Abstract
Infection with parasitic nematodes is characterized by the induction of a profound type 2 immune response. We have studied the role of glycans in the induction of the skewed type 2 response by antigens of the parasitic nematode Brugia malayi as well as the free-living nematode Caenorhabditis elegans. Lymph node cells from BALB/c mice immunized with soluble extracts of the two nematodes showed distinct antigen-specific proliferation and cytokine production; however, both nematodes induced antigen-specific interleukin 4 (IL-4) production, demonstrating that the induction of a biased type 2 response is not unique to parasitic nematodes. Sodium periodate-treated soluble extracts of both nematodes consistently induced significantly less IL-4 production than the respective mock-treated extracts, indicating that glycans play a critical role in the induction of the Th2 immune response by these nematodes. The glycan-dependent induction of the Th2-potentiating cytokine IL-4 occurs by 72 h postinoculation. Our data suggest that glycan determinants common to nematodes act as ligands, displaying distinct molecular patterns that trigger the immune system to launch a biased Th2 immune response upon exposure to these organisms or their products. Further, the similarity of our findings to those for Schistosoma mansoni egg antigen is striking considering the enormous phylogenetic distance between nematodes and trematodes. These data thus have important implications for how the mammalian host responds to widely divergent metazoan invaders and suggest that the powerful C. elegans model system can be used to address these questions.
A predominant feature of parasitic nematodes is their uniform ability to induce type 2 immune responses in their hosts, a feature that they share with other parasitic helminths (14, 33). However, despite the extensive characterization of the immune responses to helminth infections, the antigens and mechanisms responsible for driving the distinct Th2 subset bias are still unknown. A number of hypotheses have been proposed, and it is likely that a combination of factors is responsible for driving the Th2 subset bias. Although Th2 responses may occur in some circumstances as a default pathway (26), the observed profound and immediate interleukin 4 (IL-4) responses to helminths or their extracts, even in the context of Th1-inducing adjuvants (24, 42, 43), suggest the existence of pattern recognition receptors (PRR) (35) that signal early IL-4 production.
The skewed type 2 response is probably mediated by parasite antigens present on the nematode surface or in the excreted-secreted compartment of the worm. Many of the immunodominant epitopes of these type 2-inducing antigens have been shown to be glycans displaying very unique structures (10, 32, 34). We had previously observed that mice immunized with an extract of the human nematode parasite Brugia malayi from which N-linked glycans had been enzymatically removed failed to produce antigen-specific IL-4 (unpublished observation). These studies led us to hypothesize that sugars on nematode glycoproteins have the potential to act as ligands for PRR and to help drive the profound Th2 response associated with infection. Consistent with this hypothesis, glycans on the parasitic trematode Schistosoma mansoni have been shown to act as Th2-enhancing adjuvants (39, 40) as well as to be the predominant targets of the host antibody response themselves (11). However, the lack of a phylogenetic relationship (>750 million years) between trematodes and nematodes means that findings found for schistosomes cannot be extrapolated readily to nematodes. Indeed, similarities in findings would have significant implications for the evolution of the mammalian immune response.
In the present study, we set out to investigate the hypothesis that glycans on soluble proteins of the parasitic nematode B. malayi influence the type of immune response evoked by this parasite. As parasitic nematodes do not form a natural phylogenetic group (5), we also hypothesized that the molecular features determining the stereotypic Th2 response may not be restricted to parasitic nematodes but may represent a fundamental class of ligands common to many or all nematodes. To test this hypothesis, we used the free-living nematode Caenorhabditis elegans as a model for a nonparasitic nematode and examined its antigenicity as well as the role played by glycans on its soluble proteins in the immune response. Our data show that carbohydrate structures from both parasitic and nonparasitic nematodes have the capacity to induce Th2 immune responses, possibly via early induction of the Th2-potentiating cytokine IL-4. In addition to broadening the understanding of the induction of the Th2 pathway, these studies suggest that C. elegans can be used as a model system for identifying the host and parasite molecular structures involved in Th2 induction as well as for overexpressing vaccine candidates to which a Th2 response is desired.
MATERIALS AND METHODS
Mice.
Mice were bred and maintained at the animal facility of the Institute of Cell, Animal, and Population Biology, University of Edinburgh. Both female and male BALB/c mice were used for the experiments at the age of 6 weeks. Control and experimental animals were matched for age and sex.
Nematode material.
B. malayi adults were recovered from infected jirds purchased from TRS Laboratories (Athens, Ga.). Adult worms were thoroughly washed first with RPMI medium supplemented with 50 μg of gentamicin/ml and then with phosphate-buffered saline (PBS) before they were frozen at −80°C until further use.
C. elegans was grown in solid cultures on sterile agar plates (containing 0.3 g of NaCl/liter, 2.5 g of peptone/liter, and 17 g of agar/liter) seeded with Escherichia coli OP50 until they consumed virtually all of the bacteria on the plates (46). The worms were then washed off with sterile ice-cold S-basal medium (5.85 g of NaCl/liter, 1 g of K2HPO4/liter, 6 g of KH2PO4/liter, 5 mg of cholesterol/liter), washed three times with ice-cold S-basal medium, and separated from residual bacteria by sucrose flotation as previously described (46). Briefly, washed worms were suspended in 20 ml of ice-cold S-basal medium in 50-ml Falcon tubes, 20 ml of ice-cold 60% sucrose was added, and the contents were mixed by gentle inversion of the tubes. Immediately thereafter, the tubes were centrifuged at 800 × g for 5 min at 4°C. Worms separating at the top were collected by using a Pasteur pipette and immediately washed three times with excess ice-cold S-basal medium to remove any sucrose. Any residual bacteria were removed by incubating the worms overnight at room temperature in sterile S-basal medium supplemented with 1% (vol/vol) Tween 20 (T20) and 0.001% (vol/vol) ampicillin (modified S-basal medium) in 75-ml sterile culture flasks (Corning, Cambridge, Mass.) with gentle shaking. The worms were collected on the following day, washed by centrifugation, and reincubated overnight in fresh modified S-basal medium as described above. Worms cleaned in this way looked normal and were actively motile. Finally, the worms were harvested, washed three times with ice-cold PBS, and frozen at −80°C until used for antigen preparation.
Antigen preparation and quantification.
PBS-soluble extracts of mixed adult B. malayi antigen (BmA) or mixed-stage C. elegans antigen (CeA) were prepared by homogenizing each worm population in sterile ice-cold PBS on ice by using a glass-glass homogenizer (Jencons, Bedfordshire, United Kingdom). The homogenates were centrifuged at 16,000 × g for 30 min at 4°C, and the supernatants containing PBS-soluble antigens were collected and frozen at −80°C until further use. Protein concentrations were measured by using a Coomassie Plus protein assay kit (Pierce, Rockford, Ill.).
Periodate treatment.
Sodium metaperiodate-mediated modification of glycan moieties in BmA and CeA was performed by using a modification of the technique of Okano et al. (39). BmA or CeA at a concentration of 2 mg/ml was treated briefly with 50 mM (vol/vol) sodium acetate (pH 4.5; Na acetate buffer) in 1.5-ml Eppendorf tubes at room temperature. The content of each tube was divided to produce periodate-modified extracts (pBmA and pCeA) and control mock-treated extracts (mBmA and mCeA). The tubes intended for the preparation of pBmA and pCeA received 10 mM sodium metaperiodate in Na acetate buffer, while the tubes intended for the preparation of mBmA and mCeA received an equivalent volume of Na acetate buffer without sodium metaperiodate. The tubes were incubated in the dark at room temperature with gentle shaking for 1 h. The reaction was completed by further incubation of each tube with 100 mM sodium borohydride in PBS (pH 7.4) for 30 min at room temperature. Excess salt was removed by using Bio-Spin 6 columns (Bio-Rad), and the final protein concentrations were assessed.
SDS-PAGE and Western blotting.
Sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) and Western blotting were performed by standard techniques. Briefly, antigen extracts in nonreducing sample buffer were boiled for 5 min at 95°C and separated on 10% polyacrylamide gels at a concentration of 10 μg/well. Separated proteins were transferred to nitrocellulose (NC) membranes (Bio-Rad) by using a semidry Western blotting unit (Bio-Rad). NC membranes were blocked for 2 h at room temperature with 3% (wt/vol) bovine serum albumin in Tris-buffered saline supplemented with either 0.05% T20 for antibody Western blotting (Ab-blocking buffer) or 2% T20 for lectin blotting (lectin-blocking buffer). NC membranes bearing mBmA or pBmA were incubated overnight with either rabbit anti-gp15/400 (50), diluted 1:1,000 in Ab-blocking buffer, or peroxidase-labeled conconavalin A (ConA; Sigma), diluted in Tris-buffered saline supplemented with 2% T20, 1 mM CaCl2, and MgCl2 (lectin buffer), to detect B. malayi gp15/400 and N-linked glycans, respectively. The NC membranes used for the detection of gp15/400 then were washed thoroughly with Ab-blocking buffer and incubated for 2 h at room temperature with peroxidase-conjugated goat anti-rabbit antibodies (Bio-Rad) diluted 1:5,000 in Ab-blocking buffer with gentle shaking. Bound peroxidase on NC membranes was visualized by using an ECL detection kit (Amersham Pharmacia Biotech, Little Chalfont, United Kingdom).
Immunization protocols.
Groups of mice were immunized subcutaneously via the footpads with a single dose of native or chemically modified BmA or CeA in complete Freund adjuvant (CFA) (experimental groups) or with PBS in CFA (control group). Each mouse received a total dose of 10 μg of nematode extract emulsified with CFA (Sigma) in a 1:1 mixture and in a final volume of 100 μl. At different times (24 h, 48 h, 72 h, 7 days, or 10 days) postimmunization (p.i), the popliteal lymph nodes were removed and used to assay either for in vitro antigen-specific proliferation and cytokine production or for cytokine mRNA transcripts by PCR. Also, blood from animals immunized for 10 days was collected, and sera prepared from the blood of individual mice were stored at −20°C.
Proliferation assays and ELISAs for cytokines and antibodies.
Single-cell suspensions were prepared from popliteal lymph nodes from individual BALB/c mice under aseptic conditions. Cells were suspended in Dulbecco modified Eagle medium (DMEM) (Sigma) supplemented with 10% fetal calf serum, 2 mM l-glutamine, 100 U of penicillin/ml, and 100 μg of streptomycin/ml (all supplements from GibcoBRL, Life Technologies) (complete DMEM) at a concentration of 5 × 106 per ml for in vitro proliferation and cytokine production assays. Lymph node cells intended for RNA extraction were washed once in serum-free complete DMEM, suspended in Trizol (GibcoBRL) at a concentration of 107 cells/ml, homogenized, and kept at −80°C until further use. For proliferation and cytokine production assays, a 100-μl cell suspension (5 × 105 cells) was plated in each well of a 96 round-bottom microtiter plate (Nunc) with 100 μl of complete DMEM alone (control cultures) or DMEM containing 20 μg of BmA or CeA/ml, containing 4 μg of ConA/ml, or containing 2,000 U of purified protein derivative (PPD; pharmaceutical grade)/ml. Cultures were set up in quadruplicate for each stimulant and incubated for 72 h in a humid incubator at 37°C and 5% CO2. Supernatants (50 μl) for cytokine assays were collected from wells and kept at −20°C until further use. Cultures were pulsed overnight with 3[H]thymidine (1 μCi/well; Amersham), the cells were harvested by using an automatic cell harvester (Tomtec), and DNA incorporation as a measure of cell division was determined by using a liquid scintillation counter (Trilux; Wallac). Cytokine enzyme-linked immunosorbent assays (ELISAs) were performed with 11B11 (anti-IL-4) and R46A2 (anti-gamma interferon [IFN-γ]) as capture antibodies and biotin-labeled antibodies for the detection of IL-4 and IFN-γ, diluted according to protocols supplied by the manufacturer (Pharmingen). Avidin-alkaline phosphatase (Sigma) was used for the final detection step. Samples were tested in duplicate, and recombinant IL-4 or IFN-γ (Genzyme, Cambridge, Mass.) was used for the construction of standard curves. Antibody ELISAs were performed as previously described (29) with 5 μg of BmA or CeA as the coating antigen.
CFSE and intracellular cytokine staining.
Single-cell suspensions were prepared from popliteal lymph nodes from mice that had been injected 7 days previously with CeA or BmA as described above. A modification of the technique of Lyons and Parish for carboxyfluorescein diacetate succinimidyl ester (CFSE) staining was used (1, 31). Briefly, 107 cells/ml were incubated with 10 μM CFSE (Molecular Probes) in PBS for 8 min at room temperature. Staining was stopped with an equal volume of fetal calf serum, and the cells were washed three times with complete RPMI medium. Stained lymph node cells were incubated with antigen (as described above) at 5 × 106 per ml for 5 days. GolgiStop was added to the cells at a 1:1,500 dilution together with 50 ng of phorbol myristate acetate/ml and 500 ng of ionomycin/ml and incubated for 4 h at 37°C. Cells from the experimental groups then were pooled in order to obtain sufficient cells for subsequent analysis. First, the pooled cells were stained with anti-CD4 (a 1:100 dilution of anti-CD4 [Cy-chrome; Pharmingen]). Then, the cells were washed and fixed by using a Cytofix/Cytoperm PlusTM kit (Pharmingen) before intracellular cytokine staining with anti-IL-4 (11B11), anti-IFN-γ (XMG1.2; Pharmingen), or control immunoglobulin-phycoerythrin conjugate (Pharmingen) in Perm/Wash solution according to the manufacturer's instructions. After staining, the cells were analyzed by flow cytometry with a Becton Dickinson FACScan and CellQuest software. All data shown represent activated lymphocytes gated by forward and side light scattering.
RT-PCR.
Total RNA was extracted from lymph node cells from individual mice by using a Trizol RNA extraction kit (GibcoBRL). For reverse transcriptase PCR (RT-PCR), first-strand cDNA was synthesized from total RNA with oligo(dT) primers by using a Moloney murine leukemia virus reverse transcriptase kit (GibcoBRL). For detection and semiquantitative comparison of cytokine mRNA transcripts in lymph nodes from different mice, RT-PCR of the amplified first-strand cDNA products was performed by using a SYBR green kit (Roche Diagnostic Systems, Somerville, N.J.) and a LightCycler real-time PCR machine (Roche). Murine cytokine primers designed to encompass an intron were manufactured by Oswel DNA Service (Southampton, United Kingdom). These included primers for IL-4 (TAGTTGTCATCCTGCTCTT and CTACGAGTAATCCATTTGC), IL-10 (AGCCGGGAAGACAATAACTG and CATTTCCGATAAGGCTTGG), and IFN-γ (GCTCTGAGACAATGAACGCT and AAAGAGATAATCTGGCTCTGC). To control for the use of equivalent amounts of template, amplification for the control β-actin housekeeping gene was carried out with β-actin-specific primers (TGGAATCCTGTGGCATCCATGAAAC and TAAAACGCAGCTCAGTAACAGTCCG). Samples were first tested to ensure that they contained equivalent levels of β-actin transcripts and then were assayed for levels of cytokine mRNAs. PCR conditions were as follows: 30 s of denaturation at 95°C, 5 s of annealing of primers at 55°C, and 12 s of elongation at 72°C for 50 cycles; the fluorescent DNA binding dye SYBR was monitored at the end of each elongation cycle at 86°C. For quantification of cytokine mRNA transcripts, standard curves for IL-4, IL-10, and IFN-γ were generated by using RNA from T-cell clones known to express high levels of either IL-4 and IL-10 (D10.G4) (15, 28) or IFN-γ (myelin oligodendrocyte glycoprotein-specific T-cell clone; kindly provided by Steve Anderton, Institute of Cell, Animal, and Population Biology) upon stimulation with the respective specific antigens. Cytokine RT-PCR products from individual mice were expressed in arbitrary units as a percentage of the same cytokine transcripts in the control clone. RT-PCR products derived from the LightCycler (10-μl reactions) also were visualized on 1% agarose gels by ethidium bromide staining.
Statistical analyses and presentation of data.
Statistical analyses were done with Prism (GraphPad Software, San Diego, Calif.). Differences between groups of mice were determined by using a two-tailed Mann-Whitney test, with a P value of <0.05 indicating a statistically significant difference. All data shown are representative of at least two independent experiments.
RESULTS
BALB/c mice exhibit distinct lymphocyte specificities for B. malayi and C. elegans.
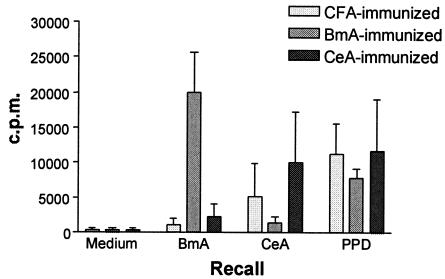
To test whether BALB/c mice can be primed in vivo to mount a specific immune response to the free-living nematode C. elegans comparable to that inducible by the parasitic nematode B. malayi, groups of mice were immunized with a single dose of native CeA or native BmA in CFA (experimental groups) or with PBS in CFA (control group). At 10 days p.i., in vitro antigen-specific proliferation and cytokine production were assessed. Lymphocytes prepared from the lymph nodes of both experimental groups consistently showed antigen-specific proliferation in response to the respective extracts (Fig. 1). The degree of cross-reactivity was relatively low, indicating that the observed proliferation in response to the antigens was attributable largely to lymphocytes with distinct specificities for either B. malayi or C. elegans soluble antigens. Responses to PPD (a protein constituent of CFA) were similar, as would be expected, since the groups received equivalent doses of CFA. PPD was used throughout the study as a control to monitor responses to the adjuvant.
FIG. 1.
Proliferation of lymph node cells from BALB/c mice immunized with nematode extracts. Lymph node cells from mice immunized 10 days previously with CFA alone or with a 1:1 suspension of CFA and BmA or CeA were stimulated in vitro with media, native BmA or CeA, or PPD. Proliferation is expressed as counts per minute (c.p.m.). The data represent the arithmetic mean and standard deviation for five animals in each group. One of four independent experiments with similar results is shown.
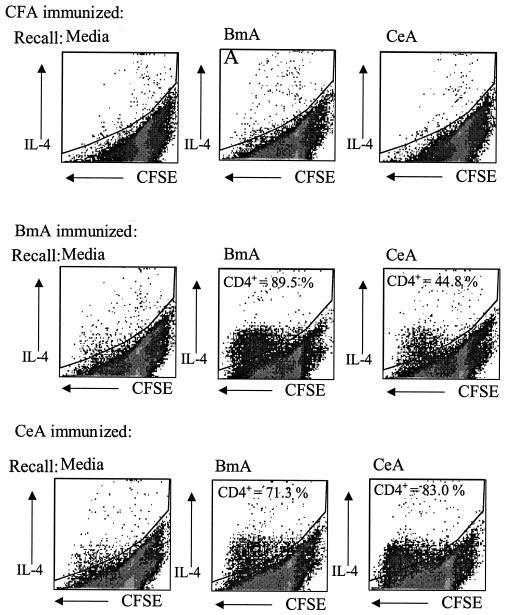
Proliferating nematode antigen-specific T lymphocytes are predominantly type 2 CD4+ cells.
In order to assess the phenotype of the antigen-specific proliferating cells, lymph node cells from BmA- and CeA-immunized mice were stained ex vivo with CFSE and cultured for 5 days in the presence of the relevant antigen. The cells then were stained for CD4 and intracellular cytokines. Internal cytokine staining was performed for IL-4 and IFN-γ as indicator cytokines for Th2 and Th1, respectively. Antigen-specific proliferating lymphocytes were predominantly CD4+ T lymphocytes secreting the type 2 cytokine IL-4 (Fig. 2) but not IFN-γ (data not shown). Considerable amounts of IL-4, but very little or no IFN-γ, also were detected in the culture supernatants of lymph node cells stimulated in vitro with either of the two nematode extracts (data not shown). Thus, soluble antigens of the nonparasitic nematode C. elegans seem to display the same property of evoking a skewed type 2 immune response as do parasitic helminths. More cross-reactivity between B. malayi and C. elegans antigens was observed in this assay system than for the proliferation of total lymph node cells. The additional 2 days required for the CFSE assay may have allowed the expansion of cross-reactive IL-4-producing cells.
FIG. 2.
Antigen-specific CD4+ lymphocytes proliferate in response to antigen and produce IL-4. Lymph node cells from mice immunized 7 days previously with CFA alone or with a 1:1 suspension of CFA and BmA or CeA were stained ex vivo with CFSE and stimulated in vitro with BmA, CeA, or PPD for 5 days. Cells stained with anti-CD4 (Cy-chrome) and anti-IL-4 (phycoerythrin) were analyzed by flow cytometry. CFSE staining is shown on the x axis (dividing cells lose fluorescence), and IL-4-positive cells are shown on the y axis. The percentages of IL-4-producing, dividing cells that were CD4+ are indicated.
Selective modification of glycans on B. malayi and C. elegans by sodium periodate treatment.
Okano et al. reported recently that glycans on soluble egg antigens of S. mansoni are inducers of the type 2 response characteristic of this parasitic trematode (39, 40). In the present study, the possible role of glycans on nematode antigens in the induction of a type 2 immune response was investigated by using B. malayi and C. elegans as models for parasitic and nonparasitic nematodes, respectively. As in the S. mansoni study (39), mild sodium periodate treatment was used to alter the three-dimensional structure of glycans on parasite molecules. Periodate oxidation, through cleavage of bonds between adjacent carbons that bear hydroxyl groups, opens sugar rings and thus destroys the integrity of carbohydrate structures. Mild periodate treatment has been demonstrated to abolish the binding of monoclonal antibodies to many carbohydrate structures, including unusual nematode N-linked glycans with highly fucosylated cores (10). The application of this treatment to BmA (pBmA) abolished the binding of the lectin ConA, whereas the mock-treated extract (mBmA) still exhibited this binding ability (Fig. 3), indicating that the glycan structures had been modified by the treatment. Periodate oxidation was selective for the glycans and apparently did not affect the peptide backbone of the parasite glycoproteins. This conclusion was evidenced by the observation that the treatment did not interfere with the binding of a rabbit antiserum produced against a nonglycosylated bacterially expressed subunit of the nematode polyprotein allergen of B. malayi, gp15/400 (Fig. 3), which is glycosylated in its native state (50). CeA was subjected to an identical mild periodate treatment, and abolition of ConA binding was confirmed, as for BmA (data not shown).
FIG. 3.
Western blot analysis of sodium periodate-treated and mock-treated soluble extracts of B. malayi. pBmA (lanes 1) and mBmA (lanes 2) prepared as detailed in Materials and Methods were separated by SDS-PAGE and transferred to NC sheets; the NC sheets were used in parallel to detect N-linked glycans with horseradish peroxidase (HRP)-conjugated ConA or the B. malayi ladder protein gp15/400, a polyclonal rabbit anti-gp15/400 antibody, and an HRP-conjugated goat anti-rabbit secondary antibody. A change in protein mobility is a common feature after treatment with sodium periodate (53).
Glycans on B. malayi and C. elegans play a major role in the induction of the skewed type 2 immune response.
To investigate whether glycans on nematode glycoproteins play a role in the induction of a predominant type 2 immune response, experimental groups of BALB/c mice were immunized with equal amounts of pBmA and mBmA in CFA, pCeA and mCeA in CFA, or PBS in CFA (control group), and the popliteal lymph nodes were assayed for in vitro antigen-specific proliferation and cytokine production 10 days later. Upon stimulation in vitro with the respective native extracts, both experimental groups showed good antigen-specific proliferative responses, comparable to those obtained for mice immunized with the native extracts, indicating that the chemical treatment did not interfere with the ability of the extracts to prime antigen-specific lymphocytes in vivo (Fig. 1 and 4). However, lymphocytes from mice immunized with the periodate-treated extracts proliferated somewhat less vigorously in response to antigenic stimulation (Fig. 4). The proliferative responses to PPD were comparable among all of the groups (Fig. 4), as were the proliferative responses to ConA (data not shown).
FIG. 4.
Proliferation of lymph node cells from BALB/c mice immunized with chemically treated nematode extracts. Lymph node cells from mice immunized with CFA alone or with a 1:1 suspension of CFA and either mBmA, pBmA, mCeA, or pCeA were stimulated in vitro with medium, native BmA, or PPD (top) or with medium, native CeA, or PPD (bottom). Proliferation is expressed as counts per minute (c.p.m.). The data represent the arithmetic mean and standard deviation for five animals in each group. One of four independent experiments with similar results is shown.
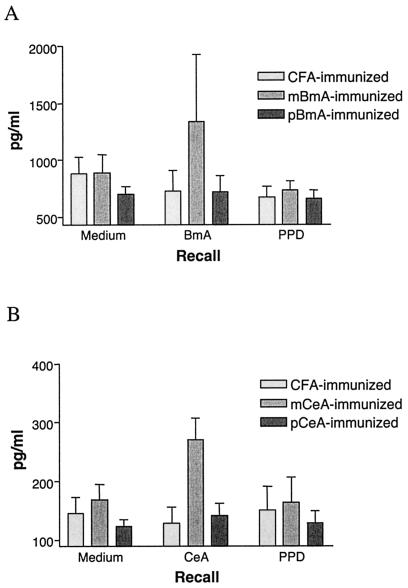
We next measured the production of IL-4 and IFN-γ in culture supernatants of lymph node cells from individual mice in the different groups. Upon antigenic stimulation, lymph node cells from mice immunized with the periodate-treated extracts produced significantly less IL-4 (P < 0.05) than did those from mice immunized with the respective mock-treated extracts (Fig. 5), while IFN-γ production by all of the groups was below the detection limit of the assay (data not shown). Lymph node cells from mice in the different groups induced equivalent amounts of IL-4 and IFN-γ in response to in vitro stimulation with ConA (data not shown).
FIG. 5.
IL-4 production by lymph node cells from BALB/c mice immunized with chemically treated nematode extracts. (A) IL-4 production in culture supernatants of lymph node cells from mice immunized with CFA, mBmA, or pBmA in response to in vitro stimulation with medium, native BmA, or PPD. Significantly more IL-4 (P < 0.05) was detected in culture supernatants of lymph node cells from mice immunized with mBmA than in those immunized with pBmA. (B) IL-4 production in culture supernatants of lymph node cells from mice immunized with CFA, mCeA, or pCeA in response to in vitro stimulation with medium, native CeA, or PPD. Significantly more IL-4 (P < 0.05) was detected in culture supernatants of lymph node cells from mice immunized with mCeA than in those immunized with pCeA. The data represent the arithmetic means and standard deviations of results for five animals in each group. One of three independent experiments with similar results is shown.
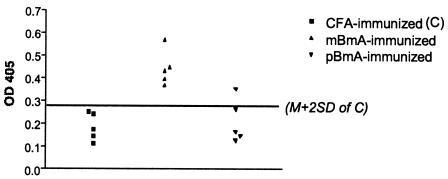
BALB/c mice in the different groups were further compared with regard to their production of antigen-specific antibodies to the respective native extracts by an ELISA 10 days after a single immunization with either periodate- or mock-treated extracts. IL-4 classically induces sequential switching to immunoglobulin G1 (IgG1) and IgE, whereas IFN-γ is associated with switching to IgG2a (13). Although overall specific antibody production (as assessed by the ELISA) was relatively low following the single immunization, sera from mice immunized with mBmA had significantly more Th2-associated isotype IgG1 than did those from mice immunized with pBmA (Fig. 6). These results are in agreement with the significantly greater IL-4 production by the former group than by the latter group. Neither of the two groups showed any antigen-specific production of Th1-associated isotype IgG2a, in agreement with the absence of antigen-specific IFN-γ production in culture supernatants of lymph node cells from these groups (data not shown). However, no antigen-specific IgG1 could be detected in sera from mice immunized with mCeA or pCeA (data not shown). Furthermore, no significant difference was detected between periodate- and mock-treated groups with regard to their total IgE production (data not shown). This finding might have been due to restricted immunoglobulin class switching during the primary immune response (49). At 10 days, it is possible that only the most stimulatory antigens induce IgG production, while IgE production may require a longer protocol with repeated immunizations.
FIG. 6.
Antigen-specific IgG1 antibodies in sera from mice immunized with CFA, mBmA, or pBmA. Significantly more IgG1 (P < 0.01) was detected in sera from BALB/c mice immunized with mBmA than in those immunized with pBmA (n = 5). The horizontal line represents the cutoff line calculated as the arithmetic mean (M) of the optical densities at 450 nm (OD 405) for the control group ±2 standard deviations (SD). One of two independent experiments with similar results is shown.
Glycans on B. malayi and C. elegans are critical in the early induction of type 2 cytokine IL-4.
It is likely that the skewed type 2 response evoked by helminth infections is spearheaded by the production of type 2 cytokines, notably IL-4, early in the course of infection (42, 43). Further, IL-10, through its inhibitory effects on Th1-cell development, may be a critical factor in the induction of Th2 responses in some settings (41, 52). This scenario may be particularly relevant to this study, where IL-10 or other down-regulatory cytokines may have been required to overcome the type 1 response-promoting effects of CFA. To test whether the type 2-promoting effects of glycans that we noted at 10 days p.i. were the result of events launched at an earlier time, we used RT-PCR to detect and quantify cytokine mRNAs in the lymph nodes of BALB/c mice immunized with native or chemically modified BmA or CeA at 24, 48, and 72 h p.i. Transcripts for IL-4, IL-10, and IFN-γ were below the detection limits at 24 h p.i., and no significant differences between the groups were detectable at 48 h p.i. (data not shown). By 72 h p.i, however, BALB/c mice immunized with either BmA or CeA showed a significantly higher level of transcription of the Th2-promoting cytokine IL-4 than did control mice immunized with CFA alone, whereas all groups displayed equivalent amounts of transcripts for IL-10 and IFN-γ (Fig. 7A). This nematode antigen-mediated induction of IL-4 was also evident 10 days p.i. (Fig. 7B), consistent with cytokine release data at this time (Fig. 5). Again, all groups induced equivalent amounts of both IL-10 and IFN-γ (Fig. 7).
FIG. 7.
Real-time RT-PCR analysis of IL-4, IL-10, and IFN-γ mRNAs in lymph node cells from BALB/c mice 72 h (A) and 10 days (B) after immunization with CFA, BmA, or CeA. The data represent the arithmetic mean and standard deviation for five mice in each group. Cytokine RT-PCR transcripts are represented in arbitrary units (A.U.) relative to the reference Th-cell clone. BALB/c mice immunized with BmA or CeA showed significantly more IL-4 mRNA in lymph node cells than control mice immunized with CFA alone but showed comparable IL-10 and IFN-γ mRNA levels. One of two independent experiments with similar results is shown.
To investigate the possible role of glycans in the early nematode antigen-driven induction of IL-4, BALB/c mice immunized with either pBmA or mBmA were assayed for cytokine transcripts at 72 h p.i. mBmA consistently induced significantly more IL-4 than did pBmA (Fig. 8). All groups induced equivalent amounts of IFN-γ, while groups immunized with either mock- or periodate-treated extracts induced more IL-10 than did the control group (Fig. 8). This increased induction of IL-10 was not seen with native extracts (Fig. 7), suggesting that chemical treatment of the extracts promoted IL-10 production.
FIG. 8.
Real-time RT-PCR analysis of IL-4, IL-10, and IFN-γ mRNAs in lymph node cells from BALB/c mice 72 h after immunization with chemically treated nematode extracts. The data represent the arithmetic mean and standard deviation for five mice in each group. Cytokine RT-PCR transcripts are represented in arbitrary units (A.U.) relative to the reference Th-cell clone. One of three independent experiments with similar results is shown.
DISCUSSION
Taken together, these data suggest that glycans on the antigens of parasitic nematodes such as B. malayi do indeed play a major role in the induction of the skewed type 2 immune response that these parasites characteristically invoke. The data further show that antigens of a nonparasitic nematode, C. elegans, also induce a predominantly type 2 immune response after a single immunization in BALB/c mice and that glycans likewise play an important role in this process. Importantly, these experiments were performed with CFA, which has a known propensity to drive Th1 responses. Previous experiments demonstrated that immunization with parasite extracts is able to overcome the Th1 bias of CFA (24). The ability of nematode extracts in this study to mount Th2 responses despite the presence of a strong type 1 adjuvant more clearly demonstrates their immune response-biasing capacity, especially in C. elegans, which had not been tested previously for Th2-inductive capacity.
An important factor in the induction of a type 2 immune response is the presence of IL-4 in the early stages of T-cell priming (17, 47). The exact origin of this early IL-4 is still a matter of contention, being ascribed to different immune cells in different experimental systems (7). Our studies indicate that periodate-sensitive structures are involved in the induction of IL-4 by 72 h. Other studies have detected helminth-induced IL-4 as early as 2 to 24 h (42, 43). Our failure to observe IL-4 at these very early times may indicate a different mechanism requiring the recruitment or development of particular cell types in this system or a difference in the antigenic composition of our parasite preparation. In particular, the presence of CFA in our preparation may necessitate additional time to overcome early type 1 responses.
It is well established that many N-linked glycans of nematodes are modified by the addition of phosphorylcholine (PC) (10, 16, 37) and that this modification underlies some of the immunological effects exerted by nematode glycoproteins on the immune system (19, 20, 25). For example, PC-containing glycoprotein ES-62 secreted by the nematode Acanthochelonema viteae is a powerful inducer of a Th2 immune response characterized by high-level induction of IL-4 synthesis (20). Our present data do not discern whether the Th2-skewing influence of nematode antigens is attributable to the carbohydrate moieties or to the attached PC residues or to both.
The molecular mechanism(s) underlying the potentiation of the Th2 arm of the immune response by protein-bound carbohydrate molecules seen in this study and in studies of schistosome egg sugars (39, 40) remains to be elucidated. One distinct possibility is that these molecules act as ligands that bind to receptors on IL-4-producing cells. A wide variety of cells, including mast cells, basophils, and eosinophils, have been implicated in the early production of IL-4 (12, 43); however, the factors that trigger IL-4 release still are not known. T cells also have been proposed as the source of early IL-4 (42), and in this scenario, the trigger may require presentation by antigen-presenting cells. For instance, when presented by CD1, the glycan moiety of a synthetic glycolipid, α-galactosylceramide, has been shown to interact directly with the T-cell receptor of NK T cells and to induce the production of IL-4 within hours of T-cell receptor engagement (27, 45). Carbohydrate recognition systems are thought to operate often as triads comprising receptors, ligands, and carrier proteins or lipids (8). It remains to be seen whether such a carrier-ligand-receptor system is the basis of the early induction of IL-4 by nematode glycans observed in the present study.
Another possible mechanism through which helminth sugars may bias the immune response is the recruitment of cells with the capacity to alter T-cell responsiveness. Terrazas et al. (48) found that schistosome oligosaccharides can recruit a population of Gr1+ cells that secrete suppressive cytokines, such as IL-10, that have the capacity to induce Th2-cell differentiation. Similarly, Loke et al. found that filarial parasites or their secreted products recruit alternatively activated macrophages that induce Th2-cell development through a transforming growth factor β-dependent pathway (30). However, the contribution of parasitic glycans to this process has not been investigated. Several studies have suggested a role for B cells in Th2-cell induction in helminth infections (2, 23), and schistosome oligosaccharides can induce B220+ cells to produce IL-10 (52). The data in the present study do not support a role for IL-10 as the key glycan-induced factor following exposure to nematode extracts, but we have not yet investigated transforming growth factor β.
Regardless of whether helminth glycans induce Th2 responses directly through triggering of IL-4 release, indirectly through antigen presentation, or through the recruitment of other cell types, there must be a specific recognition event that leads to a stereotypic response. Pathogen-associated molecular patterns (PAMPs) represent conserved molecular structures often shared by large groups of microorganisms and are thought to be essential for the survival of these organisms (35). The mammalian immune system has evolved to recognize these PAMPs through pathogen-specific receptors (PRR) and thus initiate the appropriate defensive strategy. The best-characterized PAMPs are derived from bacterial and single-celled organisms and induce proinflammatory cytokine release that leads to a Th1 response through the activation of Toll-like receptors (TLRs) (35). A role for TLRs in the induction of Th2 responses remains an area of controversy (9), but studies with MyD88-deficient mice have suggested that TLRs are not the key players in this pathway (44), and any involvement of TLRs is likely to be indirect. Consistent with these findings, schistosomal lipid moieties have been shown to induce dendritic cell maturation that leads to the induction of both Th2 cells and IL-10-producing regulatory T cells, but only the regulatory T-cell pathway is mediated by a TLR (51).
Structural studies of glycans on helminth glycoproteins are beginning to reveal that they possess many of the characteristics of PAMPs, such as structural conservation within and specificity for a certain helminth group (10, 22). Of particular significance is our finding that the antigens of the free-living nematode C. elegans invoke the type 2 bias that is a hallmark of infection with parasitic nematodes. The paradigm of “molecular signatures” (35) defined for prokaryotic or single-celled organisms thus may be applicable to metazoans. Given the complexity and diversity of metazoan organisms, defining these molecular patterns will not be an easy task. Our data bear a striking similarity to data from studies with schistosome eggs, which are among the most potent inducers of type 2 responses (39, 40, 48, 52). However, the phylogenetic distance between schistosomes and filarial parasites is greater than 750 million years, suggesting that ligands specific for helminths per se are unlikely to exist.
Glycan structures unique to nematodes may exist, and there are certainly biochemical data to support this notion (10). Further, the evolutionary drive for the mammalian immune system to rapidly identify invasion by nematodes is sufficiently great, as the nematodes represent a broad class of metazoan organisms that infect mammals. Most mammalian species carry at least one nematode parasite, and over one-quarter of the human population is infected with nematodes. However, the similarity of our findings to those obtained for schistosomes suggests that the mammalian immune system may have evolved to recognize a much broader class of metazoan invader. The divergence of mammals from both schistosomes and nematodes occurred at the protostome-deuterostome split about 1 billion years ago, and it is possible that molecular patterns exist to differentiate these two broad groups of animals. Consistent with this possibility is evidence that exposure to arthropods can lead to Th2 induction (36). Carbohydrate structures shared by many groups of protostomes have been identified (18). A third possibility is indicated by the finding that lacto-N-fucopentaose III, the predominant carbohydrate found in S. mansoni egg antigen, is also found in human milk (40). Certain carbohydrate structures may be shared by all metazoans and signal a Th2 response when they are presented in a “foreign” or invasive context.
Of particular utility in addressing these hypotheses is our finding that the antigens of the free-living nematode C. elegans induce the characteristic type 2 bias associated with parasitic helminth infections. The ability to grow unlimited quantities of the nematode, the availability of different mutants, the availability of the complete genome sequence, and the ability to alter gene expression through RNA interference (3, 4, 21) provide an unparalleled and powerful opportunity to define the structures involved in Th2 induction. Taking advantage of the ability to produce large quantities of soluble C. elegans antigen, we have used serial lectin affinity chromatography to demonstrate that the type 2 response-inducing activity lies almost exclusively in the fraction containing fucosylated glycoproteins (unpublished observation). Recent advances in the understanding of C. elegans glycans (6, 38) make this an extremely valuable system for defining and producing the structures that mediate Th2 activation. From here it should be possible to elucidate their cellular targets in the mammalian host as well as to determine whether these structures are nematode specific, protostome specific, or broadly metazoan.
Acknowledgments
We thank David Guilliano, Bill Gregory, Yvonne Harcus, Tracey Lamb, P'ng Loke, and Eva Malone for invaluable assistance and helpful discussions. We also thank Rick Maizels for critical review of the manuscript.
This work was funded by the Medical Research Council of the United Kingdom.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Bird, J. J., D. R. Brown, A. C. Mullen, N. H. Moskowitz, M. A. Mahowald, J. R. Sider, T. F. Gajewski, C. R. Wang, and S. L. Reiner. 1998. Helper T cell differentiation is controlled by the cell cycle. Immunity 9:229-237. [DOI] [PubMed] [Google Scholar]
- 2.Blackwell, N. M., and K. J. Else. 2001. B cells and antibodies are required for resistance to the parasitic gastrointestinal nematode Trichuris muris. Infect. Immun. 69:3860-3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blaxter, M. L. 1998. Caenorhabditis elegans is a nematode. Science 282:2041-2046. [DOI] [PubMed] [Google Scholar]
- 4.Blaxter, M. L. 1993. Cuticle surface proteins of wild type and mutant Caenorhabditis elegans. J. Biol. Chem. 268:6600-6609. [PubMed] [Google Scholar]
- 5.Blaxter, M. L., P. De Ley, J. Garey, L. X. Liu, P. Scheldeman, A. Vierstraete, J. Vanfleteren, L. Y. Mackey, M. Dorris, L. M. Frisse, J. T. Vida, and W. K. Thomas. 1998. A molecular evolutionary framework for the phylum Nematoda. Nature 392:71-75. [DOI] [PubMed] [Google Scholar]
- 6.Cipollo, J. F., C. E. Costello, and C. B. Hirschberg. 2002. The fine structure of Caenorhabditis elegans N-glycans. J. Biol. Chem. 277:49143-49157. [DOI] [PubMed] [Google Scholar]
- 7.Coffman, R. L., and T. Vonderweid. 1997. Multiple pathways for the initiation of T helper 2 (Th2) responses. J. Exp. Med. 185:373-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crocker, P. R., and T. Feizi. 1996. Carbohydrate recognition systems: functional triads in cell-cell interactions. Curr. Opin. Struct. Biol. 6:679-691. [DOI] [PubMed] [Google Scholar]
- 9.Dabbagh, K., and D. B. Lewis. 2003. Toll-like receptors and T-helper-1/T-helper-2 responses. Curr. Opin. Infect. Dis. 16:199-204. [DOI] [PubMed] [Google Scholar]
- 10.Dell, A., S. M. Haslam, H. R. Morris, and K. H. Khoo. 1999. Immunogenic glycoconjugates implicated in parasitic nematode diseases. Biochim. Biophys. Acta 1455:353-362. [DOI] [PubMed] [Google Scholar]
- 11.Eberl, M., J. A. Langermans, R. A. Vervenne, A. K. Nyame, R. D. Cummings, A. W. Thomas, P. S. Coulson, and R. A. Wilson. 2001. Antibodies to glycans dominate the host response to schistosome larvae and eggs: is their role protective or subversive? J. Infect. Dis. 183:1238-1247. [DOI] [PubMed] [Google Scholar]
- 12.Falcone, F. H., C. A. Dahinden, B. E. Gibbs, T. Noll, U. Amon, H. Hebestreit, O. Abrahamsen, J. Klaucke, M. Schlaak, and H. Haas. 1996. Human basophils release interleukin-4 after stimulation with Schistosoma mansoni egg antigen. Eur. J. Immunol. 26:1147-1155. [DOI] [PubMed] [Google Scholar]
- 13.Finkelman, F. D., J. Holmes, I. M. Katona, J. F. Urban, M. P. Beckmann, L. S. Park, K. A. Schooley, R. L. Coffman, T. R. Mossman, and W. E. Paul. 1990. Lymphokine control of in vivo immunoglobulin isotype selection. Annu. Rev. Immunol. 8:303-333. [DOI] [PubMed] [Google Scholar]
- 14.Finkelman, F. D., T. Shea-Donohue, J. Goldhill, C. A. Sullivan, S. C. Morris, K. B. Madden, W. C. Gause, and J. F. Urban, Jr. 1997. Cytokine regulation of host defense against parasitic gastrointestinal nematodes: lessons from studies with rodent models. Annu. Rev. Immunol. 15:505-533. [DOI] [PubMed] [Google Scholar]
- 15.Fiorentino, D. F., M. W. Bond, and T. R. Mosmann. 1989. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J. Exp. Med. 170:2081-2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gerdt, S., R. D. Dennis, G. Borgonie, R. Schnabel, and R. Geyer. 1999. Isolation, characterization and immunolocalization of phosphorylcholine-substituted glycolipids in developmental stages of Caenorhabditis elegans. Eur. J. Biochem. 266:952-963. [DOI] [PubMed] [Google Scholar]
- 17.Haas, H., F. H. Falcone, M. J. Holland, G. Schramm, K. Haisch, B. F. Gibbs, A. Bufe, and M. Schlaak. 1999. Early interleukin-4: its role in the switch towards a Th2 response and IgE-mediated allergy. Int. Arch. Allergy Immunol. 119:86-94. [DOI] [PubMed] [Google Scholar]
- 18.Haase, A., M. Stern, K. Wächtler, and G. Bicker. 2001. A tissue-specific marker of Ecdysozoa. Dev. Genes Evol. 211:428-433. [DOI] [PubMed] [Google Scholar]
- 19.Harnett, W., M. R. Deehan, K. M. Houston, and M. M. Harnett. 1999. Immunomodulatory properties of a phosphorylcholine-containing secreted filarial glycoprotein. Parasite Immunol. 21:601-608. [DOI] [PubMed] [Google Scholar]
- 20.Harnett, W., and M. M. Harnett. 1999. Phosphorylcholine: friend or foe of the immune system? Immunol. Today 20:125-129. [DOI] [PubMed] [Google Scholar]
- 21.Hashmi, S., W. Tawe, and S. Lustigman. 2001. Caenorhabditis elegans and the study of gene function in parasites. Trends Parasitol. 17:387-393. [DOI] [PubMed] [Google Scholar]
- 22.Haslam, S. M., K. M. Houston, W. Harnett, A. J. Reason, H. R. Morris, and A. Dell. 1999. Structural studies of N-glycans of filarial parasites. Conservation of phosphorylcholine-substituted glycans among species and discovery of novel chito-oligomers. J. Biol. Chem. 274:20953-20960. [DOI] [PubMed] [Google Scholar]
- 23.Hernandez, H. J., Y. Wang, and M. J. Stadecker. 1997. In infection with Schistosoma mansoni, B cells are required for T helper type 2 cell responses but not for granuloma formation. J. Immunol. 158:4832-4837. [PubMed] [Google Scholar]
- 24.Holland, M. J., Y. M. Harcus, P. L. Riches, and R. M. Maizels. 2000. Proteins secreted by the parasitic nematode Nippostrongylus brasiliensis act as adjuvants for Th2 responses. Eur. J. Immunol. 30:1977-1987. [DOI] [PubMed] [Google Scholar]
- 25.Houston, K. M., E. H. Wilson, L. Eyres, F. Brombacher, M. M. Harnett, J. Alexander, and W. Harnett. 2000. Presence of phosphorylcholine on a filarial nematode protein influences immunoglobulin G subclass response to the molecule by an interleukin-10-dependent mechanism. Infect. Immun. 68:5466-5468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jankovic, D., Z. Liu, and W. C. Gause. 2001. Th1- and Th2-cell commitment during infectious disease: asymmetry in divergent pathways. Trends Immunol. 22:450-457. [DOI] [PubMed] [Google Scholar]
- 27.Kawano, T., J. Q. Cui, Y. Koezuka, I. Toura, Y. Kaneko, K. Motoki, H. Ueno, R. Nakagawa, H. Sato, E. Kondo, H. Koseki, and M. Taniguchi. 1997. CD1d-restricted and TCR-mediated activation of v(alpha)14 NKT cells by glycosylceramides. Science 278:1626-1629. [DOI] [PubMed] [Google Scholar]
- 28.Kaye, J., S. Porcelli, J. Tite, and B. Jones. 1983. Both a monoclonal antibody and antisera specific for determinants unique to individual cloned helper T cell lines can substitute for antigen and antigen-presenting cells in the activation of T cells. J. Exp. Med. 158:836-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Le Goff, L., P. Loke, H. F. Ali, D. W. Taylor, and J. E. Allen. 2000. Interleukin-5 is essential for vaccine-mediated immunity but not innate resistance to a filarial parasite. Infect. Immun. 68:2513-2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loke, P., A. S. MacDonald, and J. E. Allen. 2000. Antigen presenting cells recruited by Brugia malayi induce Th2 differentiation of naive CD4+ T cells. Eur. J. Immunol. 30:1127-1135. [DOI] [PubMed] [Google Scholar]
- 31.Lyons, A. B., and C. R. Parish. 1994. Determination of lymphocyte division by flow cytometry. J. Immunol. Methods 171:131-137. [DOI] [PubMed] [Google Scholar]
- 32.Maizels, R. M., J. Burke, and D. A. Denham. 1987. Phosphorylcholine-bearing antigens in filarial nematode parasites: analysis of somatic extracts, in-vitro secretions and infection sera from Brugia malayi and B. pahangi. Parasite Immunol. 9:49-66. [DOI] [PubMed] [Google Scholar]
- 33.Maizels, R. M., M. J. Holland, F. H. Falcone, X. X. Zang, and M. Yazdanbakhsh. 1999. Vaccination against helminth parasites—the ultimate challenge for vaccinologists? Immunol. Rev. 170:125-147. [DOI] [PubMed] [Google Scholar]
- 34.Maizels, R. M., M. W. Kennedy, M. Meghji, B. D. Robertson, and H. V. Smith. 1987. Shared carbohydrate epitopes on distinct surface and secreted epitopes of the parasitic nematode Toxocara canis. J. Immunol. 139:207-214. [PubMed] [Google Scholar]
- 35.Medzhitov, R., and C. Janeway, Jr. 2000. Innate immune recognition: mechanisms and pathways. Immunol. Rev. 173:89-97. [DOI] [PubMed] [Google Scholar]
- 36.Mejri, N., N. Franscini, B. Rutti, and M. Brossard. 2001. Th2 polarization of the immune response of BALB/c mice to Ixodes ricinus instars, importance of several antigens in activation of specific Th2 subpopulations. Parasite Immunol. 23:61-69. [DOI] [PubMed] [Google Scholar]
- 37.Morelle, W., S. M. Haslam, V. Olivier, J. A. Appleton, H. R. Morris, and A. Dell. 2000. Phosphorylcholine-containing N-glycans of Trichinella spiralis: identification of multiantennary lacdiNAc structures. Glycobiology 10:941-950. [DOI] [PubMed] [Google Scholar]
- 38.Natsuka, S., J. Adachi, M. Kawaguchi, S. Nakakita, S. Hase, A. Ichikawa, and K. Ikura. 2002. Structural analysis of N-linked glycans in Caenorhabditis elegans. J. Biochem. (Tokyo) 131:807-813. [DOI] [PubMed] [Google Scholar]
- 39.Okano, M., A. R. Satoskar, K. Nishizaki, M. Abe, and D. A. Harn, Jr. 1999. Induction of Th2 responses and IgE is largely due to carbohydrates functioning as adjuvants on Schistosoma mansoni egg antigens. J. Immunol. 163:6712-6717. [PubMed] [Google Scholar]
- 40.Okano, M., A. R. Satoskar, K. Nishizaki, and D. A. Harn, Jr. 2001. Lacto-N-fucopentaose III found on Schistosoma mansoni egg antigens functions as adjuvant for proteins by inducing Th2-type response. J. Immunol. 167:442-450. [DOI] [PubMed] [Google Scholar]
- 41.Osborne, J., and E. Devaney. 1999. Interleukin-10 and antigen-presenting cells actively suppress Th1 cells in BALB/c mice infected with the filarial parasite Brugia pahangi. Infect. Immun. 67:1599-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Osborne, J., and E. Devaney. 1998. The L3 of Brugia induces a Th2-polarized response following activation of an IL-4-producing CD4−CD8− αβ T cell population. Int. Immunol. 10:1583-1590. [DOI] [PubMed] [Google Scholar]
- 43.Sabin, E. A., M. A. Kopf, and E. J. Pearce. 1996. Schistosoma mansoni egg-induced early IL-4 production is dependent upon IL-5 and eosinophils. J. Exp. Med. 184:1871-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schnare, M., G. M. Barton, A. C. Holt, K. Takeda, S. Akira, and R. Medzhitov. 2001. Toll-like receptors control activation of adaptive immune responses. Nat. Immunol. 2:947-950. [DOI] [PubMed] [Google Scholar]
- 45.Singh, N., S. Hong, D. C. Scherer, I. Serizawa, N. Burdin, M. Kronenberg, Y. Koezuka, and L. Van Kaer. 1999. Activation of NK T cells by CD1d and alpha-galactosylceramide directs conventional T cells to the acquisition of a Th2 phenotype. J. Immunol. 163:2373-2377. [PubMed] [Google Scholar]
- 46.Sulston, J. E., and J. Hodgkin. 1988. Methods, p. 587-606. In W. B. Wood (ed.), The nematode Caenorhabditis elegans. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 47.Swain, S. L. 1995. T-cell subsets. Who does the polarizing? Curr. Biol. 5:849-851. [DOI] [PubMed] [Google Scholar]
- 48.Terrazas, L. I., K. L. Walsh, D. Piskorska, E. McGuire, and D. A. Harn, Jr. 2001. The schistosome oligosaccharide lacto-N-neotetraose expands Gr1(+) cells that secrete anti-inflammatory cytokines and inhibit proliferation of naive CD4(+) cells: a potential mechanism for immune polarization in helminth infections. J. Immunol. 167:5294-5303. [DOI] [PubMed] [Google Scholar]
- 49.Toellner, K. M., A. Gulbranson-Judge, D. R. Taylor, D. M. Sze, and I. C. MacLennan. 1996. Immunoglobulin switch transcript production in vivo related to the site and time of antigen-specific B cell activation. J. Exp. Med. 183:2303-2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tweedie, S., W. A. Paxton, L. Ingram, R. M. Maizels, L. A. McReynolds, and M. E. Selkirk. 1993. Brugia pahangi and Brugia malayi—a surface-associated glycoprotein (gp15/400) is composed of multiple tandemly repeated units and processed from a 400-kDa precursor. Exp. Parasitol. 76:156-164. [DOI] [PubMed] [Google Scholar]
- 51.van der Kleij, D., E. Latz, J. F. Brouwers, Y. C. Kruize, M. Schmitz, E. A. Kurt-Jones, T. Espevik, E. C. de Jong, M. L. Kapsenberg, D. T. Golenbock, A. G. Tielens, and M. Yazdanbakhsh. 2002. A novel host-parasite lipid cross-talk. Schistosomal lyso-phosphatidylserine activates Toll-like receptor 2 and affects immune polarization. J. Biol. Chem. 277:48122-48129. [DOI] [PubMed] [Google Scholar]
- 52.Velupillai, P., and D. A. Harn. 1994. Oligosaccharide-specific induction of interleukin-10 production by B220+ cells from schistosome-infected mice—a mechanism for regulation of CD4+ T-cell subsets. Proc. Natl. Acad. Sci. USA 91:18-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Woodward, M. P., W. W. Young, Jr., and R. A. Bloodgood. 1985. Detection of monoclonal antibodies specific for carbohydrate epitopes using periodate oxidation. J. Immunol. Methods 78:143-153. [DOI] [PubMed] [Google Scholar]