchrotron radiation circular dichroismn spectroscopy studies, which revealed that DsrA34 did not alter the structure of HfqEc (29). In contrast, the presence of a longer RNA fragment comprising the 5′ upstream region and the immediate coding region of the hfq gene, which was shown to require the C-terminal extension of HfqEc for binding (28), did lead to an ordering of the C-terminus of HfqEc (29).
DISCUSSION
We used complementary biophysical and structural biology methods to study the arrangement of domain II of the sRNA DsrA on the surface of the HfqEc hexamer at a stoichiometry ratio as reported by Updegrove et al. (18). Previous electrophoretic mobility shift experiments (22) showed that Hfq can bind to full-length DsrA with a 2:1 ratio, although binding of a second Hfq hexamer required high concentrations of the protein. In addition, a recent biophysical study indicated likewise a 2:1 ratio between Hfq and DsrA (13). Moreover, crystallographic studies by Wang et al. (13) revealed that a short oligonucleotide A(U)6A was bound with the (U)6A segment to the proximal side of one Hfq hexamer, whereas the 5′A nucleotide was found inserted into a distal R site of a second Hfq hexamer. In our study, the increase of SAXS overall parameters for HfqEc–DsrA34 at higher concentrations could be explained by the formation of 2:1 (or 2:2) HfqEc–DsrA34 complexes, which would be in agreement with (13). As no concentration effects were observed for the HfqEc65–DsrA34 complex (Supplementary Table S1), this interaction is probably not RNA mediated and therefore could occur through interactions of C-terminal residues of HfqEc. However, given that Hfq appears to be rather limiting for riboregulation during normal growth conditions (52), the biological significance of the observed higher Hfq–RNA complexes remains uncertain. In addition, the studies by Wang et al. (13) are at variance with the observations by Updegrove et al. (18). Using different experimental approaches, the latter authors showed by mimicking the cellular environment in terms of concentration and ionic strength conditions that HfqEc and HfqEc65 form a 1:1 complex with DsrA or DsrA domain II in solution.
Similarly as observed by Updegrove et al. (18), our studies strongly suggest that HfqEc and HfqEc65 form a 1:1 complex with DsrA34. Furthermore, the NMR study identified three clusters of residues affected by binding of DsrA34 to HfqEc65. (i) The YKH motif located in the central pore of the Hfq hexamer together with the surrounding residues residing in the adjacent β-strands. In the YKH motif, K57 and H58 participate in RNA binding in S. aureus Hfq (8), whereas Y56 is involved in aromatic stacking interactions stabilizing H58 in an appropriate orientation for interaction with the RNA base. (ii) The N-terminal segment of Hfq, where structuring of aa residues 2–4 was observed. These residues precede the α-helix located on the proximal face of the Hfq hexamer (7) (Figure 1C), and their interaction with RNA might lead to concomitant perturbation of the hydrogen bonding network in the α-helix itself and to some subtle repacking of the α-helix as a whole. (iii) As a consequence, the residues spatially adjacent to the C-cap of the α-helix, i.e. the aa residues 34–37 located at the end of β2-strand and in the turn connecting to the following β2′-strand, are also affected. Although the reason(s) for these chemical shift variations is unclear, it seems possible that this loop has some conformational variability and can assume different conformations in different environments. This hypothesis is supported by our NMR studies as well as by the crystal structure (pdb1HK9 (7)) in that the 34–37 region displays some flexibility, which might result in different conformations and hence in the observed chemical shifts variations in the HfqEc65–DsrA34 complex. These NMR studies corroborate mutational analyses (9), which likewise indicated that DsrA binds to the proximal site of Hfq.
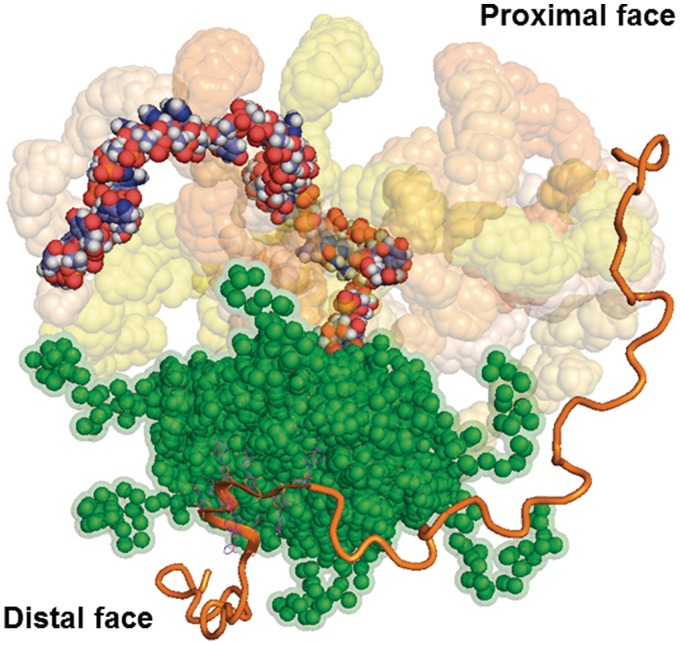
Biophysical studies (18) have recently shown that a ternary complex between Hfq, DsrA domain II and polyA18, the latter of which was shown to bind to the distal site (11), is rather unstable. However, Soper et al. (6) provided evidence that Hfq forms persistent ternary complexes when the two ligands are complemetary. These experiments also indicated that at least transient co-binding between Hfq, rpoS mRNA and DsrA contributes to riboregulation, which can be reconciled with the observed rapid binding and release of Hfq from ternary complexes during annealing (21). Hence, the two distinct binding sites on Hfq could at least transiently increase the local concentration of both the sRNA and the mRNA, which could in turn facilitate the interaction of ligands with medium affinities and circumvent the need for a high concentration of either substrate. Taken the above-mentioned studies together with the observation that stable binding of longer mRNAs by Hfq appears to involve contacts with the intrinsically flexible C-termini of Hfq (28,29) and with the structural data presented herein, we suggest the following model for the function of Hfq in riboregulation. The model entails five steps: (i) fast binding of both RNA ligands (21) to Hfq leading to their increased local concentration (Figure 6), followed by (ii) restructuring of the substrates by Hfq (25,26,28). Hereby, the Hfq-induced conformational fluctuations in both the sRNA and mRNA may occur separately. While the intrinsically unstructured region of the C-terminus of Hfq (29) may contribute to mRNA binding and may induce conformational changes in these ligands, it seems to be dispensable for doing so in sRNAs, as the C-terminally truncated HfqEc65 was proficient to alter the structure of DsrA (28). In addition, at least in the HfqEc–DsrA34 complex, the DsrA34 does not interact with the C-terminus of HfqEc (Figure 5D–F); (iii) initiation of base-pairing between the ligands (21). In this step, the inherent capacity of Hfq to present RNA in extended conformation and different spatial orientation(s) (Figures 5D–F and 6) would allow to cover a large space over Hfq, which would firstly favour the encounter and secondly the initial annealing of two cognate RNAs. In addition, it has been recently shown that Hfq can bind to the U-rich sequence following the rho-independent terminator of sRNAs (53). Thus, different binding sites of Hfq on one sRNA could be likewise important in terms of increasing the geometric variability of these ligands on the proximal face of Hfq. Moreover, not only the sRNA may be presented in different orientations on the proximal face but—given the presence of six tripartite-binding motifs (11)—also the mRNA bound on the distal side may adopt different orientations. Hence, the geometric variability of the ligands in individual complexes would facilitate annealing in a stochastic manner; (iv) ligand release from Hfq (21,22) followed by (v) stable duplex formation between the RNA substrates. At this juncture, RNA displacement from Hfq may occur by invasion of other RNAs (54). In this model, fast binding, restructuring and the presence of conformationally variable complexes would not only ensure a fast turnover of cognate RNAs but could also provide a means of proofreading for non-cognate ligands, i.e. where initial base pairing cannot take place. Similarly, Doetsch et al. (55) suggested that a human immunodeficiency virus-1 derived Tat peptide accelerates annealing of two RNA ligands by changing the population distribution of RNA structures to favour an annealing-competent RNA conformation.
Figure 6.
Model of Hfq RNA chaperone function. The sRNA displayed by the full-atom model with atoms colour-coded is shown bound to the proximal face of HfqEc (green, solvent accessible surface). Through conformational fluctuations, the sRNA can cover a larger conformational space (nine representative DsrA34 models are displayed with their solvent accessible surface coloured as in Figure 5). The mRNA is bound on the distal side (polyA9 orange flat cartoon representation) to one of the six tripartite binding motifs as shown in the crystal structure pdb3GIB (11). The model of a hypothetical mRNA chain is displayed in orange oval cartoon. HfqEc acts by restructuring the mRNA (6), which may be accomplished by the conformationally flexible C-termini (29). The structural variability of both RNAs in a transient ternary 1:1:1 complex (18) would allow to sample large spaces. In this way, HfqEc would not only act as a platform for binding and by increasing the local concentration of both ligands but would also serve to promote their flexibility and consequently successful annealing in a stochastic manner.
As mentioned above, the increased local concentration of two RNA ligands together with the structural plasticity of RNA bound to the RNA chaperone Hfq would allow the RNA to sample large spaces and therefore enhance the probability of successful annealing between RNAs. This capacity of Hfq appears to be of particular importance when the free energy of sRNA–mRNA pairing interactions is low, i.e. when the complementarity of Hfq-dependent sRNAs and their target mRNAs is non-contiguous, as it is the case for many studied sRNA–mRNA pairs in GC-rich Enterobacteriaceae (56). In contrast, Hfq is dispensable for duplex formation between the sRNA IstR-1 and tisAB mRNA in E. coli (57) as well as for base pairing between RNAIII and the target mRNA sa1000 in S. aureus (58). In both cases, the free energy of base-pairing is ∼2–3-fold higher than observed for Hfq-dependent sRNA–mRNA duplexes in E. coli. Experimental support for the link between the Hfq requirement and the free energy of sRNA–mRNA pairing has also been obtained by Woodson et al. (21). Overexpression of DsrA resulted in increased rpoS translation even in the absence of Hfq, whereas ArcZ and RprA, which are also known to stimulate RpoS synthesis, were unable to do so. These authors further showed that DsrA binds the rpoS leader more tightly in the absence of Hfq than RprA and ArcZ and concluded that the stability of the RNA duplex between rpoS mRNA and the sRNA rather than the kinetics of formation is important.
In conclusion, the proposed molecular mechanism for Hfq-mediated RNA annealing is reminiscent of that suggested for intrinsically unstructured proteins and their interactions with protein partners. In these proteins, flexible, intrinsically disordered regions are believed to provide conformational fluctuations, which can facilitate intermolecular interactions, forming complexes with high specificity and relatively low affinity. This is critical for processes in which not only specific association but also subsequent dissociation of binding partners is required (59).
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online: Supplementary Table 1 and Supplementary Figures 1–3.
FUNDING
Special Research Program (SFB17) on ‘Modulators of RNA fate and function’ by the Austrian Science Fund [F1722 to K.D.-C. and F1720 to U.B.]; Bundesministerium für Bildung und Forschung grant SYNC-LIFE [contract number 05K10YEA to D.I.S.]; European Union FP7 e-Infrastructure grant WeNMR [contract number 261572 to D.I.S.]. Funding for open access charge: Austrian Science Fund.
Conflict of interest statement. None declared.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Giuseppe Zaccai (ILL, Grenoble, France) for fruitful discussions. E.d.A.R., K.D.-C., U.B. and M.B.-F. Conceived and designed the experiments: EdAR, MB-F, UB, KDj-C, UB. Performed the experiments: EdAR, HH, GK, BV, MB-F. Analysed the data: EdAR, DS, KDj-C, UB, PK, WS, GK. Wrote the paper: EdAR, KDj-C, UB, DS, PK, WS, GK. Edited the manuscript: HP, DS, UB, KDj-C.
REFERENCES
- 1.Vogel J, Luisi BF. Hfq and its constellation of RNA. Nat. Rev. Microbiol. 2011;9:578–589. doi: 10.1038/nrmicro2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang A, Wassarman KM, Ortega J, Steven AC, Storz G. The Sm-like Hfq protein increases OxyS RNA interaction with target mRNAs. Mol. Cell. 2002;9:11–22. doi: 10.1016/s1097-2765(01)00437-3. [DOI] [PubMed] [Google Scholar]
- 3.Wilusz CJ, Wilusz J. Eukaryotic Lsm proteins: lessons from bacteria. Nat. Struct. Mol. Biol. 2005;12:1031–1036. doi: 10.1038/nsmb1037. [DOI] [PubMed] [Google Scholar]
- 4.Repoila F, Darfeuille F. Small regulatory non-coding RNAs in bacteria: physiology and mechanistic aspects. Biol. Cell. 2009;101:117–131. doi: 10.1042/BC20070137. [DOI] [PubMed] [Google Scholar]
- 5.Beisel CL, Storz G. Base pairing small RNAs and their roles in global regulatory networks. FEMS Microbiol. Rev. 2010;34:866–882. doi: 10.1111/j.1574-6976.2010.00241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soper TJ, Doxzen K, Woodson SA. Major role for mRNA binding and restructuring in sRNA recruitment by Hfq. RNA. 2011;17:1544–1550. doi: 10.1261/rna.2767211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sauter C, Basquin J, Suck D. Sm-like proteins in eubacteria: the crystal structure of the Hfq protein from Escherichia coli. Nucleic Acids Res. 2003;31:4091–4098. doi: 10.1093/nar/gkg480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schumacher MA, Pearson RF, Moller T, Valentin-Hansen P, Brennan RG. Structures of the pleiotropic translational regulator Hfq and an Hfq-RNA complex: a bacterial Sm-like protein. EMBO J. 2002;21:3546–3556. doi: 10.1093/emboj/cdf322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mikulecky PJ, Kaw MK, Brescia CC, Takach JC, Sledjeski DD, Feig AL. Escherichia coli Hfq has distinct interaction surfaces for DsrA, rpoS and poly(A) RNAs. Nat. Struct. Mol. Biol. 2004;11:1206–1214. doi: 10.1038/nsmb858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sauer E, Weichenrieder O. Structural basis for RNA 3′-end recognition by Hfq. Proc. Natl Acad. Sci. USA. 2011;108:13065–13070. doi: 10.1073/pnas.1103420108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Link TM, Valentin-Hansen P, Brennan RG. Structure of Escherichia coli Hfq bound to polyriboadenylate RNA. Proc. Natl Acad. Sci. USA. 2009;106:19292–19297. doi: 10.1073/pnas.0908744106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Someya T, Baba S, Fujimoto M, Kawai G, Kumasaka T, Nakamura K. Crystal structure of Hfq from Bacillus subtilis in complex with SELEX-derived RNA aptamer: insight into RNA-binding properties of bacterial Hfq. Nucleic Acids Res. 2011:1–12. doi: 10.1093/nar/gkr892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang W, Wang L, Zou Y, Zhang J, Gong Q, Wu J, Shi Y. Cooperation of Escherichia coli Hfq hexamers in DsrA binding. Genes Dev. 2011;25:2106–2117. doi: 10.1101/gad.16746011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lease RA, Cusick ME, Belfort M. Riboregulation in Escherichia coli: DsrA RNA acts by RNA:RNA interactions at multiple loci. Proc. Natl Acad. Sci. USA. 1998;95:12456–12461. doi: 10.1073/pnas.95.21.12456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sledjeski DD, Whitman C, Zhang A. Hfq is necessary for regulation by the untranslated RNA DsrA. J. Bacteriol. 2001;183:1997–2005. doi: 10.1128/JB.183.6.1997-2005.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Resch A, Večerek B, Palavra K, Bläsi U. Requirement of the CsdA DEAD-box helicase for low temperature riboregulation of rpoS mRNA. RNA Biol. 2010;7:796–802. doi: 10.4161/rna.7.6.13768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Resch A, Afonyushkin T, Lombo TB, McDowall KJ, Bläsi U, Kaberdin VR. Translational activation by the noncoding RNA DsrA involves alternative RNase III processing in the rpoS 5′-leader. RNA. 2008;14:454–459. doi: 10.1261/rna.603108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Updegrove TB, Correia JJ, Chen Y, Terry C, Wartell RM. The stoichiometry of the Escherichia coli Hfq protein bound to RNA. RNA. 2011;17:489–500. doi: 10.1261/rna.2452111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soper TJ, Woodson SA. The rpoS mRNA leader recruits Hfq to facilitate annealing with DsrA sRNA. RNA. 2008;14:1907–1917. doi: 10.1261/rna.1110608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soper T, Mandin P, Majdalani N, Gottesman S, Woodson SA. Positive regulation by small RNAs and the role of Hfq. Proc. Natl Acad. Sci. USA. 2010;107:9602–9607. doi: 10.1073/pnas.1004435107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woodson SA, Hopkins JF, Panja S. Rapid binding and release of Hfq from ternary complexes during RNA annealing. Nucleic Acids Res. 2011;39:5193–5202. doi: 10.1093/nar/gkr062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lease RA, Woodson SA. Cycling of the Sm-like protein Hfq on the DsrA small regulatory RNA. J. Mol. Biol. 2004;344:1211–1223. doi: 10.1016/j.jmb.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 23.Brescia CC, Mikulecky PJ, Feig AL, Sledjeski DD. Identification of the Hfq-binding site on DsrA RNA: Hfq binds without altering DsrA secondary structure. RNA. 2003;9:33–43. doi: 10.1261/rna.2570803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Majdalani N, Cunning C, Sledjeski D, Elliott T, Gottesman S. DsrA RNA regulates translation of RpoS message by an anti-antisense mechanism, independent of its action as an antisilencer of transcription. Proc. Natl Acad. Sci. USA. 1998;95:12462–12467. doi: 10.1073/pnas.95.21.12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moll I, Leitsch D, Steinhauser T, Bläsi U. RNA chaperone activity of the Sm-like Hfq protein. EMBO Rep. 2003;4:284–289. doi: 10.1038/sj.embor.embor772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geissmann TA, Touati D. Hfq, a new chaperoning role: binding to messenger RNA determines access for small RNA regulator. EMBO J. 2004;23:396–405. doi: 10.1038/sj.emboj.7600058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Udekwu KI, Darfeuille F, Vogel J, Reimegard J, Holmqvist E, Wagner EGH. Hfq-dependent regulation of OmpA synthesis is mediated by an antisense RNA. Genes Dev. 2005;19:2355–2366. doi: 10.1101/gad.354405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Večerek B, Rajkowitsch L, Sonnleitner E, Schroeder R, Bläsi U. The C-terminal domain of Escherichia coli Hfq is required for regulation. Nucleic Acids Res. 2008;36:133–143. doi: 10.1093/nar/gkm985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beich-Frandsen M, Večerek B, Konarev PV, Sjoblom B, Kloiber K, Hammerle H, Rajkowitsch L, Miles AJ, Kontaxis G, Wallace BA, et al. Structural insights into the dynamics and function of the C-terminus of the E. coli RNA chaperone Hfq. Nucleic Acids Res. 2011;39:4900–4915. doi: 10.1093/nar/gkq1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garcia De La Torre J, Huertas ML, Carrasco B. Calculation of hydrodynamic properties of globular proteins from their atomic-level structure. Biophys. J. 2000;78:719–730. doi: 10.1016/S0006-3495(00)76630-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- 32.Goddard TD, Kneller DG. San Francisco, CA: University of California; Sparky—NMR Assignment and Integration Software. [Google Scholar]
- 33.Piotto M, Saudek V, Sklenar V. Gradient-tailored excitation for single-quantum NMR spectroscopy of aqueous solutions. J. Biomol. NMR. 1992;2:661–665. doi: 10.1007/BF02192855. [DOI] [PubMed] [Google Scholar]
- 34.Kay LE, Ikura M, Tschudin R, Bax A. Three-dimensional triple-resonance NMR spectroscopy of isotopically enriched proteins. J. Magn. Reson. 1990;89:496–514. doi: 10.1016/j.jmr.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 35.Bax A, Ikura M. An efficient 3D NMR technique for correlating the proton and 15N backbone amide resonances with the alpha-carbon of the preceding residue in uniformly 15N/13C enriched proteins. J. Biomol. NMR. 1991;1:99–104. doi: 10.1007/BF01874573. [DOI] [PubMed] [Google Scholar]
- 36.Wittekind M, Mueller L. HNCACB, a high-sensitivity 3D NMR experiment to correlate amide-proton and nitrogen resonances with the alpha- and beta-carbon resonances in proteins. J. Magn. Reson. 1993;101:201–205. [Google Scholar]
- 37.Farrow NA, Muhandiram R, Singer AU, Pascal SM, Kay CM, Gish G, Shoelson SE, Pawson T, Forman-Kay JD, Kay LE. Backbone dynamics of a free and phosphopeptide-complexed Src homology 2 domain studied by 15N NMR relaxation. Biochemistry. 1994;33:5984–6003. doi: 10.1021/bi00185a040. [DOI] [PubMed] [Google Scholar]
- 38.Farrow NA, Zhang O, Szabo A, Torchia DA, Kay LE. Spectral density function mapping using 15N relaxation data exclusively. J. Biomol. NMR. 1995;6:153–162. doi: 10.1007/BF00211779. [DOI] [PubMed] [Google Scholar]
- 39.Roessle MW, Klaering R, Ristau U, Robrahn B, Jahn D, Gehrmann T, Konarev P, Round A, Fiedler S, Hermes C, et al. Upgrade of the small-angle X-ray scattering beamline X33 at the European Molecular Biology Laboratory, Hamburg. J. Appl. Crystallogr. 2007;40:S190–S194. [Google Scholar]
- 40.Roessle M, Round AR, Franke D, Moritz S, Huchler R, Fritsche M, Malthan D, Klaering R, Svergun DI. Automated sample-changing robot for solution scattering experiments at the EMBL Hamburg SAXS station X33. J. Appl. Crystallogr. 2008;41:913–917. doi: 10.1107/S0021889808021018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Konarev PV, Petoukhov MV, Volkov VV, Svergun DI. ATSAS 2.1, a program package for small-angle scattering data analysis. J. Appl. Crystallogr. 2006;39:277–286. doi: 10.1107/S0021889812007662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Konarev PV, Volkov VV, Sokolova AV, Koch MHJ, Svergun DI. PRIMUS: a Windows PC-based system for small-angle scattering data analysis. J. Appl. Crystallogr. 2003;36:1277–1282. [Google Scholar]
- 43.Svergun DI. Determination of the regularization parameter in indirect-transform methods using perceptual criteria. J. Appl. Crystallogr. 1992;25:495–503. [Google Scholar]
- 44.Svergun DI. Restoring low resolution structure of biological macromolecules from solution scattering using simulated annealing. Biophys. J. 1999;76:2879–2886. doi: 10.1016/S0006-3495(99)77443-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bernado P, Mylonas E, Petoukhov MV, Blackledge M, Svergun DI. Structural characterization of flexible proteins using small-angle X-ray scattering. J. Am. Chem. Soc. 2007;129:5656–5664. doi: 10.1021/ja069124n. [DOI] [PubMed] [Google Scholar]
- 46.Petoukhov MV, Svergun DI. Global rigid body modeling of macromolecular complexes against small-angle scattering data. Biophys. J. 2005;89:1237–1250. doi: 10.1529/biophysj.105.064154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Flores SC, Wan Y, Russell R, Altman RB. Predicting RNA structure by multiple template homology modeling. Pac. Symp. Biocomput. 2010:216–227. doi: 10.1142/9789814295291_0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Flores SC, Altman RB. Turning limited experimental information into 3D models of RNA. RNA. 2010;16:1769–1778. doi: 10.1261/rna.2112110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Caliskan G, Hyeon C, Perez-Salas U, Briber RM, Woodson SA, Thirumalai D. Persistence length changes dramatically as RNA folds. Phys. Rev. Lett. 2005;95:268303. doi: 10.1103/PhysRevLett.95.268303. [DOI] [PubMed] [Google Scholar]
- 50.Hyeon C, Dima RI, Thirumalai D. Size, shape, and flexibility of RNA structures. J. Chem. Phys. 2006;125:194905. doi: 10.1063/1.2364190. [DOI] [PubMed] [Google Scholar]
- 51.Sun X, Wartell RM. Escherichia coli Hfq binds A18 and DsrA domain II with similar 2:1 Hfq6/RNA stoichiometry using different surface sites. Biochemistry. 2006;45:4875–4887. doi: 10.1021/bi0523613. [DOI] [PubMed] [Google Scholar]
- 52.Moon K, Gottesman S. Competition among Hfq-binding small RNAs in Escherichia coli. Mol. Microbiol. 2011;82:1545–1562. doi: 10.1111/j.1365-2958.2011.07907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Otaka H, Ishikawa H, Morita T, Aiba H. PolyU tail of rho-independent terminator of bacterial small RNAs is essential for Hfq action. Proc. Natl Acad. Sci. USA. 2011;108:13059–13064. doi: 10.1073/pnas.1107050108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fender A, Elf J, Hampel K, Zimmermann B, Wagner EGH. RNAs actively cycle on the Sm-like protein Hfq. Genes Dev. 2010;24:2621–2626. doi: 10.1101/gad.591310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Doetsch M, Furtig B, Gstrein T, Stampfl S, Schroeder R. The RNA annealing mechanism of the HIV-1 Tat peptide: conversion of the RNA into an annealing-competent conformation. Nucleic Acids Res. 2011;39:4405–4418. doi: 10.1093/nar/gkq1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jousselin A, Metzinger L, Felden B. On the facultative requirement of the bacterial RNA chaperone, Hfq. Trends Microbiol. 2009;17:399–405. doi: 10.1016/j.tim.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 57.Darfeuille F, Unoson C, Vogel J, Wagner EGH. An antisense RNA inhibits translation by competing with standby ribosomes. Mol. Cell. 2007;26:381–392. doi: 10.1016/j.molcel.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 58.Boisset S, Geissmann T, Huntzinger E, Fechter P, Bendridi N, Possedko M, Chevalier C, Helfer AC, Benito Y, Jacquier A, et al. Staphylococcus aureus RNAIII coordinately represses the synthesis of virulence factors and the transcription regulator Rot by an antisense mechanism. Genes Dev. 2007;21:1353–1366. doi: 10.1101/gad.423507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tompa P, Csermely P. The role of structural disorder in the function of RNA and protein chaperones. FASEB J. 2004;18:1169–1175. doi: 10.1096/fj.04-1584rev. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.