Abstract
Analysis of T-cell subsets accumulating in the lungs of C57BL/6 mice chronically infected with Mycobacterium tuberculosis revealed that both CD4 and CD8 T-cell populations expressed a cell surface phenotype consistent with that of effector T cells and that a significant proportion of these cells were in the process of secreting gamma interferon.
An alarming aspect of the present global tuberculosis epidemic caused by Mycobacterium tuberculosis is that an estimated minimum of 100 to 200 million people carry the bacillus in an active or latent form (7-10, 17, 18, 21-24). All immunocompetent inbred mouse strains initially contain and control M. tuberculosis infection induced by aerosol exposure. Furthermore, the highly resistant strains of mice, such as C57BL/6, then exhibit a chronic or persistent disease state that can last 200 to 300 days with only minimal increases in the bacterial load (17). However, whether this chronic stage truly represents a form of bacterial latency is still a matter of debate. For example, surviving bacilli can still be killed by chemotherapeutic agents such as isoniazid (1, 5, 19), proteins recognized early in the infection such as ESAT-6 can still be recognized by T cells (25), and the restraining granulomatous pathology still has a dynamic rather than static profile (26).
Theoretically, if the M. tuberculosis infection is latent, then one would expect reactive T cells in the lung lesions to be in a state of memory, as can indeed be observed if the infection is cleared by drugs (1). However, other models of immunological memory, particularly those following more acute viral infections, have provided convincing evidence that the differentiation of naïve T cells into effector cells and subsequently into memory immune cells is a tightly controlled process (15, 16). A central determining factor is the availability of antigen, and it has been suggested by Bevan (2) that the transition of T cells from effector to memory would be unlikely in a situation such as chronic tuberculosis, where a source of antigen persists.
To address the issue of antigen persistence and its influence on the activation state of T cells during a pulmonary tuberculosis infection, T cells were harvested from the lungs of mice chronically infected with M. tuberculosis and then analyzed for their cell surface molecule expression. Throughout the chronic stage of infection, the majority of cells consistently exhibited a phenotype consistent with that of effector T cells. The data suggest that some surviving M. tuberculosis bacilli may slowly and continuously replicate during the chronic stage of infection, thus providing sufficient antigen to maintain the T-cell population in the lung lesions in a state of effector immunity.
Mice and bacterial strain.
C57BL/6 mice, 8 to 10 weeks old, were purchased from Jackson Laboratories, Bar Harbor, Maine. M. tuberculosis strain Erdman was grown to mid-log phase in Proskauer-Beck liquid medium containing 0.05% Tween 80 and then frozen in aliquots at −70°C until needed. Mice were infected by aerosol exposure procedures described previously (5, 6).
Lung cells and flow cytometry.
In order to obtain single-cell suspensions from the lungs, the organs were treated and processed strictly as described previously (11, 12). Cells were labeled with the following specific antibodies: fluorescein isothiocyanate (FITC) anti-CD44, phycoerythrin anti-CD45RB or anti-CD122, peridinin chlorophyll a protein anti-CD8 or anti-CD4, and allophycocyanin anti-CD62L. All antibodies were purchased from BD Pharmingen and were used at a concentration 0.2 μg per 106 cells in phosphate-buffered saline for 30 min at 4°C. The cells were analyzed on a Becton Dickinson FACScalibur and the data were analyzed with CellQuest software (Becton Dickinson Immmunocytometry Systems, San Jose, Calif.). All the analyses were done with an acquisition of 100,000 events.
Specific immune response.
Bone marrow macrophages (2 × 105 cells/ml) were either infected with 2.5 × 106 CFU of M. tuberculosis Erdman per ml in a final volume of 1 ml of complete Dulbecco minimum essential medium or pulsed with culture filtrate protein from M. tuberculosis (10 μg/ml; supplied by John T. Belisle) for 4 h. The plates were incubated for 18 h at 37°C in 5% CO2 with lung cell suspensions of control age-matched or infected mice. For the final 6 h of the incubation period, 3 μM monensin (Golgi Stop; BD Pharmingen) was added to the cultures. The lung cells were labeled with allophycocyanin anti-CD4 and peridinin chlorophyll a protein anti-CD8 and fixed and permeabilized according to the manufacturer's directions with a Golgi Stop kit (BD Pharmingen); cells were then stained for intracellular FITC anti-gamma interferon (IFN-γ) or FITC rat immunoglobulin G1 isotype control (BD Pharmingen). The samples were analyzed by flow cytometry.
IHC analysis.
In order to execute an immunohistochemical (IHC) analysis for CD4 and CD8 T cells, the right first lobe from each mouse was collected. The IHC analysis was done as previously described (11). All experiments were performed three times, and statistical significance between the different groups was determined using the Student t test.
Maintenance of a stable bacterial load and number of inflammatory cells in the lung.
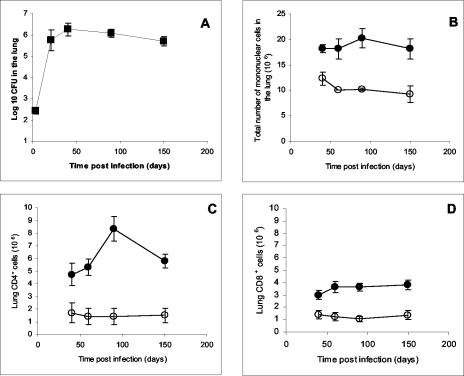
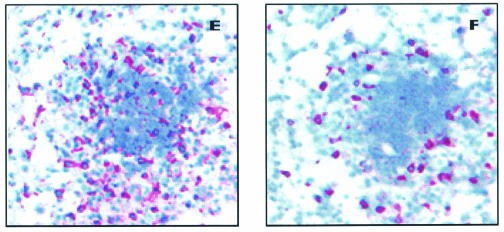
In order to follow the inflammatory response during the chronic stage of infection, mice were infected by the aerosol route with M. tuberculosis, and at various time points after exposure, the bacterial load and the total number of cells recovered from individual lungs were enumerated and analyzed by flow cytometry for CD4+and CD8+ T cells. As expected, after the initial exponential growth phase, a stable bacterial load and homeostatic maintenance of inflammatory cells in the lung were observed through 150 days of the experiment (Fig. 1A and B). The numbers of CD4+ T cells that could be isolated from the infected lungs increased up to 90 days postinfection (P = 0.01), while a smaller increase in CD8+ T cells was observed (Fig. 1C and D). IHC analysis of lung granulomas 90 days postinfection corroborated the flow results showing a prevalence of CD4 cells that were both surrounding and within the lesions (Fig. 1E), while CD8+ cells were sparsely distributed around the perimeter of the granuloma (Fig. 1F), as has been previously observed (11).
FIG. 1.
Bacterial load and influx of inflammatory cells in the lung after aerosol infection with M. tuberculosis. (A) Bacterial load in the lungs after aerosol infection. Data are expressed as the log10 CFU per lung from four mice per group (means ± standard deviations). (B) Total lung cells obtained during the chronic phase of the disease. (C and D) Total numbers of CD4+ and CD8+ T cells in the lung measured by flow cytometry. Infected mice (filled circles) were compared to uninfected control mice (open circles) to allow for known changes in T cells as animals age. Data shown are the means ± standard errors of the means (six mice per group) and are representative of three separate infections. (E and F) Representative photomicrographs of a typical C57BL/6 pulmonary granuloma stained for CD4 (E) and CD8 (F) cells at 90 days postinfection (magnification, ×100).
CD4+ T cells predominantly express an effector cell surface phenotype.
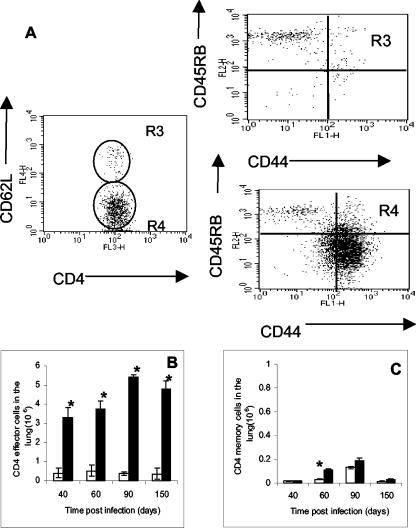
Next, we addressed whether T cells entering the sites of the pulmonary infection with M. tuberculosis transitioned from a state of effector immunity to one of memory as the bacterial load became stable during the chronic stage of disease. To discriminate between effector and memory cells, four-color flow cytometric analysis was used to characterize CD4 effector cells as CD44high, CD62Llow, and CD45RBmid and memory cells as CD44high, CD62Lhigh, and CD45RBlow. It was consistently found (Fig. 2) that most T cells present in the lungs between day 40 and day 150 were CD62Llow, and further analysis of this gated region showed that most of these cells were CD44high and CD45RBmid. Plotting these data versus the course of the infection (Fig. 2B) indicated the predominance of a stable effector CD4 cell population. Minimal numbers of memory CD4 cells could be identified (Fig. 2C).
FIG. 2.
Surface marker expression by isolated lung CD4 T cells. (A) In the representative example, lung lymphocytes were stained for CD4 and CD62L cells. CD62Lhigh (R3) and CD62Llow (R4) cells were further analyzed for the expression of CD44 and CD45RB. (B) Kinetics of effector CD4 cells (CD44high, CD45RBmid, and CD62Llow) over the chronic phase of the infection. (C) Kinetics of memory CD4 cells (CD44high, CD45RBlow, and CD62Lhigh). Data are expressed as the means ± the standard errors of the means and are from six mice per group per time point, representative of two independent experiments. Open bars, control aged-matched mice; filled bars, infected mice. Statistical significance based on a comparison of control and infected mice is indicated by an asterisk (P < 0.01).
Changes in CD8+-T-cell populations.
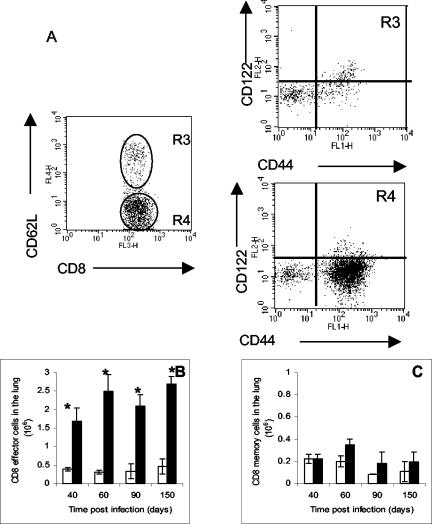
To enumerate lung CD8+ T cells, we performed flow cytometry with CD44 high, CD122 high, and CD62L high as surface markers for memory cells and CD44high, CD122low, and CD62Llow as surface markers for effector cells (Fig. 3). Similar to the results obtained for CD4 cells, the number of CD8+ effector cells in the lung predominated during the chronic phase of the disease (Fig. 3B), with only a limited number of cells expressing a memory T-cell phenotype (Fig. 3C).
FIG. 3.
Similar analysis for effector CD8+ cells isolated from the lungs during chronic infection with M. tuberculosis. As above, cells were stained for CD8 and CD62L and then further analyzed for the expression of CD44 and CD122 cells. (B) Kinetics of effector CD8+ cells (CD44 high, CD122low, and CD62Llow). (C) Kinetics of memory CD8+ cells (CD44 high, CD122 high, and CD62L high). Data are expressed as the means ± the standard errors of the means and are from six mice per group per time point, representative of two independent experiments. Open bars, control aged-matched mice; filled bars, infected mice. Statistical significance based on a comparison of control and infected mice is indicated by an asterisk (P < 0.01).
Cytokine-secreting CD4+- and CD8+-cell populations in the lung.
The critical cytokine induced during M. tuberculosis infection is IFN-γ, and its production is directly correlated with protection in the mouse model (6, 7). In addition, however, both innate and acquired mechanisms induced by pulmonary tuberculosis can drive the production of this cytokine (4, 7, 13-14, 20). As shown in Fig. 4A, an increase in CD4 T cells secreting IFN-γ was observed up to the 90-day time point, and a smaller, yet still significant, increase was seen within the CD8 cell population (Fig. 4B). In both cases, cells producing this cytokine were predominately from within the effector T-cell subsets.
FIG. 4.
T cells producing IFN-γ during the chronic stage of infection. Data are expressed as the means ± the standard errors of the means and are from eight mice per group per time point, representative of two independent experiments. Open circles, control aged-matched mice; filled circles, infected mice. Statistical significance based on a comparison of control and infected mice is indicated by an asterisk (P < 0.01).
The results of this study show that the vast majority of T cells that can be isolated from the lungs of M. tuberculosis-infected mice up to 150 days postinfection possess a CD62Llow phenotype that is associated with effector immunity. Moreover, a significant proportion of these cells are in the process of producing the key protective cytokine IFN-γ. These results are fully consistent with the recent hypothesis put forth by Bevan (2) that chronic diseases such as tuberculosis and leprosy probably create a state of immunity characterized by the continuous presence of effector T cells. This process is probably driven by antigen, because prolonged chemotherapy which clears M. tuberculosis leads to the establishment of memory immunity (1, 13, 20). If such a hypothesis is correct, then it implies that the persisting bacteria produce sufficient antigen to maintain the lung immune response in a state of effector immunity and argues against the notion that bacteria present at this time are in some state of latency.
It is not known at this point if CD4 T cells in a state of memory immunity control challenge infections directly or whether they must first revert to effector cells. Since the bacteria are contained but not cleared in animal vaccination models of tuberculosis, one must assume that prolonged control of persistent low-level infection in such models requires the sustained presence of effector T cells. One must also assume that initially establishing a population of antigen-specific memory T cells is the most efficient way to achieve this outcome, but it may also be predicated on efficient transition to a more protective phenotype, as recent results from Andersen and Smedegaard (1) tend to suggest. To complicate matters even further, the CD62Llow populations probably include some cells in a state of memory immunity (3). These findings may provide new strategies for rational tuberculosis vaccine design efforts (21).
Acknowledgments
This work was supported by National Institutes of Health grants AI-40488, AI-44072, and AI-45707.
We thank Angelo Izzo and Todd Lasco for revising the text.
Editor: F. C. Fang
REFERENCES
- 1.Andersen, P., and B. Smedegaard. 2000. CD4+ T-cell subsets that mediate immunological memory to Mycobacterium tuberculosis infection in mice. Infect. Immun. 68:621-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bevan, M. J. 2002. Immunology: remembrance of things past. Nature 420:748-749. [DOI] [PubMed] [Google Scholar]
- 3.Blander, J. M., D. B. Sant'Angelo, D. Metz, S. Kim, R. A. Flavell, K. Bottomly, and C. A. Janeway, Jr. 2003. A pool of central memory-like CD4 T cells contains effector memory precursors. J. Immunol. 170:2940-2948. [DOI] [PubMed] [Google Scholar]
- 4.Brightbill, H. D., D. H. Libraty, S. R. Krutzik, R. B. Yang, J. T. Belisle, J. R. Bleharski, M. Maitland, M. V. Norgard, S. E. Plevy, S. T. Smale, P. J. Brennan, B. R. Bloom, P. J. Godowski, and R. L. Modlin. 1999. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science 285:732-736. [DOI] [PubMed] [Google Scholar]
- 5.Cooper, A. M., J. E. Callahan, M. Keen, J. T. Belisle, and I. M. Orme. 1997. Expression of memory immunity in the lung following re-exposure to Mycobacterium tuberculosis. Tuber. Lung Dis. 78:67-73. [DOI] [PubMed] [Google Scholar]
- 6.Cooper, A. M., D. K. Dalton, T. A. Stewart, J. P. Griffin, D. G. Russell, and I. M. Orme. 1993. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J. Exp. Med. 178:2243-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper, A. M., and J. L. Flynn. 1995. The protective immune response to Mycobacterium tuberculosis. Curr. Opin. Immunol. 7:512-516. [DOI] [PubMed] [Google Scholar]
- 8.Dolin, P. J., M. C. Raviglione, and A. Kochi. 1994. Global tuberculosis incidence and mortality during 1990-2000. Bull. W. H. O. 72:213-220. [PMC free article] [PubMed] [Google Scholar]
- 9.Flynn, J. L., and J. Chan. 2001. Immunology of tuberculosis. Annu. Rev. Immunol. 19:93-129. [DOI] [PubMed] [Google Scholar]
- 10.Flynn, J. L., and J. Chan. 2001. Tuberculosis: latency and reactivation. Infect. Immun. 69:4195-4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalez-Juarrero, M., O. C. Turner, J. Turner, P. Marietta, J. V. Brooks, and I. M. Orme. 2001. Temporal and spatial arrangement of lymphocytes within lung granulomas induced by aerosol infection with Mycobacterium tuberculosis. Infect. Immun. 69:1722-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gonzalez-Juarrero, M., T. S. Shim, A. Kipnis, A. P. Junqueira-Kipnis, and I. M. Orme. 2003. Dynamics of macrophage cell populations during murine pulmonary tuberculosis. J. Immunol. 171:3128-3135. [DOI] [PubMed] [Google Scholar]
- 13.Griffin, J. P., and I. M. Orme. 1994. Evolution of CD4 T-cell subsets following infection of naive and memory immune mice with Mycobacterium tuberculosis. Infect. Immun. 62:1683-1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heldwein, K. A., and M. J. Fenton. 2002. The role of Toll-like receptors in immunity against mycobacterial infection. Microbes Infect. 4:937-944. [DOI] [PubMed] [Google Scholar]
- 15.Kaech, S. M., S. Hemby, E. Kersh, and R. Ahmed. 2002. Molecular and functional profiling of memory CD8 T cell differentiation. Cell 111:837-851. [DOI] [PubMed] [Google Scholar]
- 16.Kaech, S. M., E. J. Wherry, and R. Ahmed. 2002. Effector and memory T-cell differentiation: implications for vaccine development. Nat. Rev. Immunol. 2:251-262. [DOI] [PubMed] [Google Scholar]
- 17.Kooyk, Y., B. Appelmelk, and T. B. Geijtenbeek. 2003. A fatal attraction: Mycobacterium tuberculosis and HIV-1 target DC-SIGN to escape immune surveillance. Trends Mol. Med. 9:153-159. [DOI] [PubMed] [Google Scholar]
- 18.Manabe, Y. C., and W. R. Bishai. 2000. Latent Mycobacterium tuberculosis--persistence, patience, and winning by waiting. Nat. Med. 6:1327-1329. [DOI] [PubMed] [Google Scholar]
- 19.Orme, I. M. 2003. The mouse as a useful model of tuberculosis. Tuberculosis (Edinb). 83:112-115. [DOI] [PubMed] [Google Scholar]
- 20.Orme, I. M., P. Andersen, and W. H. Boom. 1993. T cell response to Mycobacterium tuberculosis. J. Infect. Dis. 167: 1481-1497. [DOI] [PubMed] [Google Scholar]
- 21.Orme, I. M., D. N. McMurray, and J. T. Belisle. 2001. Tuberculosis vaccine development: recent progress. Trends Microbiol. 9:115-118. [DOI] [PubMed] [Google Scholar]
- 22.Orme, M. 2001. The latent tuberculosis bacillus (I'll let you know if I ever meet one). Int. J. Tuberc. Lung Dis. 5:589-593. [PubMed] [Google Scholar]
- 23.Parrish, N. M., J. D. Dick, and W. R. Bishai. 1998. Mechanisms of latency in Mycobacterium tuberculosis. Trends Microbiol. 6:107-112. [DOI] [PubMed] [Google Scholar]
- 24.Raviglione, M. C., D. E. Snider, Jr., and A. Kochi. 1995. Global epidemiology of tuberculosis. Morbidity and mortality of a worldwide epidemic. JAMA 273:220-226. [PubMed] [Google Scholar]
- 25.Rhoades, E. R., A. A. Frank, and I. M. Orme. 1997. Progression of chronic pulmonary tuberculosis in mice aerogenically infected with virulent Mycobacterium tuberculosis. Tuber. Lung Dis. 78:57-66. [DOI] [PubMed] [Google Scholar]
- 26.Winslow, G. M., A. D. Roberts, M. A. Blackman, and D. L. Woodland. 2003. Persistence and turnover of antigen-specific CD4 T cells during chronic tuberculosis infection in the mouse. J. Immunol. 170:2046-2052. [DOI] [PubMed] [Google Scholar]