Abstract
Nontypeable Haemophilus influenzae (NTHi) is a major cause of opportunistic respiratory tract infections, including otitis media and bronchitis. The persistence of NTHi in vivo is thought to involve bacterial persistence in a biofilm community. Therefore, there is a need for further definition of bacterial factors contributing to biofilm formation by NTHi. Like other bacteria inhabiting host mucosal surfaces, NTHi has on its surface a diverse array of lipooligosaccharides (LOS) that influence host-bacterial interactions. In this study, we show that LOS containing sialic (N-acetyl-neuraminic) acid promotes biofilm formation by NTHi in vitro and bacterial persistence within the middle ear or lung in vivo. LOS from NTHi in biofilms was sialylated, as determined by comparison of electrophoretic mobilities and immunochemical reactivities before and after neuraminidase treatment. Biofilm formation was significantly reduced in media lacking sialic acid, and a siaB (CMP-sialic acid synthetase) mutant was deficient in biofilm formation in three different in vitro model systems. The persistence of an asialylated siaB mutant was attenuated in a gerbil middle ear infection model system, as well as in a rat pulmonary challenge model system. These data show that sialylated LOS glycoforms promote biofilm formation by NTHi and persistence in vivo.
Haemophilus influenzae is a fastidious gram-negative bacterium that is highly adapted to human hosts and exists primarily as a commensal in the nasopharynges and upper airways of most healthy people. Most H. influenzae isolates from patients with asymptomatic carriage and localized infections are nontypeable H. influenzae (NTHi) strains lacking capsular polysaccharides (35). NTHi causes opportunistic infections such as otitis media (25), sinusitis (23), and bronchial infections associated with chronic obstructive pulmonary disease (34, 42) and viral infections (2, 21). Bacterial factors important for the persistence of H. influenzae in vivo include pili and other protein adhesins, as well as lipooligosaccharides (LOS) (56).
The H. influenzae LOS contains many glycoforms differing in composition and structure (46, 60). LOS from many H. influenzae strains contain sialic (N-acetyl-neuraminic) acid (NeuAc) (8, 19, 29, 43). In H. influenzae and Neisseria spp., NeuAc is added to acceptor LOS forms terminating in N-acetyl-lactosamine (44). H. influenzae acquires NeuAc from environmental sources, from which it is added to a cytidine-monophosphate carrier by the CMP-NeuAc synthetase siaB (19) and linked to the oligosaccharide portion of the LOS by at least three separate sialyltransferases (encoded by lic3A, siaA, and lsgB) with differing LOS acceptor specificities (18, 19, 22).
Despite considerable genomic diversity and plasticity, NTHi populations in vivo acquire a consistent pattern of phenotypes that are still being defined (30, 59, 62, 66, 67). It is clear that the majority of NTHi isolates add NeuAc to LOS (19, 29), and a recent survey of 24 NTHi strains showed that 23 produced sialylated LOS forms (4). NeuAc may provide a competitive advantage, as asialylated H. influenzae mutants are less resistant than sialylated strains to complement-mediated killing by serum (19). Recently, Bouchet and colleagues showed that diverse NTHi strains acquire host sialic acid during colonization of the chinchilla middle ear and that sialylation is required for bacterial persistence in a chinchilla infection model (5).
H. influenzae causes otitis media, which has been cited as an example of a setting in which biofilms contribute to bacterial persistence and disease (7). Evidence for H. influenzae biofilms in vivo has included the detection of bacterial products in culture-negative patient specimens (10, 47) and direct visualization of biofilms on tympanostomy tubes from children with recurrent otitis (45) and in the middle ears of chinchillas following experimental infection with NTHi (11). An autotransporter adhesin, Hap, mediates bacterial adherence to the extracellular matrix and host cells and the formation of microcolonies, an early step in biofilm formation (12, 13, 16). However, despite data showing that the hap gene is ubiquitous among H. influenzae strains (37, 49), there is significant variation in biofilm formation by clinical NTHi isolates in vitro (36). In this study, we demonstrate that the addition of NeuAc to LOS promotes biofilm formation by NTHi in vitro and contributes to the persistence of NTHi in vivo.
MATERIALS AND METHODS
Bacteria.
The bacterial strains used in this study are listed in Table 1. H. influenzae strains were cultured on brain heart infusion (BHI) media (Difco) supplemented with hemin (ICN Biochemicals) and NAD (Sigma) or on a defined medium (26). NeuAc (20 μg/ml; Sigma) was added as indicated below.
TABLE 1.
List of strains used in this study
| Strain | Description | Reference(s) |
|---|---|---|
| H. influenzae | ||
| Rd KW-20 | Acapsular serotype d; TIGRa genomic sequence strain | 14 |
| 2019 | Nontypeable; bronchial isolate | 6 |
| 3198 | Nontypeable; bronchial isolate | 6 |
| 7502 | Nontypeable; bronchial isolate | 6 |
| 86-0298NP | Nasopharyngeal isolate | 3, 24, 57 |
| M37 | Otitis media isolate | 41 |
| LB2 | Otitis media isolate | 41 |
| LB5 | Otitis media isolate | 41 |
| mr31 | Otitis media isolate | 41 |
| 3031 | Otitis media isolate | 41 |
| P. aeruginosa | ||
| PAO1 | Laboratory strain | K. Jackson and D. Wozniak, unpublished data |
| wfpA60 | Biofilm-deficient mutant |
TIGR, The Institute for Genomic Research.
Microtiter assay.
H. influenzae strains were screened for biofilm initiation by a well-described microtiter assay (38-40). Briefly, overnight cultures of H. influenzae were diluted to ∼107 CFU per ml in supplemented BHI broth, inoculated into 96-well microtiter dishes (100 μl/well), and incubated at 37°C. At various times thereafter, the plates were removed, washed, stained with 0.1% crystal violet, washed again, and dried. The remaining crystal violet in the wells was solubilized with ethanol and quantified by determining the optical density at 540 nm. The significance of results was assessed by a standard paired t test.
Electron microscopy.
H. influenzae cells were cultured in polystyrene chamber slides (Nunc), slanted such that an air-liquid interface was established. After 24 to 48 h, the culture medium was removed and the bacteria were fixed with 2% glutaraldehyde and processed for scanning electron microscopy (SEM) analysis by using a graded acetone dehydration series and osmium tetraoxide. The chamber slides were trimmed, mounted onto stubs, and coated with palladium. Biofilm communities growing in a condensed line at the air-liquid interface were viewed on a Phillips SEM-515 scanning electron microscope.
Biofilm formation in silicon tubing.
H. influenzae biofilm formation in a continuous-flow silicon tubing system was also assessed (50, 68). At 24 to 48 h postinoculation, a 9-in. section of tubing was excised approximately 3 in. below the site of inoculation. The tubing was opened and scraped to remove adherent bacteria, which were enumerated by plate counting.
Isolation and analysis of lipooligosaccharides.
LOS was isolated from H. influenzae by a modified proteinase K procedure (17, 22) and subjected to Tricine-sodium dodecyl sulfate-16.5% polyacrylamide gel electrophoresis (20, 27). LOS was visualized by ammonia silver staining (63) or by immunochemical analysis with monoclonal antibody (MAb) 3F11, which recognizes exposed lactosamine structures (22, 29, 65).
Gerbil infection studies.
Colonization of the middle ear by NTHi was assessed by use of a Mongolian gerbil otitis media model system (15). Briefly, groups of male Mongolian gerbils (weighing 60 to 70 g each; Charles River, Wilmington, Mass.) were randomized into groups of five animals. Each gerbil was anesthetized by inhalation of sevoflurance and infected with 0.03 ml of a 6-h logarithmic-phase culture containing 7.3 to 7.4 log10 CFU of NTHi injected percutaneously into the superior posterior chamber of the left middle ear. At 24 to 72 h postinfection, the gerbils were euthanized and the left middle ear chamber was washed via injection with 0.03 ml of normal saline solution through the tympanic membrane. Bacterial counts were obtained from serial dilutions of the middle ear fluid aspirates and are expressed as numbers of CFU per milliliter of middle ear fluid. The limit of detection was ∼10 CFU/ml. All of these studies were approved by the Abbott Laboratories Institutional Animal Care and Use Committee.
Rat pulmonary infection studies.
The persistence of NTHi within the lung was assessed by use of a rat pulmonary infection model system (1, 31). Briefly, NTHi was cultured to the late logarithmic phase in supplemented BHI broth. Groups of male Sprague-Dawley rats (weighing 280 to 300 g each; Charles River) were randomized into groups of five animals and infected intratracheally with 0.5 ml of bacteria (8.0 to 8.2 log10 CFU) suspended in 5% hog gastric mucin. At various times postinfection, the rats were euthanized, their lungs were removed and homogenized, and the homogenate was serially diluted and plated on chocolate agar plates (BBL). Bacterial counts were expressed as numbers of CFU per lung pair. The limit of detection was ∼50 CFU per lung pair. These studies were also approved by the Abbott Laboratories Institutional Animal Care and Use Committee.
RESULTS
Varied levels of biofilm formation by H. influenzae strains.
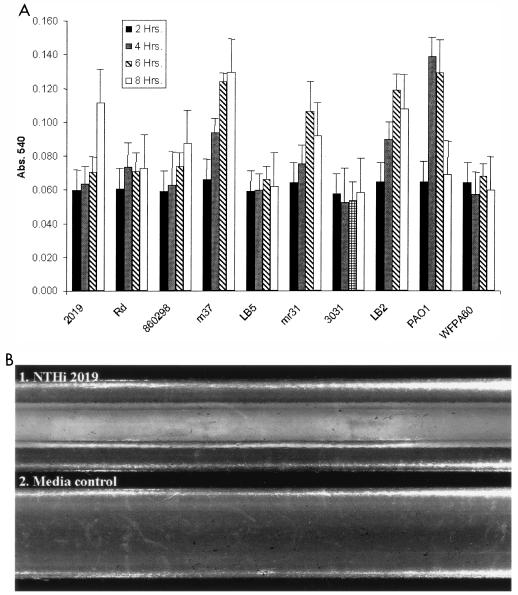
Because previous work showed that H. influenzae strains vary in levels of biofilm initiation (36), we used a polystyrene microtiter dish pellicle formation assay (38-40) to screen a set of H. influenzae strains (Fig. 1A). In addition to uninoculated wells, controls included Pseudomonas aeruginosa PAO1 and an isogenic mutant P. aeruginosa strain (wfpA60) that has a biofilm defect (K. Jackson and D. Wozniak, unpublished data). The results obtained showed that three strains (NTHi 2019, NTHi M37, and NTHi LB2) exhibited significantly greater biofilm initiation than other H. influenzae strains tested (P < 0.05). Because our mutations were in the NTHi 2019 background, we performed further analysis of biofilm formation for this strain using a continuous-flow silicon tubing assay (50, 68). After 48 h, flocculent bacterial communities were observed on the walls of the silicon tubing (Fig. 1B).
FIG. 1.
(A) Biofilm initiation by H. influenzae strains. Comparable numbers of bacteria were inoculated into microtiter dishes, washed, and stained with crystal violet. The density of bacteria remaining within the wells was estimated on the basis of the absorbance of ethanol-solubilized crystal violet at 540 nm (Abs. 540) (see Materials and Methods). P. aeruginosa PAO1 and wfpa60 were included as positive and negative controls, respectively. (B) Biofilm formation by NTHi 2019 in a continuous-flow silicon tubing system. Sterile silicon tubing was seeded with NTHi 2019 (see Materials and Methods) and incubated at 37°C with a continuous flow of supplemented BHI medium for 48 h.
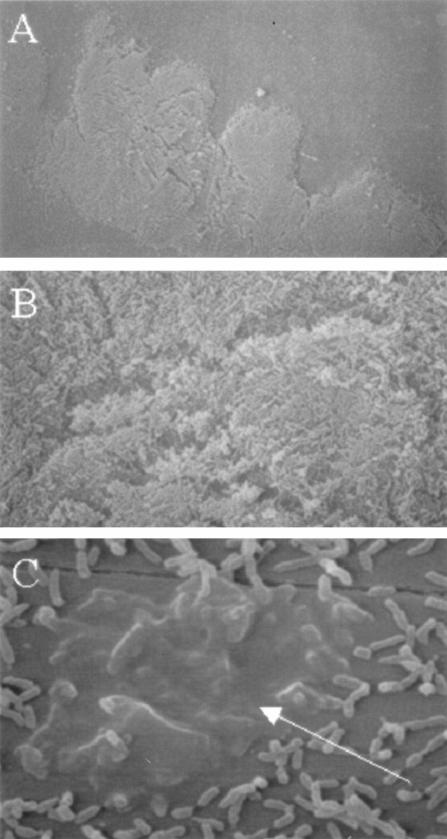
Bacterial communities that had formed at the air-liquid interface of biphasic cultures in polystyrene chamber slides were examined by using SEM (Fig. 2). NTHi 2019 formed dense bacterial communities (Fig. 2A and B) that included bacteria encased in a dense matrix material (Fig. 2C). Similar results were obtained with NTHi M37 and NTHi LB2 (data not shown).
FIG. 2.
SEM analysis of NTHi 2019 biofilms on plastic. Bacteria were grown in biphasic cultures in supplemented BHI (see Materials and Methods). (A) Low-magnification image of bacteria at air-liquid interface; (B) high-magnification image of bacteria, showing multilayered community; (C) bacteria encased in matrix material (denoted by arrow).
Asialylated mutants of NTHi 2019 have a biofilm defect.
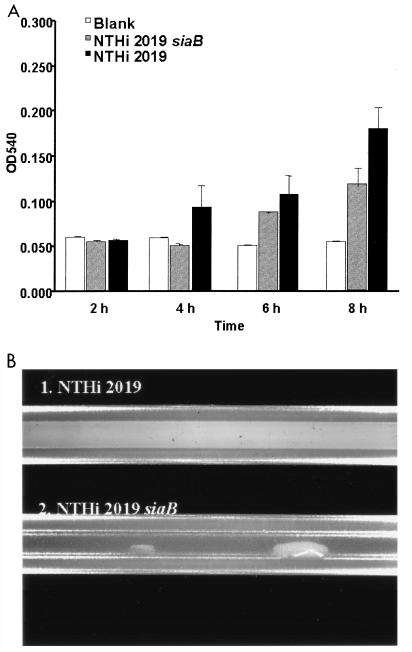
Because sialylation is a conserved phenotype among NTHi strains that is associated with carriage in vivo, we hypothesized that the addition of NeuAc to LOS promotes biofilm formation. Therefore, the biofilm formation of an NTHi 2019 siaB (CMP-NeuAc synthetase) mutant that produces exclusively asialylated LOS (19, 22) was compared with that of the parental strain by using the microtiter and continuous-flow model systems (Table 2 and Fig. 3). It should be noted that the siaB mutant has no apparent growth defect in vitro (data not shown). Biofilm formation was diminished for the siaB mutant in the microtiter assay (Fig. 3A). A more dramatic defect was observed in the continuous-flow system, in which the NTHi 2019 siaB mutant colonized significantly less than the parental strain (P < 0.001) (Fig. 3B and Table 2). These results show that sialylation contributes to biofilm initiation and the formation of biofilms in a continuous-flow model system.
TABLE 2.
Quantitation of bacteria recovered from a silicon tube flow biofilm growth assaysa
| Strain | Growth medium | Bacteria recovered (log10 no. of CFU) |
|---|---|---|
| NTHi 2019 | sBHI | 11.73 ± 2.04 |
| NTHi 2019 siaB | sBHI | 5.96 ± 1.34 |
| NTHi 2019 | CDM | 6.34 ± 1.89 |
| NTHi 2019 | CDM + NeuAc | 8.49 ± 3.46 |
Bacteria were cultured in supplemented BHI (sBHI) or a chemically defined minimal medium (CDM) (26) for 24 h. NeuAc (20 μg/ml) was added as indicated. H. influenzae was inoculated into sterile silicon tubing, through which a continuous flow of growth medium was passed (50). After 24 h, the tubing was excised and the numbers of CFU were obtained (see Materials and Methods).
FIG. 3.
(A) Comparison of levels of biofilm formation by NTHi 2019 and an isogenic siaB mutant. Bacteria were seeded into microtiter dishes, and biofilm formation was assessed at the times indicated by crystal violet staining (see Fig. 1A and Materials and Methods). OD540, optical density at 540 nm. (B) Comparison of levels of biofilm formation by NTHi 2019 and an isogenic siaB mutant in a continuous-flow silicon tube model system. A continuous flow of supplemented BHI medium was maintained, and the tubes were photographed 48 h postinoculation.
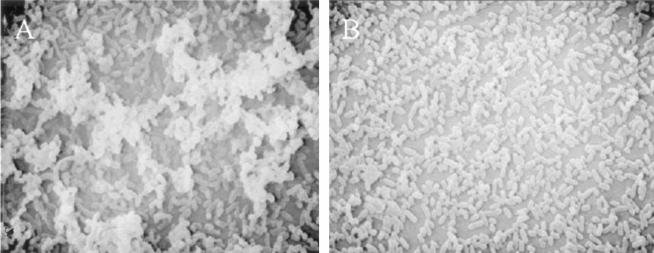
We also compared biofilm communities that had formed at an air-liquid interface in biphasic cultures of NTHi 2019 and the siaB mutant by using SEM. Although NTHi 2019 formed a multilayered bacterial community similar to that depicted in Fig. 2 (Fig. 4A), the siaB mutant formed a markedly less dense community (Fig. 4B). These results show that sialylation contributes to the formation of biofilm communities in biphasic cultures.
FIG. 4.
Role of sialylation in biofilm formation in biphasic cultures. NTHi 2019 (A) and an isogenic siaB mutant (B) were cultured in supplemented BHI (see Materials and Methods), fixed, and processed for SEM analysis. The panels show dense bacterial communities at the air-liquid interface in each culture after 24 h and are representative of multiple fields of view.
H. influenzae acquires sialic acid from environmental sources, and thus growth in the absence of sialic acid results in asialylated bacteria. We therefore compared levels of biofilm formation by NTHi 2019 in the continuous-flow model system using a defined medium with and without added NeuAc. Significantly higher bacterial counts were obtained in the presence of NeuAc than in the absence of NeuAc (Table 2). These results clearly show that NeuAc is required for the formation of biofilms by NTHi 2019.
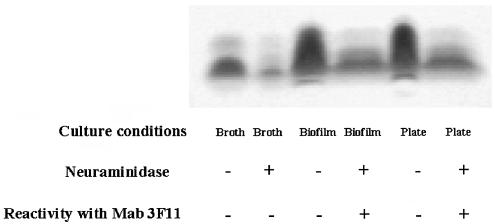
LOS from H. influenzae biofilm are sialylated.
The presence of NeuAc on LOS can be assessed on the basis of changes in electrophoretic mobility or the exposure of lactosamine following neuraminidase digestion (29). Therefore, we compared LOS from NTHi 2019 cultured under planktonic and continuous-flow biofilm conditions in silicon tubing. The electrophoretic mobility of LOS from both strains cultured in biofilms in silicon tubing and the binding of MAb 3F11, which recognizes asialylated lactosamine, were increased following neuraminidase treatment (Fig. 5). Although the interpretation of these data are complicated by the diminished synthesis of LOS glycoforms with terminal lactosamine in broth culture (22, 65), the results obtained clearly show that LOS from NTHi bacteria growing in a biofilm are sialylated. These findings are consistent with those from other recent work showing that NTHi populations become sialylated in vivo (5).
FIG. 5.
Silver stain of NTHi 2019 LOS from planktonic, biofilm, and plate cultures. LOS was prepared from broth, plate, and continuous-flow tube biofilm cultures and examined by Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis and silver staining (see Materials and Methods). Reactivity with MAb 3F11 is an index of asialylated terminal lactosamine and was determined by dot blot analysis.
Persistence of asialylated NTHi mutants in vivo.
Because NeuAc promotes NTHi biofilm formation in vitro, we hypothesized that NTHi mutants lacking NeuAc would be less persistent in vivo than the parental strain. Therefore, we tested this hypothesis by comparing the persistence of NTHi 2019 and that of an isogenic siaB mutant in the Mongolian gerbil otitis model and a rat pulmonary challenge model system. In the otitis model, the siaB mutant was less able to colonize and persist in vivo than the parental strain (P < 0.001) (Table 3). A less dramatic, but statistically significant, defect in colonization and persistence was observed in the rat pulmonary infection model (P < 0.05) (Table 4).
TABLE 3.
Role of NeuAc in the persistence of NTHi 2019 in the gerbil middle eara
| Strain | Inoculum (log10 no. of CFU) | Log10 no. of CFUb recovered on:
|
|
|---|---|---|---|
| Day 1 | Day 3 | ||
| NTHi 2019 | 7.4 | 7.0 ± 0.29 | 6.9 ± 0.21 |
| NTHi 2019 siaB | 7.5 | 1.2 ± 0.22 | 0.17 ± 0.17 |
Groups of Mongolian gerbils were infected with NTHi 2019 and an isogenic siaB mutant by injection through the tympanic membrane (described in Materials and Methods), and bacterial counts for middle ear lavage fluid were determined at days 1 and 3 postinfection. The limit of detection was 10 CFU.
Mean ± standard error of the mean for 10 animals.
TABLE 4.
Role of NeuAc in the persistence of NTHi 2019 in rat lungsa
| Strain | Inoculum (log10 no. of CFU) | Log10 no. of CFUb recovered at indicated time postinfection
|
|||
|---|---|---|---|---|---|
| 5 h | Day 1 | Day 2 | Day 3 | ||
| NTHi 2019 | 8.12 | 8.3 ± 0.06 | 7.02 ± 0.17 | 6.26 ± 0.32 | 5.67 ± 0.24 |
| NTHi 2019 siaB | 8.90 | 8.32 ± 0.02 | 6.14 ± 0.31 | 4.88 ± 0.14 | 4.62 ± 0.56 |
Groups of male Sprague-Dawley rats were infected intratracheally with NTHi 2019 and an isogenic siaB mutant. On days 1, 2, and 3 postinfection, the rats were euthanized and bacterial counts in the lung were determined. The limit of detection was 50 CFU per lung pair.
Mean ± standard error of the mean for 5 (at 5 h) or 10 (on days 1, 2, and 3) animals.
Although our data showing a defect in biofilm formation in vitro for asialylated NTHi bacteria support the conclusion that a biofilm defect is at least partially responsible for the colonization defect of the siaB mutant in both in vivo models, it is possible that the absence of sialylation diminishes the resistance of NTHi to host defenses. This alternative hypothesis is supported by prior work showing a serum-susceptible phenotype for asialylated NTHi bacteria (19). Therefore, it was important to test whether the siaB mutant was more susceptible to innate killing factors present in the respiratory tract. In additional experiments, we observed no appreciable difference in the survival of the NTHi 2019 siaB mutant from that of the NTHi 2019 siaB mutant in rat lung homogenates (data not shown). Therefore, we conclude that the addition of NeuAc enhances the persistence of NTHi in vivo at least in part by promoting biofilm formation.
DISCUSSION
Biofilms are generally defined as a community of bacteria adhering to a surface and are often encased within a polysaccharide matrix (7). Biofilm formation is a multistage process, initiated by surface attachment of individual bacteria and subsequent formation of microcolonies that develop into mature biofilm communities. The aim of this study was to define the contribution of sialylated LOS glycoforms to biofilm formation in vitro and to the persistence of NTHi in vivo.
Many H. influenzae strains, and in particular most NTHi strains, produce sialylated LOS (4, 19, 29). For most bacteria, surface NeuAc provides protection from host innate defenses by mimicry of host glycoproteins found on cell surfaces and in mucus (28, 33, 64), and previous data showed that a loss of NeuAc renders H. influenzae more susceptible to killing in human serum (19). Sialylation may thus afford resistance to killing by complementation or other innate defenses. However, it has also been shown that NeuAc has no effect on the resistance of NTHi to killing by the human beta-defensins HBD-1, HBD-2, and HBD-3 (54), which are major components of the innate defenses of the respiratory tract (53). The results of the present study clearly show that the addition of NeuAc to LOS promotes biofilm formation and suggest that this process is important for persistence in vivo. These findings are consistent with those from recent work showing that NTHi sialylates its LOS in vivo and that asialylated mutants are attenuated in a chinchilla otitis model system (5).
Host cell sialoglycoproteins and mucus serve as receptors for H. influenzae adherence (9, 32, 48, 55), and it is therefore possible that bacterial sialylation promotes bacterial aggregation in the formation of microcolonies. The data clearly show that the addition of NeuAc to LOS promotes biofilm formation and suggest that this process is important in the persistent colonization that is a hallmark of NTHi disease. Because H. influenzae produces multiple structurally distinct sialylated LOS glycoforms, our work does not address whether the addition of NeuAc in general or that of discrete sialylated glycoforms promotes biofilm formation. The recent work of another group suggests that a subpopulation of sialylated LOS glycoforms may contribute to biofilm formation (M. Apicella, personal communication).
Opportunistic infections caused by NTHi are typically associated with preceding defects that compromise innate host defenses and allow colonization by other commensals and opportunists. Included among these opportunistic infections are coinfections with other bacteria or viruses. Many respiratory bacteria and viruses produce neuraminidases that strip NeuAc from host cells, and recent work showed that pneumococcal neuraminidase desialylates H. influenzae and meningococcal LOS (52). Certainly, our data support the hypothesis that desialylation impacts how H. influenzae colonizes the host. It may be that that neuraminidases diminish biofilm formation and maturation, thus altering routes of host colonization by NTHi. It is now well established that some LOS glycoforms mediate the adherence of H. influenzae to host cells (58, 61). Prior work suggested that lactosamine-LOS forms (presumably asialyated) contribute to the adherence of H. influenzae to host cells (58). Therefore, it is possible that the desialylation of lactosamine-LOS changes how NTHi colonizes airway epithelial cells. Alternatively, desialylation may be a means for other pathogens to outcompete H. influenzae on a mucosal surface by disrupting mature biofilms.
The physiologic impact of bacteria in biofilms is complex. A hallmark of bacterial biofilms in vivo is resistance to clearance by host innate defenses and antimicrobials. Because of the prevalence of NeuAc on the surfaces of clinical isolates of NTHi and other mucosal bacteria (4, 19, 29), the efficacies of antimicrobials on persistent NTHi bacteria in the airways may be diminished by sialylation. Our data show that sialylated LOS contributes to the persistence of NTHi in the middle ear and in the lung. Because NTHi is a major cause of both respiratory infections (42, 51) and otitis media (25), these data raise important questions regarding how NTHi infections might eventually be prevented or treated.
Acknowledgments
We thank Daniel Wozniak and Kara Jackson for help with the biofilm assays and Steven Richardson and Steve Mizel for reviews of the manuscript and helpful discussions. We also thank Michael Apicella (University of Iowa) for sharing comparable data prior to their publication. Dee Shortridge, Angela Nilius, Carol Olson (all from Abbott Laboratories), and Bruce Rubin (Department of Pediatrics, WFUHS) provided helpful discussions. Ken Grant, Bilinda Dawson, and Paula Moore of the MicroMed core facility (Department of Pathology, WFUHS) provided expert assistance with electron microscopy, and Darryll Williams (WFUHS Summer Student Research Opportunities Program) provided technical assistance with the SEM work.
This work was supported by institutional funds from WFUHS, a study contract from Abbott Laboratories, and a grant from NIH/NIAID (AI50108 to W.E.S.).
Editor: J. N. Weiser
REFERENCES
- 1.Alder, J. D., P. J. Ewing, A. M. Nilius, M. Mitten, A. Tovcimak, A. Oleksijew, K. Jarvis, L. Paige, and S. K. T. Tanaka. 1998. Dynamics of clarithromycin and azithromycin efficacies against experimental Haemophilus influenzae pulmonary infection. Antimicrob. Agents Chemother. 42:2385-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bakaletz, L. O., T. M. Hoepf, T. F. DeMaria, and D. J. Lim. 1988. The effect of antecedent influenza A virus infection on the adherence of Hemophilus influenzae to chinchilla tracheal epithelium. Am. J. Otolaryngol. 9:127-134. [DOI] [PubMed] [Google Scholar]
- 3.Bakaletz, L. O., B.-J. Kennedy, L. A. Novotny, G. Duquesne, J. Cohen, and Y. Lobet. 1999. Protection against development of otitis media induced by nontypeable Haemophilus influenzae by both active and passive immunization in a chinchilla model of virus-bacterium superinfection. Infect. Immun. 67:2746-2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bauer, S. H., M. Mansson, D. W. Hood, J. C. Richards, E. R. Moxon, and E. K. Schweda. 2001. A rapid and sensitive procedure for determination of 5-N-acetyl neuraminic acid in lipopolysaccharides of Haemophilus influenzae: a survey of 24 non-typeable H. influenzae strains. Carbohydr. Res. 335:251-260. [DOI] [PubMed] [Google Scholar]
- 5.Bouchet, V., D. W. Hood, J. Li, J. R. Brisson, G. A. Randle, A. Martin, Z. Li, R. Goldstein, E. K. Schweda, S. I. Pelton, J. C. Richards, and E. R. Moxon. 2003. Host-derived sialic acid is incorporated into Haemophilus influenzae lipopolysaccharide and is a major virulence factor in experimental otitis media. Proc. Natl. Acad. Sci. USA 100:8898-8903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campagnari, A. A., M. R. Gupta, K. C. Dudas, T. F. Murphy, and M. A. Apicella. 1987. Antigenic diversity of lipooligosaccharides of nontypable [sic] Haemophilus influenzae. Infect. Immun. 55:882-887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 8.Cox, A. D., D. W. Hood, A. Martin, K. M. Makepeace, M. E. Deadman, J. Li, J. R. Brisson, E. R. Moxon, and J. C. Richards. 2002. Identification and structural characterization of a sialylated lacto-N-neotetraose structure in the lipopolysaccharide of Haemophilus influenzae. Eur. J. Biochem. 269:4009-4019. [DOI] [PubMed] [Google Scholar]
- 9.Davies, J., I. Carlstedt, A.-K. Nilsson, A. Håkansson, H. Sabharwal, L. van Alphen, M. Van Ham, and C. Svanborg. 1995. Binding of Haemophilus influenzae to purified mucins from the human respiratory tract. Infect. Immun. 63:2485-2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dingman, J. R., M. G. Rayner, S. Mishra, Y. Zhang, M. D. Ehrlich, J. C. Post, and G. D. Ehrlich. 1998. Correlation between presence of viable bacteria and presence of endotoxin in middle-ear effusions. J. Clin. Microbiol. 36:3417-3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ehrlich, G. D., R. Veeh, X. Wang, J. W. Costerton, J. D. Hayes, F. Z. Hu, B. J. Daigle, M. D. Ehrlich, and J. C. Post. 2002. Mucosal biofilm formation on middle-ear mucosa in the chinchilla model of otitis media. JAMA 287:1710-1715. [DOI] [PubMed] [Google Scholar]
- 12.Fink, D. L., A. Z. Buscher, B. Green, P. Fernsten, and J. W. St. Geme III. 2003. The Haemophilus influenzae Hap autotransporter mediates microcolony formation and adherence to epithelial cells and extracellular matrix via binding regions in the C-terminal end of the passenger domain. Cell. Microbiol. 5:175-186. [DOI] [PubMed] [Google Scholar]
- 13.Fink, D. L., B. A. Green, and J. W. St. Geme III. 2002. The Haemophilus influenzae Hap autotransporter binds to fibronectin, laminin, and collagen IV. Infect. Immun. 70:4902-4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fleischmann, R. D., M. D. Adams, O. White, R. A. Clayton, E. F. Kirkness, A. R. Kerlavage, et al. 1995. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science 269:496-512. [DOI] [PubMed] [Google Scholar]
- 15.Fulghum, R. S., J. E. Brinn, A. M. Smith, H. J. Daniel III, and P. J. Loesche. 1982. Experimental otitis media in gerbils and chinchillas with Streptococcus pneumoniae, Haemophilus influenzae, and other aerobic and anaerobic bacteria. Infect. Immun. 36:802-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hendrixson, D. R., and J. W. St. Geme III. 1998. The Haemophilus influenzae Hap serine protease promotes adherence and microcolony formation, potentiated by a soluble host protein. Mol. Cell 2:841-850. [DOI] [PubMed] [Google Scholar]
- 17.Hitchcock, P. J., and T. M. Brown. 1983. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J. Bacteriol. 154:269-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hood, D. W., A. D. Cox, M. Gilbert, K. Makepeace, S. Walsh, M. E. Deadman, A. Cody, A. Martin, M. Mansson, E. K. Schweda, J. R. Brisson, J. C. Richards, E. R. Moxon, and W. W. Wakarchuk. 2001. Identification of a lipopolysaccharide alpha-2,3-sialyltransferase from Haemophilus influenzae. Mol. Microbiol. 39:341-350. [DOI] [PubMed] [Google Scholar]
- 19.Hood, D. W., K. Makepeace, M. E. Deadman, R. F. Rest, P. Thibault, A. Martin, J. C. Richards, and E. R. Moxon. 1999. Sialic acid in the lipopolysaccharide of Haemophilus influenzae: strain distribution, influence on serum resistance and structural characterization. Mol. Microbiol. 33:679-692. [DOI] [PubMed] [Google Scholar]
- 20.Inzana, T. J. 1983. Electrophoretic heterogeneity and interstrain variation of the lipopolysaccharide of Haemophilus influenzae. J. Infect. Dis. 148:492-499. [DOI] [PubMed] [Google Scholar]
- 21.Jiang, Z., N. Nagata, E. Molina, L. O. Bakaletz, H. Hawkins, and J. A. Patel. 1999. Fimbria-mediated enhanced attachment of nontypeable Haemophilus influenzae to respiratory syncytial virus-infected respiratory epithelial cells. Infect. Immun. 67:187-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones, P. A., N. A. Samuels, N. J. Phillips, R. S. Munson, J. A. Bozue, J. A. Arseneau, W. A. Nichols, A. Zaleski, B. W. Gibson, and M. A. Apicella. 2002. Haemophilus influenzae type B strain A2 has multiple sialyltransferases involved in lipooligosaccharide sialylation. J. Biol. Chem. 277:14598-14611. [DOI] [PubMed] [Google Scholar]
- 23.Kalcioglu, M. T., B. Durmaz, E. Aktas, O. Ozturan, and R. Durmaz. 2003. Bacteriology of chronic maxillary sinusitis and normal maxillary sinuses: using culture and multiplex polymerase chain reaction. Am. J. Rhinol. 17:143-147. [PubMed] [Google Scholar]
- 24.Kennedy, B. J., L. A. Novotny, J. A. Jurcisek, Y. Lobet, and L. O. Bakaletz. 2000. Passive transfer of antiserum specific for immunogens derived from a nontypeable Haemophilus influenzae adhesin and lipoprotein D prevents otitis media after heterologous challenge. Infect. Immun. 68:2756-2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kilpi, T., E. Herva, T. Kaijalainen, R. Syrjanen, and A. K. Takala. 2001. Bacteriology of acute otitis media in a cohort of Finnish children followed for the first two years of life. Pediatr. Infect. Dis. J. 20:654-662. [DOI] [PubMed] [Google Scholar]
- 26.Klein, R. D., and G. H. Luginbuhl. 1979. Simplified media for the growth of Haemophilus influenzae from clinical and normal flora sources. J. Gen. Microbiol. 113:409-411. [DOI] [PubMed] [Google Scholar]
- 27.Lesse, A. J., A. A. Campagnari, W. E. Bittner, and M. A. Apicella. 1990. Increased resolution of lipopolysaccharides and lipooligosaccharides utilizing tricine sodium dodecyl sulfate polyacrylamide gel electrophoresis. J. Immunol. Methods 126:109-117. [DOI] [PubMed] [Google Scholar]
- 28.Mandrell, R. E., and M. A. Apicella. 1993. Lipo-oligosaccharides (LOS) of mucosal pathogens: molecular mimicry and host-modification of LOS. Immunobiology 187:382-402. [DOI] [PubMed] [Google Scholar]
- 29.Mandrell, R. E., R. McLaughlin, Y. A. Kwaik, A. Lesse, R. Yamasaki, B. Gibson, S. A. Spinola, and M. A. Apicella. 1992. Lipooligosaccharides (LOS) of some Haemophilus species mimic human glycosphingolipids, and some LOS are sialyated. Infect. Immun. 60:1322-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mason, K. M., R. S. Munson, Jr., and L. O. Bakaletz. 2003. Nontypeable Haemophilus influenzae gene expression induced in vivo in a chinchilla model of otitis media. Infect. Immun. 71:3454-3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mitten, M. J., J. Meulbroek, M. Nukkala, L. Paige, K. Jarvis, A. Oleksijew, A. Tovcimak, L. Hernandez, J. D. Alder, P. Ewing, Y. S. Or, Z. Ma, A. M. Nilius, K. Mollison, and R. K. Flamm. 2001. Efficacies of ABT-773, a new ketolide, against experimental bacterial infections. Antimicrob. Agents Chemother. 45:2585-2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miyamoto, N., and L. O. Bakaletz. 1996. Selective adherence of non-typeable Haemophilus influenzae (NTHi) to mucus or epithelial cells in the chinchilla eustachian tube and middle ear. Microb. Pathog. 21:343-356. [DOI] [PubMed] [Google Scholar]
- 33.Moran, A. P., M. M. Prendergast, and B. J. Appelmelk. 1996. Molecular mimicry of host structures by bacterial lipopolysaccharides and its contribution to disease. FEMS Immunol. Med. Microbiol. 16:105-115. [DOI] [PubMed] [Google Scholar]
- 34.Murphy, T. F. 2000. Haemophilus influenzae in chronic bronchitis. Semin. Respir. Infect. 15:41-51. [DOI] [PubMed] [Google Scholar]
- 35.Murphy, T. F., and M. A. Apicella. 1987. Nontypable Haemophilus influenzae: a review of clinical aspects, surface antigens and the human response to infection. Rev. Infect. Dis. 9:1-15. [DOI] [PubMed] [Google Scholar]
- 36.Murphy, T. F., and C. Kirkham. 2002. Biofilm formation by nontypeable Haemophilus influenzae: strain variability, outer membrane antigen expression and role of pili. BMC Microbiol. 2:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O'Neill, J. M., J. W. St. Geme III, D. Cutter, E. E. Adderson, J. Anyanwu, R. F. Jacobs, and G. E. Schutze. 2003. Invasive disease due to nontypeable Haemophilus influenzae among children in Arkansas. J. Clin. Microbiol. 41:3064-3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O'Toole, G. A., and R. Kolter. 1998. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol. Microbiol. 30:295-304. [DOI] [PubMed] [Google Scholar]
- 39.O'Toole, G. A., and R. Kolter. 1998. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: a genetic analysis. Mol. Microbiol. 28:449-461. [DOI] [PubMed] [Google Scholar]
- 40.O'Toole, G. A., L. A. Pratt, P. I. Watnick, D. K. Newman, V. B. Weaver, and R. Kolter. 1999. Genetic approaches to study of biofilms. Methods Enzymol. 310:91-109. [DOI] [PubMed] [Google Scholar]
- 41.Pettigrew, M. M., B. Foxman, C. F. Marrs, and J. R. Gilsdorf. 2002. Identification of the lipooligosaccharide biosynthesis gene lic2B as a putative virulence factor in strains of nontypeable Haemophilus influenzae that cause otitis media. Infect. Immun. 70:3551-3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pfaller, M. A., A. F. Ehrhardt, and R. N. Jones. 2001. Frequency of pathogen occurrence and antimicrobial susceptibility among community-acquired respiratory tract infections in the respiratory surveillance program study: microbiology from the medical office practice environment. Am. J. Med. 111:4S-12S. [DOI] [PubMed] [Google Scholar]
- 43.Phillips, N. J., M. A. Apicella, J. M. Griffiss, and B. W. Gibson. 1993. Structural studies of the lipooligosaccharides from Haemophilus influenzae type b strain A2. Biochemistry 32:2003-2012. [DOI] [PubMed] [Google Scholar]
- 44.Phillips, N. J., R. McLaughlin, T. J. Miller, M. A. Apicella, and B. W. Gibson. 1996. Characterization of two transposon mutants from Haemophilus influenzae type b with altered lipooligosaccharide biosynthesis. Biochemistry 35:5937-5947. [DOI] [PubMed] [Google Scholar]
- 45.Post, J. C. 2001. Direct evidence of bacterial biofilms in otitis media. Laryngoscope 111:2083-2094. [DOI] [PubMed] [Google Scholar]
- 46.Preston, A., R. E. Mandrell, B. W. Gibson, and M. A. Apicella. 1996. The lipooligosaccharides of pathogenic gram-negative bacteria. Crit. Rev. Microbiol. 22:139-180. [DOI] [PubMed] [Google Scholar]
- 47.Rayner, M. G., Y. Zhang, M. C. Gorry, Y. Chen, J. C. Post, and G. D. Ehrlich. 1998. Evidence of bacterial metabolic activity in culture-negative otitis media with effusion. JAMA 279:296-299. [DOI] [PubMed] [Google Scholar]
- 48.Reddy, M. S., J. M. Bernstein, T. F. Murphy, and H. S. Faden. 1996. Binding between outer membrane proteins of nontypeable Haemophilus influenzae and human nasopharyngeal mucin. Infect. Immun. 64:1477-1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rodriguez, C. A., V. Avadhanula, A. Buscher, A. L. Smith, J. W. St. Geme III, and E. E. Adderson. 2003. Prevalence and distribution of adhesins in invasive non-type b encapsulated Haemophilus influenzae. Infect. Immun. 71:1635-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schaefer, A. L., E. P. Greenberg, and M. R. Parsek. 2001. Acylated homoserine lactone detection in Pseudomonas aeruginosa biofilms by radiolabel assay. Methods Enzymol. 336:41-47. [DOI] [PubMed] [Google Scholar]
- 51.Sethi, S., and T. F. Murphy. 2001. Bacterial infection in chronic obstructive pulmonary disease in 2000: a state-of-the-art review. Clin. Microbiol. Rev. 14:336-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shakhnovich, E. A., S. J. King, and J. N. Weiser. 2002. Neuraminidase expressed by Streptococcus pneumoniae desialylates the lipopolysaccharide of Neisseria meningitidis and Haemophilus influenzae: a paradigm for interbacterial competition among pathogens of the human respiratory tract. Infect. Immun. 70:7161-7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Singh, P. K., H. P. Jia, K. Wiles, J. Hesselberth, L. Liu, B. A. Conway, E. P. Greenberg, E. V. Valore, M. J. Welsh, T. Ganz, B. F. Tack, and P. B. J. McCray. 1998. Production of beta-defensins by human airway epithelia. Proc. Natl. Acad. Sci. USA 95:14961-14966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Starner, T. D., W. E. Swords, M. A. Apicella, and P. B. McCray, Jr. 2002. Susceptibility of nontypeable Haemophilus influenzae to human β-defensins is influenced by lipooligosaccharide acylation. Infect. Immun. 70:5287-5289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.St. Geme, J. W., III. 1994. The HMW1 adhesin of nontypeable Haemophilus influenzae recognizes sialylated glycoprotein receptors on cultured human epithelial cells. Infect. Immun. 62:3881-3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.St. Geme, J. W., III. 2002. Molecular and cellular determinants of non-typeable Haemophilus influenzae adherence and invasion. Cell. Microbiol. 4:191-200. [DOI] [PubMed] [Google Scholar]
- 57.Suzuki, K., and L. O. Bakaletz. 1994. Synergistic effect of adenovirus type 1 and nontypeable Haemophilus influenzae in a chinchilla model of experimental otitis media. Infect. Immun. 62:1710-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Swords, W. E., B. Buscher, K. Ver Steeg, W. Nichols, A. Preston, J. N. Weiser, B. Gibson, and M. A. Apicella. 2000. Nontypeable Haemophilus influenzae adhere to and invade human bronchial epithelial cells by an interaction of lipooligosaccharide with the PAF receptor. Mol. Microbiol. 37:13-27. [DOI] [PubMed] [Google Scholar]
- 59.Swords, W. E., D. L. Chance, L. A. Cohn, J. Shao, M. A. Apicella, and A. L. Smith. 2002. Acylation of the lipooligosaccharide of Haemophilus influenzae and colonization: an htrB mutation diminishes the colonization of human airway epithelial cells. Infect. Immun. 70:4661-4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Swords, W. E., P. A. Jones, and M. A. Apicella. 2003. The lipooligosaccharides of Haemophilus influenzae: an interesting assortment of characters. J. Endotoxin Res. 9:131-144. [DOI] [PubMed] [Google Scholar]
- 61.Swords, W. E., M. R. Ketterer, J. Shao, C. A. Campbell, J. N. Weiser, and M. A. Apicella. 2001. Binding of the nontypeable Haemophilus influenzae lipooligosaccharide to the PAF receptor initiates host cell signaling. Cell. Microbiol. 8:525-536. [DOI] [PubMed] [Google Scholar]
- 62.Tong, H. H., L. E. Blue, M. A. James, Y. P. Chen, and T. F. DeMaria. 2000. Evaluation of phase variation of nontypeable Haemophilus influenzae lipooligosaccharide during nasopharyngeal colonization and development of otitis media in the chinchilla model. Infect. Immun. 68:4593-4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tsai, C.-M., and C. E. Frasch. 1982. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal. Biochem. 119:115-119. [DOI] [PubMed] [Google Scholar]
- 64.Vimr, E., and C. Lichtensteiger. 2002. To sialylate, or not to sialylate: that is the question. Trends Microbiol. 10:254-257. [DOI] [PubMed] [Google Scholar]
- 65.Vimr, E., C. Lichtensteiger, and S. Steenbergen. 2000. Sialic acid metabolism's dual function in Haemophilus influenzae. Mol. Microbiol. 36:1113-1123. [DOI] [PubMed] [Google Scholar]
- 66.Weiser, J. N., and N. Pan. 1998. Adaptation of Haemophilus influenzae to acquired and innate humoral immunity based on phase variation of lipopolysaccharide. Mol. Microbiol. 30:767-775. [DOI] [PubMed] [Google Scholar]
- 67.Weiser, J. N., N. Pan, K. L. McGowan, D. Musher, A. Martin, and J. Richards. 1998. Phosphorylcholine on the lipopolysaccharide of Haemophilus influenzae contributes to persistence in the respiratory tract and sensitivity to serum killing mediated by C-reactive protein. J. Exp. Med. 187:631-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wozniak, D. J., T. J. Wyckoff, M. Starkey, R. Keyser, P. Azadi, G. A. O'Toole, and M. R. Parsek. 2003. Alginate is not a significant component of the extracellular polysaccharide matrix of PA14 and PAO1 Pseudomonas aeruginosa biofilms. Proc. Natl. Acad. Sci. USA 100:7907-7912. [DOI] [PMC free article] [PubMed] [Google Scholar]