Abstract
The anthrax toxin protective antigen precursor is activated by proteolytic cleavage by furin or a furin-like protease. We present here data demonstrating that the small stable furin inhibitor hexa-d-arginine amide delays anthrax toxin-induced toxemia both in cells and in live animals, suggesting that furin inhibition may represent a reasonable avenue for therapeutic intervention in anthrax.
Anthrax toxin consists of three polypeptides, the protective antigen (PA), lethal factor (LF), and edema factor (EF) (reviewed in references 1, 2, 3, and 13). The PA is secreted by the anthrax bacterium as a precursor molecule which must be proteolytically activated by furin (8, 11, 18, 26) and/or furin-like proteases, such as PACE4 (9). Cleavage occurs at a surface loop and releases PA20, the N-terminal fragment of PA. Loss of this N-terminal fragment results in self-association of PA63, the C-terminal fragment of PA, forming membrane-inserting heptamers capable of binding LF and/or EF (20). These complexes are taken up into cells and enter the lumen of the acidic endosomal compartment, from which LF emerges to initiate enzymatic reactions that ultimately result in cell death (1, 12, 15).
Through the use of combinatorial chemistry techniques, workers in our laboratory have developed the compound hexa-d-arginine (D6R) as a potential therapeutically useful furin inhibitor (4). Studies that used Pseudomonas aeruginosa exotoxin A (PEA), a bacterial toxin which also requires furin processing, have shown that D6R effectively blocks PEA-induced cell death in vitro and in vivo (21). In this study, we have tested the efficacy of D6R against anthrax exotoxins (PA plus LF) in cells and in two animal models.
D6R inhibits the cytotoxicity of anthrax toxin in a murine alveolar macrophage cell line.
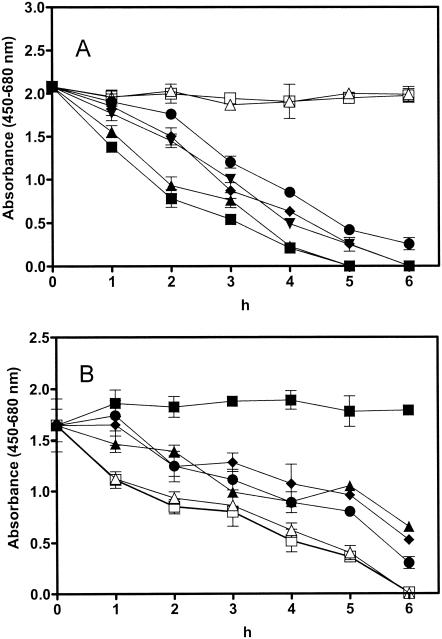
To evaluate the ability of D6R to protect the murine alveolar macrophage cell line RAW 264.7 (ATCC TIB-71) from anthrax toxin-induced cytotoxicity, we first established the dose of toxin representing the 50% effective concentration (EC50) (Fig. 1A), and we then treated cells with different concentrations of D6R in combination with this dose. A concentration of 25 ng of PA per ml in the presence of 12 ng of LF per ml was required for lysis of half of the murine alveolar macrophages after 3 h of incubation with toxins (at EC50). The inclusion of 1 μM D6R protected 16% of the cells from death at 6 h, while 100 μM D6R increased this survival rate to 36% compared with that of cells treated with anthrax toxin alone (0% survival rate) and with that of the untreated control group (100% survival rate, P < 0.0001) (Fig. 1B). To determine whether multiple administration of D6R would exhibit improved protective effects against anthrax toxin-induced cytotoxicity, we treated cells with D6R prior to administration of toxin and also tested multiple applications of D6R (10 μM) every hour after application of toxin, but no improved efficacy was detected (data not shown).
FIG. 1.
D6R protects against the cytotoxic effects of anthrax toxin in vitro. (A) Determination of the EC50 for PA. Cells from the RAW 264.7 line were grown in RPMI medium with 10% fetal bovine serum. For the determination of the EC50 for PA, cells were treated with various doses of PA (0 to 100 ng/ml each in combination with 12 ng of LF per ml) 12 h after seeding. Cytotoxicity assays were performed by using the WST-1 cell proliferation reagent kit (Roche Diagnostics GmbH, Mannheim, Germany). Assays were performed according to the manufacturer's protocol. Open squares, untreated cells; open triangles, 0 ng of PA per ml; filled circles, 5 ng of PA per ml; filled diamonds, 10 ng of PA per ml; filled inverted triangles, 25 ng of PA per ml; filled upright triangles, 50 ng of PA per ml; filled squares, 100 ng of PA per ml. The experiment was repeated three times, and the results represent the means ± standard deviations of all three experiments. (B) D6R protects against anthrax toxin intoxication of RAW cells. To investigate the protective effect of D6R against anthrax toxin-induced cytotoxicity, RAW 264.7 cells were treated with anthrax toxin (25 ng of PA and 12 ng of LF per ml) simultaneously with various concentrations of D6R (0 to 100 μM). Cytotoxicity was measured at the incubation times shown in the figure. Filled squares, untreated cells; open squares, cells treated with anthrax toxin (25 ng of PA and 12 ng of LF per ml) only; filled triangles, anthrax toxin and 100 μM D6R; filled diamonds, anthrax toxin and 10 μM D6R; filled circles, anthrax toxin and 1 μM D6R; open triangles, AT and 0.1 μM D6R. The experiment was repeated three times, and the results represent the means ± standard deviations of all three experiments. The statistical significance between groups was analyzed by Student's t test. A P value of ≤0.0001 was accepted as statistically significant.
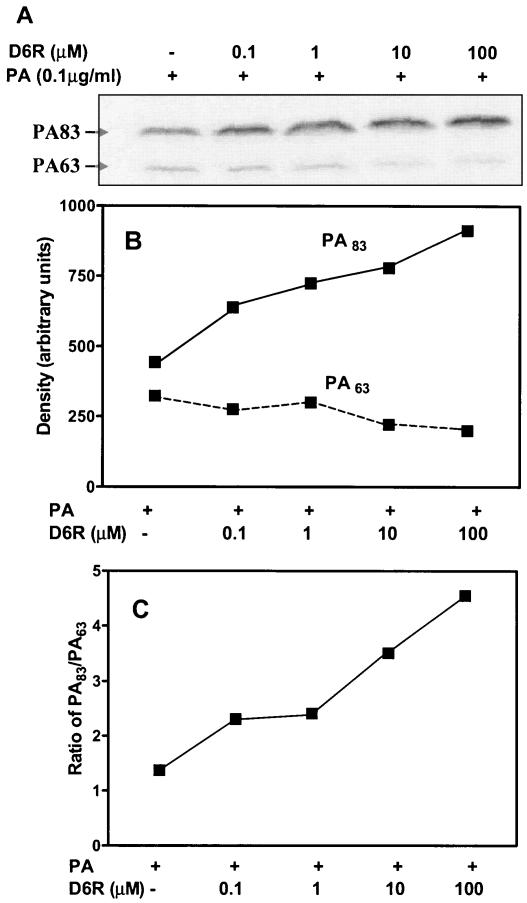
To confirm the protective effect of D6R on the cleavage of PA83, we analyzed RAW 264.7 cell cleavage of PA labeled with 125I-Bolton-Hunter reagent in the presence and absence of D6R (Fig. 2A). These data support the idea that the concentrations of D6R required to block the cleavage of PA are similar to those required to inhibit toxicity. This experiment was repeated twice with similar results; the data shown are those from a representative experiment.
FIG. 2.
(A) Protective effect of D6R on the cleavage of PA by RAW 264.7 cells. RAW 264.7 cells were grown in 12-well plates under the conditions described above. PA was radiolabeled by iodination with 125I-Bolton-Hunter reagent (Amersham Pharmacia Biotech, Inc.) to a specific activity of 4.6 × 106 cpm/μg. This method of iodination has no effect on the biological activity of PA (22). Binding experiments were performed at 37°C. Confluent wells of RAW 264.7 cells were treated for 25 min with 0.1 μg of I125-labeled PA (4.6 × 105 cpm) in the presence of increasing concentrations of D6R (0, 0.1, 1, 10, and 100 μM) in RPMI medium supplemented with 10% serum. Cell extracts were washed four times with RPMI medium supplemented with 10% serum and boiled for 5 min in 200 μl of Laemmli sample buffer prior to electrophoresis of 25-μl aliquots on a sodium dodecyl sulfate-10% acrylamide gel. The dried gel was exposed to a phosphorimage screen for 12 h and analyzed with a Typhoon 9410 variable-mode imager and ImageQuant Software (Amersham Pharmacia Biotech). Quantitation of the optical density of each band (B) and the ratio of processed PA 63 to unprocessed PA 83 (C) are shown. The experiment was repeated twice with similar results; the data shown are those from a representative experiment.
D6R inhibits anthrax toxemia in vivo.
Fisher 344 rats were used to evaluate the ability of D6R to protect animals from the lethal effects of anthrax toxin. The experiment was performed according to a standard protocol for this experiment (6, 17, 19) in which rats are challenged with 10 times the minimum lethal dose of PA and LF (40 and 8 μg/rat, respectively) with or without inhibitors in a double-blind fashion. D6R was injected intravenously immediately after the administration of toxin, and the survival rate was monitored for 24 h. During this time, animals were under observation for signs of impending morbidity, such as intensive, extremely labored breathing and prostration. Animals were sacrificed by CO2 inhalation when judged moribund per these criteria, and survival rates were recorded at specific intervals. This standard humane protocol has been used in similar anthrax studies (6).
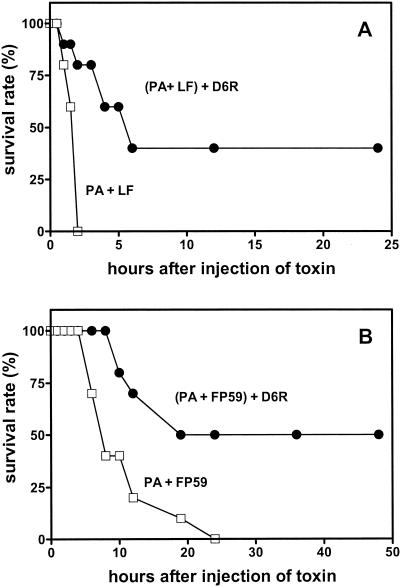
Rats treated with the combination of anthrax toxins and D6R exhibited a 40% survival rate at 5 h compared with a control group treated solely with anthrax toxin (0% survival rate). Surviving animals exhibited no symptoms of toxemia or additional deaths during the 2 weeks following the experiment. Administration of D6R also resulted in a considerable delay in the onset of anthrax toxemia compared with results of the control group treated solely with anthrax toxin (Fig. 3A).
FIG. 3.
D6R inhibits anthrax toxemia in Fisher 344 rats. (A) Twenty Fisher 344 male rats (weight, 250 to 300 g) were anesthetized by intraperitoneal injection of ketamine-xylazine and injected intravenously with a mixture of 40 μg of PA and 8 μg of LF per rat (which represents 10 times the minimal lethal dose) in a total volume of 200 μl of phosphate-buffered saline. With this dose of toxin, animals become moribund after approximately 90 to 120 min. D6R (1 mg/100 μl in physiological saline) was given intravenously to 10 rats immediately after administration of anthrax toxins; 100 μl of phosphate-buffered saline was given to the other group. The time until moribund, defined as the point at which severe symptoms of intoxication (highly labored breathing and prostration) appear, was scored at specific time intervals (every 30 min for 2 h and every hour thereafter). When moribund, animals were sacrificed by CO2 inhalation for humane reasons (6, 17, 19). Rats that did not show these symptoms survived toxin administration, recovered from anesthesia, and were sacrificed after 2 weeks of observation. The first group of 10 rats was treated with anthrax toxin alone (open squares), and the second group was treated with anthrax toxin and 1 mg of D6R (filled circles). A preliminary experiment was performed in a nonblind fashion, while the experiment shown was performed in a double-blind fashion. (B) D6R protects against anthrax toxin-induced intoxication in mice. Groups of FVB mice (10 animals per group, 6 weeks old, both genders) were also given anthrax toxin (10 μg of PA and 0.5 μg of FP59 per mouse), but intraperitoneally (open squares). One group of mice was also administered 100 μg of D6R intraperitoneally immediately after anthrax toxin (filled circles). Mice were observed for 24 h after injection. With the first signs of impending morbidity (intensive and labored breathing accompanied by prostration), mice were sacrificed by CO2 inhalation. Survival rates were assessed every half hour for the first 2 h, every 2 h thereafter for 12 h, and intermittently thereafter for 48 h. Surviving mice were sacrificed 2 weeks later. Results are presented as percent survival at the times indicated. Surviving mice were observed for 48 h after injection. Per the Fisher exact test, differences between both experimental groups achieved significance at the 6-h time point and afterward (P < 0.05).
The second animal model used to evaluate the protective effect of D6R against anthrax toxin consisted of groups of FVB mice treated with 10 μg of PA and 0.5 μg of FP59 (by intraperitoneal injection) per mouse. FP59 is a fusion protein which consists of the PA-binding portion of LF (residues 1 to 254) coupled to the cytotoxic effector PEA (domain III) (8). All mice treated solely with this toxin exhibited the first signs of severe toxemia approximately 6 h after injection. Twenty-four hours after administration of toxins, no animals survived in this group. However, 50% of the group treated with PA-FP59 toxin in combination with D6R (100 μg/mouse, also given intraperitoneally) were still alive 24 h later, indicating significant protection by this agent compared to that of the group of mice treated with anthrax toxin alone (for which the survival rate was 0% [P < 0.0001]) (Fig. 3B). No further deaths occurred in this group over the next 2 weeks.
The same protocol was used to investigate toxicity in the 129/Sv mouse strain. Twenty-four hours after toxin administration, the group treated with PA-FP59 toxin and D6R exhibited a 30% survival rate compared with 0% survival in the control group injected solely with toxins (data not shown). The FVB strain thus exhibits somewhat better resistance to anthrax toxin than does the 129/Sv strain; strain differences in response to anthrax have been previously demonstrated (25).
Few methods are presently available to combat anthrax toxin toxemia (5, 7, 14, 16, 17, 19, 23, 24). The therapeutic target in most of these cases is PA; however, downstream targets EF and LF may also be useful. We have previously shown that polyarginines, and in particular D6R, represent potent inhibitors of mouse furin (4) and can be used to block Pseudomonas exotoxin A toxicity in cells and in mice (21). In the study presented here, we have demonstrated the efficacy of this inhibitor against anthrax toxins.
In conclusion, the data presented in this paper suggest that D6R represents a reasonable lead compound for the further development of small-molecule furin inhibitors capable of inhibiting or preventing furin-related pathophysiological processes in vivo. The recent crystallization of mouse furin (10) provides a promising new template upon which to model and improve upon D6R-related compounds.
Acknowledgments
We thank Joelle Finley for assistance with cell culture and Gregory Hubbard for assistance with animal handling.
This study was supported by NIH/NIAID grant number R21 AI53517 to I.L. and a Junta de Andalucia grant to J.R.P.
Editor: J. T. Barbieri
REFERENCES
- 1.Ascenzi, P., P. Visca, G. Ippolito, A. Spallarossa, M. Bolognesi, and C. Montecucco. 2002. Anthrax toxin: a tripartite lethal combination. FEBS Lett. 531:384-388. [DOI] [PubMed] [Google Scholar]
- 2.Brossier, F., and M. Mock. 2001. Toxins of Bacillus anthracis. Toxicon 39:1747-1755. [DOI] [PubMed] [Google Scholar]
- 3.Brossier, F., M. Weber-Levy, M. Mock, and J. C. Sirard. 2000. Role of toxin functional domains in anthrax pathogenesis. Infect. Immun. 68:1781-1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cameron, A., J. Appel, R. A. Houghten, and I. Lindberg. 2000. Polyarginines are potent furin inhibitors. J. Biol. Chem. 275:36741-36749. [DOI] [PubMed] [Google Scholar]
- 5.Chaudry, G. J., M. Moayeri, S. Liu, and S. H. Leppla. 2001. Quickening the pace of anthrax research: three advances point to possible therapies. Trends Microbiol. 10:58-62. [DOI] [PubMed] [Google Scholar]
- 6.Ezzell, J. W., B. E. Ivins, and S. H. Leppla. 1984. Immunoelectrophoretic analysis, toxicity, and kinetics of in vitro production of the protective antigen and lethal factor components of Bacillus anthracis toxin. Infect. Immun. 45:761-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flick-Smith, H. C., N. J. Walker, P. Gibson, H. Bullifent, S. Hayward, J. Miller, R. W. Titball, and E. D. Williamson. 2002. A recombinant carboxy-terminal domain of the protective antigen of Bacillus anthracis protects mice against anthrax infection. Infect. Immun. 70:1653-1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friedman, T. C., V. M. Gordon, S. H. Leppla, K. R. Klimpel, N. P. Birch, and Y. P. Loh. 1995. In vitro processing of anthrax toxin protective antigen by recombinant PC1 (SPC3) and bovine intermediate lobe secretory vesicle membranes. Arch. Biochem. Biophys. 316:5-13. [DOI] [PubMed] [Google Scholar]
- 9.Gordon, V. M., A. Rehemtulla, and S. H. Leppla. 1997. A role for PACE4 in the proteolytic activation of anthrax toxin protective antigen. Infect. Immun. 65:3370-3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henrich, S., A. Cameron, G. P. Bourenkov, R. Kiefersauer, R. Huber, I. Lindberg, W. Bode, and M. E. Than. 2003. The crystal structure of the proprotein processing proteinase furin explains its stringent specificity. Nat. Struct. Biol. 10:520-526. [DOI] [PubMed] [Google Scholar]
- 11.Klimpel, K., S. Molloy, G. Thomas, and S. Leppla. 1992. Anthrax toxin protective antigen is activated by a cell surface protease with the sequence specificity and catalytic properties of furin. Proc. Natl. Acad. Sci. USA 89:10277-10281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lacy, D. B., and R. J. Collier. 2002. Structure and function of anthrax toxin. Curr. Top. Microbiol. Immunol. 271:61-85. [DOI] [PubMed] [Google Scholar]
- 13.Leppla, S. H. 1999. p. 243-263. In J. A. Alouf and J. Freer (ed.), Comprehensive sourcebook of bacterial protein toxins. Academic Press, London, United Kingdom.
- 14.Leppla, S. H. 2001. A dominant-negative therapy for anthrax. Nat. Med. 7:659-660. [DOI] [PubMed] [Google Scholar]
- 15.Leppla, S. H., N. Arora, and M. Varughese. 1999. Anthrax toxin fusion proteins for intracellular delivery of macromolecules. J. Appl. Microbiol. 87:284. [DOI] [PubMed] [Google Scholar]
- 16.Leppla, S. H., J. B. Robbins, R. Schneerson, and J. Shiloach. 2002. Development of an improved vaccine for anthrax. J. Clin. Investig. 110:141-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maynard, J. A., C. B. M. Maassen, S. H. Leppla, K. Brasky, J. L. Patterson, B. L. Iverson, and G. Georgiou. 2002. Protection against anthrax toxin by recombinant antibody fragments correlates with antigen affinity. Nat. Biotechnol. 20:597-601. [DOI] [PubMed] [Google Scholar]
- 18.Molloy, S. S., P. A. Bresnahan, S. H. Leppla, K. R. Klimpel, and G. Thomas. 1992. Human furin is a calcium-dependent serine endoprotease that recognizes the sequence Arg-X-X-Arg and efficiently cleaves anthrax toxin protective antigen. J. Biol. Chem. 267:16396-16402. [PubMed] [Google Scholar]
- 19.Mourez, M., R. S. Kane, J. Mogridge, S. Metallo, P. Deschatelets, B. R. Sellman, G. M. Whitesides, and R. J. Collier. 2001. Designing a polyvalent inhibitor of anthrax toxin. Nat. Biotechnol. 19:958-961. [DOI] [PubMed] [Google Scholar]
- 20.Petosa, C., R. J. Collier, K. R. Klimpel, S. H. Leppla, and R. C. Liddington. 1997. Crystal structure of the anthrax toxin protective antigen. Nature 385:833-838. [DOI] [PubMed] [Google Scholar]
- 21.Sarac, M. S., A. Cameron, and I. Lindberg. 2002. The furin inhibitor hexa-d-arginine blocks the activation of Pseudomonas aeruginosa exotoxin A in vivo. Infect. Immun. 70:7136-7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh, Y., K. R. Klimpel, S. Goel, P. K. Swain, and S. H. Leppla. 1999. Oligomerization of anthrax toxin protective antigen and binding of lethal factor during endocytic uptake into mammalian cells. Infect. Immun. 67:1853-1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soelaiman, S., B. Q. Wei, P. Bergson, Y. S. Lee, Y. Shen, M. Mrksich, B. K. Schoichet, and W. J. Tang. 2003. Structure-based inhibitor discovery against adenylyl cyclase toxins from pathogenic bacteria that cause anthrax and whooping cough. J. Biol. Chem. 278:25990-25997. [DOI] [PubMed] [Google Scholar]
- 24.Tonello, F., M. Seveso, O. Marin, M. Mock, and C. Montecucco. 2002. Screening inhibitors of anthrax lethal factor. Nature 418:386. [DOI] [PubMed] [Google Scholar]
- 25.Welkos, S. L., T. J. Keener, and P. H. Gibbs. 1986. Differences in susceptibility of inbred mice to Bacillus anthracis. Infect. Immun. 51:795-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang, Y., Y. Kida, K. Kuwano, Y. Misumi, Y. Ikehara, and S. Arai. 2001. Role of furin in delivery of CTL epitope of an anthrax toxin-fusion protein. Microbiol. Immunol. 45:119-125. [DOI] [PubMed] [Google Scholar]