Abstract
Background
The National Institutes of Health mandates the inclusion of ancestrally diverse populations into federally funded biomedical and clinical trials research. However, low participation of ethnic minorities in genetics-genomics research continues to be one of the most difficult aspects of conducting human subjects research.
Objective
This systematic review was conducted to document effective recruitment strategies that increase participation in genetics-genomics studies.
Methods
Extensive literature search strategies were employed to locate and appraise relevant literature reporting original data in which strategies to recruit African American adults into genetics-genomics research studies had been evaluated.
Results
Six studies published up to July, 2011 were included. Informal recruitment strategies for initial contact appeared to have a more positive impact on increasing recruitment and participation numbers than formal mailings of letters and postcards. Another key stratagem identified was participant-recruiter like-ancestry. Other methods such as monetary incentives and support of the research project by community leaders were not as effective.
Conclusions
Some strategies bolstered recruitment rates while others did not. More research is needed to determine the efficacy of recruitment strategies with African Americans.
Keywords: African Americans, Genomics, Genetic Research, Research Subject Recruitment, Systematic Review
In the United States, health disparities and inequalities among ethnic minority groups persist. Compared to European Americans, studies that track population health using national data continue to demonstrate that African Americans bear the greatest burden of chronic disease and the morbidity and mortality associated with those diseases (Frieden & Centers for Disease Control and Prevention [CDC], 2011). The increased vulnerability for disease development and the poor health outcomes associated with the most commonly observed chronic diseases such as diabetes, obesity, heart disease, and cancer is classically attributed to a combination of social, environmental and behavioral factors. However, variations in genetic architecture continue to emerge as important indicators of disease diversity and health outcomes.
The successful completion of the global Human Genome Project has supercharged genetic research. An article in BMC Genomics by Pohlhaus and Cook-Degan (2008), reported that between 2003–2007, nearly $3 billion public funding dollars were invested in genomics research worldwide. Of the 38 countries polled, the U.S. was the forerunner in spending. It was estimated that expenditures for extramural genomics research during this timeframe by the National Institutes of Health (NIH) alone was in the range of $571 million. Yet, when comparing the significant disparities associated with African American health, and the representation of African Americans in the general population, it is clear that much of the publicly funded research in genetics and genomics has been conducted with suboptimal representations of members from this population group. While African Americans approximate 13% of the total U.S. populace (U.S. Census Bureau, 2010), data suggest African Americans comprise 0% to 5% of the sample sizes in genetics research studies (Murphy & Thompson, 2009).
Similar to other health related research, effective recruitment of African American participants appears to be one of the most difficult aspects of conducting genetics-genomics research. Many of the beliefs and attitudes that critically impact the willingness of African Americans to participate in general healthcare research are also applicable to this field of research. Reasons for non-participation typically include mistrust of the scientific community, unethical conduct of investigators, and violation of human rights (Durant, Davis, St. George, Williams, Blumenthal, & Corbie-Smith, 2007; Harris, Ahluwalia, Catley, Okuyemi, Mayo, & Resnicow, 2003). Correspondingly, but unlike general health research, there are unique ethical, legal and social nuances associated with genetics-genomics research that present an additional challenge in recruiting subjects for participation (Conley, Doerr, & Vorhaus, 2010; McGuire, Colgrove, Whitney, Diaz, Bustillos, & Versalovic, 2008; Slaughter, 2008). One such example is the question of property rights with respect to genetic material and genetic information. Another consideration for prospective study participants is the potential for discrimination and marginalization that may occur as a result of study findings. The ambiguity of source compensation for the use of biological materials that lead to long-term commercial and or scientific success-advancements (e.g., immortal cell lines) may also impact ones willingness to participate in genetics-genomics research.
The severity of the recruitment problem and the research implications of low African American participation rates have not gone unnoticed. From a scientific standpoint, many studies have reported links between population (intra) and group (inter) specific genetic variation with differential disease risks, treatment options, and health outcomes (Chang et al., 2011; Crawford et al., 2006; Yang et al., 2011). Along this same vein, the lack of subjects across relevant populations can potentially jeopardize data generalizability and limit study findings. From a policy perspective several national initiatives have been implemented to address the issues of genetic testing protections and minority recruitment. Examples include the 1993 NIH Revitalization Act which mandates the inclusion of minorities in research funded by this organization. The 2008 genetic information nondiscrimination act (GINA) protects Americans against discrimination based on their genetic information when it comes to health insurance and employment. Although nationalized protections and assurances are important factors in recruiting members of ancestrally diverse groups, population-specific recruitment strategies may better serve efforts to increase the enrollment numbers for underrepresented populations.
The extant literature has suggested a plethora of determinants that facilitate the recruitment and enrollment of ancestrally diverse populations into research studies. Among these are face-to-face contact, monetary incentives, and utilization of African American research team members (Satia, Galanko, & Rimer, 2005). We must, however, move beyond basic research toward validation research. In this manner, scientists can evaluate how well factors identified as positive influences in the recruitment of minorities work. Therefore, our systematic review identified published studies reporting efficacious and/or effective recruitment strategies that increase the enrollment of African-Americans into genetics-genomic research.
METHODS
Conceptual Framework
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (Liberati, Altman, Tetzlaff, Mulrow, Gotzsche, Ioannidis et al., 2009), Centre for Reviews and Dissemination (CRD) 10 Guidelines (2009), and a modified population intervention comparison and outcomes (PICO) model, were used to guide the assessment and integration of literature for the systematic review. Authors and reviewers specified a priori: a formulated question of interest, key terms that would direct the subsequent literature search, and the protocol for identification and screening of potentially relevant studies (Khan, Kunz, Kleijnen, & Antes, 2003; Pai, McCullough, Gorman, Pai, Enanoria, Kennedy et al., 2004).
Derived from work conducted by Diers (1979) and more recently, those of Lutz, Anderson, Pridham, Riesch, and Becker (2009), a Discovery-Assessment-Intervention design component helped facilitate the comparative organization, evaluation and integration of relevant study findings into the review. Discovery studies were qualitative in nature, and identified specific phenomena or concepts affecting recruitment and retention. Assessment studies focus on indicators, correlates or predictors of participation in genetics-genomics research. Intervention studies involve case-control or cohort approaches and strategies that effect recruitment and retention.
We also determined that findings from this review may have important implications for social change and policy campaigns for genetics-genomics research with vulnerable populations. Therefore, our approach incorporated some of the techniques of interpretive synthesis described by Lomas (2005). This model supports the accumulation, integration and interpretation of knowledge beyond the summation of scientific information often associated with formal reviews of clinical interventions and outcomes research. Contrary to the search and screening protocols, the preparation (analyses) and presentation of findings are not constrained to a pre-specified design. In light of the available material, results can be iteratively tailored to accommodate all potential users of such information.
Aims
The aims of this systematic review are to (a) provide a critical interpretive synthesis of the evidence in the literature identifying approaches and stratagems that effectually influence the recruitment and retention of African Americans in genetics-genomics research; and (b) illuminate gaps in recruitment and retention strategies geared toward facilitating representation of African Americans in genetics-genomics research.
Eligibility Criteria
To be included, an article had to report original data on participatory influences in genetics-genomics research among African Americans (18+ years). In the event that a sample was comprised of ancestrally diverse individuals, findings for African Americans had to be reported separately. Outcome measures were defined as (a) a comparative numeric measurement between two or more recruitment and/or retention interventions; or (b) a comparative numeric measurement of recruitment and/or retention interventions between two or more groups; or (c) identification of factors (qualitative or quantitative) that measurably impact recruitment and retention in genetics-genomics research. Studies were excluded if their results were specific to a client group that did not meet our inclusion criteria (e.g., child or adolescent) and could not be extrapolated to adults. Editorials, letters, books or book chapters, dissertations, theses, and conference papers were also excluded. Where systematic review or meta-analysis was included, we did not examine each paper contained within it.
Definition of Genetics-Genomics Research
For the purpose of this systematic review, genetics-genomics research is defined as a systematic investigation involving humans that incorporates the collection and analysis (testing) of DNA or other gene based technology; or the collection, analysis and quantification (assay) of target materials or substances in human body fluid; or the recruitment of individuals into registries or other point of access repositories for the purpose of current and future biological or molecular testing and assaying.
Identification of Studies
In November, 2010, the search strategy was executed by a single research librarian under direction of a co-author (V.J.). An inclusive string of Medical Subjects Heading (MeSH) and free terms [(Genetic Research AND (African American* OR Minority Group* OR Ethnic Group* OR Blacks) AND (Research Subjects* OR Patient Selection OR Patient Acceptance of Health Care/ethnology OR Patient P OR Trust* OR recruitment OR retention OR retain*)) OR (MM “Genetic Research” AND (African American* OR Minority Group* OR Ethnic Group OR Blacks))] was developed to query five electronic literature databases [Medline-PubMed, (Cumulative Index for Nursing and Allied Health (CINAHL), Psychlnfo, EMBASE, Education Resources Information Center (ERIC), and The Cochran Database of Systematic Reviews (CDSR)] for reports published from January, 2000 through November, 2010. There were no restrictions on study design; however limitations included human participants and English language. In addition, the reference sections of potentially relevant studies were reviewed as was a catalogue of secondary research amassed through various contributions of team members. Citation searches were carried out for all eligible articles using Web of Knowledge and Goggle Scholar. Searches were updated periodically by all available alerts in the intervening interval to the end of July, 2011.
Study Selection
Screening of Citations
First, 325 titles and abstracts for potentially eligible citations were divided (162/163) and blind reviewed independently against the inclusion criteria by a team of two reviewers each (V.J. and Y.P.Y.) or (E.T. and M.A.). After this process was completed, 72 full text articles were obtained, abstracted in RefWorks and assessed for methodological quality for further evaluation in the review. Whenever it was not clear whether a citation was appropriate, it was included. Subsequently, any disagreements regarding citation inclusion were resolved by consensus between the authors.
Assessment of Methodological Quality
The methodological quality of the 72 full-text articles were assessed by two authors (E.T. and Y.P.Y) or (V.J. and I.J.S.). Separate criteria were used to evaluate qualitative and quantitative research. These criteria were derived from recommended approaches (Cohen & Crabtree, 2008; Duffy, 2005; Grimshaw, McAuley, Bero, Grilli, Oxman, Ramsay et al., 2003; Guyatt, Sackett, Sinclair, Hayward, Cook, & Cook, 1995; Liberati et al., 2009) and additional items specific to this review. Quantitative papers were assessed on sample size (African American); design (including clarity of hypothesis-research question, methodology, relevance of participants to aims of the review); and analysis (including plan, reporting, clarity of results). Qualitative papers were assessed on sampling, data collection, data inspection, data analysis, and use of supportive quantitative methods. Following the full text review 43 were included in the final review.
Data Abstraction
Details of the studies were abstracted by the authors and recorded on a data abstraction form. Key data elements abstracted included (1) author data, (2) year, (3) design type, (4) genetic (disease) focus, (5) purpose, (6) hypotheses-research questions, (7) recruitment-retention techniques, interventions or factors, (8) sample-recipients, (9) demographic characteristics, and (10) ratio differences.
RESULTS
Description of the Studies
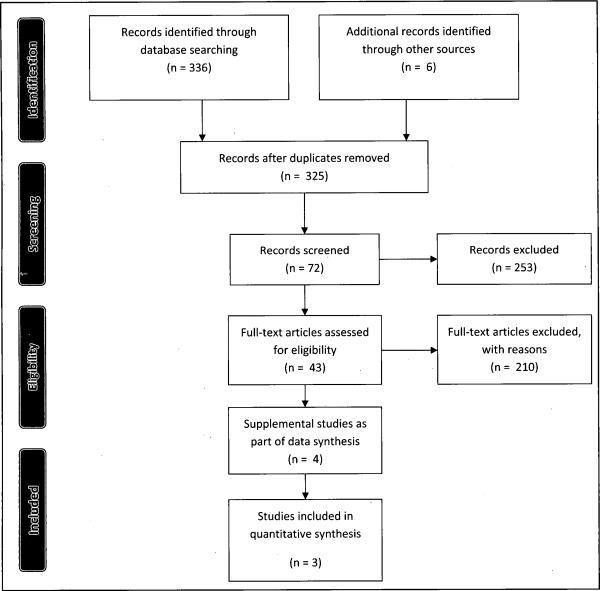
As shown in Figure 1, the vast majority of the studies identified for possible inclusion were excluded because they did not meet one or more of the established review criteria. Although six studies were used in the final analysis, only three of these studies met the criteria for inclusion in the current systematic review. Using community based recruitment strategies; Study 1 (Sadler, 2005) reported enrollment differences by group based on complexity of data collection requirements. Study 2 (Cabral, 2003) provided quantitative measures of recruitment success between case and control groups in a lung cancer study. Study 3 Olsen, 2008 described recruitment yield using an African American sorority as a community partner. The remaining studies (Crider, 2006; Aliyu, 2006; Bussey-Jones, 2010; Spruill, 2010) provided what we determined were significant insights into factors that were both specific and important to the uniqueness of recruiting African Americans into genetics-genomics research.
Figure 1.
Flow diagram of inclusion studies modified from Moher, D., Liberati, A., Tetzlaff, J., Altman, D.G., The PRISM Group (2009). Preferred reporting items for systematic review analysis: The PRISM statement. PLoS Med
Study Findings
Using population strategies
When initial contact for possible study enrollment was made by phone, recruitment success rates ranged from 63% to 91%. Study enrollment was significantly less, 3% through 19%, when the initial contact for participation was made using a mailed letter or postcard.
Using community-based strategies
Resulted in highly variable enrollment rates ranging between 1% through 82%. The most significant factor to this variability appeared to be ancestry of the recruitment team. Studies indicate that even when community leadership actively encouraged constituency involvement, impact on the decision to participate appeared to be negligible. In contrast, one study reported that 76% of the individuals contacted agreed to provide biological data for the study when African Americans solicited the consent and initiated the research protocol. Another study that had leadership buy-in but used non-African Americans as primary team members for recruitment and consent had only a 1% (3/279) enrollment.
Using supplemental strategies
Of these studies, only one offered a monetary incentive for participation. However, this incentive was not shown to make a difference in participation rates. In spite of an additional $20 incentive, only 35% of those enrolled in the study agreed to provide a biological sample. We felt that this may have been a result of having to provide a specimen for the entire family, father and any children. We hypothesized that an adult may consent to certain aspects of research that they would not necessarily allow for their children. However, comments related to refusal did not support this fear of child exploitation hypothesis. Concerns for privacy, forbidden by family, and disliked genetics were some of the reasons provided for non-participation.
Additional Factors Affecting Recruitment
Participants were significantly more likely to answer questionnaires than provide biological samples. However, the type of biological sample that was required by the study protocol appeared to significantly impact the decision to participate. A study reported that 79% of 153 participants would agree to provide a blood sample but 91% would provide a saliva sample. Compared to European Americans, African Americans consistently and to a greater extent feel that African Americans shouldn't do research until we know how the info obtained from such studies will be used (67% vs. 44%). Other areas of concerns for African Americans, comparatively, were: the government can't be trusted to regulate use of genetic information (49% vs. 35%); research participants may be deceived by researchers (44% vs. 27%); researchers do harmful experiments without patient's knowledge (40% vs. 22%); researchers want to know more than they need to know (36% vs. 15%); lose insurance coverage by taking part in study (28% vs. 19%); and medical researchers use minorities as guinea pigs (27% vs. 6%).
DISCUSSION
In the studies reviewed here, initial face-to-face contact regarding study inclusion, African American ancestry of the research team members, and the least invasive method of obtaining a biological sample appears to improve participation statistics among African American in genetics-genomics research. Studies of other factors impacting recruitment (e.g., deception, exploitation, trust) also suggest some influence on recruitment but the study designs prevented a true assessment of their impact. This is the first known review of the effect of recruitment techniques on recruitment rates in genetics-genomics research among African Americans.
STRENGTHS AND LIMITATIONS
The major strength of this review was its extensive systematic search of the published literature. The lack of studies that rigorously evaluate recruitment methods suggests that such studies are rarely conducted. Sociodemographic factors such as gender, socioeconomic status, and education level may also influence recruitment numbers. However, few studies had empirical data and/or did not report their data in a manner that facilitated comparative evaluation of recruitment outcomes, Meta-analysis would have allowed the investigators to draw conclusions by estimating both study-specific intervention effects and effect size, thereby strengthening our findings. But the current studies did not report data in a systematic way so that aggregation and evaluation could be conducted across studies.
SIGNIFICANCE OF THIS STUDY AND CONCLUSIONS
The findings of this study demonstrate the need for studies to rigorously evaluate the efficacy of recruitment methods among vulnerable populations in genetics-genomics research. Our findings indicate that strategies such as face-to-face recruitment can positively impact recruitment rates. However, other factors such as monetary incentive, consistently identified in literature to enhance recruitment, may not be as effective in recruiting African Americans into genetics-genomics research studies. Future studies should endeavor to implement studies that comparably evaluate the effectiveness of recruitment strategies among African Americans.
Acknowledgements
This publication was made possible by Grant Number T32 NR07110 from the National Institute for Nursing Research (NINR), a part of the National Institutes of Health (NIH), Janet Williams, PhD, University of Iowa, Director, The contents of the manuscript are solely the responsibility of the authors and do not necessarily represent the official views of NIH. The early contributions of Dr. Maxine Adegbola, University of Iowa Posdoctoral Fellow, and Carolyn Loper, RN, OUHSC Graduate Nursing student, are greatly appreciated.
REFERENCES
- Aliyu MH, Calkins ME, Swanson CL, Jr, Lyons PD, Savage RM, May R, PAARTNERS Study Group Project among african americans to explore risks for schizophrenia (PAARTNERS): Recruitment and assessment methods. Schizophrenia Research. 2006;87(1–3):32–44. doi: 10.1016/j.schres.2006.06.027. [DOI] [PubMed] [Google Scholar]
- Bussey-Jones J, Garrett J, Henderson G, Moloney M, Blumenthal C, Corbie-Smith G. The role of race and trust in tissue/blood donation for genetic research. Genetics in Medicine: Official Journal of the American College of Medical Genetics. 2010;12(2):116–121. doi: 10.1097/GIM.0b013e3181cd6689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral DN, Nápoles-Springer AM, Miike R, McMillan A, Sison JD, Wrensch MR, Wiencke JK. Population-and community-based recruitment of African Americans and Latinos. American Journal of Epidemiology. 2003;158(3):272. doi: 10.1093/aje/kwg138. [DOI] [PubMed] [Google Scholar]
- Centre for Reviews and Dissemination . Systematic reviews: CRD's guidance for undertaking reviews in health care. University of York NHS Centre for Reviews and Dissemination; Layerthorpe, York, UK: 2009. [Google Scholar]
- Chang MH, Ned RM, Hong Y, Yesupriya A, Yang Q, Liu T, Dowling NF. Race/Ethnic variation in the association of lipid-related genetic variants with blood lipids in the adult U.S. population. Circulation.Cardiovascular Genetics. 2011 doi: 10.1161/CIRCGENETICS.111.959577. [DOI] [PubMed] [Google Scholar]
- Cohen DJ, Crabtree BF. Evaluative criteria for qualitative research in health care: Controversies and recommendations. Annals of Family Medicine. 2008;6(4):331–339. doi: 10.1370/afm.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley JM, Doerr AK, Vorhaus DB. Enabling responsible public genomics. Health Matrix (Cleveland, Ohio : 1991) 2010;20(2):325–385. [PubMed] [Google Scholar]
- Crawford DC, Sanders CL, Qin X, Smith JD, Shephard C, Wong M, Nickerson DA. Genetic variation is associated with C-reactive protein levels in the third national health and nutrition examination survey. Circulation. 2006;114(23):2458–2465. doi: 10.1161/CIRCULATIONAHA.106.615740. [DOI] [PubMed] [Google Scholar]
- Crider KS, Reefhuis J, Woomert A, Honein MA. Racial and ethnic disparity in participation in DNA collection at the atlanta site of the national birth defects prevention study. American Journal of Epidemiology. 2006;164(8):805–812. doi: 10.1093/aje/kwj264. [DOI] [PubMed] [Google Scholar]
- Diers D. Research in nursing practice. Lippincott; Philadelphia: 1979. [Google Scholar]
- Duffy JR. Critically appraising quantitative research. Nursing & Health Sciences. 2005;7(4):281–283. doi: 10.1111/j.1442-2018.2005.00248.x. [DOI] [PubMed] [Google Scholar]
- Durant RW, Davis RB, St George DM, Williams IC, Blumenthal C, Corbie-Smith GM. Participation in research studies: Factors associated with failing to meet minority recruitment goals. Annals of Epidemiology. 2007;17(8):634–642. doi: 10.1016/j.annepidem.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frieden TR, Centers for Disease Control and Prevention (CDC) CDC health disparities and inequalities report - united states, 2011. MMWR Surveillance Summary. 2011;60(Suppl):1–116. [PubMed] [Google Scholar]
- Grimshaw J, McAuley LM, Bero LA, Grilli R, Oxman AD, Ramsay C, et al. Systematic reviews of the effectiveness of quality improvement strategies and programmes. Quality & Safety in Health Care. 2003;12(4):298–303. doi: 10.1136/qhc.12.4.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyatt GH, Sackett DL, Sinclair JC, Hayward R, Cook DJ, Cook RJ. Users guide to the medical literature. IX. A method for grading health care recommendations. JAMA. 1995;274:1800–1804. doi: 10.1001/jama.274.22.1800. [DOI] [PubMed] [Google Scholar]
- Harris KJ, Ahluwalia JS, Catley D, Okuyemi KS, Mayo MS, Resnicow K. Successful recruitment of minorities into clinical trials: The kick it at swope project. Nicotine & Tobacco Research : Official Journal of the Society for Research on Nicotine and Tobacco. 2003;5(4):575–584. doi: 10.1080/1462220031000118540. [DOI] [PubMed] [Google Scholar]
- Khan KS, Kunz R, Kleijnen J, Antes G. Five steps to conducting a systematic review. Journal of the Royal Society of Medicine. 2003;96(3):118–121. doi: 10.1258/jrsm.96.3.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. Annals of Internal Medicine. 2009;151(4):W65–94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- Lomas J. Using research to inform healthcare managers' and policy makers' questions: From summative to interpretive synthesis. Healthcare Policy = Politiques De Sante. 2005;1(1):55–71. [PMC free article] [PubMed] [Google Scholar]
- Lutz KE, Anderson LS, Pridham KA, Riesch SK, Becker PT. Furthering the understanding of parent-child relationships: A nursing scholarship review series. part 1: Introduction. Journal for Specialists in Pediatric Nursing : JSPN. 2009;14(4):256–261. doi: 10.1111/j.1744-6155.2009.00206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire AL, Colgrove J, Whitney SN, Diaz CM, Bustillos D, Versalovic J. Ethical, legal, and social considerations in conducting the human microbiome project. Genome Research. 2008;18(12):1861–1864. doi: 10.1101/gr.081653.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy E, Thompson A. An exploration of attitudes among black americans towards psychiatric genetic research. Psychiatry. 2009;72(2):177–194. doi: 10.1521/psyc.2009.72.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen SJ, Malvern KT, May BJ, Jenkins IL, Griffin CA. Partnership with an african american sorority to enhance participation in cancer genetics research. Community Genetics. 2008;11(4):201–207. doi: 10.1159/000116880. [DOI] [PubMed] [Google Scholar]
- Pai M, McCulloch M, Gorman JD, Pai N, Enanoria W, Kennedy G, et al. Systematic reviews and meta-analyses: An illustrated, step-by-step guide. The National Medical Journal of India. 2004;17(2):86–95. [PubMed] [Google Scholar]
- Pohlhaus JR, Cook-Deegan RM. Genomics research: World survey of public funding. BMC Genomics. 2008;9:472. doi: 10.1186/1471-2164-9-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler GR, Peterson M, Wasserman L, Mills P, Malcame VL, Rock C, et al. Recruiting research participants at community education sites. Journal of Cancer Education. 2005;20(4):235–239. doi: 10.1207/s15430154jce2004_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satia JA, Galanko JA, Rimer BK. Methods and strategies to recruit african americans into cancer prevention surveillance studies. Cancer Epidemiology Biomarkers & Prevention. 2005;14(3):718. doi: 10.1158/1055-9965.EPI-04-0132. [DOI] [PubMed] [Google Scholar]
- Slaughter LM. The genetic information nondiscrimination act: Why your personal genetics are still vulnerable to discrimination. The Surgical Clinics of North America. 2008;88(4):723–38. vi. doi: 10.1016/j.suc.2008.04.004. [DOI] [PubMed] [Google Scholar]
- U.S. Census Bureau 2010 census data. 2010 Retrieved September 1, 2011, from http://2010.census.gov/2010census/data/
- Yang JJ, Cheng C, Devidas M, Cao X, Fan Y, Campana D, et al. Ancestry and pharmacogenomics of relapse in acute lymphoblastic leukemia. Nature Genetics. 2011;43(3):237–241. doi: 10.1038/ng.763. [DOI] [PMC free article] [PubMed] [Google Scholar]