Abstract
In Corte de Pedra (CP), northeastern Brazil, Leishmania braziliensis causes three distinct forms of American tegumentary leishmaniasis (ATL). To test the hypothesis that strain polymorphism may be involved in this disease spectrum and accurately characterize the parasite population structure in CP, we compared one L. major, two non-CP L. braziliensis, one CP L. amazonensis, and 45 CP L. braziliensis isolates, obtained over a 10-year period from localized cutaneous, mucosal, and disseminated leishmaniasis patients, with randomly amplified polymorphic DNA (RAPD). Electrophoretic profiles were mostly unique across species. All typing protocols revealed polymorphism among the 45 CP L. braziliensis isolates, which displayed eight different RAPD patterns and greater than 80% overall fingerprint identity, attesting to the adequacy of the tools to assess strain variability in CP's geographically limited population of parasites. The dendrogram based on the sum of RAPD profiles of each isolate unveiled nine discrete typing units clustered into five clades. Global positioning showed extensive overlap of these clades in CP, precluding geographic sequestration as the mechanism of the observed structuralization. Finally, all forms of ATL presented a statistically significant difference in their frequencies among the clades, suggesting that L. braziliensis genotypes may be accompanied by specific disease manifestation after infection.
Leishmaniases encompass a spectrum of diseases that occur mostly in tropical and subtropical areas of the globe. They pose a major public health problem, with 400 million individuals at risk, plus an annual estimated worldwide incidence of 600,000 and prevalence of 12 million cases (9). Productive infections result in visceral or tegumentary disorders of potentially life-threatening or disfiguring clinical outcomes (1, 19). There is substantial variability among the etiological agents at the subgenus level, with at least 15 species described (30, 31). The strong association between Leishmania spp. and different disease forms suggests a role of the microorganism's specific genetic background on clinical manifestation and possibly prognosis.
For the last 20 years our group has focused attention on the study of American tegumentary leishmaniasis (ATL) in the area of Corte de Pedra (CP) where it is endemic (Fig. 1). Three distinct forms of ATL can be found in this region: localized cutaneous, mucosal, and disseminated leishmaniasis. Cutaneous, mucosal, and disseminated leishmaniasis are all associated with L. braziliensis in CP, differing markedly clinically and immunologically. Cutaneous leishmaniasis is usually limited to a single or a few skin ulcers more commonly found in the upper and lower limbs, being characterized by moderate and well-regulated antileishmanial Th1 immune responses (1). In disseminated leishmaniasis, multiple ulcerated and nonulcerated skin lesions are concurrently found in more than one area of the patient's body, which may be preceded by a brief period of transient low-grade fever. Individuals with disseminated leishmaniasis present with poorer gamma interferon and tumor necrosis factor alpha responses than those with cutaneous leishmaniasis (5, 7, 35). Mucosal leishmaniasis is the most severe complication of ATL, affecting mostly the mouth, nose, and pharyngeal mucosae. It is characterized by an exaggerated immune response, rich in proinflamatory cytokines like tumor necrosis factor alpha and gamma interferon, which may lead to severely disfiguring facial lesions and life-threatening oral, pharyngeal, and laryngeal destruction (2, 18). Up to 4% of cutaneous leishmaniasis and 40% of disseminated leishmaniasis patients develop mucosal leishmaniasis (18, 35). This wealth of leishmaniasis outcomes in a confined area led us to suspect that more subtle intraspecific genetic variation among the Leishmania spp. might be involved in the disease spectrum observed with ATL.
FIG. 1.
The area of Corte de Pedra (CP) where American tegumentary leishmaniasis is endemic is located in the northeastern Brazilian state of Bahia (identified by a dot and shaded municipalities on the South American and Bahian maps, respectively), being delimited by the geographical coordinates (latitude/longitude) 14°/39°, 13°/39°, 14°/40°, and13°/40°.
In order to test this hypothesis, accurate characterization of the population structure of the etiologic agents in the region was necessary. Little is known about the mode of reproduction, evolution, and polymorphism of parasites in areas of active disease transmission. This basic knowledge is of great importance not only for understanding the microorganism's biology, but also for devising more effective preventive and therapeutic interventions. Corte de Pedra's small size, steady human population dynamics, and diverse clinical forms of ATL associated with L. braziliensis make it an excellent study site to address these questions.
MATERIALS AND METHODS
Parasite isolates and genomic DNA extractions.
One L. major, two non-CP L. braziliensis, one CP L. amazonensis, and 45 CP L. braziliensis isolates were used in the study (Table 1). The samples tested were obtained over a 10-year period, between 1992 and 2001, from cutaneous, mucosal, and disseminated leishmaniasis patients by aspiration of lesions at initial consultations, before treatment. Each isolate originated from a different human subject. Parasite samples of disseminated leishmaniasis patients developing mucosal leishmaniasis were not represented in the study. Aspirates were cultivated in LIT/NNN medium at 25°C, transferred to Schneider medium, and incubated until the parasites reached the late logarithmic phase, then stored frozen in dimethyl sulfoxide at −180°C. For genomic DNA extraction, stocks of isolates were thawed, cultivated in Schneider medium for up to 107 cells/ml, and treated as previously described (22). Long-term storage DNA aliquots were kept at −70°C, while test samples were maintained at −20°C.
TABLE 1.
Description of Leishmania isolates used in this study
| Isolatesa | Species | Origin | Clinical form of ATLb |
|---|---|---|---|
| Lm | L. major | Non-CP | |
| La | L. amazonensis | CP | CL |
| Lb 1-45 | L. braziliensis | CP | 21 CL, 13 ML, 11 DL |
| Lb 46 | L. braziliensis | Non-CP | DL |
| Lb 47 | L. braziliensis | Non-CP | ML |
See Fig. 3 legend.
ATL, American tegumentary leishmaniasis; CL, cutaneous leishmaniasis; ML, mucosal leishmaniasis; DL, disseminated leishmaniasis.
ATL-endemic area characterization.
Corte de Pedra comprises 20 municipalities that developed in a rural area formerly dominated by an Atlantic rain forest. L. braziliensis-transmitting Lutzomyia (Nyssomyia) whitmany and L. (Nyssomyia) intermedia are endemic among the local fauna. This biome has not undergone any major changes during the time frame of the study. Residents of CP are mostly engaged in agriculture, often carried out within primary or secondary forests. There is little population migration in or out of this region. The study participants' average time of residence in their living addresses at the time of diagnosis and parasite sampling was 17.5 years.
Parasite genotyping and classification.
Four randomly amplified polymorphic DNA (RAPD) protocols were used for molecular genotyping of parasite isolates, employing three different primers either singly or in combination (Table 2). Reaction conditions were: 20 ng of DNA; 0.2 mM each dATP, dTTP, dGTP, and dCTP; 3.5 mM MgCl2; 4 μM each primer; and 0.5 U of Platinum Taq DNA polymerase plus standard MgCl2-free PCR buffer (Invitrogen Co.). All protocols applied one cycle of 5 min at 94°C, followed by 35 cycles of 30 s at 94°C, 1 min at 36°C, and 2 min at 72°C on a GeneAmp 9600 PCR system (Perkin Elmer Inc.). Amplicons were fractionated by 1.3% agarose gel electrophoreses performed in 0.5× TBE buffer at 120 V for 50 min. After ethidium bromide (0.5 μg/ml) staining, gels were digitally photographed with a UVP Labworks laboratory imaging and analysis system (UVP Inc.). Well-defined and reproducible bands identified in the electrophoretic profiles were explored to score for the presence or absence of characters in each tested Leishmania isolate. We defined as plesiomorphic those bands occurring in all three species and as sinapomorphic those common to all or a subset of individuals of a single species. The resulting character matrix was used to construct a dendrogram based on unweighted pair-group method with arithmetic averages (UPGMA) cluster analysis with PAUP (Sinauer Associates Inc.) and Winclada systematic analysis packages.
TABLE 2.
RAPD primers and protocols employed in this studya
| RAPD | Primer(s) |
|---|---|
| A | CCACAGCAGT |
| B | CCACAGCAGT + GTGACGTAGG |
| C | GGACTGGAGT |
| D | GGACTGGAGT + GTGACGTAGG |
Cycling conditions: one cycle of 5 min at 94°C, followed by 35 cycles of 30 s at 94°C, 1 min at 36°C, and 2 min at 72°C.
Geographic positioning of Leishmania braziliensis isolates.
High-resolution distribution of isolates in the ATL-endemic area was determined by acquisition of geographic coordinates of likely places of disease transmission, with a Brunton Multinavigator global positioning system apparatus (The Brunton Co.) with a 15-m range precision. Since disease is thought to be transmitted mostly within plantations, where residents of the region live and work, patient residences were used as reference points for standardization purposes. Collected data were then plotted onto a high-definition satellite photograph of CP (ENGESAT, PR/Brazil), with the ArcView GIS version 3.0 package (Environmental Systems Research Institute Inc.).
Statistical analysis.
Differences in distribution frequencies of cutaneous leishmaniasis, disseminated leishmaniasis, and mucosal leishmaniasis cases among clades of L. braziliensis in the sample studied (see Fig. 3) were analyzed by chi-square test, employing medians for comparisons, while evaluations of patients' ages and times of residence in their living addresses at the moment of diagnosis and parasite isolation, as well as times elapsed since parasite sampling used the Kruskal-Wallis one-way analysis of variance. In the analyses, clades enriched in cutaneous leishmaniasis (Fig. 3B and E), disseminated leishmaniasis (Fig. 3A and D) and mucosal leishmaniasis (Fig. 3C) were taken as groups 1, 2, and 3, respectively. P < 0.05 was considered significant.
FIG. 3.
UPGMA dendrogram of 49 Leishmania isolates generated according to RAPD A to D data. Labels at the end of branches show L. braziliensis isolates of American tegumentary leishmaniasis cases from Corte de Pedra (Lb 1 to 45), non-Corte de Pedra L. braziliensis isolated from ATL cases (Lb 46 and 47, also labeled NCP), an L. major isolate (Lm), an L. amazonensis isolate (La), and isolates of localized cutaneous, mucosal, and disseminated disease origin (C, M, and D, respectively). Labels at node origins: A to E, L. braziliensis clades A to E, respectively; 1 to 9, L. braziliensis clones 1 to 9, respectively. Highlighted in black is clade C diagnosed by polymorphic character 3 (see text).
RESULTS
The parasite genotypes generated by all RAPD protocols used in this study revealed polymorphism among Leishmania braziliensis isolates from CP (Fig. 2). The majority of amplicons in the RAPD electrophoretic patterns were shared among the 47 L. braziliensis isolates tested. In each typing protocol, polymorphism was usually scored by one or a few bands consistently either present or absent in subsets of the assayed sample. The frequency of individuals presenting a particular polymorphic character ranged from 21.3% to 66% (Fig. 2C and B, respectively). When the results of all typing protocols were combined and data were compared across isolates, an overall identity of greater than 80% was found among all the genomic fingerprints.
FIG. 2.
Collage of RAPD patterns obtained with protocols A to D described in Table 2. The lanes contained molecular size markers (MW), Leishmania braziliensis isolates (Lb), a Leishmania amazonensis isolate (La) and a Leishmania major isolate (Lm). Numbers beneath Lb lanes indicate the number of isolates with the corresponding pattern. L. braziliensis bands used to score for polymorphism in the systematic classification of parasites are indicated by arrowheads.
Electrophoretic profiles for the three distinct Leishmania spp. assayed were mostly unique across species, with only few shared bands (Fig. 2). On the other hand, the 47 L. braziliensis test isolates displayed a total of eight different RAPD patterns, indicating the usefulness of the typing procedures for discriminating this group of parasites at the specific and intraspecific levels. Moreover, the methods showed enough resolving power to reveal strain variability even in geographically limited populations of parasites, such as in CP.
To better evaluate the polymorphism of CP L. braziliensis and characterize its population structure, we combined the electrophoretic profiles obtained with each RAPD and classified the isolates by distance. The UPGMA dendrogram in Fig. 3 further identifies the complexity of this parasite population in CP. Nine distinguishable discrete typing units (Fig. 3, labeled 1 to 9), each comprised of 3 to 11 isolates, considered clones, were found. Mapping single characters in the final dendrogram unveiled a pattern of clone/clade acquisition/loss of polymorphic traits. For instance, polymorphic character 3 (Fig. 2, RAPD C) divides the L. braziliensis sample into marker-positive (Fig. 3, black branch) and marker-negative subpopulations. The observation of identical genotypes among isolates collected up to 6 years apart indicates that the mutations detected in these parasites are stably maintained for long periods of time in the region.
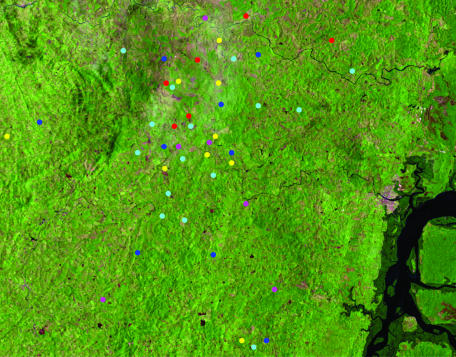
The occurrence of subpopulations of L. braziliensis with closer genetic backgrounds was evidenced by five well-defined clades of discrete typing units (Fig. 3, labeled A to E). Global positioning of tested isolates showed extensive overlap of these clades in CP (Fig. 4), precluding geographic sequestration as the mechanism of the observed structuralization. Clades A and C were more localized to the northeastern and central sectors, respectively, while B, D, and E had a wider distribution over the region.
FIG. 4.
High-resolution mapping of American tegumentary leishmaniasis cases bearing Leishmania braziliensis isolates in Corte de Pedra. Red, dark blue, yellow, pink, and light blue dots correspond to parasites from clades A, B, C, D, and E (depicted in Fig. 3), respectively. For details, refer to the Materials and Methods section.
The distribution of human disease outcomes due to infection with the tested isolates in Fig. 3 suggests a role for the L. braziliensis strain polymorphism in the course of American tegumentary leishmaniasis. Three of the five clades (A, C, and D) were dominated by either mucosal leishmaniasis or disseminated leishmaniasis, which presented twice the frequency of cutaneous leishmaniasis in A and C despite corresponding to only about half the sample input altogether. Also of note was the finding that the two non-CP isolates (Lb46 and Lb47) cosegregated with CP clones (two and five, respectively) originating from patients with the same clinical outcomes. To statistically test for the association between clades of parasites and clinical presentation of disease, we divided the sample into three groups: cutaneous leishmaniasis enriched clades B and E; disseminated leishmaniasis enriched clades A and D; and mucosal leishmaniasis enriched clade C. All forms of ATL showed a statistically significant difference in their distributions among these sets of clades (Table 3), which further suggested that L. braziliensis genotypes may be accompanied by specific disease manifestations after infection.
TABLE 3.
Frequency distribution of cutaneous (CL), mucosal (ML), and disseminated (DL) leishmaniasis cases among three groups of L. braziliensis clades (depicted in Fig. 3) from the study sample
| Clades | No. of casesa
|
|||
|---|---|---|---|---|
| CL | ML | DL | Total | |
| A + D | 5* | 0** | 8*** | 13 |
| B + E | 13* | 7** | 4*** | 24 |
| C | 3* | 7** | 0*** | 10 |
| Total | 21 | 14 | 12 | 47 |
*, P = 0.001; **, P = 0.03; ***, P = 0.02. Frequencies were compared by the nonparametric chi-square test.
On the other hand, the patients' ages (A and D, B and E, and C medians of 19, 20, and 33 years, respectively; P = 0.47) and times of residence at their living addresses at the moment of diagnosis and parasite isolation (A and D, B and E, and C medians of 16, 12, and 18 years, respectively; P = 0.19), as well as times elapsed since parasite sampling (A and D, B and E, and C medians of 1, 1, and 0.5 years, respectively; P = 0.18) were statistically similar among all clades as tested by Kruskal-Wallis one-way analysis of variance.
DISCUSSION
American tegumentary leishmaniasis is a major public health problem in the New World. ATL is often difficult to treat and presents with severe, frequently disfiguring outcomes, and extreme cases may even result in the patient's death. L. braziliensis is one of the parasites responsible for ATL in South America. The wide spectrum of disorders caused by these protozoa suggests an intraspecies variability that affects disease manifestations. Here we explored a well-defined study area where ATL is endemic (Corte de Pedra) to better evaluate the population structure among medically relevant Leishmania spp. and determine if strain differences are associated with different clinical outcomes.
We found a multiclonal population structure among the isolates obtained from clinical cases in CP. This suggests that sexual reproduction is not frequent among L. braziliensis in this region. Proliferation of the organism in the area appears to occur predominantly by binary fission, resulting in clonal expansion of individual strains, as suggested by the inability of geographically overlapping subpopulations to share genetic tags like clade C-specific polymorphic character 3. This observation is supported by recent sequencing data from L. major Friedlin chromosome one, which indicated almost complete identity between the two homologs of a diploid pair (23). This would be unexpected if sexual reproduction in Leishmania spp. were the rule. Still other systematic studies, employing strains from distant geographic origins, have reported strain polymorphism (3, 6, 8, 11, 13, 14, 16, 20, 24-27, 34) and possible clonal proliferation of Leishmania spp. (3, 8, 16, 32, 33), further strengthening our findings. Nonetheless, in vitro evidence of sexual reproduction (17, 36) and field reports describing hybrids (4, 8, 10, 12) do exist, usually of different species, suggesting rare mating events in nature.
Most efforts to link the polymorphism of Leishmania strains with clinical outcome have not been very revealing (11, 24, 27, 34). Wide geographic distribution and multiple sources (e.g., vectors, reservoirs and human immunodeficiency virus-positive and -negative human hosts) of tested isolates may be among the reasons for this lack of associations. However, two Colombian reports by Saravia et al. (25, 26) were very insightful in this respect. In one study, they detected an increased frequency of mucosal involvement among human cases by a particular L. braziliensis zymodeme (25), while in the other (26) similar findings were described for a group of serologically null strains of L. braziliensis and L. panamensis. Furthermore, the disease evolution in those infected with parasites of the zymodeme mentioned was statistically longer (P = 0.002) than that caused by other strains (25).
The multiclonal structure of L. braziliensis in CP greatly simplified our task of evaluating the role of strain polymorphism on the outcomes of ATL. All forms of tegumentary leishmaniasis showed a statistically different distribution among the clades of the dendrogram. The biological significance of the findings is reinforced by the identification of subpopulations markedly enriched in parasites drawn from mucosal leishmaniasis and disseminated leishmaniasis cases. The frequencies of such isolates in these “biased clades” reached 50% to 71% despite their overall input of about half that for cutaneous leishmaniasis in the sample. Also remarkable was the observation of discrete typing units containing only mucosal leishmaniasis or disseminated leishmaniasis clones, including those of non-CP origin. Besides strengthening the evidence for the link between microorganism strain differences and disease outcome, this last finding also suggests an interchange of L. braziliensis genotypes between CP and surrounding areas.
The absence of disseminated leishmaniasis-borne genotypes in clade C and of mucosal leishmaniasis in clades A and D was surprising, considering the high frequency of mucosal involvement in disseminated leishmaniasis. We offer two possible explanations for this observation. First, there was a lack of isolates from individuals with disseminated leishmaniasis developing mucosal leishmaniasis in the sample. Second, diverse pathways triggered by particular L. braziliensis strains exist for mucosal involvement. In fact, the lesions found in the nose of disseminated leishmaniasis patients are different from what has been classically detected for mucosal leishmaniasis. While in the former they are characterized by small nodules, in the latter there is a strong inflammatory reaction associated with deep ulcers. Furthermore, it is of note that peripheral blood mononuclear cells react to Leishmania antigen with a strong proinflammatory bias in mucosal leishmaniasis and closer to a milder Th1 with higher levels of interleukin-10 in disseminated leishmaniasis (2, 5, 7, 18, 35). This marked contrast in systemic immunity certainly reinforces the question of whether mucosal injury may be a common consequence of different pathogenetic mechanisms in ATL.
The presence of cutaneous leishmaniasis isolates in all clades was not unexpected and reflects the natural history of ATL. The most common manifestations of disseminated and mucosal leishmaniases are either immediately or historically preceded by single ulcers typical of localized cutaneous disease (5, 7, 18, 35). Thus, early diagnosis followed by effective therapy may link a parasite genotype commonly found in mucosal leishmaniasis and disseminated leishmaniasis to cutaneous leishmaniasis. Another important cautionary note regards the possibility of mixed infections previously reported in New and Old World leishmaniases (15, 21, 28, 29). In such instances, a dominant strain, possibly due to its greater relative load, immune response evasiveness, or drug resistance, may more strongly influence outcome. However, in vitro selection of less clinically relevant clones during rounds of laboratory cultivation might lead to artifactual selection during the evaluation process. It has been demonstrated that cocultures of Leishmania spp. from natural infections tend to be dominated by a single species (15). In our case, however, we expect the great majority of genotypes found reflect the predominant and medically relevant L. braziliensis strains in CP, especially because only a single or very few rounds of cultivation separated isolation from DNA extraction and also due to the extensive redundancy detected.
Finally, the identification of a subpopulation-specific marker (polymorphic character 3) in our sample was also quite revealing. It suggests the feasibility of future descriptions of parasite genetic tags with prognostic value that could be explored for the clade/clonal detection and treatment of ATL. This would enable early identification of possibly unfavorable outcomes and proper adjustment of therapy.
Acknowledgments
We are deeply indebted to the personnel at the Health Post of Corte de Pedra who made this work feasible, to Elbe Silva, Lúcia Reis, Clenildo Santos, and Jackson Lemos for their secretarial and administrative work, to Kátia Salgado and Ângela Diniz for their kind help with parasite management, and to Jeffrey J. Shaw, who was an insightful source of guidance and incentive at the beginning of this project.
This work was funded by the NIH AI-30639 and FAPESB 195710491113 research grants.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Azulay, R. D., and D. R. Azulay Junior. 1995. Immune-clinical-pathologic spectrum of leishmaniasis. Int. J. Dermatol. 34:303-307. [DOI] [PubMed] [Google Scholar]
- 2.Bacellar, O., H. Lessa, A. Schriefer, P. Machado, A. Ribeiro de Jesus, W. O. Dutra, K. J. Gollob, and E. M. Carvalho. 2002. Up-regulation of Th1-type responses in mucosal leishmaniasis patients. Infect. Immun. 70:6734-6740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banuls, A. L., M. Hide, and M. Tibayrenc. 1999. Molecular epidemiology and evolutionary genetics of Leischmania parasites. Int. J. Parasitol. 29:1137-1147. [DOI] [PubMed] [Google Scholar]
- 4.Belli, A. A., M. A. Miles, and J. M. Kelly. 1994. A putative Leishmania panamensis/Leishmania braziliensis hybrid is a causative agent of human cutaneous leishmaniasis in Nicaragua. Parasitology 109:435-442. [DOI] [PubMed] [Google Scholar]
- 5.Carvalho, E. M., A. Barral, J. M. Costa, A. Bittencourt, and P. Marsden. 1994. Clinical and immunopathological aspects of disseminated cutaneous leishmaniasis. Acta Trop. 56:315-325. [DOI] [PubMed] [Google Scholar]
- 6.Chouicha, N., G. Lanotte, F. Pratlong, C. A. Cuba Cuba, I. D. Velez, and J. P. Dedet. 1997. Phylogenetic taxonomy of Leishmania (Viannia) braziliensis based on isoenzymatic study of 137 isolates. Parasitology 115:343-348. [DOI] [PubMed] [Google Scholar]
- 7.Costa, J. M., P. D. Marsden, E. A. Llanos-Cuentas, E. M. Netto, E. M. Carvalho, A. Barral, A. C. Rosa, C. C. Cuba, A. V. Magalhães, and A. C. Barreto. 1986. Disseminated cutaneous leishmaniasis in a field clinic in Bahia, Brazil: a report of eight cases. J. Trop. Med. Hyg. 89:319-323. [PubMed] [Google Scholar]
- 8.Cupolillo, E., G. Grimaldi Jr., and H. Momen. 1997. Genetic diversity among Leishmania (Viannia) parasites. Ann. Trop. Med. Parasitol. 91: 617-626. [DOI] [PubMed] [Google Scholar]
- 9.Desjeux, P. 1992. Human leishmaniases: epidemiology and public health aspects. World Health Stat. Q. 45:267-275. [PubMed] [Google Scholar]
- 10.Dujardin, J. C., A. L. Banuls, A. Llanos-Cuentas, E. Alvarez, S. DeDoncker, D. Jacquet, D. Le Ray, J. Arevalo, and M. Tibayrenc. 1995. Putative Leishmania hybrids in the Eastern Andean valley of Huanuco, Peru. Acta Trop. 59:293-307. [DOI] [PubMed] [Google Scholar]
- 11.El Tai, N. O., M. El Fari, I. Mauricio, M. A. Miles, L. Oskam, S. H. El Safi, W. H. Presber, and G. Schonian. 2001. Leishmania donovani: intraspecific polymorphisms of Sudanese isolates revealed by PCR-based analyses and DNA sequencing. Exp. Parasitol. 97:35-44. [DOI] [PubMed] [Google Scholar]
- 12.Evans, D. A., W. P. Kennedy, S. Elbihari, C. J. Chapman, V. Smith, and W. Peters. 1987. Hybrid formation within the genus Leishmania? Parasitologia 29:165-173. [PubMed] [Google Scholar]
- 13.Gomes, R. F., A. M. Macedo, S. D. Pena, and M. N. Melo. 1995. Leishmania (Viannia) braziliensis: genetic relationships between strains isolated from different areas of Brazil as revealed by DNA fingerprinting and RAPD. Exp. Parasitol. 80:681-687. [DOI] [PubMed] [Google Scholar]
- 14.Hide, M., A. L. Banuls, and M. Tibayrenc. 2001. Genetic heterogeneity and phylogenetic status of Leishmania (Leishmania) infantum zymodeme MON-1: epidemiological implications. Parasitology 123:425-432. [DOI] [PubMed] [Google Scholar]
- 15.Ibrahim, M. E., A. J. Smyth, M. H. Ali, D. C. Barker, and A. Kharazmi. 1994. The polymerase chain reaction can reveal the occurrence of naturally mixed infections with Leishmania parasites. Acta Trop. 57:327-332. [DOI] [PubMed] [Google Scholar]
- 16.Ishikawa, E. A., F. T. Silveira, A. L. Magalhaes, R. B. Guerra Jr., M. N. Melo, R. Gomes, T. G. Silveira, and J. J. Shaw. 2002. Genetic variation in populations of Leishmania species in Brazil. Trans. R. Soc. Trop. Med. Hyg. 96(Suppl. 1):111-121. [DOI] [PubMed] [Google Scholar]
- 17.Kreutzer, R. D., J. J. Yemma, M. Grogl, R. B. Tesh, and T. I. Martin. 1994. Evidence of sexual reproduction in the protozoan parasite Leishmania (Kinetoplastida: Trypanosomatidae). Am. J. Trop. Med. Hyg. 51:301-307. [DOI] [PubMed] [Google Scholar]
- 18.Marsden, P. D. 1986. Mucosal leishmaniasis (“espundia” Escomel, 1911). Trans. R. Soc. Trop. Med. Hyg. 80:859-876. [DOI] [PubMed] [Google Scholar]
- 19.Marsden, P. D., and T. C. Jones. 1985. Clinical manifestations, diagnosis and treatment of leishmaniasis. Elsevier Science Publisher, Amsterdam, The Netherlands.
- 20.Mauricio, I. L., M. K. Howard, J. R. Stothard, and M. A. Miles. 1999. Genomic diversity in the Leishmania donovani complex. Parasitology 119:237-246. [DOI] [PubMed] [Google Scholar]
- 21.Mebrahtu, Y. B., P. G. Lawyer, L. D. Hendricks, R. Muigai, C. N. Oster, P. V. Perkins, D. K. Koech, H. Pamba, and C. R. Roberts. 1991. Concurrent infection with Leishmania donovani and Leishmania major in a Kenyan patient: Clinical description and parasite characterization. Am. J. Trop. Med. Hyg. 45:290-296. [DOI] [PubMed] [Google Scholar]
- 22.Medina-Acosta, E., and G. A. Cross. 1993. Rapid isolation of DNA from trypanosomatid protozoa with a simple 'mini-prep' procedure. Mol. Biochem. Parasitol. 59:327-329. [DOI] [PubMed] [Google Scholar]
- 23.Myler, P. J., L. Audleman, T. de Vos, G. Hixson, P. Kiser, C. Lemley, C. Magness, E. Rickel, E. Sisk, S. Sunkin, S. Swartzell, T. Westlake, P. Bastien, G. Fu, A. Ivens, and K. Stuart. 1999. Leishmania major Friedlin chromosome 1 has an unusual distribution of protein-coding genes. Proc. Natl. Acad. Sci. 96:2902-2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reiner, N. E., R. Lo, A. Llanos-Cuentas, H. Guerra, L. L. Button, and W. R. McMaster. 1989. Genetic heterogeneity in Peruvian Leishmania isolates. Am. J. Trop. Med. Hyg. 41:416-421. [DOI] [PubMed] [Google Scholar]
- 25.Saravia, N. G., I. Segura, A. F. Holguin, C. Santrich, L. Valderrama, and C. Ocampo. 1998. Epidemiologic, genetic, and clinical associations among phenotypically distinct populations of Leishmania (Viannia) in Colombia. Am. J. Trop. Med. Hyg. 59:86-94. [DOI] [PubMed] [Google Scholar]
- 26.Saravia, N. G., K. Weigle, C. Navas, I. Segura, L. Valderrama, A. Z. Valencia, B. Escorcia, and D. McMahon-Pratt. 2002. Heterogeneity, geographic distribution, and pathogenicity of serodemes of Leishmania viannia in Colombia. Am. J. Trop. Med. Hyg. 66:738-744. [DOI] [PubMed] [Google Scholar]
- 27.Schonian, G., L. Schnur, M. el Fari, L. Oskam, A. A. Kolesnikov, W. Sokolowska-Kohler, and W. Presber. 2001. Genetic heterogeneity in the species Leishmania tropica revealed by different PCR-based methods. Trans. R. Soc. Trop. Med. Hyg. 95:217-224. [DOI] [PubMed] [Google Scholar]
- 28.Shaw, J. J., and R. Lainson. 1987. Ecology and epidemiology: new world. In W. Peters and R. Killick-Kendrick (ed.), Leishmaniasis in biology and medicine. Academic Press, New York, N.Y.
- 29.Silveira, F. T., R. Lainson, J. J. Shaw, and R. M. Ribeiro. 1984. Leishmaniose cutÂnea na Amazônia. Registro do primeiro caso humano de infecção mista, determinado por duas espécies distintas de leishmanias: Leishmania braziliensis e Leishmania mexicana amazonensis. Rev. Inst. Med. Trop. São Paulo 26: 272-275. [DOI] [PubMed] [Google Scholar]
- 30.Thomaz-Soccol, V., G. Lanotte, J. A. Rioux, F. Pratlong, A. Martini-Dumas, and E. Serres. 1993. Phylogenetic taxonomy of New World Leishmania. Ann. Parasitol. Hum. Comp. 68:104-106. [PubMed] [Google Scholar]
- 31.Thomaz-Soccol, V., G. Lanotte, J. A. Rioux, F. Pratlong, A. Martini-Dumas, and E. Serres. 1993. Monophyletic origin of the genus Leishmania Ross, 1903. Ann. Parasitol. Hum. Comp. 68:107-108. [PubMed] [Google Scholar]
- 32.Tibayrenc, M., and F. J. Ayala. 1999. Evolutionary genetics of Trypanosoma and Leishmania. Microbes Infect. 1:465-472. [DOI] [PubMed] [Google Scholar]
- 33.Tibayrenc, M., F. Kjellberg, and F. J. Ayala. 1990. A clonal theory of parasitic protozoa: the population structures of Entamoeba, Giardia, Leishmania, Naegleria, Plasmodium, Trichomonas, and Trypanosoma and their medical and taxonomical consequences. Proc. Natl. Acad. Sci. 87:2414-2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toledo, A., J. Martin-Sanchez, B. Pesson, C. Sanchiz-Marin, and F. Morillas-Marquez. 2002. Genetic variability within the species Leishmania infantum by RAPD. A lack of correlation with zymodeme structure. Mol. Biochem. Parasitol. 119:257-264. [DOI] [PubMed] [Google Scholar]
- 35.Turetz, M. L., P. R. Machado, A. I. Ko, F. Alves, A. Bittencourt, R. P. Almeida, N. Mobashery, W. D. Johnson, Jr., and E. M. Carvalho. 2002. Disseminated leishmaniasis: a new and emerging form of leishmaniasis observed in northeastern Brazil. J. Infect. Dis. 186: 1829-1834. [DOI] [PubMed] [Google Scholar]
- 36.Youssef, M. Y., M. M. Eissa, and S. T. el Mansoury. 1997. Evidence of sexual reproduction in the protozoan parasite Leishmania of the Old World. J. Egypt. Soc. Parasitol. 27:651-657. [PubMed] [Google Scholar]